Abstract
We report the characterization of a type-common human recombinant monoclonal antibody previously isolated by antigen selection from a phage-displayed combinatorial antibody library established from a herpes simplex virus (HSV)-seropositive individual. Competition with well-characterized murine monoclonal antibodies and immunodetection of gD truncations revealed that this antibody recognizes the group Ib antigenic site of glycoprotein D, a highly conserved and protective type-common determinant. To our knowledge, this is the first human group Ib monoclonal antibody ever described. The antibody also displayed first-order neutralization kinetics and a high neutralization rate constant, was capable of completely inhibiting syncytium formation by a fusogenic strain of HSV type 1, and efficiently neutralized low-passage clinical isolates of both HSV serotypes. Taken together with our earlier observations of the in vivo antiviral activities of this human recombinant antibody in animal models of HSV infection, the present results support the high therapeutic potential of this antibody.
Passively administered immunoglobulins confer protection against a large number of viral and bacterial pathogens (6, 10, 17). The growing clinical problems associated with resistance to antiherpetic drugs, especially in immunosuppressed individuals, supports the importance of exploring novel potential therapeutic approaches (7, 8, 19, 35, 39).
The capacity of murine monoclonal antibodies (MAbs) to herpes simplex virus (HSV) to protect experimental animals in different paradigms has been widely demonstrated (2, 13, 24, 30). However, the efficacy of passive immunization with human immune serum is limited (23, 45). This is consistent with the notion that protective antibodies against HSV may be present only as a minor component of the natural immune response, as suggested by several lines of evidence (16, 30, 33). Therefore, it is of primary importance to clonally isolate and characterize the protective antibodies generated in the natural human immune response and to explore their protective mechanisms, as well as the means by which they can be exploited therapeutically.
Only a few human MAbs suitable for passive immunization of humans have been produced, primarily because of the limited efficacy of conventional hybridoma technologies in establishing human antibodies. Affinity-based antibody cloning from combinatorial Fab libraries displayed on the surface of bacteriophage M13 is an alternative and very effective means to isolate human MAbs against viral pathogens (46). With this approach, sequences coding for light chains and the portion of heavy chains contributing to antibody Fab fragments (Fd regions) are cloned in the same phagemid vector and expressed in Escherichia coli as a fusion protein with a filamentous phage structural protein, cpIII (reviewed in reference 6). This fusion protein is targeted to the periplasmic space, where functional Fabs assemble. Upon infection with a helper phage, the Fab-cpIII fusions are incorporated into the virions and can bind immobilized antigens, thus allowing for the selection of antibodies to the targets of interest (6). Such Fabs can later be converted to whole antibodies by recombinant DNA techniques and expressed in eukaryotic cell systems. Antibodies cloned with this strategy have proved effective against different viruses in both in vitro and in vivo paradigms (4, 9, 37, 40, 41, 46, 50) and have the potential to prove useful in the prophylaxis or therapy of uncontrolled, medically important human viral diseases (6). Furthermore, they can be valuable diagnostic tools (5, 47).
With this technology, we have also generated human monoclonal antibodies to HSV type 1 (HSV-1) and HSV-2 from HSV-seropositive individuals (4, 41, 46), including the antibody described in the present report, which was designated HSV8 (4). HSV8 is an immunoglobulin G1 (IgG1) type-common neutralizing antibody specific for HSV glycoprotein D (gD) (4). Initial in vivo experiments with this human recombinant antibody in mice demonstrated that it can be highly effective, even in immunodeficient mice, when administered either systemically or topically (40, 50). The present study was undertaken to further characterize the antiviral activities of antibody HSV8. In particular, we have determined the epitope specificity of the antibody and investigated its antiviral activities in in vitro assays designed to explore its effectiveness against both cell-free and cell-associated virus. These included neutralization kinetics, neutralization of low-passage clinical isolates, and inhibition of cell-to-cell spread by a fusogenic strain of HSV-1.
MATERIALS AND METHODS
Antibodies, cells, and virus.
The recombinant human MAb in this study was initially termed AC8 (4), but we now refer to it as HSV8. Establishment and production of whole HSV8 IgG1 in eukaryotic cells has been reported (40). Unless otherwise specified, the experiments described here utilized whole HSV8 IgG1 expressed in CHO cells. Murine MAbs to HSV gD were kindly provided by Patricia Spear, Northwestern University, Chicago, Ill. (MAb III-174); Lenore Pereira, University of California, San Francisco (MAbs H170 and HD1); and Gary Cohen and Roselyn Eisenberg, University of Pennsylvania, Philadelphia (DL11). A MAb to gB (1105) was obtained from the Goodwin Institute (Plantation, Fla.). All murine MAbs were used as divalent whole IgGs. A rabbit polyclonal antibody to HSV-1 was obtained from Dako Corporation (Carpinteria, Calif.). gD fragments were also generously provided by Gary Cohen and Roselyn Eisenberg. HSV-2 strain G and HSV-1 strain F were obtained from the American Type Culture Collection (ATCC) and propagated in Hep2 cells (ATCC). Fusogenic viral strain HSV-1(HFEM)syn was kindly provided by Patricia Spear. Serotyped low-passage primary isolates, isolated by standard techniques (1), were generously provided by Nino Manca, University of Brescia, Brescia, Italy. They were all from primary lesions of patients never exposed to antiherpetic treatments (see the legend to Fig. 6 for clinical data). Vero cells (ATCC) were used for viral titrations and all plaque reduction and syncytium inhibition experiments. All cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum.
FIG. 6.
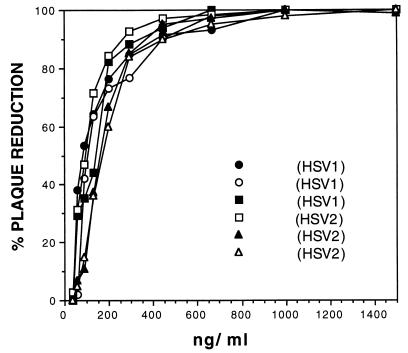
Neutralization of low-passage clinical isolates by human recombinant MAb HSV8. Fab HSV8 efficiently neutralized low-passage clinical isolates of HSV types I and II with an average of 50% inhibition at about 100 ng/ml and an average of 80% inhibition at about 200 ng/ml. These concentrations are comparable to those required for HSV8 neutralization of laboratory strains of HSV-1 and -2. The clinical localizations of lesions from which viruses were isolated were the following: ○ and ■, lip (cold sore); •, gingivostomatitis (serotype I); □, foreskin; ▴ and ▵, vagina (serotype II).
Epitope mapping. (i) Competition capture ELISA.
Different MAbs against gD were bound to enzyme-linked immunosorbent assay (ELISA) plates (Costar, Cambridge, Mass.) overnight at 4°C (0.2 μg/well in 25 μl of phosphate-buffered saline [PBS]). MAb 1105 against glycoprotein B was used in parallel as a negative control. The wells were blocked for 1 h with 3% bovine serum albumin (BSA) in PBS. A clarified total lysate of HSV-1-infected Vero cells in 1× radioimmunoprecipitation assay buffer (1% Nonidet P-40 [Sigma, St. Louis, Mo.], 1% Na deoxycholate in PBS) was then added (about 100 ng/well), and the mixture was incubated for 20 min. After washes with PBS containing 0.05% (vol/vol) Tween 20 (Sigma), the wells were incubated with either HSV8 (1 μg/well) or a rabbit anti-HSV serum (1:400) in 1% BSA in PBS for 1 h. The plates were again washed with PBS-Tween and incubated with an alkaline phosphatase-conjugated goat anti-human F(ab′)2 second antibody (Pierce, Rockford, Ill.) for 1 h more (1:1,000 in PBS). After washing, phosphatase activity was revealed with p-nitrophenyl phosphate (PNPP; 0.1%, wt/vol) in 0.1 M NaHCO3 buffer (pH 8.4). Optical density values were read at 405 nm.
(ii) Competition in direct (noncapture) ELISA.
ELISA plates were coated overnight with about 25 ng of total HSV-1-infected cell proteins and blocked with BSA as described above. To evaluate the ability of HSV8 to compete with group I anti-gD antibodies, a set of wells was preincubated with 50 μl of PBS–1% BSA with HSV8 at 5 μg/well for 30 min, while control wells were incubated for the same amount of time with 50 μl of PBS–1% BSA. Fifty nanograms of either a group I MAb (III-174) or a group VII MAb (H170) to gD was then added per well. Wells that had been preincubated with HSV8 were reacted with either MAb III-174 or MAb H170 in the presence of HSV8 (5 μg/well). The MAbs were then detected with an alkaline phosphatase-conjugated goat anti-mouse secondary antibody (Pierce) as described above.
(iii) Slot blotting of gD truncations.
Purified gD fragments (1 to 234, 1 to 275, and 1 to 306), a generous gift of Gary Cohen and Roselyn Eisenberg, were vacuum blotted onto polyvinylidene difluoride membranes in 100 μl of PBS (60 to 100 ng/well). The membranes were washed in Tris-buffered 3× saline (450 mM NaCl, 20 mM Tris HCl [pH 7.5], Tris-buffered saline [TBS]), blocked in 5% (wt/vol) nonfat milk (Bio-Rad, Hercules, Calif.) for 2 h and immunoreacted with 1 to 4 μg of either HSV8 or different MAbs to gD per ml in a casein blocker in TBS (Bio-Rad) containing 0.05% (vol/vol) Tween 20 (Tris-casein-Tween) for 4 h. The blots were then washed in TBS with 0.05% (vol/vol) Tween 20 and incubated for 1 h with a 1:20,000 dilution of either a horseradish peroxidase-conjugated goat anti-human F(ab′)2 second antibody (for HSV8) or a horseradish peroxidase-conjugated goat anti-mouse antibody (both from Pierce) in Tris-casein-Tween. The blots were developed for chemiluminescence with the SuperSignal substrate system (Bio-Rad) and exposed to Kodak BioMax MR film.
Inhibition of syncytium formation.
The ability of antibody HSV8 to inhibit the formation of syncytia was determined by using fusogenic strain HSV-1(HFEM)-syn, a generous gift of Patricia Spear, as described by Noble et al. (34). Briefly, confluent Vero cell monolayers in 24-well plates were infected with HSV-1 (HFEM)syn at a multiplicity of infection (MOI) of either 1 or 0.001. Wells infected at the higher MOI were used to determine the lowest antibody concentration that completely abolished the formation of syncytia (see Fig. 3), while the wells infected at the lower MOI were used to determine the sizes of syncytia at different antibody concentrations (see Fig. 4), given that at this MOI confluent areas of syncytium were rare. Two hours postinfection, the inoculum was removed and either medium alone or medium containing different concentrations of HSV8 was added. After overnight incubation at 37°C, the monolayers were fixed with 10% formalin in PBS for 10 min and stained in PBS for 5 min with 10-μg/ml 4′,6-diamidino-2-phenylindole (DAPI; Sigma) which selectively stains nuclei for UV fluorescence. Even small syncytia with DAPI fluorescence are very clearly detectable, allowing the quantification of the number of cells in each individual syncytium.
FIG. 3.
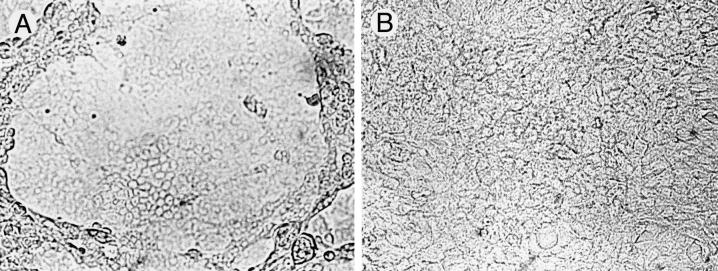
Inhibition of syncytium formation by human recombinant MAb HSV8. (A) Large areas of syncytium are apparent after infection of Vero cell monolayers with fusion-inducing strain HSV-1(HFEM)syn at an MOI of 1. (B) At a concentration of 8 μg/ml, HSV8 was effective in completely abolishing syncytium formation.
FIG. 4.
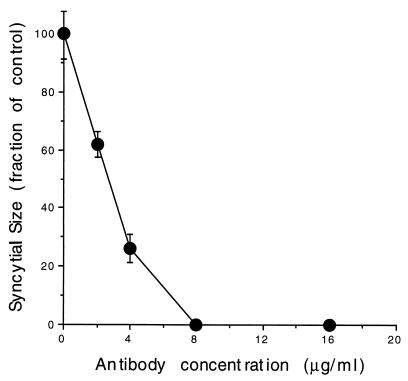
Syncytial inhibition as a function of HSV8 concentration. Vero cell monolayers were infected with fusion-inducing strain HSV-1(HFEM)syn at an MOI of 0.001 so that the number of cells contributing to individual syncytium foci could be determined by counting of DAPI-stained nuclei. A statistically significant syncytium size reduction of about 40% was observed at 2 μg/ml (P < 0.05, Scheffe F test, following significant analysis of variance), and a reduction of about 70% was seen at 4 μg/ml (P < 0.01). At the higher concentrations tested (8 and 16 μg/ml), syncytium formation was completely prevented.
Neutralization experiments.
For neutralization kinetics, HSV-1 (F) at 5,000 PFU/ml was incubated at 33°C with three different concentrations of bacterially expressed Fab HSV8 (2, 6, and 18 μg/ml) or at 37°C with 3-μg/ml Fab HSV8. At selected time points (1.25, 2.5, 5, 7.5, 10, and 15 min), 50-μl aliquots were removed from the virus-antibody mixtures and immediately added to 5 ml of prechilled serum-free medium at 4°C to terminate the reaction. Such 5-ml suspensions were then adsorbed onto confluent Vero cell monolayers in 100-mm-diameter plates for 1 h at 37°C with intermittent shaking. After removal of the inoculum, a nutrient overlay containing 0.5% agarose and 2% heat-inactivated fetal salt serum (final concentrations) in RPMI 1640 medium was added. After the appearance of plaques, the plates were fixed with 10% formalin in PBS, rinsed, and stained with crystal violet (10% [wt/vol] in 70% methanol). The log10 of the residual infectivity (number of plaques at the indicated experimental time points/initial viral inoculum [V/V0]) was plotted against time on an arithmetic scale. The neutralization rate constant was calculated with the equation (28) K = D/t · 2.3 log V0/Vt, where V0 and Vt are infectious virus at time zero and time t, respectively, and D is either the dilution factor of the antiserum or, as in the present case, the reciprocal of the molar concentration of the antibody solution (1/C). Neutralization of low-passage HSV isolates was carried out by standard techniques. Serial dilutions of Fab HSV8 were incubated for 1 h at 37°C with 100 PFU of virus; they were then adsorbed onto confluent Vero cell monolayers in six-well plates by incubation for 1 h. After removal of the inoculum, a nutrient overlay was applied as already described. Monolayers were fixed and stained as already described.
RESULTS
Epitope mapping.
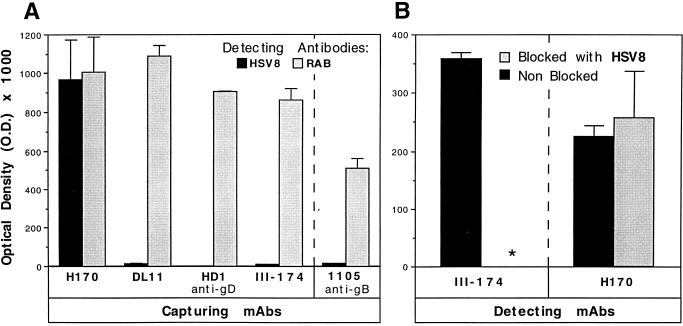
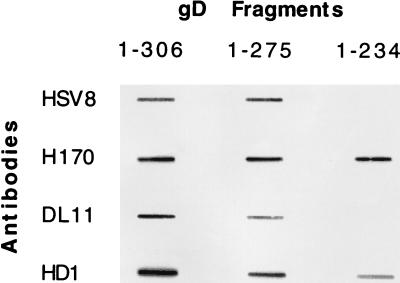
HSV8 did not react with gD in denaturing reducing Western blots, suggesting that it recognizes a nonlinear conformation-dependent epitope (data not shown). MAbs with potent complement-independent neutralizing activity and directed to type-common discontinuous epitopes on gD tend to recognize determinants clustered in antigenic group I of the polypeptide (15, 33). Therefore, we used competition with known group I MAbs to determine whether the epitope recognized by HSV8 lay within this antigenic cluster. In a competition capture ELISA, HSV8 could not detect HSV-1 gD specifically immunoadsorbed from a total viral lysate with group I MAbs to gD (Fig. 1). Conversely, HSV8 was capable of recognizing HSV-1 gD (Fig. 1) when this glycoprotein was immunoadsorbed by MAb H170, which does not compete with group I antibodies. The epitope recognized by MAb H170 is a linear epitope located within antigenic group VII (reviewed in reference 33). Detection with a rabbit polyclonal anti-HSV-1 serum was used as a positive control to demonstrate gD capture (Fig. 1). Capture with an anti-gB antibody, MAb 1105, served as a negative control (Fig. 1). In addition, in a direct (noncapture) ELISA experiment using immobilized total HSV-1-infected cell lysate as a source of antigen, HSV8 very effectively blocked detection of gD by a group I antibody (III-174) but not by a group VII antibody (H170). These observations suggest that HSV8 is a group I human MAb. Group I antibodies are further subdivided into subgroups Ia and Ib (see Discussion). All group I antibodies react with fragment 1 to 275 of gD. Subgroup Ia antibodies also react with fragment 1 to 234 of gD, while Ib antibodies do not, since they appear to require residues located between amino acids 234 and 275 for their binding. HSV8 detected recombinant gD truncations 1 to 306 and 1 to 275 in slot blots but not fragment 1 to 234, consistent with a subgroup Ib assignment (Fig. 2). Subgroup Ia antibody HD1, subgroup Ib antibody LP11, and MAb H170, specific for the group VII antigenic site, were used as controls (Fig. 2).
FIG. 1.
Since human recombinant MAb HSV8 is directed to a discontinuous determinant, epitope mapping was carried out by competition with known antibodies in two ELISA configurations. The y axis represents optical density (O.D.). (A) With known MAbs in a competition capture ELISA, HSV8 (black bars) was clearly capable of recognizing HSV-1 gD immunoadsorbed from a total viral lysate with a group VII anti-gD antibody (H170); conversely, HSV8 could not detect gD specifically immunoadsorbed with group I MAbs (DL11, HD1, and III-174). Rabbit polyclonal anti-HSV-1 serum (RAB) was used as a positive control in the same format in place of HSV8 (black bars); anti-gB antibody MAb 1105 was used as a capture antibody as a negative control (rightmost part of panel A). (B) In a direct (noncapture) ELISA experiment using immobilized total HSV-1-infected cell lysate as a source of antigen, HSV8 blocked detection of gD by a group I antibody (III-174) but not by a group VII antibody (H170). These observations suggest that HSV8 is a group I human MAb.
FIG. 2.
Epitope mapping of human recombinant MAb HSV8. While all group I antibodies react with a 1-to-306 and a 1-to-275 fragment of gD, only subgroup Ia antibodies react with a 1-to-234 truncation. HSV8 detected recombinant gD truncations 1 to 306 and 1 to 275 in slot blots but not fragment 1 to 234, consistent with a subgroup Ib assignment. Subgroup Ia antibody HD1, subgroup Ib antibody LP11, and MAb H170, specific for the continuous group VII antigenic site, were used as controls.
Inhibition of syncytium formation.
At a concentration of 8 μg/ml, HSV8 was effective in completely abolishing syncytium formation following infection with fusion-inducing strain HSV-1(HFEM)syn (34) at an MOI of 1 (Fig. 3). When Vero cell monolayers were infected at a lower MOI so that the number of cells contributing to individual syncytium foci could be determined by counting of DAPI-stained nuclei, a reduction of syncytium size of about 40% was observed at 2 μg/ml and a reduction of about 70% was seen at 4 μg/ml (Fig. 4). Analysis of variance revealed a main effect of the antibody on syncytium size; F(4.15) = 25.65, P < 0.01. Post-hoc analysis revealed that statistical significance was reached at all of the antibody concentrations tested.
Neutralization kinetics.
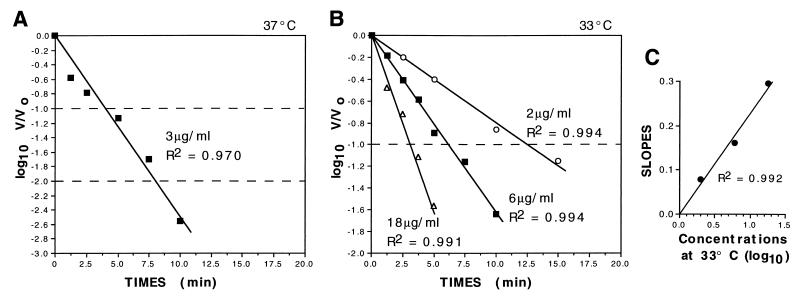
In neutralization kinetic experiments utilizing Fab HSV8 when the log10 of the residual infectivity at selected intervals (log10 V/V0) was plotted against time on an arithmetic scale, somewhat linear profiles extrapolating to the origin were obtained (Fig. 5A and B). This kind of plot, in which neutralization by an antibody proceeds linearly and is not preceded by a lag period, is indicative of first-order kinetics (11–13). Furthermore, when the slopes of the straight lines obtained at the different concentrations used (Fig. 5B) were plotted on an arithmetic scale versus the log10 antibody concentrations, they also yielded a straight line passing through the origin (Fig. 5C), which also supports first-order kinetics (13). The neutralization rate constant (± the standard error) was calculated to be 1.9 (± 0.4) × 105 M−1 s−1 at 37°C, assuming a Fab molecular mass of 50 kilodaltons. For comparison, we also determined the k values at 37°C of other potent type-common neutralizing murine MAbs to HSV, i.e., H170, H128, HD1, and III-174. In our study, H170, a group VII antibody, demonstrated a k value of 7.8 (± 0.6) × 104 M−1 s−1; H128, a subgroup Ib antibody, had a k value of 6.2 (± 0.6) × 104 M−1 s−1; HD1, a subgroup Ia MAb, had a kinetic constant of 1.8 (± 1.8) × 104 M−1 s−1; while MAb III-174, a highly protective subgroup Ib antibody, demonstrated k values of 1.25 (± 0.26) × 105 M−1 s−1 and 1.7 (± 0.15) × 105 M−1 s−1 in two independent experiments (not shown).
FIG. 5.
Neutralization kinetics of human recombinant MAb HSV8. Plots of neutralization kinetics at 37°C (A) and at 33°C (B) (note that the ordinate in panel A spans 3 logs while the ordinate in panel B spans 2) are shown. Fab HSV8 was incubated with HSV-1 strain F at the concentrations indicated (3 μg/ml at 37°C and 2, 6, and 18 μg/ml at 33°C), and the residual infectivity (V/V0) over time was determined by plaque assay. Straight kinetic profiles intercepting the ordinates at the origin were obtained when the log10 of V/V0 was plotted against time. Such linear plots are indicative of first-order kinetics. The plots obtained with 3 μg/ml at 37°C (A) and with 18 μg/ml at 33°C (B) show a slight initial dip that departs from the theoretical linear profile. This is commonly seen when this technique is used at higher antibody concentrations and/or temperatures and may be an artifact of the rapid initial mixing of the antibody-virus mixture. (C) When the slopes of the lines obtained at 33°C at the three different concentrations were plotted against the log10 of the antibody concentrations, a straight line passing through the origin was obtained; this is also indicative of first-order kinetics. The neutralization rate constant (± the standard error) was calculated to be 1.9 (± 0.4) × 105 M−1 s−1 (from two independent experiments).
Neutralization of low-passage clinical isolates.
Lastly, we investigated the ability of Fab HSV8 to neutralize low-passage clinical isolates of HSV-1 and -2. All isolates were from primary peripheral lesions, as indicated in the legend to Fig. 6. All isolates were efficiently and comparably neutralized with an average of 50% inhibition by about 100 ng/ml and an average of 80% inhibition by about 200 ng/ml (Fig. 6). These concentrations are comparable to those obtained with laboratory strains of HSV-1 and -2 (4).
DISCUSSION
Despite the availability of effective antiherpetic drugs, passive immunotherapy with human MAbs would be a valuable prophylactic tool to prevent infections in newborns and in other settings, as well as a complement to chemotherapeutic drugs for immunocompromised individuals.
The incidence of HSV infection of newborns is 1 in 2,000 to 5,000 deliveries, and in about 50% of the cases, it involves herpetic encephalitis, which is invariably followed by severe sequelae (22, 45). The most severe cases of neonatal herpes present disseminated infection with multiple visceral organs and central nervous system involvement and are usually fatal (45). Interestingly, transplacental maternal neutralizing antibodies and antibody-dependent cell-mediated cytotoxicity antibodies to HSV appear to influence both the severity of infection and the likelihood of transmission (38, 45, 48). For instance, the incidence of neonatal herpes is 1 order of magnitude higher (30 to 50%) in neonates born vaginally to mothers with primary infections than in neonates born to mothers with recurrent infections, who could transfer protective antibodies to the fetus (1 to 3%) (23). Therefore, it seems reasonable to expect that passive immunization could play a role in the prophylaxis and therapy of HSV infections in newborns, especially since antiherpetic chemotherapy during early development and in newborns should never be considered risk free (17). Beside the prevention of vertical transmission of HSV, human recombinant antibodies could be useful in preventing horizontal transmission of HSV in cases in which a sexual partner is infected with HSV-2; in rape cases; among contact sport athletes, such as wrestlers (herpes gladiatorum); etc.
HIV-infected individuals and individuals immunodepressed for other pathologic or iatrogenic reasons often exhibit extensive indolent HSV lesions (43). Although antiherpetic chemotherapy proved highly effective in the immunocompetent, significant unique problems may be encountered in immuno-depressed patients (43). These include higher toxicity (43) and the emergence of drug resistance (8). In one study, the incidence of acyclovir resistance in HSV-1 and -2 isolates exceeded 20% among individuals exposed to the drug, while clinical strains isolated before acyclovir therapy are rarely resistant (36). Resistance to both vidarabine and foscarnet has also long been known and is not infrequently encountered in immunodepressed patients receiving treatment with these drugs (3, 8, 39, 49). The emergence of drug-resistant HSV strains in the immunocompromised often correlates with treatment failure and high morbidity and mortality (7, 35, 43). Novel antiherpetic drugs are available and being tested. None, however, is potentially resistance free, and the possibility of multiple drug resistance limits the potential of combined therapy with the currently available chemotherapeutic drugs (8, 19). Although passive immunization has traditionally been considered mostly a prophylactic measure, it is reasonable to believe that it could be beneficial, possibly in combination with antimicrobial drugs, in cases in which active immunity cannot be elicited, such as in the immunocompromised. In addition, immune therapy with human MAbs could be combined with chemotherapy with no risk of developing cross-resistance because of their completely unrelated mechanisms of action. In HIV-infected patients and in patients with primary immunoglobulin deficiencies, even passive immunotherapy with sera from normal, healthy individuals proved beneficial in limiting HSV morbidity (31, 44).
Immunoglobulins of human origin have been employed for several years in clinical practice, and such a large body of experience suggests that they can be administered with a high degree of safety and that the benefits largely outweigh the risks (14, 44). Replacement therapy for patients with agammaglobulinemia has provided an opportunity to evaluate even very high doses of polyclonal human IgG for adverse reactions. In these patients, while adverse reaction were not infrequently seen with intramuscular preparations and even with early intravenous preparations, with newer intravenous formulations introduced in the 1980s, only very mild reactions are occasionally encountered, which only exceptionally require interruption of administration (44). The number one risk associated with the use of human immunoglobulins in human therapy is their potential contamination with viruses or other infectious agents. Such a risk is much lower for recombinantly generated MAbs than for immunoglobulins from pools of donors. The most desirable immunoglobulin preparations for human therapy are specific human MAbs or cocktails of human MAbs with exceptional protective qualities. These are theoretically almost devoid of untoward effects when administered either alone or in combination with other drugs. Human MAbs or cocktails of human MAbs are also more desirable than polyclonal sera, since protective antibodies may be only a minor component in the natural host response and because infection-enhancing antibodies are often present in polyclonal sera (discussed in reference 32). The use of human antibodies either chronically or in a repeated fashion may theoretically induce the emergence of anti-idiotypic and antiallotypic antibodies; however, the occurrence of serum sickness-like adverse reactions of the sort seen with animal sera in sensitized individuals has never been reported with human immunoglobulins. The emergence of low levels of antiallotypic antibodies has been reported after repeated administration of human-mouse chimeric antibodies, but with no severe clinical consequence (21). The consequences of the possible emergence of anti-idiotypic antibodies are difficult to predict and deserve close monitoring. In this regard, it has been reported that while human antimouse antibodies directed to murine IgG1 isotypic determinants resulted in rapid clearance of the administered murine antibody from the circulation and in loss of treatment efficacy, (i) levels of the transferred antibodies in the serum of patients who developed only anti-idiotypic antibodies and (ii) therapeutic efficacy were unaffected (42).
The importance of antibodies in recovery and in the prevention of infection and reinfection is widely accepted. However, while high neutralization rates, potency, and efficacy may be beneficial in prophylaxis, the specific contribution of neutralization has been a subject of debate (12). In most cases, however, a strong correlation between in vitro neutralization and in vivo protection was observed, supporting a role for neutralization in vivo (reviewed in reference 12). In the case of HSV, antibodies that are protective in in vivo experimental models are usually characterized by high potency in neutralization assays and effectiveness in limiting cell-to-cell viral spread in in vitro paradigms (15, 33). In clinical studies, high levels of both neutralizing antibodies and antibodies capable of eliciting antibody-dependent cell-mediated cytotoxicity have been shown to positively affect the severity of perinatal HSV infections in humans (23, 38, 48). Recurrences, however, are often seen despite the presence of neutralizing antibodies in the serum (27). This could be due to a number of factors. A crucial protective role may be played by a limited number of epitopes; protective antibodies against certain viral pathogens may be present only as a minor component of the natural immune response, while infection-enhancing antibodies may also be present; and lastly, a combination of antibody-mediated antiviral activities against both cell-free virus and cell-associated virus may be important in vivo. These may include nonlytic mechanisms which may be especially important in neurotropic virus infections (25, 26). In this regard, HSV8 demonstrated potent activities against cell-associated virus in a syncytium inhibition assay (Fig. 4) and in a plaque development inhibition assay (4), which are two in vitro models likely to be relevant to cell-to-cell virus spread in vivo. The antibody consistently, dramatically, and significantly prolonged survival times in vivo, when administered systemically up to 24 h postinfection, a time when the virus had already reached the peripheral nervous system (40).
Continuous epitopes on gD are grouped into three type-common groups (II, VII, and XI) and one type-specific group (V), while MAbs to discontinuous epitopes are grouped into four groups, I, III, IV, and VI, the former two being type-common determinants (15, 33). Two antigenic sites, I and VII, on gD elicit antibodies with potent protective properties (33). By use of an ELISA-based competition assay, we have shown that HSV8 can be competed by group I MAbs to gD (Fig. 1). Group I MAbs recognize epitopes within a highly conserved type-common antigenic site, have high complement-independent neutralization titers, are effective in passive immunization in animal models, and inhibit virus penetration, and some also inhibit cell-cell fusion by syncytium-inducing strains (15, 33). Consistently, HSV8 was capable of efficiently neutralizing low-passage clinical isolates of both serotypes (Fig. 6) and inhibited cell-cell fusion by a fusogenic strain of HSV-1 (Fig. 3 and 4), and we previously showed that it is effective in murine models of HSV infection both topically and systemically (40, 50). Group I epitopes can be further classified as Ia or Ib on the basis of the induction of specific neutralization escape mutations and reactivity to gD deletion mutants and gD truncations (33). We classified HSV8 as a subgroup Ib epitope on the basis of gD truncation recognition (Fig. 2). To our knowledge, the isolation of no other human group I MAb has been reported to date. Other human MAbs obtained with nonrecombinant approaches have been assigned to antigenic group III (reviewed in reference 31). Antibodies tentatively assigned to group III have also been molecularly isolated by phage display technology (our unpublished data and reference 41).
We also investigated the kinetics of HSV8 neutralization. In standard neutralization kinetic plots, HSV8 exhibited profiles extrapolating through the origin without an initial lag (Fig. 5). These plots are consistent with an apparent first-order neutralization reaction. The HSV8 neutralization rate constant (k value) was calculated to be in the range of 105 M−1 s−1. The HSV8 neutralization rate constant was higher than those of several potent HSV-neutralizing MAbs tested for comparison, although highly protective MAb III-174 had a very comparable k value (see above). k values in the range of 106 M−1 s−1 or higher were reported only for a few antibodies against other viruses; examples are an anti-poliovirus mouse MAb (20) and a mouse MAb to influenza virus type A hemagglutinin (12). The basis of the neutralization kinetic plot is still controversial and likely to be complex (11, 13). However, it is generally accepted that, in practical terms, (i) antibodies that, like HSV8, neutralize without an initial lag are more desirable for therapy than antibodies with similar potency but which do not reduce viral infectivity immediately and (ii) antibodies with higher neutralization rate constants are more desirable for clinical use (29). If prevention of infection by neutralizing antibodies is viewed as “a race between neutralization and escape of the virus particle from neutralization by entry into a host cell” (29), the importance of these factors becomes apparent. Therefore, the potential of HSV8 to be clinically effective is strengthened by the observations that it displayed apparent first-order kinetics and a neutralization rate constant higher than or comparable to that of potent anti-HSV neutralizers.
In conclusion, HSV8 was found to recognize a highly conserved antigenic site on gD known to induce highly protective type-common humoral responses. It was also found to inhibit cell-cell fusion by a syncytium-inducing HSV-1 strain and to neutralize by apparent first-order kinetics. Lastly, HSV8 efficiently neutralized low-passage clinical isolates of both serotypes. Taken together with our earlier observations of HSV8 effectiveness in passive immunization paradigms (40, 50), the present observations support the high therapeutic potential of this human recombinant antibody.
ACKNOWLEDGMENTS
We are particularly thankful to Gary Cohen, Roselyn Eisenberg (University of Pennsylvania), Lenore Pereira (University of California San Francisco), Bernard Roizman (University of Chicago), and Patricia Spear (Northwestern University), who generously provided us with viral strains, antibodies, and other indispensable tools. We also thank Gary Cohen, Roselyn Eisenberg, Lenore Pereira, and Bernard Roizman for helpful discussion and Michael Buchmeier, Peter Ghazal, and Lindsay Whitton (The Scripps Research Institute) for critical review of the manuscript.
This research was partially supported by PHS grants AI37582 (P.P.S.) and AI33292 (D.R.B.).
REFERENCES
- 1.Arvin A M, Prober C G. Herpes simplex viruses. In: Balows A, editor. Manual of clinical microbiology. Washington, D.C: American Society for Microbiology; 1991. pp. 822–828. [Google Scholar]
- 2.Balachandran N, Bacchetti S, Rawls W E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982;37:1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birch C J, Tachedjian G, Doherty R R, Hayes K, Gust I D. Altered sensitivity to antiviral drugs of herpes simplex virus isolates from a patient with the acquired immunodeficiency syndrome. J Infect Dis. 1990;162:731–734. doi: 10.1093/infdis/162.3.731. [DOI] [PubMed] [Google Scholar]
- 4.Burioni R, Williamson R A, Sanna P P, Bloom F E, Burton D R. Recombinant human Fab to glycoprotein D neutralizes infectivity and prevents cell-to-cell transmission of herpes simplex viruses 1 and 2 in vitro. Proc Natl Acad Sci USA. 1994;91:355–359. doi: 10.1073/pnas.91.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cattani P, Rossolini G M, Cresti S, Santangelo R, Burton D R, Williamson R A, Sanna P P, Fadda G. Detection and typing of herpes simplex viruses by using recombinant immunoglobulin fragments produced in bacteria. J Clin Microbiol. 1997;35:1504–1509. doi: 10.1128/jcm.35.6.1504-1509.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanock R M, Crowe J J, Murphy B R, Burton D R. Human monoclonal antibody Fab fragments cloned from combinatorial libraries: potential usefulness in prevention and/or treatment of major human viral diseases. Infect Agents Dis. 1993;2:118–131. [PubMed] [Google Scholar]
- 7.Christophers J, Sutton R N. Characterisation of acyclovir-resistant and -sensitive clinical herpes simplex virus isolates from an immunocompromised patient. J Antimicrob Chemother. 1987;20:389–398. doi: 10.1093/jac/20.3.389. [DOI] [PubMed] [Google Scholar]
- 8.Coen D M. The implications of resistance to antiviral agents for herpesvirus drug targets and drug therapy. Antiviral Res. 1991;15:287–300. doi: 10.1016/0166-3542(91)90010-o. [DOI] [PubMed] [Google Scholar]
- 9.Crowe J J, Murphy B R, Chanock R M, Williamson R A, Barbas C F, Burton D R. Recombinant human respiratory syncytial virus (RSV) monoclonal antibody Fab is effective therapeutically when introduced directly into the lungs of RSV-infected mice. Proc Natl Acad Sci USA. 1994;91:1386–1390. doi: 10.1073/pnas.91.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis B D, Dulbecco R, Eisen H N, Ginsberg H S. Microbiology. J. B. New York, N.Y: Lippincott; 1990. [Google Scholar]
- 11.Della Porta A J, Westaway E G. A multi-hit model for the neutralization of animal viruses. J Gen Virol. 1978;38:1–19. doi: 10.1099/0022-1317-38-1-1. [DOI] [PubMed] [Google Scholar]
- 12.Dimmock N J. Neutralization of animal viruses. Curr Top Microbiol Immunol. 1993;183:1–149. doi: 10.1007/978-3-642-77849-0. [DOI] [PubMed] [Google Scholar]
- 13.Dulbecco R, Vogt M S A G R. A study of the basic aspects of neutralization of two animal viruses. Virology. 1956;2:162–205. doi: 10.1016/0042-6822(56)90017-4. [DOI] [PubMed] [Google Scholar]
- 14.Eickhoff T C. Immunizations. In: Kelly W N, editor. Textbook of internal medicine. J. B. New York, N.Y: Lippincott; 1989. pp. 1813–1819. [Google Scholar]
- 15.Eisenberg R J, Cohen G H. Identification and analysis of biologically active sites of herpes simplex virus glycoprotein D. In: Crowell R, Lonberg-Holm K, editors. Virus attachment and entry into cells. Washington, D.C: American Society for Microbiology; 1986. pp. 74–84. [Google Scholar]
- 16.Eis Hubinger A, Schmidt D S, Schneweis K E. Anti-glycoprotein B monoclonal antibody protects T cell-depleted mice against herpes simplex virus infection by inhibition of virus replication at the inoculated mucous membranes. J Gen Virol. 1993;74:379–385. doi: 10.1099/0022-1317-74-3-379. [DOI] [PubMed] [Google Scholar]
- 17.Goodman-Gilman A, Goodman L S, Rall T W, Murad F. Goodman and Gilman’s the pharmacological basis of therapeutics. New York, N.Y: MacMillan Publishing Co.; 1985. [Google Scholar]
- 18.Highlander S L, Sutherland S L, Gage P J, Johnson D C, Levine M, Glorioso J C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987;61:3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang C B, Ruffner K L, Coen D M. A point mutation within a distinct conserved region of the herpes simplex virus DNA polymerase gene confers drug resistance. J Virol. 1992;66:1774–1776. doi: 10.1128/jvi.66.3.1774-1776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Icenogle J, Shiwen H, Duke G, Gilbert S, Rueckert R, Anderegg J. Neutralization of poliovirus by a monoclonal antibody: kinetics and stoichiometry. Virology. 1983;127:412–425. doi: 10.1016/0042-6822(83)90154-x. [DOI] [PubMed] [Google Scholar]
- 21.Knox S J, Levy R, Hodgkinson S, Bell R, Brown S, Wood G S, Hoppe R, Abel E A, Steinman L, Berger R G, et al. Observations on the effect of chimeric anti-CD4 monoclonal antibody in patients with mycosis fungoides. Blood. 1991;77:20–30. [PubMed] [Google Scholar]
- 22.Kohl S. Herpes virus infections. In: Kelly W N, editor. Textbook of internal medicine. J. B. New York, N.Y: Lippincott; 1989. pp. 1647–1666. [Google Scholar]
- 23.Kohl S. The role of antibody in herpes simplex virus infection in humans. Curr Top Microbiol Immunol. 1992;179:75–88. doi: 10.1007/978-3-642-77247-4_5. [DOI] [PubMed] [Google Scholar]
- 24.Lausch R N, Staats H, Metcalf J F, Oakes J E. Effective antibody therapy in herpes simplex virus ocular infection. Characterization of recipient immune response. Intervirology. 1990;31:159–165. doi: 10.1159/000150150. [DOI] [PubMed] [Google Scholar]
- 25.Levine B, Hardwick J M, Trapp B D, Crawford T O, Bollinger R C, Griffin D E. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 26.Liebert U G, Schneider S S, Baczko K, Meulen V. Antibody-induced restriction of viral gene expression in measles encephalitis in rats. J Virol. 1990;64:706–713. doi: 10.1128/jvi.64.2.706-713.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lodmell D L, Niwa A, Hayashi K, Notkins A L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973;173:706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBride W D. Antigenic analysis of polioviruses by kinetic studies of serum neutralization. Virology. 1959;7:45–58. doi: 10.1016/0042-6822(59)90176-x. [DOI] [PubMed] [Google Scholar]
- 29.McLain L, Dimmock N J. Single- and multi-hit kinetics of immunoglobulin G neutralization of human immunodeficiency virus type 1 by monoclonal antibodies. J Gen Virol. 1994;75:1457–1460. doi: 10.1099/0022-1317-75-6-1457. [DOI] [PubMed] [Google Scholar]
- 30.Mester J C, Glorioso J C, Rouse B T. Protection against zosteriform spread of herpes simplex virus by monoclonal antibodies. J Infect Dis. 1991;163:263–269. doi: 10.1093/infdis/163.2.263. [DOI] [PubMed] [Google Scholar]
- 31.Mofenson L M, Moye J J. Intravenous immune globulin for the prevention of infections in children with symptomatic human immunodeficiency virus infection. Pediatr Res. 1993;33:S80–S87. doi: 10.1203/00006450-199305001-00464. [DOI] [PubMed] [Google Scholar]
- 32.Monath T P. Flaviviruses. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press; 1990. pp. 763–814. [Google Scholar]
- 33.Muggeridge M I, Roberts S R, Isola V J, Cohen G H, Eisenberg R J. Herpes simplex virus. In: van Regenmortel M H V, Neurath A R, editors. Immunochemistry of viruses. II. The basis for serodiagnosis and vaccines. New York, N.Y: Elsevier Science Publishers; 1990. pp. 459–481. [Google Scholar]
- 34.Noble A G, Lee G T, Sprague R, Parish M L, Spear P G. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology. 1983;129:218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]
- 35.Norris S A, Kessler H A, Fife K H. Severe, progressive herpetic whitlow caused by an acyclovir-resistant virus in a patient with AIDS. J Infect Dis. 1988;157:209–210. doi: 10.1093/infdis/157.1.209. [DOI] [PubMed] [Google Scholar]
- 36.Nugier F, Colin J N, Aymard M, Langlois M. Occurrence and characterization of acyclovir-resistant herpes simplex virus isolates: report on a two-year sensitivity screening survey. J Med Virol. 1992;36:1–12. doi: 10.1002/jmv.1890360102. [DOI] [PubMed] [Google Scholar]
- 37.Parren P W H I, Ditzel H J, Gulizia R J, Binley J M, Barbas C F I, Burton D R, Mosier D E. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a monoclonal antibody against the gp120 CD4-binding site. AIDS. 1995;9:F1–F6. doi: 10.1097/00002030-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Prober C G, Sullender W M, Yasukawa L L, Au D S, Yeager A S, Arvin A M. Low risk of herpes simplex virus infections in neonates exposed to the virus at the time of vaginal delivery to mothers with recurrent genital herpes simplex virus infections. N Engl J Med. 1987;316:240–244. doi: 10.1056/NEJM198701293160503. [DOI] [PubMed] [Google Scholar]
- 39.Sacks S L, Wanklin R J, Reece D E, Hicks K A, Tyler K L, Coen D M. Progressive esophagitis from acyclovir-resistant herpes simplex. Clinical roles for DNA polymerase mutants and viral heterogeneity? Ann Intern Med. 1989;111:893–899. doi: 10.7326/0003-4819-111-11-893. [DOI] [PubMed] [Google Scholar]
- 40.Sanna P P, De L A, Williamson R A, Hom Y L, Straus S E, Bloom F E, Burton D R. Protection of nude mice by passive immunization with a type-common human recombinant monoclonal antibody against HSV. Virology. 1996;215:101–106. doi: 10.1006/viro.1996.0011. [DOI] [PubMed] [Google Scholar]
- 41.Sanna P P, Williamson R A, De Logu A, Bloom F E, Burton D R. Directed selection of recombinant human monoclonal antibodies to herpes simplex glycoproteins from phage display libraries. Proc Natl Acad Sci USA. 1995;92:6439–6443. doi: 10.1073/pnas.92.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schobert I, Renner C, Pfreundschuh M, Diehl V, Pohl C. Mimicry of the Hodgkin-associated IRAC antigen by an anti-idiotype network: potential use in active immunotherapy of Hodgkin’s lymphoma. Leuk Lymphoma. 1994;13:429–440. doi: 10.3109/10428199409049632. [DOI] [PubMed] [Google Scholar]
- 43.Schooley R T. Herpes infection in individuals with HIV infection. In: De Vita V T J, Hellman S, Rosenberg S, editors. AIDS: etiology, diagnosis, treatment, and prevention. 3rd ed. Philadelphia, Pa: J. B. Lippincott; 1990. pp. 193–207. [Google Scholar]
- 44.Skull S, Kemp A. Treatment of hypogammaglobulinaemia with intravenous immunoglobulin, 1973–93. Arch Dis Child. 1996;74:527–530. doi: 10.1136/adc.74.6.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitley R J. Herpes simplex viruses. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press; 1990. pp. 1843–1888. [Google Scholar]
- 46.Williamson R A, Burioni R, Sanna P P, Partridge L J, Barbas C F, Burton D R. Human monoclonal antibodies against a plethora of viral pathogens from single combinatorial libraries. Proc Natl Acad Sci USA. 1993;90:4141–4145. doi: 10.1073/pnas.90.9.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williamson R A, Lazzarotto T, Sanna P P, Bastidas R B, Dalla Casa B, Campisi G, Burioni R, Landini M P, Burton D R. Use of recombinant human antibody fragments for detection of cytomegalovirus antigenemia. J Clin Microbiol. 1997;35:2047–2050. doi: 10.1128/jcm.35.8.2047-2050.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeager A S, Arvin A M, Urbani L J, Kemp J., III Relationship of antibody to outcome in neonatal herpes simplex virus infections. Infect Immun. 1980;29:532–538. doi: 10.1128/iai.29.2.532-538.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida T, Suzutani T, Azuma M. Study on the apparent resistant strains of herpes simplex virus type 1 against 9-beta-d-arabinofuranosyladenine. Tohoku J Exp Med. 1988;156:279–290. doi: 10.1620/tjem.156.279. [DOI] [PubMed] [Google Scholar]
- 50.Zeitlin L, Whaley K J, Sanna P P, Moench T R, Bastidas R, De Logu A, Williamson R A, Burton D R, Cone R A. Topically applied human recombinant monoclonal IgG1 antibody and its Fab and Fab′2 fragments protect mice from vaginal transmission of HSV-2. Virology. 1996;225:213–215. doi: 10.1006/viro.1996.0589. [DOI] [PubMed] [Google Scholar]