Abstract
Background and Objectives
Neurodevelopmental evaluation of toddlers with complex congenital heart disease is recommended, but reported frequency is low. Data on barriers to attending neurodevelopmental follow-up are limited. This study aims to estimate the attendance rate for a toddler neurodevelopmental evaluation in a contemporary multicenter cohort and to assess patient and center level factors associated with attending this evaluation.
Methods
This is a retrospective cohort study of children born between September 2017 and September 2018 who underwent cardiopulmonary bypass in their first year of life at a center contributing data to the Cardiac Neurodevelopmental Outcome Collaborative and Pediatric Cardiac Critical Care Consortium clinical registries. The primary outcome was attendance for a neurodevelopmental evaluation between 11–30 months of age. Sociodemographic and medical characteristics and center factors specific to neurodevelopmental program design were considered as predictors for attendance.
Results
Among 2,385 patients eligible from 16 cardiac centers, the attendance rate was 29.0% (692/2,385), with a range of 7.8–54.3% across individual centers. In multivariable logistic regression models, hospital-initiated (vs. family-initiated) scheduling for neurodevelopmental evaluation had the largest odds ratio in predicting attendance (OR=4.24, 95% CI, 2.74–6.55). Other predictors of attendance included antenatal diagnosis, absence of Trisomy 21, higher STAT mortality category, longer postoperative length of stay, private insurance, and residing a shorter distance from the hospital.
Conclusions
Attendance rates reflect some improvement but remain low. Changes to program infrastructure and design and minimizing barriers affecting access to care are essential components for improving neurodevelopmental care and outcomes for children with congenital heart disease.
Article Summary
This study estimates the rate of attendance for a toddler cardiac neurodevelopmental evaluation and considers patient and program specific predictors to identify potentially modifiable barriers.
Introduction
Congenital heart disease (CHD) occurs in 9 per 1000 live births, a third of which are complex forms requiring interventions in the first year of life.1,2 Advances in care of patients with complex CHD have dramatically improved survival and uncovered neurodevelopmental impairment as the most common morbidity, affecting approximately 50% of children who undergo cardiac surgery in the first year of life.3–5 The developmental profile of children with CHD may include fine and gross motor impairments; speech and language disorders; challenges with social cognition and perceptual reasoning; issues with visual-motor integration, attention, and executive dysfunction; emotional and behavioral disorders; and lower intelligence quotient and academic achievement.6–8 By adolescence, nearly two-thirds of patients require special education or psychosocial services.9 Cognitive and psychosocial concerns may persist into adulthood with an additional risk of dementia, all of which can significantly impact quality of life, educational achievement, employment, and financial security.10–15
Early interventions may improve outcomes for children at risk for neurodevelopmental disorders,16,17 underscoring the importance of routine neurodevelopmental surveillance within standard pediatric care.18 In 2012, the American Heart Association (AHA) and American Academy of Pediatrics (AAP) published guidelines for neurodevelopmental screening and evaluation, and management of neurodevelopmental disorders in children with CHD, expanding upon general pediatric developmental assessment recommendations.5 Subsequently, a number of cardiac centers have established neurodevelopmental follow-up programs within their comprehensive care models for complex CHD. These programs are not only important for identifying neurodevelopmental disorders, but also for providing a conduit to early intervention referrals and specialized therapy services.19,20 Early intervention services are deemed valuable by nearly all caregivers, but initial studies report less than 20% of eligible patients are evaluated in cardiac neurodevelopmental clinics.21,22 Patients with non-private insurance and who live further from their cardiac center are less likely to attend general cardiac and neurodevelopmental visits,21,23,24 suggesting inequities in access to care may contribute to low follow-up rates. These early data emphasize a need to understand barriers and center-based differences for follow-up care to ultimately improve attendance for neurodevelopmental evaluation in children with CHD.
The current study aimed to estimate the attendance rate for a toddler neurodevelopmental evaluation in children with complex CHD requiring infant cardiopulmonary bypass (CPB) and to understand the patient and center level factors associated with attending this evaluation. We hypothesized that patient sociodemographic and medical characteristics, along with center-specific factors, would predict which children attended the evaluation.
Methods
Data Sources
The Cardiac Neurodevelopmental Outcome Collaborative (CNOC) clinical registry was developed to collect neurodevelopmental data for children with CHD to inform best practices for neurodevelopmental care. The registry was built within the same web-based platform as the Pediatric Cardiac Critical Care Consortium (PC4) data registry, a quality improvement collaborative with rigorous data auditing for pediatric cardiac intensive care units across North America.25–27 Integration of the CNOC and PC4 registries was an intentional, collaborative effort of Cardiac Networks United28 to facilitate projects spanning cardiac intensive care and neurodevelopmental follow-up. Both registries require training of data entry personnel before data entry can begin. For PC4, this includes a two-day training on the database, data entry process, and data definitions and the requirement to pass a certification examination.27 CNOC has developed a similar half-day training (due to fewer number of variables) followed by a certification examination. Both registries also host regular conference calls with data entry staff to provide clarification on definitions and processes. Institutional Review Board approval, with waiver of consent, was obtained for this project at the University of Michigan Data Coordinating Center and appropriate data use agreements were completed with each contributing site.
Inclusion Criteria
Patient-specific inclusion criteria were infant CPB, defined as bypass in the first year of life, date of birth between September 15, 2017 and September 15, 2018, and alive at 11 months of age (making them eligible for a toddler neurodevelopmental evaluation). Infant CPB was chosen as the primary cardiac inclusion criterion because it is a key risk factor for neurodevelopmental disorders in children with CHD.5 The birth dates were chosen based on when a toddler’s neurodevelopmental evaluation would have occurred relative to when the CNOC registry became available for data entry (May 2019) and when institutions halted in-person visits due to the coronavirus-19 pandemic (March 2020). To identify eligible patients and to collect sociodemographic and medical predictors of clinic attendance, eligible CNOC centers had to contribute data to the PC4 registry by 2017.
Outcome and Predictor Variables
The primary outcome was attending a toddler neurodevelopmental evaluation, defined as any visit between 11 and 30 months of age, regardless of whether formal neurodevelopmental testing was performed. This broad age range accounted for practical application of a recommended5,29 18-month (i.e., toddler) visit in clinical practice and across institutional models.
To determine whether a patient attended the neurodevelopmental evaluation, all eligible patients in the PC4 registry were cross-referenced with those in the CNOC registry. Due to potential lags in data entry in the new CNOC registry, each participating CNOC center was provided a list of patients at their site who met inclusion criteria but did not have data in the CNOC registry. Centers then queried their institutional data and entered the following information into a standardized data collection form in REDCap (Research Electronic Data Capture30,31): whether any neurodevelopmental evaluation was performed, the age of the child at the visit if it occurred, and the reason if the visit did not occur. Instructions for entering the REDCap data, including what qualified as a neurodevelopmental evaluation, were provided to each center via conference call and in writing. Because PC4 was used for all sociodemographic and medical variables (details below), data standards for these variables were not affected by reconciliation of the REDCap and clinical registry data.
Sociodemographic and medical variables were collected from the PC4 registry. Sociodemographic variables included the child’s sex, race and ethnicity, insurance type, and household zip code at the time of surgery. Household and cardiac center zip codes were used to estimate the distance patients lived from their center. Distance from center was then categorized into approximate quartiles of the distribution of the data (≤15 miles, 16–45 miles, 45–100 miles, and >100 miles). Institution-specific regulations for sharing private health information within clinical registries limited the availability of household zip codes for some patients.
Medical variables included antenatal diagnosis, gestational age at birth, genetic diagnosis, age at first cardiac surgery, history of stroke, history of clinical or electrographic seizure, history of extracorporeal membrane oxygenation (ECMO), and postoperative length of stay at the time of the first infant CPB operation. Postoperative length of stay was categorized into 2-week intervals based on increased risk of adverse outcome for stays greater than 2 weeks.5 The Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery (STAT) mortality score32 was used to categorize cardiac severity, with higher scores reflecting higher risk of mortality.
Center-specific variables were obtained via a REDCap30,31 survey developed to optimally capture variations in cardiac neurodevelopmental program design of participating centers. Survey questions addressed the year the program was established, the program’s recommended age for the first neurodevelopmental evaluation, location of the program/clinic in relation to their cardiac center, program referral criteria for neurodevelopmental evaluation (e.g., based on AHA/AAP criteria vs. only patients with single-ventricle physiology), patient access for neurodevelopmental evaluation at clinics outside of their neurodevelopmental program, and hospital or family-initiated scheduling process for neurodevelopmental evaluation. Hospital-initiated scheduling included automatic scheduling during a hospitalization or outpatient visit or scheduling by a nurse or coordinator who directly contacted the family. Family-initiated scheduling included family response to a letter, developmental screening, or clinician referral.
Statistical Analysis
Patient sociodemographic, medical, and center-specific variables were considered as predictors for attendance for a neurodevelopmental evaluation. Univariable associations compared attendance rates by categories of these predictor variables. Stepwise backward logistic regression based on p-value < 0.05 was conducted to determine independent predictors of attendance for multivariable models. Throughout, generalized estimating equations with exchangeable working correlation for adjustment due to center were used to control for center-to-center variability. Ten-fold cross-validation was performed to assess model diagnostics including sensitivity, specificity, and area under the receiver operating characteristic curve. R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses.
Results
Cohort Characteristics
Of 24 CNOC centers entering data, 16 were eligible for inclusion (Figure 1). Seventy-five percent of centers (12/16) used all AHA/AAP high-risk criteria5 for referral for neurodevelopmental evaluation, whereas the remainder used targeted criteria within these guidelines (e.g., prioritizing single ventricle patients). All centers recommended the first neurodevelopmental evaluation occur by 13 months of age, 94% (15/16) were co-located with the cardiology clinic or in a location familiar to the family (i.e., same medical campus where surgery was performed), and 75% (12/16) used hospital-initiated scheduling.
Figure 1.
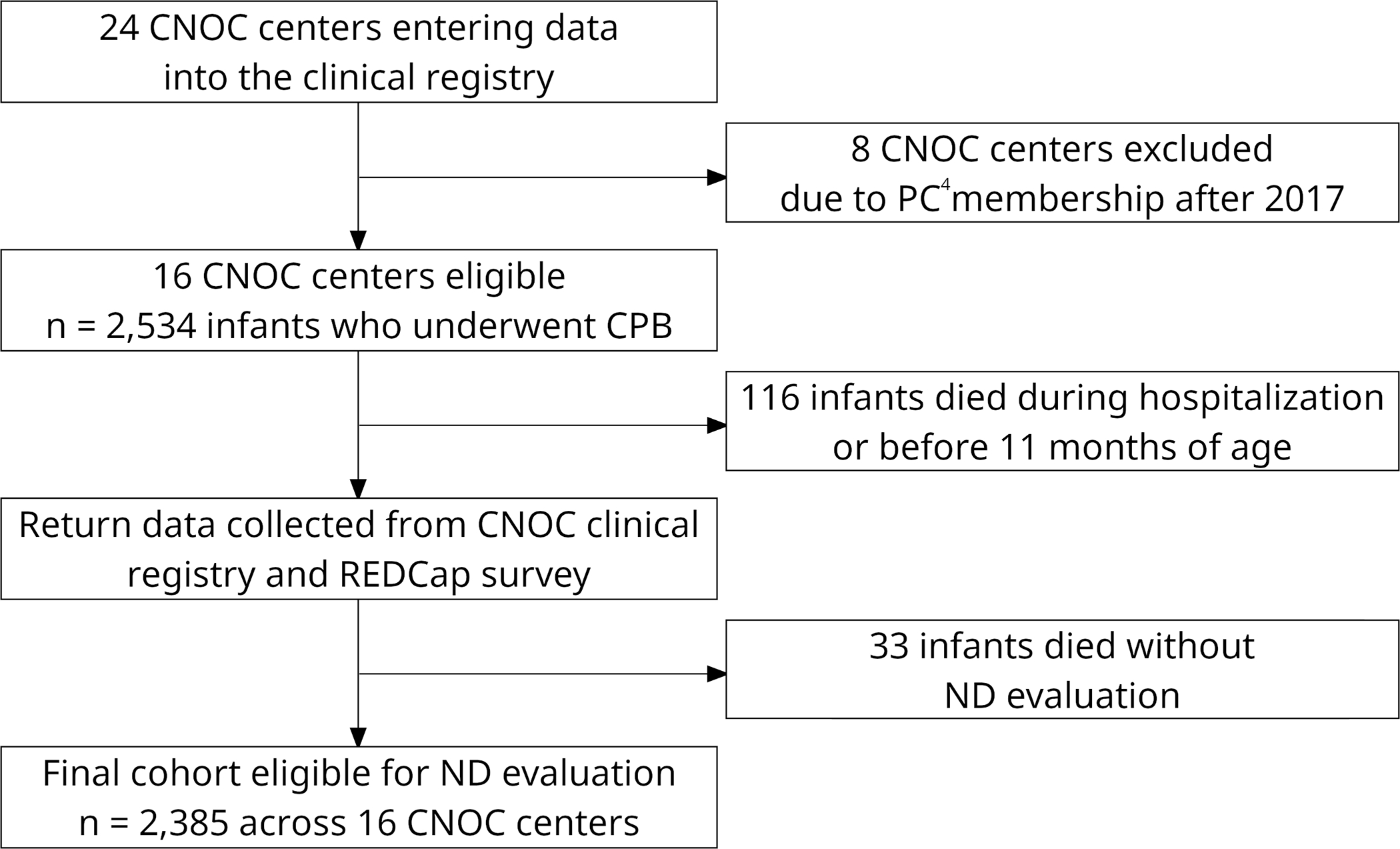
Flow diagram of CNOC center participation and patient eligibility for the study.
The median number of patients who underwent infant CPB at each site was 127 (range 78–302) during the study period, with a total of 2,385 patients eligible for a toddler neurodevelopmental evaluation (Figure 1). For the entire cohort, 53.5% (1,272/2,379) of patients were male, 44.5% (894/2,007) were non-Hispanic white, 58.7% (1,164/1,983) had public insurance, and 47.7% (726/1,522) lived over 45 miles from their cardiac center. An antenatal diagnosis was made in 54.9% (1,142/2,082), 37.4% (892/2,385) underwent neonatal surgery, and a genetic diagnosis was present in 21.1% (504/2,384), the majority of which were Trisomy 21. STAT mortality category 4 represented the largest number of patients (712/2,385, 29.9%) and category 5 the fewest (217/2,385, 9.1%). Missing data limited the sample size for some variables, which is reflected in the numbers reported above and in Table 1.
TABLE 1.
Attendance Rates for Neurodevelopmental Evaluation between 11–30 Months as a Function of Patient Medical and Sociodemographic Characteristics (2,385 Patients Across 16 Centers)
| Patient Characteristic | (Number Attended) / (Number in Category) (%) |
P-valuea |
|---|---|---|
|
| ||
| Gender | 0.64 | |
| Male | 383/1272 (30.1) | |
| Female | 308/1107 (27.8) | |
| Race and ethnicity | <0.001 | |
| Non-Hispanic White | 288/894 (32.2) | |
| Hispanic | 131/468 (28.0) | |
| Non-Hispanic Black | 93/298 (31.2) | |
| Asian | 18/76 (23.7) | |
| Native American/Pacific Islander | 3/34 (8.8) | |
| Other/multiracial | 57/237 (24.1) | |
| Insurance | 0.004 | |
| Public | 319/1164 (27.4) | |
| Private | 294/819 (35.9) | |
| Distance from hospital | <0.001 | |
| ≤ 15 miles | 135/357 (37.8) | |
| 16–45 miles | 142/439 (32.3) | |
| 46–100 miles | 97/359 (27.0) | |
| > 100 miles | 61/367 (16.6) | |
| Antenatal diagnosis | <0.001 | |
| Yes | 430/1142 (37.7) | |
| No | 208/940 (22.1) | |
| Preterm birth (< 37 weeks) | 0.40 | |
| Yes | 118/435 (27.1) | |
| No | 571/1902 (30.0) | |
| Genetic diagnosis | 0.004 | |
| No genetic diagnosis | 589/1880 (31.3) | |
| Trisomy 21 | 51/342 (14.9) | |
| Other genetic diagnosis | 52/162 (32.1) | |
| Age at first cardiac surgery | <0.001 | |
| ≤ 30 days (neonatal) | 350/892 (39.2) | |
| > 30 days | 342/1493 (22.9) | |
| STAT mortality category | <0.001 | |
| 1 | 101/592 (17.1) | |
| 2 | 111/441 (25.2) | |
| 3 | 118/423 (27.9) | |
| 4 | 261/712 (36.7) | |
| 5 | 101/217 (46.5) | |
| Stroke | 0.11 | |
| Yes | 26/63 (41.3) | |
| No | 666/2322 (28.7) | |
| Seizure | 0.12 | |
| Yes | 31/78 (39.7) | |
| No | 660/2306 (28.6) | |
| ECMO | 0.02 | |
| Yes | 35/86 (40.7) | |
| No | 657/2299 (28.6) | |
| Postoperative length of stay | <0.001 | |
| ≤ 15 days | 352/1489 (23.6) | |
| 16–30 days | 156/432 (36.1) | |
| 31–45 days | 59/163 (36.2) | |
| >45 days | 125/301 (41.5) | |
ECMO = extracorporeal membrane oxygenation, STAT = Society of Thoracic Surgeons - European Association for Cardio-Thoracic Surgery.
Univariable P-values comparing attendance rates across patient characteristics were based on generalized estimating equations with exchangeable working correlation for adjustment due to center. Patients missing that characteristic were excluded from comparisons.
Attendance Rates for Neurodevelopmental Evaluation
The overall attendance rate for a toddler neurodevelopmental evaluation between 11–30 months of age was 29.0% (692/2,385). An additional 196 patients (8.2%) attended a neurodevelopmental evaluation, but the visit was conducted outside of 11–30 months. Another 323 (13.5%) had their primary cardiac care outside the institution where the cardiac surgery was performed; thus, attendance could not be verified. For the remainder of the patients, the reason for not attending was unknown by the center. Individual center attendance rates were highly variable, ranging from 7.8% to 54.3%.
Patient- and Center-specific Predictors of Attendance for Neurodevelopmental Evaluation
Table 1 displays the number who attended the evaluation over the total sample of available data by patient sociodemographic and medical characteristics. Patients who attended a neurodevelopmental evaluation were more likely to have an antenatal diagnosis, undergo neonatal surgery, be in a higher STAT mortality category, undergo ECMO, have a longer postoperative length of stay, have private insurance, and live closer to their cardiac center. Attendance rates also differed for genetic diagnosis categories and for race and ethnicity, although race and ethnicity covaried with insurance type. Figure 2 provides graphical representation of variations in attendance with confidence intervals by selected medical factors.
Figure 2.
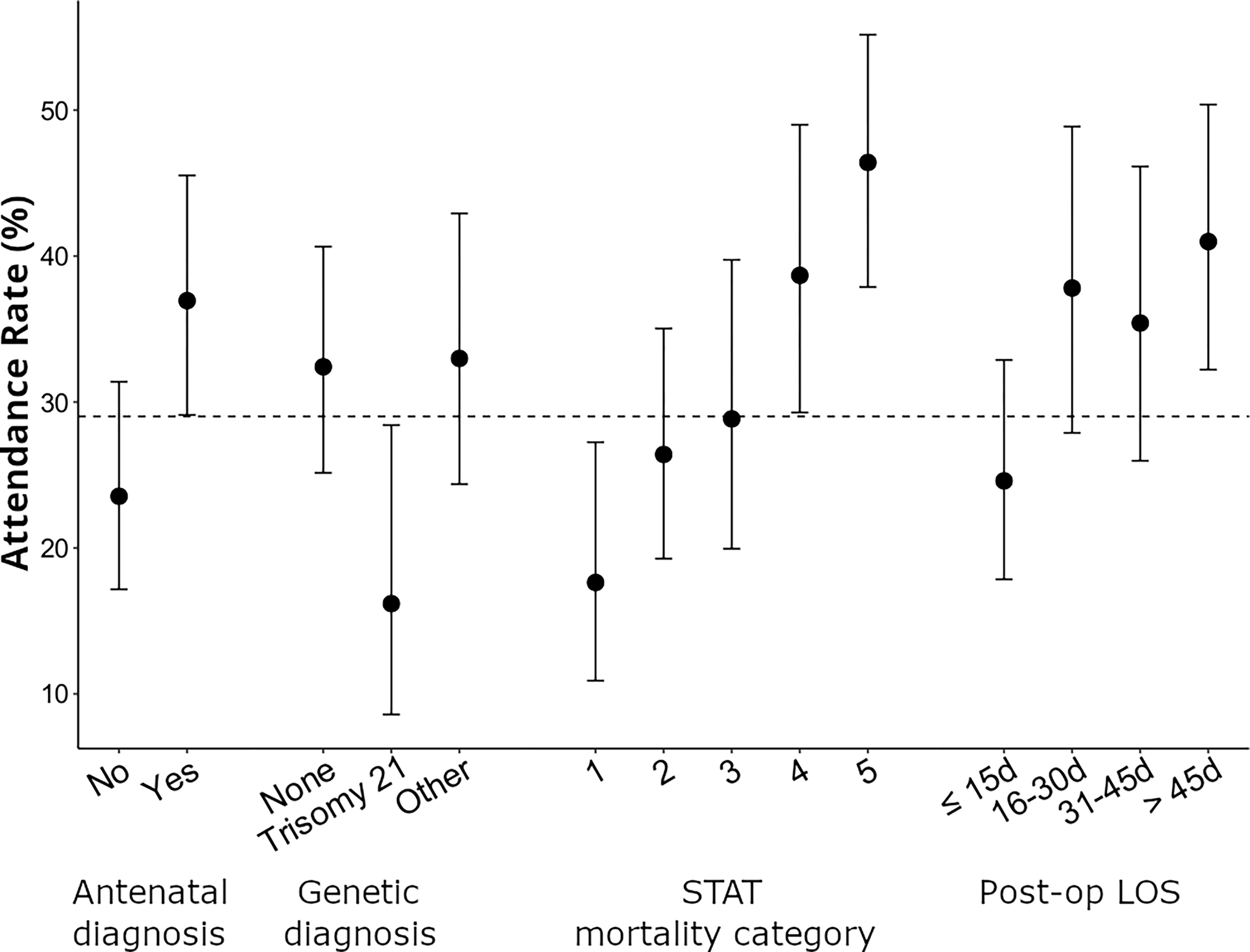
Attendance rates for neurodevelopmental evaluation between 11–30 months as a function of selected patient medical characteristics, together with 95% confidence intervals.
Centers with fewer patients undergoing infant CPB and those using a hospital-initiated scheduling process were more likely to have higher attendance rates (Figure 3). For hospital-initiated scheduling, attendance rates did not significantly differ for centers using automatic scheduling compared to those where a nurse or coordinator directly contacted the family to schedule (33.5% versus 37.6%, p=0.08). Program location and program referral criteria were also associated with higher attendance (Table 2).
Figure 3.
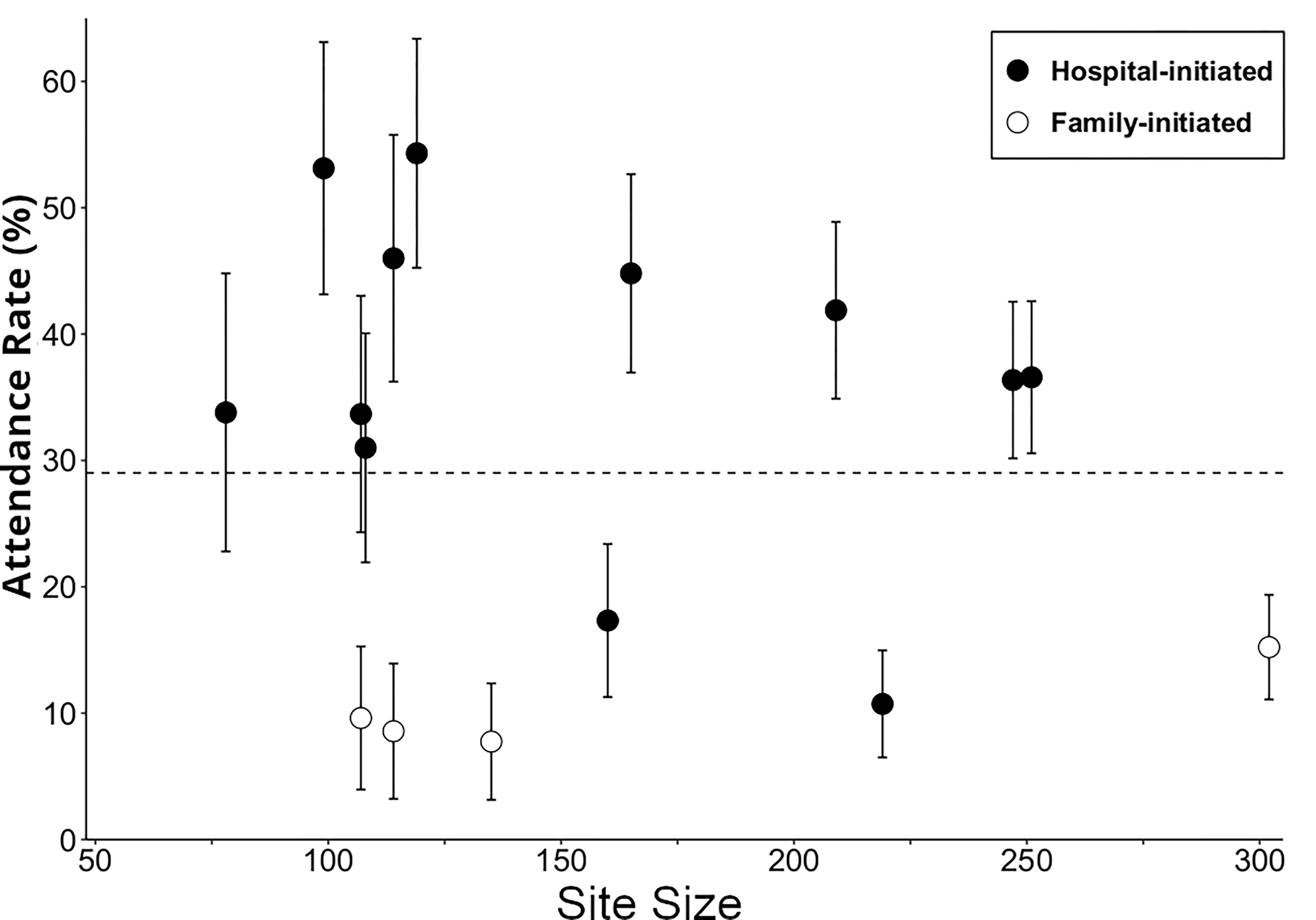
Attendance rates for neurodevelopmental evaluation between 11–30 months by center as a function of site size and follow-up scheduling process, together with 95% confidence intervals. Site size refers to number of infants with a CPB surgery in the first year of life during the time frame of this study. Follow-up scheduling was either hospital-initiated or family-initiated.
TABLE 2.
Attendance Rates for Neurodevelopmental Evaluation between 11–30 Months as a Function of Center-Specific Factors (2,385 Patients Across 16 Centers)
| Center-Specific Factor | Number of Centers | (Number Attended) / (Number in Category) (%) |
P-valuea |
|---|---|---|---|
|
| |||
| Follow-up scheduling | <0.001 | ||
| Hospital-initiated | 12 | 619/1758 (35.2) | |
| Family-initiated | 4 | 73/627 (11.6) | |
| Neurodevelopmental program location | <0.001 | ||
| Familiar or adjacent to prior clinical cardiac care |
8 | 474/1241 (38.2) | |
| Co-located with outpatient cardiology clinic |
7 | 174/855 (20.4) | |
| New to family | 1 | 44/289 (15.2) | |
| Neurodevelopmental program emphasis | <0.001 | ||
| American Heart Association high risk category |
12 | 509/1660 (30.7) | |
| Neonatal surgery | 2 | 130/331 (39.3) | |
| Infant surgery | 1 | 44/289 (15.2) | |
| Single-ventricle anatomy | 1 | 9/105 (8.6) | |
| Access to neurodevelopmental evaluation at another location | 0.57 | ||
| Yes | 10 | 459/1566 (29.3) | |
| No | 6 | 233/819 (28.4) | |
| Site size | 0.33 | ||
| Small (≤ 127 surgeries) | 8 | 267/790 (33.8) | |
| Large (> 127 surgeries) | 8 | 424/1595 (26.6) | |
Univariable P-values comparing attendance rates across center-level factors were based on generalized estimating equations with exchangeable working correlation for adjustment due to center. Site size refers to number of infants with a cardiopulmonary bypass surgery during the time frame of this study.
Multivariable models were examined in two ways due to the extent of missing data for sociodemographic characteristics. The first multivariable model (Model 1) examined patient medical and center-level characteristics. Antenatal diagnosis, genetic diagnosis, STAT mortality category, postoperative length of stay, and scheduling process for the neurodevelopmental program were all independent predictors for attending a toddler neurodevelopmental evaluation (Table 3). The second multivariable model (Model 2) added the sociodemographic characteristics. In that model, race and ethnicity, insurance type, and distance from the center were associated with attending the evaluation, in addition to the previously identified medical and center factors (Table 3). Across both models, a hospital-initiated scheduling process for the clinic had the highest odds ratio for predicting attendance (Model 1 odds ratio 4.24, 95% CI: 2.74, 6.55; Model 2 odds ratio 4.85, 95% CI: 1.80, 13.09).
TABLE 3.
Multivariable Models to Predict Attendance Rates for Neurodevelopmental Evaluation between 11–30 Months
| Model 1 Patient Medical and Center-Specific Characteristics Excluding Sociodemographic Factors (2,071 Patients Across 16 Centers) a |
Model 2 Patient Medical and Center-Specific Characteristics Including Sociodemographic Factors (1,163 Patients Across 12 Centers) b |
|||
|---|---|---|---|---|
|
| ||||
| Characteristic | Odds Ratio (95% CI) |
P-valuec | Odds Ratio (95% CI) |
P-valuec |
|
| ||||
| Antenatal diagnosis | ||||
| Yes | 1.67 (1.31, 2.14) | <0.001 | 1.54 (1.24, 1.91) | <0.001 |
| No | 1.0 | 1.0 | ||
| Genetic diagnosis | <0.001 | <0.001 | ||
| No genetic diagnosis | 1.0 | 1.0 | ||
| Trisomy 21 | 0.38 (0.22, 0.65) | 0.43 (0.22, 0.84) | ||
| Other genetic diagnosis | 0.83 (0.62, 1.11) | 1.18 (0.96, 1.61) | ||
| STAT mortality category | 0.01 | 0.04 | ||
| 1 | 1.0 | 1.0 | ||
| 2 | 1.39 (0.93, 2.08) | 1.03 (0.64, 1.64) | ||
| 3 | 1.90 (1.10, 3.28) | 1.13 (0.85, 1.50) | ||
| 4 | 2.23 (1.35, 3.67) | 1.58 (1.10, 2.28) | ||
| 5 | 2.43 (1.29, 4.58) | 1.54 (0.95, 2.49) | ||
| Postoperative length of stay | <0.001 | 0.02 | ||
| ≤ 15 days | 1.0 | 1.0 | ||
| 16–30 days | 1.53 (1.12, 2.11) | 1.71 (1.08, 2.73) | ||
| 31–45 days | 1.22 (0.82, 1.81) | 1.01 (0.54, 1.88) | ||
| > 45 days | 1.52 (1.10, 2.11) | 1.65 (1.09, 2.50) | ||
| Follow-up scheduling | <0.001 | 0.002 | ||
| Hospital-initiated | 4.24 (2.74, 6.55) | 4.85 (1.80, 13.09) | ||
| Family-initiated | 1.0 | 1.0 | ||
| Race and ethnicity | NA | 0.04 | ||
| Non-Hispanic White | 1.0 | |||
| Hispanic | 1.11 (0.88, 1.40) | |||
| Non-Hispanic Black | 0.82 (0.56, 1.19) | |||
| Otherd | 0.64 (0.47, 0.87) | |||
| Insurance | NA | 0.006 | ||
| Public | 1.0 | |||
| Private | 1.37 (1.10, 1.72) | |||
| Distance from hospital | NA | <0.001 | ||
| ≤ 15 miles | 1.0 | |||
| 16–45 miles | 0.73 (0.54, 0.98) | |||
| 46–100 miles | 0.47 (0.33, 0.66) | |||
| > 100 miles | 0.26 (0.17, 0.40) | |||
AUC = area under the receiver operating characteristic curve, CV = 10-fold cross validation, NA = not applicable, STAT = Society of Thoracic Surgeons - European Association for Cardio-Thoracic Surgery.
Model 1 has sensitivity 90% (CV sensitivity 89%), specificity 28% (CV specificity 37%), and AUC 69% (CV AUC 74%).
Model 2 has sensitivity 88% (CV sensitivity 83%), specificity 41% (CV specificity 51%), and AUC 75% (CV AUC 76%). Reduction in sample size compared to Model 1 due to demographic characteristics not available from 4 centers and also missing for some patients from the remaining 12 centers.
Multivariable P-values were based on generalized estimating equations with exchangeable working correlation for adjustment due to center.
Other race and ethnicity category includes Asian, Native American, Pacific Islander, other (not specified), and multiracial.
Discussion
In the last decade there has been significant growth in the number of congenital heart centers with neurodevelopmental follow-up programs, but each program’s design varies due to infrastructure and resources. This is the first multicenter effort to evaluate the attendance rate for cardiac neurodevelopmental evaluation and to compare patient and center level variation predicting attendance. We found an attendance rate for a toddler neurodevelopmental evaluation of 29.0% (range 7.8–54.3% across centers) for patients undergoing CPB in the first year of life. An additional 8.2% of patients were evaluated before or after 11–30 months of age. Prior single center studies report 4–17% of eligible patients are evaluated in cardiac neurodevelopmental clinics.21,22 However, these data were published in an earlier era and include different age ranges of follow-up. The attendance rate of 29.0% likely reflects some improvement since these single center studies, but may not be indicative of follow-up rates in older children. The factor providing the greatest prediction of attending the neurodevelopmental evaluation was whether the hospital or the family initiated the scheduling of the appointment. This finding emphasizes the importance of infrastructure and programmatic design as a potentially modifiable factor for optimizing cardiac neurodevelopmental follow-up and may inform models of care for other pediatric populations at risk for neurodevelopmental disorders.
Nearly a third of patients with complex CHD who undergo neurodevelopmental evaluation receive a new referral for early intervention, therapy, or ancillary medical services at their first visit.20 Throughout the course of outpatient neurodevelopmental care, over 70% of patients with CHD receive this type of referral and/or a new psychosocial or neurodevelopmental diagnosis.22 Targeted developmental services/interventions may improve cognitive and motor outcomes for preterm children,16 enhance academic performance in children with lead exposure,17 and improve inhibitory control, attention, and cognitive regulatory skills in adolescents with CHD.33 Yet, compared to preterm children, children with CHD appear to receive fewer therapy services.34 The potential benefits of neurodevelopmental intervention highlight a need to increase the proportion of children with CHD who are evaluated in neurodevelopmental programs.
One modifiable component of cardiac neurodevelopmental follow-up that could improve attendance rates is program design. CNOC centers included in the current analysis were generally well-established centers (based on number of patients undergoing CPB, age of the program, and ability for data entry into the CNOC clinical registry), but the structure of each clinic varied by organizational model and resources.35 Program-specific variation is also reported for Canadian and European cardiac centers, with 40–50% having structured cardiac neurodevelopmental programs but variation in surveillance, screening, and evaluation approaches across sites.36,37 CNOC site level differences provided an opportunity to evaluate specific program components in relation to attendance. The strongest predictor of attendance in this cohort was the clinic’s scheduling process. While standardization of scheduling across centers may be unrealistic, it seems critical that centers ease scheduling burden for the family. Hospital-initiated scheduling not only reduces burden, but provides another interaction with the family to convey the importance of neurodevelopmental follow-up and to coordinate the visit with other clinical appointments. In addition to scheduling process, patient volume and clinic location were also associated with attendance, although only location of the clinic remained in the multivariable model. The univariate association of higher attendance for sites with fewer infant cardiopulmonary bypass cases may reflect catchment area, particularly since patient volume did not remain a predictor in multivariable models including distance from center. Two additional considerations for optimizing return, which were not included in the current study, are incorporating neurodevelopmental care during hospitalization and educational initiatives. Prior data suggest neurodevelopmental surveillance while patients are hospitalized for cardiac surgery may improve follow-up rates.38 Inpatient and outpatient educational efforts geared towards families and healthcare providers could also increase follow-up. Collaborative multicenter approaches aimed at optimizing clinic design while leveraging individual institutional and international experiences will be an essential next step, including understanding resource utilization for hospital-initiated scheduling. Interval surveys of CNOC sites to understand changes in programmatic infrastructure and to incorporate newer, less established programs will be an important component.
Patient-specific factors were also associated with attending a toddler neurodevelopmental evaluation. Similar to prior data,21 greater surgical complexity and longer length of stay related to higher attendance. It is also not surprising that antenatal diagnosis was associated with higher attendance, as these patients generally have more complex cardiac disease and have been counseled about neurodevelopment. Further, antenatal diagnosis may relate to access to care and resources available to the family.23,39 Consistent with this, greater distance from the cardiac center and non-private insurance were associated with lower attendance rates. Both of these factors relate to follow-up for pediatric and adult cardiac care in other cohorts, yet healthcare utilization and cost is significantly higher for those who live in rural communities and/or further from their tertiary care.21,23,24 Minimizing barriers that affect access to care and developing new strategies to limit travel burden, such telehealth visits for initial intake screening, as outlined by the CNOC Telehealth Task Force,40,41 will likely be essential components for improving follow-up. Race and ethnicity was also associated with attendance rates. However, this relationship likely reflects non-biological, social and structural factors and differential lived experiences contributing to inequities that were not captured within this data.42,43 Finally, lower attendance was seen for those with Trisomy 21. This may be due to subspecialty follow-up clinics or other parent resources available for patients with Trisomy 21, potentially making clinic visits seem less relevant for families who have already leveraged neurodevelopmental and related community services. This may also be occurring for other groups of patients, such as those with seizures or stroke who may be followed in neurology clinics and/or by rehabilitation medicine.
The current study has several limitations. First, data were limited to centers contributing to the CNOC and PC4 registries. These centers may have the greatest infrastructure and resources for cardiac neurodevelopmental care, so our findings may overestimate attendance rates across newer programs. In addition, a proportion of follow-up evaluations could have been performed at a location not associated with the cardiac center, in specialty clinics, or at other centers closer to where the family lives, which would underestimate attendance rates. Also, completing neurodevelopmental evaluations was challenging during the coronavirus-19 pandemic, especially during lockdown periods. A specific range of birth dates was used to account for this, but the true follow-up rate when a pandemic does not disrupt care may still be underestimated. Finally, the use of REDCap to capture neurodevelopmental visits not entered in the CNOC registry may have introduced the potential for error in estimating follow-up rates. An additional limitation was the use of registry data, which provided a robust sample size but meant specific factors were unavailable, such as primary language spoken, or had higher rates of missing data, such as zip code. Missing sociodemographic data did significantly reduce the sample size for statistical modeling, but data from 1,163 patients were still available and provided an adequate sample for analysis. Nonetheless, results should be interpreted in the context of this sample size reduction and the possibility that missing data may not be random. To address this limitation in future analyses, the Cardiac Networks United clinical registries now include a Health Equity Module that uses the Decentralized Geomarker Assessment for Multi-site Studies (DeGAUSS) tool, which allows sites to enter information about where a patient lives without directly exchanging protected health information across institutions.44 A final limitation is that center factors were specific to those programs included in the current study. As CNOC membership continues to grow and individual programs develop unique strategies for neurodevelopmental care, it will be important to re-evaluate programmatic design to determine whether other factors or new approaches improve attendance rates.
Conclusion
An attendance rate of 29.0% for neurodevelopmental evaluation reflects some improvement compared to previous reports but remains low. Changes to infrastructure and program design and minimizing barriers that affect access to care are essential for improving neurodevelopmental care for children with CHD. These results lay the foundation for more detailed evaluation of variation in cardiac neurodevelopmental programs, and inclusion of additional centers, to inform program design. Evaluation of neurodevelopmental outcomes at a multisite level for those who attend neurodevelopmental appointments will also be important for understanding diagnosis-specific risks that may allow targeted neurodevelopmental care. Finally, a critical component of future efforts should involve health care policy and advocacy to ensure equitable and accessible care for all children with CHD45 and collaborative efforts with patient and family groups to understand perceived barriers. This will be important for understanding access for evaluations, as well as variations in region-specific resources such as the availability of early intervention services once a follow-up visit occurs.
What’s Known on This Subject.
Routine neurodevelopmental screening and evaluation are standard of care for children with complex congenital heart disease, but reported follow-up rates are low. There is an urgent need to understand barriers in order to improve attendance rates for outpatient neurodevelopmental care.
What This Study Adds.
Medical severity and factors related to access to care predicted attendance rates, but the most predictive factor was hospital-initiated (vs. family-initiated) follow-up care. Reducing access barriers and improving programmatic infrastructure may optimize neurodevelopmental follow-up for children with congenital heart disease.
Acknowledgements
We acknowledge the PC4 and CNOC data collection teams at the participating centers, the various Cores and Committees of CNOC that provide infrastructure for the CNOC clinical registry, and the University of Michigan Congenital Heart Center and the Heart Institute at Cincinnati Children’s Hospital for their support of Cardiac Networks United.
Funding/Support:
Cardiac Networks United, which includes the Cardiac Neurodevelopmental Outcome Collaborative and the Pediatric Cardiac Critical Care Consortium, is supported by the Children’s Heart Foundation.
Abbreviations:
- AAP
American Academy of Pediatrics
- AHA
American Heart Association
- CHD
congenital heart disease
- CNOC
Cardiac Neurodevelopmental Outcome Collaborative
- CPB
cardiopulmonary bypass
- ECMO
extracorporeal membrane oxygenation
- PC4
Pediatric Cardiac Critical Care Consortium
- REDCap
Research Electronic Data Capture
- STAT
The Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery
Footnotes
Conflict of Interest Disclosures:
The authors have no conflicts of interest relevant to this article to disclose and no financial interests to disclose.
References
- 1.van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. Nov 15 2011;58(21):2241–7. doi: 10.1016/j.jacc.2011.08.025 [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. Jun 19 2002;39(12):1890–900. doi: 10.1016/s0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- 3.Erikssen G, Liestol K, Seem E, et al. Achievements in congenital heart defect surgery: a prospective, 40-year study of 7038 patients. Circulation. Jan 27 2015;131(4):337–46; discussion 346. doi: 10.1161/CIRCULATIONAHA.114.012033 [DOI] [PubMed] [Google Scholar]
- 4.Triedman JK, Newburger JW. Trends in Congenital Heart Disease: The Next Decade. Circulation. Jun 21 2016;133(25):2716–33. doi: 10.1161/CIRCULATIONAHA.116.023544 [DOI] [PubMed] [Google Scholar]
- 5.Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. Aug 28 2012;126(9):1143–72. doi: 10.1161/CIR.0b013e318265ee8a [DOI] [PubMed] [Google Scholar]
- 6.Feldmann M, Bataillard C, Ehrler M, et al. Cognitive and Executive Function in Congenital Heart Disease: A Meta-analysis. Pediatrics. Oct 2021;148(4)doi: 10.1542/peds.2021-050875 [DOI] [PubMed] [Google Scholar]
- 7.Bolduc ME, Dionne E, Gagnon I, Rennick JE, Majnemer A, Brossard-Racine M. Motor Impairment in Children With Congenital Heart Defects: A Systematic Review. Pediatrics. Dec 2020;146(6)doi: 10.1542/peds.2020-0083 [DOI] [PubMed] [Google Scholar]
- 8.Ortinau CM, Smyser CD, Arthur L, et al. Optimizing Neurodevelopmental Outcomes in Neonates With Congenital Heart Disease. Pediatrics. Nov 1 2022;150(Suppl 2)doi: 10.1542/peds.2022-056415L [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellinger DC, Wypij D, Rivkin MJ, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. Sep 20 2011;124(12):1361–9. doi: 10.1161/CIRCULATIONAHA.111.026963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng LX, Khan AM, Drajpuch D, et al. Prevalence and correlates of post-traumatic stress disorder in adults with congenital heart disease. The American journal of cardiology. 2016;117(5):853–857. [DOI] [PubMed] [Google Scholar]
- 11.Bagge CN, Henderson VW, Laursen HB, Adelborg K, Olsen M, Madsen NL. Risk of dementia in adults with congenital heart disease: population-based cohort study. Circulation. 2018;137(18):1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. Dec 02 2008;52(23):e143–263. doi: 10.1016/j.jacc.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 13.Marelli A, Miller SP, Marino BS, Jefferson AL, Newburger JW. Brain in Congenital Heart Disease Across the Lifespan: The Cumulative Burden of Injury. Circulation. May 17 2016;133(20):1951–62. doi: 10.1161/CIRCULATIONAHA.115.019881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlosser L, Kessler N, Feldmann M, et al. Neurocognitive functioning in young adults with congenital heart disease: insights from a case-control study. Cardiol Young. May 2022;32(5):694–701. doi: 10.1017/S1047951121002705 [DOI] [PubMed] [Google Scholar]
- 15.Rometsch S, Greutmann M, Latal B, et al. Predictors of quality of life in young adults with congenital heart disease. Eur Heart J Qual Care Clin Outcomes. Apr 1 2019;5(2):161–168. doi: 10.1093/ehjqcco/qcy046 [DOI] [PubMed] [Google Scholar]
- 16.Anderson PJ, Treyvaud K, Spittle AJ. Early developmental interventions for infants born very preterm - what works? Semin Fetal Neonatal Med. Jun 2020;25(3):101119. doi: 10.1016/j.siny.2020.101119 [DOI] [PubMed] [Google Scholar]
- 17.Stingone JA, Sedlar S, Lim S, McVeigh KH. Receipt of Early Intervention Services Before Age 3 Years and Performance on Third-Grade Standardized Tests Among Children Exposed to Lead. JAMA Pediatr. Mar 7 2022;doi: 10.1001/jamapediatrics.2022.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipkin PH, Macias MM, Council On Children With Disabilities SOD, Behavioral P. Promoting Optimal Development: Identifying Infants and Young Children With Developmental Disorders Through Developmental Surveillance and Screening. Pediatrics. Jan 2020;145(1)doi: 10.1542/peds.2019-3449 [DOI] [PubMed] [Google Scholar]
- 19.Brosig C, Butcher J, Butler S, et al. Monitoring developmental risk and promoting success for children with congenital heart disease: Recommendations for cardiac neurodevelopmental follow-up programs. Clinical Practice in Pediatric Psychology. 2014;2(2):153–165. doi: 10.1037/cpp0000058 [DOI] [Google Scholar]
- 20.Monteiro SA, Serrano F, Tsang R, et al. Ancillary referral patterns in infants after initial assessment in a cardiac developmental outcomes clinic. Congenit Heart Dis. Sep 2019;14(5):797–802. doi: 10.1111/chd.12789 [DOI] [PubMed] [Google Scholar]
- 21.Loccoh EC, Yu S, Donohue J, et al. Prevalence and risk factors associated with non-attendance in neurodevelopmental follow-up clinic among infants with CHD. Cardiol Young. Apr 2018;28(4):554–560. doi: 10.1017/S1047951117002748 [DOI] [PubMed] [Google Scholar]
- 22.Glotzbach KL, Ward JJ, Marietta J, et al. The Benefits and Bias in Neurodevelopmental Evaluation for Children with Congenital Heart Disease. Pediatr Cardiol. Feb 2020;41(2):327–333. doi: 10.1007/s00246-019-02260-7 [DOI] [PubMed] [Google Scholar]
- 23.Davey B, Sinha R, Lee JH, Gauthier M, Flores G. Social determinants of health and outcomes for children and adults with congenital heart disease: a systematic review. Pediatr Res. Jan 2021;89(2):275–294. doi: 10.1038/s41390-020-01196-6 [DOI] [PubMed] [Google Scholar]
- 24.Khan AM, McGrath LB, Ramsey K, Agarwal A, Slatore CG, Broberg CS. Distance to Care, Rural Dwelling Status, and Patterns of Care Utilization in Adult Congenital Heart Disease. Pediatr Cardiol. Mar 2022;43(3):532–540. doi: 10.1007/s00246-021-02750-7 [DOI] [PubMed] [Google Scholar]
- 25.Gaies M, Cooper DS, Tabbutt S, et al. Collaborative quality improvement in the cardiac intensive care unit: development of the Paediatric Cardiac Critical Care Consortium (PC4). Cardiol Young. Jun 2015;25(5):951–7. doi: 10.1017/S1047951114001450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaies M, Donohue JE, Willis GM, et al. Data integrity of the Pediatric Cardiac Critical Care Consortium (PC4) clinical registry. Cardiol Young. Aug 2016;26(6):1090–6. doi: 10.1017/S1047951115001833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuette J, Zaccagni H, Donohue J, et al. Assessing data accuracy in a large multi-institutional quality improvement registry: an update from the Pediatric Cardiac Critical Care Consortium (PC(4)). Cardiol Young. Dec 28 2021:1–6. doi: 10.1017/S1047951121004984 [DOI] [PubMed] [Google Scholar]
- 28.Gaies M, Anderson J, Kipps A, et al. Cardiac Networks United: an integrated paediatric and congenital cardiovascular research and improvement network. Cardiol Young. Feb 2019;29(2):111–118. doi: 10.1017/S1047951118001683 [DOI] [PubMed] [Google Scholar]
- 29.Ware J, Butcher JL, Latal B, et al. Neurodevelopmental evaluation strategies for children with congenital heart disease aged birth through 5 years: recommendations from the cardiac neurodevelopmental outcome collaborative. Cardiol Young. Nov 2020;30(11):1609–1622. doi: 10.1017/S1047951120003534 [DOI] [PubMed] [Google Scholar]
- 30.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. Apr 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. Jul 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien SM, Clarke DR, Jacobs JP, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. Nov 2009;138(5):1139–53. doi: 10.1016/j.jtcvs.2009.03.071 [DOI] [PubMed] [Google Scholar]
- 33.Calderon J, Wypij D, Rofeberg V, et al. Randomized Controlled Trial of Working Memory Intervention in Congenital Heart Disease. J Pediatr. Dec 2020;227:191–198 e3. doi: 10.1016/j.jpeds.2020.08.038 [DOI] [PubMed] [Google Scholar]
- 34.Wehrle FM, Bartal T, Adams M, et al. Similarities and Differences in the Neurodevelopmental Outcome of Children with Congenital Heart Disease and Children Born Very Preterm at School Entry. J Pediatr. Nov 2022;250:29–37 e1. doi: 10.1016/j.jpeds.2022.05.047 [DOI] [PubMed] [Google Scholar]
- 35.Miller TA, Sadhwani A, Sanz J, et al. Variations in practice in cardiac neurodevelopmental follow-up programs. Cardiol Young. Nov 2020;30(11):1603–1608. doi: 10.1017/S1047951120003522 [DOI] [PubMed] [Google Scholar]
- 36.Feldmann M, Hagmann C, de Vries L, et al. Neuromonitoring, neuroimaging, and neurodevelopmental follow-up practices in neonatal congenital heart disease: a European survey. Pediatr Res. Apr 12 2022;doi: 10.1038/s41390-022-02063-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marie-Eve Bolduc JER, Gagnon Isabelle, Majnemer Annette, Brossard-Racine Marie. Canadian Developmental Follow-up Practices in Children With Congenital Heart Defects: A National Environmental Scan. CJC Pediatric and Congenital Heart Disease. 2022;1(1):3–10. doi: 10.1016/j.cjcpc.2021.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michael M, Scharf R, Letzkus L, Vergales J. Improving Neurodevelopmental Surveillance and Follow-up in Infants with Congenital Heart Disease. Congenit Heart Dis. Mar-Apr 2016;11(2):183–8. doi: 10.1111/chd.12333 [DOI] [PubMed] [Google Scholar]
- 39.Hill GD, Block JR, Tanem JB, Frommelt MA. Disparities in the prenatal detection of critical congenital heart disease. Prenat Diagn. Sep 2015;35(9):859–63. doi: 10.1002/pd.4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox SM, Butcher JL, Sadhwani A, et al. Integrating Telehealth Into Neurodevelopmental Assessment: A Model From the Cardiac Neurodevelopmental Outcome Collaborative. J Pediatr Psychol. Jun 7 2022;47(6):707–713. doi: 10.1093/jpepsy/jsac003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasparian NA, Sadhwani A, Sananes R, et al. Telehealth services for cardiac neurodevelopmental care during the COVID-19 pandemic: a site survey from the Cardiac Neurodevelopmental Outcome Collaborative. Cardiol Young. Feb 24 2022:1–8. doi: 10.1017/S1047951122000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuban KCK, Boynton-Jarrett R, Heeren T, O’Shea TM. A Consideration of Racism in Pediatric Epidemiologic Studies. J Pediatr. Dec 2021;239:225–227. doi: 10.1016/j.jpeds.2021.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright JL, Davis WS, Joseph MM, et al. Eliminating Race-Based Medicine. Pediatrics. Jul 1 2022;150(1)doi: 10.1542/peds.2022-057998 [DOI] [PubMed] [Google Scholar]
- 44.Brokamp C, Wolfe C, Lingren T, Harley J, Ryan P. Decentralized and reproducible geocoding and characterization of community and environmental exposures for multisite studies. J Am Med Inform Assoc. Mar 1 2018;25(3):309–314. doi: 10.1093/jamia/ocx128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chowdhury D, Johnson JN, Baker-Smith CM, et al. Health Care Policy and Congenital Heart Disease: 2020 Focus on Our 2030 Future. J Am Heart Assoc. Oct 19 2021;10(20):e020605. doi: 10.1161/JAHA.120.020605 [DOI] [PMC free article] [PubMed] [Google Scholar]


