Abstract
Gulf War Illness (GWI) collectively describes the multitude of central and peripheral disturbances affecting soldiers who served in the 1990–1991 Gulf War. While the mechanisms responsible for GWI remain elusive, the prophylactic use of the reversible acetylcholinesterase inhibitor, pyridostigmine bromide (PB), and war-related stress have been identified as chief factors in GWI pathology. Post-deployment stress is a common challenge faced by veterans, and aberrant cholinergic and/or immune responses to these psychological stressors may play an important role in GWI pathology, especially the cognitive impairments experienced by many GWI patients. Therefore, the current study investigated if an immobilization stress challenge would produce abnormal responses in PB-treated rats three-months later. Results indicate that hippocampal cholinergic responses to an immobilization stress challenge are impaired three months after PB administration. We also assessed if an immune or stress challenge reveals deficits in PB-treated animals during hippocampal-dependent learning and memory tasks at this delayed timepoint. Novel object recognition (NOR) testing paired with either acute saline or LPS (30 μg/kg, i.p.), as well as Morris water maze (MWM) testing was conducted approximately three months after PB administration and/or repeated restraint stress. Rats with a history of PB treatment exhibited 24-hour hippocampal-dependent memory deficits when challenged with LPS, but not saline, in the NOR task. Similarly, in the same cohort, PB-treated rats showed 24-hour memory deficits in the MWM task, irrespective of stress history. Ultimately, these studies highlight the long-term effects of PB treatment on hippocampal function and provide insight into the progressive cognitive deficits observed in veterans with GWI.
Keywords: acetylcholinesterase, pyridostigmine bromide, acetylcholine, hippocampus, lipopolysaccharide, stress
1. Introduction:
Gulf War Illness (GWI) is a multi-symptom illness that continues to affect over 250,000 American Gulf War (GW) veterans (Mawson and Croft, 2019). During and after the 1990–1991 GW, soldiers began experiencing a variety of symptoms ranging from musculoskeletal pain to respiratory impairments and neurological disturbances (Blanchard et al., 2005; Kang et al., 2009; Li et al., 2011). One of the most insidious aspects of GWI is the progressive cognitive impairments affecting patients’ memory, attention, and mood (Hubbard et al., 2014; Tillman et al., 2017; White et al., 2016). To date, the pathophysiology of GWI remains elusive, but many symptoms have been linked to a variety of environmental, physiological, and pharmacological exposures sustained during deployment (Steele, 2000). While soldiers were exposed to several hazards in the Gulf, the prophylactic use of the reversible acetylcholinesterase (AChE) inhibitor, pyridostigmine bromide (PB), and war-related stress have been identified as chief factors in GWI pathology (Golomb, 2008; Haley et al., 1997; Steele et al., 2012; Sullivan et al., 2003; White et al., 2001; White et al., 2016). PB was prescribed to soldiers as a protective measure against potential nerve gas attacks; mainly irreversible acetylcholinesterase inhibitors such as sarin gas (Gordon et al., 1978; von Bredow et al., 1991). Various studies since the GW have proposed that the physical and psychological stress associated with deployment may exacerbate the peripheral and central effects of PB as both exposures alter cholinergic signaling, a key regulator of cognition and immune responses (Steele et al., 2012; Sullivan et al., 2003; White et al., 2016).
Such observations provided the framework for our previous studies in which we established a rat model of GWI by combining 14 days of PB treatment with 10 days of repeated restraint stress (RRS) (Burzynski et al., 2022; Macht et al., 2020; Macht et al., 2018; Macht et al., 2019). The goal of these previous studies was to determine if this interaction elicits cholinergic dysfunction in the peripheral and central nervous systems (CNS) in ways that mimic the changes observed in veterans with GWI. Importantly, our studies evaluated the immediate and long-lasting effects of PB and stress as many GWI symptoms continue to worsen as veterans age (Blanchard et al., 2005; Kang et al., 2009; Li et al., 2011; Nettleman, 2015). Most recently, we found that three months after PB and stress exposure, PB-treated rats exhibit an unexpected increase in plasma cholinesterase activity and altered immune responses in both the periphery and CNS (Burzynski et al., 2022). Moreover, relative to vehicle treatment, rats with a history of PB treatment show enhanced pro-inflammatory responses to an acute immune challenge (30 μg/kg lipopolysaccharide: LPS) in plasma as well as hippocampal homogenates (Burzynski et al., 2022), an essential integration center for learning and memory (McEwen, 2012). Interestingly, at this delayed timepoint, PB-treated animals exhibit elevated hippocampal acetylcholine (ACh) efflux when responding to an acute LPS challenge (Burzynski et al., 2022). As ACh is known to exert anti-inflammatory actions (Hoover, 2017), these results highlight PB’s long-term dysregulation of the cholinergic anti-inflammatory system, particularly when stimulated by an immune challenge. Such findings are consistent with clinical studies that report greater immune activation and cognitive impairment in GWI patients following a stressor, such as an exercise challenge (Broderick et al., 2013; Broderick et al., 2011; Whistler et al., 2009).
Similar to immune responses, dysregulated stress responses have also been observed in GWI patients and are known to impair cognition (Rayhan et al., 2013; Washington et al., 2020). In view of these clinical and preclinical observations, here we used in vivo microdialysis during an acute immobilization stress to investigate if a stress challenge produces the same dysregulated cholinergic responses observed in our LPS studies. As we hypothesize that such cholinergic impairments may be mediating the progressive cognitive deficits observed in GWI patients, we also assessed hippocampal-dependent learning and memory following an immune or stress challenge. We first assessed hippocampal-dependent memory with the novel object recognition (NOR) task using an acute immune challenge and a 24-hour intertrial interval (ITI) in a delayed cohort (i.e., three months post treatment). We then assessed the long-term effects of our treatment paradigm on hippocampal-dependent learning and memory with the Morris water maze (MWM). Our objective was to assess the potential role of stressors, namely immune or stress challenges, in the development of cognitive-behavioral deficits in our GWI model, thereby providing insight into how the long-lasting deficits in the hippocampal cholinergic system may be contributing to the cognitive deficits in veterans with GWI.
2. Materials and Methods:
2.1. Animals
Adult male Sprague Dawley rats (Envigo, 200g, approximately six weeks old) were individually housed at the University of South Carolina School of Medicine animal facility with irradiated Sani-Chips wood bedding (P.J. Murphy Forest Products Corp.) and maintained on a 12/12hr light-dark cycle (lights on at 7:00A.M.) at 22°C. Rats were given ad libitum access to food and water and provided Nylabones® for enrichment. This study focused on male rats as over 95% of GWI patients are males (Nettleman, 2015). Animals were given one week to acclimate before the GWI treatment paradigm began. All procedures were performed in accordance with all guidelines and regulations of the Dorn VA Animal Care and Use Committee.
2.2. GWI paradigm
Our previously established rat model of GWI consists of a 14-day treatment paradigm of PB with 10 days of RRS (Burzynski et al., 2022; Macht et al., 2020; Macht et al., 2018; Macht et al., 2019). Specifically, animals received vehicle (sterile water) or PB (1.3 mg/kg bodyweight, prepared daily) by oral gavage for 14 consecutive days. We have previously shown this dose inhibits plasma cholinesterase activity by approximately 50%, mimicking the dose prescribed to soldiers (Marino et al., 1998). On day 5, immediately following gavage, half of the animals underwent restraint stress in mesh restrainers for six hours per day (10:00A.M.−4:00P.M.) for 10 consecutive days. Nonstressed control rats were handled daily and returned to their home cage. This 2 × 2 design created the following 4 treatment groups; 1) non-stressed control rats treated with vehicle (vehicle-NSC), 2) PB-treated, non-stressed rats (PB-NSC), 3) rats that received vehicle and underwent repeated restraint stress (vehicle-RRS), and 4) PB-treated rats subjected to repeated restraint stress (PB-RRS). Rats undergoing stress conditions were housed in a separate room for the 14-day treatment paradigm. This room was maintained at the same conditions as the room that housed non-stressed controls. Restraint stress took place in the home cage to selectively examine the effects of this stressor without adding the additional stress of a cage change. After the completion of the treatment paradigm, all animals were housed in the same room for the duration of the study.
2.3. Cholinesterase assay
Cholinesterase activity was measured in hippocampal homogenates (20 μg) with a colorimetric assay (Abcam, ab#138871) following manufacturer’s instructions. Homogenates were prepared as described previously (Burzynski et al., 2022). The plate was read on a BioTek Synergy microplate reader (BioTek Instruments Inc.).
2.4. In vivo microdialysis
The in vivo microdialysis studies presented in the current study were collected from the same animals described previously (Burzynski et al., 2022). Briefly, approximately 100 days after the treatment paradigm, rats in cohort #1 were anesthetized with isoflurane and underwent stereotaxic surgery to place a guide cannula into the dorsal hippocampus. Interlocking intracerebral guide cannula and stylets from Bioanalytical Systems Incorporated (BASi: #MD-2251) were unilaterally implanted relative to bregma: AP, - 5.5mm; L, ± 4.0mm; DV, - 3.8mm at a 10° angle. Coordinates were based on Paxinos and Watson rat brain atlas with target accuracy validated for each rat post-mortem (Paxinos et al., 1980). Left and right hemispheres were counterbalanced across groups. Rats were left undisturbed for one full day following surgery for recovery, before beginning habituation to the microdialysis bowls. No differences in surgical recovery were observed in any group. Each rat in cohort #1 underwent two sessions of microdialysis as previously described (Macht et al., 2020; Macht et al., 2019). Briefly, rats were habituated to the microdialysis bowls in the BASi Raturn system for 20 hours over the course of four days. This habituation period ensured rats received a full week of recovery from guide cannula surgery before microdialysis began. At the start of each microdialysis session, BASi probes (2 mm, MD-2200) were placed into the guide cannula and perfused with artificial cerebral spinal fluid (150 mM NaCl; 3 mM KCl; 1.7 mM CaCl2H2O; 0.183 mM MgCl26H2O; 5 mM D-glucose) with 100 nM neostigmine at a rate of 2 μL/min. The first microdialysis session included an acute LPS challenge (30 μg/kg, intraperitoneal (i.p.) injection) as described previously (Burzynski et al., 2022). Rats were given one day of rest before undergoing a second microdialysis session 48-hours later. The first three hours (8:00 am-11:00 am) of collection from both microdialysis sessions were discarded to allow for recovery from probe insertion. Collections were then taken at 15-minute intervals with the first four collections serving as baseline measurements. Rats were placed in a plexiglass restrainer at the start of the 5th collection and were removed from the restrainer at the end of the 8th collection for a total of 1-hour of immobilization. Collections continued for 1-hour after immobilization. Samples were immediately frozen and stored at −80°C at the end of each collection until analysis. It is important to note that this restrainer is different from the mesh restrainers used during the GWI treatment paradigm and this immobilization session takes place in the microdialysis bowls instead of the home cage. Therefore, this immobilization challenge is a novel stressor to all animals.
2.5. Transcardial perfusion
Following the second session of microdialysis, rats in cohort #1 were anesthetized with isoflurane and transcardially perfused with 0.1M phosphate buffered saline followed by 4% paraformaldehyde in 0.1M phosphate buffer. Brains were removed and placed in a 30% sucrose/0.1M phosphate buffer solution at 4°C for several days and then rapidly frozen using isopentane on dry ice and stored at −80°C. A sliding microtome was used to cut 40 μm coronal sections to verify probe placement in each rat as shown previously (Macht et al., 2019).
2.6. High performance liquid chromatography (HPLC)
ACh concentration in dialysate samples was measured as previously described (Burzynski et al., 2022; Calva et al., 2018; Fadel et al., 2005; Macht et al., 2019). Briefly, dialysate samples were thawed individually and 20 μL loaded onto an Eicom AC-GEL reverse-phase analytical column, where choline and ACh were isolated from other biogenic compounds in interaction with a mobile phase consisting of 50 mM potassium bicarbonate, 300 mg/L sodium decanesulfonate, and 50 mg/mL 2Na EDTA, pH 8.4. A dual enzymatic column AC-ENZYM II from Eicom metabolized ACh into hydrogen peroxide by acetylcholinesterase and choline oxidase. An applied potential of +450 mV oxidized the hydrogen peroxide at the platinum electrochemical detector. The current was read with the Eicom HT-500 detector system with a detection limit of 10 fmol and a retention time of 15 minutes. Concentration of ACh in samples was interpolated against a three-point standard curve.
2.7. Novel object recognition task
On days 24–29 (approximately three months old) and 100–110 (approximately 6 months old) of the GWI paradigm, a separate cohort of rats (cohort #2) underwent NOR testing during the early portion of the light cycle (9:00A.M.−12:00P.M.). The arena was 60 cm × 60 cm with 35 cm walls. Luminosity was maintained at 45 lux throughout the arena. All objects were made of ceramic or glass, approximately 7 cm × 12 cm and secured to the arena with magnets. Each set of familiar and novel objects had similar dimensions. The arena and objects were cleaned with 5% ammonium hydroxide between each animal. Behavior was recorded and locomotor activity was measured with EthoVision XT 15 software (Noldus, Leesburg, VA, USA).
2.7.1. Habituation
Rats were given 3 days of acclimation to the testing arena for 5 minutes per day. Animals were also habituated to an i.p. injection by receiving 1 mL/kg sterile saline (i.p.) 15 minutes before each habituation session. These habitual injections were meant to remove any injection stress during the immune challenge presented in the third NOR session.
2.7.2. Session 1
After habituation, the first NOR session occurred approximately 28 days post treatment. Animals received an i.p. injection of 1 mL/kg sterile saline 15 minutes prior to initiation of the test. The 5-minute test (trial 1) consisted of two identical objects placed in opposite corners, approximately 25 cm from wall. Twenty-four hours later, animals underwent trial 2 with no injection. This 5-minute test consisted of one familiar object from the previous day and one novel object in the same locations as trial 1. Locations of familiar and novel objects were counterbalanced across groups.
2.7.3. Session 2
Approximately three months after treatment cessation, animals in cohort #2 underwent additional NOR testing following the same habituation and testing protocol as session 1. Briefly, animals underwent three days of habituation to the arena for 5 minutes per day. Animals were also habituated to an intraperitoneal (i.p.) injection by receiving 1 mL/kg i.p. sterile saline 15 minutes before each habituation session. On the fourth day of session 2, animals received an i.p. injection of 1 mL/kg sterile saline 15 minutes before the test began. The 5-minute test (trial 1) consisted of two identical objects (different object than those used in session 1) placed in opposite corners, approximately 25 cm from wall. Twenty-four hours later, animals underwent trial 2 with no injection. This 5-minute test consisted of one familiar object from the previous day and one novel object in the same locations as trial 1. Locations of familiar and novel objects were counterbalanced across groups.
2.7.4. Session 3
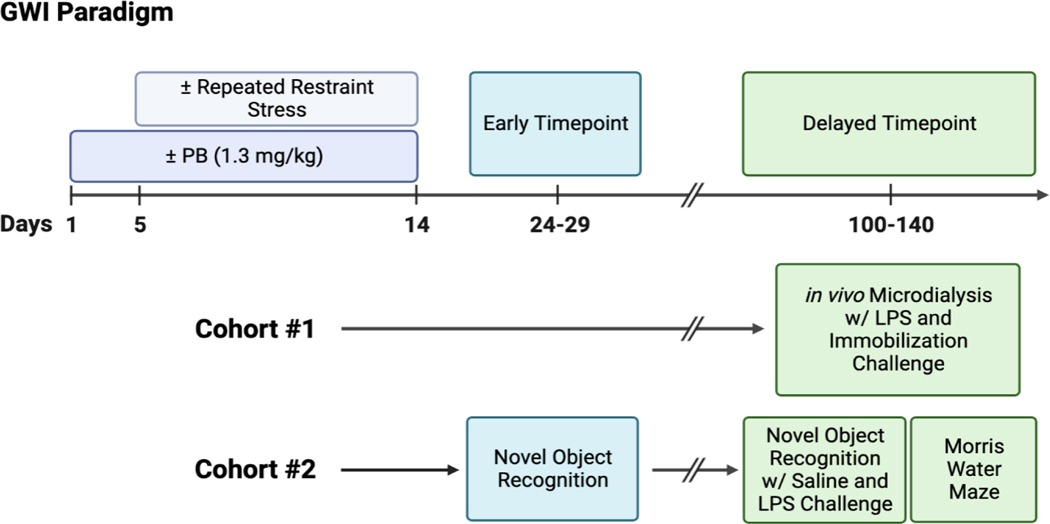
Animals in cohort #2 were given 2 days of rest after session 2 before undergoing another habituation session with 1 mL/kg i.p. saline injection. The following day, animals received an i.p. injection of 30 μg/kg LPS 15 minutes before the test began. The 5-minute test (trial 1) followed the same protocol as session 1, trial 1 with two new identical objects. Trial 2 occurred 24 hours after trial 1 and followed the same protocol as session 1, trial 2 with one familiar object from the previous day and a different novel object. Locations of familiar and novel objects were counterbalanced across groups. See Figure 1 for experimental timeline. f
Figure 1: Experimental Timeline.
All rats underwent the GWI paradigm with 2 levels of drug treatment (vehicle, PB) and 2 levels of stress (non-stressed controls, repeated restraint stress). Cohort #1 underwent in vivo microdialysis approximately 100 days after treatment cessation. The first session of microdialysis included an acute LPS challenge and is discussed in a previous study (Burzynski et al., 2022). The second session of microdialysis discussed in this study occurred 48-hours later and included a 1-hour immobilization stress challenge. Cohort #2 underwent Session 1 of NOR testing approximately 10 days after treatment cessation which included 3 days of habituation, familiar object presentation on day 4, and novel object presentation on day 5 (24-hour intertrial interval). Cohort #2 underwent an additional 8 days of NOR testing approximately 3 months later which included an acute saline challenge before familiar object presentation (Session 2) and an acute LPS challenge before familiar object presentation (Session 3) the following week. Approximately two weeks after the final NOR session, the same cohort of rats underwent Morris water maze testing for a total of 6 days. Illustration made with biorender.com.
2.7.5. Scoring
The time spent exploring each object was scored manually by two lab members blinded to the animals’ treatment histories and the other member’s analysis. Exploration was defined as the nose of the rat actively touching the object or being in close proximity (approximately 2 cm or less) while the nose was oriented toward the object. If a rat used the object to rear but was looking around the arena, this interaction was not considered exploratory. The percent of time spent exploring the novel object was calculated by dividing the time spent exploring the novel object by the total time spent exploring both the novel and familiar object, multiplied by 100.
2.8. Morris water maze testing
2.8.1. Apparatus
MWM testing took place approximately two weeks after session three of NOR testing (roughly 135 days post treatment). Testing was conducted in a 1.76 m diameter pool filled with 27°C water made opaque with non-toxic, white, tempera paint. The pool was surrounded by a white curtain that contained black geometric patterns in each quadrant that served as visual cues. Behavior was recorded and performance measures were assessed with EthoVision XT 15 software (Noldus, Leesburg, VA, USA).
2.8.2. Place learning procedure
Animals underwent four days of training to find the hidden platform, submerged 2 cm under the water in the middle of the southwest quadrant. Each trial began with the rat being placed in the pool at a different starting position (north, south, east, or west) facing the wall of the pool. Animals were given 1-minute to find the hidden platform. If animals were unable to find the platform in the allotted, time, they were guided to the platform where they remained for 15 seconds. Each day of training consisted of four trials, with 5-minutes between each trial. On the 4th day of training, animals also underwent a probe trial 1-hour after the 4th training trial. During the probe trial, the platform was lowered to the bottom of the pool, requiring the rat to swim for the entire 1-minute trial. After 1-minute, the platform was raised to allow the animal to escape and reinforce the trained behavior. A second probe trial was conducted 24-hours later, but animals did not undergo any training trials on this day. During training trials, escape latency was used to evaluate performance while the time spent swimming in the target quadrant was the metric used to evaluate performance during the probe trials. Swim speed was measured during all trials to ensure that there were no physical limitations of any animal or group.
2.8.3. Cue training procedure
Following training and probe trials, animals in cohort #2 underwent cue training to exclude any animals that may have visual impairments. All animals underwent six cue trials in which the platform was raised 1 cm above the water, making it fully visible. The position of the raised platform alternated between three quadrants (northwest, northeast, southeast) and rats were placed at a different starting position (north, south, east, or west) for each trial. Escape latency and path length were recorded for each cue trial.
2.9. Statistical analysis
In vivo microdialysis data were assessed with a 2 × 2 × 12 mixed ANOVA as previously described (Macht et al., 2020; Macht et al., 2019). Briefly, for between-subjects factors, this study had 2 levels of drug treatment (vehicle, PB) and 2 levels of stress (NSC, RRS). Within-subjects repeated measures consisted of 12 levels as there were 12 consecutive collections during the microdialysis session. Comparisons of basal levels of acetylcholine between the LPS and immobilization microdialysis sessions were analyzed by unpaired t-test. While efflux measurements were not corrected for probe recovery, previous studies have found these microdialysis probes to have a reliable recovery rate of 10–15% (Fadel et al., 2001) and any variations in probe recovery are accounted for by representing ACh efflux as a mean percent change from baseline. Successful discrimination between the familiar and novel object during NOR testing was assessed in each group by paired t-test. Comparisons of NOR performance between groups during the first session, as well as locomotor activity and total exploration, were assessed with a 2 × 2 ANOVAs. NOR performance, locomotor activity and total exploration in sessions 2 and 3 were assessed with 2 × 2 × 2 mixed ANOVAs. Between-subjects factors consisted of 2 levels of drug treatment and 2 levels of stress. Within-subjects repeated measures consisted of 2 levels, representing the immune challenge presented prior to testing (saline, LPS). MWM performance during the training trials was assessed with a 2 × 2 × 4 mixed ANOVA with between-subjects factors consisting of 2 levels of drug treatment and 2 levels of stress and the 4 days of training representing the within-subjects repeated measures. Performance in the probe trials was first assessed with a 2 × 2 × 4 ANOVA and the 4 quadrants representing the within-subjects repeated measures. One-sample t-tests were performed as follow-up to determine if the time spent in the target quadrant was significantly different than chance (15 seconds or 25% of trial) in each group. Performance in the probe trials was then analyzed by a 2 × 2 ANOVA to observe group differences. Locomotion during the probe trials and cue trials was also assessed by a 2 × 2 ANOVA. For all analyses, statistical significance was set at α= 0.05. Unless otherwise stated, following a significant interaction, post-hoc follow-ups were assessed with a Bonferroni-corrected simple main effects analyses. Post-hoc tests assessed all levels of drug treatment within each level of stress, and all levels of stress within each level of drug treatment across each level of time (in vivo microdialysis, MWM) or immune challenge (NOR sessions 2 and 3) when applicable.
3. Results
3.1. Prior history of PB treatment impairs recovery of hippocampal acetylcholine from stress challenge
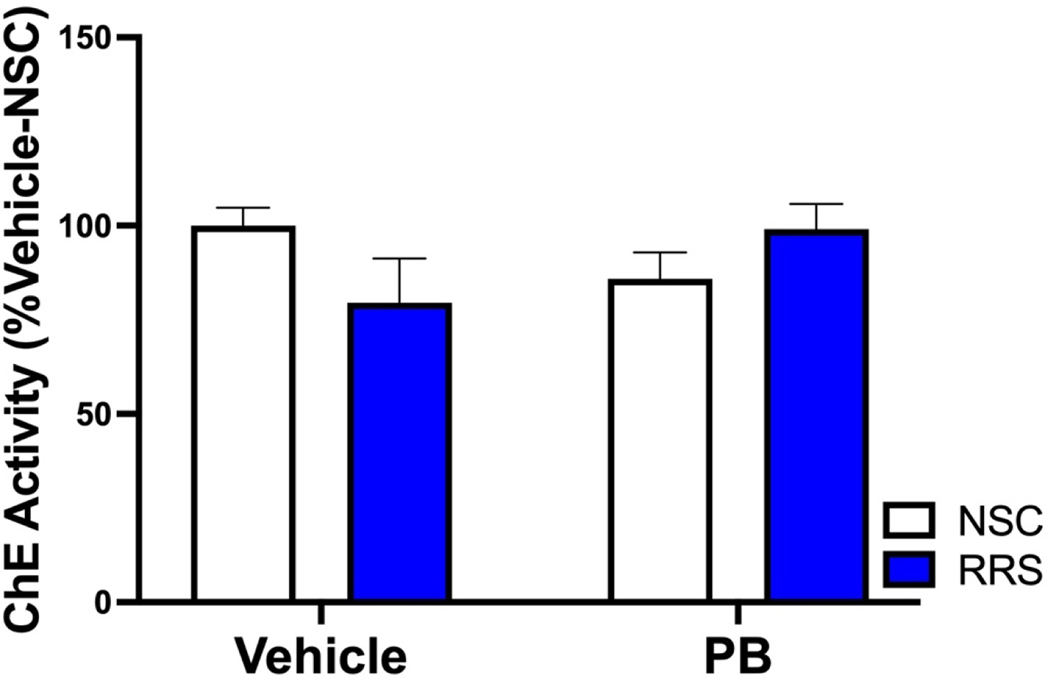
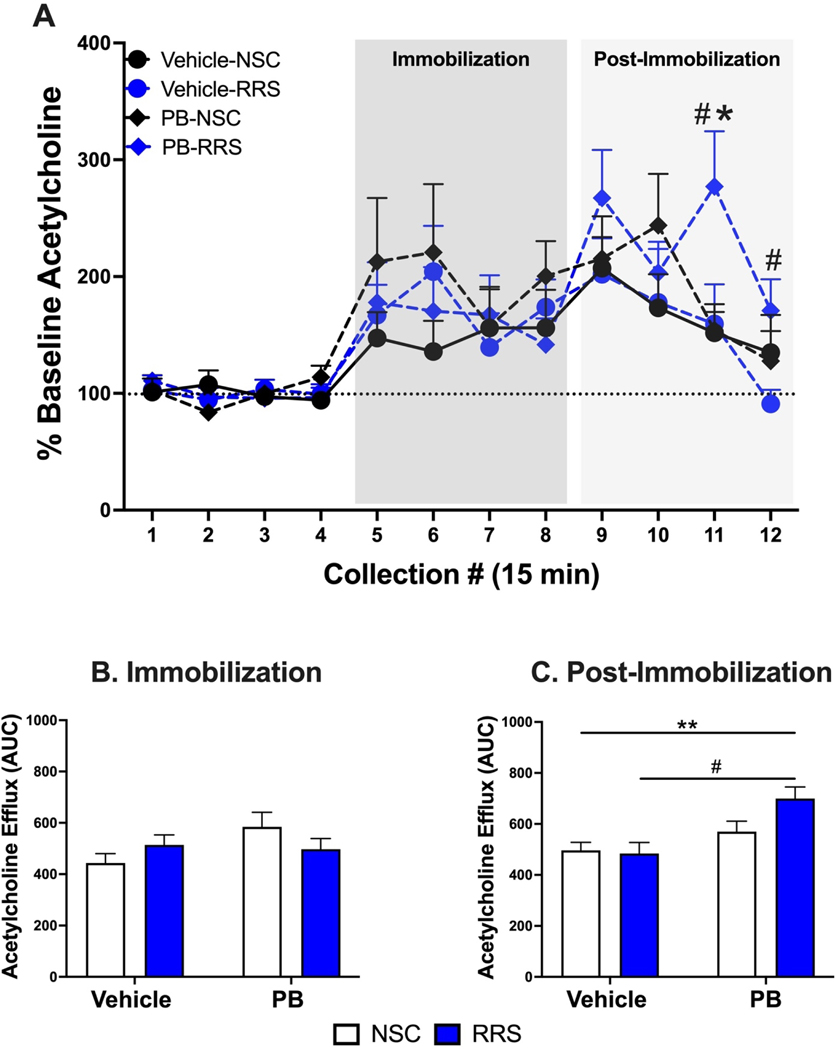
Using in vivo microdialysis, we have previously shown that over time, PB-treated rats exhibit enhanced hippocampal cholinergic responses to an acute LPS challenge (Burzynski et al., 2022), but we have yet to assess such responses to a stress challenge. Two days after the in vivo microdialysis session paired with an acute LPS challenge, the delayed cohort used in our previous study underwent a second session of in vivo microdialysis paired with a 1-hour immobilization stress and 1-hour recovery period. We previously reported that basal levels of ACh in the hippocampus did not differ between our treatment groups prior to LPS administration. Importantly, the prior administration of LPS did not alter basal levels of ACh prior to immobilization stress 48-hours later (Table 1). Additionally, neither a history of PB treatment nor stress alters hippocampal cholinesterase activity 3 months after treatment cessation (Figure 2). In response to immobilization stress, there was a significant interaction of PB and RRS to potentiate the hippocampal cholinergic response at this delayed timepoint (Figure 3A) [2 × 2 × 12 mixed ANOVA, F(11, 209) = 2.058, p = 0.025] but there were no significant main effects of PB [2 × 2 × 12 mixed ANOVA, F(11,209) = 0.849, p = 0.591] or RRS [2 × 2 × 12 mixed ANOVA, F(11,209) = 0.896, p = 0.545]. Bonferroni corrected post-hoc follow-up measures revealed that prior exposure to PB in RRS rats significantly increased ACh levels in the post-stress period, namely at collections 11 (p = 0.018) and 12 (p = 0.027), relative to Vehicle-RRS rats. The potentiated cholinergic response observed in PB-RRS rats was also significantly greater than cholinergic responses of PB-NSC rats at collection 11 (p = 0.017).
Table 1:
Basal levels of hippocampal Acetylcholine (ACh) prior to LPS administration or immobilization stress challenge
| Group | Basal ACh prior to LPS Rx (pmols/20 μL) | Basal ACh prior to stress Rx (pmols/20 μL) | p value |
|---|---|---|---|
|
| |||
| Vehicle-NSC | 0.071 ± 0.02 | 0.075 ± 0.02 | 0.896 |
| Vehicle-RRS | 0.108 ± 0.03 | 0.059 ± 0.01 | 0.124 |
| PB-NSC | 0.099 ± 0.02 | 0.056 ± 0.02 | 0.106 |
| PB-RRS | 0.114 ± 0.02 | 0.078 ± 0.02 | 0.232 |
All data expressed as mean ± SEM, n = 5–9/group.
Figure 2: Hippocampal cholinesterase activity 3 months after treatment cessation.
Neither a history of PB nor RRS altered hippocampal cholinesterase activity 3 months after treatment cessation. All data are represented as a percent of vehicle-NSC animals and are expressed as mean +SEM, n = 7–8/group.
Figure 3: Hippocampal cholinergic responses to immobilization stress 3 months after treatment cessation.
Within rats previously subjected to RRS, a history of PB treatment potentiates the cholinergic response to immobilization stress during the 1-hour recovery period (collections 11 and 12) compared to Vehicle-RRS rats (Panel A). This potentiated response in PB-RRS rats at collection 11 is also significantly greater than the responses of PB-NSC rats. Area under the curve analysis revealed that hippocampal acetylcholine efflux during the 1-hour immobilization stress does not differ between groups (Panel B). However, prior history of PB treatment produces significant elevations in hippocampal acetylcholine efflux in the 1-hour post-stress recovery period (Panel C). All data are expressed as mean + SEM, n = 5–7/group. [#: Significant effect of PB in RRS rats, p < 0.05. *: Significant effect of RRS in PB-treated rats, p <0.05. **: Significant effect of PB, p < 0.01].
Given the interesting differences in ACh levels during the post-stress period, we next conducted area under the curve analysis of ACh efflux during the 1-hour of immobilization stress and the 1-hour following stress. During immobilization stress, there was an increase in ACh efflux above baseline in all groups, but there were no significant differences between groups (Figure 3B). While all groups continued to have elevated ACh efflux in the post-stress period, a two-way ANOVA indicated that rats with a history of PB treatment exhibit potentiated hippocampal ACh levels during the post-stress period relative to vehicle-treated rats (Figure 3C) [F(1,19) = 11.62, p = 0.003]. There was not a significant main effect of stress history [F(1,19) = 1.925, p = 0.181] or a significant interaction of PB and RRS [F(1,19) = 2.814, p = 0.110]. These findings illustrate that PB treatment has lasting effects on hippocampal cholinergic responses to stress, specifically during the recovery of an acute stress challenge.
3.2. LPS administration elicits delayed impairments in NOR performance in PB-treated rats
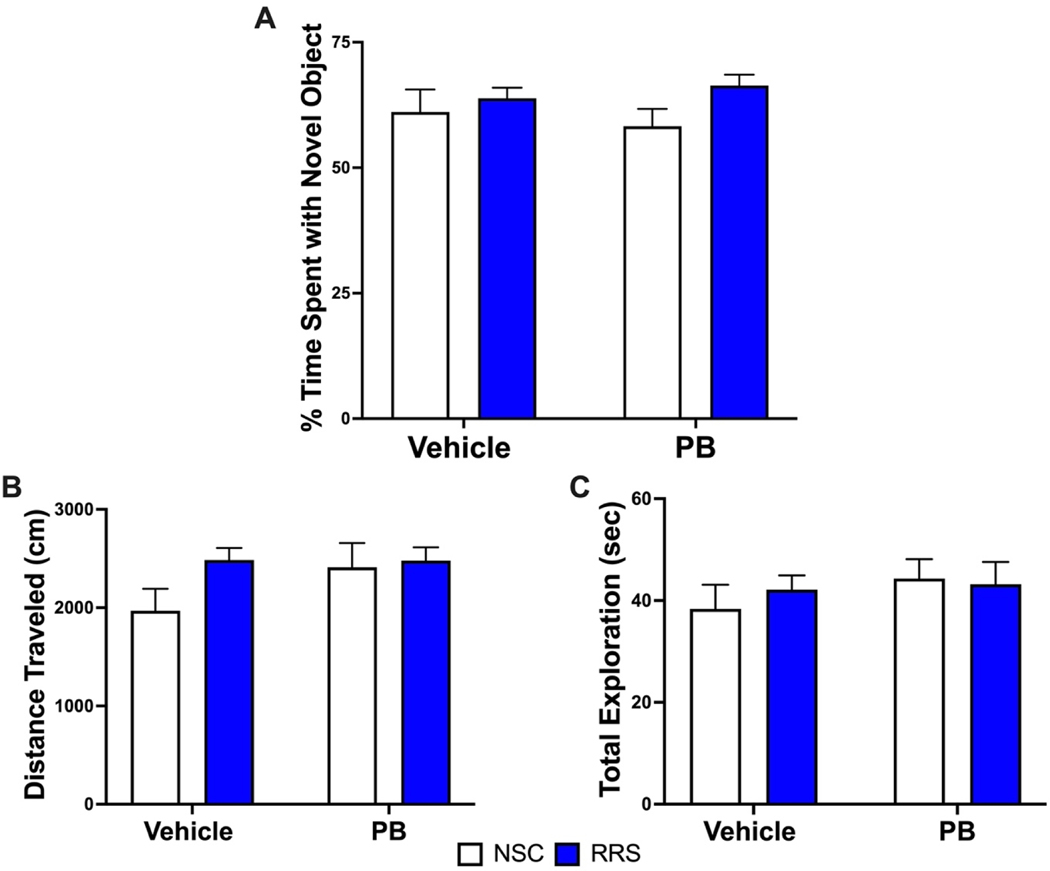
Since we see robust differences in immune and stress responses between our treatment groups at a delayed timepoint, we examined whether a mild immune challenge would adversely affect performance of a hippocampal-dependent learning and memory task, namely the NOR task. We first conducted NOR testing in a separate cohort of rats (cohort #2) approximately 10 days after the cessation of the treatment paradigm to ensure all animals were capable of completing this task. When given saline (1 mL/kg, i.p.) and a 24-hour ITI, a two-way ANOVA revealed that there were no significant differences in novel object exploration observed between treatment groups at this early timepoint (Figure 4A) and paired t-tests confirmed that all groups successfully exhibited recognition of the novel object (p < 0.05). In addition, neither PB nor stress impacted the total distance traveled (Figure 4B) or total exploration time (Figure 4C) during the session when assessed by two-way ANOVA.
Figure 4: Novel object recognition performance 10 days after treatment cessation.
PB administration alone and in combination with RRS did not elicit any deficits in novel object recognition when animals were tested approximately 10 days after treatment with a 24-hour intertrial interval (Panel A). The total distance traveled (Panel B) and total exploration time did not differ between groups (Panel C). All data are expressed as mean + SEM, n = 12–15/group.
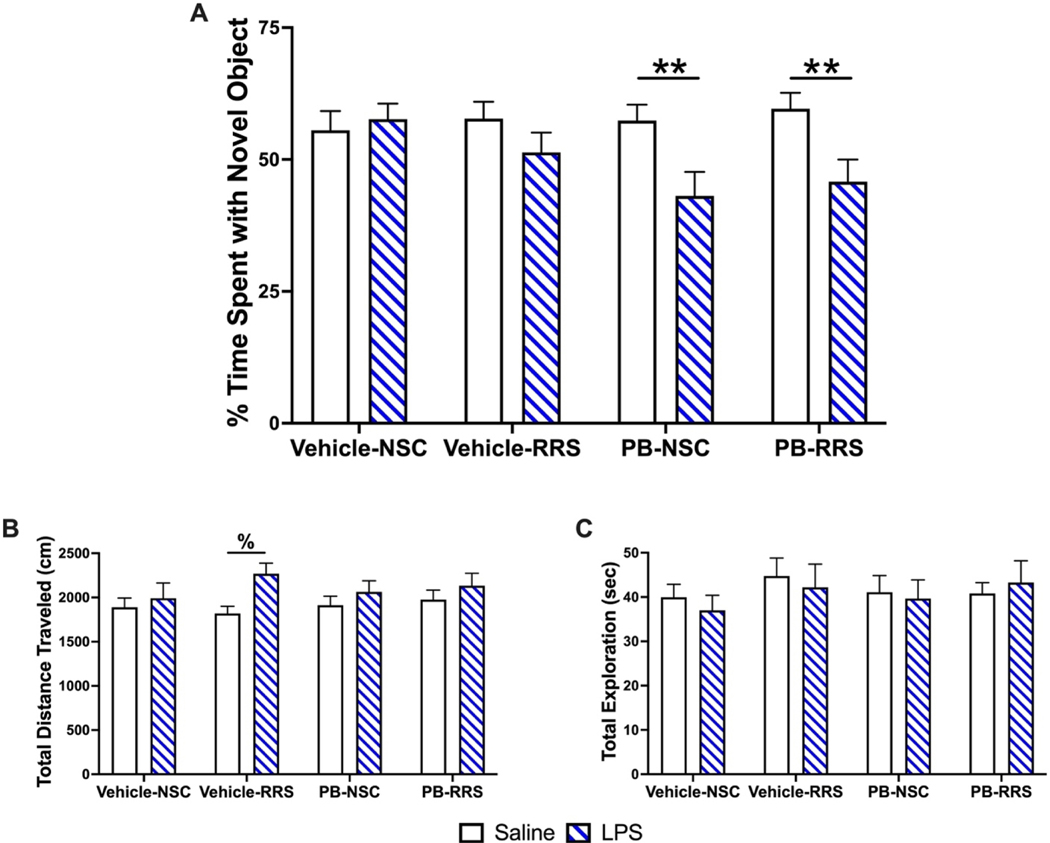
As we hypothesize that the interaction of PB and stress produces delayed effects on hippocampal-dependent memory, the same cohort of rats underwent additional NOR testing 3 months after the cessation of treatment. When rats were given saline (1 mL/kg, i.p.) and assessed following a 24-hour ITI, paired t-tests revealed that the Vehicle-RRS, PB-NSC and PB-RRS groups exhibited recognition (p < 0.05), but the Vehicle-NSC group failed to reach statistical significance (p = 0.16). Importantly, there were no significant differences in the time spent exploring the novel object observed between groups when assessed by two-way ANOVA (Figure 5A). Two-way ANOVAs also revealed that there was no effect of treatment history on locomotion (Figure 5B) or total exploration time (Figure 5C) during this session. Interestingly, when rats were challenged with acute LPS administration (30 μg/kg, i.p.) one-week later, we observed a significant main effect of LPS [2 × 2 × 2 mixed ANOVA, F(1,49) = 8.950, p = 0.004] and significant interaction of LPS and PB [2 × 2 × 2 mixed ANOVA, F(1,49), = 8.232, p = 0.006] to decrease the time spent with the novel object 24 hours later (Figure 5A). Post-hoc pairwise comparisons revealed that PB-treated rats, irrespective of stress history, spent significantly less time with the novel object when challenged with LPS compared to their performance during the saline session (PB-NSC: p = 0.007, PB-RRS: p = 0.003). There were no significant interactions between LPS and RRS [2 × 2 × 2 mixed ANOVA, F(1,49) = 0.361, p = 0.551] or LPS, PB and RRS [2 × 2 × 2 mixed ANOVA, F(1,49) = 0.202, p = 0.655]. This LPS-induced deficit in PB-treated rats was not due to any locomotor impairments as there was a significant main effect of LPS to increase the distance traveled in all groups, relative to the saline session [2 × 2 × 2 mixed ANOVA, F(1,47) = 11.297, p = 0.002] (Figure 5B). Post-hoc pairwise comparisons show that Vehicle-RRS rats travel significantly greater distances during the LPS NOR session compared to their total distance traveled during the saline NOR session (p = 0.002). The total exploration time (Figure 5C) during the LPS session did not differ between groups nor between the saline session.
Figure 5: Novel object recognition performance with acute immune challenge at delayed timepoint.
Three months after treatment cessation, PB-treated rats spent significantly less time with the novel object when challenged with acute LPS (Blue hatched bars) compared to their performance when challenged with saline (Open bars; Panel A). All animals traveled more during the LPS session relative to the saline session, with the Vehicle-RRS animals reaching significance (Panel B). There was no effect of treatment history or LPS on the total exploration time of any group (Panel C). All data are expressed as mean + SEM, n = 12–14/group. [**: Significant effect of LPS in PB-treated rats, p < 0.01. %: Significant effect of LPS in Vehicle-RRS rats, p < 0.01].
3.3. Prior history of PB treatment produces deficits in long-term retention in hippocampal-dependent learning and memory
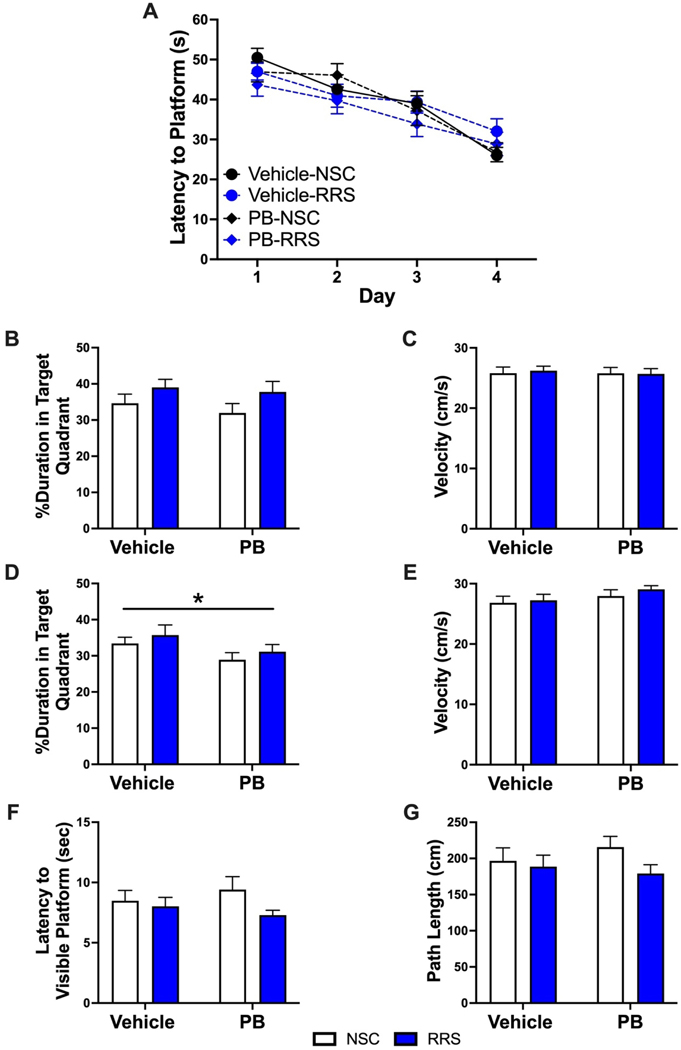
Beyond NOR, we also investigated if PB and stress produce delayed deficits in hippocampal-dependent learning and memory. Approximately two weeks after the final NOR session (approximately 4 months after PB and stress), the rats in cohort #2 underwent six days of Morris water maze testing. After completing four training trials per day for four consecutive days, a 2 × 2 × 4 mixed ANOVA revealed that there was a significant effect of time on the latency to find the platform (Figure 6A) [F(3,62) = 32.402, p < 0.001], although there was no effect of prior PB treatment or stress on acquisition throughout the training trials. When the platform was removed 1-hour after the last training session, there was no effect of treatment history on the time spent swimming in the target quadrant (Figure 6B). A 2 × 2 × 4 mixed ANOVA revealed a significant effect of quadrant [F(3, 162) = 63.02, p < 0.0001] and one-sample t-tests revealed that the time spent swimming in the target quadrant was significantly greater than chance (15 seconds) for all groups (p < 0.05). Additionally, there were no differences in swim speed observed in any group during the 1-hour probe trial (Figure 6C). Similar to our NOR findings, a 24-hour probe trial conducted on day five revealed that PB-treated rats spent significantly less time in the target quadrant relative to vehicle-treated rats (Figure 6D) [2 × 2 ANOVA, F(1,54) = 4.347, p = 0.042]. A 2 × 2 × 4 mixed ANOVA revealed a significant effect of quadrant [F(2.079, 112.30) = 35.95, p < 0.0001]. One-sample t-tests revealed that the time spent swimming in the target quadrant was significantly greater than chance (15 seconds) in Vehicle-NSC, Vehicle-RRS and PB-RRS animals (p < 0.05), but PB-NSC animals failed to reach statistical significance (p = 0.070). This 24-hour memory deficit observed in PB-treated rats was not due to any locomotor deficits as swim speed was not different between groups (Figure 6E). Cue training conducted the following week validated that treatment history did not result in any visual impairments as there were no differences in escape latency (Figure 6F) or path length (Figure 6G) when the platform was visible.
Figure 6: Morris water maze performance 3 months after treatment cessation.
There was no effect of treatment history on acquisition across 4 training days at the delayed timepoint (Panel A). There is no effect of PB or RRS history on the time spent swimming in the target quadrant (Panel B) or swimming velocity (Panel C) during the 1-hour probe trial. Rats with a history of PB treatment spent significantly less time in the target quadrant during the 24-hour probe trial (Panel D) but swim speed was not different between groups (Panel E). When the platform was visible, there was no effect of treatment history on escape latency (Panel F) or path length (Panel G). All data are expressed as mean + SEM, n = 13–15/group. [*: Significant effect of PB, p < 0.05].
4. Discussion
The results of the current study demonstrate that PB produces lasting effects on hippocampal neurochemistry and behavior, particularly in response to an immune or stress challenge. These findings have critical implications for GWI, which is characterized by its persistent and progressive nature. Specifically, three months after treatment cessation, PB-treated rats show exaggerated hippocampal ACh efflux following an acute immobilization stress, which is consistent with our previous findings using an acute immune challenge (Burzynski et al., 2022). In addition to neurochemical alterations, male rats exhibit 24-hour memory deficits in NOR three months after PB administration, but only after an immune challenge. A history of PB treatment also produced 24-hour memory impairments in MWM performance but did not have any effect on learning. Collectively, these findings demonstrate that PB treatment alters hippocampal cholinergic responses to mild stressors and impairs hippocampal-dependent memory processes. Our current and prior observations have important translational implications as mild stressors and immune threats are a part of daily life and the dysregulated responses of GWI patients likely worsen with time, contributing to the progressive nature of this disease.
4.1. Disrupted memory consolidation in PB-treated rats
Memory deficits are considered a hallmark feature of GWI as such impairments were first reported during the GW (Fukuda et al., 1998; Group, 1997; Haley et al., 1997; Steele, 2000; Sullivan et al., 2003; White et al., 2001), and many veterans continue to experience cognitive disturbances over 30 years later (Blanchard et al., 2005; Dursa et al., 2016; Kang et al., 2009; Mawson and Croft, 2019; Nettleman, 2015; White et al., 2016). These lasting memory impairments may be due to long-term disruptions in the central cholinergic system as ACh is a critical component of memory formation. The role of ACh in cognition is dynamic, and the optimal levels of ACh required for memory encoding and consolidation differ greatly as increased ACh efflux is necessary for memory acquisition, but consolidation requires low levels of ACh (Hasselmo, 1999; Kametani and Kawamura, 1990; Marrosu et al., 1995). Previous in vivo microdialysis studies from our group have shown that hippocampal ACh efflux increases when exploring both the familiar and novel objects during NOR testing and there is a significant correlation between hippocampal ACh efflux and novel object exploration time (Stanley et al., 2012). These findings are consistent with clinical studies that have shown intravenous administration of the muscarinic antagonist scopolamine impairs memory acquisition but does not affect retention (Ghoneim and Mewaldt, 1977; Petersen, 1977). Building upon these studies, Rogers and Kesner refined the role of ACh in learning and memory by selectively manipulating ACh efflux in the rat hippocampus. They found that intrahippocampal injections of scopolamine impaired spatial acquisition using a modified Hebb-Williams maze, but retention was not affected. Conversely, when the AChE inhibitor physostigmine was injected into the rat hippocampus, memory consolidation was significantly impaired (Rogers and Kesner, 2003).
These findings, along with our previous (Burzynski et al., 2022) and current in vivo microdialysis studies, provide neurochemical insight into the 24-hour memory impairments we observe in PB-treated rats at a delayed timepoint. We have shown that over time, PB-treated rats exhibit larger elevations in hippocampal ACh efflux when responding to an acute LPS challenge or immobilization stress challenge. While repeated in vivo microdialysis sessions require multiple probe insertions that likely create tissue damage, it has been well established that repeated in vivo microdialysis sessions, separated by 48 hours, do not affect basal or stimulated ACh efflux (Johnson and Bruno, 1995; Moore et al., 1995). The immune and immobilization sessions were not counterbalanced in this study to allow for any carryover effects of LPS to be observed. As basal ACh efflux was not changed between the two sessions, which is consistent with our previous studies measuring both hippocampal ACh (Macht et al., 2019) and glutamate (Macht et al., 2020), we do not believe that responses to the immobilization challenge 48 hours later are influenced by lasting immune responses.
Given our neurochemical findings, it is interesting to speculate that this PB-induced elevation of ACh after a stressor may be responsible for the retention deficits observed in NOR (when paired with an acute LPS challenge) and the MWM. Importantly, PB treatment does not produce acquisition impairments in these behavioral assays, nor did we observe any acquisition deficits in our previous contextual fear conditioning studies (Macht et al., 2018), further supporting Rogers and Kesner’s findings. Taken together, our studies suggest that over time, PB treatment potentiates the hippocampal cholinergic response to stressors, which may explain the 24-hour memory deficits observed in our delayed model of GWI, as well as the progressive cognitive deficits observed in GWI patients.
4.2. PB effects on the hippocampal cholinergic anti-inflammatory pathway
An early hypothesis regarding the underlying causes of GWI revolved around the concept that stress altered the pharmacokinetic profile of PB to allow the drug to cross the blood-brain barrier and inhibit brain AChE activity. While some studies have demonstrated exposure to GW-related chemicals, including PB, inhibits brain AChE activity (Beck et al., 2003; Friedman et al., 1996; Kaufer et al., 1998; Tian et al., 2002) other studies failed to replicate these findings (Amourette et al., 2009; Grauer et al., 2000; Kant et al., 2001; Song et al., 2002; Tian et al., 2002). Moreover, our previous neurochemical studies do not support the concept that PB acts in the CNS to modulate brain ACh levels. Specifically, we did not observe changes in basal levels of ACh in the hippocampus and the prefrontal cortex in PB-treated rats approximately 10 days after PB administration (Macht et al., 2019) or at the delayed time point (Burzynski et al., 2022). Additionally, the hippocampal cholinesterase activity of PB-treated rats does not differ from vehicle-treated rats at the delayed timepoint. Taken together, these findings do not support the concept that the neurological complications of GWI result from central effects of PB on AChE activity. Our studies provide an alternative explanation for the effects of PB in the CNS, namely that alterations in cholinergic anti-inflammatory network leads to a pro-inflammatory state in the brain that impairs cholinergic function and cognition. Indeed, along with memory encoding and consolidation, ACh also plays a key role in the anti-inflammatory response (Hoover, 2017). The cholinergic anti-inflammatory pathway utilizes ACh to suppress the release of pro-inflammatory cytokines by binding to alpha 7 nicotinic ACh receptors (α7 nAChRs) expressed on macrophages, microglia, and astrocytes (Wu et al., 2021). We have previously shown that PB treatment dysregulates this critical response long after treatment cessation. Specifically, three months after treatment, PB-treated rats have potentiated hippocampal ACh efflux in response to an acute LPS challenge, but this is not accompanied by a suppressed inflammatory response. Instead, rats with a history of PB treatment show exaggerated levels of pro-inflammatory cytokines in the hippocampus following this acute LPS challenge (Burzynski et al., 2022).
One such pro-inflammatory cytokine that is elevated in the hippocampus of PB-treated rats following LPS administration is IL-1β (Burzynski et al., 2022). IL-1β is thought to be a key contributor to hippocampal neuroinflammation and subsequent cognitive decline due to its dense receptor expression in this region (Farrar et al., 1987). While it has been shown that low levels of IL-1β facilitate hippocampal-dependent learning and memory, high levels of IL-1β can inhibit these processes (Lynch, 2015; Yirmiya and Goshen, 2011). For example, clinical studies have found an upregulation of hippocampal IL-1β in post-mortem tissue of Alzheimer’s patients (Cacabelos et al., 1994). From a synaptic perspective, electrophysiology studies have demonstrated that IL-1β blocks long-term potentiation, a cellular correlate of learning and memory, in hippocampal slices from both mice (Katsuki et al., 1990) and rats (Bellinger et al., 1993). Moreover, in vivo studies have found that intrahippocampal injections of IL-1β impair memory consolidation in contextual fear conditioning (Gonzalez et al., 2009) and intracerebroventricular injections of IL-1β produced retention deficits in the MWM but did not impair acquisition (Oitzl et al., 1993). These findings provide evidence that the exaggerated IL-1β response seen in the hippocampus of PB-treated rats may contribute to the PB-induced NOR impairments seen 24-hours after LPS administration.
Collectively, our studies suggest that PB treatment has lasting effects on the hippocampal cholinergic system, specifically as it relates to memory consolidation. When challenged by a stressor, it is unclear whether the potentiated ACh efflux observed in PB-treated rats is due to a hyperactive cholinergic system or is a compensatory measure to dampen the exaggerated pro-inflammatory responses. Regardless, the inability of ACh to suppress the pro-inflammatory response indicates an impairment in the cholinergic anti-inflammatory pathway. While our previous studies reported that hippocampal α7 nAChR expression is not changed in PB-treated rats (Burzynski et al., 2022), it is possible that PB administration elicits functional deficits in α7-containing receptors. While future studies are needed to fully elucidate this mechanism, it is likely that both the exaggerated hippocampal cholinergic and IL-1β responses observed in PB-treated rats are impairing memory consolidation. Importantly, these altered responses emerge long after treatment cessation, highlighting the enduring effects of PB treatment and how it may contribute to the progressive cognitive deficits seen in GWI patients.
4.3. Potential role of stressors in behavioral deficits in GWI rodent studies
While many laboratories have assessed hippocampal-dependent learning and memory in rodent models of GWI, the results from these studies are varied. One group observed deficits in water maze performance in a rat model of GWI as early as 16 days after treatment cessation and these impairments remained at days 52, 113 and 199 (Lamproglou et al., 2009). Another group saw similar deficits in water maze performance in their rat model of GWI approximately 3 months after treatment cessation, but did not observe any deficits in novel object recognition (Parihar et al., 2013). Conversely, a different rat model of GWI exhibited significant impairments in both novel object recognition and novel place recognition more than 3 months after treatment cessation (Hattiangady et al., 2014). Other studies using a mouse model of GWI saw water maze performance decline over time, as impairments were not seen at days 20–30 post-treatment (Abdullah et al., 2012), but emerged by day 115 (Abdullah et al., 2011). Another study from this group observed similar changes in Barnes maze performance, only seeing deficits 106 days post-treatment, but not at day 14 (Zakirova et al., 2015b). Interestingly, when this group performed behavioral tests 22.5 months after treatment cessation, this mouse model of GWI did not show deficits in Barnes maze performance (Zakirova et al., 2015a). Such inconsistencies have made it difficult to identify the mechanisms responsible for the progressive cognitive deficits seen in GWI and hinders the development of potential therapeutics for affected individuals.
One potential explanation for these disparate results is the use of various experimental models of GWI in each study, including different chemical exposures, stress paradigms and treatment durations. However, another important factor that we believe is responsible for the equivocal results in GWI behavioral studies is the absence of the administration of stressors during such assays. Our studies and others (Broderick et al., 2013; Broderick et al., 2011), suggest that many aspects of GWI are latent, and the long-lasting effects of PB treatment are only visible in response to an immune or stressful stimulus. For example, three months after treatment cessation, basal levels of hippocampal ACh are not affected by PB treatment, but the cholinergic response to an acute immune or stress challenge increases over time. Furthermore, when challenged with saline, PB-treated rats do not exhibit elevated plasma or hippocampal cytokines relative to vehicle-treated rats, but their pro-inflammatory response to LPS is significantly greater. Additionally, rats with a history of PB treatment do not exhibit deficits in novel object recognition when challenged with saline, but an acute LPS challenge significantly impairs their performance in this task. It is worth noting that the more consistent findings in GWI studies assessed hippocampal-dependent memory with the MWM (Abdullah et al., 2011; Lamproglou et al., 2009; Parihar et al., 2013), which further supports the concept that stressors (i.e., swimming) serve as a necessary stimulus to unmask hippocampal-dependent learning and memory deficits in GWI. Importantly, the stimulus-dependent cognitive impairments observed in our rodent model of GWI are consistent with clinical studies in which an exercise challenge is used to exacerbate cognitive deficits in GWI patients (Broderick et al., 2013; Broderick et al., 2011; Whistler et al., 2009).
4.4. Conclusions
Taken together, our findings across multiple studies are consistent with many others (Broderick et al., 2013; Broderick et al., 2011; Rayhan et al., 2013; Washington et al., 2020) that suggest GWI may be characterized as having a latent phenotype, and the pathophysiology can be better understood when stressors are presented. We have shown that PB-treatment creates lasting deficits in hippocampal cholinergic signaling that emerge long after treatment cessation, but only under conditions when the CNS is stimulated with either an immune or stress challenge. We believe that including such stressors in GWI research, especially during behavioral studies, is necessary to fully elucidate the mechanistic basis of GWI and thereby identify potential sites of intervention that could be used in the treatment of the cognitive deficits observed in veterans with GWI.
Highlights:
PB treatment elicits long-term disruptions in the central cholinergic system
Cholinergic disruptions emerge when stimulated by physiological stressor
Aberrant cholinergic responses to stressors produce cognitive deficits in GWI
Daily life stressors likely exacerbate memory deficits in GWI
Acknowledgments
Funding and Disclosure:
Financial support for these studies was provided by the Department of Veterans Affairs grant numbers I21 BX002085 (LPR), IO1 BX001804 (LPR) and VISN7 Research Development Award (FH), the National Institute of Health grant numbers R01AG050518 (JRF), K01AG061263, P20GM109091 (JAM), P20GM109091 (FH) and F31DK131773 (HEB), and the National Science Foundation grant number IOS-1656626 (CAG). Figures 1 was made with Biorender.com.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the U.S. Department of Veterans Affairs, the United States Government, or the other stated funding agencies.
Footnotes
Credit authorship contribution statement
LPR: Conceptualization, Formal analysis, Writing, Review & Editing, Supervision, Funding Acquisition. HEB: Investigation, Conceptualization, Formal analysis, Writing, Review & Editing, Funding Acquisition. KEA, MAF, HAD, JLW, BRE: Investigation. VAM: Investigation, Conceptualization, Formal analysis, Review & Editing. FH, JAM, CAG, JRF: Conceptualization, Formal analysis, Review & Editing, Supervision. LPR is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors declare no competing financial interests.
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullah L, Crynen G, Reed J, Bishop A, Phillips J, Ferguson S, Mouzon B, Mullan M, Mathura V, Ait-Ghezala G, Crawford F, 2011. Proteomic CNS profile of delayed cognitive impairment in mice exposed to Gulf War agents. Neuromolecular Med 13, 275–288. [DOI] [PubMed] [Google Scholar]
- Abdullah L, Evans JE, Bishop A, Reed JM, Crynen G, Phillips J, Pelot R, Mullan MA, Ferro A, Mullan CM, Mullan MJ, Ait-Ghezala G, Crawford FC, 2012. Lipidomic profiling of phosphocholine-containing brain lipids in mice with sensorimotor deficits and anxiety-like features after exposure to Gulf War agents. Neuromolecular Med 14, 349–361. [DOI] [PubMed] [Google Scholar]
- Amourette C, Lamproglou I, Barbier L, Fauquette W, Zoppe A, Viret R, Diserbo M, 2009. Gulf War illness: Effects of repeated stress and pyridostigmine treatment on blood–brain barrier permeability and cholinesterase activity in rat brain. Behavioural brain research 203, 207–214. [DOI] [PubMed] [Google Scholar]
- Beck KD, Brennan FX, Moldow RL, Ottenweller JE, Zhu G, Servatius RJ, 2003. Stress interacts with peripheral cholinesterase inhibitors to cause central nervous system effects. Life Sci 73, 41–51. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Madamba S, Siggins GR, 1993. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res 628, 227–234. [DOI] [PubMed] [Google Scholar]
- Blanchard MS, Eisen SA, Alpern R, Karlinsky J, Toomey R, Reda DJ, Murphy FM, Jackson LW, Kang HK, 2005. Chronic Multisymptom Illness Complex in Gulf War I Veterans 10 Years Later. American Journal of Epidemiology 163, 66–75. [DOI] [PubMed] [Google Scholar]
- Broderick G, Ben-Hamo R, Vashishtha S, Efroni S, Nathanson L, Barnes Z, Fletcher MA, Klimas N, 2013. Altered immune pathway activity under exercise challenge in Gulf War Illness: an exploratory analysis. Brain, behavior, and immunity 28, 159–169. [DOI] [PubMed] [Google Scholar]
- Broderick G, Kreitz A, Fuite J, Fletcher MA, Vernon SD, Klimas N, 2011. A pilot study of immune network remodeling under challenge in Gulf War Illness. Brain, behavior, and immunity 25, 302–313. [DOI] [PubMed] [Google Scholar]
- Burzynski HE, Macht VA, Woodruff JL, Crawford JN, Erichsen JM, Piroli GG, Grillo CA, Fadel JR, Reagan LP, 2022. Pyridostigmine bromide elicits progressive and chronic impairments in the cholinergic anti-inflammatory pathway in the prefrontal cortex and hippocampus of male rats. Neurobiol Stress 18, 100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacabelos R, Alvarez XA, Fernández-Novoa L, Franco A, Mangues R, Pellicer A, Nishimura T, 1994. Brain interleukin-1 beta in Alzheimer’s disease and vascular dementia. Methods Find Exp Clin Pharmacol 16, 141–151. [PubMed] [Google Scholar]
- Calva CB, Fayyaz H, Fadel JR, 2018. Increased acetylcholine and glutamate efflux in the prefrontal cortex following intranasal orexin‐A (hypocretin‐1). Journal of neurochemistry 145, 232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursa EK, Barth SK, Schneiderman AI, Bossarte RM, 2016. Physical and mental health status of Gulf War and Gulf era veterans. Journal of occupational and environmental medicine 58, 41–46. [DOI] [PubMed] [Google Scholar]
- Fadel J, Pasumarthi R, Reznikov LR, 2005. Stimulation of cortical acetylcholine release by orexin A. Neuroscience 130, 541–547. [DOI] [PubMed] [Google Scholar]
- Fadel J, Sarter M, Bruno JP, 2001. Basal forebrain glutamatergic modulation of cortical acetylcholine release. Synapse 39, 201–212. [DOI] [PubMed] [Google Scholar]
- Farrar WL, Kilian PL, Ruff MR, Hill JM, Pert CB, 1987. Visualization and characterization of interleukin 1 receptors in brain. J Immunol 139, 459–463. [PubMed] [Google Scholar]
- Friedman A, Kaufer D, Shemer J, Hendler I, Soreq H, Tur-Kaspa I, 1996. Pyridostigmine brain penetration under stress enhances neuronal excitability and induces early immediate transcriptional response. Nature medicine 2, 1382–1385. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, Noah DL, Barrett DH, Randall B, Herwaldt BL, 1998. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. Jama 280, 981–988. [DOI] [PubMed] [Google Scholar]
- Ghoneim MM, Mewaldt SP, 1977. Studies on human memory: The interactions of diazepam, scopolamine, and physostigmine. Psychopharmacology 52, 1–6. [DOI] [PubMed] [Google Scholar]
- Golomb BA, 2008. Acetylcholinesterase inhibitors and Gulf War illnesses. Proc Natl Acad Sci U S A 105, 4295–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez PV, Schiöth HB, Lasaga M, Scimonelli TN, 2009. Memory impairment induced by IL-1β is reversed by α-MSH through central melanocortin-4 receptors. Brain, behavior, and immunity 23, 817–822. [DOI] [PubMed] [Google Scholar]
- Gordon J, Leadbeater L, Maidment MP, 1978. The protection of animals against organophosphate poisoning by pretreatment with a carbamate. Toxicology and applied pharmacology 43, 207–216. [DOI] [PubMed] [Google Scholar]
- Grauer E, Alkalai D, Kapon J, Cohen G, Raveh L, 2000. Stress does not enable pyridostigmine to inhibit brain cholinesterase after parenteral administration. Toxicology and applied pharmacology 164, 301–304. [DOI] [PubMed] [Google Scholar]
- Group, I.P.G.S., 1997. Self-reported illness and health status among Gulf War veterans: a population-based study. J. Am. Med. Assoc 277, 238–245. [PubMed] [Google Scholar]
- Haley RW, Kurt TL, Hom J, 1997. Is there a Gulf war syndrome?: searching for syndromes by factor analysis of symptoms. Jama 277, 215–222. [PubMed] [Google Scholar]
- Hasselmo ME, 1999. Neuromodulation: acetylcholine and memory consolidation. Trends in cognitive sciences 3, 351–359. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Mishra V, Kodali M, Shuai B, Rao X, Shetty AK, 2014. Object location and object recognition memory impairments, motivation deficits and depression in a model of Gulf War illness. Frontiers in behavioral neuroscience 8, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DB, 2017. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol Ther 179, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard NA, Hutchison JL, Motes MA, Shokri-Kojori E, Bennett IJ, Brigante RM, Haley RW, Rypma B, 2014. Central Executive Dysfunction and Deferred Prefrontal Processing in Veterans with Gulf War Illness. Clin Psychol Sci 2, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BJ, Bruno JP, 1995. Dopaminergic modulation of striatal acetylcholine release in rats depleted of dopamine as neonates. Neuropharmacology 34, 191–203. [DOI] [PubMed] [Google Scholar]
- Kametani H, Kawamura H, 1990. Alterations in acetylcholine release in the rat hippocampus during sleep-wakefulness detected by intracerebral dialysis. Life sciences 47, 421–426. [DOI] [PubMed] [Google Scholar]
- Kang HK, Li B, Mahan CM, Eisen SA, Engel CC, 2009. Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years. Journal of occupational and environmental medicine, 401–410. [DOI] [PubMed] [Google Scholar]
- Kant GJ, Bauman RA, Feaster SR, Anderson SM, Saviolakis GA, Garcia GE, 2001. The combined effects of pyridostigmine and chronic stress on brain cortical and blood acetylcholinesterase, corticosterone, prolactin and alternation performance in rats. Pharmacology Biochemistry and Behavior 70, 209–218. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M, 1990. Interleukin-1 beta inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol 181, 323–326. [DOI] [PubMed] [Google Scholar]
- Kaufer D, Friedman A, Seidman S, Soreq H, 1998. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature 393, 373–377. [DOI] [PubMed] [Google Scholar]
- Lamproglou I, Barbier L, Diserbo M, Fauvelle F, Fauquette W, Amourette C, 2009. Repeated stress in combination with pyridostigmine: Part I: Long-term behavioural consequences. Behavioural brain research 197, 301–310. [DOI] [PubMed] [Google Scholar]
- Li B, Mahan CM, Kang HK, Eisen SA, Engel CC, 2011. Longitudinal health study of US 1991 Gulf War veterans: changes in health status at 10-year follow-up. Am J Epidemiol 174, 761–768. [DOI] [PubMed] [Google Scholar]
- Lynch MA, 2015. Neuroinflammatory changes negatively impact on LTP: A focus on IL-1β. Brain research 1621, 197–204. [DOI] [PubMed] [Google Scholar]
- Macht VA, Woodruff JL, Burzynski HE, Grillo CA, Reagan LP, Fadel JR, 2020. Interactions between pyridostigmine bromide and stress on glutamatergic neurochemistry: Insights from a rat model of Gulf War Illness. Neurobiol Stress 12, 100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macht VA, Woodruff JL, Grillo CA, Wood CS, Wilson MA, Reagan LP, 2018. Pathophysiology in a model of Gulf War Illness: Contributions of pyridostigmine bromide and stress. Psychoneuroendocrinology 96, 195–202. [DOI] [PubMed] [Google Scholar]
- Macht VA, Woodruff JL, Maissy ES, Grillo CA, Wilson MA, Fadel JR, Reagan LP, 2019. Pyridostigmine bromide and stress interact to impact immune function, cholinergic neurochemistry and behavior in a rat model of Gulf War Illness. Brain Behav Immun 80, 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MT, Schuster BG, Brueckner RP, Lin E, Kaminskis A, Lasseter KC, 1998. Population pharmacokinetics and pharmacodynamics of pyridostigmine bromide for prophylaxis against nerve agents in humans. The Journal of Clinical Pharmacology 38, 227–235. [DOI] [PubMed] [Google Scholar]
- Marrosu F, Portas C, Mascia MS, Casu MA, Fà M, Giagheddu M, Imperato A, Gessa GL, 1995. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain research 671, 329–332. [DOI] [PubMed] [Google Scholar]
- Mawson AR, Croft AM, 2019. Gulf War Illness: Unifying Hypothesis for a Continuing Health Problem. International Journal of Environmental Research and Public Health 16, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 2012. Endocrine effects on the brain and their relationship to behavior. Basic neurochemistry. Elsevier, pp. 945–962. [Google Scholar]
- Moore H, Stuckman S, Sarter M, Bruno JP, 1995. Stimulation of cortical acetylcholine efflux by FG 7142 measured with repeated microdialysis sampling. Synapse 21, 324–331. [DOI] [PubMed] [Google Scholar]
- Nettleman M, 2015. Gulf War Illness: Challenges Persist. Trans Am Clin Climatol Assoc 126, 237–247. [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, van Oers H, Schöbitz B, de Kloet ER, 1993. Interleukin-1 beta, but not interleukin-6, impairs spatial navigation learning. Brain Res 613, 160–163. [DOI] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Shuai B, Shetty AK, 2013. Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus. Neuropsychopharmacology 38, 2348–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson CR, Emson PC, 1980. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. Journal of neuroscience methods 3, 129–149. [DOI] [PubMed] [Google Scholar]
- Petersen RC, 1977. Scopolamine induced learning failures in man. Psychopharmacology 52, 283–289. [DOI] [PubMed] [Google Scholar]
- Rayhan RU, Stevens BW, Raksit MP, Ripple JA, Timbol CR, Adewuyi O, VanMeter JW, Baraniuk JN, 2013. Exercise challenge in Gulf War Illness reveals two subgroups with altered brain structure and function. PLoS One 8, e63903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Kesner RP, 2003. Cholinergic modulation of the hippocampus during encoding and retrieval. Neurobiol Learn Mem 80, 332–342. [DOI] [PubMed] [Google Scholar]
- Song X, Tian H, Bressler J, Pruett S, Pope C, 2002. Acute and repeated restraint stress have little effect on pyridostigmine toxicity or brain regional cholinesterase inhibition in rats. Toxicological Sciences 69, 157–164. [DOI] [PubMed] [Google Scholar]
- Stanley EM, Wilson MA, Fadel JR, 2012. Hippocampal neurotransmitter efflux during one-trial novel object recognition in rats. Neurosci Lett 511, 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele L, 2000. Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. American journal of epidemiology 152, 992–1002. [DOI] [PubMed] [Google Scholar]
- Steele L, Sastre A, Gerkovich MM, Cook MR, 2012. Complex factors in the etiology of Gulf War illness: wartime exposures and risk factors in veteran subgroups. Environmental health perspectives 120, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K, Krengel M, Proctor SP, Devine S, Heeren T, White RF, 2003. Cognitive Functioning in Treatment-Seeking Gulf War Veterans: Pyridostigmine Bromide Use and PTSD. Journal of Psychopathology and Behavioral Assessment 25, 95–103. [Google Scholar]
- Tian H, Song X, Bressler J, Pruett S, Pope CN, 2002. Neither forced running nor forced swimming affect acute pyridostigmine toxicity or brain-regional cholinesterase inhibition in rats. Toxicology 176, 39–50. [DOI] [PubMed] [Google Scholar]
- Tillman GD, Calley CS, Buhl VI, Chiang HS, Haley RW, Hart J Jr., Kraut MA, 2017. Electrophysiological correlates of semantic memory retrieval in Gulf War Syndrome 2 patients. J Neurol Sci 373, 66–72. [DOI] [PubMed] [Google Scholar]
- von Bredow JD, Adams NL, Groff WA, Vick JA, 1991. Effectiveness of oral pyridostigmine pretreatment and cholinolytic-oxime therapy against soman intoxication in nonhuman primates. Fundamental and Applied Toxicology 17, 761–770. [PubMed] [Google Scholar]
- Washington SD, Rayhan RU, Garner R, Provenzano D, Zajur K, Addiego FM, VanMeter JW, Baraniuk JN, 2020. Exercise alters cerebellar and cortical activity related to working memory in phenotypes of Gulf War Illness. Brain Commun 2, fcz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler T, Fletcher MA, Lonergan W, Zeng XR, Lin JM, Laperriere A, Vernon SD, Klimas NG, 2009. Impaired immune function in Gulf War Illness. BMC Med Genomics 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF, Proctor SP, Heeren T, Wolfe J, Krengel M, Vasterling J, Lindem K, Heaton KJ, Sutker P, Ozonoff DM, 2001. Neuropsychological function in Gulf War veterans: relationships to self-reported toxicant exposures. Am J Ind Med 40, 42–54. [DOI] [PubMed] [Google Scholar]
- White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, Bloom FE, Bunker JA, Crawford F, Graves JC, 2016. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex 74, 449–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.-j., Wang L, Ji C.-f., Gu S.-f., Yin Q, Zuo J, 2021. The role of α7nAChR-mediated cholinergic anti-inflammatory pathway in immune cells. Inflammation 44, 821–834. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I, 2011. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 25, 181–213. [DOI] [PubMed] [Google Scholar]
- Zakirova Z, Crynen G, Hassan S, Abdullah L, Horne L, Mathura V, Crawford F, Ait-Ghezala G, 2015a. A Chronic Longitudinal Characterization of Neurobehavioral and Neuropathological Cognitive Impairment in a Mouse Model of Gulf War Agent Exposure. Front Integr Neurosci 9, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakirova Z, Tweed M, Crynen G, Reed J, Abdullah L, Nissanka N, Mullan M, Mullan MJ, Mathura V, Crawford F, 2015b. Gulf War agent exposure causes impairment of long-term memory formation and neuropathological changes in a mouse model of Gulf War Illness. PloS one 10, e0119579. [DOI] [PMC free article] [PubMed] [Google Scholar]