Abstract
Acid sphingomyelinase deficiency (ASMD) is a severe lipid storage disorder caused by the diminished activity of the acid sphingomyelinase enzyme. ASMD is characterized by the accumulation of sphingomyelin in late endosomes and lysosomes leading to progressive neurological dysfunction and hepatosplenomegaly. Our objective was to investigate the utility of synthetic apolipoprotein A-I (ApoA-I) mimetics designed to act as lipid scavengers for the treatment of ASMD. We determined the lead peptide, 22A, could reduce sphingomyelin accumulation in ASMD patient skin fibroblasts in a dose dependent manner. Intraperitoneal administration of 22A formulated as a synthetic high-density lipoprotein (sHDL) nanodisc mobilized sphingomyelin from peripheral tissues into circulation and improved liver function in a mouse model of ASMD. Together, our data demonstrates that apolipoprotein mimetics could serve as a novel therapeutic strategy for modulating the pathology observed in ASMD.
Keywords: Apolipoprotein mimetics, Acid Sphingomyelinase Deficiency
Graphical Abstract
Synthetic high-density lipoprotein nanodiscs accept excess sphingomyelin from peripheral tissues in a mouse model of ASMD.
Introduction
Acid sphingomyelinase deficiency (ASMD), is an autosomal recessive disorder resulting from loss of function mutations in SMPD1, leading to lysosomal accumulation of sphingomyelin and other lipids.1-3 Disease progression can lead to hepatosplenomegaly and reduced pulmonary function, and, in the most severe cases, neurological deterioration, leading death typically at 2-3 years of age.4
Prior studies have shown ASMD patients have reduced levels of high-density lipoproteins (HDL) in circulation that are enriched in sphingomyelin content.5,6 When apolipoprotein A-1 (ApoA-1) is secreted by the liver, it interacts with the ATP binding cassette transporter A1 (ABCA1) on cell membranes to form nascent HDL particles from phospholipids.7,8 Recent work has taken advantage of ApoA-1 function for the development of synthetic HDL as therapeutics for cardiovascular disease.9-12 Because of the difficulty producing significant quantities of recombinant ApoA-1, several short ApoA-1 mimetic peptides have been developed.13-15 These peptides efflux cellular lipids via the ABCA1 transporter and increase circulating HDL levels. Based on this existing data, we decided to investigate if redistribution of accumulated sphingomyelin by apolipoprotein mimetics could serve as a new treatment strategy for ASMD using a genetic mouse model of the disease.16
Materials and Methods
Detailed materials and methods can be found in the supplementary information.
Results and Discussion
We compared 3 different apolipoprotein-mimetic peptides in their ability to solubilize sphingomyelin lipid vesicles. The 18A peptide contains 18 amino acids designed to mimic the amphipathic a-helix found in ApoA-1 with no sequence homology to the full-length protein.15 The 5A peptide contains two helices based on 18A, with 5 alanine substitutions in the second helix.14 The 22A peptide is based on the most prevalent amino acid present at each position in the a-helices of ApoA-1, with modifications to improve lipid binding.13 Full length ApoA-1 was used as a reference, and it induced moderate solubilization of sphingomyelin (~25% reduction in turbidity) but was surpassed by 22A and 18A (Figure 1A). We next investigated the ability of the peptides to remove sphingomyelin from ASMD patient fibroblasts (Figure 1B), as accumulation of sphingomyelin is a hallmark feature of ASMD. At 10μM, 22A, 18A, and 5A all showed similar efflux capabilities (~15%). Treatment at 100μM resulted in enhanced efflux, however, this came at the cost of cytotoxicity for 18A and 5A (Figure 1C). This cytotoxicity is likely due in part to disruption of the cell membrane, as the peptide with the strongest lipid binding, 18A, showed the most severe drop in cell viability. From these experiments, 22A stood out as the most promising peptide due to its dose-dependent increase in sphingomyelin efflux and superior safety profile compared to the other treatments.
Figure 1:
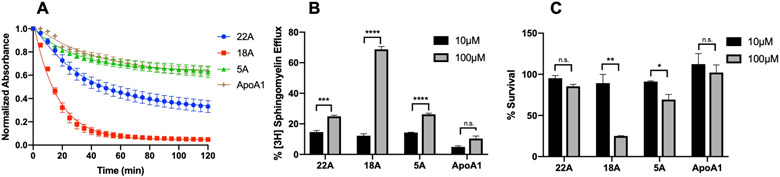
Evaluation of apolipoprotein mimetic peptides in vitro. Solubilization of sphingomyelin vesicles during 2-hour incubation with 5mg/mL apolipoprotein mimetic peptides measured by reduction in turbidity at 600nm (A). Sphingomyelin efflux from ASMD fibroblasts after 24-hour treatment with 10μM or 100μM peptide (B). Viability of ASMD patient cells following 24-hour treatment with peptides. (C). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, n.s. not significant. N=3
Most apolipoprotein mimetics in development are dosed as synthetic high-density lipoprotein nanodiscs (sHDL) instead of free peptide. Prior studies from our lab have demonstrated sHDL formulations of 22A have higher areas under the curve than free peptide.17 With this in mind, we determined the differences between sHDL and free 22A in vitro to further inform the best formulation for in vivo experiments. We prepared the sHDL by combining the 22A peptide with the phospholipid DMPC at a 2:1 ratio. The resulting particles were 6-8 nm in size and possessed disc-like morphology as determined by DLS and TEM respectively (Figure 2A-B). The sHDL nanodiscs showed slightly reduced sphingomyelin efflux capabilities compared to the free peptide when used at 10μM peptide concentration (15% vs 11%) (Figure 2C). This difference is likely attributable to effects of the peptide complex with DMPC, which reduces the amount of exposed peptide able to directly bind sphingomyelin. Despite the small change in efflux, the sHDL formulation of 22A possessed a superior safety profile in ASMD fibroblasts compared to free peptide, showing no decrease in survival at concentrations up to 500μM. From this, we decided to investigate the effects of the sHDL formulation of 22A in a mouse model of ASMD.
Figure 2:
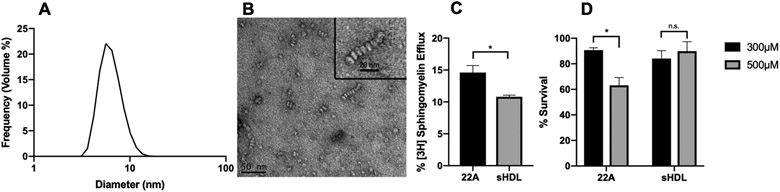
Comparing sHDL to free peptide in vitro. Size distribution of sHDL nanodiscs measured by dynamic light scattering (DLS) (A). Morphology of sHDL nanodiscs observed using negative-stain TEM (B). Sphingomyelin efflux comparison in ASMD skin fibroblasts following 24-hour treatment with free 22A peptide or 22A-DMPC (2:1) sHDL at 10μM peptide concentration (C). Cell viability following 24-hour treatment with free 22A peptide or sHDL at 300μM and 500μM (D). *p<0.05, n.s. not significant. N=3
Given the increased sphingomyelin content as a result of ASMD, we sought to determine if sHDL would serve as an acceptor of excess sphingomyelin from tissues and mobilize it into the bloodstream. Despite the absence of ASM activity in this mouse model, the presence of neutral sphingomyelinases localized to the Golgi, ER, and plasma membrane represented an opportunity for remobilized sphingomyelin to be metabolized.18,19 WT and Smpd1−/− mice were administered a single injection of 100 mg/kg sHDL nanodiscs intraperitoneally (I.P.), and serum was collected for sphingomyelin quantification. Both WT and Smpd1−/− mice had similar amounts of sphingomyelin present in the serum prior to treatment. Following sHDL injection, Smpd1−/− mice showed a ~20% increase in circulating sphingomyelin that persisted through the 24-hour time point (Figure 3A). A similar increase was observed in the WT mice 24-hours after treatment. In addition to serum sphingomyelin levels, we compared the lipoprotein profiles of WT and Smpd1−/− mice before and after sHDL injection. Prior to injection, Smpd1−/− mice had similar levels of very-low-density lipoproteins (VLDL), low-density lipoproteins (LDL), and HDL compared to WT (Figure 3B). This deviates from what is observed in humans, as the enriched sphingomyelin content of immature HDL particles has been shown to inhibit the enzymes required for HDL maturation.5 Six hours after sHDL injection in WT and Smpd1−/− mice, we observed a small decrease in HDL and a substantial increase in VLDL (Figure 3C). This change in VLDL levels is the result of rapid exchange of cholesterol and phospholipids from endogenous lipoproteins to sHDL and is consistent with previous reports on lipoprotein profiles following sHDL administration.17 Twenty-four hours post-injection, we observed a drop in VLDL and slight increase in HDL levels relative to baseline for both WT and Smpd1−/− mice (Figure 3D). Post-column analysis of lipoprotein fractions revealed the VLDL species appearing after sHDL nanodisc injection to be enriched in sphingomyelin and cholesterol content, with 30-40% of the total amount of sphingomyelin and cholesterol contained in the VLDL (not shown).
Figure 3:
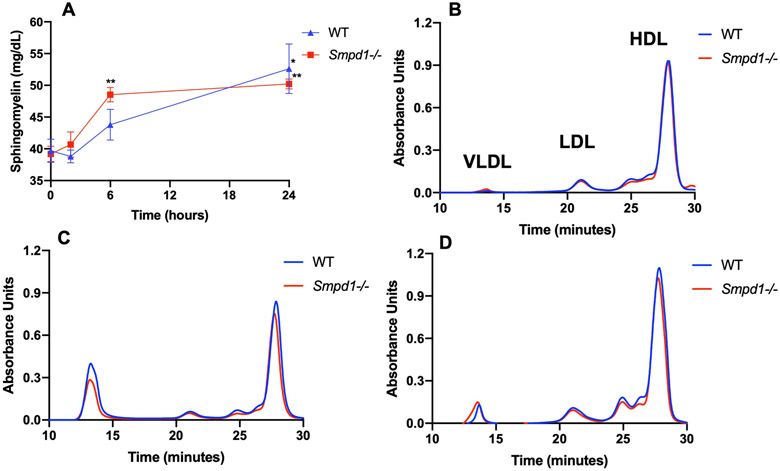
Single dose of sHDL nanodiscs increases circulating levels of sphingomyelin and alters lipoprotein profile in WT and Smpd1−/− mice. Mice were injected I.P. with 100mg/kg of sHDL and serum was analyzed at 2, 6, and 24 hours post-injection for sphingomyelin levels (A). Distribution of lipoproteins in serum for WT and Smpd1−/− mice prior to treatment with sHDL (B), 6 hours after sHDL injection (C), and 24 hours after injection (D) was evaluated using SEC with detection at 265nm. *p<0.05. **p<0.01 compared to initial SM levels. N=3
We next performed a 4-week study in which Smpd1−/− mice received daily I.P. injections of 100 mg/kg sHDL nanodiscs starting at 6-weeks of age. Smpd1−/− mice demonstrated deficits in motor performance and increased body weight compared to WT mice, which were not affected by sHDL treatment. (Figure 4A-B). This is likely due to limited quantities of peripherally administered sHDL nanodiscs able to cross the BBB (data not shown). Serum was collected at the end of treatment and analyzed for markers of liver function. Treatment of Smpd1−/− mice with sHDL nanodiscs resulted in significant reductions in serum alkaline phosphatase (ALKP) and aspartate aminotransferase (AST), two common markers of healthy liver function, rescuing them to levels observed in WT mice (Figure 4C-E).
Figure 4:
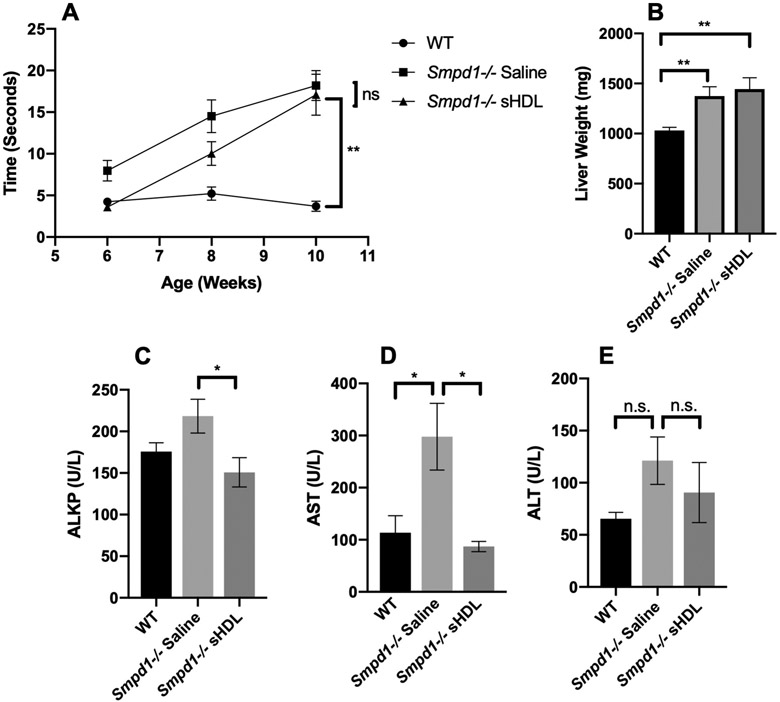
Effects of repeated dosing of sHDL in Smpd1−/− mice. Smpd1−/− or WT mice were given daily injections of saline or 100mg/kg sHDL I.P. for 4 weeks starting at 6 weeks of age. The motor deficits of mice were evaluated via time to traverse a balance beam (A). Livers were weighed at the end of treatment (B). Serum was collected at the end of treatment and analyzed for ALKP (C), AST (D), and ALT (E). N=6-11 mice per group. Error bars are SEM. *p<0.05, **p<0.01
Overall, our findings represent the first investigations of apolipoprotein mimetics in ASMD. Their ability to remove sphingomyelin from ASMD patient fibroblasts and mobilize sphingomyelin in vivo are both positive effects, however these changes were not able to halt disease progression in the complete absence of acid sphingomyelinase activity. Future experiments in models possessing residual enzyme activity, such as the murine model of non-neurological ASMD that features low levels of lysosomal sphingomyelinase activity developed by Marathe et al. 20, could reveal a more prominent role for apolipoprotein mimetics in managing ASMD.
Supplementary Material
Acknowledgements:
This work was funded by the NIH (R01 NS122746 to APL; K01 DK124450 to MLS; R21 NS11191 to AS), Niemann-Pick Canada (to MLS), and the PhRMA Foundation (Predoctoral Fellowship in Drug Discovery to TAH).
Footnotes
Conflicts of Interest:
Dr. Schwendeman declares financial interests for board membership, as a paid consultant, for research funding, and/or as equity holder in EVOQ Therapeutics. The University of Michigan has a financial interest in EVOQ Therapeutics, Inc.
References:
- 1.Brady RO, Kanfer JN, Mock MB, Fredrickson DS The metabolism of sphingomyelin. II. Evidence of an enzymatic deficiency in Niemann-Pick disease. Proc. Natl. Acad. Sci. U. S. A 55, 366–369 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider PB, Kennedy EP Sphingomyelinase in normal human spleens and in spleens from subjects with Niemann-Pick disease. J. Lipid Res 8, 202–209 (1967). [PubMed] [Google Scholar]
- 3.Wasserstein MP, Desnick RJ, Schuchman EH, Hossain S, Wallenstein S, Lamm C, McGovern MM The natural history of type B Niemann-Pick disease: Results from a 10-year longitudinal study. Pediatrics 114, (2004). [DOI] [PubMed] [Google Scholar]
- 4.Schuchman EH, Wasserstein MP Types A and B Niemann-Pick disease. Best Pract. Res. Clin. Endocrinol. Metab 29, 237–247 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Ching YL, Lesimple A, Denis M, Vincent J, Larsen A, Mamer O, Krimbou L, Genest J, Marcil M Increased sphingomyelin content impairs HDL biogenesis and maturation in human Niemann-Pick disease type B. J. Lipid Res 47, 622–632 (2006). [DOI] [PubMed] [Google Scholar]
- 6.McGovern MM, Pohl-Worgall T, Deckelbaum RJ, Simpson W, Mendelson D, Desnick RJ, Schuchman EH, Wasserstein MP Lipid abnormalities in children with types A and B Niemann Pick disease. J. Pediatr 145, 77–81 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Kuai R, Li D, Chen YE, Moon JJ, Schwendeman A High-Density Lipoproteins: Nature’s Multifunctional Nanoparticles. ACS Nano 10, 3015–3041 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Briggs MR HDL: The Metabolism, Function, and Therapeutic Importance. Chem. Rev 104, 119–137 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Tardif JC, Ballantyne CM, Barter P, Dasseux JL, Fayad ZA, Guertin MC, et al. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: A randomized trial. Eur. Heart J 35, 3277–3286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholls SJ, Andrews J, Kastelein JJP, Merkely B, Nissen SE, Ray KK, et al. Effect of serial infusions of CER-001, a pre-β High-density lipoprotein mimetic, on coronary atherosclerosis in patients following acute coronary syndromes in the CER-001 atherosclerosis regression acute coronary syndrome trial: A randomized clinical trial. JAMA Cardiol. 3, 815–822 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, et al. Effect of Recombinant ApoA-I Milano on Coronary Atherosclerosis in Patients with Acute Coronary Syndromes: A Randomized Controlled Trial. J. Am. Med. Assoc 290, 2292–2300 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Gibson CM, Kastelein JJP, Phillips AT, Aylward PE, Yee MK, Tendera M, et al. Rationale and design of ApoA-I Event Reducing in Ischemic Syndromes II (AEGIS-II): A phase 3, multicenter, double-blind, randomized, placebo-controlled, parallel-group study to investigate the efficacy and safety of CSL112 in subjects after acute myocardial infarction. Am. Heart J 231, 121–127 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Di Bartolo BA, Nicholls SJ, Bao S, Rye KA, Heather AK, Barter PJ, Bursill C The apolipoprotein A-I mimetic peptide ETC-642 exhibits anti-inflammatory properties that are comparable to high density lipoproteins. Atherosclerosis 217, 395–400 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Amar MJ, D’Souza W, Turner S, Demosky S, Sviridov D Stonik J., et al. 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J. Pharmacol. Exp. Ther 334, 634–641 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datta G, White CR, Dashti N, Chaddha M, Palgunachari MN, Gupta H, et al. Anti-inflammatory and recycling properties of an apolipoprotein mimetic peptide, Ac-hE18A-NH2. Atherosclerosis 208, 134–141 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horinouchi K, Erlich E, Perl DP, Ferlinz K, Bisgaier CL, Sandhoff K, et al. Acid sphingomyelinase deficient mice: A model of types A and B Niemann–Pick disease. Nat. Genet 10, 288–293 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Tang J, Li D, Drake L, Yuan W, Deschaine S, Morin ME, et al. Influence of route of administration and lipidation of apolipoprotein A-I peptide on pharmacokinetics and cholesterol mobilization. J. Lipid Res 58, 124–136 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan RD Alkaline sphingomyelinase: An old enzyme with novel implications. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1761, 281–291 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Xiang H, Jin S, Tan F, Xu Y, Lu Y, Wu T Physiological functions and therapeutic applications of neutral sphingomyelinase and acid sphingomyelinase. Biomed. Pharmacother. 139, 111610 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Marathe S, Miranda SRP, Devlin C, Johns A, Kuriakose G, Williams KJ, et al. Creation of a mouse model for non-neurological (type B) Niemann–Pick disease by stable, low level expression of lysosomal sphingomyelinase in the absence of secretory sphingomyelinase: relationship between brain intra-lysosomal enzyme activity and central nervous system function. Human Molecular Genetics. 9, 1967–1976 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


