Abstract
Background and Aims:
The lack of easily measurable biomarkers remains a challenge in executing clinical trials for diabetic neuropathy (DN). Plasma Neurofilament light chain (NFL) concentration is a promising biomarker in immune-mediated neuropathies. Longitudinal studies evaluating NFL in DN have not been performed.
Methods:
A nested case-control study was performed in participants with youth-onset type 2 diabetes enrolled in the prospective Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study. Plasma NFL concentrations were measured at 4-year intervals from 2008–2020 in 50 participants who developed DN and 50 participants with type 2 diabetes who did not develop DN.
Results:
NFL concentrations were similar in the DN and no DN groups at first assessment. Concentrations were higher in DN participants at all subsequent assessment periods (all p<0.01). NFL concentrations increased over time in both groups, with higher degrees of change in DN participants (interaction p=0.045). A doubling of the NFL value at Assessment 2 in those without DN increased the odds of ultimate DN outcome by an estimated ratio of 2.86 (95% CI: (1.30, 6.33), p=0.0046). At the final study visit, positive Spearman correlations (controlled for age, sex, diabetes duration, and BMI) were observed between NFL and HbA1c (0.48, p<0.0001), total cholesterol (0.25, p=0.018), and LDL (0.30, p=0.0037). Negative correlations were observed with measures of heart rate variability (−0.42 to −0.46, p=<0.0001).
Interpretation:
The findings NFL concentrations are elevated in individuals with youth-onset type 2 diabetes, and increase more rapidly in those who develop DN, suggest NFL could be a valuable biomarker for DN.
Keywords: diabetic neuropathy, cardiac autonomic neuropathy, biomarkers, type 2 diabetes, youth-onset diabetes
Introduction:
Diabetic neuropathy (DN) is a highly morbid complication of diabetes for which no disease modifying treatments are currently available. Recent studies have shown that people with youth-onset type 2 diabetes develop both peripheral neuropathy and cardiac autonomic neuropathy (CAN) early in the course of their disease, with a reported 15-year cumulative incidence of DN of 32.4%1–5. Currently employed clinical outcome assessments lack the sensitivity needed to detect gradual neuropathy progression or regression6, and objective measures of DN severity, including nerve conduction studies (NCS) and intra-epidermal nerve fiber density (IENFD), also have important limitations. There is therefore a critical need for novel, non-invasive biomarkers for DN and CAN.
Neurofilament light chain (NFL) is a cytoskeletal protein released in response to axonal injury increasingly recognized as a promising biomarker for a variety of neurological disorders7,8. NFL is abundant in large, myelinated axons and present in both the central and peripheral nervous system. Elevations in blood NFL concentrations have been demonstrated in multiple acquired and inherited peripheral nerve disorders9–12. Specifically, NFL concentrations were found to serve as an independent prognostic marker in patients with Guillain Barre Syndrome (GBS)13, and to decline following treatment of vasculitic neuropathy, Chronic Inflammatory Demyelinating Polyneuropathy (CIDP), and Hereditary Transthyretin Amyloidosis Polyneuropathy (haTTR)9–11.
A recent cross-sectional study in individuals with type 1 and type 2 diabetes demonstrated higher NFL concentrations in those with clinical and electrophysiological evidence of neuropathy, however, there have been no longitudinal studies addressing the predictive value of NFL in people with diabetes14. Among candidate plasma biomarkers for peripheral neuropathy, NFL has several notable advantages, including high concentrations in axons, availability in biofluids15, and stability in stored samples16. We performed a case-control study evaluating plasma NFL concentrations over a 12-year period in participants of the Treatment Options for Type 2 Diabetes in Adolescents and Youth and observational follow-up (TODAY/TODAY2) study, which includes the largest cohort of young people with youth-onset type 2 diabetes to date1,3,17. The TODAY cohort shows rapid progression of insulin resistance and beta cell failure and high incidence of diabetes-associated complications at a young age, including peripheral neuropathy and CAN1,5. The objectives of our study were to retrospectively evaluate cross-sectional and longitudinal associations of plasma NFL concentrations with the presence of peripheral and autonomic neuropathy, in the context of established metabolic risk factors for DN in youth-onset type 2 diabetes.
Materials and Methods:
Participants:
The TODAY study (www.clinicaltrial.gov: NCT00081328) involved 699 participants with type 2 diabetes (American Diabetes Association 2002 criteria) diagnosed before the age of 18, with duration of diabetes <2 years, BMI≥85th percentile for age and sex, negative islet cell antibodies, and C-peptide >0.6 ng/mL randomized to receive metformin alone, metformin plus rosiglitazone, or metformin plus an intensive lifestyle intervention program at 15 participating centers17. After the initial TODAY trial ended in 2011, 572 participants were enrolled in the TODAY2 observational follow-up study17. All blood and urine samples were processed at the Northwest Lipid Metabolism and Diabetes Research Laboratories (NWRL) at the University of Washington. Frozen plasma samples from select participants at 4-year intervals between 2008 and 2020 were sent to the University of Colorado for determination of NFL concentrations.
TODAY and TODAY2 were approved by the institutional review boards and all participants and guardians provided written informed assent and/or consent as appropriate for age and local guidelines. Additional information on the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY), clinical treatment trial, Clinical Trials.gov Identifier NCT00081328, is available at: https://clinicaltrials.gov/ct2/show/NCT00081328.
Plasma samples for external control participants without diabetes were obtained from the observational control cohort of the Crnic Institute Human Trisome Project Biobank (COMIRB #15–2170) and provided to study staff in a de-identified manner. Informed consent was obtained from all participants for their use. Diabetes was excluded based on review of medical records and plasma samples were obtained at a single time point.
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Assessment of DN:
Michigan Neuropathy Screening Instrument (MNSI) scores for TODAY/TODAY2 participants were evaluated at four-year intervals between 2008 and 2020 - subsequently referred to as Assessments 1, 2, 3 and 4. The MNSI is a validated screening tool for DPN in adults and has been used in large cohort studies evaluating neuropathy in youth-onset diabetes3,18–20 The instrument consists of a 15-item self-administered questionnaire (MNSI-Q) and a 4-item examination score (MNSI-E)21. Measures of HR variability in the TODAY study were available at Assessments 2 and 4 only and obtained using methods previously described by Shah et al5. Briefly, heartbeats from an electrocardiogram (SphygmoCor device; AtCor Medical, Naperville, IL) were recorded for 10 minutes to determine HR variability. Using Fast Fourier analysis, the SphygmoCor device separates the heart rate spectrum into various components and allows the quantification of sympathetic and vagal influences on the heart. Measures obtained included the standard deviation of the R-R (NN) intervals (SDNN), an overall measure of HR variability, as well as the root mean square differences of successive NN intervals (RMSSD), percent of NN beats that differ by more than 50 ms (PNN50), and low-frequency (LF) power domain, high-frequency (HF) power domain, and their ratio (LF:HF). DN status was defined as an MNSI-E score >2 (a threshold previously validated in adult studies of DN) at the final assessment in 2019–202019,20. 50 participants with the highest MNSI-E scores were selected for the DN group in this study. All DN participants had an MNSI-E score of <2 (consistent with the absence of DN) at the first assessment in 2008. TODAY participants without DN had MNSI-E scores ≤ 2 at all assessments, including the final assessment in 2019–2020 and were matched to DN participants by sex, age, and disease duration. If multiple controls without DN matched a DN case equally well in sex, age, and disease duration, then the control with the lowest MNSI-E score was selected to maximize the difference between cases and controls.
Measurement of NFL:
Plasma samples from 100 participants from the TODAY/TODAY 2 study (50 with DN and 50 without DN) who were evaluated between 2008 and 2020 at four-year intervals (Assessments 1, 2, 3 and 4). Plasma was isolated from whole blood by centrifugation at 1500×g for 20 minutes followed by centrifugation at 400×g for 10 minutes to further deplete cells. Plasma was divided into 150 μl aliquots and frozen at −80°C. Individual aliquots of plasma were thawed overnight at 4°C and diluted 1:4 with Quanterix Sample Diluent from the Simoa® NF-light™ Advantage (SR-X) Kit (Quanterix catalog # 103400). Diluted samples were centrifuged at 10,000×g for 10 minutes at 4°C to pellet any residual debris and processed following the two-step assay instructions from the manufacturer and as previously reported22–24. Additionally, 39 external control subjects without type 2 diabetes were evaluated at a single time point for comparison. Blood samples were collected into BD Vacutainer® K2 EDTA tubes (BD, 366643). After centrifugation at 700×g for 15 mins at room temperature, EDTA plasma was separated and spun again at 2200×g for 15 mins and then used for SIMOA® measurements of neurodegeneration and neuroinflammation biomarkers. The Simoa® N4PB Kit (Quanterix catalog # 103345) was utilized and all samples were diluted and handled in the same manner as those assessed using the Simoa® NF-light™ Advantage (SR-X) Kit following the same two-step approach outlined above.
The Simoa® technology is highly sensitive for biomarker detection and is specifically designed to isolate and count single molecule immunocomplexes in its 50 femtoliter sized microarray wells, allowing for early detection of possible neuropathology and neurodegeneration21,22. The Simoa® NF-light™ Advantage assay is highly reproducible and has a coefficient of variation (CV) range within the run of 3–8%, between runs of 6–12% and between instruments of 1–6%23.
Statistical Methods:
The case-control data were analyzed on an available case basis using SAS 9.4. Summary statistics are presented, with means for continuous or scale variables and percentages for categorical variables. Differences between DN and no DN groups were tested using two-sample T-tests for continuous or scale variables, and with chi-square/Fisher’s exact association tests for categorical variables. MNSI scale components were examined descriptively with frequency tables. Spearman correlations and partial Spearman correlations were run between NFL and clinical characteristics and neuropathy outcome measures, including measures of overall metabolic and cardiorenal health. MNSI-E, MNSI-Q, LN[NFL], and LN[HbA1C] were analyzed using longitudinal linear mixed models, including repeated measures over time. To model predicted change in NFL over time with respect to DN, a categorical time variable and an interaction parameter between time and DN status at Assessment 4 were added to the linear mixed model. Cross sectional ANOVA/ANCOVA type models also compared TODAY DN and non-DN participants at the third and final assessments, 2016–2017 and 2019–2020 respectively, to external controls. T and F tests were performed on linear combinations of model parameters. Effect estimates for DN status were back transformed to geometric means. Logistic regression models were used to investigate the ability of NFL, in combination with other factors, to predict DN, and we examined resulting ROC curves to assess the predictive power of the models. Separate models were fit for each assessment. Covariates in the linear and logistic models included sex, age, type 2 diabetes duration, and BMI. HbA1C (log scale), an established predictor of microvascular complications in type 2 diabetes, was included as a covariate in selective models to assess the degree of association between NFL and DN independent of HbA1C. Covariates were selected at different assessments depending on the models. Unless otherwise stated, covariate values were selected at the time of the response for cross sectional models. In longitudinal models, values for BMI and HbA1C were time varying, selected from the same assessment as the response, while age and type 2 diabetes duration were selected at an end point, either Assessment 1 or Assessment 4, because assessment was also an explanatory variable in the model. For models predicting future outcomes at a previous assessment, the covariate values were selected at the previous assessment. Univariate alpha was set to 0.05 unless otherwise stated. The sample was selected so NFL and neuropathy data were complete at the beginning and end of the study.
Results:
Participants
50 TODAY participants with DN (as defined by an MNSI-E>2 at Assessment 4) and 50 participants without DN (MNSI-E<2 at all assessments) were evaluated. DN cases and controls were matched for self-reported gender (56% female), age (27 years) and duration of type 2 diabetes at the final visit (Assessment 4), and did not differ with respect to weight, BMI, or TODAY randomization group (TABLE 1). At Assessment 1, mean age for both groups was 16 years, and mean type 2 diabetes duration was 2.5 years. HbA1C levels among TODAY participants with and without DN did not differ at Assessments 1–3, however, participants with DN had higher HbA1C levels at Assessment 4 (geometric means 9.6% vs. 8.1%, p=0.008). There was no evidence of association between NFL and TODAY randomization group.
Table 1 –
Clinical characteristics, neuropathy and autonomic function measures.
| - | Type 2 diabetes without DN | Type 2 diabetes with DN |
|---|---|---|
|
| ||
| N=50 | N=50 | |
|
| ||
| Female (%) | 28 (56%) | 28 (56%) |
|
| ||
| Age (years) | ||
| Assessment 4 | 27.22 ± 2.68 | 27.30 ± 2.83 |
|
| ||
| Type 2 diabetes duration (years) | ||
| Assessment 4 | 13.48 ± 1.41 | 13.5 ± 1.47 |
|
| ||
| Race/Ethnicity: | ||
|
| ||
| Non-Hispanic Black (%) | 15 (30%) | 15 (30%) |
|
| ||
| Hispanic (%) | 21 (42%) | 25 (50%) |
|
| ||
| Non-Hispanic White (%) | 13 (26%) | 7 (14%) |
|
| ||
| Other (%) | 1 (2%) | 3 (6%) |
|
| ||
| Weight (kg) | ||
| Assessment 4 | 105.49 ± 25.31 (n=49) | 106.71 ± 29.41 |
|
| ||
| BMI (kg/m2) | ||
| Assessment 4 | 36.33 ± 8.46 (n=48) | 38.25 ± 10.43 (n=49) |
|
| ||
| SBP (mmHg) | ||
| Assessment 4 | 119.72 ± 13.32 | 122.80 ± 12.06 |
|
| ||
| Total cholesterol (mg/dL) | ||
| Assessment 4 | 181.68 ± 49.29 | 184.76 ± 42.96 |
|
| ||
| HDL (mg/dL) | ||
| Assessment 4 | 45.52 ± 12.31 | 46.28 ± 14.23 |
|
| ||
| LDL (mg/dL) | ||
| Assessment 4 | 101.70 ± 32.97 | 109.70 ± 30.73 |
|
| ||
| TG (mg/dL) | ||
| Assessment 4 | 182.10 ± 227.32 | 148.86 ± 111.13 |
|
| ||
| Serum Cystatin-C (mg/L) | ||
| Assessment 4 | 0.74 ± 0.25 | 0.69 ± 0.14 |
|
| ||
| eGFR (FAS) (mL/min/1.73m2) | ||
| Assessment 4 | 121.00 ± 28.27 | 129.40 ± 28.72 |
|
| ||
| Diabetes medication use (yes) | ||
| Assessment 4 | 39 (78%) | 35 (70%) |
|
| ||
| Metformin use (yes) | ||
| Assessment 4 | 31 (62%) | 21 (42%)* |
|
| ||
| Insulin (yes) | ||
| Year 4 | 24 (48%) | 27 (54%) |
|
| ||
| Statin use (yes) | ||
| Assessment 4 | 7 (14%) | 6 (12%) |
|
| ||
| Hypertension Medication (yes) | ||
| Assessment 4 | 22 (44%) | 14 (28%) |
|
| ||
| Neuropathy Measures | ||
|
| ||
| MNSI-Q | ||
| Assessment 4 | 1.36 ± 1.56 | 2.46 ± 2.65* |
|
| ||
| MNSI-E | ||
| Assessment 4 | 0.97 ± 0.87 | 3.38 ± 1.22*** |
|
| ||
| HbA1C concentrations (%) (n) | ||
|
| ||
| Assessment 4 | 8.05 (7.39, 8.76) (48) | 9.59 (8.80, 10.44) (49)** |
|
| ||
| Neurofilament (NFL) concentration (pg/mL) | ||
|
| ||
| Assessment 4 | 8.44 (6.94, 10.26) (50) | 12.48 (9.98, 15.60) (50)** |
|
| ||
| Cardiac Autonomic Neuropathy measures (n=37) | ||
|
| ||
| SDNN (ms) | ||
| Assessment 4 | 51.76 ± 25.00 | 42.79 ± 30.59 |
|
| ||
| RMSSD (ms) | ||
|
| ||
| Assessment 4 | 45.70 ± 35.17 | 35.95 ± 33.33 |
|
| ||
| PNN50 (%) | ||
| Assessment 4 | 20.16 ± 22.53 | 14.90 ± 22.24 |
|
| ||
| LF Power (n.u.) | ||
| Assessment 4 | 51.85 ± 22.26 | 54.02 ± 20.81 |
|
| ||
| HF Power (n.u.) | ||
| Assessment 4 | 48.15 ± 22.26 | 45.98 ± 20.81 |
|
| ||
| LF-HF Power | ||
| Assessment 4 | 1.74 ± 1.64 | 1.92 ± 2.18 |
Values are expressed as mean ± standard deviation (SD) or frequency (percent). Geometric means (95% CI) (N) for NFL and %HbA1C concentrations, calculated using a longitudinal regression model, presented.
Participants were matched for gender, age and duration of type 2 diabetes at Assessment 4.
statistically significant (p value: *p < 0.05, **p < 0.01, ***p <0.001). BMI = body mass index, SBP = systolic blood pressure, HBA1C = hemoglobin A1C, HDL = high-density lipoprotein, LDL = low-density lipoprotein, TG = triglyceride, eGFR (FAS) = estimated glomerular filtration rate (full age spectrum), MNSI-Q = Michigan Neuropathy Screening Instrument Questionnaire, MNSI-E = Michigan Neuropathy Screening Instrument Examination, SDNN = Standard deviation of NN intervals, RMSSD = Root mean square of successive RR interval differences, PNN50 = proportion of consecutive RR intervals that differ by more than 50ms, LF Power = power of the low-frequency band (0.04–0.15 Hz), HF Power = power of the high-frequency band (0.15–0.4 Hz). *Of note, for LF and HF, only normalized values are provided as absolute values were not available.
MNSI-E and MNSI-Q
Mean MSNI-E and MNSI-Q scores were similar in the two groups at Assessment 1 and indicated the absence of DN (for MNSI-E 0.62 points for DN and 0.36 points for no DN, p=0.11; for MNSI-Q 1.14 points for DN and 0.75 points for non DN, p=0.08). Between Assessments 1 and 4, MNSI-E and MNSI-Q increased in both the DN and non-DN groups [MNSI-E: 2.76 (p<0.0001) for DN, 0.61 (p<0.0001) for non-DN; MNSI-Q: 1.32 (p=0.002) for DN, and 0.61 (p=0.013) for non-DN]. MNSI-E increased more in the DN group than in the non-DN group (p<0.0001), however there was no between group difference in change for MNSI-Q (data not shown). Specific items of the MNSI-E that worsened in the DN group included ankle reflexes, vibration perception at the great toe, foot appearance, and dry skin and callus.
NFL concentrations are higher in TODAY participants with ultimate DN.
There was no significant difference in NFL between DN and no DN for TODAY participants at Assessment 1, however the geometric means for the DN group were significantly higher than in the no DN group for all subsequent time points [all p<0.01, adjusted for gender, age, and type 2 diabetes duration, and BMI (time varying)]. At Assessment 4, the geometric mean for NFL concentrations in TODAY participants with DN was 12.5 pg/mL as compared to 8.4 pg/mL in those without DN (Tukey-Kramer adjusted p<0.0001) (FIGURE 1A). In contrast to NFL, there were no significant group differences in HbA1C levels until Assessment 4 (p=0.004) (FIGURE 3B). Furthermore, if HbA1C (log scale; time varying) was further added as a covariate to the model with NFL as the response, group differences for NFL concentrations remained statistically significant (p<0.01) for both Assessment 2 and Assessment 3 while a trend remained at Assessment 4 (p=0.066) (data not shown).
Figure 1:
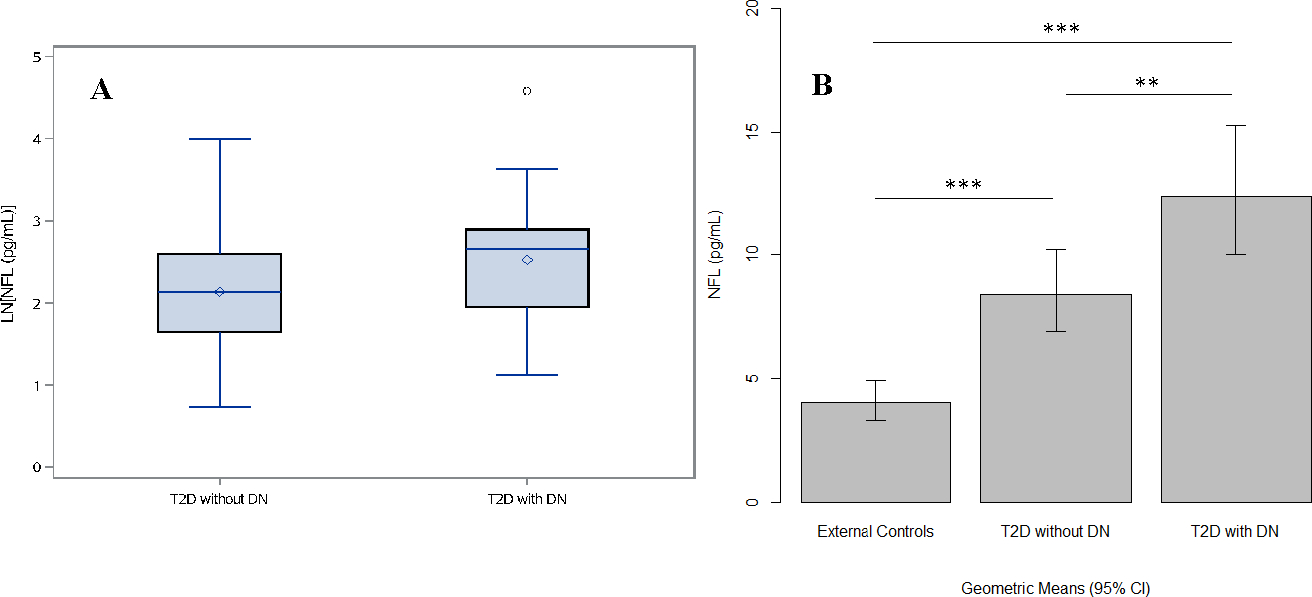
Plasma neurofilament light chain (NFL) concentration values in the TODAY cohort and external control cohort without diabetes.
Panel A--Boxplots of LN[NFL] (pg/mL) values at Assessment 4 in the TODAY/TODAY2 participants with and without DN. Panel B – Bar graph of NFL concentrations (pg/ml) in TODAY/TODAY2 participants compared to external controls without diabetes at Assessment 4. Statistically significant (p value: *p < 0.05, **p < 0.01, ***p <0.001).
Figure 3:
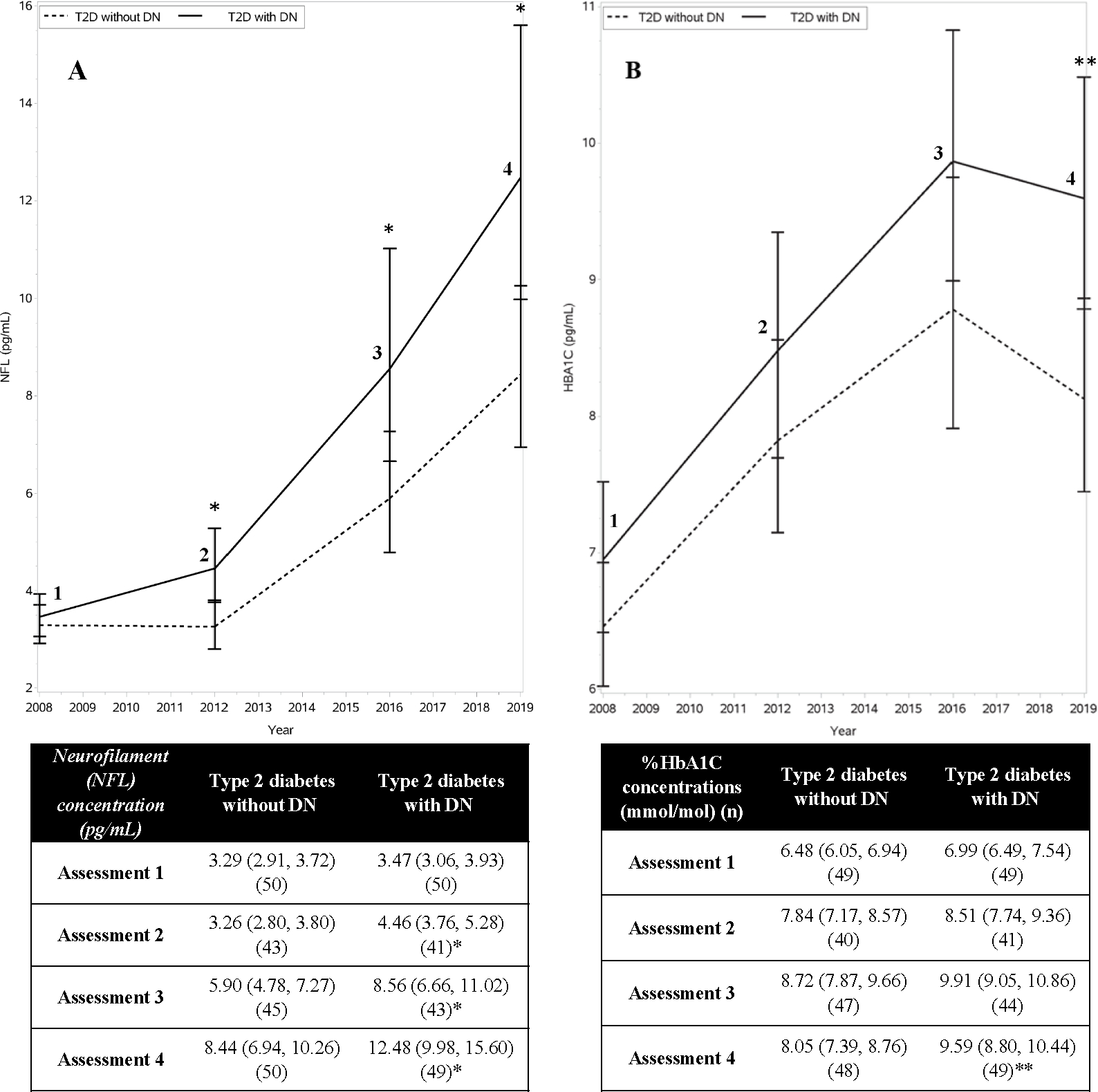
Neurofilament (NFL) concentration and HBA1C values over time.
Panel A = NFL concentrations increased over time in both the TODAY/TODAY2 DN and no DN groups, with higher degrees of change in the DN group.
Panel B = HBA1C concentrations over time. Statistically significant (p value: *p < 0.05, **p < 0.01). No difference in HBA1C concentrations between the DN and no DN groups was observed prior to Assessment 4.
NFL concentrations are higher in the TODAY cohort than those without type 2 diabetes.
NFL concentrations from TODAY participants were also compared to those of 39 external control subjects from a convenience cohort without type 2 diabetes. There was no gender difference between the external control participants and the TODAY participants. At Assessment 4, the external control participants were younger than the TODAY participants (mean 22 versus 27 years, p < 0.0001); and had lower BMIs (mean 23 kg/m2 for controls versus 36 kg/m2 for TODAY participants without DN and 38 kg/m2 for TODAY participants with DN, p < 0.0001). NFL concentrations in TODAY participants with and without DN were increased compared to those of the external controls (geometric means 12.5 and 8.4 pg/mL for T2D with and without DN respectively, as compared to 4.1 pg/mL for controls; Tukey-Kramer adjusted p < 0.0001 for both comparisons) (FIGURE 1). Results were qualitatively the same after adjusting for gender, age, and BMI. The estimated NFL geometric means at study Assessment 4, for both the DN and non-DN groups in TODAY were also substantially greater than the 90th percentile for NFL (7.57 pg/mL) in healthy controls at age 30, as established by an age adjusted model in a prior study25. NFL concentrations of the external control group were also compared to those at Assessment 3 when the TODAY participants were closest in age to the external control subjects [mean age 24 years for both TODAY groups versus 22 years for external controls (p = 0.071)]. The geometric means for NFL were 8.86 and 6.08 pg/mL for the TODAY DN and non-DN groups respectively, versus 4.10 pg/mL for the external control group (Tukey-Kramer adjusted p values < 0.0001 and equal to 0.008 respectively).
NFL increased over time in all participants with type 2 diabetes, with more rapid increases in the DN group.
We evaluated change over time in NFL concentrations among the TODAY participants using longitudinal regression using data from all four assessments (FIGURE 3A). Geometric means increased over time for both the DN and no DN groups (p<0.0001), with higher magnitude increases in those with DN (group by time interaction p = 0.045). Between Assessments 1 and 4, the geometric mean of the DN group increased by an estimated ratio of 3.60 (95% CI: (2.91, 4.45), p<0.0001), as compared to 2.567 (95% CI: (2.07, 3.178), p<0.0001) for the non-DN group (p value for the group difference in change = 0.026). HbA1C levels also increased within both DN and no DN patients (p<0.0001 for both). In contrast to NFL, however, there were no significant group differences in change in HbA1C levels (FIGURE 3B).
Predicting DN outcome from NFL levels
Logistic regression models for DN case-control status were adjusted for gender, age, type 2 diabetes duration, and BMI. At Assessment 4, the time of DN diagnosis, a doubling of NFL increased the odds of DN by a ratio of 1.86 [95% CI: (1.19, 2.91)], p =0.004). Despite the significant association of proportional differences in NFL with DN outcome, the predictive power of NFL at Assessment 4 was relatively low, with an ROC of 0.664 [95% CI: (0.56, 0.77)] (FIGURE 2A). There was no evidence for association between NFL concentrations (log scale) and DN at Assessment 1. At Assessment 2, when logistic models were restricted to participant with MNSI-E scores ≤2 (meaning no DN), a doubling of the NFL value at Assessment 2 increased the odds of DN outcome by an estimated ratio of 2.87 (95% CI: (1.30, 6.34), p=0.005), ROC = 0.719 [95% CI: (0.59, 0.85)].
Figure 2:
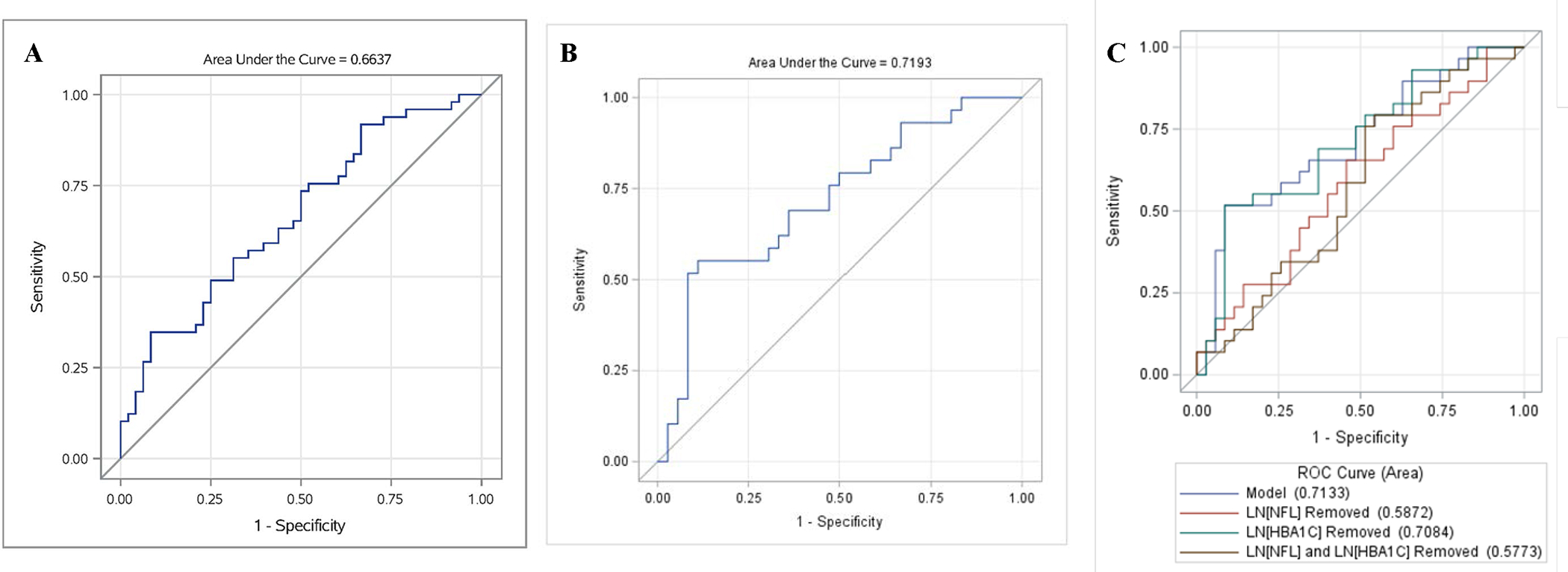
Predicting DN status using plasma neurofilament light chain concentrations (NFL)
Panel A = Cross-sectional predictive value of NFL at Assessment 4. Receiver operating characteristic (ROC) curve of DN at Assessment 4 with value of LN[NFL] and gender, age, Type 2 diabetes duration and BMI as explanatory variables. Cross sectional NFL concentrations show modest predictive power for DN status.
Panel B = Predictive value of NFL at Assessment 2 on future DN status. ROC for logistic regression model for DN status (Assessment 4), using LN[NFL], sex, age, duration, and BMI all at Assessment 2 for participants MNSIE <= 2). NFL concentrations at Assessment 2 show moderate predictive power for future DN status.
Panel C = Marginal contributions of NFL and HbA1C individually and combined to the predictive power of NFL at Assessment 2 on future DN status. ROC for logistic regression model for DN status (Assessment 4), using LN[NFL], LN[HbA1C], sex, age, duration, and BMI all at Assessment 2 for participants with MNSIE <= 2. NFL contributes more substantially to the predictive power of DN than HbA1C.
Given that HbA1C is an established risk factor for DN, we evaluated whether NFL provided added value in predicting future DN. We found that NFL remained a predictor of future DN when HbA1C (log scale) was added to the model for Assessment 2 (p value = 0.007), and its marginal effect was greater than the marginal effect of HbA1C, which was not statistically significant. The area under the ROC for the full model was 0.71, compared to 0.59 when NFL was removed, versus 0.71 when HbA1C was removed (FIGURE 2C).
NFL level associations with known metabolic risk factors for DN at Assessment 4
We examined the relationship between NFL concentrations and measures of multiple common metabolic risk factors and complications associated with type 2 diabetes, and with DN (SUPPLEMENTAL TABLE 1). Among all TODAY participants at Assessment 4, there was no evidence for association between NFL concentrations and either age or type 2 diabetes duration, potentially resulting from matching for these variables. Positive Spearman correlations (controlled for age, sex, type 2 diabetes duration and BMI at Assessment 4) were observed between NFL and HbA1C (0.48, p<0.0001), total cholesterol (0.25, p=0.018), LDL (0.30, p=0.004) and EGFR-FAS (0.26, p=0.013). In contrast, a negative correlation was observed for NFL with weight and BMI (−0.30, p=0.003 and −0.28, p=0.006 respectively). No evidence for association was observed for NFL with systolic BP, HDL, TG or Cystatin-C.
NFL level associations with measures of neuropathy at Assessment 4
Among all TODAY participants at Assessment 4, we observed positive partial Spearman correlations (controlled for age, sex, type 2 diabetes duration and BMI) between NFL concentrations and the MNSI-E (0.34, p=0.001), however no evidence for association was observed between NFL and MNSI-Q. Given the small sample size and limited range of MNSI-E scores in the DN group (1–5 points), these results should be interpreted with caution.
NFL levels associated with measures of autonomic function
Autonomic measures in our cohort were available at Assessments 2 and 4 only. Aside from a lower RMSSD and PNN50 at Assessment 2 in the type 2 diabetes with DN as compared to type 2 diabetes without DN group (p=0.048 and p=0.038, respectively), no differences in the measures were observed between groups. Negative Spearman correlations were observed between NFL concentrations at Assessment 4 (controlled for age, sex, type 2 diabetes duration and BMI) and SDNN, RMSSD, and PNN50 (−0.46, p<0.0001, −0.42, p=0.0001, and −0.42, p=0.0001 respectively) (FIGURE 4). When additionally controlling for HbA1C, weaker but still significant correlations were seen with SDNN and RMSSD (−0.26, p=0.019 and −0.24, p=0.032 respectively) while correlation with PNN50 was no longer significant (−0.22, p=0.055). No correlations were seen between NFL and the low-frequency (LF), and high-frequency (HF) power domains, or their ratios (SUPPLEMENTAL TABLE 1). Levels of NFL at Assessment 2 (controlled for age, sex, type 2 diabetes duration, and BMI at Assessment 2) negatively correlated with levels of SDNN, RMSSD, and PNN50 at Assessment 4. (−0.50, p<0.0001, −0.43, p=0.0004, and −0.44, p=0.0003 respectively). Change scores were not significantly correlated.
Figure 4:
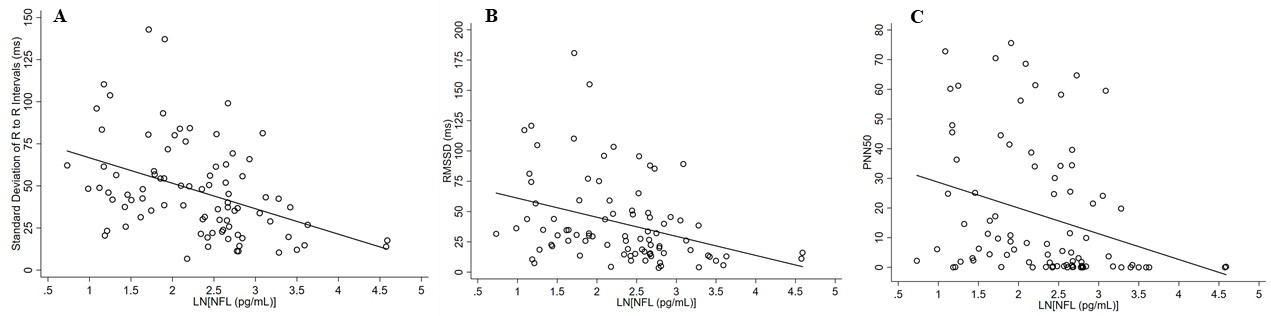
Correlation of Neurofilament (NFL) to Heart Rate Variability Measures at Assessment 4
Panel A = Standard deviation (SD) of the NN intervals compared to natural logarithm of Neurofilament light chain (NFL).
Panel B = Root mean square differences of successive NN intervals compared to natural logarithm of Neurofilament light chain (NFL).
Panel C = Percent of adjacent NN intervals with a difference >50 msec compared to natural logarithm of Neurofilament light chain (NFL). Data presented is unadjusted.
Discussion:
The TODAY/TODAY2 involves the largest longitudinal cohort with detailed physiological assessments in youth onset type 2 diabetes. This nested case-control study demonstrated elevated cross-sectional NFL concentrations in individuals with youth-onset type 2 diabetes, with greater increases in NFL concentrations over time in those who ultimately developed DN. Cross-sectional NFL concentrations did not adequately discriminate between participants with and without DN; however, a doubling of the NFL value at Assessment 2 in participants who had not yet developed DN increased the odds of DN outcome by an estimated 2.87 (ROC AUC of 0.721). Increases in NFL concentrations also preceded elevations in HbA1C in the DN group, suggesting NFL could have value as an earlier predictor of future DN. NFL positively correlated with multiple established neuropathy risk factors including HbA1C, total cholesterol, and LDL, as well as with multiple measures heart rate variability. Taken together, these findings suggest NFL concentration could provide value as a plasma biomarker of neuronal injury in type 2 diabetes.
The degree of NFL elevation observed in the TODAY participants aligns with levels reported in other chronic neuropathies including CIDP and Charcot Marie Tooth disease, but is lower than those reported with more acute and/or aggressive neuropathies15. The degree of relative increase in NFL concentrations associated with DN in the TODAY cohort is also comparable to that reported in adults with diabetes14. The lower absolute NFL concentrations in the TODAY participants with DN as compared to those reported by Maalmi et al. may reflect the age of the participants (mean age of 27 years in TODAY versus 49 years for participants in the adult cohort, respectively).
The finding that NFL was already elevated early in the disease course of type 2 diabetes in TODAY participants is concerning and suggests neuronal injury is occurring long before clinical manifestations of peripheral neuropathy. Given NFL is a non-specific marker of damage to large, myelinated axons, we cannot be certain whether these early elevations reflect central versus peripheral nervous system injury. There is mounting evidence that hyperglycemia is associated with structural brain changes and neurocognitive dysfunction in both adults and youth with type 2 diabetes26–29, and serum NFL concentrations were previously shown to associate with stroke incidence in adults with diabetes30. Underscoring the specific relevance of NFL to nerve injury in diabetes is the recent work of Maalmi et al., showing that higher NFL concentrations correlate with slower motor and sensory nerve conduction velocities, as well as lower sural sensory amplitudes14.
The TODAY participants were, at most, two years out from their diagnosis of type 2 diabetes at study entry and no participant had developed clinically detectable neuropathy at the first assessment. Our analyses of NFL concentrations were therefore likely to coincide with the onset of axonal degeneration. In keeping with this consideration is the observation NFL concentrations changed the least over the first 4 years of the study and then increased more rapidly, particularly in the DN group. Our findings pose an interesting contrast to those of a prior study in adult patients with inherited neuropathies (mean age 46 years), which revealed baseline elevations in NFL without changes in concentrations over a 6-year period12. The importance of the timing of NFL measurement to disease activity has been underscored previously, including the observation serum NFL concentrations are higher in patients with a recent relapse of Multiple Sclerosis versus those with clinically stable disease31.
Geometric mean NFL concentrations were higher in TODAY participants with type 2 diabetes with DN versus type 2 diabetes without DN at all visits except at assessment 1, and the magnitude of the differences increased across the visits. Only a minority of the 50 participants that eventually went on to develop DN during the study period met the MNSI-E criteria for DN at Assessments 2 and 3 (N=8 and 21, respectively) consistent with elevated NFL levels at least four years prior to diagnosis of clinical DN. Furthermore, a doubling in change in NFL from Assessment 1 to Assessment 2 substantially increased the odds of ultimate DN. NFL has shown promise as a pre-symptomatic marker of nerve injury in multiple central nervous system disorders, however, its potential to predict onset of DN will require further investigation8. The potential of NFL concentrations to track disease severity (as seen in chemotherapy-associated neuropathy), and to respond to therapeutic intervention9–11,32, could also notably enhance the design and sensitivity of clinical trials if similar findings are identified in patients with DN.
We did not observe a correlation between NFL concentrations and age in TODAY/TODAY2 participants. These findings contrast with those of prior studies, which have shown that NFL concentrations increase with age, as a likely consequence of neuronal degeneration associated with normal aging33. The absence of a relationship between age and NFL in TODAY may be explained by the young age of the participants at study entry, the limited age range in this cohort, and the fact that participants in the DN and no DN group were matched by age at the final study visit. Most prior longitudinal studies of NFL have also examined older individuals, and Hayer et al. previously found no increase in NFL with age in healthy controls aged <30 years34. It is also possible that the degree of NFL elevation in people with active neurodegeneration masks the normal age-related increase in NFL.
HbA1C is an established risk factor for DN, so it was important to consider whether NFL provides added benefit as a biomarker. In this cohort, controlling for HbA1C at Assessment 4, when DN status was determined, attenuated the difference in NFL concentrations, as well as in the change in NFL concentrations between participants with and without the ultimate outcome of DN. It is important to note, however, that the change in NFL trajectory was already apparent at Assessment 2, prior to divergence of HbA1C trajectories. As such, differences in glycemic control cannot explain the diverging NFL levels in the two groups. Rather, it is possible the DN group was metabolically distinct in ways that made these participants more vulnerable to early neuronal injury and deterioration in glycemic control. Furthermore, in the logistic model at Assessment 2, NFL contributed more to the predictive power of future DN than HbA1C (FIGURE 2C). The modest negative correlation observed between NFL concentrations and BMI is surprising given that obesity is a well-established risk factor for neuropathy in adults, and that the same relationship has been reported in youth3,35. The participants of our study had a high prevalence of obesity (mean BMI of 36 kg/m2), and this finding will therefore need to be readdressed in larger cohorts that include lower BMI participants with diabetes. Of particular interest are the observed negative correlations between NFL concentrations and autonomic measures. Autonomic dysfunction is a common and underdiagnosed in patients with type 2 diabetes, and cardiac autonomic neuropathy (CAN) is associated with a 5-fold increase in mortality risk36. We identified negative correlations between NFL and SDNN, a measure of overall HRV, as well as with several measures of parasympathetic function. While adjusting for HbA1C weakened the correlation between NFL and measures of HRV, it remained significant, suggesting glycemic control partially accounts for the association, yet does not fully explain the relationship between NFL and the observed autonomic changes. Our findings suggest plasma NFL levels could potentially aid in identifying patients at risk for CAN.
Our study is limited by small sample size, though the notable difference in the rate of change in NFL between participants with and without DN in this sample further underscores the potential relevance of NFL to DN. Though rising in prevalence, type 2 diabetes in youth remains an uncommon disease and the TODAY2 represents the largest natural history study in youth onset type 2 diabetes. Plasma samples for control participants without diabetes were also obtained from a different study and detailed phenotyping for metabolic risk factors and neuropathy was not available. Another important limitation is the lack of objective neuropathy-specific measures in the TODAY participants, who were rigorously phenotyped for type 2 diabetes complications, but given their young age at study entry, did not undergo NCS or skin biopsies for IENFD. Given the limitations of the MNSI we cannot be confident participants in the type 2 diabetes without DN group were not experiencing early nerve injury. Finally, the TODAY cohort was disproportionately Black and Hispanic, and “non-white” race has been reported to associate with higher plasma concentrations of NFL30. An important strength of our study was the availability of longitudinal data over a 12-year period, including detailed autonomic measures.
Taken together, our findings suggest that NFL may have a role as an objective biomarker of neuronal injury in type 2 diabetes and could add value to future prevention and treatment trials. Future studies will need to examine NFL across the age spectrum of type 2 diabetes in conjunction with currently available neuropathy assessments including IENFD to better define its relevance in DN.
Supplementary Material
Acknowledgements:
The TODAY study was conducted by the TODAY Investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The data and samples from the TODAY study reported here were supplied by the NIDDK Central Repository and the Lifecourse Epidemiology of Adiposity & Diabetes Center at the University of Colorado Anschutz Medical Campus.
We would like to thank the participants of the TODAY study and all of the participating centers, participants in the Crnic Institute Human Trisome Project. We also wish to acknowledge the TODAY Study Group, including the leadership and the staff at the coordinating center, for their support and guidance. We would like to thank Eva Feldman, Brian Callaghan and Rodica Pop-Busui, University of Michigan, for helpful discussions addressing DN and CAN specific outcome assessments, and Sean Selva, University of Colorado, for assisting with sample preparation. We would like to thank our funders including from NIH grants U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, U01-DK61254, 1K23DK118202–01A1, R01AI150305 (JME), UL1TR002535, the University of Colorado School of Medicine Doris Duke Fund to Retain Clinical Scientists Program Award, the Linda Crnic Institute for Down Syndrome, the Global Down Syndrome Foundation and VA CX001532, BX002046, UL1 TR000154, P30 DK116073 (JEBR).
Dr. Vera Fridman is the guarantor of this manuscript and takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
V.F. was responsible for the study conception, oversaw study design and data analysis, wrote the first draft of the manuscript and revised the manuscript. S.S. performed the statistical analysis and contributed the statistical analysis and graphs for the manuscript. A.R. performed the NFL analysis for TODAY participants. J.B. assisted with manuscript preparation and submission and edited drafts of the manuscript. C.C. performed NFL analysis for the external control samples and edited drafts of the manuscript. P.A. processed external control samples and edited drafts of the manuscript. J.M.E. processed control samples and edited drafts of the manuscript. K.S. processed control samples. E.M.L. contributed to the statistical analysis and edited drafts of the manuscript. L.A.L. contributed to study design and edited drafts of the manuscript. L.E. oversaw data collection and data transfer and edited drafts of the manuscript. K.L.D. oversaw data collection and data transfer and edited drafts of the manuscript. P.Z oversaw data collection and data transfer and edited drafts of the manuscript. J.E.B.R. facilitated collaboration with the TODAY study, was involved in the study design, and edited drafts of the manuscript.
Footnotes
All authors report no relevant financial conflicts of interest to disclose.
References
- 1.Group TS, Bjornstad P, Drews KL, et al. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med. Jul 29 2021;385(5):416–426. doi: 10.1056/NEJMoa2100165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaiswal M, Divers J, Dabelea D, et al. Prevalence of and Risk Factors for Diabetic Peripheral Neuropathy in Youth With Type 1 and Type 2 Diabetes: SEARCH for Diabetes in Youth Study. Diabetes Care. Sep 2017;40(9):1226–1232. doi: 10.2337/dc17-0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Group TS. Risk Factors for Diabetic Peripheral Neuropathy in Adolescents and Young Adults With Type 2 Diabetes: Results From the TODAY Study. Diabetes Care. Oct 29 2021;doi: 10.2337/dc21-1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaiswal M, Divers J, Urbina EM, et al. Cardiovascular autonomic neuropathy in adolescents and young adults with type 1 and type 2 diabetes: The SEARCH for Diabetes in Youth Cohort Study. Pediatr Diabetes. Jun 2018;19(4):680–689. doi: 10.1111/pedi.12633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah AS, El Ghormli L, Vajravelu ME, et al. Heart Rate Variability and Cardiac Autonomic Dysfunction: Prevalence, Risk Factors, and Relationship to Arterial Stiffness in the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study. Diabetes Care. Nov 2019;42(11):2143–2150. doi: 10.2337/dc19-0993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zilliox LA, Ruby SK, Singh S, Zhan M, Russell JW. Clinical neuropathy scales in neuropathy associated with impaired glucose tolerance. J Diabetes Complications. Apr 2015;29(3):372–7. doi: 10.1016/j.jdiacomp.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petzold A Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci. Jun 15 2005;233(1–2):183–98. doi: 10.1016/j.jns.2005.03.015 [DOI] [PubMed] [Google Scholar]
- 8.Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. Aug 2019;90(8):870–881. doi: 10.1136/jnnp-2018-320106 [DOI] [PubMed] [Google Scholar]
- 9.Bischof A, Manigold T, Barro C, et al. Serum neurofilament light chain: a biomarker of neuronal injury in vasculitic neuropathy. Ann Rheum Dis. Jul 2018;77(7):1093–1094. doi: 10.1136/annrheumdis-2017-212045 [DOI] [PubMed] [Google Scholar]
- 10.Fukami Y, Iijima M, Koike H, Yamada S, Hashizume A, Katsuno M. Association of serum neurofilament light chain levels with clinicopathology of chronic inflammatory demyelinating polyneuropathy, including NF155 reactive patients. J Neurol. Oct 2021;268(10):3835–3844. doi: 10.1007/s00415-021-10537-2 [DOI] [PubMed] [Google Scholar]
- 11.Ticau S, Sridharan GV, Tsour S, et al. Neurofilament Light Chain as a Biomarker of Hereditary Transthyretin-Mediated Amyloidosis. Neurology. Jan 19 2021;96(3):e412–e422. doi: 10.1212/WNL.0000000000011090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossor AM, Kapoor M, Wellington H, et al. A longitudinal and cross-sectional study of plasma neurofilament light chain concentration in Charcot-Marie-Tooth disease. J Peripher Nerv Syst. Dec 1 2021;doi: 10.1111/jns.12477 [DOI] [PubMed] [Google Scholar]
- 13.Martin-Aguilar L, Camps-Renom P, Lleixa C, et al. Serum neurofilament light chain predicts long-term prognosis in Guillain-Barre syndrome patients. J Neurol Neurosurg Psychiatry. Nov 5 2020;doi: 10.1136/jnnp-2020-323899 [DOI] [PubMed] [Google Scholar]
- 14.Maalmi H, Strom A, Petrera A, et al. Serum neurofilament light chain: a novel biomarker for early diabetic sensorimotor polyneuropathy. Diabetologia. Mar 2023;66(3):579–589. doi: 10.1007/s00125-022-05846-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wieske L, Smyth D, Lunn MP, Eftimov F, Teunissen CE. Fluid Biomarkers for Monitoring Structural Changes in Polyneuropathies: Their Use in Clinical Practice and Trials. Neurotherapeutics. Oct 2021;18(4):2351–2367. doi: 10.1007/s13311-021-01136-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altmann P, Ponleitner M, Rommer PS, et al. Seven day pre-analytical stability of serum and plasma neurofilament light chain. Sci Rep. May 26 2021;11(1):11034. doi: 10.1038/s41598-021-90639-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Group TS, Zeitler P, Epstein L, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. Apr 2007;8(2):74–87. doi: 10.1111/j.1399-5448.2007.00237.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaiswal M, Lauer A, Martin CL, et al. Peripheral neuropathy in adolescents and young adults with type 1 and type 2 diabetes from the SEARCH for Diabetes in Youth follow-up cohort: a pilot study. Diabetes Care. Dec 2013;36(12):3903–8. doi: 10.2337/dc13-1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman WH, Pop-Busui R, Braffett BH, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. Jul 2012;29(7):937–44. doi: 10.1111/j.1464-5491.2012.03644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg. Jul 2006;108(5):477–81. doi: 10.1016/j.clineuro.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 21.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. Nov 1994;17(11):1281–9. doi: 10.2337/diacare.17.11.1281 [DOI] [PubMed] [Google Scholar]
- 22.Mariotto S, Gajofatto A, Zuliani L, et al. Serum and CSF neurofilament light chain levels in antibody-mediated encephalitis. J Neurol. Jul 2019;266(7):1643–1648. doi: 10.1007/s00415-019-09306-z [DOI] [PubMed] [Google Scholar]
- 23.Bettcher BM, Olson KE, Carlson NE, et al. Astrogliosis and episodic memory in late life: higher GFAP is related to worse memory and white matter microstructure in healthy aging and Alzheimer’s disease. Neurobiol Aging. Jul 2021;103:68–77. doi: 10.1016/j.neurobiolaging.2021.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potter H, Woodcock JH, Boyd TD, et al. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer’s disease. Alzheimers Dement (N Y). 2021;7(1):e12158. doi: 10.1002/trc2.12158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harp C, Thanei GA, Jia X, et al. Development of an age-adjusted model for blood neurofilament light chain. Ann Clin Transl Neurol. Apr 2022;9(4):444–453. doi: 10.1002/acn3.51524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pourabbasi A, Tehrani-Doost M, Qavam SE, Arzaghi SM, Larijani B. Association of diabetes mellitus and structural changes in the central nervous system in children and adolescents: a systematic review. J Diabetes Metab Disord. 2017;16:10. doi: 10.1186/s40200-017-0292-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. Jun 16 2012;379(9833):2291–9. doi: 10.1016/S0140-6736(12)60360-2 [DOI] [PubMed] [Google Scholar]
- 28.Callisaya ML, Beare R, Moran C, Phan T, Wang W, Srikanth VK. Type 2 diabetes mellitus, brain atrophy and cognitive decline in older people: a longitudinal study. Diabetologia. Mar 2019;62(3):448–458. doi: 10.1007/s00125-018-4778-9 [DOI] [PubMed] [Google Scholar]
- 29.Redel JM, Dolan LM, DiFrancesco M, Vannest J, Shah AS. Youth-Onset Type 2 Diabetes and the Developing Brain. Curr Diab Rep. Jan 21 2019;19(1):3. doi: 10.1007/s11892-019-1120-y [DOI] [PubMed] [Google Scholar]
- 30.Korley FK, Goldstick J, Mastali M, et al. Serum NfL (Neurofilament Light Chain) Levels and Incident Stroke in Adults With Diabetes Mellitus. Stroke. Jul 2019;50(7):1669–1675. doi: 10.1161/STROKEAHA.119.024941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Disanto G, Barro C, Benkert P, et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol. Jun 2017;81(6):857–870. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SH, Choi MK, Park NY, et al. Serum neurofilament light chain levels as a biomarker of neuroaxonal injury and severity of oxaliplatin-induced peripheral neuropathy. Sci Rep. May 14 2020;10(1):7995. doi: 10.1038/s41598-020-64511-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vagberg M, Norgren N, Dring A, et al. Levels and Age Dependency of Neurofilament Light and Glial Fibrillary Acidic Protein in Healthy Individuals and Their Relation to the Brain Parenchymal Fraction. PLoS One. 2015;10(8):e0135886. doi: 10.1371/journal.pone.0135886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayer SN, Liepelt I, Barro C, et al. NfL and pNfH are increased in Friedreich’s ataxia. J Neurol. May 2020;267(5):1420–1430. doi: 10.1007/s00415-020-09722-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callaghan BC, Xia R, Banerjee M, et al. Metabolic Syndrome Components Are Associated With Symptomatic Polyneuropathy Independent of Glycemic Status. Diabetes Care. May 2016;39(5):801–7. doi: 10.2337/dc16-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. Jun 2003;26(6):1895–901. doi: 10.2337/diacare.26.6.1895 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


