Abstract
A vast array of αβ T cell receptors (TCRs) is generated during T cell development in the thymus through V(D)J recombination, which involves the rearrangement of multiple V, D, and J genes and the pairing of α and β chains. These diverse TCRs provide protection to the human body against a multitude of foreign pathogens and internal cancer cells. The entirety of TCRs present in an individual’s T cells is referred to as the TCR repertoire. Despite an estimated 4 × 1011 T cells in the adult human body, the lower bound estimate for the TCR repertoire is 3.8 × 108. While the number of circulating T cells may slightly decrease with age, the changes in the diversity of the TCR repertoire is more apparent. Here, I review recent advancements in TCR repertoire studies, the methods used to measure it, how richness and diversity change as humans age, and some of the known consequences associated with these changes.
Keywords: αβ TCR, TCR repertoire, diversity index, species richness, aging, CD4+ T cells, CD8+ T cells, IAV, CMV
1. αβTCR: generation, specificity, and dynamics
The antigen-specific T cell receptor (TCR) is a heterodimer composed of an α and a β chain (αβTCR or TCR), accounting for 90% of circulating T cells. The TCR recognizes antigenic peptides presented by MHC complexes on dendritic cells or other antigen-presenting cells with high specificity (1). The interaction between the TCR-peptide and the MHC complex triggers a signaling cascade that leads to T cell activation, differentiation, and various functions (2). This specific recognition by the TCR of its antigen defines one of the key features of cell-mediated immunity. Exposure to a wide range of environmental microorganisms and internal cancer cells necessitates a diverse repertoire of TCR-expressing T cells to ensure immune protection throughout an individual’s life.
T cells are generated in the thymus through an orderly process of rearrangement. Firstly, the TCRβ chain is generated by combining variable (V), diversity (D), and joining (J) gene segments to form a functional VDJ domain. Secondly, the TCRα chain is generated by combining variable (V) and joining (J) gene segments to form a functional VJ domain. Lastly, the TCRα and TCRβ chains pair up for the selection of a functional αβTCR. In humans, there are 39–46 functional V genes, 2 D genes, 13 J genes, and 2 C genes for TCRβ (3, 4). Additionally, there are 45 functional V genes, 58 J genes, and one C gene for TCRα (5, 6). The recombination of these gene segments, along with imprecise joining (deletion and insertion of nucleotides) and pairing of αβ chains, collectively give rise to an estimated 1014-1019 unique TCR sequences (7, 8, 9, 10) (Figure 1).
Figure 1.
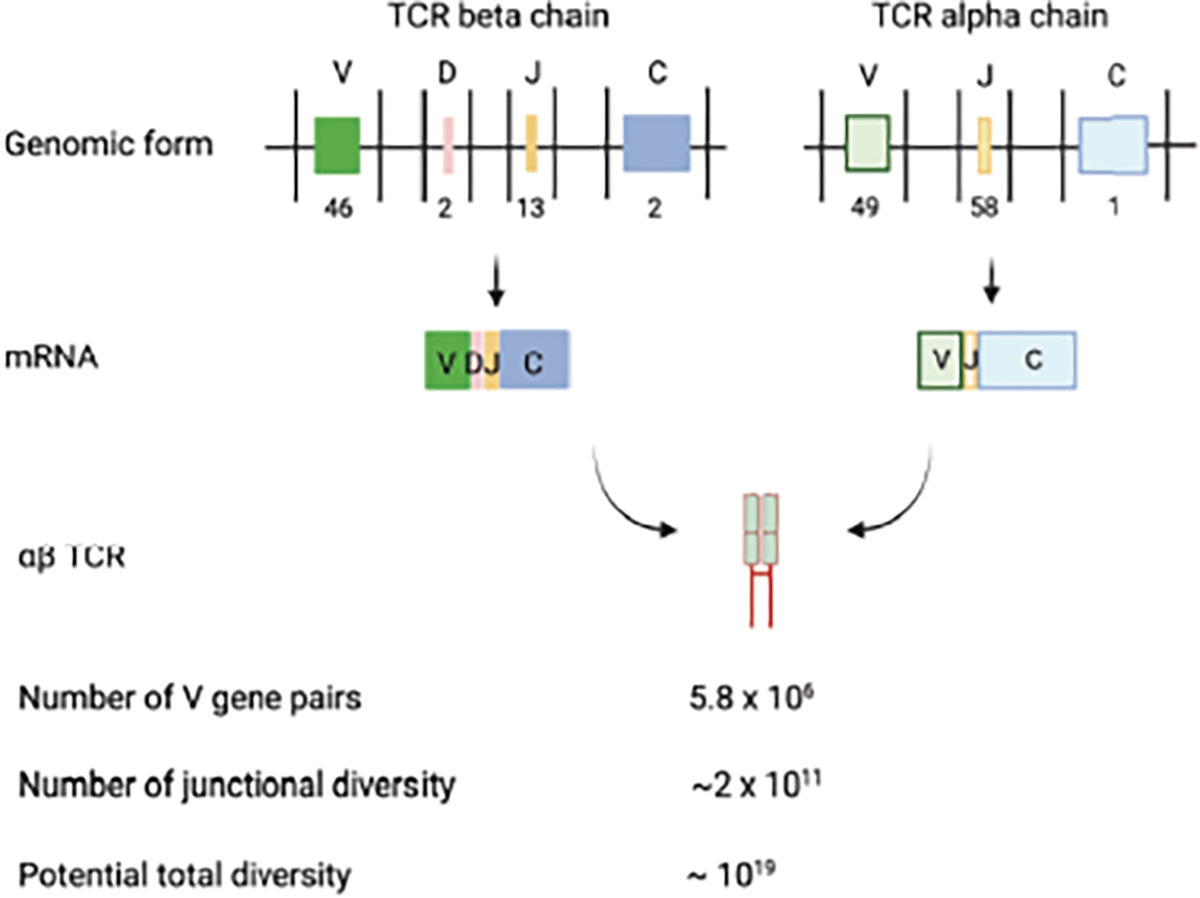
Generation of TCR and estimated size of TCR repertoire. Human TCR b and a locus contains multiple functional V (46), D (2), J (13) and V (49), J (58) gene segments, respectively (3, 4, 5, 6). The recombination of V(D)J, imprecise at the joining, and TCR pairing create a potential number of 1019 different TCR in human (7, 8, 9, 10). Image created using BioRender.com.
An adult human is estimated to have approximately 4 × 1011 T cells, which includes both naïve T cells and various subsets of memory T cells (11, 12). The maintenance of T cells relies on thymic output and peripheral proliferation. The thymus is responsible for producing an estimated 107-108 naïve T cells per day in young adults (13, 14). However, thymic output significantly diminishes after puberty and continues to decline with aging (15). This reduction in thymic output represents one of the two major factors contributing to fewer unique TCRs with age.
Activation of naïve T cells leads to the generation of memory T cells, which are long-lived and provide sustained protection against pathogens upon subsequent encounters (16, 17, 18). Both the initial activation and subsequent activations throughout an individual’s life contribute to the accumulation of memory T cells with age (19, 20), resulting in clonally expanded T cell populations alongside with loss of some unused naïve T cells as part of T cell pool maintenance. Furthermore, naïve T cells are maintained by the homeostatic proliferation in the absence of antigen stimulation which could lead to biased expansion of certain naïve T cells (21, 22). Under some conditions, this type of proliferation also leads to phenotype change from naïve to memory (virtual memory T cells) (23). These programs operating over a lifetime and their cumulative changes contribute to the diminishing the number of unique TCRs and selective expansion of certain TCRs (Figure 2). Such processes could be accelerated under persistent or chronic infection {Colonna-Romano, 2007 #20}. The TCR repertoire encompasses the entire collection of unique TCRs and their varying degrees of clonal distribution within the body. However, the precise number of unique TCRs and the rate of loss of unique TCRs with age are not yet fully understood.
Figure 2.

TCR repertoire dynamics of selective clonal expansion and loss during T cell activation and homeostatic proliferation throughout a person’s life. The content of the TCR repertoire is dynamic, influenced by the production of new TCRs and selective expansion of certain TCRs during T cell activation (16, 17, 18) and homeostatic proliferation (21, 22). The image shows 1) activation of a selected TCR-T cell (dark red), which undergoes activation, expansion, and maintenance phases resulting in change of the content of the overall TCR repertoire, and 2) homeostatic proliferation of selected four TCRs resulting in various expansion. Homeostatic expansion also could induce virtual memory T cells (cells within the light blue frame) (23). Image created using BioRender.com.
2. TCR repertoire: measurements, estimations, and features.
Precisely measuring the TCR repertoire in humans has been challenging due to the small sample size compared to the vast number of T cells and the difficulty of analyzing a matching number of TCR sequences. Recent advancements in next-generation sequencing (NGS) have partially overcome the technical challenges associated with the latter difficulty, but the high cost remains a hurdle that needs to be addressed. Our current understanding of the TCR repertoire is primarily based on the analysis of many TCRβ sequences (24, 25, 26). Various methods, including genomic DNA-based and mRNA-based approaches, have been developed to analyze TCR sequences (27). However, as the majority of TCR sequences are unknown, the accumulation of errors from PCR and sequencing in these methods makes it challenging to distinguish between genuine rare TCR sequences and errors. The application of unique molecular identifiers (UMI) in mRNA-based methods allows for molecular identifier group-based error correction, resulting in high-quality TCR sequence repertoire (28, 29).
Determining individual TCRβ and TCRα sequences provides an initial glimpse of TCR diversity, but it is not a complete picture as a real TCR is an αβ TCR dimer. Single-cell-based TCR sequence analysis methods (scTCRseq) provide paired αβ TCR sequences for each T cell, revealing the true level of complexity and TCR repertoire diversity (30, 31). However, the currently available methods for scTCRseq are still too expensive to conduct large-scale analyses compared with the relative affordability of individual TCR chain sequencing.
Currently, TCR sequence analysis is performed using a sample containing a fraction of circulating (0.1–10 × 106) T cells, and several diversity measures have been employed to quantify and estimate the size of the TCR repertoire (32). Two main features of the TCR repertoire, borrowed from ecological diversity measures, have been measured: the total number of clonal types or species richness (33), and diversity indices such as Shannon entropy and Simpson index (34, 35) to measure clonal distribution/expansion (Figure 3). Experimental analyses of TCRβ sequences suggest that the lower bound predicted TCRβ repertoire size is 108 (36, 37, 38). In our analysis, using both TCRβ and TCRα sequences from a total of 108 T cells from 30 human donors, we conclude that the lower bound of the αβ TCR repertoire is 3.8 × 108, based on the ratio of TCRα and TCRβ pairing frequency in a large dataset of αβ paired TCRs (39).
Figure 3.
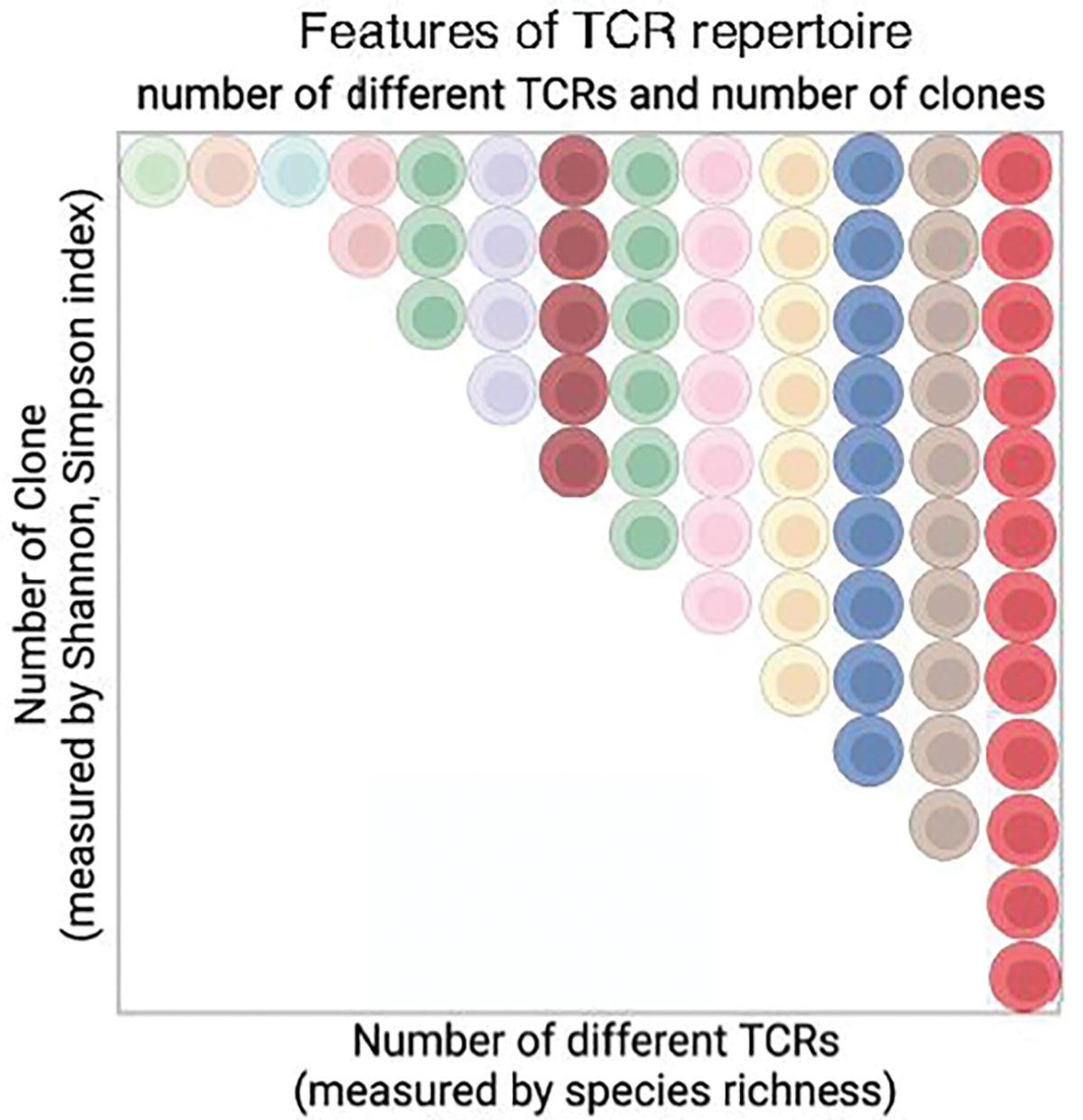
Features of TCR repertoire. The TCR repertoire includes the total number of distinct TCR clonotypes and clonal distribution of each distinct TCR. The total number of distinct TCRs is estimated by Species Richness (33) and the clonal distribution/expansion is measured by various diversity indexes such as the Shannon index (32). Each color represents a distinct TCR, and the number of cells represents the clonal size of a TCR. Image created using BioRender.com.
These studies confirm that the TCR repertoire differs among different subsets of T cells and among individuals. Overall, the TCR repertoire richness is greater in CD4+ T cells compared to CD8+ T cells, and naïve T cells exhibit greater richness than memory T cells (37, 39). Furthermore, TCRβ (CDR3β) sequences show distinctions between CD4+ and CD8+ T cells (38), as well as between conventional T (Tconv) cells and regulatory T (Treg) cells in healthy neonates, adults (40), and tumor microenvironments (41). These differences reflect variations in peptide and MHC (Class I vs. Class II) features, as well as their development and selection processes, thereby establishing a link between TCR specificity and the function of different T cell subtypes (42). Analysis of TCR sequences also reveals that shared or public TCR sequences are more common than expected by chance among different individuals. One study shows that three donors share 8% of TCRβ or 11% of TCRα clonotypes among all T cells, and 5% of naïve CD4+ and 3.5% of naïve CD8+ T cell subsets share their TCRβ clonotypes. In contrast, memory CD4+ and CD8+ T cell subsets share 2.3% and 0.4% of their TCRβ clonotypes in four donors, respectively (43). Similar public TCRβ and TCRα sequences are also observed in our study of 30 donors with mixed HLA haplotypes (39). These analyses are based on individual TCRβ and TCRα sequences; therefore, the extent of true paired public αβ TCRs is expected to be lower. In a recent analysis of 965,523 αβ paired TCRs from 15 healthy donors, the common public TCRα and TCRβ sequences were found in these donors at 0.1–0.2% of unique CD4+ TCRs (44). These public TCR clonotypes may be involved in normal immunity against pathogens, cancer, or autoimmunity (45).
In addition to general TCR repertoire analyses, studies of antigen- or pathogen-specific TCR repertoires reveal additional details regarding distinct features of both TCRs and antigens. Analysis of CD8+ TCR repertoires for two dominant viral epitopes, pp65495–503 (NLV) of cytomegalovirus (CMV) and M158–66 (GIL) of influenza A virus (IAV), shows highly individualized TCRα and TCRβ repertoires comprising thousands of unique TCRα and TCRβ sequences, as well as dozens of distinct complementary determining region 3 (CDR3α and CDR3β) motifs per person (46). Collectively, the estimated size of TCR repertoires for these two dominant epitopes ranges from a few hundreds to thousands per person (47) to several thousand in the human population (46). Further annotation of TCRα and TCRβ pairs with known antigen specificities reveals that approximately a third of the T cells possess α and β chains recognizing different antigens (48). This suggests that αβ pairing plays a critical role in TCR-peptide/MHC-specific recognition. CMV infection alters the pp65-specific TCR repertoire by substantially expanding a few CMV-specific TCRs, characterized by dominant public clones (49, 50).
Compared to CD8+ T cells, the antigen specific CD4+ T cell repertoire is less well characterized, partly due to the limited availability of MHC class II tetramers. An analysis of CD4+ T cell response to Varicella zoster virus (VZV) vaccine observed an expansion of infrequent VZV antigen-reactive TCRβ repertoires, suggesting vaccination-induced diversification of the VZV-specific CD4+ TCR repertoire, which can be used for monitoring immune protection (51).
The development of antigen-specific MHC-peptide tetramers has made tremendous progress in understanding TCR sequences and specificity (52). Characterization of epitope specific TCR repertoires of CD8+ T cells has been achieved using distance measures in TCR space, allowing for clustering and classification of previously unknown TCR specificities (53). The application of machine learning tools has further flourished TCR sequence-based characterization and classification, yielding increasingly promising results (54, 55, 56). One notable algorithm, GLIPH (grouping of lymphocyte interactions by paratope hotspots), clusters TCRs with a high probability of sharing specificity based on conserved motifs and global similarity of CDR3 sequences (54). Structure-based models and deep learning techniques are also being used for TCR-peptide-MHC interaction specificity prediction, offering a generalizable approach (55, 56).
Lastly, the TCR repertoire is highly individualized (39, 57) and is shaped by genetic differences in major histocompatibility complex (MHC) polymorphism. Recent analyses have explored the associations between MHC alleles, antigenic epitopes, and shared TCRs among humans (58). A better characterization of MHC genotyping and peptide-TCR relationships will enhance our understanding of the characteristics of individual-specific TCR repertoires and their past, present, and potential future.
3. Alteration of general TCR repertoire with age
With advancing age, there is a reduced proportion of naïve T cells and an increased proportion of memory T cells (consisting of major portion of antigen-induced and minor portion of non-cognitive antigen induced virtual memory cells), which is particularly evident in CD8+ T cells compared to CD4+ T cells (59, 60). Cross-sectional analyses of TCRβ repertoires in donors spanning from newborns to centenarians demonstrate a roughly linear reduction in TCRβ richness in blood T cells with age. This reduction is negatively correlated with the percentage of naïve T cells (61, 62). Direct analysis of TCRβ repertoire richness in naïve CD4+ and CD8+ T cells reveals a noticeable decline in healthy elderly individuals, reducing to 50%−20% of the repertoire size seen in young individuals. However, the reduction in TCRβ repertoire richness is less pronounced in memory CD4+ and CD8+ T cells (37).
Nevertheless, in naïve T cells of older donors, an inequality in clonal sizes, as measured by a modified Gini-Simpson index, is observed. This indicates clonal expansion of naïve T cells due to uneven homeostatic proliferation (37, 39, 63). Furthermore, analysis of CDR3β regions reveals age-associated significant decreases in CDR3 length, NDN insertions, and the number of non-templates added N nucleotides. Additionally, there are changes in the physicochemical properties of the central part of the CDR3β loop observed in various types of T cells, including CD4+, CD8+, and naïve T cells (64).
Considering the highly individualized nature of the TCR repertoire, longitudinal analyses have been reported to measure age-related changes more precisely in the TCR repertoire. The first study analyzed TCRβ repertoires of blood CD4+ and CD8+ T cells from six donors (equal sex distribution, aged 23–65 years old) over three visits spanning 19–25 years (65). It was found that the CDR3β diversity (number of unique CDR3β sequences divided by the total number of cells) of CD8+ T cells decreased significantly with age (p<0.001), and there was an increase in the frequencies of clonal populations (p=0.003). However, no significant changes were observed in CD4+ T cells. Approximately 10–30% of CDR3β sequences in CD4+ T cells and 30–80% in CD8+ T cells were found to be present across different visits within the same donor. The retention of certain TCRβ sequences over time was reported over the course of one year (66) and even up to 20 years (65). As the oldest age of this study donor is 65, the age changes of the TCR repertoire in 70–90 years of age as well as in the naïve and memory T cell subsets were not analyzed.
To overcome these shortcomings, the second longitudinal study analyzed both TCRβ and TCRα repertoires in CD4+ and CD8+ T cell subsets (naïve and memory cells) from 30 healthy donors. The donors had an equal sex distribution, their ages ranged from late 20s to early 80s at the first visit with an average of 9.2 years until the second visit). This study utilized a UMI-based RNAseq method and projected the TCR repertoire richness based on the actual circulating T cell number (39). Several interesting findings emerged from this study: 1) there was a significant reduction in TCRβ repertoire richness, but not TCRα repertoire richness, in both CD4+ and CD8+ T cells with age; 2) a significant reduction in TCRβ repertoire richness was observed in naïve CD4+ and CD8+ T cells, but not in memory CD4+ and CD8+ T cells, with age; and 3) a significant reduction in estimated αβ paired TCR repertoire richness was observed in CD8+ T cells (−2.4%/year), naïve CD8+ T cells (−2.8%/year), and memory CD8+ T cells (−2.0%/year), but not in total, naïve, or memory CD4+ T cells (Figure 4). This longitudinal study provides evidence of distinct changes in TCR repertoire richness with age in naïve and memory CD4+ and CD8+ T cells, with age-associated reductions in TCR repertoire richness occurring specifically in naïve CD4+ and CD8+ T cells. However, because a minor portion of memory T cells is non-antigen-induced virtual memory T cells, it is currently unknown whether the age-related TCR repertoire change is similar or different between antigen-induced memory and virtual memory T cells.
Figure 4.
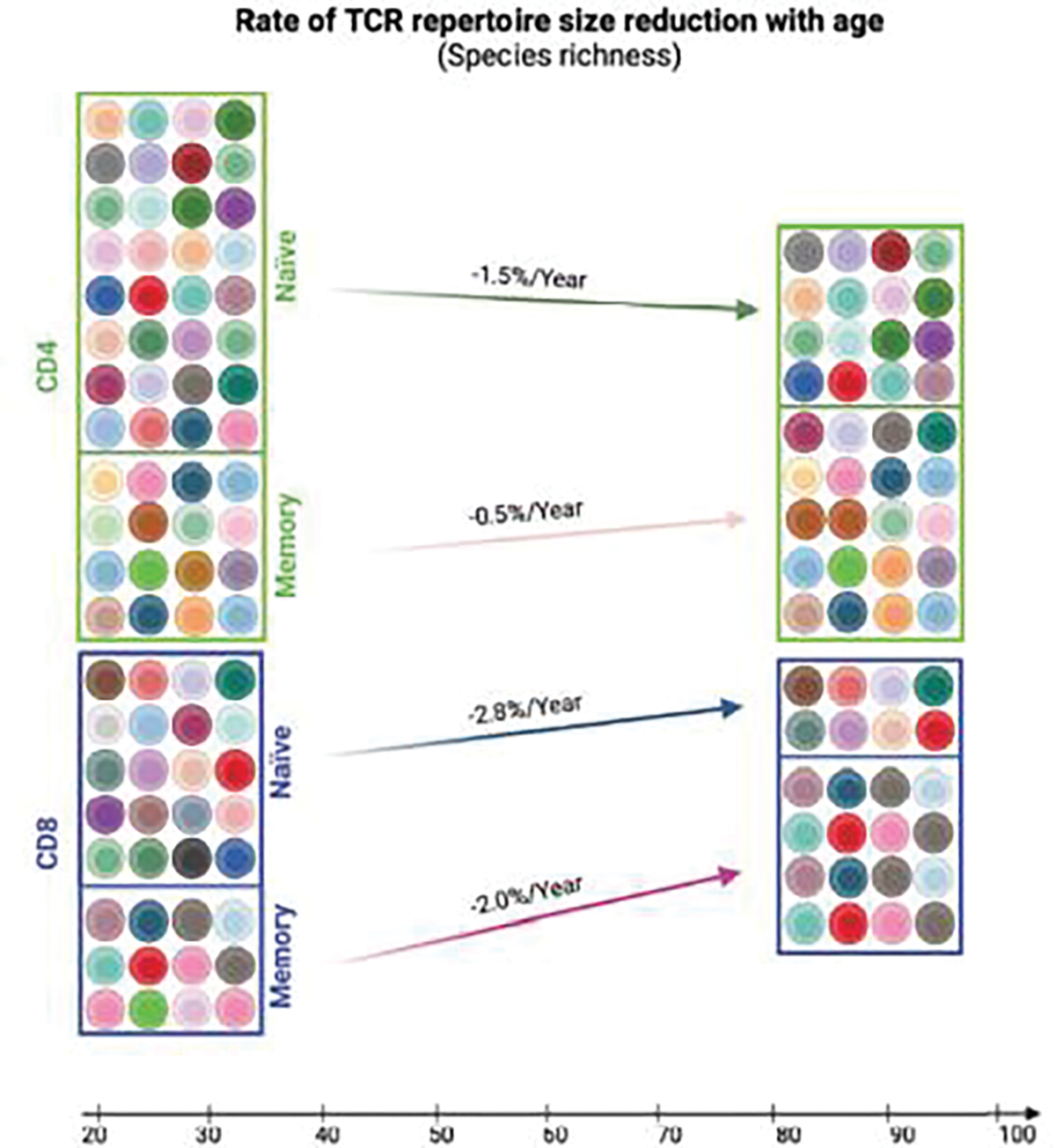
Estimated rate of TCR repertoire reduction in human CD4+ and CD8+ naïve and memory T cells with age. The rates are calculated based on linear regression of the estimated αβ-paired TCR repertoire reduction of human CD4+ and CD8+ naïve and memory T cells with age (presented as change of the percentage of the starting size per year) (39). Image created using BioRender.com.
This longitudinal αβ TCR sequence analysis further revealed the following: 1) TCRα and TCRβ sequences are increasingly retained with age, meaning the same TCRs are found in both visits of the same donor, in both CD4+ and CD8+ T cells. The degree of retention is more prominent in CD8+ cells than in CD4+ cells with age, and 2) the retention of TCRα and TCRβ sequences is more pronounced in memory T cells compared to naïve T cells, with the highest retention observed in memory CD8+ T cells. This suggests that the reduction in TCR repertoire size with age leads to a more stabilized TCR repertoire, particularly in memory CD8+ T cells, as individuals age. These findings indicate that the age-related reduction in TCR repertoire size contributes to an increasingly stable TCR repertoire, especially in memory CD8+ T cells, as individuals get older. This age-associated reduction in TCR repertoire may provide a potential explanation for the reduced ability to respond to novel antigens in older adults. It is essential to identify the precise loss of antigen specific TCRs and its association with reduced specific T cell immunity in older adults.
4. Antigen-specific TCR repertoire changes with age:
Studies investigating the changes in antigen-specific TCR repertoires with aging provide valuable insights into age-associated alterations in specific T cell immunity. Analysis of CD8+ TCR repertoires specific to the influenza M158–66 epitope has revealed the following observations: 1) there is a correlation between increasing age and a narrowing of the TCR repertoire, potentially leading to enhanced cross-reactivity in older individuals (67, 68) and 2) the TCR repertoire specific to the M1 epitope undergoes changes in older adults, characterized by an increase in private M1-specific CD8+ TCRs and a loss of public CD8+ TCRs (69). Prior to the current NGS methods, clonally expanded CD8+ TCRs against CMV-specific epitopes such as pp65 were reported in older adults (70). However, more recent analysis of CD8+ TCRβ sequences using NGS techniques has shown many expanded TCRβ clones in CMV seropositive donors, with these clones gradually increasing with age (71). Interestingly, despite the accumulation of CMV-induced TCRβ clones, the overall CD8+ TCRβ diversity appears to be retained in older adults. Therefore, the impact of the CMV specific TCR repertoire changes on the functional competency of CD8+ T cell immunity against CMV and other pathogens in older adults remains to be determined.
The content of the TCR repertoire is highly individualized, influenced by the unique history of interactions with the environment. Aging causes reduction of naïve T cells and the accumulation of terminally differentiated memory T cells, which contributes to a generalized weakening of T cell function. However, loss of certain TCR specificities with aging is highly individualized and explains different degree of susceptibility to different pathogens in older adults. The challenge lies in determining which individuals are susceptible to which pathogens as both the TCR contents and its change with age are highly individualized. Therefore, the identification and characterization of age-related antigen specific TCR repertoire changes are crucial next steps in fully understanding the impact of aging on T cell immunity in older adults, both at the population level and the individual level. By gaining insights into these changes, we can better assess the susceptibility of older adults to specific pathogens and develop targeted interventions to enhance their immune responses.
5. Conclusion:
The analysis of the TCR repertoire presents significant technical challenges due to its complexity and vast size. More accurate measurements of the TCR repertoire should adopt paired αβ TCR methods, increase sample sizes, and conduct longitudinal measurements involving a larger pool of donors. As we progress, achieving a more precise measurement of TCR repertoire size and clonal distribution is feasible. This advancement could potentially lead to the integration of TCR repertoire assessment as a routine blood test for evaluating T cell function once the costs become more affordable. Periodic assessments would provide more data points, addressing the challenge of small sampling and improving the measurement of TCR repertoire size. By implementing these approaches, we can further our understanding of the TCR repertoire and its implications for immune health.
While the general TCR repertoire provides important insights into overall T cell immunity, analyzing antigen specific TCR repertoires offers a direct measure of specific T cell responses against particular pathogens. By examining changes in antigen specific TCR repertoires, we can more accurately assess the impact of aging on specific T cell responses. It is important to recognize that the content of TCR repertoires and their age-related changes are highly individualized. Therefore, characterizing antigen specific TCR repertoires and precisely identifying age-related changes can provide valuable information for better understanding the dynamics and size of TCR repertoires, both in terms of their biology and potential clinical applications.
The analysis of antigen specific TCR repertoires has the potential to yield valuable clinical insights. It can aid in assessing the status of past and present infections, guiding the selection of active and passive vaccination programs, and determining the optimal timing for administering boost vaccines. By leveraging TCR repertoire analysis, we can gain a deeper understanding of T cell immunity and explore potential clinical interventions. Overall, TCR repertoire analysis holds great promise in advancing our understanding of T cell immunity and its application in clinical settings.
Acknowledgments
This work was supported by the Intramural Research Programs of the NIH (AG000499). The author would like to thank Xiaoping Sun, Thomas Nguyen, and Achouak Achour for their excellent work, and Beverly Baptiste for proofreading the manuscript.
Footnotes
Conflict of interest
The authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334(6181):395–402. [DOI] [PubMed] [Google Scholar]
- 2.Nicolas P, Ollier J, Mori D, Voisinne G, Celis-Gutierrez J, Gregoire C, et al. Systems-level conservation of the proximal TCR signaling network of mice and humans. J Exp Med. 2022;219(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folch G, Lefranc MP. The human T cell receptor beta diversity (TRBD) and beta joining (TRBJ) genes. Exp Clin Immunogenet. 2000;17(2):107–14. [DOI] [PubMed] [Google Scholar]
- 4.Folch G, Lefranc MP. The human T cell receptor beta variable (TRBV) genes. Exp Clin Immunogenet. 2000;17(1):42–54. [DOI] [PubMed] [Google Scholar]
- 5.Scaviner D, Lefranc MP. The human T cell receptor alpha joining (TRAJ) genes. Exp Clin Immunogenet. 2000;17(2):97–106. [DOI] [PubMed] [Google Scholar]
- 6.Scaviner D, Lefranc MP. The human T cell receptor alpha variable (TRAV) genes. Exp Clin Immunogenet. 2000;17(2):83–96. [DOI] [PubMed] [Google Scholar]
- 7.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4(2):123–32. [DOI] [PubMed] [Google Scholar]
- 8.Murugan A, Mora T, Walczak AM, Callan CG, Jr. Statistical inference of the generation probability of T-cell receptors from sequence repertoires. Proc Natl Acad Sci U S A. 2012;109(40):16161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarnitsyna VI, Evavold BD, Schoettle LN, Blattman JN, Antia R. Estimating the diversity, completeness, and cross-reactivity of the T cell repertoire. Front Immunol. 2013;4:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupic T, Marcou Q, Walczak AM, Mora T. Genesis of the alphabeta T-cell receptor. PLoS Comput Biol. 2019;15(3):e1006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins MK, Chu HH, McLachlan JB, Moon JJ. On the composition of the preimmune repertoire of T cells specific for Peptide-major histocompatibility complex ligands. Annu Rev Immunol. 2010;28:275–94. [DOI] [PubMed] [Google Scholar]
- 12.Jiang N, Malone M, Chizari S. Antigen-specific and cross-reactive T cells in protection and disease. Immunol Rev. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazenberg MD, Borghans JA, de Boer RJ, Miedema F. Thymic output: a bad TREC record. Nat Immunol. 2003;4(2):97–9. [DOI] [PubMed] [Google Scholar]
- 14.den B I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36(2):288–97. [DOI] [PubMed] [Google Scholar]
- 15.Palmer DB. The effect of age on thymic function. Front Immunol. 2013;4:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–62. [DOI] [PubMed] [Google Scholar]
- 17.Akondy RS, Fitch M, Edupuganti S, Yang S, Kissick HT, Li KW, et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature. 2017;552(7685):362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Broek T, Borghans JAM, van Wijk F. The full spectrum of human naive T cells. Nat Rev Immunol. 2018;18(6):363–73. [DOI] [PubMed] [Google Scholar]
- 19.Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol. 2007;81(14):7759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colonna-Romano G, Akbar AN, Aquino A, Bulati M, Candore G, Lio D, et al. Impact of CMV and EBV seropositivity on CD8 T lymphocytes in an old population from West-Sicily. Exp Gerontol. 2007;42(10):995–1002. [DOI] [PubMed] [Google Scholar]
- 21.Yanes RE, Gustafson CE, Weyand CM, Goronzy JJ. Lymphocyte generation and population homeostasis throughout life. Semin Hematol. 2017;54(1):33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lythe G, Molina-Paris C. Some deterministic and stochastic mathematical models of naive T-cell homeostasis. Immunol Rev. 2018;285(1):206–17. [DOI] [PubMed] [Google Scholar]
- 23.Drobek A, Moudra A, Mueller D, Huranova M, Horkova V, Pribikova M, et al. Strong homeostatic TCR signals induce formation of self-tolerant virtual memory CD8 T cells. EMBO J. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goronzy JJ, Qi Q, Olshen RA, Weyand CM. High-throughput sequencing insights into T-cell receptor repertoire diversity in aging. Genome Med. 2015;7(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114(19):4099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman JD, Warren RL, Webb JR, Nelson BH, Holt RA. Profiling the T-cell receptor beta-chain repertoire by massively parallel sequencing. Genome Res. 2009;19(10):1817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barennes P, Quiniou V, Shugay M, Egorov ES, Davydov AN, Chudakov DM, et al. Benchmarking of T cell receptor repertoire profiling methods reveals large systematic biases. Nat Biotechnol. 2021;39(2):236–45. [DOI] [PubMed] [Google Scholar]
- 28.Shugay M, Britanova OV, Merzlyak EM, Turchaninova MA, Mamedov IZ, Tuganbaev TR, et al. Towards error-free profiling of immune repertoires. Nat Methods. 2014;11(6):653–5. [DOI] [PubMed] [Google Scholar]
- 29.Ma KY, He C, Wendel BS, Williams CM, Xiao J, Yang H, et al. Immune Repertoire Sequencing Using Molecular Identifiers Enables Accurate Clonality Discovery and Clone Size Quantification. Front Immunol. 2018;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Simone M, Rossetti G, Pagani M. Single Cell T Cell Receptor Sequencing: Techniques and Future Challenges. Front Immunol. 2018;9:1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pai JA, Satpathy AT. High-throughput and single-cell T cell receptor sequencing technologies. Nat Methods. 2021;18(8):881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thierry Mora AMW. Quantifying lymphocyte receptor diversity. In: Jayajit Das CJ, editor. Systems Immunology. 1st Edition ed. Boca Raton: CRC Press; 2019. p. 16. [Google Scholar]
- 33.Laydon DJ, Bangham CR, Asquith B. Estimating T-cell repertoire diversity: limitations of classical estimators and a new approach. Philos Trans R Soc Lond B Biol Sci. 2015;370(1675). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venturi V, Kedzierska K, Turner SJ, Doherty PC, Davenport MP. Methods for comparing the diversity of samples of the T cell receptor repertoire. J Immunol Methods. 2007;321(1–2):182–95. [DOI] [PubMed] [Google Scholar]
- 35.Chiffelle J, Genolet R, Perez MA, Coukos G, Zoete V, Harari A. T-cell repertoire analysis and metrics of diversity and clonality. Curr Opin Biotechnol. 2020;65:284–95. [DOI] [PubMed] [Google Scholar]
- 36.Robins HS, Srivastava SK, Campregher PV, Turtle CJ, Andriesen J, Riddell SR, et al. Overlap and effective size of the human CD8+ T cell receptor repertoire. Sci Transl Med. 2010;2(47):47ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee JY, et al. Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci U S A. 2014;111(36):13139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li HM, Hiroi T, Zhang Y, Shi A, Chen G, De S, et al. TCRbeta repertoire of CD4+ and CD8+ T cells is distinct in richness, distribution, and CDR3 amino acid composition. J Leukoc Biol. 2016;99:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun X, Nguyen T, Achour A, Ko A, Cifello J, Ling C, et al. Longitudinal analysis reveals age-related changes in the T cell receptor repertoire of human T cell subsets. J Clin Invest. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golding A, Darko S, Wylie WH, Douek DC, Shevach EM. Deep sequencing of the TCRbeta repertoire of human forkhead box protein 3 (FoxP3)+ and FoxP3− T cells suggests that they are completely distinct and non-overlapping. Clin Exp Immunol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmadzadeh M, Pasetto A, Jia L, Deniger DC, Stevanovic S, Robbins PF, et al. Tumor-infiltrating human CD4(+) regulatory T cells display a distinct TCR repertoire and exhibit tumor and neoantigen reactivity. Sci Immunol. 2019;4(31). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasatskaya SA, Ladell K, Egorov ES, Miners KL, Davydov AN, Metsger M, et al. Functionally specialized human CD4(+) T-cell subsets express physicochemically distinct TCRs. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soto C, Bombardi RG, Kozhevnikov M, Sinkovits RS, Chen EC, Branchizio A, et al. High Frequency of Shared Clonotypes in Human T Cell Receptor Repertoires. Cell Rep. 2020;32(2):107882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanno H, Gould TM, McDaniel JR, Cao W, Tanno Y, Durrett RE, et al. Determinants governing T cell receptor alpha/beta-chain pairing in repertoire formation of identical twins. Proc Natl Acad Sci U S A. 2020;117(1):532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madi A, Poran A, Shifrut E, Reich-Zeliger S, Greenstein E, Zaretsky I, et al. T cell receptor repertoires of mice and humans are clustered in similarity networks around conserved public CDR3 sequences. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen G, Yang X, Ko A, Sun X, Gao M, Zhang Y, et al. Sequence and Structural Analyses Reveal Distinct and Highly Diverse Human CD8+ TCR Repertoires to Immunodominant Viral Antigens. Cell Rep. 2017;19(3):569–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song I, Gil A, Mishra R, Ghersi D, Selin LK, Stern LJ. Broad TCR repertoire and diverse structural solutions for recognition of an immunodominant CD8+ T cell epitope. Nat Struct Mol Biol. 2017;24(4):395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carter JA, Preall JB, Grigaityte K, Goldfless SJ, Jeffery E, Briggs AW, et al. Single T Cell Sequencing Demonstrates the Functional Role of alphabeta TCR Pairing in Cell Lineage and Antigen Specificity. Front Immunol. 2019;10:1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toya T, Taguchi A, Kitaura K, Misumi F, Nakajima Y, Otsuka Y, et al. T-cell receptor repertoire of cytomegalovirus-specific cytotoxic T-cells after allogeneic stem cell transplantation. Sci Rep. 2020;10(1):22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Link CS, Eugster A, Heidenreich F, Rucker-Braun E, Schmiedgen M, Oelschlagel U, et al. Abundant cytomegalovirus (CMV) reactive clonotypes in the CD8(+) T cell receptor alpha repertoire following allogeneic transplantation. Clin Exp Immunol. 2016;184(3):389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi Q, Cavanagh MM, Le Saux S, NamKoong H, Kim C, Turgano E, et al. Diversification of the antigen-specific T cell receptor repertoire after varicella zoster vaccination. Sci Transl Med. 2016;8(332):332ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274(5284):94–6. [DOI] [PubMed] [Google Scholar]
- 53.Dash P, Fiore-Gartland AJ, Hertz T, Wang GC, Sharma S, Souquette A, et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature. 2017;547(7661):89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glanville J, Huang H, Nau A, Hatton O, Wagar LE, Rubelt F, et al. Identifying specificity groups in the T cell receptor repertoire. Nature. 2017;547(7661):94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bradley P Structure-based prediction of T cell receptor:peptide-MHC interactions. Elife. 2023;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin R, Ribeiro-Filho HV, Lin V, Gowthaman R, Cheung M, Pierce BG. TCRmodel2: high-resolution modeling of T cell receptor recognition using deep learning. Nucleic Acids Res. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhuo Y, Yang X, Shuai P, Yang L, Wen X, Zhong X, et al. Evaluation and comparison of adaptive immunity through analyzing the diversities and clonalities of T-cell receptor repertoires in the peripheral blood. Front Immunol. 2022;13:916430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeWitt WS 3rd, Smith A, Schoch G, Hansen JA, Matsen FAt, Bradley P. Human T cell receptor occurrence patterns encode immune history, genetic background, and receptor specificity. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin Y, Kim J, Metter EJ, Nguyen H, Truong T, Lustig A, et al. Changes in blood lymphocyte numbers with age in vivo and their association with the levels of cytokines/cytokine receptors. Immun Ageing. 2016;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alpert A, Pickman Y, Leipold M, Rosenberg-Hasson Y, Ji X, Gaujoux R, et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat Med. 2019;25(3):487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Britanova OV, Putintseva EV, Shugay M, Merzlyak EM, Turchaninova MA, Staroverov DB, et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol. 2014;192(6):2689–98. [DOI] [PubMed] [Google Scholar]
- 62.Britanova OV, Shugay M, Merzlyak EM, Staroverov DB, Putintseva EV, Turchaninova MA, et al. Dynamics of Individual T Cell Repertoires: From Cord Blood to Centenarians. J Immunol. 2016;196(12):5005–13. [DOI] [PubMed] [Google Scholar]
- 63.de Greef PC, Oakes T, Gerritsen B, Ismail M, Heather JM, Hermsen R, et al. The naive T-cell receptor repertoire has an extremely broad distribution of clone sizes. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Egorov ES, Kasatskaya SA, Zubov VN, Izraelson M, Nakonechnaya TO, Staroverov DB, et al. The Changing Landscape of Naive T Cell Receptor Repertoire With Human Aging. Front Immunol. 2018;9:1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshida K, Cologne JB, Cordova K, Misumi M, Yamaoka M, Kyoizumi S, et al. Aging-related changes in human T-cell repertoire over 20years delineated by deep sequencing of peripheral T-cell receptors. Exp Gerontol. 2017;96:29–37. [DOI] [PubMed] [Google Scholar]
- 66.Chu ND, Bi HS, Emerson RO, Sherwood AM, Birnbaum ME, Robins HS, et al. Longitudinal immunosequencing in healthy people reveals persistent T cell receptors rich in highly public receptors. BMC Immunol. 2019;20(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gil A, Yassai MB, Naumov YN, Selin LK. Narrowing of human influenza A virus-specific T cell receptor alpha and beta repertoires with increasing age. J Virol. 2015;89(8):4102–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clark F, Gil A, Thapa I, Aslan N, Ghersi D, Selin LK. Cross-reactivity influences changes in human influenza A virus and Epstein Barr virus specific CD8 memory T cell receptor alpha and beta repertoires between young and old. Front Immunol. 2022;13:1011935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sant S, Grzelak L, Wang Z, Pizzolla A, Koutsakos M, Crowe J, et al. Single-Cell Approach to Influenza-Specific CD8(+) T Cell Receptor Repertoires Across Different Age Groups, Tissues, and Following Influenza Virus Infection. Front Immunol. 2018;9:1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169(4):1984–92. [DOI] [PubMed] [Google Scholar]
- 71.Lindau P, Mukherjee R, Gutschow MV, Vignali M, Warren EH, Riddell SR, et al. Cytomegalovirus Exposure in the Elderly Does Not Reduce CD8 T Cell Repertoire Diversity. J Immunol. 2019;202(2):476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


