Abstract
Introduction:
Kaposi sarcoma herpes virus (KSHV) is associated with several diseases including Kaposi sarcoma, a form of multicentric Castleman’s disease, primary effusion lymphoma, and an inflammatory cytokine syndrome. These KSHV-associated diseases (KAD) can present with heterogenous signs and symptoms that are often associated with cytokine dysregulation that may result in multiorgan dysfunction. Inability to promptly diagnose and treat these conditions can result in long term complications and mortality.
Areas covered:
Existing epidemiological subtypes of existing KSHV-associated diseases, specifically Kaposi sarcoma as well as the incidence of several KSHV-associated disorders are described. We review the KSHV latent and lytic phases as they correlate with KSHV-associated diseases. Given the complicated presentations, we discuss the clinical manifestations, current diagnostic criteria, existing treatment algorithms for individual KAD, and when they occur concurrently. With emerging evidence on the virus and host interactions, we evaluate novel approaches for treatment of KAD. An extensive literature search was conducted to support these findings.
Expert opinion:
KSHV leads to complex and concurrent disease processes that are often underdiagnosed both in the United States and worldwide. New therapies that exist for many of these conditions focus on chemotherapy-sparing options that seek to target the underlying viral pathogenesis or immunotherapy strategies.
Keywords: Kaposi-sarcoma herpesvirus, KSHV-associated diseases (KAD), multicentric Castleman disease (MCD), primary effusion lymphoma (PEL), Kaposi sarcoma (KS), KSHV-inflammatory cytokine syndrome (KICS)
1. Introduction
Kaposi sarcoma herpesvirus (KSHV) is an oncogenic virus that is associated with the several KSHV-associated diseases (KAD) that cause inflammation and cytokine dysregulation: Kaposi sarcoma (KS), a plasmablastic form of multicentric Castleman disease (MCD), primary effusion lymphoma (PEL), KSHV-associated large cell lymphoma (KSHV-LCL), and an inflammatory cytokine syndrome (KICS) [1–4]. These conditions can present as singular entities but can also occur together. Concurrent KAD present with inflammatory signs and symptoms, often resulting in multiorgan dysfunction and death if not appropriately diagnosed and treated [5]. Within the United States, KAD often occur among people with HIV (PWH).
The prevalence of KSHV varies by geography with highest prevalence in areas of sub-Saharan Africa and along the Mediterranean[6]. Within the United States, KSHV is prevalent among men who have sex with men (MSM)[7]. KAD manifestations vary worldwide and conditions such as MCD or PEL are underdiagnosed, particularly in sub-Saharan Africa where KS is one of the most common tumors overall due to the high prevalence of both KSHV and HIV[8]. In 2020, KS was the leading cause of cancer incidence among men less than 65 years of age in Malawi, Mozambique, and Uganda, and the leading cause of cancer mortality among men in Mozambique and Uganda[9]. Worldwide, KAD often present in individuals with human immunodeficiency virus (HIV) and can emerge despite well-controlled HIV and varying degrees of immunosuppression. Treatment of HIV with antiretroviral therapy (ART) is paramount among those with diagnosed with KAD and HIV. Beyond this distinction, those with and without HIV who have KAD undergo the same diagnostic procedures and have similar treatment options.
The diagnosis and treatment of KSHV-associated disease can be challenging, especially when these conditions occur together and among individuals who also have a risk of opportunistic infections. KAD may be underdiagnosed or undertreated in the immunodeficient population. As an example, fevers and night sweats in a patient with KS and HIV may be attributed to HIV rather than a concurrent diagnosis of MCD or PEL. In a study from our center investigating patients with MCD, a proportion of patients were initially referred for a diagnosis of KS and we diagnosed concurrent MCD[5]. In such cases, existing diagnostic work-up is rigorous, potentially requiring imaging, endoscopic evaluation, tissue biopsies, and always requires expert pathology review (Figure 1), which can be problematic in low-resource settings. Here, we provide an overview of each of these KAD, considerations for management and the rationale for selected ongoing clinical trial options.
Figure 1.
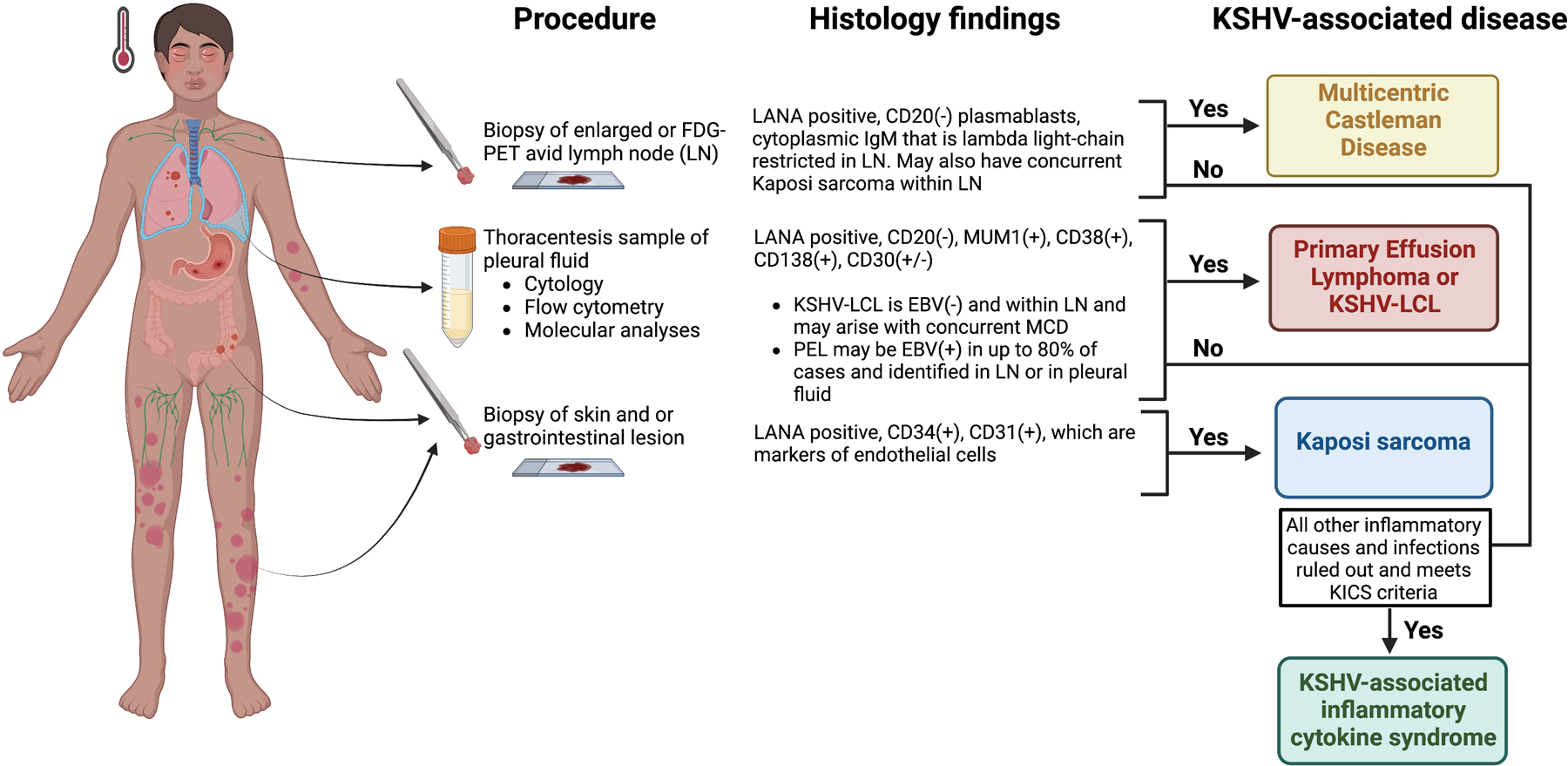
Diagnostic criteria for KSHV-associated disorders.
2. Methods
We searched PubMed search for clinical trials, meta-analyses and guidelines of major professional societies on the diagnosis and management of KSHV-associated diseases. Terms that were used consisted of, “Kaposi sarcoma,” “Multicentric Castleman disease,” “KSHV associated diseases,” “primary effusion lymphoma,” “KICS,” “HHV-8,” and “KSHV lytic and latent life cycle.”
3. KSHV life cycle
The discovery of KSHV, the necessary etiologic agent of KS and other KAD, was discovered in 1994 by Chang and colleagues[10]. KSHV is a life-long persistent infection that manifests with varying disease presentations. This depends upon viral genetic invasion of host cells affecting cell proliferation, apoptosis, angiogenesis, and subsequent reactive host inflammatory reactions leading to further immunosuppression and worsening viral oncogenesis[11]. KSHV has 2 distinct infection programs: latency and lytic replication.
KSHV may bind and infect several cell types including B cells, epithelial cells, monocytes and fibroblasts[12]. To facilitate entry into endothelial cells, KSHV may bind to several host cell surface receptors such as integrins, heparan sulfate and tyrosine protein kinase receptor EPHA2[13]. During latency, which is thought to be the default pathway, viral gene expression is tightly regulated, and the viral genome expresses a minimal number of genes required to maintain infection[12]. These genes are clustered together in a locus known as the latency transcription unit. The viral genome on entry to the nucleus can remain latent as an episome that is tethered to host chromosomes via the latency-associated nuclear protein (LANA) protein. This results in replication of the viral genome alongside the host genome during cell division[14].
The second state is lytic replication where all viral genes are expressed, and this results in the progeny of virions. In PEL and KS, the infected cells are predominantly in a latent state, with up to 5% of cells undergoing lytic replication at any time[15]. In MCD, KSHV is present in a more lytic state; a large proportion of plasmablasts express KSHV-encoded homolog viral interleukin 6 (vIL6, a viral homolog of human interleukin (IL)-6) and a proportion of these also express the lytic gene repertoire[16,17]. For all KAD, paracrine effects of infected cells and neighboring immune cells add further complexity to the emergence of disease, symptomatic course, and outcomes.
4. Kaposi sarcoma (KS)
4.1. Epidemiology
Kaposi sarcoma (KS) was first described in 1872 by Moritz Kaposi. Initially observed among elderly men of Mediterranean origin, Kaposi noted “nodules the size of shot, peas, or hazelnuts and brown red to blue-red in colour develop in the skin”[18]. Since these initial descriptions of classic KS, four other epidemiologic subtypes have been identified. This includes endemic KS seen among adults and children in sub-Saharan Africa[19], iatrogenic KS observed in those with who have received organ transplantation[20], epidemic KS is associated with HIV, and finally a subtype described in 2008 among MSM who are HIV-negative[21]. A recent study from Uganda studying 200 participants with epidemic and endemic forms of KS identified that the course of endemic KS is likely to take a more indolent course as compared to HIV-associated KS[22]. Disease manifestations, course, and severity may vary across subtypes of KS, but cutaneous disease (particularly manifesting in the lower extremities) is a common feature irrespective of subtype (Table 1). However, in patients with HIV-associated or post-transplant KS, there may be cases where there is visceral involvement of lymph nodes, gastrointestinal (GI), or respiratory systems without cutaneous disease.
Table 1:
Epidemiologic subtypes of Kaposi sarcoma and their disease manifestations
| Disease Manifestations | Populations Impacted | |
|---|---|---|
| Classic KS | Lesions on the skin and specifically occur in distal extremities. Rarer occurrence in mucus membranes, viscera or lymph nodes. |
Middle aged to older men; Mediterranean or Central/Eastern European ancestry Cisgender men more commonly affected |
| Endemic KS | Nodular lesions typically affecting the lower extremities that can be aggressive. Lymphedema is common. |
Individuals from sub-Saharan Africa Impacts adults and children |
| HIV-associated KS | Cutaneous involvement may occur over lower extremities, but can affect other parts of the body Visceral involvement, lesions may appear raised, discolored and/or ulcerated: • Oral – hard and soft palate • GI - lesions can be asymptomatic or present as nausea/vomiting, bleeding, weight loss • Pulmonary - dyspnea, cough, or hemoptysis. Lymphedema is common |
Persons living with HIV. Severity of disease may be related to underlying immunosuppression, with lower CD4 count associated with more sever disease. |
| Iatrogenic/Transplant- associated KS | Lesions are often localized to the skin with visceral organ involvement less commonly involved | Solid organ and hematopoietic stem cell transplant recipients |
| HIV-negative (non-epidemic KS) | Lesions limited to the skin that are localized Less associated with lymphedema |
Younger, non-immunocompromised MSM |
4.2. Diagnosis
Confirmation of KS requires a biopsy of the skin and histological features include the presence of spindle cells that are positive for KSHV latency-associated nuclear antigen staining by immunohistochemistry. If patients with KS present with respiratory symptoms (such as dyspnea, cough or hemoptysis) or GI concerns (including melaena, weight loss), this should prompt additional diagnostic assessments. Bronchoscopy is essential for patients with respiratory symptoms, particularly among those who are substantially immunosuppressed, to diagnose co-existent opportunistic infections and to visualize pulmonary KS which are characteristically red or violet macules or papules. KS has been found to have varying radiological findings from hilar and mediastinal adenopathy to bronchial thickening and irregular recticular peribronchovascular opacities[23]. As KS lesions are inherently vascular and may bleed, biopsy of possible pulmonary lesions is not recommended. Conversely, suspected KS lesions in the GI tract should be biopsied by upper or lower GI endoscopy, as in some cases, these lesions can resemble infectious processes such as cytomegalovirus or other neoplasms[24]. Therefore, it is important to obtain histopathology and immunohistochemistry testing to differentiate these processes. Positron emission tomography/computed tomography using F-18-Fluorodeoxyglucose (FDG PET/CT) may have a role in noting the extent of KS in some sites such lymph node, lung and bone[25]. However, such imaging modalities may also indicate concurrent KSHV-associated processes such as MCD, which may guide other diagnostic procedures such as lymph node sampling.
If patients present with concurrent systemic symptoms or deteriorating multiorgan dysfunction, other KAD or other opportunistic infections should be considered. Among those initiating ART, KS immune reconstitution inflammatory syndrome (KS-IRIS) may occur when there is the suppression of HIV VL and/or increase in CD4 T cell count by more than 50 cells/µL compared to pre-ART levels within the first 12 weeks of ART initiation[26,27]. Currently, there are no specific biomarkers to clearly delineate between KS-IRIS and other KAD. However, a worsening burden of KS with associated symptoms, particularly when there is visceral involvement should prompt urgent oncological treatment of KS and investigation of other KAD or causes of multi-organ dysfunction so appropriate treatment can be given. Patients with KS limited to the skin and/or lymph nodes and/or minimal oral disease are classified as having stage T0 disease, and patients with edema, ulceration, extensive oral disease, or visceral KS as having stage T1 disease. Specifically, for those with HIV-associated KS, a stage-stratified approach to treatment is feasible where patients with minimal disease receive ART alone and those with T1 disease and extensive cutaneous disease or who otherwise require rapid disease resolution receive ART and specific KS-directed treatment, such as chemotherapy[27].
4.3. Treatment
Despite the varying epidemiologic subtypes, systemic therapies directed for KS are largely similar. There are specific considerations to considerations depending on epidemiologic subtype that guide KS management[28]. Individuals with HIV-associated KS must receive ART. Those with post-transplant associated KS may require reduction of immunosuppressive therapy that may contribute to the onset of KS. There are three Untied States Food and Drug Administration-approved therapies for the oncologic management of KS that are commonly in use; interferon-alpha was approved during the beginning of the HIV epidemic but is rarely used now because of a poorer activity and toxicity profile. Pegylated liposomal doxorubicin (LD) is a form of doxorubicin, an anthracycline chemotherapy, which is encapsulated in long-circulating stealth liposomes that prolong circulation times. Randomized controlled trials in the mid-1990s comparing single agent LD against combination chemotherapy regimens demonstrated response rates of 40–50%. Among individuals with HIV, many of these studies were done in an era prior to combination ART. Subsequent studies combining ART with LD as compared to ART alone resulted in a response rate of 76%[29–31]. In 1997, the taxane chemotherapy, paclitaxel, was licensed for use in HIV-associated KS. Response rates vary from 56–69% in individuals with HIV[32–36]. In a recent study of 11 trial sites including areas of sub-Saharan Africa and Brazil, a randomized non-inferiority study demonstrated paclitaxel with ART had superiority to oral etoposide plus ART and bleomycin and vincristine plus ART[37]. The most recently approved treatment is pomalidomide, a third-generation immunomodulatory oral agent. It was approved for KS patients with and without HIV[38]. Pomalidomide, which has antiangiogenic properties, may enhance T and NK cell activation, or work through other mechanisms such as effects on angiogenesis [39]. Findings from an early phase study of 28 participants with and without HIV identified responses in both groups, and in 2020, pomalidomide was approved by the FDA for KS based on this study. Twelve of 18 HIV-positive (67%; 95% CI, 41–87%) and 8 of 10 HIV-negative participants (80%; 95% CI, 44%−97%) had a KS response [40]. Though the approval of pomalidomide was made based on a Phase I/II study, a confirmatory study in the US and in Sub-Saharan Africa is underway. The effects observed in the pomalidomide study may also be a class effect as a study of lenalidomide in HIV-associated KS led by the AIDS Malignancy Consortium (AMC) demonstrated clinical benefit [41]. Among 25 evaluable participants receiving lenalidomide at the recommended phase II dose, 60% of these individuals had a partial response. It is important to note that unlike other cancers, there is no fixed set of cycles of treatment for KS. Instead, oncologists will be guided by the treatment response to KS and impact on other symptomatic parameters such as edema and pain. Specifically for the management of transplant-associated KS, it is important to consider reduction or discontinuation of immunosuppressive therapies, which must be carefully balanced with the risk of graft rejection. In a study of 15 patients who had received renal transplantation, the initiation of sirolimus, an mTOR inhibitor, led to resolution of cutaneous KS lesions within 3 months[42].
In patients with HIV-associated KS who are newly diagnosed and present with low CD4+ T cell counts at baseline, patients may have improvement in their KS with appropriate treatment, immune reconstitution, and incrementation in their CD4+ T cell count. Though KS generally responds to first line treatment, following cessation of treatment, KS may be recurrent irrespective of epidemiologic subtypes as existing therapies do not eradicate the underlying cause – KSHV infection. Among patients with HIV, KS may recur even at high CD4+ T cell counts[43]. The factors that lead to recurrence of KS are largely unknown. One study has observed that individuals with classic KS and those who present with HIV-associated KS at normal or minimally suppressed CD4+ T cell counts who are aviremic may have a similar and possibly a more indolent course as compared to those with HIV-associated viremic KS[44]. Therefore, there may be several host and viral mechanisms that contribute to KS onset and recurrence. If patients have recurrent KS, the same standard treatments can be used again to treat KS. However, recurrent chemotherapy treatment can lead to long-term toxicities such treatment-related myelodysplastic syndrome associated with liposomal doxorubicin or neurotoxicity associated with paclitaxel[45]. Treatment with chemotherapy can also lead to decreases in CD4+ T cell counts, which can contribute to KS persistence and increase the risk of opportunistic infections.
Several chemotherapy-sparing immunotherapies that address the mechanisms by which KSHV evades immune surveillance are currently being evaluated in KS. Irrespective of HIV status[46], in prospective clinical trials among patients with KS, PD-1 inhibitors have led to some KS responses without excessive immune-related adverse events[47,48]. The class of agents such as proteosome inhibitors has been shown to result in KSHV-lytic replication. In a recent dose-escalation study of bortezomib of 15 evaluable participants with HIV-associated KS within the AMC, the overall response rate was 60% with responses at higher doses of bortezomib[49]. An ongoing study evaluating an oral proteosome inhibitor, ixazomib, will determine the effect of this therapy in KS. Other treatments in development for KS include cytokine-based therapies that aim to target KS pathogenesis by addressing angiogenesis, increasing T cell responses or CD4+ T cell lymphocytes. Examples include a new subcutaneous form of interleukin (IL)12, known as NHS-IL12. Earlier studies of IL12 in patients with KS demonstrated that this agent was active against KS with a response rate of 71% at higher doses[50]. IL12 promotes the development of Th1-type helper T cells, facilitate cytotoxic T-cell responses, enhance the NK-cell lytic activity, and induce production of interferon-gamma[51,52]. Additionally, IL12 administration was shown in KS patients to induce increases in inducible protein-10, which can downregulate activity of a constitutively active viral G-protein– coupled receptor that is encoded by KSHV ORF74 and induces production of vascular endothelial growth factor and an angiogenic state. IL-12 development was abandoned overall, and it was not further evaluated for KS. However, there is now active study of NHSIL-12 in KS and other diseases. IL7, which is associated with T cell proliferation in initial studies led to increases in CD4+ T cell counts among those with HIV [53] and presents a potentially promising option for individuals with KS; an initial study is ongoing.
5. Multicentric Castleman Disease (MCD)
5.1. Epidemiology
The term Castleman disease refers to a spectrum of conditions with varying pathologic descriptions. This ranges from an indolent localized hyperplasia known as unicentric Castleman disease to a multicentric lymphoproliferation associated with inflammatory symptoms[54]. KSHV-associated MCD is a form of multicentric Castleman disease. It is a polyclonal B cell lymphoproliferative disorder that is most common among those with HIV but there are cases observed in HIV-negative individuals[55,56]. The incidence of MCD caused by KSHV is largely unknown worldwide as it is difficult to diagnose given the similarity to other causes of inflammation and multiorgan dysfunction in PWH, such as tuberculosis[57]. Moreover, imaging and expert pathology services required to diagnose MCD may be difficult to obtain in many parts of sub-Saharan Africa where KSHV and HIV co-infection are highly prevalent. A study from the United Kingdom estimated the incidence of MCD in PWH is 4.3 cases per 10,000 person-years and has been increasing over time with widespread ART availability[58]. A recent study from Malawi found that 15% of cases of lymphoproliferative disease in PWH were MCD caused by KSHV. MCD is often observed in individuals with higher CD4 T cell counts and suppressed HIV viral load. In up to 50% of cases of MCD, patients may present with concurrent KS[59]. In a study within our center, 7 of 62 patients (12.5%) with MCD also had both PEL and KS. In these cases, the diagnosis of PEL emerged within a year of the MCD diagnosis and was associated with lower CD4+ T cell counts[5].
5.2. Pathophysiology
The pathogenesis of MCD is believed to result from interactions between the virus and the host immune system that leads to its unique presentation. A case report of an infant with X-linked lymphoproliferative disease with an absence of invariant natural killer T cells (iNKT) cells who presented with KSHV-associated hemophagocytic lymphohistiocytosis resembling MCD highlighted the possible role of iNKT in MCD pathogenesis[60]. Analyses of patients with HIV and MCD demonstrated decreases in the frequency and function of iNKT cells, which is an integral component of the innate immune system and response to herpesviruses[61]. The role of oral KSHV transmission and infection of tonsillar B cells may also contribute to MCD pathogenesis. In vitro experiments of human tonsillar B cells have shown that KSHV infection of these cells led to proliferation and transformation to plasmablasts that had high levels of IgM and IL6 receptor that are morphologically similar to MCD[62]. Other possible components of pathogenesis include spliced X-box binding protein 1, which is found in high levels in developing B cells and may induce KSHV lytic replication also also upregulate viral IL6[63]. HHV-8 encodes for an analog of Interluekin-6 (IL-6), viral IL-6 (vIL-6), which can activate human IL-6 in other cells and contributes to MCD pathogenesis [64]. It has been shown that symptoms related to MCD are associated with high levels of serum C-reactive protein, as well as high KSHV viral load, KSHV vIL-6 high human IL-6 and IL-10 levels in the peripheral blood[65].
5.3. Clinical Presentation
MCD is a relapsing and remitting condition marked by flares of severe non-specific inflammatory signs and symptoms that can mimic the signs and symptoms of sepsis or other inflammatory disorders[66]. The symptoms include fevers, night sweats, weight loss, edema, rashes, and respiratory or gastrointestinal symptoms. Patients have widespread lymphadenopathy and hepatosplenomegaly and often concurrent KS. Laboratory findings during an active flare of MCD include cytopenias, hyponatremia, hypoalbuminemia, elevated C-reactive protein, and KSHV viremia. Patients may experience signs and symptoms of an hemophagocytic lymphohistiocytosis (HLH)-like syndrome marked by hyperferritenemia and pancytopenia. These signs and symptoms of MCD can occur irrespective of HIV status[55]. Serum and plasma levels of IL6 and IL10 are increased at periods of flare, and they decrease following successful treatment[4,5]. The KSHV viral load in the plasma or peripheral blood may be elevated during periods of MCD flare and decreases during periods of remission. Imaging of patients with suspected MCD may show extensive lymphadenopathy, splenomegaly and/or effusions. An 18-fluorodeoxyglucose positron emission tomography may show extensive avid lymph nodes. This may be used to select the best lymph node for excision or core biopsy for the highest diagnostic yield.
5.4. Diagnosis
Histopathological confirmation in a lymph node is required for diagnosis. MCD lymph nodes demonstrate an expansion of KSHV-infected plasmablasts in the mantle zone of B cell follicles positive for latent nuclear antigen-1 (LANA), viral IL6, exhibit a cytoplasmic IgM that is lambda light-chain restricted, and express a polyclonal immunoglobulin gene arrangement[67]. Extra-nodal sites such as bone marrow may be involved in MCD, which may show plasmacytosis with lymphoid aggregates and occasionally KSHV-infected mononuclear cells. Patients with MCD may also have effusions with elevated KSHV viral load in their pleural fluid. A recent study demonstrated a distinct lambda-restricted plasmablastic (LRP) population following flow cytometry analyses of effusions among a subset of patients with MCD[68]. Assessment of effusions is also important as patients may have concurrent PEL at the time of MCD diagnosis. Data from Fremont and colleagues have also noted IgM+λ+CD38 cells plasmablasts that were KSHV infected and present in the peripheral blood of 14 of 18 individuals with active MCD. Thus, it is possible that these circulating cells may help with the diagnosis of active MCD [17].
5.5. Treatment
The standard therapy for KSHV-associated MCD irrespective of HIV status is generally rituximab, a monoclonal antibody targeting CD20 positive B cells; while not FDA-approved for this indication, it has been incorporated into national guidelines for MCD treatment[69]. Several prospective studies have demonstrated MCD response and survival benefit following four doses of weekly rituximab. The CastlemaB study in 2007 resulted in an overall survival rate of 92% with 71% remission at 1 year[70]. In 21 patients from the United Kingdom with previously untreated MCD who received rituximab had a 2-year overall survival of 95%[71]. Long-term results from the largest cohort study of patients in North America with MCD alone or with other concurrent KSHV-associated conditions were recently published. Within this cohort, among individuals with MCD alone who received rituximab, the 5- and 10-year survival was 90% and 73%, respectively[5]. The mechanism by which KSHV-MCD responds to rituximab is unclear, and his may be an area of future research. The activity of rituximab in MCD may be due to off-target effects on KSHV-uninfected CD20 positive B cells within the microenvironment that secrete inflammatory cytokines and potentially harbor a reservoir of KSHV infection and replication as the MCD plasmablasts themselves are generally CD20 negative.
A major side effect associated with rituximab administration in patients with MCD is the onset or progression of KS among 35–67% of patients[59]. In these cases, a standard treatment for KS can be administered with rituximab. A prospective study of liposomal doxorubicin with rituximab resulted in a 3-year overall survival of 81%[72]. The addition of etoposide, a topoisomerase II inhibitor, to rituximab has also been studied retrospectively in cases of multiorgan dysfunction in a stage-stratified approach[73]. However, etoposide is not a particularly effective therapy in cases of concurrent KS with MCD. Most patients who receive rituximab will have remission of their MCD-related signs and symptoms. A subset of patients experience subsequent flares and rituximab can be used again successfully in these cases, but there is no known clinical benefit to giving maintenance rituximab to prevent subsequent flares[74]. Rituximab also reduces the risk of the onset of non-Hodgkin lymphoma in the population of patients with MCD, who are at high risk for the development of KSHV-associated lymphomas[75].
Therapies such as ganciclovir and zidovudine (AZT) that are activated by herpesviruses to toxic moieties have also been investigated in MCD. KSHV ORF36 and ORF21 are two lytic genes that encode enzymes that may phosphorylate ganciclovir and zidovudine, respectively, and lead to accumulation of triphosphate moieties that are toxic to KSHV-infected cells. A pilot study investigating high-dose AZT and valganciclovir (VGC) in 14 patients with MCD resulted in clinical benefit in 12 patients; however, the median progression-free survival (PFS) was 6 months[76]. From our larger cohort of patients with MCD, the 5-year PFS was 26% for those treated with AZT/VGC, whereas those receiving rituximab alone had a 5-year PFS of 71%[5]. Interestingly, within this same cohort patients who received rituximab followed by AZT/VGC maintenance following an MCD flare had a 5-year PFS of 87% in these retrospective analyses.
Another approach that has been explored for treating MCD has been targeting IL6 given the elevated levels of this specific cytokine in active MCD flares and that this is a major contributor to these symptoms. For individuals with idiopathic MCD, siltuximab, a monoclonal antibody targeting IL6, is FDA-approved but has not been studied extensively in KSHV-associated MCD[77]. Tocilizumab, a monoclonal antibody against IL6 receptor alone and in combination with AZT/VGC has been studied in 8 patients with KSHV-associated MCD (2 with concurrent KS)[78]. In these patients, the response rate was 63% but the response was short-lived with the median progression-free survival of 3.2 months. For subsequent MCD flares, the majority of participants in this study required rituximab. The limited duration of response may be due to the effects of the KSHV-encoded viral homolog of IL6 (vIL6), which can directly bind to and signal through the gp130 co-receptor, thus bypassing the inhibition of the IL6 receptor and activating the IL6 pathway. Targeted approaches with MCD in the future may include anti-CD38 monoclonal antibodies or Janus kinase (JAK)-signal transducer and activator of transcription (STAT). These options may offer rituximab-sparing options that reduce the risk of KS progression in patients with MCD and improve our understanding of the pathogenesis of this complex condition.
6. Primary Effusion Lymphoma (PEL) and KSHV-associated diffuse large cell lymphoma (KSHV-LCL)
6.1. Pathophysiology
HIV-associated lymphomas are often caused by oncogenic viruses, such as EBV and KSHV[79]. Both PEL and KSHV diffuse large B cell lymphoma not otherwise specified (KSHV-LCL) are B cell lymphomas caused by KSHV. The malignant B cells have a plasmacytic differentiation and generally lack many traditional B cell markers such as CD19, CD20, CD22, CD79a and PAX5, but show clonal rearrangement of the immunoglobulin gene[80,81]. The tumor cells are positive for MUM1, CD38 and CD138 as well as activation-associated markers, such as CD30, and do not express germinal center markers, such as CD10 and BCL6 (Figure 2). Several KSHV latent viral genes are associated with lymphomagenesis. This includes LANA, which is expressed on all KSHV-infected cells, and dysregulates cell growth and survival by binding to key tumor suppressor proteins. Other KSHV-encoded viral proteins mimic host functions such as viral cyclins, which activate cell growth, and viral FLICE-inhibitory protein (vFLIP), which upregulates NF-κB leading to increased expression of antiapoptotic proteins and inflammatory cytokines[82,83]. vFLIP can also interact with c-myc to promote lymphomagenesis. In up to 80% of cases, PEL is also co-infected with EBV[2]. Though PEL tumors that are positive for EBV may confer better prognostic outcomes, the role of EBV in the pathogenesis of PEL is unclear.
Figure 2.
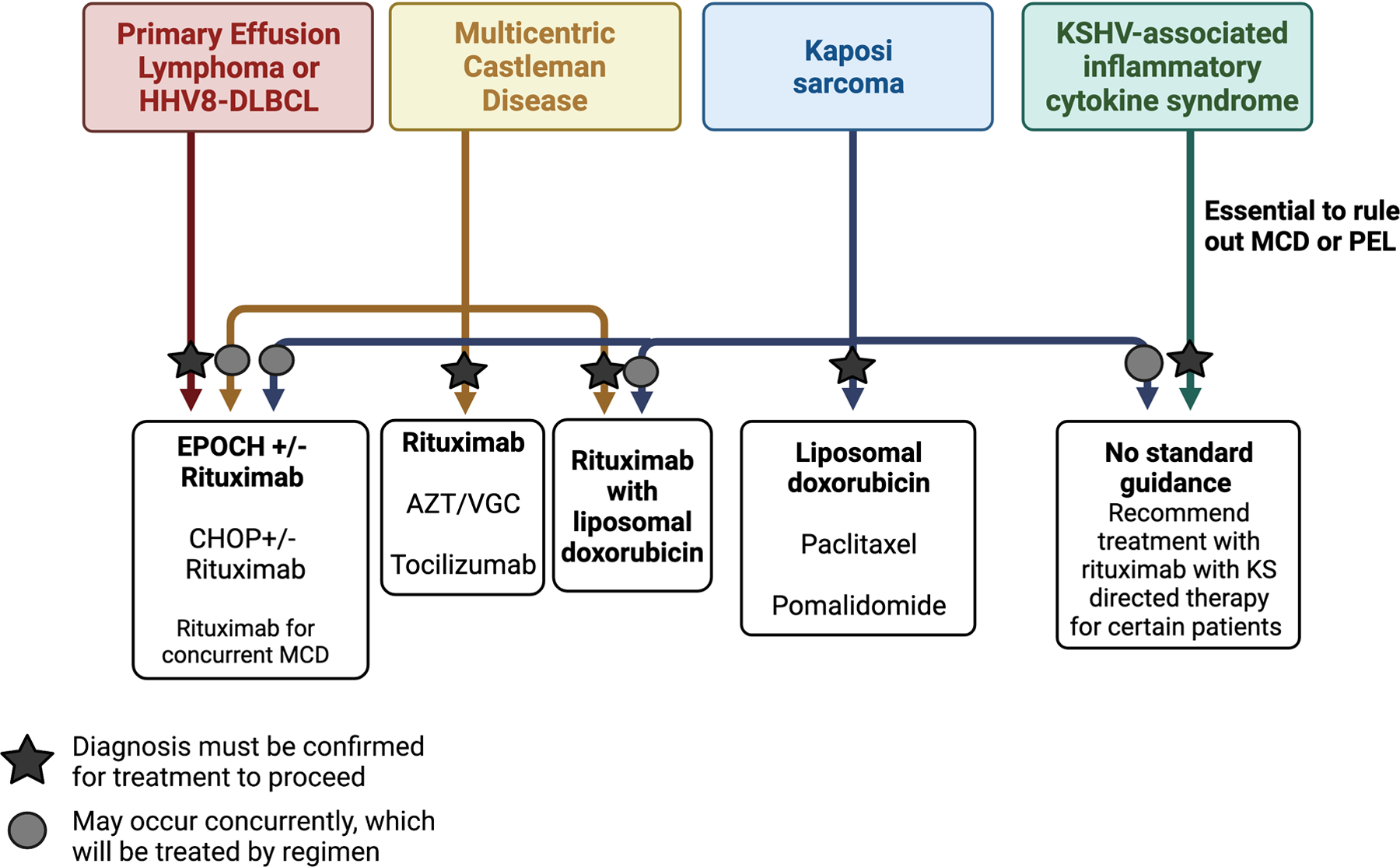
Procedures and pathologic criteria for diagnosis of KSHV-associated disorders.
6.2. Epidemiology
PEL accounts for between 2–4% of HIV-associated lymphomas and in rare cases can occur in HIV-negative individuals who are older and HIV-negative individuals from KSHV endemic regions. In the modern ART era, the incidence of PEL among all HIV-associated lymphoma has increased[84]. Patients with PEL generally present with malignant effusions within cavitary spaces such as the abdominal, pleural, and/or pericardial spaces. An extracavitary solid form is a variant of PEL that arises in the lymph nodes or extranodal areas, such as the gastrointestinal tract and skin. In a cohort of 20 patients with PEL from our center, 6 (30%) had concurrent MCD and 15 (75%) had concurrent KS[2].
KSHV-LCL, not otherwise specified, is a rare entity that usually emerges in the setting of MCD. Unlike PEL, KSHV-LCL presents more typically in the lymph nodes or the spleen. It consists of confluent EBV-negative large plasmablastic cells that express LANA, vIL6, IRF4 and cytoplasmic IgMλ[80,85]. Signs and symptoms are similar to those seen in MCD and PEL and are associated with cytokine excess and KSHV viremia. KS may be present in patients with KSHV-LCL.
6.3. Treatment
There are no FDA-approved therapies for KSHV-associated lymphomas. However, current guidelines indicate that PEL and KSHV-LCL should generally be treated with curative intent anthracycline-containing chemotherapy regimens. One often used therapy consists of infusional etoposide, vincristine, doxorubicin, cyclophosphamide and prednisone (EPOCH)[69]. Up to 30% of individuals with PEL may have MCD[2]. Despite the CD20 negative status of PEL, rituximab in these cases has been found to be of value. The possible mechanism of action may be the rapidly decreasing cytokine dysregulation and depleting the KSHV infected B cell reservoir. However, clinicians should be mindful of the risk of worsening KS. Patients with HIV should continue ART as immune reconstitution aids not only in prevention of opportunistic infections but also lymphoma control. One study showed that every log increase in HIV viral load following lymphoma therapy increases the risk of mortality[86]. All patients should receive opportunistic infection prophylaxis over the course of treatment regardless of baseline CD4 T cell count to reduce the risk of infectious complications as T cell counts will decrease with therapy.
Among a cohort of 20 participants with PEL from our center who were treated with curative-intent EPOCH with or without rituximab, the medial overall survival was 22 months. The 3-year cancer specific survival was 47%[2]. The median progression-free survival among an overlapping larger cohort of 36 patients who received EPOCH for PEL was 16.4 months[87]. In vitro studies have shown that immunomodulatory agents such as lenalidomide and pomalidomide, which are approved in multiple myeloma, can lead to PEL cell death by targeting IRF4[39,88]. Additionally, KSHV and EBV are known to downregulate immune cell surface markers, agents such as pomalidomide (an immunomodulatory agent approved in multiple myeloma) may reverse this issue and render these cells visible to the immune system [39]. Given the potential benefit of immunomodulatory agents, an ongoing prospective study combining lenalidomide with EPOCH in the front-line setting is underway within our group for patients with PEL and KSHV-LCL. The phase I portion has led to a 50% complete remission rate and 66.7% 2-year overall survival in 6 patients[89].
Patients with refractory or recurrent PEL may have chemotherapy-sparing therapies as second-line options. Similar to KS, the use of chemotherapy-sparing options in this setting may reduce the risk of worsening CD4+ T cell counts that can predispose to other complications such as infection or persistence of concurrent KS. These include immunotherapy-based options that target chronic viral antigenemia of KSHV and HIV that result in loss of cytotoxic T-cell function and exhaustion via upregulation of immune-checkpoint proteins, including PD-1. PD-1 inhibitors such as pembrolizumab and nivolumab have been studied in several prospective studies in patients with HIV-associated cancers including patients with KS and lymphoma[47]. There may be synergy between immune checkpoint inhibitors and immunomodulatory agents. Moreover, these agents are known to penetrate the central nervous system, which is not the case with many chemotherapies used in the front-line treatment of lymphoma. In one study of patients with HIV-associated lymphomas, including 5 patients with relapsed or refractory PEL, patients were administered pembrolizumab with or without pomalidomide[90]. Among the subset of patients with KSHV and/or EBV-associated tumors in this study, the response rate was 50% with no excess immune-related adverse events.
Targeted therapies for PEL and KSHV-LCL may also specifically address the phenotypic characteristics of these lymphomas or exploit its viral pathogenesis. Monoclonal antibodies that target CD30 (brentuximab) or CD38 (daratumumab) are rational to consider where PEL tumors express these markers. Several case reports of daratumumab in PEL have demonstrated its clinical benefit in the relapsed setting both as a single agent or in combination with pomalidomide [91,92]. With respect to targeting the viral etiology of PEL, in vitro studies have demonstrated that PEL cells have an increased dependency to cyclin D[93], which impacts cell growth, and may be a possible target for therapy. CDK4/6 inhibitors, which are approved in metastatic breast cancer, have been shown to arrest cell growth and upregulate immune cell surface markers in KSHV-infected B cells and endothelial cells; thus, rendering these cells more visible to the host immune system[94].
7. KSHV-associated Inflammatory Cytokine Syndrome (KICS)
KICS was described initially as a syndrome consisting of KSHV-associated inflammation and multiorgan dysfunction, without a diagnosis of MCD despite lymph node evaluation or at follow up. These patients with HIV often had KSHV viremia in the peripheral blood, elevated IL10 levels, and both elevated human and viral IL6 levels in the circulation. Many of the patients with KICS had concurrent KS. In the initial retrospective cohort of 6 patients, 3 patients died, and the cause of deaths included progressive KS[95]. In a subsequent prospective characterization of 10 patients with KICS, inflammatory cytokines were compared to 2 control cohorts, first those with KSHV and HIV co-infection and the other with HIV infection alone [3]. Within each control cohort, participants were divided into those with and without controlled HIV levels. Among those with KICS, patients had severe symptoms, lower hemoglobin and albumin, higher C-reactive protein, higher IL6 and IL10 levels as compared to any of the the control groups. None of the patients with KICS had MCD, even at the time of autopsy, and the median survival following a diagnosis of KICS was 13.6 months.
7.1. Diagnosis
The challenge of KICS is its diagnostic difficulty in the setting of critically unwell patients and is a diagnosis of exclusion of both PEL, MCD, or other conditions that can cause similar symptoms and elevated cytokines. Initially, patients with PEL and signs and symptoms of KICS were included the working definition of KICS. However, every PEL patient studied in our group also met criteria for KICS, and these patients are now excluded from the working definition of KICS. A recent study evaluating multiparametric flow cytometry of effusions has identified a unique LANA positive lambda-restricted plasmablastic population (LRP) in 8 of 11 patients with MCD confirmed in lymph node biopsies[68]. LRP was also noted in the effusions of 7 of 19 patients diagnosed with KICS. Interestingly, the clinical characteristics of those with KICS+LRP resembled those with MCD rather than those with KICS alone without LRP. Therefore, this suggests that a subset of patients with KICS may have a form of MCD despite no evidence lymph node involvement on biopsy. KICS also has similar characteristics to KS-IRIS. However, KS-IRIS generally occurs in conjunction with the initiation of ART, whereas those with KICS have progressive symptoms long after theinitiation of ART or treatment for KS alone. Patients with PEL are a separate cohort of patients who should be treated with curative-intent combination chemotherapy and should not be classified as having KICS. This is an important distinction as the treatment paradigm for PEL is well-known whereas the ideal therapy for KICS is currently unknown.
7.2. Treatment
The investigation of optimal therapies for KICS is a question under active investigation. Given the possible overlap seen in analyses of flow cytometry in a subset of effusions from patients with KICS that resembles those in MCD, it is possible that MCD-specific therapies may be helpful in KICS. In a larger prospective study of 44 patients with KICS from our center, 18 patients received rituximab with standard chemotherapies for KS[96]. Ten of these 18 participants had a clinical benefit in the symptoms and signs of KICS. Although this is lower than the response rate for rituximab-based therapies in MCD, it suggests that rituximab-based therapies can be successful in patients with KICS. More needs to be learned about the pathogenesis of KICS. As we better understand the sources of the inflammatory cytokines in this condition and the interactions between the host and virus, there may be more treatment options that are identified.
8. Concurrent KSHV-associated diseases
Despite the descriptions of these independent conditions caused by KSHV, these diseases may frequently occur concurrently in the same individual. In our cohort of patients with MCD, 44% of all these patients were only diagnosed following referral and evaluation at our center[5]. Many of these patients had systemic symptoms concerning for lymphoma or recurrent KS not responsive to therapy. Up to 50% of patients with MCD have KS and 97% of patients with KICS have KS. It is also possible to have KS, MCD and PEL occur at the same time, which was observed in 7 out of 62 patients (11%)[5]. It is essential to diagnose these conditions as the treatments can vary following their identification (Figure 2 and 3). In patients with KS, cytokine dysregulation, inflammation and KSHV viremia led to the consideration of one or more concurrent disease processes such as MCD, KICS or PEL. However, a histological diagnosis is required to confirm MCD or PEL in these patients and it is vital to have this reviewed by expert pathologists. It is not generally advised to pre-emptively treat patients with EPOCH based upon suspicion without biopsy-proven evidence of PEL due to the intensity and risk of infectious complications with combination chemotherapy. Therefore, it is imperative to promptly seek and diagnose these conditions to appropriately treat and improve the patient’s outcome.
Figure 3.
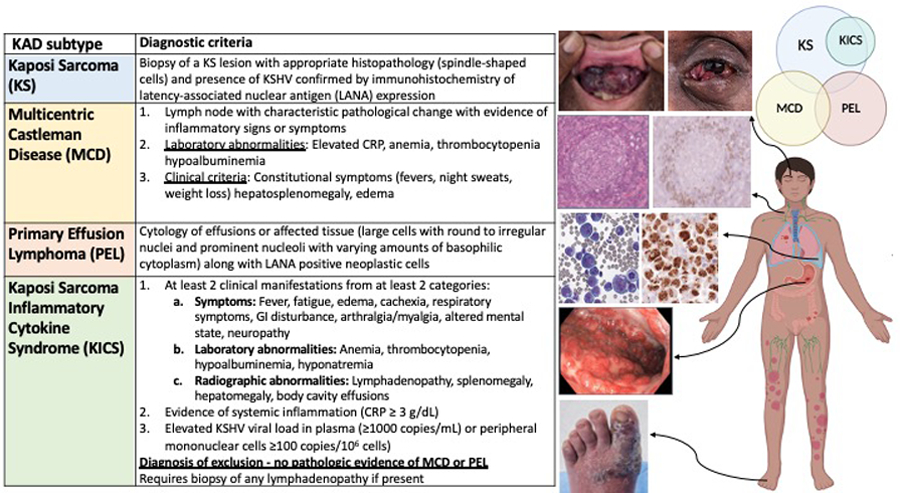
Treatment algorithm for KSHV-associated disorders.
We studied 47 patients admitted to our intensive care unit (ICU) with KSHV-associated diseases[97]. In 22 patients with KICS, a diagnosis of PEL or MCD had been excluded prior to ICU entry. In addition to providing supportive care and antimicrobial therapies, during the ICU admission, additional investigations, including additional thoracentesis, paracentesis and lymph node biopsies were pursued. In 8 of the 22 patients with previously presumed KICS at ICU admission, MCD and/or PEL were later identified. In this retrospective study, patients tolerated the administration of chemotherapy or targeted therapies for KAD, emphasizing the need for appropriate diagnosis and treatment even in the critical care setting. In limited resource settings, it is extremely challenging to identify concurrent KAD among patients with KS. A recent study has identified elevated KSHV viremia, and lower CD4 T cell counts to be associated with poorer outcomes despite treatment for KS.[22] However, it was not feasible to do further investigations to identify MCD or PEL. Developing algorithms and to easily stratify patients that may need additional investigations or therapies may be the first step in delineating these populations that may have concurrent KAD.
9. Future directions
KSHV causes a spectrum of diseases which are significant causes of morbidity and mortality worldwide. Improved access to ART has reduced the incidence if HIV-associated KS, but it continues to be a major problem in certain regions. While supportive care and KSHV-directed treatments have improved outcomes for many patients with KAD, lack of awareness among clinicians or the lack of resources to confirm the diagnoses still contribute to excess mortality in these conditions. It is particularly challenging as the need for diagnoses and treatment of KAD may occur in unwell and rapidly deteriorating patients. As KSHV can infect and impact several organ systems and the circulation, it is important to improve our understanding of the interaction between the virus and host. Specifically, the evasion of immune surveillance, inflammation, and aberrant angiogenesis are important hallmarks of cancer that are implicated in KADs that require further evaluation. Currently, our understanding of this interaction arises from study of KSHV-infected cell lines as there are no good animal models that exist to approximate these conditions. These models may serve to increase the understanding of disease pathogenesis but also help with therapeutic targets.
10. Expert opinion
Despite decreases in KS incidence, there is evidence for increasing incidence rates of other KSHV-associated diseases (KAD) such as MCD and PEL in the current antiretroviral therapy era worldwide. KAD persists as a significant challenge for people with and without HIV. Among individuals without HIV, it is paramount that the field is able to identify the key drivers that lead to KAD in this population and the mechanisms of immune dysfunction that lead to KAD. Though the presentations of many KAD are the same irrespective of the underlying epidemiologic subtype, the course and outcomes of different KAD between those with and without HIV may vary.
KAD may be rarer in the United States as compared to sub-Saharan Africa where KSHV is endemic. However, irrespective of geographic location, KAD can occur concurrently and are often difficult to diagnose especially when patients can experience multiorgan dysfunction that can occur with some KAD. Medical professionals caring for patients with these diseases, such as KS, may often miss the presence of concurrent KAD. They may attribute signs and symptoms associated with inflammation to the presence of infections specifically in people with HIV who may also experience immune reconstitution inflammatory syndrome. The diagnostic process to identify KAD currently requires imaging, procedures to obtain tissue and expert pathology services. Patients with these conditions can often deteriorate rapidly if all KAD are not appropriately identified and treated promptly. This is often challenging in cases where people with HIV may have opportunistic infections that lead to systemic signs or symptoms that can mimic KAD. It is important to note that even in the critical care setting, if these disorders are appropriately diagnosed and treated, this may lead to long-term benefit for these patients.
Though there are no FDA-approved therapies for many of these diseases, there are standard treatments from other cancer paradigms with rational use in KAD. Approved KS therapy consists of chemotherapy-based regimens and recently an immunomodulatory drug. Increasingly, there are immunotherapy options to consider that target the mechanisms of KSHV-driven immune evasion and T cell exhaustion. For people with HIV, these options for KS may be T-cell sparing and may reduce the risk of persistent KS. Due to the prevalence of KS in the United States, it may not be feasible to conduct randomized clinical trials in KAD. However, smaller phase studies with correlative endpoints may help advance our knowledge of the pathogenesis and treatment of these conditions. Moreover, identifying rational and inexpensive diagnostic tests or therapies may help advance the care of these diseases in limited resource settings where these conditions are highly prevalent. As these conditions are prevalent in limited resource settings, it is essential to develop clinical trial infrastructure for patients with these conditions as well as research support for investigators from these regions to study these disorders. It is critical that we improve our understanding of the interaction between host and virus that may provide insights into viral oncogenesis. Understanding these interactions may also lead to the development of a vaccine for KSHV, which is of interest to the field. The lessons learnt from KAD may be applicable to other cancers caused by oncogenic viruses and may also provide options for treatment worldwide.
Article highlights.
Kaposi sarcoma herpes virus (KSHV), also known as HHV-8, is associated with Kaposi sarcoma, multicentric Castleman disease, primary effusion lymphoma, and KSHV-inflammatory cytokine syndrome and these conditions can occur alone or concurrently in the same patient.
The viral life cycle of KSHV exists in two distinct phases: latency and lytic replication. While PEL and KS are considered to exist primarily in a state of latency, there is more lytic replication occurring among those with MCD.
Diagnosis of concurrent KAD requires careful assessment and diagnostic tests that may be resource intensive, which poses a challenge in resource-limited setting where these diseases are prevalent but underdiagnosed.
The diagnosis of concurrent KAD will impact the treatment outcomes as many patients with KS often have undiagnosed MCD or PEL that will require different treatment paradigms.
The management of KICS, which is a the most recent KAD described, is a diagnosis of exclusion but does not yet have a standard management. Treatment approaches may include the rituximab-based regimens.
Funding
This research was supported by the intramural program of the National Cancer Institute.
Footnotes
Declaration of interests
K Lurain, R Yarchoan, and R Ramaswami report receiving research support from Bristol Myers Squibb and CTI Bioscience through CRADAs with the NCI and receiving drugs for clinical trials from Merck, EMD-Serono, Eli Lilly, and CTI BioPharma through CRADAs with the NCI. R Yarchoan reports receiving drug supply for laboratory research from Janssen Pharmaceuticals and CTI Biopharma. R Yarchoan is co-inventor on US Patent 10,001,483 entitled “Methods for the treatment of Kaposi’s sarcoma or KSHV-induced lymphoma using immunomodulatory compounds and uses of biomarkers.” An immediate family member of R Yarchoan is a co-inventor on patents or patent applications related to internalization of target receptors, epigenetic analysis, and ephrin tyrosine kinase inhibitors. All rights, title, and interest to these patents have been assigned to the U.S. Department of Health and Human Services; the government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L. 99–502). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Ramaswami R, Lurain K, Yarchoan R. Oncologic Treatment of HIV-Associated Kaposi Sarcoma 40 Years on. J Clin Oncol 2022. Jan 20;40(3):294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lurain K, Polizzotto MN, Aleman K, et al. Viral, immunologic, and clinical features of primary effusion lymphoma. Blood 2019;133(16):1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polizzotto MN, Uldrick TS, Wyvill KM, et al. Clinical Features and Outcomes of Patients With Symptomatic Kaposi Sarcoma Herpesvirus (KSHV)-associated Inflammation: Prospective Characterization of KSHV Inflammatory Cytokine Syndrome (KICS). Clinical Infectious Diseases 2016;62(6):730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polizzotto MN, Uldrick TS, Wang V, et al. Human and viral interleukin-6 and other cytokines in Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Blood 2013;122(26):4189–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramaswami R, Lurain K, Polizzotto MN, et al. Characteristics and outcomes of KSHV-associated multicentric Castleman disease with or without other KSHV diseases. Blood Advances 2021;5(6):1660–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dollard SC, Butler LM, Jones AM, et al. Substantial regional differences in human herpesvirus 8 seroprevalence in sub-Saharan Africa: insights on the origin of the “Kaposi’s sarcoma belt”. International journal of cancer 2010. Nov 15;127(10):2395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labo N, Miley W, Benson CA, et al. Epidemiology of Kaposi’s sarcoma-associated herpesvirus in HIV-1-infected US persons in the era of combination antiretroviral therapy. Aids 2015. Jun 19;29(10):1217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamiwe T, Matthew SP, Nathan DM, et al. A prospective description of HIV-associated multicentric Castleman disease in Malawi. Haematologica 2019. May/01;104(5):e215–e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 10.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma [ 10.1126/science.7997879]. Science 1994;266(5192):1865. [DOI] [PubMed] [Google Scholar]
- 11.Douglas JL, Gustin JK, Moses AV, et al. Kaposi Sarcoma Pathogenesis: A Triad of Viral Infection, Oncogenesis and Chronic Inflammation. Transl Biomed 2010;1(2). [PMC free article] [PubMed] [Google Scholar]
- 12.Bechtel Jill T, Liang Y, Hvidding J, et al. Host Range of Kaposi’s Sarcoma-Associated Herpesvirus in Cultured Cells. Journal of Virology 2003. 2003/06/01;77(11):6474–6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerur N, Veettil MV, Sharma-Walia N, et al. Characterization of entry and infection of monocytic THP-1 cells by Kaposi’s sarcoma associated herpesvirus (KSHV): Role of heparan sulfate, DC-SIGN, integrins and signaling. Virology 2010. 2010/10/10/;406(1):103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballestas ME, Chatis PA, Kaye KM. Efficient Persistence of Extrachromosomal KSHV DNA Mediated by Latency-Associated Nuclear Antigen. Science 1999. 1999/04/23;284(5414):641–644. [DOI] [PubMed] [Google Scholar]
- 15.Broussard G, Damania B. Regulation of KSHV Latency and Lytic Reactivation. Viruses 2020. [cited. DOI: 10.3390/v12091034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du M-Q, Liu H, Diss TC, et al. Kaposi sarcoma–associated herpesvirus infects monotypic (IgMλ) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood 2001;97(7):2130–2136. [DOI] [PubMed] [Google Scholar]
- 17.Martin de Frémont G, Vanjak A, Sbihi Z, et al. Characteristics of circulating KSHV-infected viroblasts during active KSHV+ multicentric Castleman disease. Blood Advances 2023;7(9):1682–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaposi. Idiopathisches multiples Pigmentsarkom der Haut. Archiv für Dermatologie und Syphilis 1872. 1872/06/01;4(2):265–273. [Google Scholar]
- 19.Cook-Mozaffari P, Newton R, Beral V, et al. The geographical distribution of Kaposi’s sarcoma and of lymphomas in Africa before the AIDS epidemic. British Journal of Cancer 1998. 1998/12/01;78(11):1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grulich AE, Vajdic CM. The Epidemiology of Cancers in Human Immunodeficiency Virus Infection and After Organ Transplantation. Seminars in Oncology 2015. 2015/04/01/;42(2):247–257. [DOI] [PubMed] [Google Scholar]
- 21.Lanternier F, Lebbé C, Schartz N, et al. Kaposi’s sarcoma in HIV-negative men having sex with men. AIDS 2008;22(10). [DOI] [PubMed] [Google Scholar]
- 22.Phipps W, Adams SV, Mooka P, et al. A prospective study of clinical outcomes of HIV-associated and HIV-negative Kaposi sarcoma in Uganda. AIDS 2023;37(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang L, Schnapp LM, Gruden JF, et al. Presentation of AIDS-related pulmonary Kaposi’s sarcoma diagnosed by bronchoscopy. American Journal of Respiratory and Critical Care Medicine 1996. 1996/04/01;153(4):1385–1390. [DOI] [PubMed] [Google Scholar]
- 24.Burkes RL, Gal AA, Stewart ML, et al. Simultaneous Occurrence of Pneumocystis carinii Pneumonia, Cytomegalovirus Infection, Kaposi’s Sarcoma, and B-Immunoblastic Sarcoma in a Homosexual Man. JAMA 1985;253(23):3425–3428. [PubMed] [Google Scholar]
- 25.Pesqué L, Delyon J, Lheure C, et al. Yield of FDG PET/CT for Defining the Extent of Disease in Patients with Kaposi Sarcoma. Cancers 2022. [cited. DOI: 10.3390/cancers14092189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letang E, Naniche D, Bower M, et al. Kaposi Sarcoma–Associated Immune Reconstitution Inflammatory Syndrome: In Need of a Specific Case Definition. Clinical Infectious Diseases 2012;55(1):157–158. [DOI] [PubMed] [Google Scholar]
- 27.Bower M, Nelson M, Young AM, et al. Immune Reconstitution Inflammatory Syndrome Associated With Kaposi’s Sarcoma. Journal of Clinical Oncology 2005. 2005/08/01;23(22):5224–5228. [DOI] [PubMed] [Google Scholar]
- 28.Lebbe C, Garbe C, Stratigos AJ, et al. Diagnosis and treatment of Kaposi’s sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC). European Journal of Cancer 2019. 2019/06/01/;114:117–127. [DOI] [PubMed] [Google Scholar]
- 29.Cianfrocca M, Lee S, Von Roenn J, et al. Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy. Cancer 2010. Aug 15;116(16):3969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart S, Jablonowski H, Goebel FD, et al. Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi’s sarcoma. International Pegylated Liposomal Doxorubicin Study Group. Journal of Clinical Oncology 1998. 1998/02/01;16(2):683–691. [DOI] [PubMed] [Google Scholar]
- 31.Harrison M, Tomlinson D, Stewart S. Liposomal-entrapped doxorubicin: an active agent in AIDS-related Kaposi’s sarcoma. J Clin Oncol 1995. Apr;13(4):914–20. [DOI] [PubMed] [Google Scholar]
- 32.Cooley T, Henry D, Tonda M, et al. A randomized, double-blind study of pegylated liposomal doxorubicin for the treatment of AIDS-related Kaposi’s sarcoma. Oncologist 2007. Jan;12(1):114–23. [DOI] [PubMed] [Google Scholar]
- 33.Gill PS, Tulpule A, Espina BM, et al. Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi’s sarcoma. J Clin Oncol 1999. Jun;17(6):1876–83. [DOI] [PubMed] [Google Scholar]
- 34.Stebbing J, Wildfire A, Portsmouth S, et al. Paclitaxel for anthracycline-resistant AIDS-related Kaposi’s sarcoma: clinical and angiogenic correlations. Ann Oncol 2003. Nov;14(11):1660–6. [DOI] [PubMed] [Google Scholar]
- 35.Welles L, Saville MW, Lietzau J, et al. Phase II trial with dose titration of paclitaxel for the therapy of human immunodeficiency virus-associated Kaposi’s sarcoma. J Clin Oncol 1998. Mar;16(3):1112–21. [DOI] [PubMed] [Google Scholar]
- 36.Saville MW, Lietzau J, Pluda JM, et al. Treatment of HIV-associated Kaposi’s sarcoma with paclitaxel. Lancet 1995. Jul 1;346(8966):26–8. [DOI] [PubMed] [Google Scholar]
- 37.Krown SE, Moser CB, MacPhail P, et al. Treatment of advanced AIDS-associated Kaposi sarcoma in resource-limited settings: a three-arm, open-label, randomised, non-inferiority trial. The Lancet 2020. 2020/04/11/;395(10231):1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polizzotto MN, Uldrick TS, Wyvill KM, et al. Pomalidomide for Symptomatic Kaposi’s Sarcoma in People With and Without HIV Infection: A Phase I/II Study. J Clin Oncol 2016. Dec;34(34):4125–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis DA, Mishra S, Anagho HA, et al. Restoration of immune surface molecules in Kaposi sarcoma-associated herpes virus infected cells by lenalidomide and pomalidomide. Oncotarget; Vol 8, No 31. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramaswami R, Polizzotto MN, Lurain K, et al. Safety, Activity, and Long-term Outcomes of Pomalidomide in the Treatment of Kaposi Sarcoma among Individuals with or without HIV Infection. Clin Cancer Res 2022. Mar 1;28(5):840–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reid EG, Shimabukuro K, Moore P, et al. AMC-070: Lenalidomide Is Safe and Effective in HIV-Associated Kaposi Sarcoma. Clinical Cancer Research 2022;28(12):2646–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stallone G, Schena A, Infante B, et al. Sirolimus for Kaposi’s Sarcoma in Renal-Transplant Recipients. New England Journal of Medicine 2005. 2005/03/31;352(13):1317–1323. [DOI] [PubMed] [Google Scholar]
- 43.Palich R, Veyri M, Valantin M-A, et al. Recurrence and Occurrence of Kaposi’s Sarcoma in Patients Living With Human Immunodeficiency Virus (HIV) and on Antiretroviral Therapy, Despite Suppressed HIV Viremia. Clinical Infectious Diseases 2020;70(11):2435–2438. [DOI] [PubMed] [Google Scholar]
- 44.Séverin D, Bessaoud F, Meftah N, et al. A comparative study of classic and HIV-viremic and aviremic AIDS Kaposi sarcoma. AIDS 2021;35(3). [DOI] [PubMed] [Google Scholar]
- 45.National Comprehensive Cancer Network. AIDS-Related Kaposi Sarcoma (Version 1.2018) [March 4, 2018]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/kaposi.pdf [DOI] [PubMed] [Google Scholar]
- 46.Lurain K, Yarchoan R, Ramaswami R. Immunotherapy for KSHV-associated diseases. Current Opinion in Virology 2022. 2022/08/01/;55:101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uldrick TS, Goncalves PH, Abdul-Hay M, et al. Assessment of the Safety of Pembrolizumab in Patients With HIV and Advanced Cancer-A Phase 1 Study. JAMA Oncol 2019. Sep 1;5(9):1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delyon J, Biard L, Renaud M, et al. PD-1 blockade with pembrolizumab in classic or endemic Kaposi’s sarcoma: a multicentre, single-arm, phase 2 study. The Lancet Oncology 2022;23(4):491–500. [DOI] [PubMed] [Google Scholar]
- 49.Reid EG, Suazo A, Lensing SY, et al. Pilot Trial AMC-063: Safety and Efficacy of Bortezomib in AIDS-associated Kaposi Sarcoma. Clinical Cancer Research 2020;26(3):558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Little RF, Pluda JM, Wyvill KM, et al. Activity of subcutaneous interleukin-12 in AIDS-related Kaposi sarcoma. Blood 2006;107(12):4650–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Little RF, Pluda JM, Wyvill KM, et al. Activity of subcutaneous interleukin-12 in AIDS-related Kaposi sarcoma. Blood 2006. Jun 15;107(12):4650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Little RF, Aleman K, Kumar P, et al. Phase 2 study of pegylated liposomal doxorubicin in combination with interleukin-12 for AIDS-related Kaposi sarcoma. Blood 2007. Dec 15;110(13):4165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sereti I, Dunham RM, Spritzler J, et al. IL-7 administration drives T cell–cycle entry and expansion in HIV-1 infection. Blood 2009;113(25):6304–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fajgenbaum DC, Uldrick TS, Bagg A, et al. International, evidence-based consensus diagnostic criteria for HHV-8–negative/idiopathic multicentric Castleman disease. Blood 2017;129(12):1646–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dossier A, Meignin V, Fieschi C, et al. Human Herpesvirus 8–Related Castleman Disease in the Absence of HIV Infection. Clinical Infectious Diseases 2013;56(6):833–842. [DOI] [PubMed] [Google Scholar]
- 56.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s Sarcoma-Associated Herpesvirus-Like DNA Sequences in Multicentric Castleman’s Disease. Blood 1995. 1995/08/15/;86(4):1276–1280. [PubMed] [Google Scholar]
- 57.Blumenthal MJ, Schutz C, Barr D, et al. The Contribution of Kaposi’s Sarcoma–Associated Herpesvirus to Mortality in Hospitalized Human Immunodeficiency Virus–Infected Patients Being Investigated for Tuberculosis in South Africa. The Journal of Infectious Diseases 2019;220(5):841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Powles T, Stebbing J, Bazeos A, et al. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castleman’s disease. Annals of Oncology 2009;20(4):775–779. [DOI] [PubMed] [Google Scholar]
- 59.Bower M How I treat HIV-associated multicentric Castleman disease. Blood 2010. 2010/11/25/;116(22):4415–4421. [DOI] [PubMed] [Google Scholar]
- 60.Pasic S, Cupic M, Lazarevic I. HHV-8-related Hemophagocytic Lymphohistiocytosis in a Boy With XLP Phenotype. Journal of Pediatric Hematology/Oncology 2012;34(6). [DOI] [PubMed] [Google Scholar]
- 61.Sbihi Z, Dossier A, Boutboul D, et al. iNKT and memory B-cell alterations in HHV-8 multicentric Castleman disease. Blood 2017. 2017/02/16/;129(7):855–865. [DOI] [PubMed] [Google Scholar]
- 62.Hassman LM, Ellison TJ, Kedes DH. KSHV infects a subset of human tonsillar B cells, driving proliferation and plasmablast differentiation. The Journal of Clinical Investigation 2011. February/01/;121(2):752–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang V, Davis David A, Deleage C, et al. Induction of Kaposi’s Sarcoma-Associated Herpesvirus-Encoded Thymidine Kinase (ORF21) by X-Box Binding Protein 1. Journal of Virology 2020;94(5):e01555–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aoki Y, Narazaki M, Kishimoto T, et al. Receptor engagement by viral interleukin-6 encoded by Kaposi sarcoma–associated herpesvirus: Presented in part at the 42nd annual meeting of the American Society of Hematology, December 4, 2000, in San Francisco, CA. Blood 2001;98(10):3042–3049. [DOI] [PubMed] [Google Scholar]
- 65.Oksenhendler E, Carcelain G, Aoki Y, et al. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric Castleman disease in HIV-infected patients. Blood 2000;96(6):2069–2073. [PubMed] [Google Scholar]
- 66.Lurain K, Yarchoan R, Uldrick TS. Treatment of Kaposi Sarcoma Herpesvirus–Associated Multicentric Castleman Disease. Hematology/Oncology Clinics of North America 2018. 2018/02/01/;32(1):75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H-W, Pittaluga S, Jaffe ES. Multicentric Castleman disease: Where are we now? Seminars in Diagnostic Pathology 2016. 2016/09/01/;33(5):294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou T, Yuan CM, Lurain K, et al. A novel approach for characterization of KSHV-associated multicentric Castleman disease from effusions [ 10.1111/bjh.18518]. British Journal of Haematology 2023. 2023/02/01;200(4):462–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.National Comprehensive Cancer Network. B-cell lymphomas - Version 4.2021-May 5, 2021. [March 4, 2018]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf [Google Scholar]
- 70.Gérard L, Bérezné A, Galicier L, et al. Prospective Study of Rituximab in Chemotherapy-Dependent Human Immunodeficiency Virus–Associated Multicentric Castleman’s Disease: ANRS 117 CastlemaB Trial. Journal of Clinical Oncology 2007. 2007/08/01;25(22):3350–3356. [DOI] [PubMed] [Google Scholar]
- 71.Bower M, Powles T, Williams S, et al. Brief Communication: Rituximab in HIV-Associated Multicentric Castleman Disease. Annals of Internal Medicine 2007. 2007/12/18;147(12):836–839. [DOI] [PubMed] [Google Scholar]
- 72.Uldrick TS, Polizzotto MN, Aleman K, et al. Rituximab plus liposomal doxorubicin in HIV-infected patients with KSHV-associated multicentric Castleman disease. Blood 2014. Dec 4;124(24):3544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bower M, Newsom-Davis T, Naresh K, et al. Clinical Features and Outcome in HIV-Associated Multicentric Castleman’s Disease. Journal of Clinical Oncology 2011. 2011/06/20;29(18):2481–2486. [DOI] [PubMed] [Google Scholar]
- 74.Pria AD, Pinato D, Roe J, et al. Relapse of HHV8-positive multicentric Castleman disease following rituximab-based therapy in HIV-positive patients. Blood 2017;129(15):2143–2147. [DOI] [PubMed] [Google Scholar]
- 75.Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcoma–associated herpesvirus–related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood 2002. 2002/04/01/;99(7):2331–2336. [DOI] [PubMed] [Google Scholar]
- 76.Uldrick TS, Polizzotto MN, Aleman K, et al. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus-associated multicentric Castleman disease: a pilot study of virus-activated cytotoxic therapy. Blood 2011;117(26):6977–6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Rhee F, Wong RS, Munshi N, et al. Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. The Lancet Oncology 2014;15(9):966–974. [DOI] [PubMed] [Google Scholar]
- 78.Ramaswami R, Lurain K, Peer CJ, et al. Tocilizumab in patients with symptomatic Kaposi sarcoma herpesvirus–associated multicentric Castleman disease. Blood 2020;135(25):2316–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lurain K, Ramaswami R, Yarchoan R. The role of viruses in HIV-associated lymphomas. Seminars in Hematology 2022. 2022/10/01/;59(4):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cesarman E, Chadburn A, Rubinstein PG. KSHV/HHV8-mediated hematologic diseases. Blood 2022;139(7):1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Calvani J, Gérard L, Fadlallah J, et al. A Comprehensive Clinicopathologic and Molecular Study of 19 Primary Effusion Lymphomas in HIV-infected Patients. The American Journal of Surgical Pathology 2022;46(3). [DOI] [PubMed] [Google Scholar]
- 82.Chang Y, Moore PS, Talbot SJ, et al. Cyclin encoded by KS herpesvirus. Nature 1996. 1996/08/01;382(6590):410–410. [DOI] [PubMed] [Google Scholar]
- 83.Chaudhary PM, Jasmin A, Eby MT, et al. Modulation of the NF-κB pathway by virally encoded Death Effector Domains-containing proteins. Oncogene 1999. 1999/10/01;18(42):5738–5746. [DOI] [PubMed] [Google Scholar]
- 84.Ramaswami R, Chia G, Dalla Pria A, et al. Evolution of HIV-Associated Lymphoma Over 3 Decades. JAIDS Journal of Acquired Immune Deficiency Syndromes 2016;72(2). [DOI] [PubMed] [Google Scholar]
- 85.Klein U, Gloghini A, Gaidano G, et al. Gene expression profile analysis of AIDS-related primary effusion lymphoma (PEL) suggests a plasmablastic derivation and identifies PEL-specific transcripts. Blood 2003;101(10):4115–4121. [DOI] [PubMed] [Google Scholar]
- 86.Gopal S, Patel MR, Yanik EL, et al. Association of early HIV viremia with mortality after HIV-associated lymphoma. AIDS 2013;27(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shanmugasundaram K, Ramaswami R, Widell A, et al. Treatment Outcomes and Prognostic Factors in 40 Patients with Primary Effusion Lymphoma. Blood 2021. 2021/11/23/;138:1437. [Google Scholar]
- 88.Gopalakrishnan R, Matta H, Tolani B, et al. Immunomodulatory drugs target IKZF1-IRF4-MYC axis in primary effusion lymphoma in a cereblon-dependent manner and display synergistic cytotoxicity with BRD4 inhibitors. Oncogene 2016. 2016/04/01;35(14):1797–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lurain K, Ramaswami R, Widell A, et al. Phase I/II Study of Lenalidomide Combined with DA-EPOCH and Rituximab (DA-EPOCH-R2) in Primary Effusion Lymphoma in Patients with or without HIV. Blood 2019;134(Supplement_1):4096–4096. [Google Scholar]
- 90.Lurain K, Ramaswami R, Mangusan R, et al. Use of pembrolizumab with or without pomalidomide in HIV-associated non-Hodgkin’s lymphoma. Journal for ImmunoTherapy of Cancer 2021;9(2):e002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shah NN, Singavi AK, Harrington A. Daratumumab in Primary Effusion Lymphoma. New England Journal of Medicine 2018. 2018/08/16;379(7):689–690. [DOI] [PubMed] [Google Scholar]
- 92.Shrestha P, Astter Y, Davis DA, et al. Daratumumab induces cell-mediated cytotoxicity of primary effusion lymphoma and is active against refractory disease. Oncoimmunology 2023;12(1):2163784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Manzano M, Patil A, Waldrop A, et al. Gene essentiality landscape and druggable oncogenic dependencies in herpesviral primary effusion lymphoma. Nature Communications 2018. 2018/08/15;9(1):3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu Y, Shrestha P, Heape NM, et al. CDK4/6 inhibitors sensitize gammaherpesvirus-infected tumor cells to T-cell killing by enhancing expression of immune surface molecules. Journal of Translational Medicine 2022. 2022/05/13;20(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uldrick TS, Wang V, O’Mahony D, et al. An Interleukin-6-Related Systemic Inflammatory Syndrome in Patients Co-Infected with Kaposi Sarcoma-Associated Herpesvirus and HIV but without Multicentric Castleman Disease. Clinical Infectious Diseases 2010;51(3):350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramaswami R, Lurain K, Polizzotto MN, Widell A, Mangusan R, Ekwede I, Carey T, Lu C, George J, Whitby D, Uldrick TS, Yarchoan R, editor Study of characteristics and outcomes of KSHV Inflammatory Cytokine Syndrome (KICS). Conference on Retroviruses and Opportunistic Infections; 2023; Seattle, WA. [Google Scholar]
- 97.Hansen ME, Mangusan R, Lurain K, et al. Characteristics of patients admitted to the ICU with Kaposi sarcoma herpesvirus-associated diseases. Aids 2022. Nov 15;36(14):1969–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


