Abstract
Background:
Hepatectomy is the cornerstone of curative-intent treatment for intrahepatic cholangiocarcinoma (ICC). However, in patients unable to be resected, data comparing efficacy of alternatives including thermal ablation and radiation therapy (RT) remain limited. Herein, we compared survival between resection and other liver-directed therapies for small ICC within a national cancer registry.
Methods:
Patients with clinical stage I-III ICC <3 cm diagnosed 2010 - 2018 who underwent resection, ablation, or RT were identified in the National Cancer Database (NCDB). Overall survival (OS) was compared using Kaplan-Meier and multivariable Cox proportional hazards methods.
Results:
Of 545 patients, 297 (54.5%) underwent resection, 114 (20.9%) ablation, and 134 (24.6%) RT. Median OS was similar between resection and ablation (50.5 months, 95% CI 37.5 - 73.9; 39.5 months, 95% CI 28.7 - 58.4, p=0.14), both exceeding that of RT (20.9 months, 95% CI 14.1 - 28.3). RT patients had high rates of stage III disease (10.4% RT vs. 1.8% ablation vs. 11.8% resection, p<0.001), but the lowest rates of chemotherapy utilization (9.0% RT vs. 15.8% ablation vs. 38.7% resection, p<0.001). In multivariable analysis, resection and ablation were associated with reduced mortality compared to RT (HR 0.44, 95% CI 0.33 - 0.58; HR 0.53, 95% CI 0.38 - 0.75, p<0.001; respectively).
Conclusion:
Resection and ablation were associated with improved survival in patients with ICC <3 cm compared to RT. Acknowledging confounders, anatomic constraints of ablation, limitations of available data, and need for prospective study, these results favor ablation in small ICC where resection is not feasible.
Introduction:
Intrahepatic cholangiocarcinoma (ICC) is an aggressive malignancy arising from the biliary epithelium within the liver, and has risen in incidence by more than 2.3% annually since the 1970s.1 Despite advances in surgical technique and systemic therapies, outcomes remain poor with a 5-year overall survival of 18%.2,3 Hepatectomy is the only curative treatment for ICC, though is feasible in less than a third of patients due to local extension, extensive biliary ductal and portal venous involvement, metastatic disease, or poor functional status.4,5
For patients with unresectable ICC, National Comprehensive Cancer Network (NCCN) guidelines suggest considering systemic therapy, external beam radiation therapy (EBRT), arterially directed therapy, or ablation. However, randomized data comparing these modalities do not exist.6,7 Indeed, the majority of recommendations for locoregional therapies in ICC are extrapolated from studies in other malignancies such as hepatocellular carcinoma (HCC).6,8 Ablation has been proposed for small tumors <5 cm with appropriate anatomy, though retrospective analyses suggest it may be inferior to resection for all but the smallest tumors (<3 cm).9–11 Work from our own group suggests that overall, resection is associated with superior outcomes compared to ablation, but that outcomes for the two modalities converge for primary tumors <3 cm (E. Kanu, unpublished data). Ablative radiotherapy has emerged as an alternative strategy with fewer anatomic limitations and improved survival compared to no treatment in stage I and III disease.9,10,12–16
While primary use of liver-directed therapies has been studied in HCC, there is a paucity of literature comparing liver-directed therapies such as ablation and RT for ICC, and how these may compare with resection in select populations.8 We therefore aimed to compare survival following resection, ablation, and radiotherapy (RT) in patients with small (<3 cm) ICC within a national cancer registry.
Methods:
Data Source
Analyses were conducted using the National Cancer Database (NCDB). The NCDB is a large, clinical oncology dataset maintained by the American College of Surgeons Commission on Cancer, capturing patient demographics, tumor characteristics, first course treatments, perioperative outcomes, and overall survival for over 70% of incident cancer cases, across 1,500 accredited centers in the United States.17,18 The NCDB contains de-identified data and therefore this study was deemed exempt by the Duke University Health System Institutional Review Board (Pro00111050).
Patient Selection
The 2019 NCDB Intraductal Biliary Participant User File was queried for patients with clinical stage I-III ICC <3 cm diagnosed from 2010 - 2018 who underwent surgical resection, ablation, or RT. The primary site code for intrahepatic biliary (C22.1) and histology for cholangiocarcinoma (ICD-0-3 8160) were used to identify cases. Patients younger than 18 years old, those with metastatic disease, or missing survival were excluded. Patients undergoing liver transplant were also excluded. Ablation was defined by the NCDB site-specific surgery code for Heat-Radio-frequency ablation (code 16), which captures both microwave and radio-frequency techniques. Demographic variables were extracted, including age, sex, race, insurance status, treatment in a Medicaid expansion state, population density, distance traveled to treating hospital, education level, and income. Clinical variables included Charlson Deyo (CD) comorbidity score, year of diagnosis, clinical stage prior to treatment initiation, tumor size and grade, and receipt of neoadjuvant and/or adjuvant chemotherapy.
Statistical analysis
Patients were stratified by management with resection, ablation, or RT. Descriptive statistics were obtained for baseline demographics and tumor characteristics, and compared using the Pearson’s chi-squared and Kruskal-Wallis tests for categorical and continuous variables, respectively. Fischer’s exact test was utilized for categorical variables with small proportions where the assumptions for Pearson’s chi-squared analysis were not satisfied.
The primary outcome was overall survival (OS), which was defined as the interval from the date of the intervention until death or most recent follow-up. Kaplan-Meier methods were used to evaluate OS and survival curves were compared using the log-rank test. Multivariable Cox proportional hazards methods were used to ascertain the association between treatment modality and mortality, while adjusting for patient (age, sex, race, insurance coverage, CD score, and year of diagnosis), tumor (clinical stage, receipt of chemotherapy), and facility (academic vs. nonacademic) characteristics. Adjusted models incorporated known covariates designated a priori. Missing data was handled with complete case analysis. All statistical tests were two-tailed and considered to be significant at p<0.05. Analyses were performed using R Version 4.1.1 for Mac (Vienna, Austria).
Results:
Demographics
Altogether, 545 patients with stage I-III ICC measuring under 3 cm met inclusion criteria (Figure 1). Of these, 297 (54.5%) underwent resection, 114 (20.9%) ablation, and 134 (24.6%) RT. Baseline demographics and tumor characteristics are shown in Table 1. By patient race, the analytic cohort was composed of 463 (85.0%) white, 39 (7.2%) black, and 43 (7.9%) other patients. Between modalities, there were no significant differences with respect to age, sex, patient insurance status, education or income quartile, treatment in an academic hospital, rural versus metropolitan area, relative distance to treating center, or location in a Medicaid expansion state. Resected patients were more likely to report white (86.5% resection vs. 81.6% ablation vs. 84.3% RT) or “other” race (9.1% resection vs. 8.8% ablation vs. 4.5% RT) (p=0.04), and least likely to identify as black (4.4% resection vs. 9.6% ablation vs. 11.2% RT, p=0.04). Slight differences in median year of diagnosis between treatment modalities were observed (resection: 2015 vs. ablation: 2016 vs. RT: 2016, p<0.001).
Figure 1.
Study CONSORT diagram.
Table 1.
Baseline demographics and tumor characteristics of patients with clinical stage I-III intrahepatic cholangiocarcinoma <3 cm in size, receiving radiation (RT), ablation, or surgical resection.
| Overall N=545 |
RT N=134 |
Ablation N=114 |
Surgical Resection N=297 |
p-value | |
|---|---|---|---|---|---|
| Age (median [IQR]) | 67 [60, 74] | 69 [61, 77] | 66 [59, 74] | 67 [60, 73] | 0.134 |
| Female Sex (%) | 250 (45.9) | 59 (44.0) | 48 (42.1) | 143 (48.1) | 0.483 |
| Race (%) | 0.038 | ||||
| White | 463 (85.0) | 113 (84.3) | 93 (81.6) | 257 (86.5) | |
| Black | 39 (7.2) | 15 (11.2) | 11 (9.6) | 13 (4.4) | |
| Other | 43 (7.9) | 6 (4.5) | 10 (8.8) | 27 (9.1) | |
| Year of Diagnosis (median [IQR]) | 2015 [2013, 2017] | 2016 [2014, 2017] | 2016 [2013, 2017] | 2015 [2012, 2017] | 0.004 |
| Charlson Deyo Comorbidity Score (%) |
<0.001 | ||||
| 0 | 348 (63.9) | 82 (61.2) | 48 (42.1) | 218 (73.4) | |
| 1 | 100 (18.3) | 21 (15.7) | 30 (26.3) | 49 (16.5) | |
| 2 | 45 (8.3) | 17 (12.7) | 11 (9.6) | 17 (5.7) | |
| ≥3 | 52 (9.5) | 14 (10.4) | 25 (21.9) | 13 (4.4) | |
| Insurance Status (%) | 0.10 | ||||
| None | 16 (3.0) | 3 (2.2) | 4 (3.5) | 9 (3.1) | |
| Private | 176 (32.8) | 43 (32.1) | 26 (23.0) | 107 (37.0) | |
| Government | 344 (64.2) | 88 (65.7) | 83 (73.5) | 173 (59.9) | |
| Medicaid Expansion State (%) | 338 (62.8) | 87 (65.4) | 72 (63.7) | 179 (61.3) | 0.701 |
| Population Density (%) | 0.324 | ||||
| Rural | 6 (1.2) | 1 (0.8) | 1 (0.9) | 4 (1.5) | |
| Urban | 63 (12.5) | 13 (10.6) | 9 (8.2) | 41 (15.2) | |
| Metropolitan | 433 (86.3) | 109 (88.6) | 100 (90.9) | 224 (83.3) | |
| Distance Traveled (median [IQR]) | 20.9 [8.1, 56.3] | 17.6 [8.1, 42.0] | 18.3 [8.3, 50.3] | 22.1 [8.1, 67.6] | 0.676 |
| Academic Center (%) | 359 (65.9) | 89 (66.4) | 73 (64.0) | 197 (66.3) | 0.897 |
| Education Quartile (% not graduating high school) | 0.470 | ||||
| Q1 | 89 (19.1) | 17 (14.4) | 21 (24.1) | 51 (19.5) | |
| Q2 | 104 (22.3) | 31 (26.3) | 18 (20.7) | 55 (21.0) | |
| Q3 | 136 (29.1) | 33 (28.0) | 28 (32.2) | 75 (28.6) | |
| Q4 | 138 (29.6) | 37 (31.4) | 20 (23.0) | 81 (30.9) | |
| Income Quartile (%) | 0.827 | ||||
| Q1 | 83 (17.8) | 19 (16.1) | 19 (21.8) | 45 (17.2) | |
| Q2 | 92 (19.7) | 24 (20.3) | 13 (14.9) | 55 (21.0) | |
| Q3 | 111 (23.8) | 26 (22.0) | 21 (24.1) | 64 (24.4) | |
| Q4 | 181 (38.8) | 49 (41.5) | 34 (39.1) | 98 (37.4) | |
| Clinical Stage (%) | <0.001 | ||||
| I | 349 (64.0) | 68 (50.7) | 85 (74.6) | 196 (66.0) | |
| II | 145 (26.6) | 52 (38.8) | 27 (23.7) | 66 (22.2) | |
| III | 51 (9.4) | 14 (10.4) | 2 (1.8) | 35 (11.8) | |
| Clinical T Stage (%) | <0.001 | ||||
| T1 | 350 (65.2) | 69 (51.5) | 85 (74.6) | 196 (67.8) | |
| T2 | 144 (26.8) | 53 (39.6) | 27 (23.7) | 64 (22.1) | |
| T3 | 38 (7.1) | 10 (7.5) | 1 (0.9) | 27 (9.3) | |
| T4 | 5 (0.9) | 2 (1.5) | 1 (0.9) | 2 (0.7) | |
| Clinical N1 (%) | 11 (2.1) | 2 (1.5) | 0 (0.0) | 9 (3.2) | 0.113 |
| Tumor Size cm (median [IQR]) | 2.2 [1.7, 2.5] | 2.2 [1.8, 2.5] | 2.3 [2.0, 2.7] | 2.1 [1.5, 2.5] | 0.006 |
| Grade (%) | 0.976 | ||||
| Well Differentiated | 78 (23.9) | 10 (22.7) | 15 (26.8) | 53 (23.3) | |
| Moderately Differentiated | 160 (48.9) | 21 (47.7) | 26 (46.4) | 113 (49.8) | |
| Poorly/Undifferentiated | 89 (27.2) | 13 (29.5) | 15 (26.8) | 61 (26.9) | |
| Chemotherapy (%) | <0.001 | ||||
| None | 400 (73.4) | 122 (91.0) | 96 (84.2) | 182 (61.3) | |
| Neoadjuvant | 26 (4.8) | 2 (1.5) | 8 (7.0) | 16 (5.4) | |
| Adjuvant | 110 (20.2) | 9 (6.7) | 10 (8.8) | 91 (30.6) | |
| Neoadjuvant and Adjuvant | 9 (1.7) | 1 (0.7) | 0 (0.0) | 8 (2.7) |
With respect to tumor characteristics, patients undergoing resection had smaller tumors compared with those undergoing ablation or RT (2.1 cm resection vs. 2.3 cm ablation vs. 2.2 cm RT, p=0.006), while also exhibiting higher rates of clinical stage III disease (11.8% resection vs. 1.8% ablation vs. 10.4% RT, p<0.001) (Table 1). Patients in the resection group reported fewer comorbidities pre-treatment (Charlson Deyo scores ≥3: 4.4% resection vs. 21.9% ablation vs. 10.4% RT, p<0.001), and accordingly were more likely to receive chemotherapy (38.7% resection vs. 15.8% ablation vs. 9.0% RT, p<0.001). Comparatively low rates of chemotherapy administration in RT patients were observed both before (1.5% RT vs. 7.0% ablation vs. 5.4% resection) and after primary therapy (6.7% RT vs. 8.8% ablation vs. 30.6% resection, p<0.001).
Primary Outcome: Overall Survival
In the overall cohort, median OS was 34.1 months (95% CI 30 - 40.6 months), with a 5-year OS of 36.8% (95% CI 32.2 - 42.0%). When stratified by treatment group, median OS was 50.5 months for resection (95% CI 37.5 - 73.9 months), 39.5 months for ablation (95% CI 28.7 - 58.4 months), and 20.9 months for RT (95% CI 14.1 - 28.3 months); 5-year OS was 46% for resection (95% CI 39.8 - 53.1%), 36.4% for ablation (95% CI 27.2 - 48.7%), and 13.1% for RT (95% CI 7.0 - 24.5%) (Figure 2). On pairwise comparison, there was no significant difference in median OS for surgical resection and ablation (log-rank p=0.14), though both had superior survival compared to RT (both log-rank p<0.0001).
Figure 2.
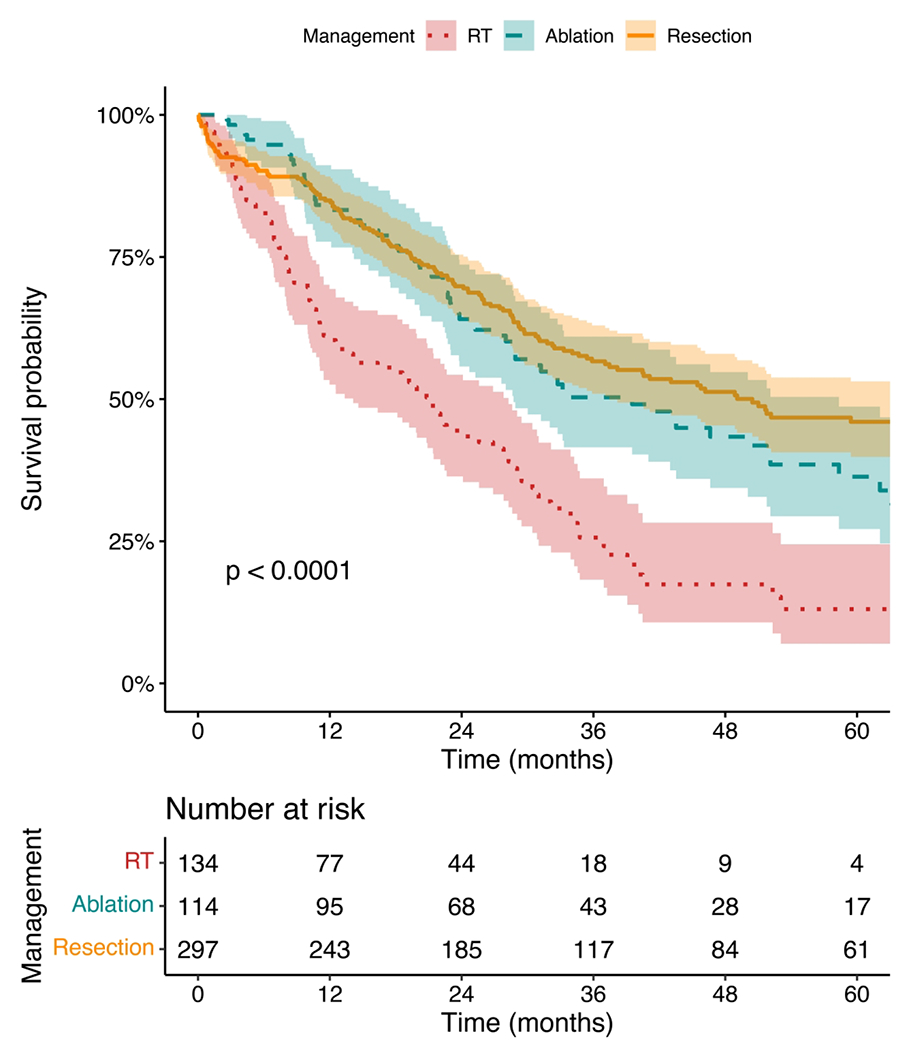
Kaplan-Meier survival curves for ICC <3 cm stratified by management by radiotherapy (RT), ablation, or resection. Survival curves were compared using the log-rank test.
In multivariable analysis, resection and ablation were both independently associated with reduced mortality compared to RT (HR 0.44, 95% CI 0.33 - 0.58; HR 0.53, 95% CI 0.38 - 0.75, all p<0.001) (Table 2). Increasing age (HR 1.02, 95% CI 1.01 - 1.04, p<0.01), clinical stage II (HR 1.76, 95% CI 1.35 - 2.31, p<0.001) and stage III disease (HR 1.96, 95% CI 1.31 - 2.92, p<0.001) were associated with greater hazard of mortality (Table 2). Both female sex (HR 0.70, 95% CI 0.55 - 0.89, p<0.01) and more recent year of diagnosis (HR 0.94, 95% CI 0.89 - 0.99, p=0.01) were associated with reduced hazard of mortality.
Table 2.
Multivariable Cox proportional hazards model for independent factors associated with mortality among patients undergoing radiation (RT), ablation, or surgical resection for clinical stage I-III intrahepatic cholangiocarcinoma <3 cm in size.
| Variable | Adjusted HR | 95% CI | p-value |
|---|---|---|---|
| Age (per year) | 1.02 | 1.01-1.04 | <0.01 |
| Female Sex (ref: male) | 0.70 | 0.55-0.89 | <0.01 |
| Race (ref: white) | |||
| Black | 0.69 | 0.40-1.18 | 0.17 |
| Other | 0.77 | 0.48-1.23 | 0.27 |
| Insurance Status (ref: none) | |||
| Private | 1.17 | 0.58-2.36 | 0.66 |
| Government | 1.14 | 0.57-2.28 | 0.71 |
| Charlson Deyo Comorbidity Score (ref: 0) | |||
| 1 | 1.35 | 0.99-1.84 | 0.06 |
| 2 | 1.00 | 0.60-1.64 | 0.99 |
| ≥3 | 1.37 | 0.93-2.01 | 0.11 |
| Year of Diagnosis (per year) | 0.94 | 0.89-0.99 | 0.01 |
| Academic Center (ref: nonacademic) | 0.90 | 0.70-1.15 | 0.39 |
| Clinical Stage (ref: stage I) | |||
| Stage II | 1.76 | 1.35-2.31 | <0.001 |
| Stage III | 1.96 | 1.31-2.92 | <0.001 |
| Chemotherapy (ref: none) | 1.11 | 0.84-1.45 | 0.46 |
| Management (ref: RT) | |||
| Ablation | 0.53 | 0.38-0.75 | <0.001 |
| Surgical Resection | 0.44 | 0.33-0.58 | <0.001 |
Discussion:
In this analysis of a national cancer registry, outcomes for patients with ICC <3 cm were compared following management with resection, ablation, or radiation therapy. Among this cohort, median and 5-year overall survival were comparable following resection and ablation, while both were superior to RT in multivariable analysis. Our findings suggest that for appropriately selected patients where resection is not considered, ablation may be prioritized as the preferred alternative over RT.
In a similar analysis of the Surveillance, Epidemiology, and End Results (SEER) database, Xiang et al compared surgical resection with radiofrequency ablation (RFA) in T1 ICC <5 cm.11 Therein, both OS and cholangiocarcinoma-specific survival were superior in resected patients, findings which persisted in a multivariate Cox model, and were recapitulated in subgroups of patients with tumors <4.5 and <4.0 cm.11 For the subgroup of 70 patients with tumors <3 cm, the authors reported 5-year OS was 66.2% and 58.3% for resection and RFA, and median survival was 38 and 39 months, respectively, though neither demonstrated significant between-group differences.11 Our study included a larger cohort of 411 resected and ablated patients with tumors <3 cm, and continued to show no significant survival difference. Though not significant, it is worth noting an 11-month disparity in median survival (50.5 months for resection vs. 39.5 months for ablation). Ablation and resection also exhibited a similar survival benefit in multivariable analysis, but it is possible that continued statistical power limitations and a selection bias arising from higher stage I disease in ablated patients mask an otherwise significant difference.
Whereas ablation and resection demonstrated comparable survival in ICC <3 cm, irradiated patients exhibited the poorest prognosis. A recent systematic review comprising 93 studies of liver-directed therapies in ICC parallel our findings, with pooled median survival estimates of 30.2 and 18.9 months after ablation and RT, respectively.22 Of note, RT group patients in this study had higher rates of stage III disease and the lowest rates of adjuvant chemotherapy. It is possible that observed survival differences between modalities may be increasingly influenced by candidacy for and receipt of systemic therapy. Indeed, the BILCAP trial demonstrated improved overall survival in a per-protocol analysis for adjuvant capecitabine in a mixed cohort of biliary tract cancers.23 Use of adjuvant chemotherapy for ICC has concomitantly increased from 18% to 52% between 2010 and 2018.24 Advanced disease, overall poorer performance status, and differential systemic therapy may therefore play a role in dictating both the use of RT and decreased prognosis, agnostic of its therapeutic efficacy.
Race may play an additional factor in selection of therapy. Black patients accounted for only 4.4% of patients undergoing resection. This is concordant with findings by Ransome et al, who reported 5.1% of resected patients identifying as black, with half the hazard of white patients for undergoing resection, lower even than Hispanic and Asian/Pacific Islander populations.20 While specific race-based biological mediators of ICC have not been elucidated, differential comorbidity, Medicaid or under-insurance, and lack of access to teaching hospitals may partly explain these findings.20 In addition, hospitals serving higher proportions of Hispanic and black patients demonstrate lower rates of NCCN guideline-concordant care.21 Amelioration of any such socioeconomic differences between patient populations is crucial to appraising liver-directed therapies, and ensuring best outcomes for ICC.19,21
Despite potential clinical and social confounders, multivariable analysis controlling for comorbidities, stage, and systemic therapy continued to demonstrate that both resection and ablation were independently associated with improved survival when compared to radiation. While prospective assessment of RT efficacy in unresectable ICC is the subject of an ongoing phase 3 clinical trial (NCT0220004), literature to date suggests a continued role for RT in select patients. Kolarich stratified outcomes by stage for ICC treated with RT and RFA, and while the latter carried a wider survival advantage over no therapy in stage I disease, RT alone continued to demonstrate superiority over no therapy for higher stage III disease.10 These findings are concordant with earlier studies which demonstrate limited effectiveness of ablation for tumors exceeding 5 cm in diameter, while also suggesting improved local control and survival rates for larger tumors treated with RT.9,14 Moreover, anatomic selection factors not captured by NCDB may render tumors less amenable to ablation; these include proximity to major portal triads, which may place patients at risk of biliary leak, biliary stricture, and reduced efficacy secondary to a heat-sink effect.25
This analysis is not without limitations. Within the NCDB, sources of potential bias include imperfect capture of descriptive data, unmeasured confounders, institutional factors dictating treatment assignment, patient preference, and even the data collection and coding process itself. Observed differences between treatment groups, such as resected patients receiving chemotherapy at substantially higher rates than either ablation or RT patients, may be attributable to these patients having the superior functional status to tolerate chemotherapy. These same patients would more likely be pathologically upstaged after surgical excision and lymphadenectomy, whereas in ablated and irradiated patients, stage III disease was more likely detected by imaging alone.23 There was also limited clinical granularity with respect to ICC. This includes the lack of information regarding anatomic location of tumor within the liver parenchyma, which may distinguish small but higher T3 or T4 stage lesions in the study. Similarly, tumor location with respect to the biliary tree may inform the use of RT over ablation.25 Evaluation of multifocality and extent of tumor was also unavailable for the majority of the cohort. Within the ablation group, we were unable to distinguish whether patients received RFA or MWA; the latter modality may benefit from greater efficacy near hepatic vasculature from a reduced heat-sink effect.26,27 Likewise, the absence of specific variables or agents for transcatheter arterial chemoembolization (TACE) and only five patients who specifically underwent Y90 radioembolization (TARE) ultimately precluded our evaluation of these therapies, which have demonstrated efficacy in small non-randomized studies.25,28,29 Critically, we were unable to assess recurrence and local disease control within the NCDB, especially salient when ICC mortality more often results from local biliary complications, vascular compromise, or hepatic failure.30 Finally, despite including only patients who received RT as primary therapy for ICC, significant missingness of dosing data meant we are unable to completely discern goals of treatment. Palliative intent for older, more comorbid patients, for instance, may have informed use of less invasive modalities such as RT and the obviation of systemic therapy altogether.
Acknowledging these limitations, within a retrospective cohort of patients with ICC <3 cm from the National Cancer Database, ablation therapy demonstrated similar 5-year and median overall survival to surgical resection, while both were superior to radiation therapy. These findings persisted in a multivariable analysis. Multidisciplinary evaluation remains important, particularly in cases where standard-of-care resection is not feasible. We advocate considering ablation therapy in unresectable lesions with favorable anatomy, while recognizing the need for direct comparison with radiotherapy. Further prospective investigation of liver-directed therapies would bolster the findings of this study, quantify efficacy with respect to local control endpoints, and guide appropriate patient selection.
Synopsis:
In a national analysis of patients with intrahepatic cholangiocarcinoma <3cm, ablation was associated with comparable survival to surgical resection, and superior survival to radiotherapy. In select patients unable to undergo resection, with appropriate anatomy, ablation may be considered over radiotherapy.
Acknowledgments:
The National Cancer Database (NCDB) is the result of a collaboration between the Commission on Cancer (CoC) of the American College of Surgeons American College of Surgeons Commission on Cancer and the American Cancer Society. Data are obtained from Commission on Cancer hospitals and in turn the NCDB. Statistical analysis and the resulting conclusions are the sole the responsibility of the study authors, and independent of these bodies.
Financial Disclosures:
K. Rhodin is supported by NIH 1R38AI140297 and by the Duke Cancer Institute as part of the P30 Cancer Center Support Grant (Grant ID: P30 CA014236)._M. Palta declares UpToDate- royalties- section editor; Varian- research funding to institution; Merck-research funding to institution; Syntactx- clinical trial adjudication.
Footnotes
Meeting: Oral presentation, Society of Surgical Oncology Advanced Cancer Therapies 2023, San Diego, CA, February 18, 2023.
References
- 1.Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist. May 2016;21(5):594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan S, Toledano M, Taylor-Robinson S. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. Hpb. 2008;10(2):77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown KM, Parmar AD, Geller DA. Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. Apr 2014;23(2):231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. Jul 2008;248(1):84–96. [DOI] [PubMed] [Google Scholar]
- 5.Burke EC, Jarnagin WR, Hochwald SN, Pisters PW, Fong Y, Blumgart LH. Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. Sep 1998;228(3):385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Hepatobiliary cancers, version 4; 2022. National Comprehensive Cancer Network Website. Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson AB, D’Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network. 2021;19(5):541–565. [DOI] [PubMed] [Google Scholar]
- 8.Takayama T, Hasegawa K, Izumi N, Kudo M, Shimada M, Yamanaka N, et al. Surgery versus Radiofrequency Ablation for Small Hepatocellular Carcinoma: A Randomized Controlled Trial (SURF Trial). Liver Cancer. Jun 2022;11(3):209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JH, Won HJ, Shin YM, Kim KA, Kim PN. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. Feb 2011;196(2):W205–209. [DOI] [PubMed] [Google Scholar]
- 10.Kolarich AR, Shah JL, George TJ Jr., Hughes SJ, Shaw CM, Geller BS, et al. Non-surgical management of patients with intrahepatic cholangiocarcinoma in the United States, 2004-2015: an NCDB analysis. J Gastrointest Oncol. Jun 2018;9(3):536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiang X, Hu D, Jin Z, Liu P, Lin H. Radiofrequency ablation vs. surgical resection for small early-stage primary intrahepatic cholangiocarcinoma. Frontiers in oncology. 2020;10:540662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. Feb 1 2008;26(4):657–664. [DOI] [PubMed] [Google Scholar]
- 13.Chen YX, Zeng ZC, Tang ZY, Fan J, Zhou J, Jiang W, et al. Determining the role of external beam radiotherapy in unresectable intrahepatic cholangiocarcinoma: a retrospective analysis of 84 patients. BMC Cancer. Sep 14 2010;10:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol. Feb 10 2016;34(5):460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen ZT, Zhou H, Li AM, Li B, Shen JS, Zhu XX. Clinical outcomes and prognostic factors of stereotactic body radiation therapy for intrahepatic cholangiocarcinoma. Oncotarget. Nov 7 2017;8(55):93541–93550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yousaf A, Kim JU, Eliahoo J, Taylor-Robinson SD, Khan SA. Ablative Therapy for Unresectable Intrahepatic Cholangiocarcinoma: A Systematic Review and Meta-Analysis. J Clin Exp Hepatol. Nov-Dec 2019;9(6):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. Jun 15 2009;99(8):488–490. [DOI] [PubMed] [Google Scholar]
- 18.Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol. Dec 1 2017;3(12):1722–1728. [DOI] [PubMed] [Google Scholar]
- 19.Antwi SO, Mousa OY, Patel T. Racial, Ethnic, and Age Disparities in Incidence and Survival of Intrahepatic Cholangiocarcinoma in the United States; 1995-2014. Ann Hepatol. July - August 2018;17(4):604–614. [DOI] [PubMed] [Google Scholar]
- 20.Ransome E, Tong L, Espinosa J, Chou J, Somnay V, Munene G. Trends in surgery and disparities in receipt of surgery for intrahepatic cholangiocarcinoma in the US: 2005-2014. J Gastrointest Oncol. Apr 2019;10(2):339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsilimigras DI, Dalmacy D, Hyer JM, Diaz A, Abbas A, Pawlik TM. Disparities in NCCN Guideline Compliant Care for Resectable Cholangiocarcinoma at MinorityServing Versus Non-Minority-Serving Hospitals. Ann Surg Oncol. Dec 2021;28(13):8162–8171. [DOI] [PubMed] [Google Scholar]
- 22.Edeline J, Lamarca A, McNamara MG, Jacobs T, Hubner RA, Palmer D, et al. Locoregional therapies in patients with intrahepatic cholangiocarcinoma: A systematic review and pooled analysis. Cancer Treat Rev. Sep 2021;99:102258. [DOI] [PubMed] [Google Scholar]
- 23.Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. May 2019;20(5):663–673. [DOI] [PubMed] [Google Scholar]
- 24.Rhodin KE, Liu A, Bartholomew A, Kramer R, Parameswaran A, Uronis H, et al. Trends in Receipt of Adjuvant Chemotherapy and its Impact on Survival in Resected Biliary Tract Cancers. Ann Surg Oncol. May 15 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koay EJ, Odisio BC, Javle M, Vauthey JN, Crane CH. Management of unresectable intrahepatic cholangiocarcinoma: how do we decide among the various liver-directed treatments? Hepatobiliary Surg Nutr. Apr 2017;6(2):105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hare AE, Makary MS. Locoregional Approaches in Cholangiocarcinoma Treatment. Cancers (Basel). Nov 28 2022;14(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giorgio A, Gatti P, Montesarchio L, Santoro B, Dell’Olio A, Crucinio N, et al. Intrahepatic Cholangiocarcinoma and Thermal Ablation: Long-term Results of An Italian Retrospective Multicenter Study. J Clin Transl Hepatol. Dec 28 2019;7(4):287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mouli S, Memon K, Baker T, Benson AB 3rd, Mulcahy MF, Gupta R, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol. Aug 2013;24(8):1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhlmann JB, Euringer W, Spangenberg HC, Breidert M, Blum HE, Harder J, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol. Apr 2012;24(4):437–443. [DOI] [PubMed] [Google Scholar]
- 30.Tao R, Krishnan S, Bhosale PR, Javle MM, Aloia TA, Shroff RT, et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients With Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J Clin Oncol. Jan 20 2016;34(3):219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]


