Colorectal cancer (CRC) is a leading cause of cancer death in the United States.1 Colonoscopy performed for primary screening or follow-up of positive screening tests may reduce CRC incidence and deaths through removal of precancerous polyps (adenomas) and detection of treatable early-stage cancers, although the extent of the beneficial outcomes of primary screening colonoscopy is uncertain and requires further study.2, 3 Physician adenoma detection rate (ADR), the percentage of screening colonoscopies at which one or more adenomas is detected, is an established colonoscopy quality metric. ADR is strongly inversely associated with patients’ risk of post-colonoscopy CRC (PCCRC) diagnosis and death; these are cancers diagnosed after a colonoscopy that did not detect cancer.4–6 Calculating ADRs using only screening colonoscopies was proposed to provide an “apples to apples” comparison between physicians within and across settings. However, substantial ADR differences by indication do not exist in all settings and, conversely, screening ADRs can have substantial variation between settings, including between high-quality programs.2, 7, 8 Methods for ascertaining colonoscopy indication include manual review, electronic medical record queries, and text string searches of colonoscopy reports; methods are resource intensive and subject to misclassification. These barriers have impeded universal adoption of screening ADR reporting and suggest the need for a simpler, valid alternative to screening ADR.9 Indeed, 2016 UK guidelines addressing key performance indicators and quality assurance standards for colonoscopy recommended ADRs be measured for all ages and across all indications,10 although at the time there were minimal data supporting these recommendations.
The current study addressed two questions: 1) Do ADRs by different indications (especially overall ADR using all colonoscopies vs. screening ADR) have comparable associations with PCCRC? And 2) Does calculating ADR based on all examinations vs. just screening examinations comparably identify the same endoscopists within each method’s lowest ADR quartile?
Using a retrospective cohort design, we evaluated four large, demographically diverse, community-based health care systems with 45 endoscopy centers across three states and including approximately 3% of the United States population (i.e., Kaiser Permanente Northern California, Kaiser Permanente Southern California, Kaiser Permanente Washington, and Parkland Hospital/University of Texas Southwestern). Among 487 endoscopists who performed 1,046,916 cancer-negative colonoscopies in January 2011 through June 2019, we evaluated associations between each colonoscopy patient’s PCCRC risk and the performing physician’s ADR, calculated from colonoscopies performed in the prior calendar year. For each colonoscopy, the endoscopist had their prior year’s ADRs calculated for screening, surveillance, diagnostic (including fecal immunochemical test (FIT) positive), and all colonoscopy indications (overall ADR) (Supplementary Table). CRC diagnosis data were available through December 31, 2019.
Median ADRs and interquartile ranges were as follows: overall ADR: 36.3% (29.2–44.4%); screening ADR: 29.7% (22.4–38.1%); diagnostic ADR: 37.1% (30.6–44.5%); and surveillance ADR: 48.6% (38.8–58.5%) (Figure 1 panel A). Comparing paired ADR values for each physician, the median overall ADR was an absolute 6.6% higher than the median screening ADR (p<0.01).
Figure 1.
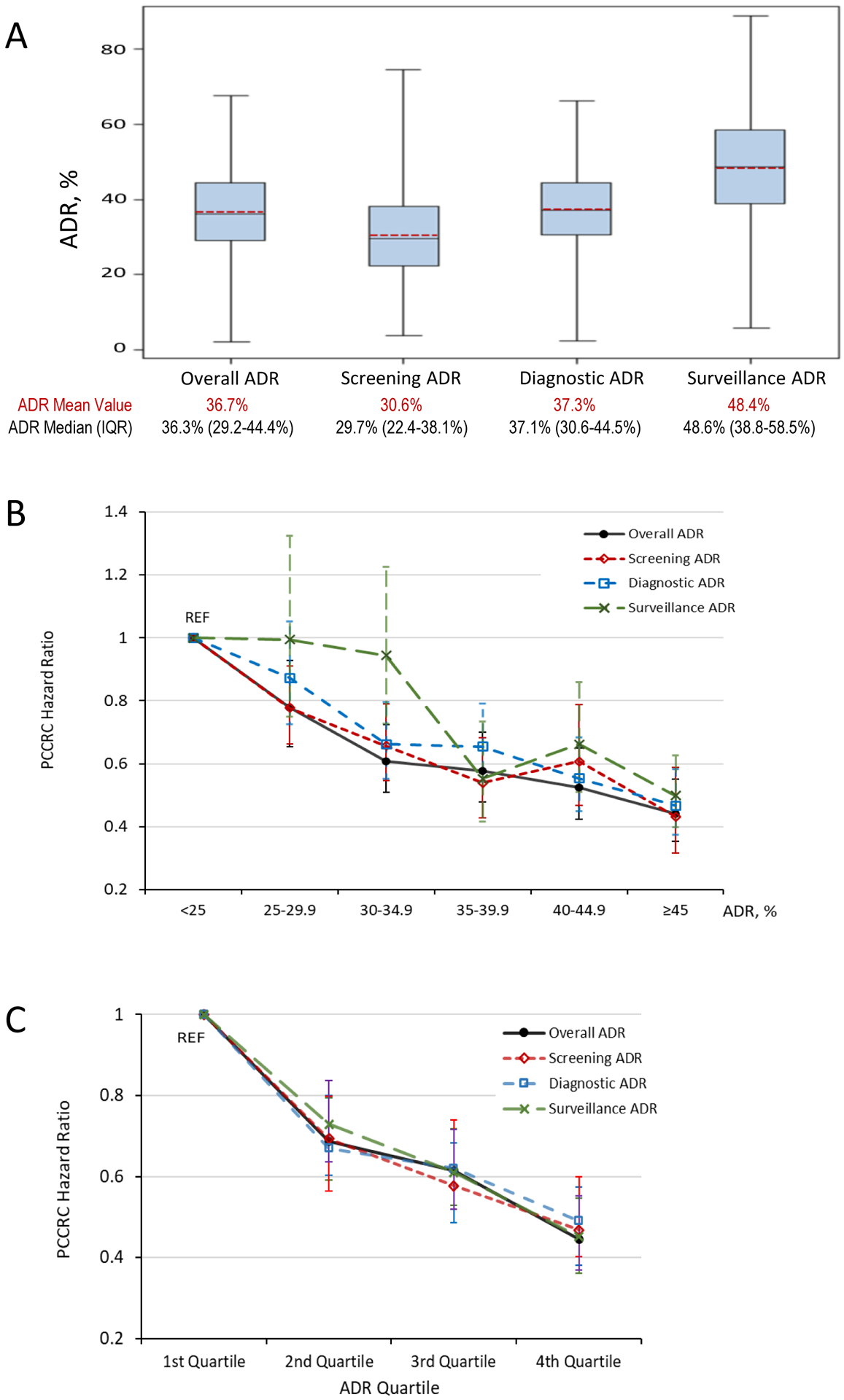
Adenoma detection rate (ADR) distributions based on colonoscopies performed in 2010–2018 (panel A) and associations between ADR categories and quartiles and risk of postcolonoscopy colorectal cancer (PCCRC) (panel B and panel C).
ADR, adenoma detection rate; IQR, interquartile range; PCCRC, post-colonoscopy colorectal cancer; REF, reference group.
Panel A: The blue boxes represent the second and third ADR quartiles or interquartile range; the horizontal black solid line inside the boxes represents the ADR median; the red dashed line inside the boxes represent the ADR mean value; the whiskers extend to the minimum and maximum ADR values. See Supplementary Table for additional ADR calculation details.
Panel B and C: Hazard ratios were adjusted for health system, sex, race, ethnicity, age, body mass index, Charlson comorbidity score, and colonoscopy year. Error bars represent 95% confidence intervals.
ADRs across colonoscopy indications were similarly inversely associated with PCCRCs by both absolute ADR categories and ADR quartiles. For example, for patients of physicians with ADRs of ≥45% vs. <25% (reference), PCCRC risk hazard ratios and 95% confidence intervals for overall and screening ADRs were 0.44 (0.35–0.55) and 0.43 (0.32–0.59), respectively (Figure 1 panel B). Similarly, although ADR ranges within quartiles varied by indication, comparable 4th vs. 1st quartile associations with PCCRC risk were found across indications (e.g., overall ADR vs. screening ADR 0.45 (0.36–0.55) vs. 0.47 (0.38–0.57), respectively) (Figure 1 panel C). Quartile of overall ADR vs. screening ADR also had similar overall predictive ability for PCCRC, with a c-statistic for each of 0.71. Surveillance ADRs were higher than ADRs for other indications and, as a result, comparatively fewer endoscopists had surveillance ADRs less than 25%, which was the reference group. This resulted in wider confidence intervals for the other surveillance ADR categories.
Two centers separately reported FIT results (KPNC and KPSC). For colonoscopies with a FIT-positive indication, the mean, median, and interquartile ADR values were 51.6%, 51.7%, and 43.5%–60.8%, respectively, whereas for diagnostic examinations not including FIT-positive colonoscopies, they were 36.0%, 35.7%, and 29.1%–42.9%, respectively. For these two centers, for a FIT-positive indication, the hazard ratios and 95% confidence intervals for associations between 4th vs. 1st quartile ADR and PCCRC were 0.62 (0.49–0.79) and, for absolute ADRs ≥45% vs. <25%, 0.55 (0.37–0.83). In these centers, for diagnostic examinations not including FIT-positive colonoscopies, the hazard ratios and 95% confidence intervals for associations between 4th vs. 1st quartile ADR and PCCRC were 0.50 (0.41–0.61) and, for absolute ADRs ≥45% vs. <25%, 0.40 (0.31–0.52).
Overall ADR vs. screening ADR comparably identified physicians’ performance by quartile groupings, particularly for the lowest quartile. Among 293 endoscopists who performed colonoscopies in 2017–2018, for example, 62 of the 73 providers (84.9%) in the lowest quartile for screening ADR were also in the lowest quartile of the overall ADR and no providers moved more than one quartile (Supplementary Figure). For all quartiles, 204 of 293 (69.6%) endoscopists had identical quartile rankings for overall ADR vs. screening ADR; 86 (29.4%) differed by 1 quartile; and only 3 (1.0%) differed by 2 quartiles.
These findings supplement and confirm prior reports evaluating ADR by indication. The differences in ADRs by indication are similar to some centers but contrast with two Veterans Affairs centers that reported no difference between overall and screening ADRs (49% vs. 50%, respectively; p=.55).7 The disparate findings likely represent different methods for classifying the screening indication, population demographics, and/or cancer screening histories, given the ADR variation by indication reported in multiple other settings.2, 7, 8 However, given the large differences in screening ADRs between settings, and the potential for indication misclassification, it is not clear that restricting ADR calculations to screening colonoscopies allows for a more “apples-to-apples” comparison across settings than methods that include all colonoscopies (i.e., overall ADR). Indeed, contrasting the Veterans Affairs study and the current large, community-based, multi-center study demonstrates that differences in screening ADR estimates between settings with high overall quality can be larger than differences between screening ADR and overall ADR estimates within settings.
In addition to simplifying the ADR calculation, eliminating misclassification by indication, and easing implementation, overall ADR is also not susceptible to potential provider-related biases, in settings that use indications from colonoscopy reports, given these indications are assigned after examination completion.11 Endoscopists may have practices that vary with respect to colonoscopy indication mix and patient demographic mix; however, the latter has demonstrably little influence on most provider’s ADR rankings.12 Nevertheless, overall ADR measurements may be less generalizable for physicians with patient populations highly skewed by demographics (e.g., age) or indication (e.g., surveillance).
Given the higher ADRs for colonoscopies with FIT-positive indications in the current study and in other settings,8 and that FIT screening is commonly used in CRC screening programs worldwide, consideration could be given to using the overall ADR vs. FIT-positive ADR for programs with high-level use of FIT. Use of FIT-positive ADR would, however, add additional complexity, as it still requires identifying examinations for a FIT-positive indication. In addition, the current study’s centers, which have high use of FIT, did not find a difference, compared with screening ADR, in using overall ADR (which includes FIT-positive indications) vs. screening or other specific indications as regards prediction for PCCRC or in identifying physicians in the lowest quartile of ADR.
In conclusion, the current study found that overall ADR as a quality metric performed similarly to screening ADR for predicting PCCRC and identifying the same providers in the lowest quartile. In addition, it is simpler to calculate, not susceptible to indication misclassification or potential provider-related biases, and is more precise (as it includes many more colonoscopies). These findings, given their multi-center derivation in community-based populations with diverse demographics, can inform potential normative values for overall ADR compared with society guideline targets for screening ADR.
Supplementary Material
ACKNOWLEDEMENT:
Corporate Authorship (PROSPR PRECISE Consortium)
Aruna Kamineni, PhD;2 Celette Sugg-Skinner, PhD;4 Wei K. Zhao, MPH;1 Rebecca A. Ziebell, BS;2 Richard Contreras, MS;5 Eric J. Kim, MS;4 Jeffrey K. Lee, MD, MPH;1 Theodore R. Levin, MD;1 Nirupa R. Ghai; PhD, MPH;6 Bruce H. Fireman, MA;1 Charles P. Quesenberry, PhD.1
Corporate Authorship Institutions
1Division of Research, Kaiser Permanente Northern California, Oakland, CA.
2Kaiser Permanente Washington Health Research Institute, Kaiser Permanente Washington, Seattle, WA.
3Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, CA.
4Simmons Comprehensive Cancer Center and Department of Population & Data Sciences, University of Texas Southwestern Medical Center, Dallas, TX.
5Research and Evaluation, Kaiser Permanente Southern California, Pasadena, CA.
6Department of Quality and Systems of Care, Kaiser Permanente Southern California, Pasadena, CA.
GRANT SUPPORT:
The study was conducted within the National Cancer Institute-funded Population-based Research to Optimize the Screening Process II (PROSPR II) consortium (UM1 CA222035).
ABBREVIATIONS:
- CRC
colorectal cancer
- ADR
adenoma detection rate
- PCCRC
post-colonoscopy colorectal cancer
- FIT
fecal immunochemical test
- KPNC
Kaiser Permanente Northern California
- KPSC
Kaiser Permanente Southern California
- KPWA
Kaiser Permanente Washington
- UTSW
Parkland Hospital/University of Texas Southwestern
- SEER
Surveillance, Epidemiology, and End Results
- SNOMED
systematized nomenclature of medicine
- ICD-O-3
International Classification of Diseases for Oncology, third edition
Footnotes
CONFLICT OF INTEREST STATEMENT:
The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors report no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA TRANSPARENCY STATEMENT:
Researchers may formally request access to the data through the Population-based Research to Optimize the Screening Process II (PROSPR II) consortium.
References
- 1.Siegel RL, et al. Ca Cancer J Clin 2021; 71: 7–33. [DOI] [PubMed] [Google Scholar]
- 2.Bretthauer M, et al. N Engl J Med 2022; 387: 1547–1556. [DOI] [PubMed] [Google Scholar]
- 3.Dominitz JA, et al. New Engl J Med 2022; 387: 1609–1611. [DOI] [PubMed] [Google Scholar]
- 4.Corley DA, et al. New Engl J Med 2014; 370: 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaminski MF, et al. Gastroenterology 2017; 153: 98–105. [DOI] [PubMed] [Google Scholar]
- 6.Schottinger JE, Jensen CD, et al. JAMA 2022; 327: 2114–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaltenbach T, et al. Clin Gastroenterol Hepatol 2021; 19: 1883–1889 e1. [DOI] [PubMed] [Google Scholar]
- 8.Wisse PHA, et al. Ann Intern Med 2022; 175: 1366–1373. [DOI] [PubMed] [Google Scholar]
- 9.Lee JK, et al. Gastrointestinal Endoscopy 2015; 81: 575–582 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rees CJ, et al. Gut 2016; 65: 1923–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rex DK, et al. Endoscopy 2017; 49: 1069–1074. [DOI] [PubMed] [Google Scholar]
- 12.Jensen CD, et al. Clin Gastroenterol Hepatol 2015; 13: 739.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers may formally request access to the data through the Population-based Research to Optimize the Screening Process II (PROSPR II) consortium.


