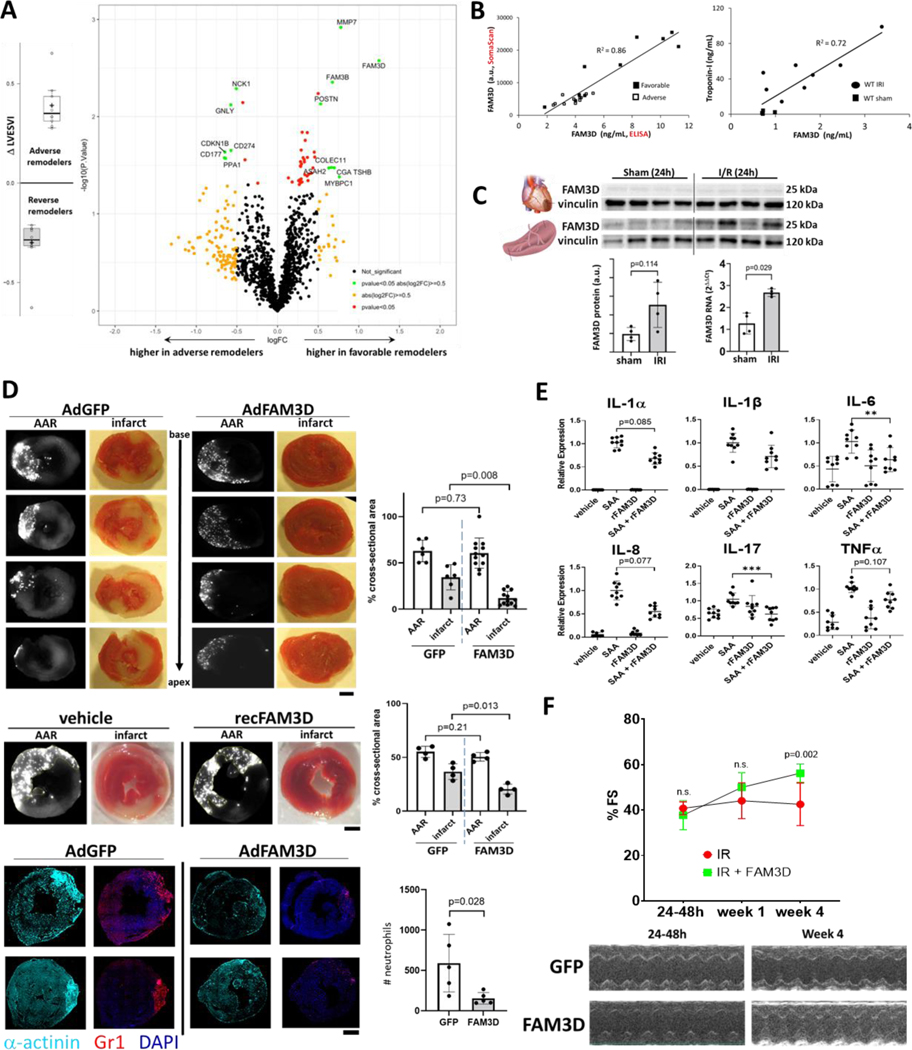
Pathophysiological mechanisms underlying adverse cardiac remodeling after myocardial ischemia-reperfusion injury (IRI) are incompletely understood. Although large infarct size and impaired function at the time of myocardial infarction (MI) are strong predictors of eventual heart failure (HF), even patients with initially preserved function can experience late adverse remodeling that leads to HF1. Here we performed plasma proteomics in a subgroup of patients enrolled in the OMEGA-REMODEL trial2 presenting with acute ST-elevation MI and successfully reperfused with primary PCI. Adverse or favorable cardiac remodeling after MI was defined as a 20% increase or decrease, respectively, in left ventricular end-systolic volume index (LVESVI) from 2–4 weeks to 6 months after MI as assessed by cardiac MRI. We selected 11 adverse and 10 favorable remodelers who were matched for initial left ventricular mass, function, and infarct size, and shared similar demographic and clinical characteristics. Plasma samples taken at the time of the initial MRI were analyzed with the 1.3K SomaScan platform. The volcano plot [A] shows the 14 candidate molecules (green dots) whose difference in abundance between favorable and adverse remodelers satisfied threshold values for significance (unadjusted p<0.05) and fold-change (FC>1.4). The cytokine FAM3D (Family with sequence similarity 3D) was elevated in favorable remodelers and exhibited the highest overall fold change. This secreted factor binds to formyl-peptide receptors (FPR) expressed predominantly on neutrophils and monocytes, and regulates their trafficking3. ELISA for FAM3D closely correlated with SomaScan measurements and confirmed the difference between the two groups [B].
Based on our human findings, we looked at FAM3D (or Oit1) in adult mice after 30 minutes of left anterior descending (LAD) coronary artery ligation followed by various periods of reperfusion. Plasma FAM3D levels after 8h of reperfusion correlated with troponin-I levels [B]. Comprehensive organ harvest 24h after reperfusion revealed the spleen as a likely source of increased circulating FAM3D [C], consistent with prior studies showing cross-talk between ischemic myocardium and hematopoietic tissues4, although the difference in protein did not reach statistical significance.
We tested the effects of FAM3D overexpression both before and after IRI. Mice were injected with adenovirus encoding CMV-driven FAM3D (1e12 ifu/mouse), which is taken up by multiple organs and achieves FAM3D plasma levels (~1nM) about three-fold higher than our human favorable remodelers, one week prior to IRI. They showed dramatically reduced infarcts 24h after reperfusion despite identical areas of underperfusion as control animals (AAR, area at risk) [D, top]. Infarct reduction was also observed in mice receiving recombinant FAM3D 30 minutes before IRI [D, middle]. Neutrophils constitute the initial inflammatory response to reperfusion injury and FAM3D overexpression significantly decreased their myocardial infiltration 12h after reperfusion [D, bottom]. Neutrophils treated in vitro with serum amyloid A (SAA), an acute phase reactant elevated in MI that binds to FPR5, displayed a marked induction of pro-inflammatory cytokines which trended downwards with co-administered FAM3D [E]. Finally, in order to assess the efficacy of a more clinically relevant intervention, mice were injected with FAM3D 24 hours after reperfusion. While initial fractional shortening (%FS) was similarly impaired in both groups, FAM3D enhanced cardiac functional recovery by four weeks [F]. Taken together, these data show that FAM3D plays an important role in limiting cardiac injury, curbing inflammation, and promoting recovery of heart function after ischemic injury.
Figure.
A, Stratification of post-MI remodelers by change in LVESVI as assessed by serial cardiac MRI. Volcano plot showing differentially expressed proteins between adverse and favorable remodelers. B, Correlations between aptamer-based SomaScan and ELISA (Abnova) measurements of FAM13D and between plasma FAM3D and troponin-I levels 8h after cardiac reperfusion. C, Western blots of murine heart and splenic FAM3D 24h after cardiac IRI, with quantification of splenic FAM3D protein (normalized to vinculin, Image Lab software, Biorad) and RNA. n=4 mice per group. p-values calculated with Mann-Whitney test. D, Mice were injected with either adenoviral GFP (n=6) or FAM3D (n=12) (1e12 ifu/mouse) one week prior to IRI, or vehicle (n=4) or recombinant FAM3D (0.4μg/gBW, n=4) 30 minutes prior to IRI. Heart sections collected after 24h of reperfusion were quantified for areas of underperfusion lacking fluorescent microspheres (area-at-risk, AAR, grayscale images) and infarct areas (red 2,3,5-triphenyltetrazolium chloride stained images). Hearts collected after 12h of reperfusion were stained for neutrophils in red (anti-mouse Gr-1 (Ly-6G/Ly-6C)). Blue = DAPI; turquoise=mouse alpha-actinin. (Leica LAS X, ImageJ software). n=5 per group. scale bars = 1mm. E, Human peripheral neutrophils were treated with 100nM serum amyloid A and 100nM recombinant FAM3D, either alone or in combination, for 3 hours. **p<0.01, ***p<0.001 using Kruskal-Wallis test. F, Fractional shortening and M-mode echocardiographs (GE VividE90) from mice injected with recombinant FAM3D (1μg/gBW i.p., daily for three days) and adenoviral FAM3D 24h after reperfusion. n=8 per group.
Sources of Funding:
This research was supported by grants from the NIH (A.Rosenzweig [R01AG061034, R35HL155318], J.Rhee [K08HL140200]), the American Heart Association (A. Rosenzweig [AHA MERIT Award]), and the Foundation for Anesthesia Education and Research (J.Rhee [FAER Mentored Research Training Grant]).
Footnotes
Disclosures: None.
Data Availability
All methods and reagents are available upon request. Proteomic datasets, analysis, and human study details can be found at Zenodo.org (https://zenodo.org/record/8132248). All animal studies (14- to 16-week-old male C57BL/6J mice were purchased from Jackson Laboratory) complied with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the MGH Animal Care and Use Committee. All surgeries and analyses were performed by investigators blinded to treatment. For SOMAscan, median normalized relative abundances of the 1305 analytes were imported into R (version 3.3.2) using the SomaDataIO package, and were analyzed using empirical Bayesian Analysis (LIMMA, Bioconductor package) that accounted for distribution of global protein expression, outliers, and likely false positives. Data are presented as mean±S.D. and analyzed using GraphPad Prism 8. p<0.05 considered significant using Student’s t-test unless otherwise indicated.
References
- 1.Schachinger V, Assmus B, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Yu J, Corti R, Mathey DG, Hamm CW, et al. Intracoronary infusion of bone marrow-derived mononuclear cells abrogates adverse left ventricular remodelling post-acute myocardial infarction: insights from the reinfusion of enriched progenitor cells and infarct remodelling in acute myocardial infarction (REPAIR-AMI) trial. Eur J Heart Fail. 2009;11:973–979. doi: 10.1093/eurjhf/hfp113 [DOI] [PubMed] [Google Scholar]
- 2.Heydari B, Abdullah S, Pottala JV, Shah R, Abbasi S, Mandry D, Francis SA, Lumish H, Ghoshhajra BB, Hoffmann U, et al. Effect of Omega-3 Acid Ethyl Esters on Left Ventricular Remodeling After Acute Myocardial Infarction: The OMEGA-REMODEL Randomized Clinical Trial. Circulation. 2016;134:378–391. doi: 10.1161/CIRCULATIONAHA.115.019949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He L, Fu Y, Deng J, Shen Y, Wang Y, Yu F, Xie N, Chen Z, Hong T, Peng X, et al. Deficiency of FAM3D (Family With Sequence Similarity 3, Member D), A Novel Chemokine, Attenuates Neutrophil Recruitment and Ameliorates Abdominal Aortic Aneurysm Development. Arterioscler Thromb Vasc Biol. 2018;38:1616–1631. doi: 10.1161/ATVBAHA.118.311289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maier W, Altwegg LA, Corti R, Gay S, Hersberger M, Maly FE, Sutsch G, Roffi M, Neidhart M, Eberli FR, et al. Inflammatory markers at the site of ruptured plaque in acute myocardial infarction: locally increased interleukin-6 and serum amyloid A but decreased C-reactive protein. Circulation. 2005;111:1355–1361. doi: 10.1161/01.CIR.0000158479.58589.0A [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All methods and reagents are available upon request. Proteomic datasets, analysis, and human study details can be found at Zenodo.org (https://zenodo.org/record/8132248). All animal studies (14- to 16-week-old male C57BL/6J mice were purchased from Jackson Laboratory) complied with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the MGH Animal Care and Use Committee. All surgeries and analyses were performed by investigators blinded to treatment. For SOMAscan, median normalized relative abundances of the 1305 analytes were imported into R (version 3.3.2) using the SomaDataIO package, and were analyzed using empirical Bayesian Analysis (LIMMA, Bioconductor package) that accounted for distribution of global protein expression, outliers, and likely false positives. Data are presented as mean±S.D. and analyzed using GraphPad Prism 8. p<0.05 considered significant using Student’s t-test unless otherwise indicated.