Abstract
Objective:
There is a strong association between sleep disturbance and negative affect. However, the day-to-day directional connections between sleep and negative affect remain unclear. We examined day-to-day relationships between sleep duration and negative affect in community adults.
Methods:
Participants were two subsamples of the Midlife in the United States Study (Sample 1: n = 2,022; Sample 2: n = 782). Daily negative affect and previous night sleep duration were assessed via end-of-day telephone interviews for eight days. Random intercept cross-lagged panel models tested sleep duration as a predictor of next-day negative affect and vice versa, controlling for age, gender, and race.
Results:
In both samples, shorter sleep duration predicted higher next-day negative affect, but daily negative affect was not a significant predictor of upcoming-night sleep duration. Follow-up analyses indicated that the relationship between sleep duration and negative affect was nonlinear. Sleeping fewer than 7.5 hours or more than 10.5 hours was associated with greater next-day negative affect than sleeping between 7.5 and 10.5 hours.
Conclusions:
In two large samples of community adults, sleep duration unidirectionally predicted higher next-day negative affect, and this relationship was nonlinear. Sleeping at least 7.5 hours and no more than 10.5 hours appeared to be an optimal range associated with lowest next-day negative affect.
Keywords: sleep, negative affect, daily diary, experience sampling, prospective
1. Introduction
There is a well-documented connection between poor sleep and heightened negative affect [1–4]. Disrupted sleep is linked to mental health problems characterized by negative affect, such as depression and anxiety [5–9]. Furthermore, experimental findings suggest a relationship between inadequate sleep and negative emotionality [10–12]. Insufficient sleep may be both an antecedent and consequence of negative affect [13–15]. However, sleep and negative affect fluctuate from day to day [16, 17], and the daily relationships between the two are not entirely clear. Thus, the present study examined whether and to what extent daily variations in negative affect and sleep duration predict one another.
Emotion regulation theories posit that sleep is critical for affective functioning. According to these models, inadequate sleep can influence emotion regulation by impairing emotional appraisal, reactivity, and management [12, 18–20]. For instance, inadequate sleep can skew negative perceptions of stimuli and lead to a negative emotional bias [1, 2, 13]. Further, experimentally induced sleep deprivation intensifies affective reactivity to negative or stressful experiences [21–24]. Moreover, as sleep restriction is associated with diminished cognitive performance and self-control, intentionally regulating negative emotions may be more difficult following a night of poor sleep [25, 26]. Thus, shorter sleep could be an antecedent of elevated negative affect.
Just as shorter sleep may influence affect, daily affect may influence sleep [27]. Stress and anxiety are associated with heightened physiological arousal, which could interfere with sleep quality and shorten sleep duration [20, 28]. Emotion regulation may also contribute to the relationship between negative affect and sleep disruption [18, 29]. Cognitive activity from engaging in maladaptive emotion regulation strategies (e.g., worry, rumination) might increase arousal and delay sleep onset [1, 14]. Therefore, negative affect and poor sleep may have a reciprocally influencing relationship.
As sleep and affect both fluctuate daily, the relationship between insufficient sleep and negative affect could play out on a day-to-day level. Laboratory studies showed that sleep deprivation heightened negative affect [10, 30, 31], but whether these findings reflect the relationship between sleep loss and negative affect in a naturalistic context is unknown. Furthermore, these experiments were limited to testing only the unidirectional association between sleep loss and future negative affect. Experience sampling designs, like daily diary studies, offer a more ecologically valid way to investigate the temporal connections between sleep and daily affect [32]. These methods gather repeated information on participants’ everyday experiences and allow for the examination of bidirectional associations. Thus, experience sampling could provide a naturalistic picture of the day-to-day relationships between sleep and negative affect.
Experience sampling research has supported the connection between sleep and daily negative affect, but the directionality of this association remains unclear. Some studies suggested a bidirectional relationship between sleep and affect. For instance, a study of adults with a depressive or anxiety disorder found a reciprocal association, such that enhanced sleep quality led to lower negative affect the following day, and higher daily negative affect predicted worse sleep that night [33]. In adolescents, elevated daily negative affect had bidirectional prospective connections with lower sleep quality [34] and shorter sleep time [35]. Similarly, higher daily stress levels were bidirectionally associated with shorter sleep duration among young adults [36] and high school students [37]. Thus, it is possible that poor sleep and negative affect have a mutually-influencing relationship on a daily basis.
On the other hand, many experiencing sampling studies suggested a unidirectional relationship of insufficient sleep predicting higher next-day negative affect, but not vice versa. One study of female twins found that poor sleep predicted elevated negative affect the following day, but daily affect was not associated with subsequent sleep [38]. McCrae, et al. [39] reported a similar pattern in a 14-day diary study of older adults: shorter sleep duration was associated with worse next-day mood, but not vice versa. Shorter sleep duration was also linked to worse emotional well-being the following morning in adolescents and adults [40]. Similarly, shorter sleep duration unidirectionally predicted higher next-day negative affect in a small female-only sample [41]. In contrast, one study suggested that negative affect may unidirectionally influence sleep. Heightened feelings of sadness and fear predicted shorter sleep duration in college-aged women, but sleep time did not predict next-day negative affect [42]. Thus, of studies documenting unidirectional daily sleep-affect relationships, the majority to our knowledge have found poorer sleep to predict greater next-day negative affect, but not vice-versa.
Of note, it is possible that the relationship between sleep and subsequent negative affect is nonlinear. In other words, although greater sleep, in general, might be related to lower next-day negative affect, sleeping for an extreme duration could predict greater negative affect. Considering behavioral theories of mood, very high levels of sleep could reduce engagement with responsibilities and rewarding activities and depress mood [43]. Support for this possibility comes from epidemiological studies documenting an increased rate of oversleeping in mood disorders [44], as well as evidence that both lack of sleep and excessive sleep prospectively predicted mood disorder onset [45]. Additionally, in a daily diary study, sleeping either below or well above (e.g., ≥ 2 hours) one’s usual level of sleep predicted poorer next-day mood among adults [40]. Therefore, the literature also calls for more fine-grained evidence on the possibly nonlinear nature of the relationship between sleep and next-day affect.
The reviewed literature provides evidence for an association between shorter sleep and negative affect. Nevertheless, more research is necessary to understand the directionality and shape of the day-to-day relationships between sleep duration and negative affect. Past research has examined this question utilizing specific samples (e.g., adolescent, clinical outpatient) and yielded heterogeneous results, which might indicate unique sleep-affect relationships in these populations. As sleep characteristics are different in non-clinical adults as compared to adolescents [46] or adults with a mental health condition [47], these past findings have limited generalizability to the broader adult population. Further, studies that have examined sleep and affect in non-clinical adult samples have not assessed daily bidirectional associations [30]. Additionally, very few studies have tested nonlinear relationships between sleep and affect. Thus, we sought to extend prior research by evaluating the day-to-day links between sleep duration and negative affect in a large community adult sample with a wide age range. The connections between sleep and affect are particularly important to examine in adulthood, as sleep habits in this period are critical for optimal health [48].
The purpose of the present study was to test relationships between self-reported sleep duration and daily negative affect over an eight-day period among community-dwelling adults. To bolster confidence in results, analyses were conducted in two separate cohorts of adults from the Midlife in the United States Study [MIDUS; 49]. We hypothesized that (1) shorter sleep duration would predict higher levels of negative affect the following day, and (2) elevated daily negative affect would predict shorter sleep duration that night. Additionally, we conducted follow-up analyses to test for nonlinearity in the relationship between sleep and next-day negative affect. In line with findings from one prior study [40], we predicted that greater sleep on a given night would be associated with lower next-day negative affect for most levels of sleep, but that this association would weaken at higher levels of sleep and reverse at extreme levels of sleep (more than 10.5 hours).
2. Methods
2.1. Participants
2.1.1. Sample 1 Participants
Data were from the National Study of Daily Experiences [NSDE; 50], a daily diary subproject of MIDUS. NSDE consisted of daily telephone interviews for eight consecutive days. Sample 1 included 2,022 community-dwelling adults from the first wave of MIDUS data collection (MIDUS 1, collected between 1995-1996) who participated in the NSDE subproject of the second wave of MIDUS data collection (MIDUS 2, collected between 2004–2009). Participants were recruited using random digit dialing. Eligibility criteria for MIDUS 1 included age between 25-75, living in the conterminous U.S., English-speaking, and non-institutionalized. Of the MIDUS 2 NSDE participants, 57% were female and the majority identified their race as white (84%). At the time of data collection for MIDUS 2, the average age of the sample was 56.2 (SD = 12.2; range = 33–84).
2.1.2. Sample 2 Participants
Sample 2 included the 782 adults who took part in the NSDE daily diary subproject of the MIDUS Refresher study [51]. Data for this sample were collected between 2012–2014 as a replication study for the main MIDUS/NSDE projects [52], using the same recruitment procedures and eligibility criteria as Sample 1. Of the participants in Sample 2, 56% identified their gender as female and 84% identified their race as white. The average age was 47.9 (SD = 12.7; range = 25–74).
2.2. Procedures
The University of Wisconsin-Madison Institutional Review Board (IRB) approved all study protocols. Written informed consent was obtained from all participants. Additional IRB approval or consent was not required for this report, which was a secondary analysis of publicly available, de-identified data.
Data collection for both Samples 1 and 2 consisted of evening telephone interviews for eight consecutive days. Participants were asked a series of questions related to daily experiences, including questions about their affect that day and sleep the previous night.
2.3. Measures
2.3.1. Sleep Duration
As part of the telephone interviews, participants were asked, “Since this time yesterday, how much time did you spend sleeping, not including time you may have spent napping?” and researchers recorded the total amount of time the participant reported sleeping. Participants’ responses were coded as hours and minutes. We calculated daily sleep duration as decimal hours (e.g., 6 hrs and 30 min as 6.5 hrs). It was not possible to calculate internal consistency for this measure given that it comprised one item, though other studies have documented strong correlations between similar one-item sleep duration measures and objectively assessed sleep duration (e.g., assessed via actigraphy) [33, 53]. Thus, we judged this to be a valid measure of sleep duration.
2.3.1. Negative Affect
Negative affect was assessed using a measure developed for the MIDUS study [54]. Items were selected from the Positive and Negative Affect Schedule [PANAS; 55] and Non-Specific Psychological Distress Scale [56]. The negative affect scale included 14 negatively-valenced emotions: restless or fidgety, nervous, worthless, so sad nothing could cheer you up, everything was an effort, hopeless, lonely, afraid, jittery, irritable, ashamed, upset, angry, and frustrated. Participants rated how often they experienced each emotion over the previous 24 hours using a 5-point scale ranging from 0 (none of the time) to 4 (all of the time). The questionnaire was scored by calculating the average score across all 14 items. In the present work, this scale demonstrated good internal consistency in both sample 1 (within-person omega = .82; between-person omega = .92) and sample 2 (within-person omega = .82; between-person omega = .94), as calculated using the multilevelTools package in R [57].
2.4. Data Analyses
Data analyses were conducted using R (Version 4.0.3) via the package lavaan [58]. Random-intercept cross-lagged panel models [RI-CLPM; 59] were used to examine prospective bidirectional relationships between sleep duration and negative affect. Through the inclusion of a random intercept, the RI-CLPM approach enables dividing the variance of observed variables into trait-like between-person components and state-like within-person components. The random intercept captures trait-like components of variables (i.e., between-person differences on average levels of variables), and levels of the variables at each time point are modeled as latent variables reflecting state-like components (i.e., within-person deviations on the variables relative to each participant’s average level). This modeling strategy thus yields separate and unconfounded estimates of between-person and within-person levels on variables of interest, akin to person-mean centering in multilevel models [60, 61].
The RI-CLPMs were implemented following the recommendations of [59]. We modeled two random intercept latent factors to represent the trait-like components of sleep duration and negative affect. The observed scores of sleep duration and negative affect were the indicators of their respective random intercept factors, and factor loadings were constrained at 1. Observed sleep duration and negative affect scores were regressed onto their own latent factors with factor loadings constrained at 1. Residual variances of observed variables were constrained at 0 to capture all the within- and between-person variance. Autoregressive and cross-lagged paths were specified between these latent, state-like factors. To ease model interpretability, all cross-lagged and autoregressive effects for a given association were constrained to be equal across time. We adjusted for age, gender, and race by modeling them as predictors of random intercepts for both sleep duration and negative affect, following the guidelines of Mulder and Hamaker [60]. Please see Figure 1 for a plot of the RI-CLPM used in this study.
Figure 1.
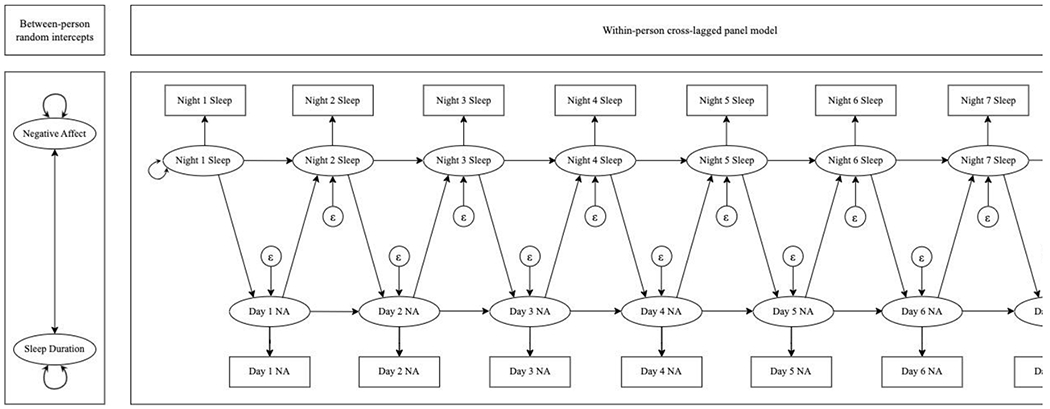
Path diagram of random intercept cross-lagged panel model used in this study. The between-person panel (left) depicts variables that reflect trait-like components of sleep duration and negative affect. The two-headed arrow between these variables demonstrates that the correlation between these variables was estimated. The two-headed arrows next to negative affect and sleep duration demonstrate that these variables had estimated variances. The within-person panel (right) depicts modeled relationships between state-like sleep duration and negative affect assessed daily. The two-headed arrow next to Night 1 Sleep demonstrates that the variance of night 1 sleep duration was estimated. Epsilons with arrows directed at subsequent sleep and negative affect assessments demonstrate that these variables each had estimated residual variances. One-headed arrows reflect autoregressive relationships between sleep duration, subsequent sleep duration, and negative affect and subsequent negative affect, as well as cross-lagged relationships between sleep duration and subsequent negative affect and negative affect and subsequent sleep duration.
The models included several parameters of substantive interest. The correlations between random intercepts reflected how stable between-person differences in sleep duration were linked with stable between-person differences in level of negative affect. Autoregressive paths indicated the extent to which state-like levels of sleep duration on a given night predicted state-like levels of sleep duration on the subsequent night, as well as the extent to which state-like levels of negative affect on one day predicted state-like levels of negative affect on the subsequent day. Finally, the cross-lagged paths reflected to what extent state-like sleep duration on one night predicted state-like negative affect on the subsequent day and the extent to which state-like negative affect on one day predicted state-like sleep duration that night.
Models were fit using maximum likelihood robust (MLR) estimation, an appropriate method for modeling daily negative affect, which tends to be right-skewed [55, 62, 63]. Model fit was assessed using the robust comparative fit index (rCFI) [64, 65], robust Tucker-Lewis index (rTLI) [64], robust root mean square error of approximation (rRMSEA) [66, 67], and standardized root mean square residual (SRMR) [68]. Models were determined to have good fit if rCFI ≥ 0.95, rTLI ≥ 0.95, rRMSEA ≤ 0.05, and SRMR ≤ 0.05 [65]. Missing data were handled using full information maximum likelihood (FIML). FIML is appropriate when data are missing at random (MAR), meaning that missingness depends only on observed (but not unobserved) variables, MAR is rendered more feasible when background variables are included in analytical models [69]. This way, if missingness is predicted by any background variable, it is accounted for in the model. Given that models adjusted for gender, age, and race, and data were missing at a very low rate, we judged MAR to be an appropriate assumption.1 In line with recommendations for multilevel data, we calculated standardized regression coefficients (ß) as an effect size metric to complement unstandardized coefficients (B) [70].
We also conducted follow-up analyses to explore the possibility of a nonlinear association between sleep duration and next-day negative affect. To do this, we combined the two samples and fit a multilevel model in which daily negative affect was regressed on a categorical variable reflecting the number of hours a person slept the previous night: less than 4.5, 4.5 to 5.5, 5.5 to 6.5, 6.5 to 7.5, 7.5 to 8.5, 8.5 to 9.5, 9.5 to 10.5, or more than 10.5 hours. The lower bound of these groupings was inclusive, whereas the upper bound was exclusive (except for the final two groupings, such that 10.5 was included in “9.5 to 10.5” and the highest bound only included values greater than 10.5; this was done for the sake of interpretability). The model adjusted for age, gender, race, and sample (Sample 1 vs. Sample 2) and incorporated an AR(1) residual structure to account for the fact that negative affect on one day was predicted by negative affect on the previous day. The model also accommodated missing data using FIML. The model was fit using the R package nlme [71].
3. Results
3.1. Sample 1
Descriptive statistics for Sample 1 are in Table 1. In Sample 1, participants slept an average of 7.13 hours per night (SD = 1.48). The average daily negative affect score was 0.19 (SD = 0.33). There were 1,283 (7.9%) missing data days for sleep duration and 1,279 missing days (7.9%) for negative affect. These missing data were accommodated by FIML estimation.
Table 1.
Sample Demographics and Descriptive Statistics of Study Variables
| M or n | SD or % | Min | Max | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|
|
|
||||||
| Sample 1 | ||||||
| Age | 56.24 | 12.20 | 33 | 84 | 0.19 | −0.82 |
| Female (vs. male) | 1157 | 57.2% | ||||
| Caucasian (vs. non-Caucasian) | 1696 | 83.9% | ||||
| Negative Affect | 0.19 | 0.33 | 0 | 3.5 | 3.17 | 14.10 |
| Sleep Duration (hours) | 7.13 | 1.48 | 0 | 18 | −0.71 | 3.45 |
| Sample 2 | ||||||
|
| ||||||
| Age | 47.9 | 12.7 | 25 | 75 | 0.24 | −0.88 |
| Female (vs. male) | 435 | 55.6% | ||||
| Caucasian (vs. non-Caucasian) | 659 | 84.3% | ||||
| Negative Affect | 0.22 | 0.35 | 0 | 3.5 | 3.19 | 15.00 |
| Sleep Duration (hours) | 7.17 | 1.51 | 0 | 18 | −0.72 | 4.23 |
Note. N = 2,022 in Sample 1, 782 in Sample 2.
The RI-CLPM for Sample 1 fit well (rCFI = 0.98; rTLI = 0.98; rRMSEA = 0.03; SRMR = 0.04). Please see Table 2 for the full set of regression parameter estimates, as well as random intercept variance and covariance estimates. The between-person association between the random intercept factors of sleep duration and negative affect was negative and significant, indicating that on average participants who reported more hours of sleep also reported lower negative affect. On the within-person level, sleep duration had a significant positive autoregressive effect, indicating that within-person deviations from average sleep duration on one night positively predicted deviations from average sleep duration the subsequent night. The autoregressive path for negative affect was also significant, indicating that within-person deviations from average negative affect were positively associated with deviations from average negative affect level the following day. Importantly, there was a significant negative cross-lagged effect of sleep duration on next-day negative. This finding indicated that, in line with hypotheses, sleeping less than average on a given night predicted experiencing higher than average negative affect on the subsequent day, and sleeping more than average on a given night predicted experiencing lower than average negative affect on the subsequent day. The within-person cross-lagged path from negative affect to same-night sleep duration was not significant. Thus, in contrast to hypotheses, the association between negative affect on a given day and sleep duration that night was not significant.
Table 2.
RI-CLPM Results
| Sample 1 |
Sample 2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | Z | p | β | B | SE | Z | p | β | |
|
|
||||||||||
| Random Intercept Variance | ||||||||||
| Sleep Duration | 0.75 | 0.04 | 18.92 | <.001 | 0.66 | 0.06 | 10.31 | <.001 | ||
| Negative Affect | 0.05 | 0.01 | 8.77 | <.001 | 0.06 | 0.01 | 5.24 | <.001 | ||
| Random Intercept Covariance | ||||||||||
| Sleep Duration & Negative Affect | −0.02 | 0.01 | −2.44 | .015 | −0.10 | −0.03 | 0.02 | −1.37 | .172 | −0.13 |
|
| ||||||||||
| Within-person Autoregressive Effects | ||||||||||
| Sleep Duration | 0.10 | 0.02 | 6.08 | <.001 | 0.10 | 0.08 | 0.03 | 2.86 | .004 | 0.09 |
| Negative Affect | 0.19 | 0.02 | 9.02 | <.001 | 0.18 | 0.11 | 0.03 | 3.74 | <.001 | 0.11 |
| Within-person Cross-lagged Effects | ||||||||||
| Sleep Duration → Negative Affect | −0.02 | 0.00 | −6.35 | <.001 | −0.08 | −0.02 | 0.00 | −4.42 | <.001 | −0.09 |
| Negative Affect → Sleep Duration | 0.04 | 0.08 | 0.49 | .623 | 0.01 | −0.10 | 0.10 | −1.03 | .306 | −0.02 |
|
| ||||||||||
| Covariate Effects on Random Intercepts | ||||||||||
| Sleep Duration | ||||||||||
| Age | 0.00 | 0.00 | 1.96 | .050 | 0.05 | 0.01 | 0.00 | 2.28 | .023 | 0.09 |
| Gender | 0.02 | 0.05 | 0.33 | .740 | 0.01 | 0.28 | 0.07 | 4.17 | <.001 | 0.17 |
| Race | −0.43 | 0.07 | −6.15 | <.001 | −0.18 | −0.21 | 0.10 | −2.11 | .035 | −0.09 |
| Negative Affect | ||||||||||
| Age | −0.01 | 0.00 | −7.09 | <.001 | −0.16 | 0.00 | 0.00 | −3.67 | <.001 | −0.13 |
| Gender | 0.03 | 0.01 | 3.01 | .003 | 0.07 | 0.03 | 0.02 | 1.65 | .099 | 0.06 |
| Race | 0.05 | 0.02 | −2.69 | .007 | −0.08 | −0.02 | 0.03 | −0.73 | .464 | −0.03 |
Note. N = 2,022 in Sample 1, N = 782 in Sample 2. RI-CLPM = random-intercept cross-lagged panel model. Boldface indicates statistical significance with alpha of .05.
3.2. Sample 2
Descriptive statistics for Sample 2 are in Table 1. Participants in Sample 2 reported an average sleep duration of 7.17 hours per night during the data collection period (SD = 1.51) and an average negative affect score of 0.22 (SD = 0.35). For Sample 2, there were 90 (1.5%) missing data days for sleep duration and 88 (1.5%) for negative affect, and these missing observations were accommodated in the analysis via FIML estimation.
The RI-CLPM for Sample 2 fit well (rCFI = 0.96; rTLI = 0.95; rRMSEA = 0.04; SRMR = 0.06). Please see Table 2 for the full set of regression parameter estimates, as well as random intercept variance and covariance estimates for Sample 2. Unlike in Sample 1, the between-person association between the random intercept factors of sleep duration and negative affect was not significant, indicating no significant between-person relationship between average sleep and average negative affect.2 There were significant positive autoregressive effects for sleep duration and negative affect, indicating that for these variables, within-person deviations from average levels on one day were related to same-direction deviations from average levels the next day. Importantly, consistent with Sample 1, the within-person cross-lagged path from sleep duration to next-day negative affect was significant, and the within-person association between negative affect and same-night sleep duration was not significant. Thus, within-person results from Sample 2 converged with results from Sample 1 by indicating that sleep duration on a given night was inversely associated with negative affect on the subsequent day, whereas negative affect on a given day was not significantly associated with sleep duration on that night.3
3.3. Follow-up analysis: Nonlinear associations between sleep and next-day affect
Results suggested that the relationship between sleep and next-day affect was nonlinear. Specifically, as the model output indicates in Table 3, ranges of sleep below or above 7.5 to 10.5 hours were associated with significantly greater next-day negative affect. There was no relationship between sleep and negative affect between 7.5 and 10.5 hours of sleep. Thus, when participants slept fewer than 7.5 hours, or above 10.5 hours, sleeping less or more predicted higher next-day negative affect. The optimal window of sleep appeared to be from 7.5 to 10.5 hours. This pattern is plotted in Figure 2.
Table 3.
Nonlinear Model Results
| Term | B | SE | t | df | p | β |
|---|---|---|---|---|---|---|
| Intercept | 0.23 | 0.01 | 40.99 | 17,837 | <.001 | |
| Sleep hours: less than 4.5 vs. 4.5 to 5.5 | −0.06 | 0.01 | −4.90 | 17,837 | <.001 | −0.17 |
| Sleep hours: 4.5 to 5.5 vs. 5.5 to 6.5 | −0.03 | 0.01 | −4.07 | 17,837 | <.001 | −0.10 |
| Sleep hours: 5.5 to 6.5 vs. 6.5 to 7.5 | −0.02 | 0.01 | −3.90 | 17,837 | <.001 | −0.07 |
| Sleep hours: 6.5 to 7.5 vs. 7.5 to 8.5 | −0.02 | 0.00 | −3.99 | 17,837 | <.001 | −0.06 |
| Sleep hours: 7.5 to 8.5 vs. 8.5 to 9.5 | −0.01 | 0.01 | −1.08 | 17,837 | .279 | −0.02 |
| Sleep hours: 8.5 to 9.5 vs. 9.5 to 10.5 | 0.01 | 0.01 | 0.80 | 17,837 | .423 | 0.03 |
| Sleep hours: 9.5 to 10.5 vs. more than 10.5 | 0.09 | 0.02 | 4.01 | 17,837 | <.001 | 0.26 |
| Sample | 0.00 | 0.01 | 0.22 | 2,799 | .826 | 0.01 |
| Age | 0.00 | 0.00 | −7.56 | 2,799 | <.001 | −0.12 |
| Gender | 0.03 | 0.01 | 3.31 | 2,799 | .001 | 0.10 |
| Race | −0.03 | 0.01 | −2.30 | 2,799 | .022 | −0.09 |
Note: N = 2,804 combined across Samples 1 and 2. All between-person difference variables (sample, age, gender, race) were grand-mean centered. Boldface indicates statistical significance with alpha of .05.
Figure 2.
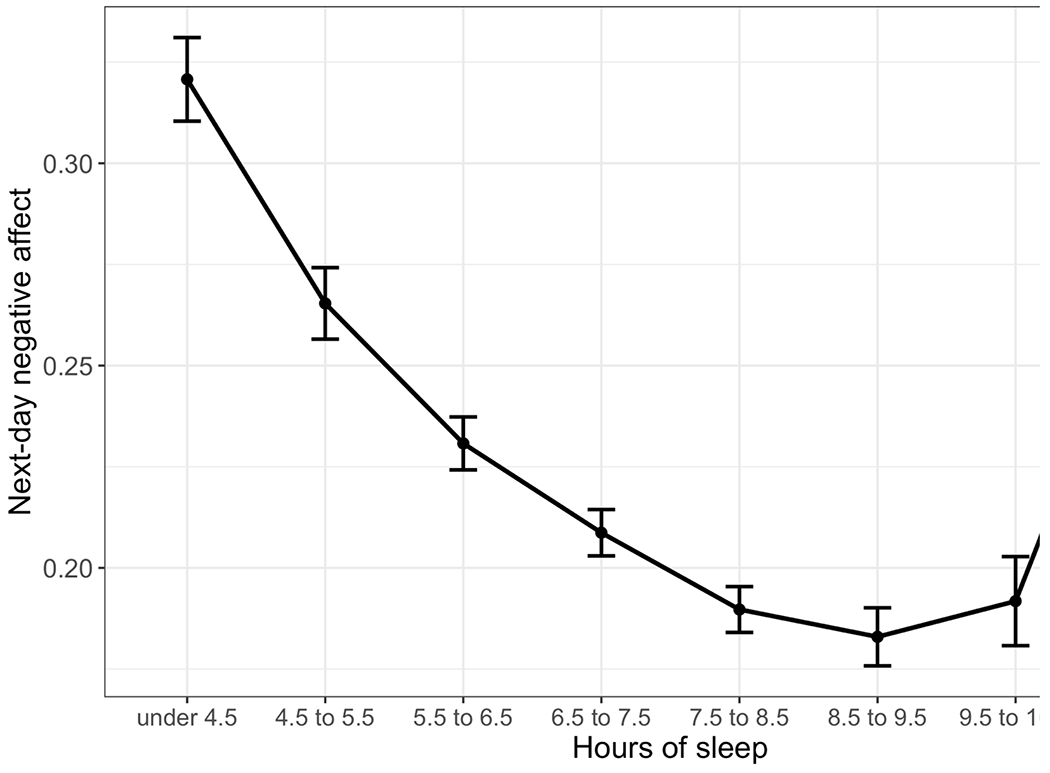
Model-predicted negative affect levels as a function of sleep duration. Error bars represent standard errors.
4. Discussion
The present work examined the sequential associations between sleep duration and daily negative affect in two large samples of middle-aged adults across eight days. Findings from both samples indicated that shorter than usual sleep duration predicted higher next-day negative affect, supporting our first hypothesis. These results align with prior research demonstrating a prospective relationship between shorter sleep and elevated negative affect on a day-to-day basis. Past experience sampling studies similarly found that shorter sleep was associated with higher next-day negative affect among older adults [39], young adult women [38, 41], and adults with depressive disorders [72]. Moreover, these results may suggest that findings from laboratory-based research indicating that sleep loss predicts elevated negative affect [e.g., 10, 30] might translate to a naturalistic, everyday context. Additionally, follow-up analyses showed that the relationship between sleep and next-day negative affect attenuated to non-significance between 7.5 hours and 10.5 hours of sleep and reversed at extremely low or high levels of sleep (less than 7.5 hours or more than 10.5 hours). In other words, the optimal amount of sleep associated with lowest next-day negative affect was between 7.5 and 10.5 hours.
In contrast to our second hypothesis, variations in daily negative affect did not predict upcoming-night sleep duration in either sample. This finding is somewhat inconsistent with results from prior studies supporting a bidirectional association between sleep and negative affect. These discrepancies may be explained by differences in the exact constructs being examined. For instance, specific emotions, like sadness [42], may have unique relationships with sleep duration compared to overall negative affect; such a possibility should be examined in future research. Age differences in samples could also contribute to these differing results, as some studies suggesting a bidirectional relationship between sleep and affect utilized adolescent samples [34, 37, 73]. Considering the present data, it is possible that shorter sleep heightens next-day negative affect for both adolescents and adults, but compared to adolescents, adults are less susceptible to sleep disruptions after a day of elevated negative affect.
In Sample 1 only, the between-person association of sleep duration and negative affect was negative and significant; this between-person association was only significant in Sample 2 under certain model specifications. Thus, the within-person association between sleep and affect was more consistent in this study than the between-person association. Some previous studies exploring sleep and affect found stronger within-person associations compared to between-person associations [36, 74]. Thus, although there is some evidence from this study that sleeping more on average was associated with lower average negative affect, affect levels on a given day appeared to also be related to a person’s prior-night sleep duration relative to his or her usual sleep duration. Nevertheless, the consistent within-person relationship between sleep and next-day negative affect underscores the important role of within-person deviations in sleep duration in predicting affect on a given day.
We found significant within-person autoregressive paths for both negative affect and sleep duration. In both samples, deviations from typical level of negative affect on one day predicted deviations from negative affect the following day. Similarly, past studies showed that negative affect can carry over from one day to the next [75, 76]. Our results also showed autoregressive effects for sleep duration: lower or higher variations in sleep duration on one night predicted similar variations in sleep duration the next night. Previous research also found significant day-to-day associations on measures of sleep [77, 78]. It thus appears that falling out of one’s typical sleep rhythm can disrupt sleep the following night.
In this study, shorter duration predicted higher negative affect the next day, although this relationship was attenuated beyond sleeping 7.5 hours and reversed at extreme levels of sleep (more than 10.5 hours). The generally negative association between sleep duration and next-day negative affect may be explained by emotion regulation theories, which posit that sleep plays a key role in the perception, reactivity, and modulation of negative emotional experiences [12, 18–20]. Less sleep may negatively skew the perception and evaluation of emotional information, leading to a negative emotional bias [14]. Indeed, restricted sleep has been consistently linked to heightened attention to negative stimuli [13, 79, 80]. Further, individuals reacted less to positive stimuli following sleep restriction [81], and neutral stimuli were rated more negatively [2, 82]. Poor sleep may also impede the consolidation of positive and neutral memories, while enhancing memory for unpleasant events [1, 80, 83]. Therefore, after a night of less sleep, pessimistic perceptions of everyday situations and a negative memory bias could increase negative affect. The finding of an optimal sleep range of at least 7.5 hours and no more than 10.5 hours corresponds with research on the relationship between oversleeping and mood. Oversleeping is a feature of mood disorders [44], and in one prior study of healthy adults, extremely long sleep duration predicted poorer next-day mood compared to sleeping one’s usual amount [40]. Public health recommendations suggest that adults sleep at least 7 hours per day but have not specified a recommended maximum sleep duration [84]. The results from this study generally converge with these recommendations, suggesting that sleeping at least 7.5 hours was associated with lower next-day negative affect relative to sleeping less than this amount; moreover, results suggested that sleeping more than 10.5 hours conferred risk for poorer mood the next day.
For most sleep durations up to a generally healthy level of sleep for adults (7.5 hours), less sleep predicted greater next-day negative affect. An additional possible mechanism for this finding is negative emotional reactivity. While high-quality, restorative sleep is hypothesized to help downregulate negative emotions, poor sleep is linked to heightened emotional responses to aversive stimuli [13, 14, 20, 85]. Indeed, laboratory studies have demonstrated the connection between sleep restriction and exaggerated negative emotional reactions [11, 21, 23, 24, 30, 82]. Illustrating this association in a naturalistic context, an experience sampling study of medical students found that shorter sleep predicted more intense negative emotions in response to stressors the following day [86]. Similarly, adults who experienced nightly sleep disruptions reacted more negatively to unpleasant situations in a seven-day experience sampling study [87]. Considering these findings, shorter than usual sleep duration might intensify negative emotional reactions and subsequently increase overall negative affect, as seen in the present study.
Another possible explanation for our results is that utilizing adaptive emotion regulation strategies to moderate negative feelings is more challenging following a night of reduced sleep. The cognitive-energy model of emotion regulation postulates that shorter sleep depletes mental resources necessary to regulate emotional reactions through strategies like cognitive reappraisal [1, 86]. Lending credence to this idea, laboratory studies demonstrated that sleep restriction worsened cognitive reappraisal ability in response to negative emotional stimuli [2, 88–90]. Moreover, Minaeva et al. [75] found a stronger carry-over effect of negative affect from one day to the next in individuals with lower sleep quality and duration. At the same time, this effect was exacerbated by current or past depression. Further, poor sleep can impede goal-directed behavior and self-control, which could pose barriers to adaptively coping with negative emotional experiences [26, 91, 92]. For example, engaging in mood-enhancing activities or reaching out for social support may feel more effortful and burdensome after sleeping fewer hours than usual. Individuals may consequently avoid these potentially mood-improving behaviors and maintain a state of heightened negative affect [23, 25, 93].
The present findings may have implications for optimizing daily emotional well-being. Our results suggest sleep could be a potential intervention target to limit next-day negative affect. Prior research found that behavioral interventions were effective in promoting sleep duration [94], which may help prevent subsequent negative affect in individuals who are vulnerable to fluctuations in sleep time. Given the nonlinear relationship between sleep and next-day affect, another implication of this study is that sleeping beyond 7.5 hours does not appear to confer any additional benefit for next-day mood, and sleeping more than 10.5 hours appears to predict poorer mood; mood may therefore be optimized if people get up and moving after they have achieved an ideal level of sleep (likely between 7.5 and 10.5 hours for most adults). Future studies should investigate whether promoting optimal sleep duration leads to lower levels of negative affect in daily life.
These results should be interpreted in the context of certain limitations. First, prior night sleep duration and daily negative affect were assessed in the same evening telephone interview. This shared assessment method and timing could have inflated the association between sleep and negative affect relative to using different methodologies (e.g., objective sleep assessment and self-reported affect) or assessing the constructs at separate times (e.g., assessing sleep immediately after awakening and affect at end of day). However, as sleep duration is inherently a more concrete construct than more subjective aspects of sleep such as quality, self-reported sleep duration may have been less subject to influence by the participants’ general mood or other factors. Additionally, sleep duration was measured with a single self-report item, which may be less reliable and valid than multiple-item scales. Despite these limitations, previous research has shown strong correlations between single-item self-reports of sleep duration and objectively measured sleep duration, lending confidence to the validity of the sleep assessment [33, 53]. Nevertheless, for a more complete picture of the connections between negative affect and sleep duration, future research should incorporate objective sleep assessments such as polysomnography. Furthermore, we cannot determine whether aspects of sleep beyond duration (e.g., efficiency, quality) would have similar relationships with negative affect. Future investigations could include measures capturing other components of sleep to explore their associations with negative affect. The present study is also limited in its eight-day assessment period. Although this represents an improvement over many studies assessing sleep for even fewer days [for a review, see 14], future studies with longer assessment durations would more accurately characterize participants’ typical sleep patterns. Furthermore, for the primary data analytic strategy, this study employed RI-CLPM, which is a gold-standard method for capturing reciprocal relationships among repeatedly assessed variables but does not afford certain modeling capacities such as random slopes that could characterize between-person differences in autoregressive and cross-lagged sleep-affect relationships. Advances in modeling software could help elucidate these important between-person differences in future research. Lastly, the MIDUS dataset is comprised mostly of White American participants; thus, replication in more culturally diverse samples is warranted.
Despite these limitations, this study has notable strengths. The use of daily diary data offers a more fine-grained understanding of how day-to-day variations in sleep duration relate to negative affect in a naturalistic context [32]. Our study is also strengthened by the use of two large samples of community-dwelling adults, with replication of key findings across both samples. This strength bolsters the reliability of the findings and extends previous research examining sleep and daily affect in adolescents [34, 35, 37, 95], older adults [39, 96], clinical populations [33, 97], and female-only samples [38, 41, 42]. Given these strengths, the findings substantially advance the literature on the association between sleep and negative affect.
In summary, the present study tested the day-to-day connections between sleep duration and negative affect. In two large national samples of adults, shorter sleep duration predicted higher negative affect the following day, but daily negative affect did not predict same-night sleep duration. Follow-up analyses revealed that sleep duration’s relationship with next-day affect was nonlinear, such that sleeping less than 7.5 hours or more than 10.5 hours was associated with greater next-day negative affect than sleeping within this range. Future research should explore whether efforts to promote optimal sleep duration are effective in promoting daily emotional well-being.
Supplementary Material
Highlights.
The study examined daily connections between sleep duration and negative affect.
Shorter sleep duration unidirectionally predicted higher next-day negative affect.
Level of daily negative affect was not associated with subsequent sleep time.
Follow-up analyses showed a nonlinear relationship between sleep and affect.
Sleeping fewer than 7.5 or more than 10.5 hours led to greater negative affect.
Declaration of Interest Statement
The authors have no conflicts of interest or financial disclosures.
Data analysis and writing for this study was supported by National Institute of Mental Health grant R01MH115128. Data collection was funded by John D. and Catherine T. MacArthur Foundation, Research Network on Successful Midlife Development, W. K. Kellogg Foundation, National Institute on Aging (AG16731, AG19239, AG210166), National Institute of Mental Health (MH53372, MH19734). The funding agencies, and the researchers who originally collected and shared the data, are not responsible for any data analysis or interpretation in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Logistic regression suggested that, in Sample 1, being younger and not being White predicted higher odds of having at least one daily diary observation (age: OR = 0.98, 95% CI [0.97, 0.98]; race: OR = 0.50, 95% CI [0.39, 0.64]). No other variable in Sample 1 or 2 predicted missing data. Because these variables were modeled, it does not pose a problem for the MAR assumption.
This non-significant correlation was unexpected. We suspected it could be due to the conservative MLR estimator, which adjusts standard errors for non-normality. We re-fit the model with the non-robust maximum likelihood estimator and found that the between-person relationship between sleep and negative affect was negative and significant using this estimator, in line with Sample 1 and our expectations. No other results differed with this estimator in either Sample 1 or Sample 2. Because the MLR estimator is robust to non-normality, we have chosen to report our main results based on this estimator, though the non-robust results are available in the Supplementary Materials.
As results showed gender differences in sleep duration in Sample 1 and gender difference in negative affect in both Samples 1 and 2, we also conducted separate RI-CLPMs for female and male participants in each sample. Results showed the same pattern of within-person unidirectional associations between sleep duration and negative affect for both female and male participants when analyzed separately. Please see Supplementary Materials.
References
- [1].Kahn M, Sheppes G, Sadeh A, Sleep and emotions: Bidirectional links and underlying mechanisms, Int J Psychophysiol 89(2) (2013) 218–228. 10.1016/j.ijpsycho.2013.05.010 [DOI] [PubMed] [Google Scholar]
- [2].Watling J, Pawlik B, Scott K, Booth S, Short MA, Sleep loss and affective functioning: More than just mood, Behav Sleep Med 15(5) (2017) 394–409. 10.1080/15402002.2016.1141770 [DOI] [PubMed] [Google Scholar]
- [3].Tomaso CC, Johnson AB, Nelson TD, The effect of sleep deprivation and restriction on mood, emotion, and emotion regulation: Three meta-analyses in one, Sleep 44(6) (2020) 1–30. 10.1093/sleep/zsaa289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].LeBlanc M, Beaulieu-Bonneau S, Mérette C, Savard J, Ivers H, Morin CM, Psychological and health-related quality of life factors associated with insomnia in a population-based sample, J Psychosom Res 63(2) (2007) 157–166. 10.1016/jjpsychores.2007.03.004 [DOI] [PubMed] [Google Scholar]
- [5].Jackson ML, Sztendur EM, Diamond NT, Byles JE, Bruck D, Sleep difficulties and the development of depression and anxiety: A longitudinal study of young Australian women, Arch Womens Ment Health 17(3) (2014) 189–198. 10.1007/s00737-014-0417-8 [DOI] [PubMed] [Google Scholar]
- [6].Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, Riemann D, Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies, J Affect Disord 135(1) (2011) 10–19. 10.1016/jjad.2011.01.011 [DOI] [PubMed] [Google Scholar]
- [7].Zhai L, Zhang H, Zhang D, Sleep duration and depression among adults: A meta-analysis of prospective studies, Depress Anxiety 32(9) (2015) 664–670. 10.1002/da.22386 [DOI] [PubMed] [Google Scholar]
- [8].Alvaro PK, Roberts RM, Harris JK, A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression, Sleep 36(7) (2013) 1059–1068. 10.5665/sleep.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Foley D, Ancoli-Israel S, Britz P, Walsh J, Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey, J Psychosom Res 56(5) (2004) 497–502. 10.1016/jjpsychores.2004.02.010 [DOI] [PubMed] [Google Scholar]
- [10].Babson KA, Trainor CD, Feldner MT, Blumenthal H, A test of the effects of acute sleep deprivation on general and specific self-reported anxiety and depressive symptoms: an experimental extension, J Behav Ther Exp Psychiatry 41(3) (2010) 297–303. 10.1016/jjbtep.2010.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Minkel JD, Banks S, Htaik O, Moreta MC, Jones CW, McGlinchey EL, Simpson NS, Dinges DF, Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived, Emotion 12(5) (2012) 1015–1020. 10.1037/a0026871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Palmer CA, Alfano CA, Sleep and emotion regulation: An organizing, integrative review, Sleep Med Rev 31 (2017) 6–16. 10.1016/j.smrv.2015.12.006 [DOI] [PubMed] [Google Scholar]
- [13].Ben Simon E, Vallat R, Barnes CM, Walker MP, Sleep loss and the socio-emotional brain, Trends Cogn Sci 24(6) (2020) 435–450. 10.1016/j.tics.2020.02.003 [DOI] [PubMed] [Google Scholar]
- [14].Konjarski M, Murray G, Lee VV, Jackson ML, Reciprocal relationships between daily sleep and mood: A systematic review of naturalistic prospective studies, Sleep Med Rev 42 (2018) 47–58. 10.1016/j.smrv.2018.05.005 [DOI] [PubMed] [Google Scholar]
- [15].Lee S, Crain TL, McHale SM, Almeida DM, Buxton OM, Daily antecedents and consequences of nightly sleep, J Sleep Res 26(4) (2017) 498–509. 10.1111/jsr.12488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Peeters F, Berkhof J, Delespaul P, Rottenberg J, Nicolson NA, Diurnal mood variation in major depressive disorder, Emotion 6(3) (2006) 383–391. 10.1037/1528-3542.6.3.383 [DOI] [PubMed] [Google Scholar]
- [17].Sin NL, Rush J, Buxton OM, Almeida DM, Emotional vulnerability to short sleep predicts increases in chronic health conditions across 8 years, Ann Behav Med 55(12) (2021) 1231–1240. 10.1093/abm/kaab018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vandekerckhove M, Wang Y-L, Emotion, emotion regulation and sleep: An intimate relationship, AIMS neuroscience 5(1) (2017) 1–17. 10.3934/Neuroscience.2018.L1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Walker MP, The role of sleep in cognition and emotion, Ann N Y Acad Sci 1156(1) (2009) 168–197. 10.1111/j.1749-6632.2009.04416.x [DOI] [PubMed] [Google Scholar]
- [20].Fairholme CP, Manber R, Sleep, emotions, and emotion regulation: An overview, in: Babson KA, Feldner MT (Eds.), Sleep and Affect, Academic Press, San Diego, 2015, pp. 45–61. 10.1016/B978-0-12-417188-6.00003-7 [DOI] [Google Scholar]
- [21].Gujar N, Yoo S-S, Hu P, Walker MP, Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences, J Neurosci 31(12) (2011) 4466–4474. 10.1523/JNEUROSa.3220-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Minkel J, Moreta M, Muto J, Htaik O, Jones C, Basner M, Dinges D, Sleep deprivation potentiates HPA axis stress reactivity in healthy adults, Health Psychol 33(11) (2014) 1430–1434. 10.1037/a0034219 [DOI] [PubMed] [Google Scholar]
- [23].Anderson C, Platten CR, Sleep deprivation lowers inhibition and enhances impulsivity to negative stimuli, Behav Brain Res 217(2) (2011) 463–466. 10.1016/j.bbr.2010.09.020 [DOI] [PubMed] [Google Scholar]
- [24].Franzen PL, Buysse DJ, Dahl RE, Thompson W, Siegle GJ, Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults, Biol Psychol 80(3) (2009) 300–305. 10.1016/j.biopsycho.2008.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Johnson KT, Williams PG, Aspinwall LG, Curtis BJ, Resilience to stress-related sleep disturbance: Examination of early pandemic coping and affect, Health Psychol 41(4) (2022) 291–300. 10.1037/hea0001169 [DOI] [PubMed] [Google Scholar]
- [26].Liu J, Zhu L, Liu C, Sleep quality and self-control: The mediating roles of positive and negative affects, Front Psychol 11 (2020) 607548. 10.3389/fpsyg.2020.607548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Steptoe A, O’Donnell K, Marmot M, Wardle J, Positive affect, psychological well-being, and good sleep, J Psychosom Res 64(4) (2008) 409–415. 10.1016/j.jpsychores.2007.ll.008 [DOI] [PubMed] [Google Scholar]
- [28].Hantsoo L, Khou CS, White CN, Ong JC, Gender and cognitive–emotional factors as predictors of pre-sleep arousal and trait hyperarousal in insomnia, J Psychosom Res 74(4) (2013) 283–289. 10.1016/j.jpsychores.2013.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vandekerckhove M, Kestemont J, Weiss R, Schotte C, Exadaktylos V, Haex B, Verbraecken J, Gross JJ, Experiential versus analytical emotion regulation and sleep: breaking the link between negative events and sleep disturbance, Emotion 12(6) (2012) 1415–1421. 10.1037/a0028501 [DOI] [PubMed] [Google Scholar]
- [30].Franzen PL, Siegle GJ, Buysse DJ, Relationships between affect, vigilance, and sleepiness following sleep deprivation, J Sleep Res 17(1) (2008) 34–41. 10.llll/j.1365-2869.2008.00635.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Talbot LS, McGlinchey EL, Kaplan KA, Dahl RE, Harvey AG, Sleep deprivation in adolescents and adults: Changes in affect, Emotion 10(6) (2010) 831–841. 10.1037/a0020138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shiffman S, Stone AA, Hufford MR, Ecological momentary assessment, Annual Review of Clinical Psychology 4(1) (2008) 1–32. 10.1146/annurev.clinpsy.3.022806.091415 [DOI] [PubMed] [Google Scholar]
- [33].Difrancesco S, Penninx BWJH, Antypa N, van Hemert AM, Riese H, Lamers F, The day-to-day bidirectional longitudinal association between objective and self-reported sleep and affect: An ambulatory assessment study, J Affect Disord 283 (2021) 165–171. 10.1016/jjad.2021.01.052 [DOI] [PubMed] [Google Scholar]
- [34].van Zundert RM, van Roekel E, Engels RC, Scholte RH, Reciprocal associations between adolescents’ night-time sleep and daytime affect and the role of gender and depressive symptoms, J Youth Adolesc 44(2) (2015) 556–569. 10.1007/s10964-013-0009-3 [DOI] [PubMed] [Google Scholar]
- [35].Shen L, Wiley JF, Bei B, Sleep and affect in adolescents: Bidirectional daily associations over 28-day ecological momentary assessment, J Sleep Res 31(2) (2021) e13491. 10.1111/jsr.13491 [DOI] [PubMed] [Google Scholar]
- [36].Yap Y, Slavish DC, Taylor DJ, Bei B, Wiley JF, Bi-directional relations between stress and self-reported and actigraphy-assessed sleep: a daily intensive longitudinal study, Sleep 43(3) (2019) 1–10. 10.1093/sleep/zsz250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Doane LD, Thurston EC, Associations among sleep, daily experiences, and loneliness in adolescence: Evidence of moderating and bidirectional pathways, J Adolesc 37(2) (2014) 145–154. 10.1016/j.adolescence.2013.11.009 [DOI] [PubMed] [Google Scholar]
- [38].de Wild-Hartmann JA, Wichers M, van Bemmel AL, Derom C, Thiery E, Jacobs N, van Os J, Simons CJP, Day-to-day associations between subjective sleep and affect in regard to future depressionin a female population-based sample, Br J Psychiatry 202(6) (2013) 407–412. 10.1192/bjpbp.112.123794 [DOI] [PubMed] [Google Scholar]
- [39].McCrae CS, McNamara JP, Rowe MA, Dzierzewski JM, Dirk J, Marsiske M, Craggs JG, Sleep and affect in older adults: using multilevel modeling to examine daily associations, J Sleep Res 17(1) (2008) 42–53. 10.1111/j1365-2869.2008.00621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wrzus C, Wagner GG, Riediger M, Feeling good when sleeping in? Day-to-day associations between sleep duration and affective well-being differ from youth to old age, Emotion 14(3) (2014) 624–628. 10.1037/a0035349 [DOI] [PubMed] [Google Scholar]
- [41].Li DX, Romans S, De Souza MJ, Murray B, Einstein G, Actigraphic and self-reported sleep quality in women: associations with ovarian hormones and mood, Sleep Med 16(10) (2015) 1217–1224. [DOI] [PubMed] [Google Scholar]
- [42].Kalmbach DA, Pillai V, Roth T, Drake CL, The interplay between daily affect and sleep: A 2-week study of young women, J. Sleep Res 23(6) (2014) 636–645. 10.1111/jsr12190 [DOI] [PubMed] [Google Scholar]
- [43].Ferster CB, A functional analysis of depression, American Psychologist 28(10) (1973) 857–870. 10.1037/h0035605 [DOI] [PubMed] [Google Scholar]
- [44].Kaplan KA, Harvey AG, Hypersomnia across mood disorders: a review and synthesis, Sleep Med Rev 13(4) (2009) 275–285. 10.1016/jsmrv.2008.09.001 [DOI] [PubMed] [Google Scholar]
- [45].Scott J, Kallestad H, Vedaa O, Sivertsen B, Etain B, Sleep disturbances and first onset of major mental disorders in adolescence and early adulthood: A systematic review and meta-analysis, Sleep Med Rev 57 (2021) 101429. 10.1016/j.smrv.2021.101429 [DOI] [PubMed] [Google Scholar]
- [46].Colrain IM, Baker FC, Changes in sleep as a function of adolescent development, Neuropsychol Rev 21(1) (2011) 5–21. 10.1007/s11065-010-9155-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Difrancesco S, Lamers F, Riese H, Merikangas KR, Beekman ATF, van Hemert AM, Schoevers RA, Penninx B, Sleep, circadian rhythm, and physical activity patterns in depressive and anxiety disorders: A 2-week ambulatory assessment study, Depress Anxiety 36(10) (2019) 975–986. 10.1002/da.22949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E, Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society, Sleep 38(6) (2015) 843–844. 10.5665/sleep.4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ryff CD, Almeida DM, Ayanian JZ, Carr DS, Cleary PD, Coe C, Davidson RJ, Krueger RF, Lachman ME, Marks NF, Mroczek DK, Seeman TE, Seltzer MM, Singer BH, Sloan RP, Tun PA, Weinstein M, Williams DR, Midlife in the United States (MIDUS 2), 2004-2006, Inter-university Consortium for Political and Social Research [distributor], 2021.
- [50].Ryff CD, Almeida DM, Midlife in the United States (MIDUS 2): Daily Stress Project, 2004-2009., Inter-university Consortium for Political and Social Research, Ann Arbor, MI, 2017. [Google Scholar]
- [51].Ryff CD, Almeida DM, Midlife in the United States (MIDUS Refresher 1): Daily Diary Project, 2012-2014, Inter-university Consortium for Political and Social Research [distributor], 2020. [Google Scholar]
- [52].Ryff C, Almeida DM, Ayanian JZ, Binkley N, Carr DS, Coe C, Davidson R, Grzywacz J, Karlamangla A, Krueger R, Lachman ME, Love G, Mailick M, Mroczek DK, Radler B, Seeman TE, Sloan R, Thomas D, Weinstein M, Williams DR, Midlife in the United States (MIDUS Refresher 1), 2011-2014, Inter-university Consortium for Political and Social Research [distributor], 2017. [Google Scholar]
- [53].Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ, Self-reported and measured sleep duration: how similar are they?, Epidemiology 19(6) (2008) 838–845. 10.1097/EDE.0b013e318187a7b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mroczek DK, Kolarz CM, The effect of age on positive and negative affect: A developmental perspective on happiness, Journal of Personality and Social Psychology 75(5) (1998) 1333–1349. 10.1037/0022-3514.75.5.1333 [DOI] [PubMed] [Google Scholar]
- [55].Watson D, Clark LA, Tellegen A, Development and validation of brief measures of positive and negative affect: The PANAS scales, Journal of personality and social psychology 54(6) (1988) 1063–1070. 10.1037//0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- [56].Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, Walters EE, Zaslavsky AM, Short screening scales to monitor population prevalences and trends in non-specific psychological distress, Psychol. Med 32(6) (2002) 959–976. [DOI] [PubMed] [Google Scholar]
- [57].Wiley JF, multilevelTools: Multilevel and Mixed Effects Model Diagnostics and Effect Sizes, 2020. https://cran.r-project.org/package=multilevelTools. [Google Scholar]
- [58].Rosseel Y, Lavaan: An R package for structural equation modeling, J Stat Softw 48(2) (2012) 1–36. 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- [59].Hamaker EL, Kuiper RM, Grasman RPPP, A critique of the cross-lagged panel model, Psychol Methods 20(1) (2015) 102–116. 10.1037/a0038889 [DOI] [PubMed] [Google Scholar]
- [60].Mulder JD, Hamaker EL, Three extensions of the random intercept cross-lagged panel model, Structural Equation Modeling: A Multidisciplinary Journal 28(4) (2021) 638–648. 10.1080/10705511.2020.1784738 [DOI] [Google Scholar]
- [61].Usami S, Murayama K, Hamaker EL, A unified framework of longitudinal models to examine reciprocal relations, Psychol Methods 24(5) (2019) 637–657. 10.1037/met0000210 [DOI] [PubMed] [Google Scholar]
- [62].Merz EL, Malcarne VL, Roesch SC, Ko CM, Emerson M, Roma VG, Sadler GR, Psychometric properties of Positive and Negative Affect Schedule (PANAS) original and short forms in an African American community sample, J Affect Disord 151(3) (2013) 942–949. 10.1016/jjad.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Rivera NV, Parmelee PA, Smith DM, The impact of social interactions and pain on daily positive and negative affect in adults with osteoarthritis of the knee, Aging Ment Health 24(1) (2020) 8–14. 10.1080/13607863.2018.1506744 [DOI] [PubMed] [Google Scholar]
- [64].Brosseau-Liard PE, Savalei V, Adjusting incremental fit indices for nonnormality, Multivar Behav Res 49(5) (2014) 460–470. 10.1080/00273171.2014.933697 [DOI] [PubMed] [Google Scholar]
- [65].Hu L.t., Bentler PM, Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives, Struct Equ Modeling 6(1) (1999) 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- [66].Brosseau-Liard PE, Savalei V, Li L, An investigation of the sample performance of two nonnormality corrections for RMSEA, Multivar Behav Res 47(6) (2012) 904–930. 10.1080/00273171.2012.715252 [DOI] [PubMed] [Google Scholar]
- [67].Savalei V, On the computation of the RMSEA and CFI from the mean-and-variance corrected test statistic with nonnormal data in SEM, Multivar Behav Res 53(3) (2018) 419–429. 10.1080/00273171.2018.1455142 [DOI] [PubMed] [Google Scholar]
- [68].Hu L.-t., Bentler PM, Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification, Psychol. Methods 3(4) (1998) 424–453. 10.1037/1082-989X.3.4.424 [DOI] [Google Scholar]
- [69].Graham JW, Missing data analysis: Making it work in the real world, Annu Rev Psychol 60(1) (2009) 549–576. 10.1146/annurev.psych.58.110405.085530 [DOI] [PubMed] [Google Scholar]
- [70].Lorah J, Effect size measures for multilevel models: definition, interpretation, and TIMSS example, Large-Scale Assessments in Education 6(8) (2018) 1–11. 10.1186/s40536-018-0061-2 [DOI] [Google Scholar]
- [71].Pinheiro J, Bates D, R Core Team, nlme: Linear and Nonlinear Mixed Effects Models, 2023. https://CRAN.R-project.org/package=nlme. [Google Scholar]
- [72].Bouwmans MEJ, Bos EH, Hoenders HJR, Oldehinkel AJ, de Jonge P, Sleep quality predicts positive and negative affect but not vice versa. An electronic diary study in depressed and healthy individuals, J Affect Disord 207 (2017) 260–267. 10.1016/jjad.2016.09.046 [DOI] [PubMed] [Google Scholar]
- [73].Le F, Yap Y, Tung NYC, Bei B, Wiley JF, The associations between daily activities and affect: A compositional isotemporal substitution analysis, Int J Behav Med (2021). 10.1007/s12529-021-10031-z [DOI] [PubMed] [Google Scholar]
- [74].Sin NL, Almeida DM, Crain TL, Kossek EE, Berkman LF, Buxton OM, Bidirectional, temporal associations of sleep with positive events, affect, and stressors in daily life across a week, Ann Behav Med 51(3) (2017) 402–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Minaeva O, George SV, Kuranova A, Jacobs N, Thiery E, Derom C, Wichers M, Riese H, Booij SH, Overnight affective dynamics and sleep characteristics as predictors of depression and its development in women, Sleep 44(10) (2021). 10.1093/sleep/zsab129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Koval P, Kuppens P, Allen NB, Sheeber L, Getting stuck in depression: The roles of rumination and emotional inertia, Cognition and Emotion 26(8) (2012) 1412–1427. 10.1080/02699931.2012.667392 [DOI] [PubMed] [Google Scholar]
- [77].Kim Y, Umeda M, Lochbaum M, Sloan R, Examining the day-to-day bidirectional associations between physical activity, sedentary behavior, screen time, and sleep health during school days in adolescents, PLoS ONE 15(9) (2020) e0238721. 10.1371/journal.pone.0238721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pesonen A-K, Kahn M, Kuula L, Korhonen T, Leinonen L, Martinmäki K, Gradisar M, Lipsanen J, Sleep and physical activity – the dynamics of bi-directional influences over a fortnight, BMC Public Health 22(1) (2022) 1160. 10.1186/s12889-022-13586-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Nota JA, Coles ME, Shorter sleep duration and longer sleep onset latency are related to difficulty disengaging attention from negative emotional images in individuals with elevated transdiagnostic repetitive negative thinking, J Behav Ther Exp Psychiatry 58 (2018) 114–122. 10.1016/j.jbtep.2017.10.003 [DOI] [PubMed] [Google Scholar]
- [80].Gobin C, Banks J, Fins A, Tartar J, Poor sleep quality is associated with a negative cognitive bias and decreased sustained attention, J Sleep Res 24(5) (2015) 535–542. 10.Ill1/jsr.12302 [DOI] [PubMed] [Google Scholar]
- [81].Pilcher JJ, Callan C, Posey JL, Sleep deprivation affects reactivity to positive but not negative stimuli, J Psychosom Res 79(6) (2015) 657–662. 10.1016/jjpsychores.2015.05.003 [DOI] [PubMed] [Google Scholar]
- [82].Yoo S-S, Gujar N, Hu P, Jolesz FA, Walker MP, The human emotional brain without sleep — a prefrontal amygdala disconnect, Curr Biol 17(20) (2007) R877–R878. 10.1016/j.cub.2007.08.007 [DOI] [PubMed] [Google Scholar]
- [83].Tempesta D, De Gennaro L, Natale V, Ferrara M, Emotional memory processing is influenced by sleep quality, Sleep Med 16(7) (2015) 862–870. 10.1016/j.sleep.2015.01.024 [DOI] [PubMed] [Google Scholar]
- [84].Consensus Conference P, Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E, Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion, J. Clin. Sleep Med 11(8) (2015) 931–952. 10.5664/jcsm.4950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Babson KA, Feldner MT, Sleep and affect: An integrative synthesis and future directions, in: Babson KA, Feldner MT (Eds.), Sleep and affect: Assessment, theory, and clinical implications, Elsevier Academic Press, San Diego, CA, 2015, pp. 463–483. 10.1016/B978-0-12-417188-6.00021-9 [DOI] [Google Scholar]
- [86].Zohar D, Tzischinsky O, Epstein R, Lavie P, The effects of sleep loss on medical residents’ emotional reactions to work events: a cognitive-energy model, Sleep 28(1) (2005) 47–54. 10.1093/sleep/28.L47 [DOI] [PubMed] [Google Scholar]
- [87].O’Leary K, Small BJ, Panaite V, Bylsma LM, Rottenberg J, Sleep quality in healthy and mood-disordered persons predicts daily life emotional reactivity, Cognition and Emotion 31(3) (2017) 435–443. 10.1080/02699931.2015.1126554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mauss IB, Troy AS, LeBourgeois MK, Poorer sleep quality is associated with lower emotion-regulation ability in a laboratory paradigm, Cognition and Emotion 27(3) (2013) 567–576. 10.1080/02699931.2012.727783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhang J, Lau EYY, Hsiao J.H.-w., Using emotion regulation strategies after sleep deprivation: ERP and behavioral findings, Cogn Affect Behav Neurosci 19(2) (2019) 283–295. 10.3758/s13415-018-00667-y [DOI] [PubMed] [Google Scholar]
- [90].Tamm S, Nilsonne G, Schwarz J, Golkar A, Kecklund G, Petrovic P, Fischer H, Åkerstedt T, Lekander M, Sleep restriction caused impaired emotional regulation without detectable brain activation changes: A functional magnetic resonance imaging study, Royal Society Open Science 6(3) (2019) 181704. 10.1098/rsos.181704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Dahl RE, The regulation of sleep and arousal: Development and psychopathology, Dev Psychopathol 8(1) (1996) 3–27. 10.1017/S0954579400006945 [DOI] [Google Scholar]
- [92].Chen J, Liang J, Lin X, Zhang Y, Zhang Y, Lu L, Shi J, Sleep deprivation promotes habitual control over goal-directed control: Behavioral and neuroimaging evidence, The Journal of Neuroscience 37(49) (2017) 11979–11992. 10.1523/JNEUROSCI.1612-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].van Peer JM, Gladwin TE, Nieuwenhuys A, Effects of threat and sleep deprivation on action tendencies and response inhibition, Emotion 19(8) (2019) 1425–1436. 10.1037/emo0000533 [DOI] [PubMed] [Google Scholar]
- [94].Baron KG, Duffecy J, Richardson D, Avery E, Rothschild S, Lane J, Technology assisted behavior intervention to extend sleep among adults with short sleep duration and prehypertension/stage 1 hypertension: A randomized pilot feasibility study, J Clin Sleep Med 15(11) (2019) 1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Tavernier R, Choo SB, Grant K, Adam EK, Daily affective experiences predict objective sleep outcomes among adolescents, J Sleep Res 25(1) (2016) 62–69. 10.1111/jsr.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Gould CE, Karna R, Jordan J, Kawai M, Hirst R, Hantke N, Pirog S, Cotto I, Schussler-Fiorenza Rose SM, Beaudreau SA, O’Hara R, Subjective but not objective sleep is associated with subsyndromal anxiety and depression in community-dwelling older adults, The American Journal of Geriatric Psychiatry 26(7) (2018) 806–811. 10.1016/jjagp.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Talbot LS, Stone S, Gruber J, Hairston IS, Eidelman P, Harvey AG, A test of the bidirectional association between sleep and mood in bipolar disorder and insomnia, J Abnorm Psychol 121(1) (2012) 39–50. 10.1037/a0024946 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


