Abstract
Objectives
Prolonged postoperative opioid use increases risk for new post-surgical opioid use disorder. We evaluated preoperative phenotypic factors predicting prolonged postoperative opioid use.
Methods
We performed a secondary analysis of a prospective observational cohort (n=108) undergoing total knee arthroplasty (TKA) for osteoarthritis with 6-week and 6-month follow-up. Current opioid use and psychosocial, pain, and opioid-related characteristics were assessed at preoperative baseline. Primary outcomes were days/week of opioid use at follow-up.
Results
At 6 weeks, preoperative opioid use and greater cumulative opioid exposure, depression, catastrophizing, anxiety, pain interference, sleep disturbance, and central sensitization were significantly associated with more days/week of opioid use after controlling for contemporaneous pain intensity. These predictors, and prior euphoric response to opioids, were also significant predictors at 6 months. All 6-week predictors except anxiety remained significant after controlling for preoperative opioid use; at 6 months, cumulative opioid exposure, catastrophizing, pain interference, and sleep disturbance remained significant after this adjustment (p’s <0.05). In multivariable models, a psychosocial factor reflecting negative affect, sleep, and pain accurately predicted 6-week opioid use (AUC=0.84). A combined model incorporating psychosocial factor scores, opioid-related factor scores, and preoperative opioid use showed near-perfect predictive accuracy at 6 months (AUC=0.97).
Discussion
Overall, preoperative psychosocial, pain-related, and opioid-related phenotypic characteristics predicted prolonged opioid use following TKA.
Keywords: persistent postoperative opioid use, pain, opioid, psychosocial, precision medicine
Introduction
About 25% of the nearly 70,000 U.S. opioid overdose deaths in 2020 involved prescription opioids.[1] Post-surgical care is often patients’ first opioid exposure,[2] and 4–10% of opioid-naïve surgical patients receiving postoperative opioids develop new persistent opioid use.[3–7] Opioid overdose or a new OUD diagnosis occurs in 0.1–0.7% of surgical patients prescribed opioids during the first postoperative year.[8, 9] Extrapolating based on surgical and dental prescribing estimates, 1.8–4.5 million U.S patients develop post-procedural new persistent opioid use and 45,000–315,000 develop post-procedural OUD annually.[10] Persistent postoperative opioid use increases risk for OUD (88% increased hazard) and overdose (78% increased hazard).[9]
Although large electronic health record and insurance database studies examining persistent post-surgical opioid use rates are available,[11] the dearth of systematic prospective data examining risk factors for persistent opioid use represents a significant research gap.[12] Prospective studies of well-phenotyped patients are needed to examine predictors of prolonged postoperative opioid use in granular detail, potentially enabling future risk stratification algorithms and personalized postsurgical care (e.g., minimizing opioids and maximizing non-opioid therapies for at-risk patients, increased surveillance for opioid misuse). Preoperative opioid use is a well-known predictor of delayed opioid cessation postoperatively.[5, 13–22]. Distant prior opioid use also predicts persistent postoperative opioid use.[23]
Biopsychosocial factors also may drive persistent postoperative opioid use. For example, persistent postoperative pain (incidence up to 50% depending on surgery[24]) is a predictor of persistent postoperative opioid use.[12, 25–27] Central sensitization, which may drive postoperative pain intensity and chronicity, may also affect postoperative opioid use, but has been little studied in this context.[28, 29] Beyond preoperative opioid use and postoperative pain, prior prospective work has identified psychosocial factors associated with prolonged postoperative opioid use, including catastrophizing,[5, 16, 17, 30, 31] anxiety,[25, 31, 32] and depression.[15, 31] These studies often comprise heterogeneous samples reflecting surgeries varying in degree of tissue trauma and expected postoperative pain course, potentially confounding interpretation. Moreover, absence of prior prospective work evaluating history of positive subjective responses to opioids (e.g., euphoria) as a risk factor for persistent postoperative opioid use is a potentially important gap, as these responses may drive continued opioid use due to operant reinforcement mechanisms.[33–35]
Given heterogeneous post-surgical pain trajectories,[36] known relationships between preoperative and persistent postoperative opioid use, and evidence that phenotypic characteristics unrelated to pain may contribute to prolonged postoperative opioid use, we comprehensively evaluated preoperative phenotypic factors predicting extent of subacute and chronic postoperative opioid use while (1) controlling for contemporaneous pain intensity (a confound rarely addressed) and (2) with and without controlling for preoperative opioid use. This approach permits evaluating whether psychosocial and pain/opioid-related phenotypes (including central sensitization and euphoric response to opioids) predict prolonged opioid use beyond what can be accounted for by preoperative opioid use and pain intensity itself. This is critical for developing optimal precision pre-surgical opioid risk stratification approaches. The current sample was a homogeneous cohort of patients undergoing unilateral total knee arthroplasty (TKA) for osteoarthritis, a population with substantial rates of prolonged postoperative opioid use (20%)[5, 13, 14, 16, 37] and persistent pain (25%),[38]
Materials and Methods
Design
A mixed between/within-subjects longitudinal design was used including assessment of pain/opioid-related and psychosocial phenotypes at preoperative baseline, and assessment of opioid use outcomes at 6 weeks and 6 months post-TKA. These are secondary analyses of data from a project examining perioperative oxidative stress mechanisms of post-TKA pain.[39–41]
Participants
Participants (n=108) were individuals of both sexes, age ≥55, undergoing primary unilateral TKA for osteoarthritis by one of the two surgeon co-authors (GGP, AAS). Participants were recruited in-person during preoperative group education sessions or (post-COVID) telephone calls to potentially qualifying patients by the research coordinator. Individuals who were interested in study participation were then screened for eligibility and consented. All procedures were Institutional Review Board-approved. Participants enrolled between August 2017-June 2021. The n=120 sample size for the parent study was chosen to achieve >0.80 power to detect associations between the primary oxidative stress measure (F2-isoprostanes) and pain outcomes.
Inclusion criteria were: 1) intact cognitive status and ability to provide informed consent (Mini Mental State Examination score ≥24),[42] 2) ability to understand English sufficiently to complete study questionnaires, 3) age ≥55 years, 4) undergoing unilateral TKA, 5) osteoarthritis diagnosis. Exclusion criteria were: 1) complex regional pain syndrome diagnosis, 2) TKA revision, 3) presence of lower extremity vascular disease, inflammatory or autoimmune disorders, or malignancy, and 4) presence of other conditions making participation unsafe. Participants received $200 for completing the study.
There were 985 potential participants identified, with 303 interested and screened. Of these, 120 qualified and consented, with 7 subsequently disqualified due to other medical issues noted (cognitive dysfunction, medical exclusions). The final sample was n=113 (no participants withdrew or were lost to follow-up). The final analyzed sample included all participants (n=108) with primary opioid outcome data available at follow-up. Table 1 summarizes sample characteristics.
Table 1.
Summary of preoperative demographic, clinical characteristics, and phenotype predictors.
| Mean (SD) or % (n = 108) | |
|---|---|
|
| |
| Demographic Characteristics: | |
| Age (years) | 67.4 (6.91) |
| Gender (% female) | 63.9 |
| Race/Ethnicity: | |
| Non-Hispanic White | 96.3 |
| African-American | 3.7 |
| BMI | 31.9 (5.30) |
| Diabetes Status (% yes) | 19.8 |
| Anesthesia Type (% spinal) | 87.0 |
| ASA Status: | |
| 2 | 32.4 |
| 3 | 67.6 |
| Current Smoking Status (% yes) | 3.7 |
| Kellgren and Lawrence Grade | |
| 2 | 1.1 |
| 3 | 7.4 |
| 4 | 91.6 |
| Preoperative Phenotype Predictors: | |
| Preoperative Opioid Use (% Yes) | 9.3 |
| HOME-Euphoric Response | 3.1 (1.22) |
| HOME-Opioid Exposure | 2.4 (1.44) |
| CES-D | 18.9 (7.14) |
| CATS | 14.2 (11.53) |
| STAI | 32.4 (9.47) |
| MBM | 4.5 (3.44) |
| PROMIS Pain Interference | 61.9 (7.50) |
| PROMIS Sleep Disturbance | 52.7 (9.14) |
| Temporal Summation of Pain | 1.7 (1.39) |
Note: BMI = Body Mass Index, HOME = History of Opioid Medical Exposure, CES-D = Center for Epidemiological Studies-Depression Scale, CATS = Catastrophizing Scale, STAI = State Trait Anxiety Inventory, MBM = Michigan Body Map. Temporal summation of pain values reflect the greatest increase in 0–10 NRS pain ratings after 10 repeated punctate stimuli (100g von frey hair) relative to a single stimulus.
Measures
Preoperative Phenotype
Preoperative phenotype measures were chosen based on prior work suggesting associations of each construct with opioid responsiveness or opioid use.[28, 43–49]
Prior Euphoric Responses to Opioids and Cumulative Opioid Exposure
The Euphoric Response and Cumulative Opioid Exposure subscales of the History of Opioid Medical Exposure measure (HOME) were used to assess prior positive subjective responses to opioids as well as the lifetime amount of opioid taken by subjects.[43]
Negative Affect
The Center for Epidemiological Studies-Depression scale (CES-D; 20 item version) was used to assess depressive symptoms. This is a widely used standardized measure validated for use in older populations[50, 51] and those with chronic medical conditions including arthritis.[52] Higher scores indicate greater depressive symptoms.
Anxiety symptoms were assessed using the trait form of the State-Trait Anxiety Inventory (STAI), a commonly used self-report measure with well-established psychometric properties.[53] Higher STAI scores denote an increased tendency toward anxiety symptoms.
Pain Catastrophizing
Pain catastrophizing was assessed using the Pain Catastrophizing Scale (CATS).[54] This is a 13-item measure that assesses the degree to which individuals have negative thoughts and feelings when experiencing pain. Higher scores indicate greater pain catastrophizing.
Centralized Pain
The Michigan Body Map (MBM) assesses how many of 19 discrete sites subjects experience pain at. It is one of two components of the Fibromyalgia Survey Criteria used for fibromyalgia diagnosis. The MBM is used in the research setting to evaluate the degree of widespread pain (a marker of centralized pain) experienced by subjects.[55]
We also included results of a dynamic quantitative sensory testing (QST) protocol, temporal summation of punctate pain (TSP), that indexes central nervous system pain facilitation (i.e., central sensitization).[56] TSP to punctate mechanical stimuli was assessed using procedures based on Goodin et al.[56] The stimulus used was a 100g monofilament, with this stimulus weight determined based on weights shown to elicit TSP in prior literature (e.g., ranging from 25g to 300g).[56, 57] A single stimulus was first applied for one second to the affected knee (4 cm above the patella, 8 cm towards the midline), with pain intensity rated using a 0–10 Numeric Rating Scale (NRS) anchored with “no pain” and “worst possible pain.” Then, using the same procedures as above, a series of 10 punctate stimuli were delivered at a rate of one stimulus per second to the affected knee, with an NRS pain intensity rating then obtained to describe the highest pain intensity experienced. This procedure was repeated twice. The change in pain intensity between the first stimulus and the stimulus eliciting the highest pain rating (mean of the two trials) was used to index preoperative TSP in the affected knee (larger positive values indicated greater TSP). This punctate TSP measure was the only QST measure used to maximize clinical feasibility of the protocol.
Sleep Disturbance
The PROMIS Sleep Disturbance – Short Form 8a instrument assesses sleep quality, sleep depth and restoration associated with sleep over the past week. Higher scores indicate greater levels of sleep disturbance. This measure has been found to have superior precision compared with other commonly used self-reported sleep measures.[58]
Function
The PROMIS Pain Interference– Short Form 8a instrument (PROMIS Interference) assesses pain-related life interference over the prior week (the impact of pain on the ability to carry out daily social, emotional, physical, and recreational activities).[59] Higher scores indicate greater levels of interference.
Opioid Use
Opioid use outcomes were self-reported number of days (0–7) of opioid use in the week prior to each assessment (6-week and 6-month follow-up). Self-reported opioid use over 2-week periods corresponds highly (intraclass correlation=0.85) with opioid use determined via electronic pill cap.[60] For use in exploratory combined predictor models evaluating Receiver Operating Characteristic (ROC) curves, a dichotomous opioid use outcome was created reflecting daily vs. non-daily opioid use at each follow-up (1/0). This secondary dichotomous outcome is clinically relevant and maintains adequate statistical power. Opioid use immediately before surgery (yes/no; confirmed against patient medication list) was also assessed for inclusion as a preoperative predictor.
Postoperative Pain
Postoperative pain was assessed using an 11-point numeric rating of past 24-hour worst pain intensity in the operative knee at each postoperative assessment (0 = “No Pain” and 10 = “Worst Possible Pain”).
Procedure
After providing informed consent, baseline preoperative assessments were conducted by the research coordinator in the participant’s home (median=3.0 days preoperatively). Assessment included demographics and opioid use, phenotype measures, and standardized TSP assessment using established protocols.[61] Anesthesia and postoperative care protocols are described in Supplemental Digital Content 1. At 6 weeks (6–8-week window) and 6 months (24–28-week window) postoperatively, the research coordinator assessed opioid use and postoperative pain outcomes in person in the participant’s home.
Statistical Analysis
Analyses were conducted using SPSS (IBM SPSS Statistics for Windows, Version 28.0, Armonk, NY). Except where noted, sample descriptive statistics included mean (SD) and percentages for continuous and categorical variables, respectively. Associations among preoperative predictors were examined using Spearman nonparametric correlations. Given the right-skewed and highly dispersed nature of the opioid use outcomes, primary analyses used generalized linear models (GLM) specifying a negative binomial distribution. All GLM models adjusted for sex, age, body mass index (BMI), and past 24-hour worst pain intensity contemporaneous with opioid use outcomes at follow-up (the latter to evaluate predictive effects beyond that attributable to postoperative pain intensity). Primary analyses examined preoperative opioid use and phenotype measures as predictors of days per week of opioid use at both follow-ups. Then, these analyses were repeated, including preoperative opioid use as an additional control variable to evaluate phenotype predictive effects beyond those accounted for simply by preoperative opioid use.
To evaluate combined predictor models in exploratory analyses while addressing multicollinearity issues, significant phenotype predictors in primary analyses were subjected to principal components analysis (varimax rotation) resulting in extraction of two factors accounting for 57.1% of variance across measures: 1) a psychosocial factor (negative affect, sleep, and pain-related), and 2) an opioid-related factor (past euphoric opioid responses and cumulative opioid exposure from the HOME). Two factor scores (regression method) reflecting the factors above were generated for each patient and then tested (in addition to preoperative opioid use) as predictors of daily versus non-daily opioid use in ROC curve analyses, with area under the curve (AUC) the predictive accuracy criterion. All analyses included the maximum number of cases available. A two-sided p<0.05 significance level defined statistical significance. The sample size resulted in power of 0.80 to detect effect sizes of r≥0.27 (i.e., moderate or larger effects).[62]
Results
Opioid Use Outcomes Post-TKA
Preoperatively, 9.3% (n=10) of patients had recently used an opioid analgesic. The standard discharge analgesic, oxycodone 10 mg, was received by 96.3% (n=104) of patients, with 2.8% (n=3) receiving hydrocodone and 0.9% (n=1) receiving hydromorphone for continuity with presurgical opioid regimens. Changes in state law regarding postoperative opioid prescribing that occurred in early 2019 did not significantly alter discharge prescription size or our primary opioid use outcome. Refills were provided as clinically indicated. Postoperatively, 54.2% (n=58) of patients requested opioid refills. Median (IQR) number of refills was 1.0 (1.0), with number of refills ranging from 0–4. Median days per week of opioid use at both post-TKA follow-ups was zero, with wide variability at both follow-ups (range: 0–7 days; see Figures, Supplemental Digital Content 2–3). At 6-week follow-up, 11.3% (n=12) were using opioids daily with 79.2% (n=84) no longer using any opioids. At 6-month follow-up, 5.6% (n=6) were using opioids daily with the remainder using no opioids.
Associations Among Preoperative Predictors
Table 2 displays intercorrelations among preoperative predictors. Preoperative opioid use was associated with higher HOME Cumulative Opioid Exposure scores, and greater widespread pain (MBM) and sleep disturbance. Greater cumulative opioid exposure was associated with higher depression (CES-D), pain interference and sleep disturbance (PROMIS measures), and more widespread pain. Depression, anxiety (STAI), and catastrophizing (CATS) were significantly intercorrelated, reflecting a common underlying negative affect (NA) construct. Greater TSP was correlated with higher depression, catastrophizing, and sleep disturbance. The HOME Euphoric Response subscale that assesses prior positive subjective opioid responses was not associated with any other measures.
Table 2.
Zero-order Spearman nonparametric correlations among preoperative predictors of postoperative opioid use.
| Preoperative Predictor | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1. Preoperative Opioid Use | 0.11 | 0.39*** | 0.08 | 0.13 | 0.02 | 0.20* | 0.15 | 0.28** | 0.12 |
| 2. HOME-Euphoric Response | -- | 0.09 | −0.10 | −0.01 | −0.17 | 0.00 | 0.00 | −0.03 | −0.11 |
| 3. HOME-Opioid Exposure | -- | -- | 0.26** | 0.19† | 0.14 | 0.24* | 0.25* | 0.26** | 0.00 |
| 4. CES-D | -- | -- | -- | 0.66*** | 0.53*** | 0.14 | 0.52*** | 0.52*** | 0.28** |
| 5. CATS | -- | -- | -- | -- | 0.59*** | 0.22* | 0.63*** | 0.42*** | 0.31** |
| 6. STAI | -- | -- | -- | -- | -- | 0.22* | 0.38*** | 0.38*** | 0.13 |
| 7. MBM | -- | -- | -- | -- | -- | -- | 0.22* | 0.18† | 0.15 |
| 8. PROMIS Pain Interference | -- | -- | -- | -- | -- | -- | -- | 0.39*** | 0.12 |
| 9. PROMIS Sleep Disturbance | -- | -- | -- | -- | -- | -- | -- | -- | 0.21* |
| 10. Temporal Summation of Pain | -- | -- | -- | -- | -- | -- | -- | -- | -- |
p<.10
p<.05
p<.01
p<.001
Note: TKA = Total Knee Arthroplasty, HOME = History of Opioid Medical Exposure, CES-D = Center for Epidemiological Studies Depression Scale, CATS = Catastrophizing Scale, STAI = State Trait Anxiety Inventory, MBM = Michigan Body Map
Prospective Predictors of Postoperative Opioid Use
Table 3 summarizes GLM analyses examining preoperative predictors of subsequent postoperative opioid use at 6-week and 6-month follow-up (controlling for contemporaneous pain intensity). As expected, preoperative opioid use significantly predicted days per week of postoperative opioid use at both follow-ups. At 6-week follow-up, a variety of preoperative phenotype characteristics predicted greater extent of opioid use, including greater cumulative opioid exposure; higher depression, anxiety, and catastrophizing; more pain-related life interference and sleep disturbance; and greater TSP (central sensitization). All predictors of postoperative opioid use at 6 weeks were also significant at 6-month follow-up. In addition, at 6 months post-TKA, higher scores on the Euphoric Response subscale of the HOME also predicted more prolonged opioid use. All of the predictors above remained significant after applying the Benjamini-Hochberg procedure to address Type I error rate inflation (FDR = 0.25).
Table 3.
GLM analyses of prospective preoperative predictors of days per week of opioid use at post-TKA follow-up.
| 6-Week Follow-Up | 6-Month Follow-Up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative Predictor | Beta | SE | P value | Adj OR | 95% CI | Beta | SE | P value | Adj OR | 95% CI |
|
| ||||||||||
| Preoperative Opioid Use | 1.344 | 0.452 | .003 | 3.83 | 1.58, 9.31 | 5.727 | 1.283 | <.001 | 307.19 | 24.86, 3796.07 |
| HOME-Euphoric Response | −0.020 | 0.163 | .902 | 0.98 | 0.71, 1.35 | 0.615 | 0.276 | .026 | 1.85 | 1.08, 3.18 |
| HOME-Opioid Exposure | 0.417 | 0.113 | <.001 | 1.52 | 1.22, 1.89 | 2.958 | 0.892 | <.001 | 19.26 | 3.36, 110.54 |
| CES-D | 0.101 | 0.022 | <.001 | 1.11 | 1.06, 1.15 | 0.130 | 0.035 | <.001 | 1.14 | 1.06, 1.22 |
| CATS | 0.053 | 0.013 | <.001 | 1.05 | 1.03, 1.08 | 0.091 | 0.024 | <.001 | 1.10 | 1.05, 1.15 |
| STAI | 0.031 | 0.015 | .035 | 1.03 | 1.01, 1.06 | 0.060 | 0.023 | .009 | 1.06 | 1.02, 1.11 |
| MBM | −0.030 | 0.047 | .520 | 0.97 | 0.89, 1.06 | 0.095 | 0.071 | .183 | 1.10 | 0.96, 1.26 |
| PROMIS Pain Interference | 0.065 | 0.023 | .005 | 1.07 | 1.02, 1.12 | 0.108 | 0.040 | .007 | 1.11 | 1.03, 1.21 |
| PROMIS Sleep Disturbance | 0.063 | 0.020 | .001 | 1.07 | 1.03, 1.11 | 0.163 | 0.039 | <.001 | 1.18 | 1.09, 1.27 |
| Temporal Summation of Pain | 0.360 | 0.105 | <.001 | 1.43 | 1.17, 1.76 | 0.786 | 0.204 | <.001 | 2.20 | 1.47, 3.28 |
Note: Analyses of the continuous days per week of opioid use outcome specified a negative binomial distribution.
Significant results are highlighted in bold text. TKA = Total Knee Arthroplasty, HOME = History of Opioid
Medical Exposure, CES-D = Center for Epidemiological Studies Depression Scale, CATS = Catastrophizing
Scale, STAI = State Trait Anxiety Inventory, MBM = Michigan Body Map
To evaluate extent to which phenotype measures predicted postoperative opioid use beyond that accounted for simply by preoperative opioid use, we repeated the analyses above in second analyses, adjusting for preoperative opioid use (see Table, Supplemental Digital Content 4). At 6-week follow-up, all phenotype predictors identified above except anxiety remained significant even after controlling for preoperative opioid use. At 6-month follow-up, cumulative opioid exposure, catastrophizing, pain interference, and sleep disturbance remained significant predictors after adjusting for influence of preoperative opioid use.
Potential for Clinical Utility
To address issues of potential clinical utility in precision pain medicine algorithms, we evaluated in exploratory analyses the accuracy of combined models reflecting the two factor scores described above (psychosocial and opioid-related factors), as well as preoperative opioid use, for predicting daily versus non-daily use of opioids at each follow-up. At 6-week follow-up (Figure 1), scores on the psychosocial factor were the most accurate predictor of daily opioid use (AUC=0.84). Both preoperative opioid use (AUC=0.64) and opioid-related factor scores (AUC=0.59) displayed similar but lower accuracy. Only scores on the psychosocial factor produced a good predictive model (i.e., AUC>0.70). A combination of all three predictors (standardized) did not improve accuracy (AUC=0.83) beyond the psychosocial factor alone.
Figure 1.
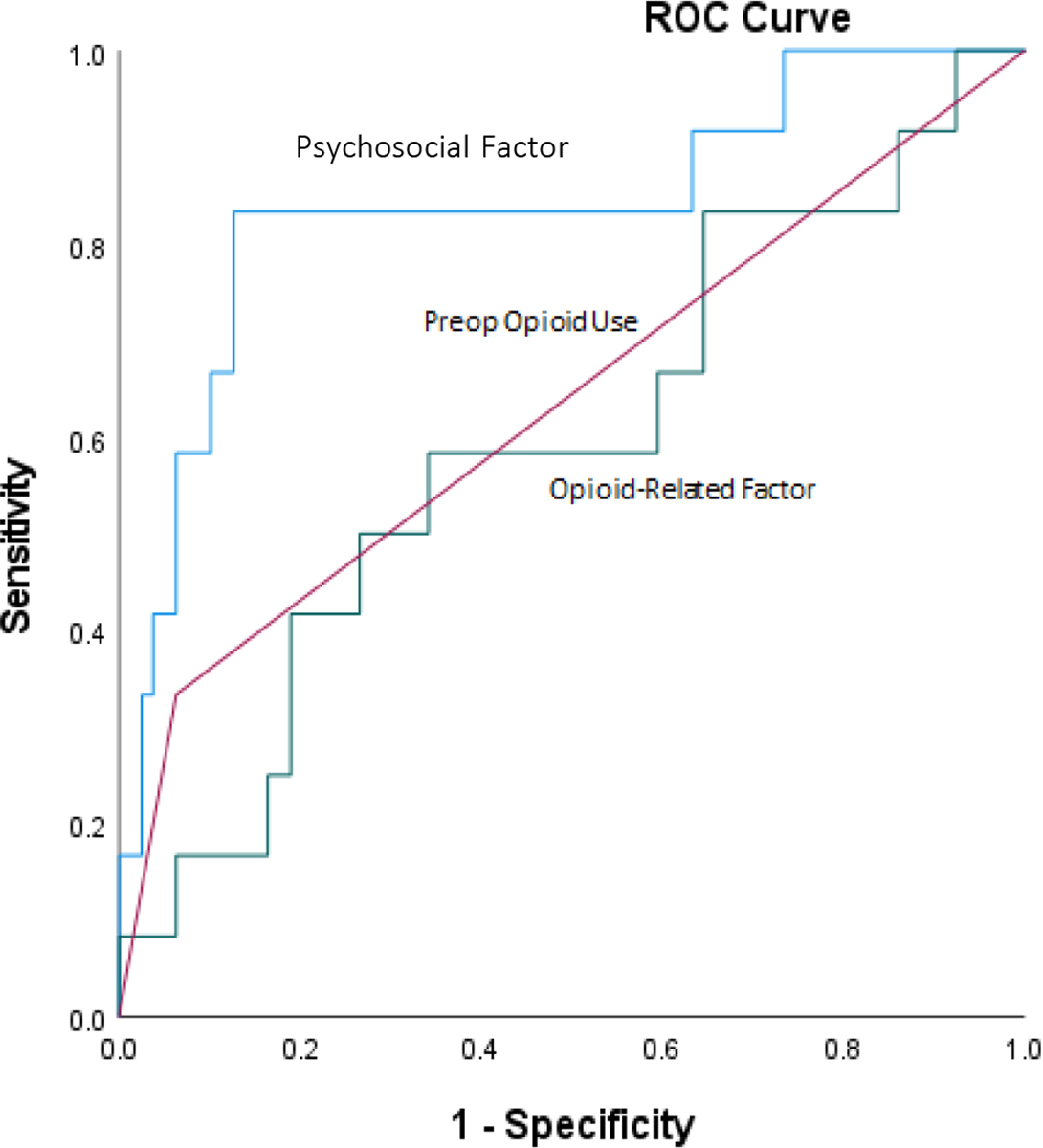
ROC curves displaying classification accuracy of preoperative opioid use and factor analysis-derived combined phenotype predictors of subsequent daily opioid use versus non-daily opioid use at 6 weeks post-TKA.
At 6-month follow-up (Figure 2), preoperative opioid use was the most accurate predictor (AUC=0.90). Scores on the psychosocial factor (AUC=0.83) and opioid-related factor (AUC=0.77) displayed somewhat lower accuracy, but all resulted in good predictive models. Combining all three predictors improved accuracy of predicting daily opioid use at 6-month follow-up to near-perfect prediction (AUC=0.97).
Figure 2.
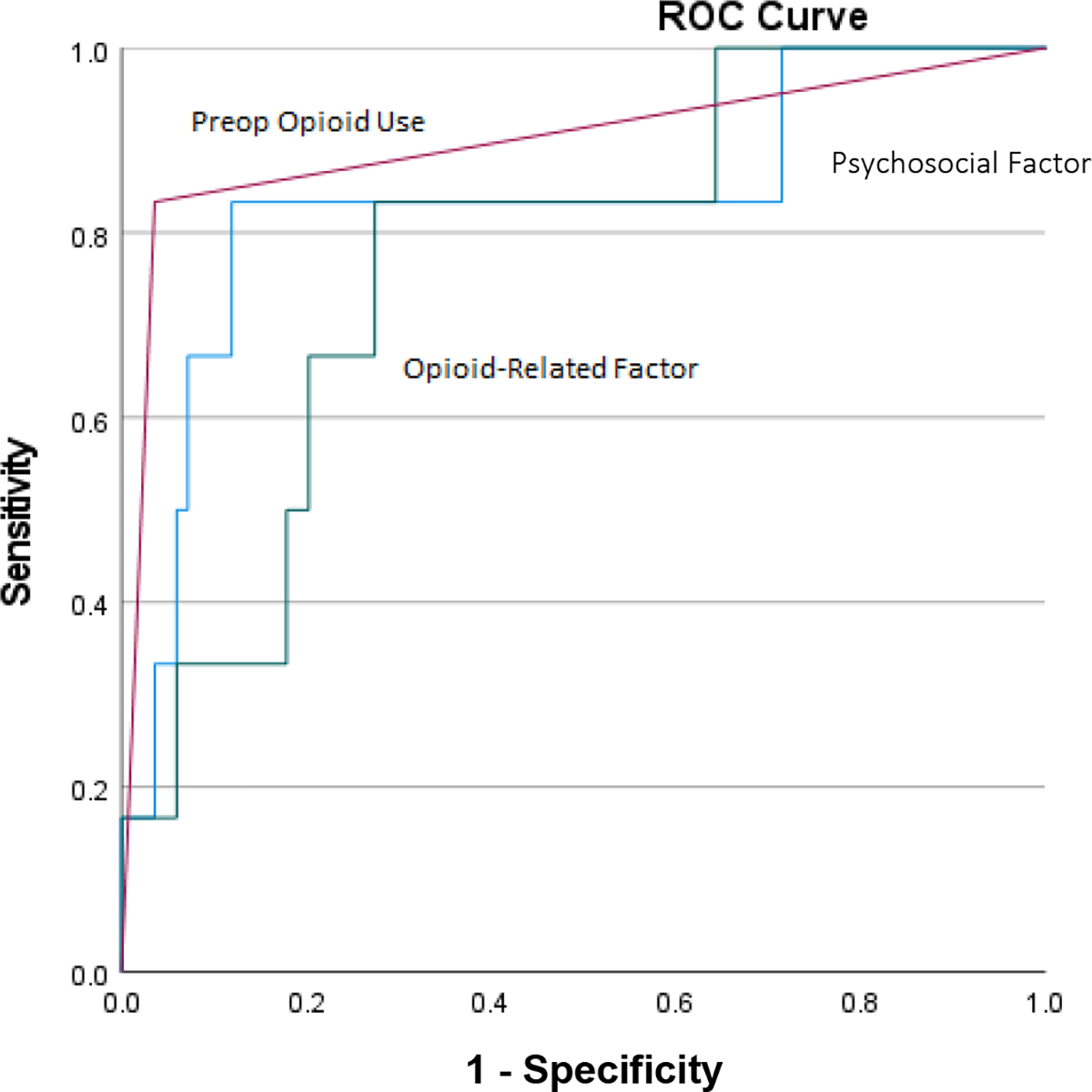
ROC curves displaying classification accuracy of preoperative opioid use and factor analysis-derived combined phenotype predictors of subsequent daily opioid use versus non-daily opioid use at 6 months post-TKA.
Discussion
Multiple studies have highlighted the high incidence of persistent postoperative opioid use.[3–7] However, much of this work has been carried out utilizing insurance claims databases and other large-scale retrospective cohorts. Progressing from problem identification to clinical precision medicine approaches to opioid risk mitigation requires prospective studies with more granular data.[12] In this study, we identified a preoperative phenotype that identifies patients at risk for prolonged opioid use following TKA. As the heterogeneity of definitions for persistent postoperative opioid use has been criticized,[11] we utilized a granular outcome of days per week of opioid use to identify phenotypic predictors in univariate models and a stringent definition of daily opioid use to construct ROC curves in exploratory combined predictor models.
As in many prior studies,[5, 13–22] preoperative opioid use predicted extent of opioid use postoperatively. Our results further indicated that even when controlling for contemporaneous pain intensity, phenotypic characteristics including cumulative prior opioid exposure, depression, anxiety, catastrophizing, pain interference, sleep disturbance, and central sensitization all independently predicted extent of opioid use at 6 weeks post-TKA. These same predictors, as well as past euphoric subjective opioid responses, predicted opioid use at 6 months (all of which represented daily opioid use). Several of these predictors replicate findings from prior studies, including catastrophizing,[5, 16, 17, 30, 31] anxiety,[25, 31, 32] and depression.[15, 31] However, our results are inconsistent with findings from a recent single-center cohort study of similar size that only preoperative patient fatigue, but not pain catastrophizing, depression, or anxiety, were associated with duration of subacute opioid use following total knee and hip arthroplasty. Reasons for this discrepancy are unclear but may potentially be related to differences in opioid outcomes or the specific measures of negative affect examined.[63] Our finding of an association between self-reported cumulative lifetime opioid use and prolonged postoperative use is similar to the association between distant prior opioid use and persistent opioid use reported recently.[23] Central sensitization has been previously associated with immediate postoperative opioid utilization; we extend this finding to a much longer timeframe.[28, 29] Surprisingly, we did not identify the expected association between prolonged opioid use and extent of widespread pain.[55] The reasons for this finding are unclear. Finally, our novel finding that preoperative patient report of a euphoric response to first-ever opioid exposure was significantly associated with persistent daily opioid use at 6 months suggests a potential role for subjective opioid reinforcing effects in the transition from routine postoperative opioid use to persistent use. While this association is consistent with reinforcement models of OUD, this study did not directly assess OUD.[33–35] The role of reinforcing subjective opioid effects has been little studied in the perioperative context and warrants further investigation.
In a secondary analysis controlling for influence of preoperative opioid use, all predictive factors above (except anxiety) remained significant at 6 weeks, with cumulative opioid exposure, catastrophizing, pain interference, and sleep disturbance remaining significant at 6 months. Many large cross-sectional predictive algorithm studies confirm that prior opioid use is frequently the strongest predictor of prolonged postoperative use.[64–67] Our prospective findings indicate that patient psychosocial and pain phenotypic characteristics predict greater opioid use at postoperative follow-up above and beyond that accounted for by preoperative opioid use. These findings have implications for developing clinically useful opioid stratification algorithms.
Our factor analytic-derived combined phenotype measures (psychosocial and opioid-related) as well as preoperative opioid use each displayed high accuracy for predicting daily opioid use at 6 months postoperatively. Combining all three predictors yielded an AUC approaching 1.0 for this outcome, implying that we are unlikely to have missed unstudied patient characteristics that drive extent of persistent postoperative use. For the shorter-term 6-week daily use outcome, the psychosocial factor was a more accurate predictor than either preoperative opioid use or the opioid-related factor, and a combined model did not improve accuracy. These data may point to a potential transition in factors driving prolonged use between the subacute and chronic periods and warrant further study in larger cohorts. Previously published predictive models for extended postoperative opioid use—all based on retrospective data—show an average AUC of 0.76 for preoperative opioid use.[64–75] Our prospective models show that at 6 weeks post-TKA, preoperative opioid use is a less accurate predictor (AUC=0.64) than prior retrospective models indicate, highlighting the importance of psychosocial and pain-related predictors at this time point. In contrast, at 6-month follow-up, preoperative opioid use is an even better prospective predictor than prior work would suggest (AUC=0.90), and the addition of the above phenotypic characteristics improves it even further.
Our study findings can be interpreted in context of our prior laboratory work indicating that elevated depression, anxiety, catastrophizing, and pain-related disability are each associated with increased analgesic responsiveness to a weight-adjusted opioid dose (morphine).[44] These associations are mediated in part by low endogenous opioid analgesic activity observed in individuals with higher levels of these phenotypic characteristics.[44] In light of these prior mechanistic findings, the current results suggest the possibility that risk for more extensive and persistent postoperative opioid use may be elevated in those with lower ability to naturally inhibit pain (i.e., low endogenous opioids). Notably, our prior work also indicates that low endogenous opioid function is associated with elevated drug liking and euphoric responses to opioids,[76] factors linked to OUD risk. These issues merit further exploration.
Clinical utility of the current findings remains to be proven. However, the risk factors identified in this study for more extensive opioid use could potentially serve as preoperative indicators for increased monitoring of patients’ postoperative opioid use. Surgeons and anesthesiologists could consider adjusting standard perioperative analgesic techniques in such patients to incorporate alternative strategies such as careful expectation-setting for postoperative pain, the use of regional techniques, and more frequent follow-up in the weeks after surgery. The potential clinical value of this precision pain medicine approach warrants further investigation.
This study has several limitations. Our rate of persistent opioid use at 6 months (5.6%) was substantially lower than that observed at 6 months post-TKA in some prior studies (11–32%).[37] This may be due to differences in the stringency of the persistent use definition (daily use in our study versus filling a single subsequent prescription 3–6 months postoperatively in some studies) or possibly heightened opioid prescribing caution related to the opioid crisis. While this low number of patients (n=6) using opioids at 6 months could potentially result in unreliable predictive models, the fact that the same predictors were significant at 6 weeks with a sample twice as large argues against this possibility. It is possible that negative findings for some predictors (widespread pain at 6 weeks and 6 months; euphoric response to opioids at 6 weeks) would have been positive had our study had greater statistical power. Reported accuracy of combined models may be inflated; these data should be used solely for comparing relative accuracy across models. Our cohort was also overwhelmingly non-Hispanic white, which limits generalizability of our data. These data require replication in more diverse samples. We also did not have data on the quantity and duration of preoperative opioid use in patients taking opioids prior to surgery (i.e., daily oral morphine equivalent dose), which may have influenced observed associations between preoperative and postoperative opioid use. Finally, reliance on participant self-reporting of postoperative opioid use may have led to over- or under-reporting of actual consumption at the two postoperative assessment points.
Conclusion
Utilizing a prospective design in a homogeneous population of patients undergoing primary unilateral TKA, we: (1) replicated preoperative opioid use as a predictor of prolonged postoperative opioid use and (2) identified multiple psychosocial and pain/opioid-related phenotypic characteristics that prospectively predict persistent postoperative opioid use independent of pain intensity. Our findings warrant replication, and future risk stratification algorithms should use both sources to predict risks for persistent postoperative opioid use.
Supplementary Material
Acknowledgements
The authors would like to express their appreciation to Alan Hendon, C.R.N.A., M.S. (Vanderbilt University Medical Center) and Jordan Ernst, M.D. (Baylor College of Medicine, formerly Vanderbilt University Medical Center) for their assistance on this project.
SOURCE OF FUNDING:
This work was supported by the National Institute on Aging (R01AG048915, P30AG024968), the National Institute on Drug Abuse (R01DA050334, K23DA057387), the National Institute of General Medical Sciences (R01GM112871, T32GM108554), and the National Center for Advancing Translational Sciences (UL1TR000445).
Footnotes
CONFLICTS OF INTEREST: The authors have no conflicts of interest to declare.
References
- 1.Ahmad F, Rossen L and Sutton P. Provisional drug overdose death counts. National Center for Health Statistics 2021. [Google Scholar]
- 2.Larach DB, Waljee JF, Hu HM, Lee JS, Nalliah R, Englesbe MJ and Brummett CM. Patterns of Initial Opioid Prescribing to Opioid-Naive Patients. Annals of surgery 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, Bohnert ASB, Kheterpal S and Nallamothu BK. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA surgery 2017;152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN and Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med 2012;172:425–30. [DOI] [PubMed] [Google Scholar]
- 5.Goesling J, Moser SE, Zaidi B, Hassett AL, Hilliard P, Hallstrom B, Clauw DJ and Brummett CM. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain 2016;157:1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harbaugh CM, Lee JS, Hu HM, McCabe SE, Voepel-Lewis T, Englesbe MJ, Brummett CM and Waljee JF. Persistent Opioid Use Among Pediatric Patients After Surgery. Pediatrics 2018;141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CR, Chen Z, Khurshan F, Groeneveld PW and Desai ND. Development of Persistent Opioid Use After Cardiac Surgery. JAMA Cardiol 2020;5:889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jivraj NK, Raghavji F, Bethell J, Wijeysundera DN, Ladha KS, Bateman BT, Neuman MD and Wunsch H. Persistent Postoperative Opioid Use: A Systematic Literature Search of Definitions and Population-based Cohort Study. Anesthesiology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aalberg JJ, Kimball MD, McIntire TR and McCullen GM. Long-Term Outcomes of Persistent Post-Operative Opioid Use: A Retrospective Cohort Study. Annals of surgery 2022; 10.1097/sla.0000000000005372. [DOI] [PubMed] [Google Scholar]
- 10.Guy GP Jr. and Zhang K. Opioid Prescribing by Specialty and Volume in the U.S. Am J Prev Med 2018;55:e153–e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jivraj NK, Raghavji F, Bethell J, Wijeysundera DN, Ladha KS, Bateman BT, Neuman MD and Wunsch H. Persistent Postoperative Opioid Use: A Systematic Literature Search of Definitions and Population-based Cohort Study. Anesthesiology 2020;132:1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kharasch ED and Clark JD. Persistent Postoperative Opioid Use: Perception, Progress, and Promise. Anesthesiology 2020;132:1304–1306. [DOI] [PubMed] [Google Scholar]
- 13.Kluger MT, Rice DA, Borotkanics R, Lewis GN, Somogyi AA, Barratt DT, Walker M and McNair PJ. Factors associated with persistent opioid use 6–12 months after primary total knee arthroplasty. Anaesthesia 2022;77:882–891. [DOI] [PubMed] [Google Scholar]
- 14.Sheth DS, Ho N, Pio JR, Zill P, Tovar S and Namba RS. Prolonged Opioid Use After Primary Total Knee and Total Hip Arthroplasty: Prospective Evaluation of Risk Factors and Psychological Profile for Depression, Pain Catastrophizing, and Aberrant Drug-Related Behavior. The Journal of arthroplasty 2020;35:3535–3544. [DOI] [PubMed] [Google Scholar]
- 15.Carroll I, Barelka P, Wang CK, Wang BM, Gillespie MJ, McCue R, Younger JW, Trafton J, Humphreys K, Goodman SB, Dirbas F, Whyte RI, Donington JS, Cannon WB and Mackey SC. A pilot cohort study of the determinants of longitudinal opioid use after surgery. Anesthesia and analgesia 2012;115:694–702. [DOI] [PubMed] [Google Scholar]
- 16.Giordano NA, Highland KB, Nghiem V, Scott-Richardson M and Kent M. Predictors of continued opioid use 6 months after total joint arthroplasty: a multi-site study. Arch Orthop Trauma Surg 2021. [DOI] [PubMed] [Google Scholar]
- 17.Kent ML, Giordano NA, Rojas W, Lindl MJ, Lujan E, Buckenmaier CC 3rd, Kroma R and Highland KB. Multidimensional Perioperative Recovery Trajectories in a Mixed Surgical Cohort: A Longitudinal Cluster Analysis Utilizing National Institutes of Health Patient-Reported Outcome Measurement Information System Measures. Anesthesia and analgesia 2022;134:279–290. [DOI] [PubMed] [Google Scholar]
- 18.Raebel MA, Newcomer SR, Bayliss EA, Boudreau D, DeBar L, Elliott TE, Ahmed AT, Pawloski PA, Fisher D, Toh S and Donahoo WT. Chronic opioid use emerging after bariatric surgery. Pharmacoepidemiol Drug Saf 2014;23:1247–57. [DOI] [PubMed] [Google Scholar]
- 19.Raebel MA, Newcomer SR, Reifler LM, Boudreau D, Elliott TE, DeBar L, Ahmed A, Pawloski PA, Fisher D, Donahoo WT and Bayliss EA. Chronic use of opioid medications before and after bariatric surgery. Jama 2013;310:1369–76. [DOI] [PubMed] [Google Scholar]
- 20.Zarling BJ, Yokhana SS, Herzog DT and Markel DC. Preoperative and Postoperative Opiate Use by the Arthroplasty Patient. The Journal of arthroplasty 2016;31:2081–4. [DOI] [PubMed] [Google Scholar]
- 21.Anderson JT, Haas AR, Percy R, Woods ST, Ahn UM and Ahn NU. Chronic Opioid Therapy After Lumbar Fusion Surgery for Degenerative Disc Disease in a Workers’ Compensation Setting. Spine 2015;40:1775–84. [DOI] [PubMed] [Google Scholar]
- 22.Kulshrestha S, Barrantes F, Samaniego M and Luan FL. Chronic opioid analgesic usage post-kidney transplantation and clinical outcomes. Clin Transplant 2014;28:1041–6. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal S, Shah A, Gunaseelan V, Sulich C, McAfee J, Urquhart AG, As-Sanie S, Lin J, Waljee JF and Brummett CM. New persistent opioid use after surgery in patients with a history of remote opioid use. Surgery 2022;171:1635–1641. [DOI] [PubMed] [Google Scholar]
- 24.Kehlet H, Jensen TS and Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- 25.Stark N, Kerr S and Stevens J. Prevalence and predictors of persistent post-surgical opioid use: a prospective observational cohort study. Anaesthesia and intensive care 2017;45:700–706. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbloom BN, McCartney CJL, Canzian S, Kreder HJ and Katz J. Predictors of Prescription Opioid Use 4 Months After Traumatic Musculoskeletal Injury and Corrective Surgery: A Prospective Study. The journal of pain : official journal of the American Pain Society 2017;18:956–963. [DOI] [PubMed] [Google Scholar]
- 27.Hah JM, Cramer E, Hilmoe H, Schmidt P, McCue R, Trafton J, Clay D, Sharifzadeh Y, Ruchelli G, Goodman S, Huddleston J, Maloney WJ, Dirbas FM, Shrager J, Costouros JG, Curtin C, Mackey SC and Carroll I. Factors Associated With Acute Pain Estimation, Postoperative Pain Resolution, Opioid Cessation, and Recovery: Secondary Analysis of a Randomized Clinical Trial. JAMA network open 2019;2:e190168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreiber KL, Zinboonyahgoon N, Xu X, Spivey T, King T, Dominici L, Partridge A, Golshan M, Strichartz G and Edwards RR. Preoperative Psychosocial and Psychophysical Phenotypes as Predictors of Acute Pain Outcomes After Breast Surgery. The journal of pain : official journal of the American Pain Society 2019;20:540–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abrecht CR, Cornelius M, Wu A, Jamison RN, Janfaza D, Urman RD, Campbell C, Smith M, Haythornthwaite J, Edwards RR and Schreiber KL. Prediction of Pain and Opioid Utilization in the Perioperative Period in Patients Undergoing Primary Knee Arthroplasty: Psychophysical and Psychosocial Factors. Pain medicine (Malden, Mass) 2019;20:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glogovac G, Kennedy M, Parman MD, Bowers KA, Colosimo AJ and Grawe BM. Opioid Requirement following Arthroscopic Knee Surgery: Are There Predictive Factors Associated with Long-Term Use. J Knee Surg 2021;34:810–815. [DOI] [PubMed] [Google Scholar]
- 31.Helmerhorst GT, Vranceanu AM, Vrahas M, Smith M and Ring D. Risk factors for continued opioid use one to two months after surgery for musculoskeletal trauma. The Journal of bone and joint surgery American volume 2014;96:495–9. [DOI] [PubMed] [Google Scholar]
- 32.Shah RF, Gwilym SE, Lamb S, Williams M, Ring D and Jayakumar P. Factors associated with persistent opioid use after an upper extremity fracture. Bone Jt Open 2021;2:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fields HL and Margolis EB. Understanding opioid reward. Trends Neurosci 2015;38:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bechara A, Berridge KC, Bickel WK, Morón JA, Williams SB and Stein JS. A Neurobehavioral Approach to Addiction: Implications for the Opioid Epidemic and the Psychology of Addiction. Psychol Sci Public Interest 2019;20:96–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volkow ND, Koob GF and McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. New England Journal of Medicine 2016;374:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh JA, Lemay CA, Nobel L, Yang W, Weissman N, Saag KG, Allison J and Franklin PD. Association of Early Postoperative Pain Trajectories With Longer-term Pain Outcome After Primary Total Knee Arthroplasty. JAMA network open 2019;2:e1915105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tay HP, Wang X, Narayan SW, Penm J and Patanwala AE. Persistent postoperative opioid use after total hip or knee arthroplasty: A systematic review and meta-analysis. Am J Health Syst Pharm 2022;79:147–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashoorion V, Sadeghirad B, Wang L, Noori A, Abdar M, Kim Y, Chang Y, Rehman N, Lopes LC, Couban RJ, Aminilari M, Malektojari A, Ghazizadeh S, Rehman Y, Ghasemi M, Adili A, Guyatt GH and Busse JW. Predictors of Persistent Post-Surgical Pain Following Total Knee Arthroplasty: A Systematic Review and Meta-Analysis of Observational Studies. Pain medicine (Malden, Mass) 2023;24:369–381. [DOI] [PubMed] [Google Scholar]
- 39.Bruehl S, Milne G, Schildcrout J, Shi Y, Anderson S, Shinar A, Polkowski G, Mishra P and Billings FTt. Perioperative oxidative stress predicts subsequent pain-related outcomes in the 6 months following total knee arthroplasty. Pain 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruehl S, Billings FTt, Anderson S, Polkowski G, Shinar A, Schildcrout J, Shi Y, Milne G, Dematteo A, Mishra P and Harden RN. Preoperative Predictors of Complex Regional Pain Syndrome Outcomes in the 6 Months Following Total Knee Arthroplasty. The journal of pain : official journal of the American Pain Society 2022;23:1712–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruehl S, Milne G, Schildcrout J, Shi Y, Anderson S, Shinar A, Polkowski G, Mishra P and Billings FTt. Oxidative stress is associated with characteristic features of the dysfunctional chronic pain phenotype. Pain 2022;163:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Folstein MF, Folstein SE and McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 43.Bruehl S, Stone AL, Palmer C, Edwards DA, Buvanendran A, Gupta R, Chont M, Kennedy M and Burns JW. Self-reported cumulative medical opioid exposure and subjective responses on first use of opioids predict analgesic and subjective responses to placebo-controlled opioid administration. Reg Anesth Pain Med 2019;44:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burns JW, Bruehl S, France CR, Schuster E, Orlowska D, Buvanendran A, Chont M and Gupta RK. Psychosocial factors predict opioid analgesia through endogenous opioid function. Pain 2017;158:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brummett CM, Janda AM, Schueller CM, Tsodikov A, Morris M, Williams DA and Clauw DJ. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: a prospective, observational cohort study. Anesthesiology 2013;119:1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhon DI, Snodgrass SJ, Cleland JA and Cook CE. Comorbid Insomnia and Sleep Apnea are Associated with Greater Downstream Health Care Utilization and Chronic Opioid Use after Arthroscopic Hip Surgery. Pain physician 2019;22:E351–e360. [PubMed] [Google Scholar]
- 47.De Cosmo G, Congedo E, Lai C, Primieri P, Dottarelli A and Aceto P. Preoperative psychologic and demographic predictors of pain perception and tramadol consumption using intravenous patient-controlled analgesia. Clin J Pain 2008;24:399–405. [DOI] [PubMed] [Google Scholar]
- 48.Geha H, Nimeskern N and Beziat JL. Patient-controlled analgesia in orthognathic surgery: evaluation of the relationship to anxiety and anxiolytics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:e33–6. [DOI] [PubMed] [Google Scholar]
- 49.Wasan AD, Davar G and Jamison R. The association between negative affect and opioid analgesia in patients with discogenic low back pain. Pain 2005;117:450–461. [DOI] [PubMed] [Google Scholar]
- 50.Berkman LF, Berkman CS, Kasl S, Freeman DH Jr., Leo L, Ostfeld AM, Cornoni-Huntley J and Brody JA. Depressive symptoms in relation to physical health and functioning in the elderly. Am J Epidemiol 1986;124:372–88. [DOI] [PubMed] [Google Scholar]
- 51.Haringsma R, Engels GI, Beekman AT and Spinhoven P. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry 2004;19:558–63. [DOI] [PubMed] [Google Scholar]
- 52.Martens MP, Parker JC, Smarr KL, Hewett JE, Slaughter JR and Walker SE. Assessment of depression in rheumatoid arthritis: a modified version of the center for epidemiologic studies depression scale. Arthritis Rheum 2003;49:549–55. [DOI] [PubMed] [Google Scholar]
- 53.Spielberger CD, Gorsuch RL and Lushene RE. STAI manual for the Stait-Trait Anxiety Inventory (“self-evaluation questionnaire”). [Palo Alto, Calif: Consulting Psychologists Press, 1970. [Google Scholar]
- 54.Sullivan MJL, Bishop SR and Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment 1995;7:524–532. [Google Scholar]
- 55.Brummett CM, Bakshi RR, Goesling J, Leung D, Moser SE, Zollars JW, Williams DA, Clauw DJ and Hassett AL. Preliminary validation of the Michigan Body Map. Pain 2016;157:1205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goodin BR, Bulls HW, Herbert MS, Schmidt J, King CD, Glover TL, Sotolongo A, Sibille KT, Cruz-Almeida Y, Staud R, Fessler BJ, Redden DT, Bradley LA and Fillingim RB. Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and older with knee osteoarthritis: ethnic differences. Psychosom Med 2014;76:302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurien T, Arendt-Nielsen L, Petersen KK, Graven-Nielsen T and Scammell BE. Preoperative Neuropathic Pain-like Symptoms and Central Pain Mechanisms in Knee Osteoarthritis Predicts Poor Outcome 6 Months After Total Knee Replacement Surgery. The journal of pain : official journal of the American Pain Society 2018;19:1329–1341. [DOI] [PubMed] [Google Scholar]
- 58.Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, Johnston KL and Pilkonis PA. Development of short forms from the PROMIS™ sleep disturbance and Sleep-Related Impairment item banks. Behavioral sleep medicine 2011;10:6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L and Lai JS. Development of a PROMIS item bank to measure pain interference. Pain 2010;150:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schirle LM, Dietrich MS, Lam L, Stone AL, Bruehl S and Osmundson SS. Accuracy of patient-reported versus real-time electronic postoperative opioid use outcomes. Am J Obstet Gynecol MFM 2021;3:100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruehl S, Billings FTt, Anderson S, Polkowski G, Shinar A, Schildcrout J, Shi Y, Milne G, Dematteo A, Mishra P and Harden RN. Preoperative Predictors of Complex Regional Pain Syndrome Outcomes in the 6 Months Following Total Knee Arthroplasty. The journal of pain : official journal of the American Pain Society 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen J. Statistical Power Analysis for the Behavioral Sciences. L. Erlbaum Associates, 1988. [Google Scholar]
- 63.Debbi EM, Krell EC, Sapountzis N, Chiu YF, Lyman S, Joseph AD, Mandl LA and Gonzalez Della Valle A. Predicting Post-Discharge Opioid Consumption After Total Hip and Knee Arthroplasty in the Opioid-Naïve Patient. The Journal of arthroplasty 2022;37:S830–S835.e3. [DOI] [PubMed] [Google Scholar]
- 64.Karhade AV, Schwab JH and Bedair HS. Development of Machine Learning Algorithms for Prediction of Sustained Postoperative Opioid Prescriptions After Total Hip Arthroplasty. The Journal of arthroplasty 2019;34:2272–2277.e1. [DOI] [PubMed] [Google Scholar]
- 65.Katakam A, Karhade AV, Schwab JH, Chen AF and Bedair HS. Development and validation of machine learning algorithms for postoperative opioid prescriptions after TKA. J Orthop 2020;22:95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klemt C, Harvey MJ, Robinson MG, Esposito JG, Yeo I and Kwon YM. Machine learning algorithms predict extended postoperative opioid use in primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2022;30:2573–2581. [DOI] [PubMed] [Google Scholar]
- 67.Anderson AB, Grazal CF, Balazs GC, Potter BK, Dickens JF and Forsberg JA. Can Predictive Modeling Tools Identify Patients at High Risk of Prolonged Opioid Use After ACL Reconstruction? Clin Orthop Relat Res 2020;478:0–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gabriel RA, Harjai B, Prasad RS, Simpson S, Chu I, Fisch KM and Said ET. Machine learning approach to predicting persistent opioid use following lower extremity joint arthroplasty. Reg Anesth Pain Med 2022;47:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu Y, Forlenza E, Wilbur RR, Lavoie-Gagne O, Fu MC, Yanke AB, Cole BJ, Verma N and Forsythe B. Machine-learning model successfully predicts patients at risk for prolonged postoperative opioid use following elective knee arthroscopy. Knee Surg Sports Traumatol Arthrosc 2022;30:762–772. [DOI] [PubMed] [Google Scholar]
- 70.Hur J, Tang S, Gunaseelan V, Vu J, Brummett CM, Englesbe M, Waljee J and Wiens J. Predicting postoperative opioid use with machine learning and insurance claims in opioid-naïve patients. Am J Surg 2021;222:659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ward A, Jani T, De Souza E, Scheinker D, Bambos N and Anderson TA. Prediction of Prolonged Opioid Use After Surgery in Adolescents: Insights From Machine Learning. Anesthesia and analgesia 2021;133:304–313. [DOI] [PubMed] [Google Scholar]
- 72.Kunze KN, Polce EM, Alter TD and Nho SJ. Machine Learning Algorithms Predict Prolonged Opioid Use in Opioid-Naïve Primary Hip Arthroscopy Patients. J Am Acad Orthop Surg Glob Res Rev 2021;5:e21.00093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grazal CF, Anderson AB, Booth GJ, Geiger PG, Forsberg JA and Balazs GC. A Machine-Learning Algorithm to Predict the Likelihood of Prolonged Opioid Use Following Arthroscopic Hip Surgery. Arthroscopy 2022;38:839–847.e2. [DOI] [PubMed] [Google Scholar]
- 74.Yen HK, Ogink PT, Huang CC, Groot OQ, Su CC, Chen SF, Chen CW, Karhade AV, Peng KP, Lin WH, Chiang H, Yang JJ, Dai SH, Yen MH, Verlaan JJ, Schwab JH, Wong TH, Yang SH and Hu MH. A machine learning algorithm for predicting prolonged postoperative opioid prescription after lumbar disc herniation surgery. An external validation study using 1,316 patients from a Taiwanese cohort. The spine journal : official journal of the North American Spine Society 2022;22:1119–1130. [DOI] [PubMed] [Google Scholar]
- 75.Karhade AV, Cha TD, Fogel HA, Hershman SH, Tobert DG, Schoenfeld AJ, Bono CM and Schwab JH. Predicting prolonged opioid prescriptions in opioid-naïve lumbar spine surgery patients. The spine journal : official journal of the North American Spine Society 2020;20:888–895. [DOI] [PubMed] [Google Scholar]
- 76.Bruehl S, Burns JW, Morgan A, Koltyn K, Gupta R, Buvanendran A, Edwards D, Chont M, Kingsley PJ, Marnett L, Stone A and Patel S. The association between endogenous opioid function and morphine responsiveness: a moderating role for endocannabinoids. Pain 2019;160:676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


