Graphical Abstract
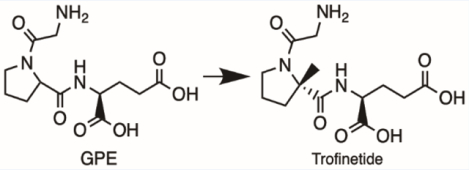
STRUCTURE: Trofinetide ((2S)-2-{[(2S)-1-(2-aminoacetyl)-2-methylpyrrolidine-2-carbonyl]amino}pentanedioic acid) is derived from the tripeptide glycine-proline-glutamic acid (GPE), which is an endogenous tripeptide of the N-terminal domain of Insulin-like Growth Factor 1 (IGF-1). The compound differs from endogenous GPE by a methyl (-CH3) substitution at the α-carbon of the proline residue. This methyl substitution assists in resistance to protease cleavage and improves oral bioavailability. The molecular formula of trofinetide is C13H21N3O6, and the molecular weight is 315.33 g/mol.
Graphical Abstract
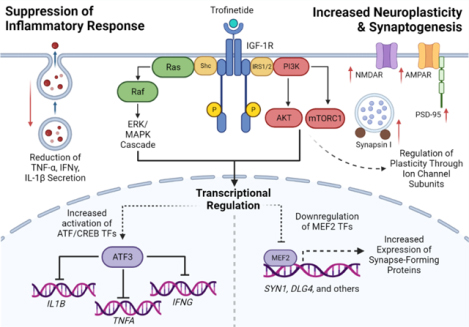
MECHANISM OF ACTION: Rett syndrome is a neurodevelopmental disease caused by mutations in the X-linked Methyl CpG Binding Protein 2 (MECP2) gene, affecting primarily females. MECP2 mutations cause a diverse range of phenotypes and disrupt various cellular signalling pathways. While the mechanism of action of trofinetide has yet to be fully elucidated, due to its homology with the N-terminus of Insulin-Like Growth Factor-1 (IGF-1) it is hypothesized that it acts through the IGF-1 receptor on both neurons and glia. IGF-1 receptor activation stimulates downstream pathways including Mitogen-Associated Protein Kinase (MAPK), Phosphoinositide-3-Kinase (PI3K) and Mammalian Target of Rapamycin (mTOR) to induce transcriptional changes. Evidence suggests that trofinetide induces upregulation of Activating Transcription Factor 3 (ATF3), which attenuates expression of inflammatory cytokine genes encoding proteins such as Interleukin-1β (IL-1β), Interferon γ (IFNγ), and Tumor Necrosis Factor α (TNF-α). These cytokines are upregulated in Rett syndrome and contribute to aberrant systemic activation of the inflammatory response. IGF-1 has also been reported to be an essential component of synaptic formation, maturation, and neuroplasticity during CNS development, particularly through modulation of α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid (AMPA) and N-methyl-D -aspartate (NMDA) receptor subunit composition and downregulation of the Myocyte Enhancer Factor-2 (MEF2) transcriptional repressor. Reduced activity of MEF2 increases expression of genes involved in synaptic formation and plasticity, such as SYN1 (Synapsin 1) and DLG4 (PSD-95) and trofinetide may also act through this pathway to enhance synaptic formation and plasticity. Trofinetide improved numerous psychomotor symptoms in Rett syndrome patients during clinical trials, which were quantified through clinician-assessed composite scores. Interestingly, clinical trials for recombinant human IGF-1 (mecasermin) in Rett syndrome patients yielded inconclusive results, which may reflect unique pharmacology or pharmacokinetics of trofinetide, warranting further investigation. Although the exact mechanism of action of the drug is still unknown, trofinetide’s efficacy in clinical trials suggests that the drug is an important step forward in the management of patients with Rett syndrome.
NAME:
Trofinetide (brand name: Daybue; also known as NNZ-2566).
DRUG CLASS:
Trofinetide is a first-in-class drug to treat multiple psychomotor symptoms in Rett syndrome. It is an orally bioavailable small molecule and received orphan drug designation from the FDA in 2015.
CLINICAL USE:
Trofinetide is indicated to treat patients 2 years and older diagnosed with Rett syndrome, including males and patients with mutations in loci other than MECP2. It is the only FDA-approved drug for Rett syndrome. Dosing information for adults of 50 kg or more: 12,000 mg twice daily. Dosage for children is weight-dependent.
DEVELOPED BY:
Acadia Pharmaceuticals Inc and Neuren Pharmaceuticals Ltd.
ADVERSE EFFECTS:
Clinical trials reported the most common adverse effects as: diarrhea (82%), vomiting (29%), fever (9%), seizure (9%), anxiety (8%), decreased appetite (8%), fatigue (8%), and nasopharyngitis (5%). Trofinetide is also a weak CYP3A4 inhibitor.
TIMELINE:
2009: First publication of preclinical efficacy of trofinetide in traumatic brain injury (NNZ-556); first publication supporting the efficacy of IGF-1 in animal model of Rett syndrome.
2010–2012: Phase I trials for Multiple Sclerosis (NCT01420042, NCT00961779).
2013–2017: Phase II trials for use in Rett syndrome (NCT02715115, NCT01703533).
2015: Trofinetide receives orphan drug designation.
2018: Acadia Pharmaceuticals enters into licensing agreement with Neuren Pharmaceuticals.
2019-Present: Phase III trials conducted in females with documented MECP2 mutations (NCT04181723, NCT04279314, NCT04776746, NCT04988867)
2022: NDA for approval in Rett Syndrome submitted.
March 2023: FDA approval granted for trofinetide.
Acknowledgments
This work was supported by grants from the NIH (MH124671, MH062646, NS031373) and the International Rett Syndrome Foundation. We also thank Dr. Rocco Gogliotti for reviewing this information.
Declaration of interests
C.N. has received royalties and research funding from Acadia Pharmaceuticals for a program other than trofinetide. H.P. and A.F. have no interests to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- Silva-Reis SC et al. (2023) Concise Overview of Glypromate Neuropeptide Research: From Chemistry to Pharmacological Applications in Neurosciences. ACS Chem. Neurosci 14, 554–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neul JL et al. (2023) Trofinetide for the treatment of Rett syndrome: a randomized phase 3 study. Nat. Med 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Santamaría V et al. (2016) IGF-1 deficiency causes atrophic changes associated with upregulation of VGluT1 and downregulation of MEF2 transcription factors in the mouse cochlear nuclei. Brain Struct. Funct 221, 709–734 [DOI] [PubMed] [Google Scholar]
- Cartagena CM et al. (2013) Mechanism of Action for NNZ-2566 Anti-inflammatory Effects Following PBBI Involves Upregulation of Immunomodulator ATF3. NeuroMolecular Med 15, 504–514 [DOI] [PubMed] [Google Scholar]
- Zalosnik MI et al. (2021) MeCP2 deficiency exacerbates the neuroinflammatory setting and autoreactive response during an autoimmune challenge. Sci. Rep 11, 10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini G et al. (2016) Illness Severity, Social and Cognitive Ability, and EEG Analysis of Ten Patients with Rett Syndrome Treated with Mecasermin (Recombinant Human IGF-1). Autism Res. and Treat 5073078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shovlin S et al. (2022) Molecular Signatures of Response to Mecasermin in Children With Rett Syndrome. Front Neurosci. 16, 868008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS and Standridge S (2021) Rett Syndrome: A Timely Review From Recognition to Current Clinical Approaches and Clinical Study Updates. Semin. Pediatr. Neurol 37, 100881. [DOI] [PubMed] [Google Scholar]
- Dyer AH et al. (2016) The role of Insulin-Like Growth Factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience 325, 89–99 [DOI] [PubMed] [Google Scholar]
- Banerjee A et al. (2019) Towards a better diagnosis and treatment of Rett syndrome: a model synaptic disorder. Brain 142, 239–248 [DOI] [PMC free article] [PubMed] [Google Scholar]


