Abstract
Growth and survival are key determinants of bacterial fitness. However, how resource allocation of bacteria could reconcile these two traits to maximize fitness remains poorly understood. Here, we find that the resource allocation strategy of Bacillus subtilis does not lead to growth maximization on various carbon sources. Survival-related pathways impose strong proteome constraints on B. subtilis. Knockout of a master regulator gene, spo0A, triggers a global resource reallocation from survival-related pathways to biosynthesis pathways, further strongly stimulating the growth of B. subtilis. However, the fitness of spo0A-null strain is severely compromised because of various disadvantageous phenotypes (e.g., abolished sporulation and enhanced cell lysis). In particular, it also exhibits a strong defect in peptide utilization, being unable to efficiently recycle nutrients from the lysed cell debris to maintain long-term viability. Our work uncovers a fitness trade-off between growth and survival that governed by Spo0A-mediated proteome allocation constraints in B. subtilis, further shedding light on the fundamental design principle of bacteria.
The resource allocation strategy of B. subtilis leads to balanced investment on growth and survival.
INTRODUCTION
Rapid growth is a fundamental property of various types of cells including bacterial cells and eukaryotic cells such as yeast cells and tumor cells (1–3) and is, in principle, advantageous for bacterial fitness (1, 3, 4). Therefore, growth rate maximization has been proposed to be an important objective of gene regulation in bacterial cells (1, 3, 5–7). In many circumstances, growth rate is used as a convenient parameter to evaluate microbial fitness (8–10). Recent quantitative studies have suggested that optimal proteome resource allocation enables bacteria to achieve growth rate maximization in various conditions (1, 7, 11–16). Molecularly, global signaling pathways mediated by guanosine tetra- and pentaphosphate [(p)ppGpp] and cyclic adenosine monophosphate (cAMP) enable bacteria to tightly coordinate the biomass growth with proteome resource allocation (1, 12, 15, 17).
A fundamental question is whether faster growth simply means higher fitness. Given that the environments in the natural niches of bacteria are often highly fluctuating, bacterial cells must be capable of surviving under stressful conditions such as nutrient deprivation (18, 19). Therefore, surviving capability should constitute another key part of fitness in addition to growth. In this sense, understanding the relationship between growth and survival is of great importance for understanding the fundamental design principles of microbes and is thus a fundamental direction of microbiology. From traditional perspectives, the regulation of growth and survival might, to a large extent, occur at separate life cycle stages and do not necessarily interfere with each other. For example, the rpoS-mediated general stress response in Escherichia coli is mainly triggered during stationary phase instead of exponential phase (18). However, it has recently been found that slow-growing E. coli cells (in poor nutrients) could sustain longer during carbon starvation than their fast-growing counterparts (in rich nutrients) due to a decreased maintenance rate (20), suggesting that the proteome response during slow growth under nutrient limitation could facilitate the long-term survival of bacterial cells after entry into stationary phase.
It has recently been found that a substantial proteome constraint of inactive ribosomes and other nonflux carrying proteins in E. coli might serve other objectives beyond supporting exponential growth, e.g., adaption to nutrient upshift, motility (1, 21–23). The implementations of these alternative objectives, which require additional proteome resources, might limit the cellular budget of proteome resources (e.g., ribosomes and metabolic proteins) that are required for supporting biomass growth. Hence, investment on these alternative objectives could occur at the expense of exponential growth, leading to a potential fitness trade-off (improve trait A at the expense of trait B) (24). In this sense, the existence of the two key determinants of fitness, growth and survival, raises the important question of whether resource allocation principles of bacteria could simultaneously optimize growth and survival or have to weigh them two against each other.
In this work, we focus on the growth physiology of the model firmicute Bacillus subtilis, a master of social behaviors and cell differentiations (25–27). We find that the strategy of proteome resource allocation in B. subtilis leads to balanced investment on growth-trait and survival-trait instead of growth maximization on various carbon sources including the preferred carbon, glucose. Knockout of spo0A, encoding a master regulator protein, substantially increases the growth capacity (both rate and yield) that results from the resource reallocation from survival-related pathways to biosynthesis pathways. Nevertheless, such a type of resource reallocation improves growth performance at the expense of survival capability of B. subtilis, supporting the existence of a fitness trade-off between growth and survival that governed by Spo0A-mediated proteome allocation constraints in B. subtilis.
RESULTS
Spo0A-mediated trade-off between growth and survival in B. subtilis
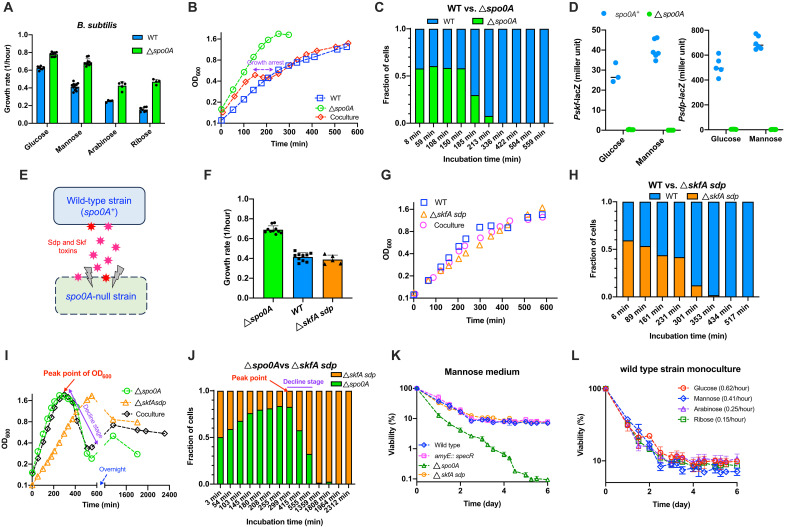
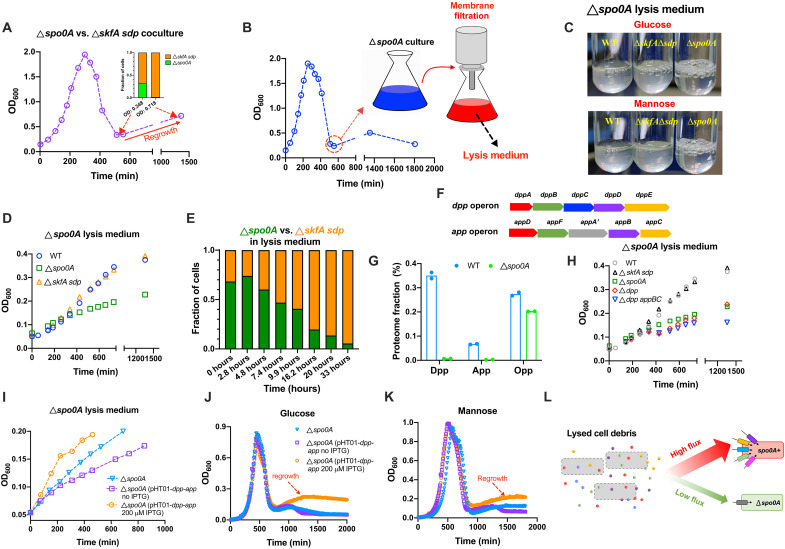
We first characterized the exponential growth rates of B. subtilis 168 wild-type strain and its spo0A-null derivative strain (spo0A encodes the master regulator of sporulation). The growth rates of these two strains were comparable to each other in rich medium (fig. S1). Notably, spo0A-null strain had substantially higher growth rates than wild-type strain in minimal media of various carbon sources including glucose, mannose, arabinose and ribose, respectively (Fig. 1A, see typical log-scale exponential growth curves in fig. S2). These results suggest that B. subtilis wild-type strain does not pursue growth maximization even on the preferred carbon source, glucose. To explore whether the faster growth of spo0A-null strain could confer a fitness advantage, we performed a competitive experiment between spo0A-null strain and wild-type strain by coculturing them in the same medium, which is a standard way to compare the relative fitness between two strains (19, 28, 29). As shown in Fig. 1B, spo0A-null strain (green circle) grew much faster than wild-type strain (blue square) when cultured alone in mannose minimal medium. However, during coculture in mannose medium (Fig. 1B, red diamond), it was quickly outcompeted by wild-type strain at early growth stage (150 min to 213 min) and soon completely disappeared during coculture (Fig. 1C). In consistent with this result, the growth of the coculture was arrested at optical density at 600 nm (OD600) ~0.5 at 150 min to 213 min (marked in purple in Fig. 1B) before restoring, suggesting that spo0A-null strain had undergone massive cell death during this short period. Therefore, the faster growth of spo0A-null strain failed to confer a fitness advantage. It is known that Spo0A is a key regulator of the cannibalism process that carried out by the skf and sdp operons in B. subtilis (30). Cannibalism is a social behavior that occurs during nutrient-limited conditions in which a fraction of cells that begin to enter into sporulation (Spo0A active cells) secrete toxins to kill nonsporulating sibling cells (Spo0A inactive cells) so that the viable cells could feed on the nutrients released by the dead cells to delay the commitment to spore formation (30, 31). β-galactosidase (LacZ) reporter assay showed that the expression of the two cannibalism operons, skf and sdp, were indeed completely abolished in the spo0A-null strain (Fig. 1D). Therefore, spo0A-null strain might be directly killed by the cannibalistic toxins secreted by wild-type cells (which are largely immune to toxins as spo0A+ background) during coculture (Fig. 1E). We then conducted a competitive experiment between the wild-type strain and the cannibalism-deficient skfA sdp–null strain, in which the skfA (encoding the spore-killing peptide) as well as the entire sdpABCDIR operon were deleted. The skfA sdp–null strain had a comparable growth rate to wild-type strain when cultured alone in mannose minimal medium (Fig. 1F); however, it was also quickly outcompeted by wild-type strain during coculture (Fig. 1H; growth curve of the coculture shown in Fig. 1G, pink circles), supporting that cannibalism could be an important factor that cause the fitness defect of spo0A-null strain relative to wild-type strain.
Fig. 1. Spo0A-mediated trade-off between growth and survival in B. subtilis.
Individual data points and SDs of biological replicates were shown in related panels. (A) The exponential growth rates of wild-type (WT) and spo0A-null strain. n = 7, 10, 4, and 7 for WT strain and n = 9, 10, 5, and 4 for spo0A-null strain growing on glucose, mannose, arabinose, and ribose, respectively. (B) The growth curves of WT, spo0A-null strain and their coculture in mannose medium. (C) The competition experiment of WT versus spo0A-null strain during coculturing in mannose medium. The growth curve of coculture corresponds to (B). (D) The lacZ reporter activities of the two cannibalism operons, skf and sdp. For Pskf-lacZ reporter, n = 3 and 6 for spo0A+ background and n = 5 and 4 for spo0A-null background in glucose and mannose media, respectively. For Psdp-lacZ reporter, n = 5 and 6 in glucose and mannose media, respectively, for both spo0A+ and spo0A-null backgrounds. (E) Schematic illustration of the interaction between WT and spo0A-null strain. (F) The exponential growth rates of WT (n = 10), spo0A-null (n = 10), and skfA sdp–null strains (n = 5) in mannose medium. (G) The growth curves of WT, skfA sdp–null strain, and their coculture in mannose medium. (H) The competition experiment of WT versus skfA sdp–null strain during coculturing in mannose medium. (I) The growth curves of spo0A-null, skfA sdp–null strain, and their coculture in mannose medium. (J) The competition experiment of spo0A-null versus skfA sdp–null strain during coculturing in mannose medium. (K) Long-term viability (measured by colony forming units) of four B. subtilis strains after nutrient depletion in mannose medium (n = 3). Viability at time zero (when OD600 reaches peak point) is set as 100%. (L) Long-term viability of WT strain after nutrient depletion on four carbon sources (n = 3). Viability at time zero is set as 100% (n = 3).
We next conducted a competitive experiment between spo0A-null and skfA sdp–null strains, for which the interference of cannibalism could be eliminated, to see whether the fast-growing spo0A-null strain can regain the fitness advantage in this case. The entire coculturing process lasted for more than 36 hours (Fig. 1I, black diamonds). When cultured alone in mannose medium, spo0A-null strain again grew much faster than skfA sdp–null strain (Fig. 1I, green circles versus orange triangles), and the coculture displayed a similar growth pattern with the monoculture of spo0A-null strain (black versus green symbols in Fig. 1I). During the initial ~300 min, spo0A-null strain exhibited a fitness advantage over skfA sdp–null strain with its population fraction steadily increasing from 50% to more than 80% (Fig. 1J) and OD600 of the coculture increased from ~0.15 to the peak point, ~1.95 (black diamonds of Fig. 1I), being consistent with its higher growth capacity and again supporting that fitness defect of spo0A-null strain relative to wild-type strain is likely to be related to its devoid of cannibalism.
Notably, the OD600 of all the three cultures exhibited a substantial decline immediately after reaching the peak point (Fig. 1I, marked in purple). This phenomenon, resulting from the massive cell lysis of B. subtilis population, will be elaborated again later. Although spo0A-null strain predominated in the coculture when OD600 reached the peak point, its fraction dropped substantially during the abrupt decline stage (marked in purple in Fig. 1, I and J), and furthermore, it was outcompeted by skfA sdp–null strain and almost completely disappeared later (Fig. 1J). These results indicate that spo0A-null strain might suffer from a long-term survival defect compared with skfA sdp–null strain during nutrient deprivation. To test this hypothesis, we investigated the long-term viability of various B. subtilis monocultures after nutrient depletion (initiated when OD600 reached peak point). When cultured alone in mannose medium, spo0A-null strain indeed exhibited a much stronger loss of viability than skfA sdp–null strain, wild-type strain, as well as the amyE::specR control strain expressing the spectinomycin-resistance gene (Fig. 1K and table S1), further demonstrating that spo0A-null strain indeed suffers a long-term survival defect. Such a survival defect of spo0A-null strain here could be partially attributed to its inability of forming spores after nutrient depletion (fig. S3). To further test whether the long-term survival defect of spo0A-null strain is related to the spo0A gene itself or the indirect effect of its fast growth, we measured the long-term survival of wild-type strain growing on four carbon sources for which the growth rates were varied four times (from 0.15/hour to 0.62/hour). As shown in Fig. 1L, the viability curves of wild-type cells growing on four carbon sources followed similar trends and the cell viability could be maintained constantly at nearly 10% during long-term incubation, supporting that the survival defect of spo0A-null strain is related to the spo0A gene itself instead of its faster growth than wild-type strain. Overall, the above results demonstrate that spo0A knockout stimulated the growth of B. subtilis at the expense of long-term survival capability and, hence, led to a fitness trade-off between growth and survival here.
The strategy of proteome resource allocation in B. subtilis
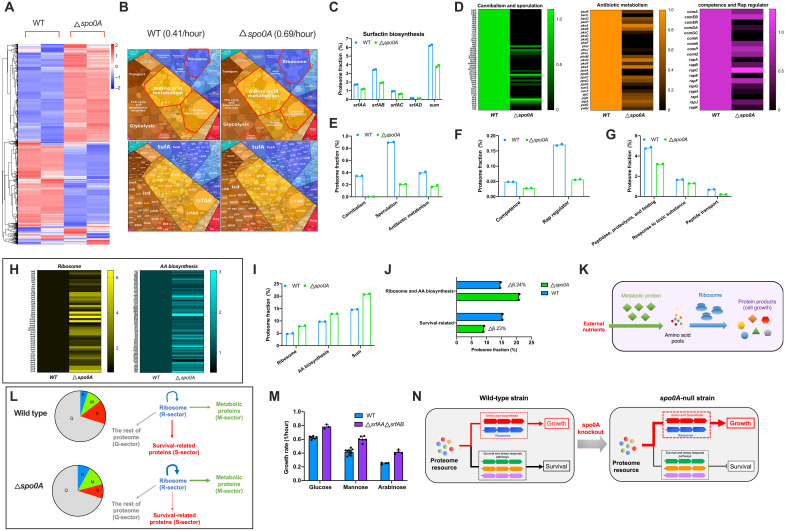
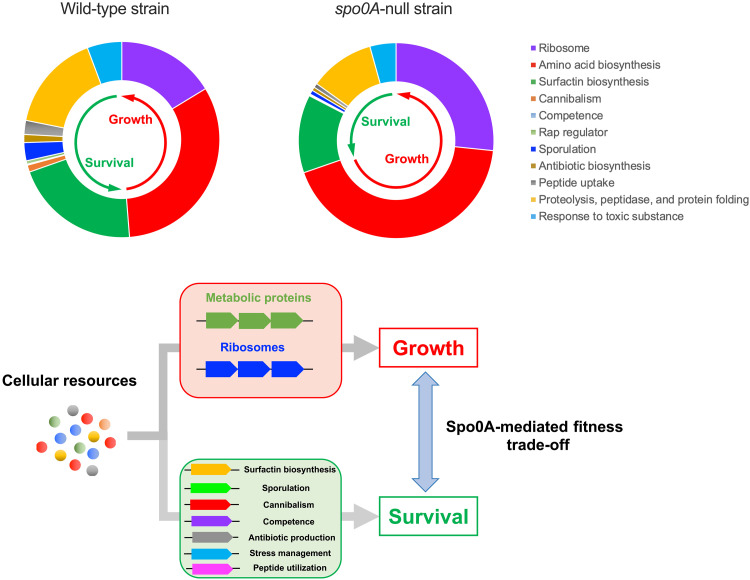
Recent quantitative studies have revealed a profound role of proteome resource allocation in controlling bacterial growth (1, 2, 7, 11, 14, 15). To gain a mechanistic insight into the growth acceleration of spo0A-null strain and the growth-survival trade-off described above, we next investigated the proteome resource allocation of B. subtilis using quantitative mass spectrometry. Our four-dimensional (4D) label-free mass spectrometry approach captured more than 2500 individual proteins of B. subtilis with high reproducibility (tables S2 and S3 and fig. S4). Heatmap and cluster analysis demonstrate a substantial difference in the global gene expression pattern between wild-type and spo0A-null strains (Fig. 2A). A first glimpse of the proteome allocation using proteomaps website (https://proteomaps.net) (32) shows that spo0A-null strain has increased expressions of central biosynthetic pathways including ribosomes and amino acid biosynthetic proteins but a decreased expression of surfactin biosynthesis pathways (25, 33) (belong to the category of cofactor biosynthesis) (Fig. 2B). We further manually quantified the proteome fractions of various specific sectors of social behaviors and cell differentiations, e.g., sporulation, competence, cannibalism, quorum sensing, and antibiotic biosynthesis, which could facilitate the survival and adaption of B. subtilis in the harsh natural environments (25, 30, 34) (table S4). The biosynthetic proteins of surfactin, which participates in regulating cell differentiations and social behaviors (e.g., biofilm, swarming, and quorum sensing) that are crucial for B. subtilis to adapt to some stressful environments (19, 25, 35, 36), accounted for a larger fraction of the proteome in wild-type strain (~6.25%) than spo0A-null strain (~3.85%) (Fig. 2C). Proteins belonging to cannibalism and sporulation groups displayed the most pronounced declining trend in spo0A-null background (Fig. 2, D and E), supporting the dominant role of Spo0A in regulating sporulation and cannibalism (30). The levels of many other social behavior–related proteins involved in antibiotic production (34), competence (25), and Rap regulators (regulate competence, sporulation, and surfactin biosynthesis) (25) also decreased strongly in spo0A-null background (Fig. 2, D to F). Beyond all of this, we also observed a down-regulation in the levels of many specific stress-responsive proteins involved in proteolysis and protein folding, response to toxic substance (Fig. 2, D and G). Collectively, spo0A knockout triggers a global down-regulation of various survival-related pathways that are involved in social behavior, cell differentiation, and stress response of B. subtilis.
Fig. 2. Proteome resource allocation of B. subtilis WT strain and spo0A-null strain.
The two strains were growing in mannose minimal medium. Data correspond to the average of two biological replicates. (A) Heatmap illustration of the proteomes of the two strains. (B) Visualization of the proteomic resource allocation of B. subtilis by proteomaps website. The subsection term of “mitochondrial biogenesis” inside of the large category “translation” based on Kyoto Encyclopedia of Genes and Genomes (KEGG) categorization (includes both prokaryotes and eukaryotes). The readers should treat these proteins here as translation factors. tRNA, transfer RNA. (C) The proteome fractions of surfactin biosynthesis proteins. (D) The relative abundances of the typical individual proteins belonging to various social behaviors, cell differentiations, and stress response pathways. (E to G) The proteome fractions of various survival-related pathways. (H) The relative abundances of individual ribosomal proteins and amino acid biosynthesis proteins. (I) The proteome fractions of ribosomal proteins and amino acid biosynthesis proteins. (J) The changes in the proteome fractions of ribosome and amino acid biosynthesis and survival-related proteins. The survival-related proteins contain all the proteins in (C) and (E) to (G). (K) Illustration of the central metabolic pathways. The external nutrients are absorbed and processed by metabolic proteins into amino acid pools, which are further polymerized into proteins by ribosomes to support cell growth. (L) The coarse-grained illustration of the proteome resource allocations of WT strain and spo0A-null strain. (M) Growth rates of WT strain and srfAA srfAB–null strain on different carbon sources. Individual data points and SDs of biological replicates were shown (n = 7, 10, and 4 for WT strain growing on glucose, mannose, and arabinose, respectively; n = 3, 6, and 4 for srfAA srfAB–null strain growing on glucose, mannose, and arabinose, respectively). (N) spo0A knockout triggers a global proteome resource reallocation from survival pathways to biosynthesis pathways, boosting growth at the expense of long-term survival.
Two major proteome sectors, ribosome and amino acid biosynthesis, exhibited the most pronounced up-regulation of the proteome fraction in spo0A-null background, increasing from 4.9 to 8% and from 9.7 to 12.9%, respectively (Fig. 2, H and I, and table S4). Coincidently, the increase in the proteome fraction of ribosome and amino acid biosynthesis (6.2% of the proteome mass) is the same as the decrease in the proteome fraction of survival-related proteins (6.2% of the proteome mass) (Fig. 2J and table S4). To support high protein synthesis rates and rapid growth, both biosynthesis proteins and ribosomes are essential to support high metabolic fluxes with the former one engaging in synthesizing raw materials such as amino acids and the later one being responsible for proteins synthesis (Fig. 2K) (1, 2). The abundance of ribosome, a key determinant of growth rate (2, 37), increased by more than 60% and thus seemed to be sufficient to account for the 60% increase in the growth rate of spo0A-null strain relative to the wild-type strain. Moreover, quantitative insight into the proteome allocation strategy of E. coli and yeast growing during carbon limitation (23) further suggests that the up-regulation of both ribosomes and amino acid biosynthesis (~6.2% of the proteome mass) is sufficient to account for the faster growth of spo0A-null strain (0.69/hour) than wild-type strain (0.41/hour) here (fig. S5). On the basis of the recent established phenomenological resource allocation model of bacterial growth, the bacterial proteome can be dissected into three coarse-grained sectors (1, 11, 12, 38–40), the metabolic protein sector including enzymes for biosynthesis (M-sector), the ribosome sector (R-sector) and a constant sector (Q-sector) that is not linked to biomass growth (including proteins for metabolic maintenance). The mass fraction of M plus R-sectors is crucial in determining bacterial growth (1, 11, 38). Overexpression of an unnecessary protein (not useful for biomass growth) imposes a proteome burden and compromises the cellular budget of R-sector and M-sector, further inhibiting bacterial growth (11, 40). In light of our study here, we propose a fourth coarse-grained sector, S-sector, that is, the survival-related pathways. Here, the expression of S-sector imposes a proteome burden and limits the cellular budget of R-sector and M-sector of B. subtilis. Knockout of spo0A triggers a resource reallocation from S-sector to both R-sector and M-sector (accounting for ~6.2% of the proteome, Fig. 2J) as less ribosomes are required for making S-sector so that more ribosomes are available to increase the levels of R-sector and M-sector (Fig. 2L). The faster growth of spo0A-null strain than wild-type strain could thus be quantitatively explained by the proteome resource reallocation model (fig. S5). We next deleted the highly expressed surfactin biosynthesis genes (srfAA and srfAB) (25, 33) to release the proteome burden of surfactin biosynthesis (~5% of proteome mass, Fig. 2C) and found that the growth of B. subtilis was indeed boosted (Fig. 2M), further supporting our resource allocation model here. Collectively, these results support that spo0A knockout triggers a global proteome resource reallocation from survival pathways to biosynthesis pathways, boosting bacterial growth at the expense of long-term survival (Fig. 2N).
Cannibalism and cell lysis shape the growth curve of B. subtilis
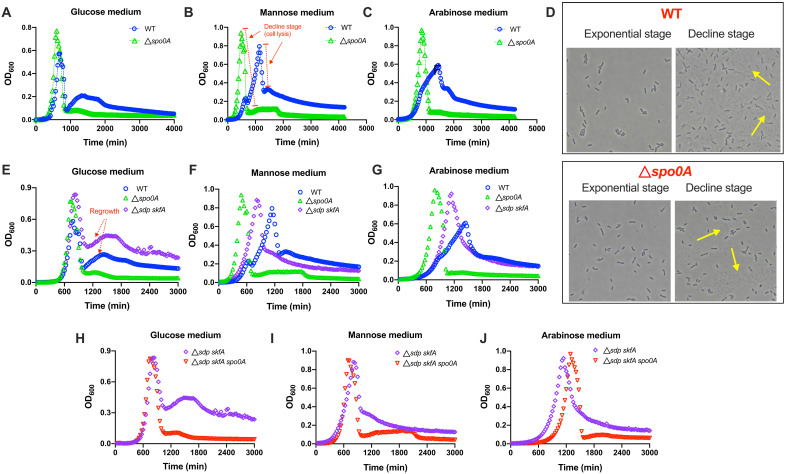
In the growth curves of spo0A-null strain (e.g., Fig. 1I), we noted that the OD600 exhibited an abrupt decline after growth cessation. Because dry mass/OD600 changes very weakly across strains and growth stages (fig. S6), OD600 is a reliable reflection of biomass in our condition. Therefore, the abrupt decline of OD600 means a substantial decrease of population biomass. The abrupt decline stage also occurred in wild-type strain but at a lower extent than spo0A-null strain (figs. S6A and S7, A and B). To gain a more accurate insight into the abrupt decline of population mass, we systematically characterized the growth kinetics of B. subtilis using the automatic microplate reader. As shown in Fig. 3 (A to C), the abrupt decline stages (exemplified in Fig. 3B) of growth curves were observed for both spo0A-null strain and wild-type strain growing on different carbon sources. Microscopic imaging of bacterial cells at this stage showed large quantities of cell debris, confirming the occurrence of massive cell lysis (Fig. 3D, yellow arrow). During the lysis stage, spo0A-null strain and wild-type strain could lose nearly 90 and 60 to 70% of population mass (OD600) in e.g., mannose medium, respectively (Fig. 3B and figs. S6A and S7, A and B). Being consistent with the drop of biomass, cell viability of B. subtilis also dropped strongly during this lysis stage (fig. S8). Cell lysis of wild-type strain also occurred strongly in glucose casamino acid (cAA)–rich medium but weakly in LB-rich medium (blue circles in fig. S9, A to D), and moreover, cell lysis was again aggravated in spo0A-null strain in both of the two rich media (green triangles in fig. S9, A and B).
Fig. 3. Cannibalism and cell lysis shape the growth curves of B. subtilis.
(A to C) Growth curves of WT and spo0A-null strains in glucose, mannose and arabinose minimal media monitored automatically by a microplate reader. The decline stage (cell lysis) is marked in red in (B). Note that the OD600 values obtained from a spectrophotometer (figs. S6A and S7, A and B) and the microplate reader here have systematic deviations from each other (for the same cell sample, the OD600 value of microplate reader is lower than that obtained by spectrophotometer), but the general trends of growth curves are quite consistent with each other. (D) Cell images of WT strain and spo0A-null strain during exponential growth stage and decline (cell lysis) stage. The yellow arrow points to some lysed cell debris. (E to G) The growth curves of WT, spo0A-null, and skfA sdp–null strains in glucose, mannose, and arabinose media monitored automatically by a microplate reader. (H to J) The growth curves of skfA sdp–null and skfA sdp spo0A-null strains in glucose, mannose, and arabinose media monitored automatically by a microplate reader.
In addition to more severe cell lysis compared with wild-type strain, spo0A-null strain also displayed a higher growth yield (the peak point of OD600) than wild-type strain on various carbon sources (Fig. 3, A to C, and figs. S3, S6A, and S7, A and B). We wondered whether the difference in growth yield is related to the effect of cannibalism, which could lead to the killing of a fraction of wild-type cells but is completely abolished in spo0A-null strain (Figs. 1D and 2E). In support of this assumption, we found that cannibalism-deficient skfA sdp–null strain (purple diamonds, Fig. 3, E to G, and fig. S6A) had similar growth yields with spo0A-null strain (green triangles, Fig. 3, E to G, and fig. S6A) but higher growth yields than wild-type strain (blue circles, Fig. 3, E to G, and also figs. S6A and S10) on different carbon sources. Moreover, knockout of spo0A in the skfA sdp–null background did not further increase the growth yields (red triangles, Fig. 3, H to J). These results suggest that cannibalism compromises the growth yield of wild-type strain via killing a fraction of B. subtilis cells during late-exponential phase (legend of fig. S10). In support of this notion, in the competitive experiments shown in Fig. 1 (C and H), the killing of the cannibalism-deficient strain (skfA sdp–null or spo0A-null) by wild-type strain did indeed occur during exponential phase. Hence, a fraction of wild-type cells could also be killed by their counterparts during this stage when cultured alone, further compromising the growth yield.
Cell lysis of B. subtilis during nutrient depletion is mediated by autolysins
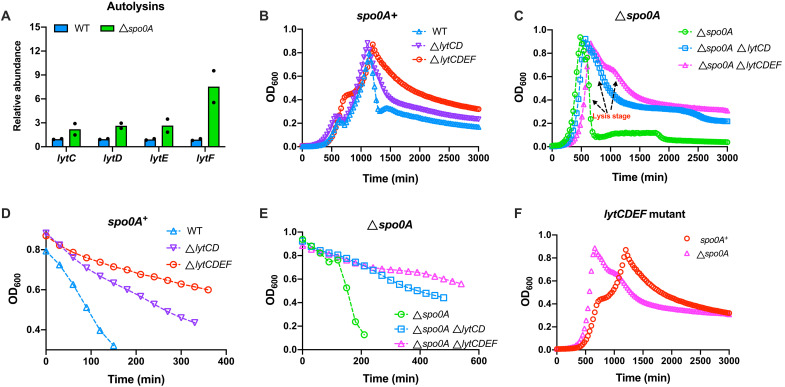
The massive cell lysis of B. subtilis after growth cessation is unusual as this phenomenon were not observed in two fast-growing Gram-negative bacterial species, E. coli and Vibrio natriegens (fig. S11) (41). It is clear that cannibalism is not related to cell lysis here because the cell lysis extent of the cannibalism-deficient skfA sdp–null strain was still comparable to that of wild-type strain (fig. S10 and Fig. 3, E to G), not to mention the even more severe lysis that occurred in the cannibalism-deficient spo0A-null strain. In addition, the cell lysis extent of skfA sdp–null strain also became more severe when its spo0A gene was deleted (Fig. 3, H to J), suggesting that the enhanced cell lysis caused by spo0A knockout originates from other mechanisms. Given that a robust cell wall is crucial for maintenance of the cell structural integrity, we hypothesized that the cell lysis was mediated by some cell wall autolytic enzymes. We then sought to explore the roles of various autolysins including two major ones, LytC and LytD (42, 43), and two minor ones, LytE and LytF (44, 45) which are involved in cell wall degradation during vegetative growth of B. subtilis. As shown in Fig. 4A, the levels of all the four autolysins were much higher in spo0A-null strain than in wild-type strain. Knockout of these autolysin genes strongly reduced the cell lysis for both spo0A+ and spo0A-null backgrounds (Fig. 4, B and C, and figs. S6B and S7C; also see fig. S9, D to F, for rich media). The cell lysis rate was substantially alleviated in both lytCD-null and lytCDEF-null backgrounds (Fig. 4, D and E). Moreover, when the lytCDEF genes had been removed, the residual cell lysis of spo0A+ and spo0A-null strains became comparable to each other (Fig. 4F), suggesting that the more severe cell lysis of spo0A-null strain could attribute to its higher levels of autolysins than wild-type strain.
Fig. 4. Cell lysis of B. subtilis mediated by autolytic enzymes.
(A) The relative abundances of autolysins in WT strain and spo0A-null strain. Data correspond to the average of two biological replicates. (B and C) The growth curves of autolysin-deficient strains in spo0A+ and spo0A-null backgrounds. Growth curves were determined automatically by a microplate reader. (D and E) The effect of autolysin deficiency on the cell lysis of B. subtilis spo0A+ and spo0A-null strains. (F) The growth curves of lytCDEF-null strains in both spo0A+ and spo0A-null backgrounds. Growth curves were determined automatically by a microplate reader.
Peptide uptake is crucial for B. subtilis to feed on the lysed cell debris
The cell lysis process observed here was so substantial that we expected the surviving cells could feed on the nutrients released from the lysed cells. We noted that the mass of bacterial population in some growth curves underwent a notable second-time increment (regrowth) stage after the abrupt lysis stage (e.g., red arrow, Fig. 3E). Recent studies on E. coli have shown that the capability of recycling nutrients released from the dead cells is important for the maintenance of long-term viability of bacterial population (20, 46). We next went back to the competitive experiment between spo0A-null strain and skfA sdp–null strain (Fig. 1, I and J). We noted an interesting phenomenon here: During the lysis stage of the coculture, the fraction of spo0A-null strain dropped quickly from 82% (299 min, peak point of Fig. 1J) to 32% (555 min, Fig. 1J), suggesting that most spo0A-null cells had undergone cell lysis during this stage. Then, after a dozen of hours, the mass (OD600) of the coculture exhibited a regrowth, increasing from 0.35 to 0.72; however, the fraction of spo0A-null cells dropped further from 32% to only 1% during this period (replotted in Fig. 5A). This result is counterintuitive as spo0A-null strain should have a higher growth capacity than skfA sdp–null strain when nutrients (released from cell lysis) become available again. Moreover, when cultured alone in mannose medium, the viability of spo0A-null strain could actually be maintained for a dozen of hours after the lysis stage (fig. S12). We wondered whether the puzzle here could be due to that spo0A-null strain had defects in competing for the nutrients released from the lysed cells against skfA sdp–null strain during coculture. We then collected the supernatant of the spo0A-null culture after lysis and filtered it to obtain the lysis medium, which contains all the nutrient released from the lysed spo0A-null population (Fig. 5B). Both the overnight yield (Fig. 5C) and the growth rate (Fig. 5D) of spo0A-null strain were indeed much lower than that of wild-type and skfA sdp–null strains when growing in the lysis medium. Being consistent with this observation, spo0A-null strain was continuously outcompeted by skfA sdp–null strain during coculturing in the lysis medium, even though it had initially been inoculated at a higher fraction (at ~70%) than skfA sdp–null strain (at ~30%) (Fig. 5E).
Fig. 5. Peptide transport is crucial for B. subtilis to feed on the lysed cell debris.
(A) The coculture experiment of spo0A-null strain and skfA sdp–null strain (replotted from Fig. 1, I and J). The relative fractions of these two strains at OD600 = 0.348 and 0.718 are shown as subpanels. (B) The lysis medium was obtained by filtering the spo0A-null cultures at the end of cell lysis stage (the red circle). (C) The overnight cultures of WT strain, spo0A-null strain, and skfA sdp–null strain in the spo0A glucose- and mannose-lysis media [means that spo0A-null strain had been cultured in glucose or mannose medium before collecting lysis medium as (B)]. (D) The growth kinetics of WT strain, spo0A-null strain, and skfA sdp–null strain in the mannose lysis medium. (E) The competition experiment between spo0A-null strain and skfA sdp–null strain when cocultured in the mannose lysis medium. (F) Schematics of the two peptide transporter operons in B. subtilis, the dpp operon and the app operon. (G) The proteome fractions of the three peptide transporter systems in WT strain and spo0A-null strain. (H) The growth kinetics of WT strain, spo0A-null strain, skfA sdp–null strain, dpp-null strain, and dpp appBC–null strain in the mannose lysis medium. (I) The effect of overexpression of dpp and app operons on the growth kinetics of spo0A-null strain in the mannose lysis medium. The expression of dpp and app operons was driven by the grac01 promoter [isopropyl-β-d-thiogalactopyranoside (IPTG)–inducible] of pHT01 vector. (J and K) The growth curves of spo0A-null strain and its dpp-app–overexpressing strain in normal glucose and mannose medium determined by a microplate reader. (L) Schematics of the coculture of spo0A-null strain and spo0A+ strain using the lysis medium. The higher levels of peptide transporters and utilization proteins in spo0A+ strain confer it a fitness advantage over spo0A-null strain.
The lower growth capacity of spo0A-null strain than wild-type and skfA sdp–null strains in the lysis medium suggests that spo0A-null strain might be lack of some proteins that are essential for the utilization of nutrients contained in the lysis medium. Proteomics studies showed that the expression of the peptide transport proteins (dpp, app, and opp operons) (47–49) decreased strongly in spo0A-null strain with the expressions of Dpp and App proteins being almost completely abolished (Fig. 5, F and G). In addition, spo0A knockout also caused reduced levels of many peptidases (for peptide degradation) (fig. S13). We next investigated the role of dpp and app systems in nutrient acquisition from the lysis medium by B. subtilis. Notably, the dpp-null strain and the dpp appBC–null strain exhibited similar growth defects with spo0A-null strain in the lysis medium (Fig. 5H), supporting that the peptide uptake indeed acts as an important bottleneck for the utilization of nutrient sources in the lysis medium. Because proteins account for most of bacterial biomass (50), the lysis medium is likely to contain many short peptides generated during the process of translation and protein degradation so that peptide transporters and peptidases could enable bacteria to use them. We further found that overexpression of dpp and app operons could stimulate the growth rate of spo0A-null strain in the lysis medium (Fig. 5I), and moreover, Dpp-App overexpression resulted in a notable regrowth of spo0A-null strain after the lysis stage in normal glucose and mannose minimal media (orange symbols in Fig. 5, J and K). Collectively, these results demonstrate that after the abrupt lysis stage, skfA sdp–null strain (which is spo0A+) has a fitness advantage over spo0A-null strain during coculture (Fig. 5A) as the former strain harbors more proteins engaged in peptide uptake and metabolism to facilitate the utilization of the nutrients provided by the lysed cell debris. In such scenario, the limitation of peptide utilization imposes a fitness cost on spo0A-null strain (Fig. 5L).
DISCUSSION
Growth is crucial for bacterial fitness and recent studies have revealed a profound role of proteome resource allocation in growth control of microbes including bacteria and yeasts (1, 7, 11–16). Meanwhile, increasing evidences show that some other objectives such as adaptation to fluctuating environments (4, 51) and chemotaxis (52) are also important. The relation between growth and survival is of special importance as the two traits are key determinants of fitness. However, whether and how resource allocation could reconcile them remains poorly understood. Here, we report a fitness trade-off between growth and survival in B. subtilis that is governed by Spo0A-mediated proteome resource reallocation. As an important term in evolutionary biology, the definition of a fitness trade-off is that improving one trait (trait A) at the expense of another trait (trait B) (24, 53–55). In our study, there exists a direct competition between growth trait and survival trait for the finite proteome resources. The proteome allocation strategy of wild-type B. subtilis strain allows balanced (bet-hedging) investment on growth and survival instead of growth rate maximization on various carbon sources. Such a strategy could allow B. subtilis to be better prepared for the upcoming harsh environments after nutrient depletion. Knockout of spo0A leads to the proteome resource reallocation from survival and stress response to biosynthesis pathways, thus strongly promoting growth at the expense of long-term survival (Fig. 6). The substantial effect of spo0A knockout on stimulating growth of B. subtilis is notable given studies on E. coli suggest that the growth rates could be nearly maximized in various conditions by global regulatory pathways such as (p)ppGpp and cAMP signaling via optimizing proteome resource allocation (1, 5, 6, 15). The proteome burden of survival and stress response is generally believed to be low during exponential stage and thus might not have a notable effect on growth. For example, knockout of rpoS, encoding the master regulator of general stress response and stationary phase (18), does not have notable effects on the exponential growth rates of E. coli under various nutrient conditions (fig. S14) (20). The high proteome investment of B. subtilis on survival and stress response during exponential growth might be partially related to the unique life cycle of B. subtills, which is often dominated by various types of complex social behaviors during adapting to harsh environments (25, 30, 36).
Fig. 6. Spo0A-mediated fitness trade-off between growth and survival that governed by proteome allocation constraints in B. subtilis.
The cellular resource of bacteria is finite, and B. subtilis attempts to balance its proteome investment on biomass growth and survival. In another word, there exists a direct competition between growth trait and survival trait for the finite proteome resources in B. subtilis. Spo0A is a key regulator of the trade-off between growth and survival in B. subtilis. Compared with WT strain, spo0A-null strain has a lower investment on survival-related proteome but a higher investment on growth-related proteome; as a consequence, spo0A-null strain has a substantially higher exponential growth rate but a lower long-term fitness than its spo0A+ counterpart (WT strain or skfA sdp–null strain). In this sense, Spo0A is an important governor of the fitness trade-off between growth and survival in B. subtilis.
Our study shows that Spo0A could act as an important governor of the trade-off relation between growth and survival in B. subtilis via shaping the global resource allocation (Fig. 6). Mechanistically, Spo0A might regulate the expressions of downstream target genes in multiple manners. Spo0A is known to be the master regulator of both sporulation and cannibalism processes (26, 30). In the case of sporulation, Spo0A-P (phosphorylated form) is known to directly activate the transcription of various genes including sigE and sigF, which encode the two major sporulation σ factors (26, 56). In the case of cannibalism, Spo0A-P can directly activate the transcription of skf operon and, alternatively, indirectly activate the expressions of both skf and sdp operons via inhibiting the expression of abrB, which encodes the repressor of cannibalism (30). For many other target genes that up-regulated by Spo0A, much less are known regarding the underlying regulatory mechanisms. For example, the regulatory effect of Spo0A on surfactin biosynthesis may require the relay of many intermediate regulators such as AbrB, SigH, and PhrC (35); The expressions of dpp and app operons are known to be repressed by CodY via direct binding (57). It is possible that there exist some unknown interconnections between Spo0A and CodY regulatory pathways. In addition, the effect of Spo0A on gene expression could also occur in a more general, indirect way via resource allocation. In such scenario, spo0A knockout lowers the expressions of survival and stress responsive genes, releasing higher fractions of RNA polymerases and ribosomes for transcribing and translating those ribosome proteins and amino acid biosynthetic proteins. Such an indirect way of gene regulation via resource allocation has previously been shown in the (p)ppGpp signaling pathway of E. coli (15, 38).
Our study provides an example of growth-survival trade-off relation that is governed by the Spo0A-mediated proteome constraint in B. subtilis. Recent studies of E. coli have investigated the effect of the growth status of bacteria on the long-term survival (20, 58). For example, when growth is limited by nutrient quality, proteome response during slow growth could promote the survival of bacteria and slow growth is then accompanied by a higher survival capability (20, 58). However, when growth is limited by other types of stress such as useless protein overexpression or chloramphenicol treatment, slow growth is accompanied with a lower survival capability because the proteome budget related to survival is also likely to be curtailed during such types of growth limitations (58). Therefore, the negative relation between growth and survival does not always hold and should depend on the specific proteome response.
Trade-off lies at the core of a cross-kingdom research discipline that seeks to investigate the occurrence of the high degree of biodiversity existing in our ecosystem (59, 60). The trade-off between growth and survival could potentially explain why there exist so many slow-growing bacterial species in nature (e.g., the mycobacterial species) (61, 62). It is known that in the natural environment of bacterial kingdom (e.g., soil), fast-growing species are often not the dominant species (63). Moreover, for the same bacterial species, a lot of slow-growing isolates are selected in their natural niches (64, 65). Because bacteria living in their natural niches are often exposed to highly fluctuating environments such as famine-feast cycles (66) and a variety of abiotic stresses (18), survival instead of growth could be preferentially selected in many cases in which a substantial fraction of cellular resource inside bacteria is allocated to the survival-related proteome to prepare for future uncertainties, thus compromising the growth capacity. Insight into the trade-off mechanism between growth and survival allows us to artificially modulate the fitness landscape of microbes across conditions. Our finding could also have direct implications for the practice of synthetic biology and microbial biotechnology, in which an artificially designed genetic circuit could also have a trade-off relation with the native system of the host bacteria as its operation could take the cellular resources away from supporting cell growth of the host bacteria (67, 68). In this sense, lessons from the trade-off relation between growth and survival in the native bacterial system could be useful in guiding the design of a fitness-robust strain.
Our study has also systematically characterized the growth kinetics of B. subtilis. In a classical microbiology textbook, it is generally proposed that bacterial growth follows a four-stages curve: lag phase, exponential phase, stationary phase, and decline phase (sometimes including a fifth stage called long-term stationary phase) (69). When bacteria like E. coli enter into stationary phase, the population biomass could stay more or less constant for a certain period (fig. S11). However, the growth of B. subtilis does not follow the standard four-stage curve and is strongly affected by cannibalism and cell lysis. During late exponential phase, a fraction of wild-type cells is killed by cannibalism, which compromises the growth yield of wild-type cells. More notably, an abrupt cell lysis stage mediated by autolysins occurs after growth cessation; during this period, 60 to 90% of cells (especially for spo0A-null strain) in the population are lysed. Physiologically, such a massive cell lysis of the population could facilitate the long-term fitness of the rest small portion of viable cells by using the nutrients released from lysed cells to sustain for a longer time. Collectively, the cell lysis phenomenon could be regarded as an important survival-related physiological process of B. subtilis.
Last, we highlight the physiological role of peptide uptake and metabolism in facilitating bacterial survival under harsh environments such as nutrient starvation. The peptide uptake systems have been widely identified among various bacterial species for a long time (70). However, its physiological role in bacteria is less well understood relative to those well-characterized carbon or amino acid transporters (70, 71). Here, we show that for B. subtilis, the peptide uptake system is required for recycling the nutrients released from the lysed cells by the rest viable cells, which is crucial for the fitness of B. subtilis during nutrient depletion. The lack of peptide uptake systems in spo0A-null strain severely compromises its capability of regrowth in the lysis medium. It has recently been shown that the recycling of nutrients released from the dead cells is crucial for the long-term survival of E. coli population during stationary phase (20, 46). Given that peptide uptake systems are widely distributed in bacteria, it could be a general mechanism for bacterial cells to recycle nutrients from cell carcasses to maintain viability during stationary phase (nutrient deprivation).
MATERIALS AND METHODS
Strain construction
Strains in the studies were all derivatives of B. subtilis 168 (trpC2) (72). The gene knockout process of B. subtilis was based on homologous recombination with the assistance of pDG1730–Sal I (SpcR) integration vector [with an additional Sal I site at the downstream region of amyE’ of pDG1730 (73)]. The flanking regions (usually at the range of 600 to 1000 bp) of both downstream and upstream of a specific gene were polymerase chain reaction (PCR) amplified using GoldenStar T6 super PCR mix (Beijing Tsingke Biotech Co., Ltd.) and inserted into the flanking regions of spectinomycin-resistance gene (spcR) of pDG1730–Sal I (Aat II/Bam HI sites for upstream fragment; Eco RV/Sal I sites for downstream fragment). The resultant vector was linearized by Xho I or Spe I endonuclease digestion and then transformed into the wild-type strain using natural competence. The double-crossover gene knockout transformants were further screened for by colony PCR using 2× GS Taq PCR mix (Genesand Biotech Co., Ltd). The DNA marker (GoldBand DL5,000 DNA Marker) and related electrophoresis reagents such as 50× tris-acetate-EDTA buffer and agarose were purchased from Yeasen Biotech. To construct multiple gene knockout strains, the spcR of pDG1730 vector was replaced with catR (chloramphenicol resistance), ermR (erythromycin resistance), kanR (kanamycin resistance), and tetR (tetracycline resistance), respectively, to obtain gene knockout vectors with different antibiotic markers. Therefore, mutants of multiple gene deletions were constructed sequentially by homologous recombination with different antibiotic resistance markers (e.g., ∆skfA::catR, ∆sdpABCDIR::ermR double-knockout strain; ∆lytC::catR, ∆lytD::ermR, ∆lytE::kanR, ∆lytF::tetR quadruple-knockout strain; dpp::catR, appBC::kanR double-knockout strain). The amyE::spcR control strain was constructed by directly transforming the linearized pDG1730–Sal I vector to wild-type strain to screen for the strain with spcR gene inserted into the amyE locus of B. subtilis. The concentrations of antibiotics were as below: spectinomycin (Solarbio Life Sciences), 100 μg/ml; chloramphenicol (Coolaber), 5 μg/ml; erythromycin (Solarbio Life Sciences), 2 μg/ml; kanamycin (Coolaber), 7.5 μg/ml; and tetracycline (Coolaber), 15 μg/ml.
To construct the Psdp-lacZ and Pskf-lacZ reporter strain, the lacZ gene of E. coli was PCR amplified and inserted into the Bam HI site of the pAX01 integration vector (74). The promoter regions of sdp operon and skf operon were PCR amplified using the B. subtilis genome as the template, being further moved into the Xho I/Spe I site of pAX01-lacZ vector. The resultant lacZ reporter vectors were then transformed and integrated into the genome of B. subtilis to obtain the lacZ reporter strains.
To construct the peptide transporter overexpression strain, the entire dpp operon and app operon were PCR amplified (Ultra HiFidelity PCR mix, TianGen) and assembled together into the Bam HI/Sma I site of pHT01 (75) using the Gibbon assembly kit, 2X MultiF Seamless Assembly Mix (RK21020) (ABclonal), generating the pHT01-dpp-app vector and further transformed into B. subtilis.
Medium
B. subtilis was cultured in a modified C-minimal medium (76) or LB broth (Coolaber). The basic recipe of C-minimal medium is as follows: K2HPO4 (16 g/liter), KH2PO4 (4 g/liter), MnSO4·4H2O (2.32 mg/liter), MgSO4·7H2O (0.123 g/liter), 12.5 μM ZnCl2, tryptophan (50 mg/liter), and ferric ammonium citrate (22 mg/liter). For all the C-minimal media used, 20 mM NH4Cl was used as the nitrogen source. Carbon sources used included 0.4% glucose, 0.4% mannose, 0.4% arabinose, and 0.4% ribose. In addition to 0.4% glucose, glucose plus cAA medium also contained 0.4% cAA.
Cell growth measurement
Cell growth was performed either in an air bath shaker (200 rpm, 37°C) or in a 24-well microplate inside a microplate reader. A standard cell culturing procedure contains three steps: seed culture, preculture, and final experimental culture (12, 39, 77). For the seed culture, cells from a fresh colony were inoculated into LB broth and grew at 37°C to OD600 ≈ 1. The seed culture was then washed and transferred into C-minimal medium supplemented with different carbon sources (the same as the final experimental culture) and grew overnight as precultures. The overnight preculture was inoculated into the same minimal medium at an initial OD600 ≈ 0.02 to 0.03 as the final experimental culture (if the overnight preculture was still within exponential growth stage at OD600 from 0.2 to 0.6, then the final culture could be initiated at a higher initial OD600 ≈ 0.05). The growth curve was either manually determined by a Genesys 50 spectrophotometer (Thermo Fisher Scientific) or automatically by an Agilent Biotek Synergy H1 microplate reader. For measuring the exponential growth rate, five to eight OD600 points (generally at the range of 0.05 to 0.7) were measured by a Genesys 50 spectrophotometer to obtain the growth curve, from which the growth rate was calculated on the basis of the exponential range (the linear range at log-scale exponential curve).
Two-strain competitive experiment
Two B. subtilis strains were first cultured separately in mannose medium to exponential phase (OD600 ≈ 0.6) and then mixed at a 1:1 ratio (OD600) into the same minimal medium at an initial OD600 ≈ 0.1. At the same time, single strain monoculture was also inoculated at an initial OD600 ≈ 0.1 for comparing the growth curves of monoculture and coculture. The growth curves of the cultures were then determined by a Genesys 50 spectrophotometer (Thermo Fisher Scientific). At different OD600 points, the coculture was pipetted into two types of LB solid agars: one with no antibiotics for counting the total cell number and the other one supplemented with specific antibiotics for counting the number of one strain. For example, for the coculture of wild-type strain and spo0A-null strain, LB plates with spectinomycin (100 μg/ml) were used to count the cell number of spo0A-null strain; for the coculture of wild-type strain and ∆skfA::catR, ∆sdpABCDIR::ermR double-mutant strain, LB plates with chloramphenicol (5 μg/ml) and erythromycin (2 μg/ml) were applied to count the cell number of ∆skfA::catR, ∆sdpABCDIR::ermR double-mutant strain.
β-galactosidase assay
The β-galactosidase (LacZ) activities of Psdp-lacZ and Pskf-lacZ reporter strains were determined by the classical o-nitrophenyl β-d-galactopyranoside (ONPG, purchased from GLPBIO) colorimetric method (12).
Dry mass measurement
About 100 to 250 ml of cell culture at different growth stages were collected by centrifugation with the supernatant medium being discarded. The cell pellets were then washed by 50 ml of double-distilled H2O (ddH2O) and collected again by centrifugation. The OD600 of the discarded supernatants was measured to calibrate the loss of cells. The cell pellets were then suspended in 2 ml of ddH2O and transferred to aluminum pans for baking overnight until reaching a constant weight. The weights of cell pellets were then normalized by the calibrated OD600.
Determination of sporulation efficiency
The sporulation efficiency (spore fraction) of B. subtilis was assayed by the heat shock method (78). The total viable cell count (Ntotal) of B. subtilis was determined by plating the serially diluted samples on LB solid agar media. For counting the spores of B. subtilis, the cultures were first subject to 80°C heating for 20 min to kill the vegetative cells and then counted by plating to get the Nspore (the number of spores). The sporulation efficiency thus equals to Nspore/Ntotal.
Proteomics
The proteomic process generally followed the same procedure as described previously for E. coli (51) and detailed again below. Exponential culture of B. subtilis (30 ml; OD600, 0.6) was collected by centrifugation (4°C, 8500 rpm for 5 min) in a prechilled 50-ml centrifuge tube (Nest Biotech). The cell pellets were then washed twice by phosphate-buffered saline, dried by a speed vacuum concentrator (CV600, Beijing JM Technology Co., Ltd.), and stored at a −80°C freezer before proteomic analysis. The 4D label-free proteomic experiment (79) was performed by Jingjie PTM Biolabs (Hang Zhou). For details: The cell pellets were first subject to ultrasonication in lysis buffer [8 M urea, 1% Triton X-100, 10 mM dithiothreitol (DTT), 1% protease inhibitor cocktail, and 2 mM EDTA]. The cell debris was then removed by centrifugation (4°C, 12,000g for 10 min) and the supernatant was transferred into a new centrifuge tube for measuring protein concentrations using a The bicinchoninic acid assay (BCA) kit. Protein samples were next pelleted by 20% trichloroacetic acid (TCA) at 4°C for 2 hours and then collected with centrifugation (4500 g/min) for 5 min. The precipitates were washed for twice by precooled acetone and further supplemented with 200 mM triethylammonium bicarbonate (TEAB). Trypsin was then added at 1:50 trypsin-to-protein mass ratio for digestion overnight. The solution was further reduced with 5 mM DTT for 30 min at 56°C and alkylated with 11 mM iodoacetamide for 15 min at room temperature in darkness. Last, the peptides were desalted by a C18 SPE column. Solvent A (0.1% formic acid, 2% acetonitrile) and solvent B (0.1% formic acid, 100% acetonitrile) were then used for the following peptides separation and Ultra Performance Liquid Chromatography (UPLC) procedures. The peptides were dissolved in solvent A and separated by the NanoElute UPLC system. The flow setting of UPLC was as follows: 0 to 43 min, 6 to 22% B; 43 to 55 min, 22 to 30% B; 55 to 58 min, 30 to 80% B; 58 to 61 min, 80% B; flow rate, 450 nl/min. After UPLC separation, the peptide was set into capillary ionization source for ionization and further analyzed by the timsTOF Pro mass spectrometry system. The electrospray voltage applied was set at 1.6 to 1.8 kV. Both the original peptide ion and its secondary fragments were detected and analyzed by high-resolution time of flight (TOF). The mass/charge ratio scan range was 100 to 1700 for full scan. Precursors with charge states 0 to 5 were selected for fragmentation, and 10 PASEF-MS/MS scans were acquired per cycle. The dynamic exclusion was set to 30 s. The mass spectra data were searched against the SwissProt B. subtilis 168 databases and analyzed by Maxquant software (80), which gave the information of both label-free quantification (LFQ) intensity and intensity based absolute quantification (iBAQ) intensity. The information of the relative abundance of each protein across different conditions was given by LFQ intensity. The mass proteome fraction (absolute abundance) of individual proteins was obtained using the iBAQ intensity of each protein to multiply the molecular weight (we referred to as “iBAQ mass” in table S3) and further normalized by the sum of the whole proteome (as iBAQ is a proxy of the copy number of each protein). The iBAQ mass of individual proteins together with the gene locus-tag were submitted to proteomaps website to obtain the Kyoto Encyclopedia of Genes and Genomes (KEGG) resource allocation map of B. subtilis cells (32).
Statistical analysis
The SDs (error bars) of data were analyzed with GraphPad Prism 8.0. Replicate numbers are specified in the captions of the figures where they appear. Data points for each biological replicates are displayed in the related figures.
Acknowledgments
We thank members of the Terry Hwa laboratory (UCSD) for useful discussions during various stages of the work. We thank J. Zhang (Shanghai Jiao Tong University), Z. Zhou (Jiangnan University), and M. Sun (Huazhong Agricultural University) for providing the pHT01 vector, the pAX01 vector, and the pDG1730 vector, respectively.
Funding: This study was supported by the National Natural Science Foundation of China, grants 32022001 and 32270034 (M.Z.); the National Natural Science Foundation of China, grant 31970027 (X.D.); the Changjiang Young Scholar Program of Chinese Ministry of Education (X.D.); the National Key Research and Development Program of China, grant 2022YFF1000400 (X.D. and M.Z.); the Natural Science Funds for Distinguished Young Scholar of Hubei Province, 2022CFA044 (M.Z.); and the Fundamental Research Funds for the Central Universities (M.Z. and X.D.).
Author contributions: Conceptualization: X.D. and M.Z. Methodology: M.Z. and X.D. Investigation: M.Z., Q.W., H.M., F.H., Y.W., and X.D. Supervision: X.D. Writing—original draft: X.D. and M.Z. Writing—review and editing: X.D. and M.Z.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD039192.
Supplementary Materials
This PDF file includes:
Table S1
Legends for tables S2 to S4
Figs. S1 to S14
Other Supplementary Material for this manuscript includes the following:
Tables S2 to S4
REFERENCES AND NOTES
- 1.M. Scott, T. Hwa, Shaping bacterial gene expression by physiological and proteome allocation constraints. Nat. Rev. Microbiol. 21, 327–342 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.X. Dai, M. Zhu, Coupling of ribosome synthesis and translational capacity with cell growth. Trends Biochem. Sci. 45, 681–692 (2020). [DOI] [PubMed] [Google Scholar]
- 3.D. Molenaar, R. van Berlo, D. de Ridder, B. Teusink, Shifts in growth strategies reflect tradeoffs in cellular economics. Mol. Syst. Biol. 5, 323 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.M. Basan, T. Honda, D. Christodoulou, M. Horl, Y. F. Chang, E. Leoncini, A. Mukherjee, H. Okano, B. R. Taylor, J. M. Silverman, C. Sanchez, J. R. Williamson, J. Paulsson, T. Hwa, U. Sauer, A universal trade-off between growth and lag in fluctuating environments. Nature 584, 470–474 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.X. P. Hu, H. Dourado, P. Schubert, M. J. Lercher, The protein translation machinery is expressed for maximal efficiency in Escherichia coli. Nat. Commun. 11, 5260 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.S. Kostinski, S. Reuveni, Ribosome composition maximizes cellular growth rates in E. coli. Phys. Rev. Lett. 125, 028103 (2020). [DOI] [PubMed] [Google Scholar]
- 7.J. Bjorkeroth, K. Campbell, C. Malina, R. Yu, F. Di Bartolomeo, J. Nielsen, Proteome reallocation from amino acid biosynthesis to ribosomes enables yeast to grow faster in rich media. Proc. Natl. Acad. Sci. U.S.A. 117, 21804–21812 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.M. Eames, T. Kortemme, Cost-benefit tradeoffs in engineered lac operons. Science 336, 911–915 (2012). [DOI] [PubMed] [Google Scholar]
- 9.G. I. Lang, A. W. Murray, D. Botstein, The cost of gene expression underlies a fitness trade-off in yeast. Proc. Natl. Acad. Sci. U.S.A. 106, 5755–5760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.C. F. Pope, T. D. McHugh, S. H. Gillespie, Methods to determine fitness in bacteria. Methods Mol. Biol. 642, 113–121 (2010). [DOI] [PubMed] [Google Scholar]
- 11.M. Scott, C. W. Gunderson, E. M. Mateescu, Z. Zhang, T. Hwa, Interdependence of cell growth and gene expression: Origins and consequences. Science 330, 1099–1102 (2010). [DOI] [PubMed] [Google Scholar]
- 12.C. You, H. Okano, S. Hui, Z. Zhang, M. Kim, C. W. Gunderson, Y. P. Wang, P. Lenz, D. Yan, T. Hwa, Coordination of bacterial proteome with metabolism by cyclic AMP signalling. Nature 500, 301–306 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.G. W. Li, D. Burkhardt, C. Gross, J. S. Weissman, Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell 157, 624–635 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Z. Tomáš, Quantitative insights into the cyanobacterial cell economy. eLife 8, e42508 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.M. Zhu, X. Dai, Growth suppression by altered (p)ppGpp levels results from non-optimal resource allocation in Escherichia coli. Nucleic Acids Res. 47, 4684–4693 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.J. Xia, B. J. Sánchez, Y. Chen, K. Campbell, S. Kasvandik, J. Nielsen, Proteome allocations change linearly with the specific growth rate of Saccharomyces cerevisiae under glucose limitation. Nat. Commun. 13, 2819 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.C. Wu, R. Balakrishnan, N. Braniff, M. Mori, G. Manzanarez, Z. Zhang, T. Hwa, Cellular perception of growth rate and the mechanistic origin of bacterial growth law. Proc. Natl. Acad. Sci. U.S.A. 119, e2201585119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A. Battesti, N. Majdalani, S. Gottesman, The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 65, 189–213 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.A. Oslizlo, P. Stefanic, I. Dogsa, I. Mandic-Mulec, Private link between signal and response in Bacillus subtilis quorum sensing. Proc. Natl. Acad. Sci. U.S.A. 111, 1586–1591 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.E. Biselli, S. J. Schink, U. Gerland, Slower growth of Escherichia coli leads to longer survival in carbon starvation due to a decrease in the maintenance rate. Mol. Syst. Biol. 16, e9478 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.M. Mori, S. Schink, D. W. Erickson, U. Gerland, T. Hwa, Quantifying the benefit of a proteome reserve in fluctuating environments. Nat. Commun. 8, 1225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R. Balakrishnan, R. T. de Silva, T. Hwa, J. Cremer, Suboptimal resource allocation in changing environments constrains response and growth in bacteria. Mol. Syst. Biol. 17, e10597 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.M. Mori, Z. Zhang, A. Banaei-Esfahani, J. B. Lalanne, H. Okano, B. C. Collins, A. Schmidt, O. T. Schubert, D. S. Lee, G. W. Li, R. Aebersold, T. Hwa, C. Ludwig, From coarse to fine: The absolute Escherichia coli proteome under diverse growth conditions. Mol. Syst. Biol. 17, e9536 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.T. Garland Jr., Trade-offs. Curr. Biol. 24, R60–R61 (2014). [DOI] [PubMed] [Google Scholar]
- 25.M. Kalamara, M. Spacapan, I. Mandic-Mulec, N. R. Stanley-Wall, Social behaviours by Bacillus subtilis: Quorum sensing, kin discrimination and beyond. Mol. Microbiol. 110, 863–878 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D. Higgins, J. Dworkin, Recent progress in Bacillus subtilis sporulation. FEMS Microbiol. Rev. 36, 131–148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Y. Qin, L. L. Angelini, Y. Chai, Bacillus subtilis cell differentiation, biofilm formation and environmental prevalence. Microorganisms 10, 1108 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.M. J. Wiser, R. E. Lenski, A comparison of methods to measure fitness in Escherichia coli. PLOS ONE 10, e0126210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.J. N. Barber, A. L. Sezmis, L. C. Woods, T. D. Anderson, J. M. Voss, M. J. McDonald, The evolution of coexistence from competition in experimental co-cultures of Escherichia coli and Saccharomyces cerevisiae. ISME J. 15, 746–761 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.J. E. González-Pastor, Cannibalism: A social behavior in sporulating Bacillus subtilis. FEMS Microbiol. Rev. 35, 415–424 (2011). [DOI] [PubMed] [Google Scholar]
- 31.J. E. González-Pastor, E. C. Hobbs, R. Losick, Cannibalism by sporulating bacteria. Science 301, 510–513 (2003). [DOI] [PubMed] [Google Scholar]
- 32.W. Liebermeister, E. Noor, A. Flamholz, D. Davidi, J. Bernhardt, R. Milo, Visual account of protein investment in cellular functions. Proc. Natl. Acad. Sci. U.S.A. 111, 8488–8493 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.F. Hu, Y. Liu, S. Li, Rational strain improvement for surfactin production: Enhancing the yield and generating novel structures. Microb. Cell Fact. 18, 42 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.T. Stein, Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 56, 845–857 (2005). [DOI] [PubMed] [Google Scholar]
- 35.F. B. Rahman, B. Sarkar, R. Moni, M. S. Rahman, Molecular genetics of surfactin and its effects on different sub-populations of Bacillus subtilis. Biotechnol. Rep. (Amst.) 32, e00686 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.T. Tian, B. Sun, H. Shi, T. Gao, Y. He, Y. Li, Y. Liu, X. Li, L. Zhang, S. Li, Q. Wang, Y. Chai, Sucrose triggers a novel signaling cascade promoting Bacillus subtilis rhizosphere colonization. ISME J. 15, 2723–2737 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.N. M. Belliveau, G. Chure, C. L. Hueschen, H. G. Garcia, J. Kondev, D. S. Fisher, J. A. Theriot, R. Phillips, Fundamental limits on the rate of bacterial growth and their influence on proteomic composition. Cell Systems 12, 924–944.e2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.M. Scott, S. Klumpp, E. M. Mateescu, T. Hwa, Emergence of robust growth laws from optimal regulation of ribosome synthesis. Mol. Syst. Biol. 10, 747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.X. Dai, M. Zhu, M. Warren, R. Balakrishnan, V. Patsalo, H. Okano, J. R. Williamson, K. Fredrick, Y. P. Wang, T. Hwa, Reduction of translating ribosomes enables Escherichia coli to maintain elongation rates during slow growth. Nat. Microbiol. 2, 16231 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.M. Scott, T. Hwa, Bacterial growth laws and their applications. Curr. Opin. Biotechnol. 22, 559–565 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.H. H. Lee, N. Ostrov, B. G. Wong, M. A. Gold, A. S. Khalil, G. M. Church, Functional genomics of the rapidly replicating bacterium Vibrio natriegens by CRISPRi. Nat. Microbiol. 4, 1105–1113 (2019). [DOI] [PubMed] [Google Scholar]
- 42.S. A. Blackman, T. J. Smith, S. J. Foster, The role of autolysins during vegetative growth of Bacillus subtilis 168. Microbiology 144, 73–82 (1998). [DOI] [PubMed] [Google Scholar]
- 43.T. J. Smith, S. A. Blackman, S. J. Foster, Autolysins of Bacillus subtilis: Multiple enzymes with multiple functions. Microbiology 146, 249–262 (2000). [DOI] [PubMed] [Google Scholar]
- 44.H. Yamamoto, S. Kurosawa, J. Sekiguchi, Localization of the vegetative cell wall hydrolases LytC, LytE, and LytF on the Bacillus subtilis cell surface and stability of these enzymes to cell wall-bound or extracellular proteases. J. Bacteriol. 185, 6666–6677 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.R. Chen, S. B. Guttenplan, K. M. Blair, D. B. Kearns, Role of the sigmaD-dependent autolysins in Bacillus subtilis population heterogeneity. J. Bacteriol. 191, 5775–5784 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.S. J. Schink, E. Biselli, C. Ammar, U. Gerland, Death rate of E. coli during starvation is set by maintenance cost and biomass recycling. Cell Syst. 9, 64–73.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 47.F. J. Slack, J. P. Mueller, M. A. Strauch, C. Mathiopoulos, A. L. Sonenshein, Transcriptional regulation of a Bacillus subtilis dipeptide transport operon. Mol. Microbiol. 5, 1915–1925 (1991). [DOI] [PubMed] [Google Scholar]
- 48.A. Koide, J. A. Hoch, Identification of a second oligopeptide transport system in Bacillus subtilis and determination of its role in sporulation. Mol. Microbiol. 13, 417–426 (1994). [DOI] [PubMed] [Google Scholar]
- 49.A. Zaprasis, J. Brill, M. Thüring, G. Wünsche, M. Heun, H. Barzantny, T. Hoffmann, E. Bremer, Osmoprotection of Bacillus subtilis through import and proteolysis of proline-containing peptides. Appl. Environ. Microbiol. 79, 576–587 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.S. Klumpp, M. Scott, S. Pedersen, T. Hwa, Molecular crowding limits translation and cell growth. Proc. Natl. Acad. Sci. U.S.A. 110, 16754–16759 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.M. Zhu, X. Dai, Stringent response ensures the timely adaptation of bacterial growth to nutrient downshift. Nat. Commun. 14, 467 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.B. Ni, R. Colin, H. Link, R. G. Endres, V. Sourjik, Growth-rate dependent resource investment in bacterial motile behavior quantitatively follows potential benefit of chemotaxis. Proc. Natl. Acad. Sci. U.S.A. 117, 595–601 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D. Reznick, K. King, Antibiotic resistance: Evolution without trade-offs. Nat. Ecol. Evol. 1, 66 (2017). [DOI] [PubMed] [Google Scholar]
- 54.C. Michaux, S. Ronneau, R. T. Giorgio, S. Helaine, Antibiotic tolerance and persistence have distinct fitness trade-offs. PLOS Pathog. 18, e1010963 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.A. F. Bennett, R. E. Lenski, An experimental test of evolutionary trade-offs during temperature adaptation. Proc. Natl. Acad. Sci. U.S.A. 104, 8649–8654 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.V. Molle, M. Fujita, S. T. Jensen, P. Eichenberger, J. E. Gonzalez-Pastor, J. S. Liu, R. Losick, The Spo0A regulon of Bacillus subtilis. Mol. Microbiol. 50, 1683–1701 (2003). [DOI] [PubMed] [Google Scholar]
- 57.B. R. Belitsky, A. L. Sonenshein, Genetic and biochemical analysis of CodY-binding sites in Bacillus subtilis. J. Bacteriol. 190, 1224–1236 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.S. Schink, C. Ammar, Y. F. Chang, R. Zimmer, M. Basan, Analysis of proteome adaptation reveals a key role of the bacterial envelope in starvation survival. Mol. Syst. Biol. 18, e11160 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.F. Farahpour, M. Saeedghalati, V. S. Brauer, D. Hoffmann, Trade-off shapes diversity in eco-evolutionary dynamics. eLife 7, e36273 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.T. Ferenci, Trade-off mechanisms shaping the diversity of bacteria. Trends Microbiol. 24, 209–223 (2016). [DOI] [PubMed] [Google Scholar]
- 61.J. L. Weissman, S. Hou, J. A. Fuhrman, Estimating maximal microbial growth rates from cultures, metagenomes, and single cells via codon usage patterns. Proc. Natl. Acad. Sci. U.S.A. 118, e2016810118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.G. M. Cook, M. Berney, S. Gebhard, M. Heinemann, M. Niederweis, Physiology of mycobacteria. Adv. Microb. Physiol. 55, 81–182 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.B. J. Campbell, D. L. Kirchman, Bacterial diversity, community structure and potential growth rates along an estuarine salinity gradient. ISME J. 7, 210–220 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.J. Li, R. L. Mau, P. Dijkstra, B. J. Koch, E. Schwartz, X. A. Liu, E. M. Morrissey, S. J. Blazewicz, J. Pett-Ridge, B. W. Stone, M. Hayer, B. A. Hungate, Predictive genomic traits for bacterial growth in culture versus actual growth in soil. ISME J. 13, 2162–2172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.R. Mikkola, C. G. Kurland, Selection of laboratory wild-type phenotype from natural isolates of Escherichia coli in chemostats. Mol. Biol. Evol. 9, 394–402 (1992). [DOI] [PubMed] [Google Scholar]
- 66.B. B. Jorgensen, A. Boetius, Feast and famine--microbial life in the deep-sea bed. Nat. Rev. Microbiol. 5, 770–781 (2007). [DOI] [PubMed] [Google Scholar]
- 67.C. Liao, A. E. Blanchard, T. Lu, An integrative circuit-host modelling framework for predicting synthetic gene network behaviours. Nat. Microbiol. 2, 1658–1666 (2017). [DOI] [PubMed] [Google Scholar]
- 68.A. J. Lopatkin, J. J. Collins, Predictive biology: Modelling, understanding and harnessing microbial complexity. Nat. Rev. Microbiol. 18, 507–520 (2020). [DOI] [PubMed] [Google Scholar]
- 69.S. E. Finkel, Long-term survival during stationary phase: Evolution and the GASP phenotype. Nat. Rev. Microbiol. 4, 113–120 (2006). [DOI] [PubMed] [Google Scholar]
- 70.J. W. Payne, M. W. Smith, Peptide transport by micro-organisms. Adv. Microb. Physiol. 36, 1–80 (1994). [DOI] [PubMed] [Google Scholar]
- 71.P. Garai, K. Chandra, D. Chakravortty, Bacterial peptide transporters: Messengers of nutrition to virulence. Virulence 8, 297–309 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.P. R. Burkholder, N. H. Giles, Induced biochemical mutations in Bacillus subtilis. Am. J. Bot. 34, 345–348 (1947). [PubMed] [Google Scholar]
- 73.A. M. Guérout-Fleury, N. Frandsen, P. Stragier, Plasmids for ectopic integration in Bacillus subtilis. Gene 180, 57–61 (1996). [DOI] [PubMed] [Google Scholar]
- 74.B. Härtl, W. Wehrl, T. Wiegert, G. Homuth, W. Schumann, Development of a new integration site within the Bacillus subtilis chromosome and construction of compatible expression cassettes. J. Bacteriol. 183, 2696–2699 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.T. T. P. Phan, L. T. Tran, W. Schumann, H. D. Nguyen, Development of Pgrac100-based expression vectors allowing high protein production levels in Bacillus subtilis and relatively low basal expression in Escherichia coli. Microb. Cell Fact. 14, 72 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.M. Zhu, H. Mu, F. Han, Q. Wang, X. Dai, Quantitative analysis of asynchronous transcription-translation and transcription processivity in Bacillus subtilis under various growth conditions. iScience 24, 103333 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.M. Basan, M. Zhu, X. Dai, M. Warren, D. Sevin, Y. P. Wang, T. Hwa, Inflating bacterial cells by increased protein synthesis. Mol. Syst. Biol. 11, 836 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Z. Chen, P. Srivastava, B. Zarazúa-Osorio, A. Marathe, M. Fujita, O. A. Igoshin, Bacillus subtilis histidine kinase KinC activates biofilm formation by controlling heterogeneity of single-cell responses. MBio 13, e0169421 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.F. Meier, A. D. Brunner, S. Koch, H. Koch, M. Lubeck, M. Krause, N. Goedecke, J. Decker, T. Kosinski, M. A. Park, Online parallel accumulation–serial fragmentation (PASEF) with a novel trapped ion mobility mass spectrometer. Mol. Cell. Proteomics 17, 2534–2545 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.S. Tyanova, T. Temu, J. Cox, The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 11, 2301–2319 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Legends for tables S2 to S4
Figs. S1 to S14
Tables S2 to S4