Abstract
Introduction:
Myocarditis associated with immune checkpoint inhibitors presents with an often-severe clinical phenotype with arrhythmias and concurrent myositis. This condition tends to occur early after treatment onset and is associated with a high fatality rate. Diagnosis may be challenging, and treatment algorithms are still evolving.
Areas covered:
This review will provide an overview of immune checkpoint inhibitor mechanism of action and how it relates to myocarditis pathophysiology, diagnostic algorithms and potential pitfalls, and emerging treatment approaches published until May 2023. We will focus on the state of the field and potential new directions in research and patient care. We will also provide consensus based diagnostic and therapeutic algorithms endorsed by major societies.
Expert Opinion:
The field needs more evidence-based approaches to risk stratification so that therapy can be tailored towards less cardiotoxic alternatives in high-risk patients. For diagnostic and therapeutic approaches, data from animal models are unlikely to provide conclusive evidence given the complexity of the human immune system. We strongly invite practitioners in the field to contribute every case to the ongoing multi-center registries.
Keywords: Immune checkpoint inhibitors, ICI, cardiotoxicity, myocarditis, immune-related adverse events, irAEs, immunotherapy
1. Introduction
Immune checkpoint inhibitors (ICI) have changed the outlook for many cancers with grim prognosis. ICIs are monoclonal antibodies that target immune cell receptors and ligands, namely CTLA-4, PD-1, and its ligand PD-L1, and LAG-3. These checkpoints promote immune-tolerance to cancer cells by attenuating the T-cell activation that should occur upon encountering tumor antigens. Ipilimumab, the first ICI to achieve FDA approval in 2011, was the only CTLA-4 targeting therapy in the US market until tremelimumab was approved in November 2022. These CTLA-4 inhibitors are now largely used in combination with either anti-PD-1 or anti-PD-L1. Anti-PD-1 directed agents include nivolumab, pembrolizumab, cemiplimab, and dostarlimab, whereas anti-PD-L1 therapies include atezolizumab, avelumab and durvalumab. In November 2022, another ICI class, the anti-LAG-3 relatlimab, was approved for use in conjunction with nivolumab in treatment of unresectable or metastatic melanoma [1]. As of March 2023, indications for ICIs include more than 20 cancer types (Table 1). ICIs are used as first or second line therapy, in adjuvant, and neo-adjuvant settings, alone or in combination with other ICIs, chemotherapy, targeted therapy, or radiotherapy [2]. These combination strategies have been shown to provide synergistic effects and decrease resistance, especially in metastatic melanoma, renal cell cancer, and microsatellite instability-high or mismatch repair deficient colon adenocarcinoma [3][4]. Therefore, a multidisciplinary approach should be developed individualized to the characteristics of the tumor and risk factors in a given patient.
Table 1.
Types of Immune Checkpoint Inhibitors and their Indications
| Targeted Immune Checkpoint | Anti-CTLA-4 | Anti-PD-1 | Anti-PD-L1 | Anti-LAG-3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Indicated Cancer Types | Ipilimumab in combination with Nivolumab | Tremelimumab in combination with Durvalumab | Pembrolizumab | Nivolumab | Cemiplimab | Dostarlimab | Atezolizumab | Avelumab | Durvalumab | Relatlimab in combination with Nivolumab |
| Melanoma | X | X | X | X | X | |||||
| RCC | X | X | X | X | ||||||
| CRC | X | X | X | |||||||
| HCC | X | X | X | X | X | X | ||||
| NSCLC | X | X | X | X | X | X | ||||
| PM | X | X | ||||||||
| SCLC | X | X | X | X | ||||||
| HNSCC | X | X | ||||||||
| cHL | X | X | ||||||||
| PMBCL | X | |||||||||
| Urothelial Ca | X | X | X | X | X | |||||
| Gastric Ca | X | X | ||||||||
| Esophageal Ca | X | X | ||||||||
| Cervical Ca | X | |||||||||
| MCC | X | X | ||||||||
| Endometrial | X | X | ||||||||
| cSCC | X | X | ||||||||
| TNBC | X | X | ||||||||
| Sarcoma | X | |||||||||
| BTC | X | X | ||||||||
| BCC | X | |||||||||
| Ovarian Ca | X | |||||||||
| Prostate Ca | X | |||||||||
RCC: Renal cell carcinoma; CRC: Colorectal cancer; HCC: Hepatocellular carcinoma; NSCLC: Non-small cell lung cancer; PM: Pleural mesothelioma; SCLC: Small cell lung cancer; HNSCC: Head and neck squamous cell carcinoma; cHL: Classical Hodgkin’s lymphoma; PMBCL: Primary mediastinal large B-cell lymphoma; MCC: Merkel cell carcinoma; cSCC: Cutaneous Squamous Cell Carcinoma; TNBC: Triple-Negative Breast Cancer; BTC: Biliary tract cancer; BCC: Basal cell carcinoma
2. How do immune checkpoint inhibitors work?
T-cells are the major players in anti-cancer immune response. Self-tolerance mechanisms during development and later life ensure appropriate T-cell activation. After T-cells mature in the thymus during development, they move to secondary lymphoid organs (spleen, lymph nodes, mucosal lymphoid tissue) as naïve T-cells that need priming. Priming occurs when an antigen is presented to the T-cell receptor (TCR) by antigen presenting cells (APC) [5]. To limit autoimmunity, priming also requires co-stimulatory signals through binding of co-stimulatory ligands, CD80 (B7–1) and CD86 (B7–2) on APCs to the co-stimulatory receptors that are constitutively present on T-cells (CD28). Once primed, the T-cells exert effector functions (secreting cytokines by CD4+ helper T-cells and killing target cells by CD+8 cytotoxic T-cells) in the periphery when they encounter their specific antigens [5]. Effector T-cells must separate self from foreign antigens; this could develop in the thymus by negative selection during T-cell maturation [6] or in the periphery [7]. One mechanism for peripheral tolerance is decreased expression of co-stimulatory receptors on T-cells or decreased co-stimulatory ligands on APCs for weak antigens (those with low degree of “foreignness”) such as in cancer [8]. Another mechanism is through upregulation of co-inhibitory receptors such as CTLA-4 and PD-1 on T-cells [7]. These receptors which render antigen-specific T-cells functionally unresponsive are known as immune checkpoints. CTLA-4 is homologous to CD28 and bind to CD80/86 albeit with a higher affinity and results in the opposite effect, T-cell inhibition [9]. PD-1, on the other hand, does not interfere with co-stimulation, but inhibits T-cell effector functions by suppressing T-cell receptor signaling. Its ligands PD-L1 and PD-L2 are expressed on many cell types including cancer cells and engage with PD-1 when upregulated on T-cells upon TCR engagement of [9]. The blocking of CTLA-4 is more proximal and occurs in the lymph node causing global disinhibition of the immune response, whereas blockage of the PD-1 and PD-L1 exerts its effects within the tumor microenvironment where the tumor cells and T-cells interact. The more central the disinhibition is, the more likely it is to develop irAEs outside of the tumor microenvironment which explains the increased cardiotoxicities seen with combined ICI regimens. Immune checkpoint inhibitors are monoclonal antibodies that exhibit anti-tumor effects by blocking the immune evasion brought on by these co-inhibitory receptors, CTLA-4, PD-1 and PD-L1.
2.1. Anti-CTLA-4 antibodies:
CTLA-4 was discovered due to its amino acid similarity to the CD28 and was shown to oppose the effects of CD28 [10]. Similar to CD28, CTLA-4 recognizes C80/86 expressed on APCs but remains sequestered in intracellular vesicles until T-cell activation [11]. On the contrary, CTLA-4 is constitutively expressed in regulatory T-cells (Treg) that are required for self-tolerance [12]. One of the ways Tregs induce immune tolerance is by transendocytosis of CD80/86 on APCs through CTLA-4 engagement [13]. Genetic or functional defects on pathways ablating the CTLA-4 function in Tregs result in autoimmunity similar to the autoimmune activation observed in patients who receive anti-CTLA-4 therapy [14]. Upon binding of ipilimumab to Tregs on the Fab domain and to myeloid and NK cells on the Fc domain, Tregs are depleted. The effector T-cells in the tumor also express increased amount of CTLA-4, a marker of chronic stimulation. Binding of ipilimumab to cytotoxic effector T-cells (Teff) results in activation of the effector T-cell through engagement of APCs via the Fc domain of ipilimumab. Therefore, the net result in the tumor upon treatment with ipilimumab is an increased Teff/Treg ratio [15,16].
2.2. Anti-PD1/PD-L1 antibodies:
In 1996, Nobel laureate Honjo and colleagues reported a protein that was induced on T- and B-cells upon antigen receptor stimulation [17]. Subsequently the same group reported that PD-1 deficient mice showed augmented T-cell proliferation upon stimulation and cardiomyopathy mediated by cardiac troponin 1 autoantibody [18,19]. PD-L1, a homologue of CD80/86 was then identified as the ligand for PD-1 and together they played a role in T-cell inhibition [20]. Chen and colleagues then showed that PD-L1 was expressed on a variety of human cancer cells and their expression enabled tumor survival through increased apoptosis of T-cells [21]. This paved the way for development of anti-PD-1 and PD-L1 antibodies as cancer immunotherapies [22]. It is now well established that blocking PD-1 restores cytotoxic activity of CD8 T-cells against tumor cells and that tumor-specific PD-L1 expression correlates with immunotherapy response [23]. PD-1, as well as PD-L1 and PD-L2, are also expressed on tumor resident macrophages and dendritic cells which support tumor growth. Therefore, blocking PD-1 and its ligands may also result in decreased tumor growth, independent of T-cell activation, by increased tumor-infiltrating macrophage activity [24,25]. As noted, combination PD-1/CTLA-4 blockade, and PD-1/LAG-3 blockade are approved in numerous cancers and improve survival compared with monotherapy [26–32]. ICIs are commonly combined with traditional chemotherapy, targeted therapies such as VEGF inhibitors or tyrosine kinase inhibitors, and radiotherapy, and routinely used in numerous cancers [2].
3. Immune Related Adverse Effects
Given that ICIs are used to overcome immune self-tolerance, autoimmune-like events known as immune-related adverse effects (irAEs) may complicate therapy. These irAEs can be acute or chronic and can involve almost any organ, most commonly skin, thyroid, colon, lungs, and liver (i.e., those with environmental interfaces or high rates of autoimmunity) [2,33]. Overall, the most common irAE involves the skin and occur in up to 30% of patients, followed by colitis and endocrine dysfunction [34]. Immune-related adverse events can be fatal in 0.4–1.2% of the patients [35]. The most common fatal irAE is colitis (70% of all fatal irAEs) with a mortality of 2–5% and whereas the irAE with the highest fatality is myocarditis with a mortality of 40% [35]. Therefore, clinical vigilance and early diagnosis are of paramount significance to be able to decrease mortality with timely treatment. Moreover, recently, data have suggested that ICIs could alter the natural course of other immune mediated diseases such as atherosclerosis, hepatic steatosis, or hypertension [36–39]. Specifically, [anonymised]et al. showed in melanoma treated with the PD-1 inhibitor nivolumab and the CTLA-4 inhibitor ipilimumab as combination therapy, systolic blood pressure (BP) increased by 5.5 mmHg, while there were no statistically significant changes in systolic BP or diastolic BP in patients treated with the PD-1 blockers alone [34]. Park et al. showed decreases in liver enzymes in patients with pre-existing steatosis/steatohepatitis [36]. Finally, very recently, [anonymised]et al. showed decreased volume of calcification in aortic atherosclerotic plaques after ICI therapy [37].
4. ICI Myocarditis
4.1. Epidemiology
ICI myocarditis occurs in up to 0.3–1.4% of the cases treated with ICIs [40,41]. However, the true incidence is difficult to define due to distinctive methodologies for screening, reporting, and diagnoses. The incidence we quote is mostly based on post-marketing surveillance studies and registries which started accumulating after anonymized et al. published the first two cases of ICI myocarditis [40]. Moreover, cases of subacute or smoldering myocarditis are recently reported [42,43]. These milder forms may be reported to the registries not as myocarditis but as demand ischemia since low-level troponin elevations are frequently seen with comorbidities such as anemia, atrial fibrillation, hypotension, sepsis, obstructive coronary artery disease that are common in cancer patients either due to shared risk factors or cancer treatment [44]. ICI myocarditis seems to have a male preference, with largest cohort studies reporting a 67–71% of male preponderance [41][45–47]. Older age appears to be more commonly represented with 65–67 years of age being the most commonly reported mean age [45–47]. However, the population of patients with cancer also tends to skew towards older and male.
4.2. Risk Factors
Based on reported myocarditis cases, combination ICI is a more frequent culprit compared to ICI monotherapy. In their disease defining paper, anonymized et al. found that the relative risk of myocarditis in those who received ipilimumab and nivolumab combination compared to nivolumab alone was 4.5 [40]. Similarly, a study using the World Health Organization’s (WHO) pharmacovigilance database (VigiBase) found a reporting odds ratio of 4.3 for combined therapy as opposed to monotherapy [45]. As explained above, this could be due to more central and robust inhibition of immune-tolerance when anti-CTLA-4 antibodies are added to anti-PD-(L)1. In addition to these observational studies, more recently a randomized clinical trial of nivolumab vs nivolumab with relatlimab 0.6% vs. 1.7% [48]. Most commonly reported risk factors in comparison with controls, appear to be diabetes mellitus, hypertension, and smoking [41,45–47]. However, Zhang et al found that 28% of their cohort of 103 patients had no identifiable any risk factors [47]. Thus, identifying a high-risk population remains challenging. In the most recent European Society of Cardio-Oncology Guidelines, high-risk patients for ICI myocarditis were defined as those who will undergo combination ICI therapy, regimens combined with other cardiotoxic therapies, patients with baseline cardiovascular disease, and patients with ICI-related non-CV events or prior cancer therapy related cardiotoxicity (due to anthracyclines, HER-2 targeted therapies, VEGF, BCR-ABL, RAF and MAK inhibitors) [49].
4.3. Pathophysiology:
Axelrod et al. recently published an elegant study elucidating the pathophysiological basis for myocarditis. Authors sequenced the RNA and T-cell receptors of the T-cells infiltrating the myocardium in a genetically engineered mouse model of ICI myocarditis. These T-cells were clonally expanded effector CD8+ T cells that were capable of inducing myocarditis upon adoptive transfer to healthy mice. The cardiac-specific protein α-myosin was identified as the recognized antigen in mice with fulminant myocarditis [50]. Axelrod’s study also tested relevance of α-myosin as a potential autoantigen in 3 patients with histologically proven ICI myocarditis. They found that α-myosin specific T-cell receptors were expanded in the diseased heart and skeletal muscles of these patients. Gergely et al. provided an alternative explanation for the pathologic basis of myocarditis. They treated C57BL6J mice with anti-PD1 antibody and found that 9 genes related to cardiac structure, signaling and inflammation were differentially regulated. The changes in expression were induced increased IL-17A signaling in thymus and myocarditis was prevented by pharmacological inhibition of IL-17 [51].
4.4. Timing and Clinical Presentation
Myocarditis, like other irAEs, generally occurs within the first several weeks of therapy initiation. Median time of onset appears to be 27–34 days after starting ICI (inter-quartile range: 18 to 75 days) [41,45]. However, irAEs can present with delayed onset including after cessation of therapy [52,53]. Recently, Nguyen et al. reported a case of autopsy diagnosed ICI myocarditis that developed 141 weeks after initiating ICI and 33 weeks after drug discontinuation [54]. Late occurrences of irAEs are increasingly being recognized and may be clinically distinct: late onset cases more often have heart failure whereas early cases have higher incidence of arrhythmias [46]. This delayed heart failure could reflect remodeling in the setting of smoldering inflammation and has important implications in long term monitoring as the mortality rate remains similarly high in early and late onset cases [46].The clinical presentation of ICI myocarditis is variable and could depend on the time of onset after ICI initiation. Dolladille et al. reported late onset cases (median time to onset 304 days from start of ICI) presented with HF symptoms 50% of the time vs. 5% in early onset cases (median time to onset of 14 days from start of ICI). Presentation with arrhythmias showed an opposite distribution [46]. Arrhythmias could present with tachyarrhythmia or bradyarrhythmia ranging from SVT and VT to CHB [46,55,56]. Overall, the most common presenting symptoms appear to be shortness of breath in 55–68%, palpitations in 5–33%, and chest pain in 13–28% [41,46][47]. The presence of LV systolic dysfunction appears to increase in late onset cases; one series reported 37% and 74% of early-onset and late-onset cases, respectively, had LV dysfunction explaining higher occurrence of heart failure symptoms in later presenting cases. Patients can also present with vague symptoms such as fatigue or weakness [57]. Suzuki et al found that 33% and 25% co-occurrence of myositis and myocarditis in their cohort of patients with nivolumab induced myasthenia like syndrome [58]; therefore, it is important to consider myocarditis even with non-specific symptoms. Lastly, myocarditis may be diagnosed incidentally during routine troponin surveillance in the absence of symptoms [59]. Heightened suspicion and early diagnosis are of utmost significance as the mortality is in the range of 30–50% [41,45,46][60][61].
4.5. Diagnosis
The Society of Immunotherapy in Cancer (SITC), European Society of Medical Oncology (ESMO), and the recent European Society of Cardiology (ESC) guidelines recommend obtaining baseline basic cardiac biomarkers such as troponin I, BNP, ECG [57] [62][49] prior to ICI start to develop an accurate baseline for comparison, whereas American Society of Clinical oncology (ASCO) guidelines do not; stating “there is no clear evidence regarding the efficacy or value of routine ECGs or troponin measurements in patients receiving checkpoint inhibitor therapy” [63]. We believe obtaining a baseline level of cardiac biomarkers is important for future diagnostic evaluation. ESC Guidelines also recommend obtaining a baseline echocardiogram in high-risk patients as defined under the section on risk factors. As for diagnostic tests, Mahmood et al. reported troponin was the most sensitive biomarker of myocarditis, followed by ECG abnormalities in a cohort of 35 cases, with sensitivity of 94% and 89%, respectively [41]. In a cohort of 101 myocarditis patients, Awadalla et al. reported 98% had elevated troponin. Though BNP is likely non-specific, the incidence of elevated BNP was significantly higher in myocarditis compared with controls who received ICI without myocarditis [64]. On the contrary, troponin was elevated only in 63% of early-onset and 50% of late-onset myocarditis in a separate cohort of 37 cases [46]. Similarly, ECG abnormalities were also lower at 50% and 55% in the cohorts of Dolladille et al. [46] and Zhang et al. [47], respectively. Notably, 50–60% of patients had a normal left ventricular ejection fraction (LVEF) both by echocardiogram and by MRI [41,47]. The ECHO modality of global longitudinal strain has long been utilized as a sensitive marker of cardiotoxicity in patients receiving cardiotoxic chemotherapy or targeted therapy [49]. Awadalla et al. investigated the utility of strain in 101 ICI myocarditis cases [64]. They saw a mean 14.1 ± 2.8% decrease in strain in myocarditis cases from pre-ICI ECHO to that done during ICI myocarditis, a decrease not observed in age, sex and cancer type matched controls. The significant decrease in strain was present in both decreased-LVEF and preserved-LVEF cases and predicted major adverse cardiovascular events (MACE) in both groups. Cardiac MRI is the gold standard for non-invasive tissue characterization in cases of myocarditis [65]. Zhang et al. investigated its diagnostic value in 103 myocarditis patients in 56 of whom, myocarditis diagnosis was confirmed by endomyocardial biopsy, and by ESC diagnostic criteria in the rest [47][66]. Late gadolinium enhancement (LGE) was present in 48% of cases, with similar rates in both preserved LVEF and reduced LVEF groups. LGE had varying patterns, most commonly midmyocardial (49%), subepicardial (27%) and diffuse (18%) enhancement. Of note, when MRI was performed after four days of admission, its sensitivity increased to 72% compared with 22% when done within the first four days of admission [47]. Moreover, LGE presence did not predict occurrence of major adverse cardiac events of cardiogenic shock, cardiac arrest, or complete heart block. Thus, neither normal LVEF nor absence of LGE rule out ICI myocarditis, a condition for which timely treatment is of paramount importance. Regardless, a repeat MRI in a few days can increase the diagnostic yield in uncertain clinical cases. Recently, Thavendiranathan et al. reported the results of MRI parametric mapping in 31 biopsy proven ICI myocarditis patients. Presence of non-ischemic myocardial injury by abnormal T1 mapping, myocardial extracellular volume fraction (ECV) or LGE was present in all patients and 63% had myocardial edema by T2 mapping or T2 weighted images [67]. Another non-invasive diagnostic tool, PET-CT, has a limited role in diagnosing ICI myocarditis. A recent study by Ederhy et al. showed that 18F-fluorodeoxyglucose PET/CT was positive only in 2 of 21 (9.5%) of patients with definite myocarditis [68,69]. Molecular PET imaging merits future attention. In a recent small study, PET-CT with 68Ga-DOTATOC (radio-tagged somatostatin analogue) was shown to be a sensitive diagnostic method for ICI myocarditis [70]. Endomyocardial biopsy is indicated in patients presenting with acute cardiogenic shock in the absence of coronary artery disease [71]. It is also frequently considered in suspected cases of myocarditis in the absence of hemodynamic instability. Even though endomyocardial biopsy has been considered the gold standard for diagnosis of myocarditis and has a relatively low complication rate of about ~1% [71], its sensitivity in ICI myocarditis is limited due to the patchy nature of the disease [72][73]. Nevertheless, it should be considered when hemodynamic instability or non-MRI compatible implants preclude performing non-invasive testing. To summarize the diagnostic modalities with the most comprehensive and up-to-date guidelines on the subject, the ESC Guidelines [49], echocardiogram and cardiac-MRI (CMR) with parametric mapping are recommended in all patients with suspected ICI myocarditis. Although it has lower sensitivity, cardiac-PET may be considered if CMR is not available or contraindicated, and endomyocardial biopsy (EMB) should be considered in cases where the diagnosis is suspected but not confirmed non-invasively. The field most commonly follows the consensus criteria initially developed by Bonaca et al. to adjudicate the probability of ICI myocarditis [68]. This set of criteria was also presented by the ESC in their 2022 Guidelines (Figure 2)[49].
Figure 2.
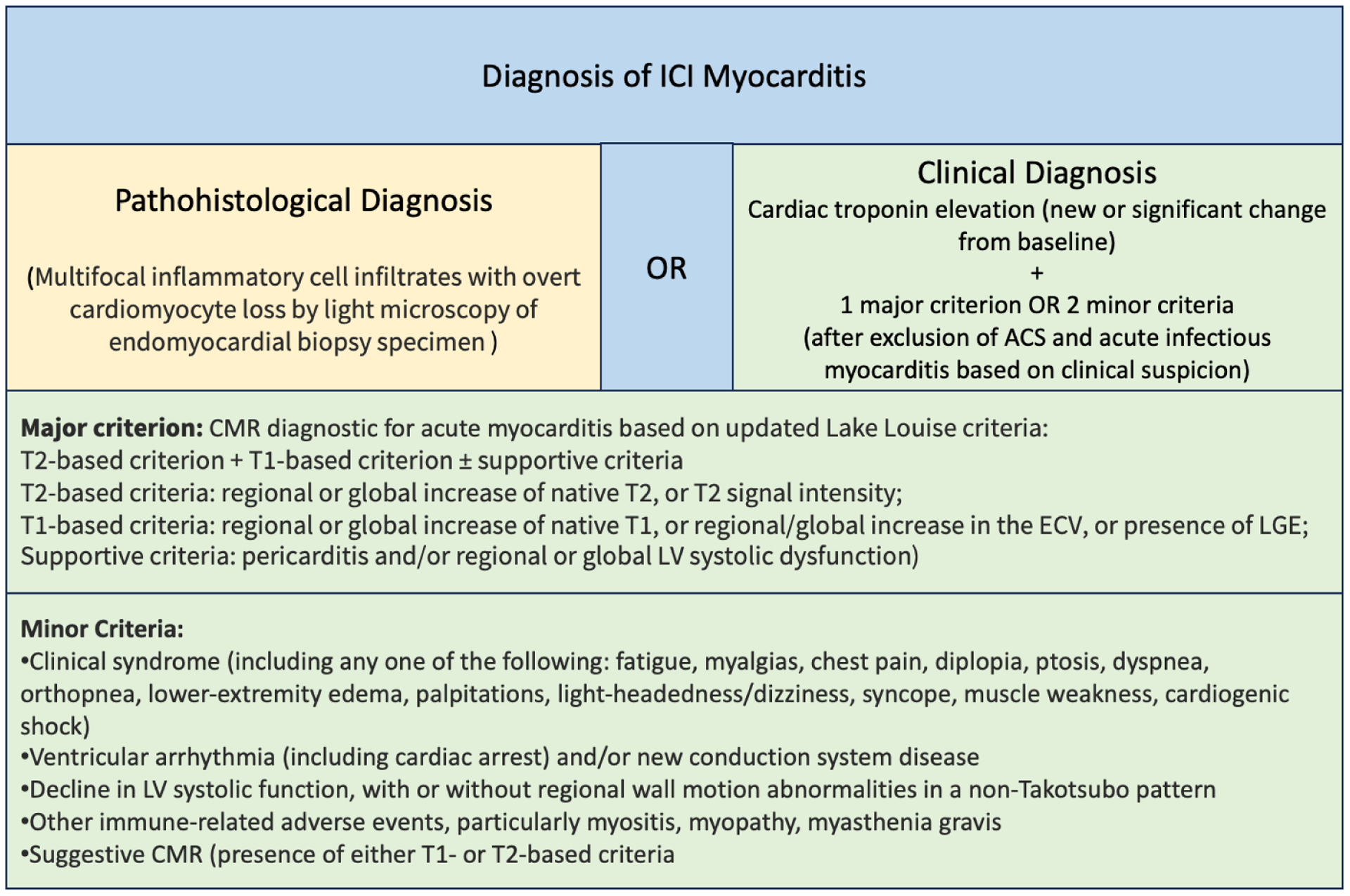
Diagnostic Criteria for ICI Myocarditis Adapted from Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology [48]
4.6. Co-occurring irAEs
Other irAEs frequently accompany myocarditis. Pharmacovigilance studies have reported that one-half to two-thirds of patients with ICI-myocarditis have accompanying irAEs [45,64]. Of the accompanying irAEs, most common was myositis in 25%, followed by pneumonitis in 11–29%, then colitis in 7–10%. Interestingly, myasthenia gravis was reported 11% of the time concurrently with myocarditis [45]. In another study looking at 62 myositis cases in VigiBase, 16% presented with myocarditis and 16% with myasthenia gravis-like symptoms, whereas both myocarditis and myasthenia gravis–like symptoms occurred in 3.3% of those with myositis [74]. Myositis can accompany myocarditis or precede it. Myositis mainly presents as weakness of the proximal extremities as commonly encountered in other autoimmune myopathies. It can involve the diaphragm resulting in respiratory complications and can also present as myasthenia gravis. In a recent study, Aldrich et al. reported that more than half of ICI myocarditis patients had accompanying myasthenia graves or myocarditis. These patients tended to have worse prognosis with 79% of them developing respiratory failure [75]. The common occurrence of distinct myotoxic phenotypes points to a shared antigen between skeletal and cardiac muscle and should prompt work up for myocarditis [50]. It is notable that endocrine and skin irAEs, the most common irAEs in general, do not commonly co-occur with myocarditis (co-occurrence 5% and 2–6%, respectively).
4.7. Other CV irAEs
Pharmacovigilance studies have also suggested that ICIs are associated with other cardiovascular adverse events besides myocarditis (Reporting Odds Ratios (ROR) for myocarditis 11.2, pericardial disease 3.8, supraventricular arrhythmias 1.7, vasculitis 1.6, where the confidence intervals of all of these excluded the null value). Amongst these cardiovascular irAEs, only myocarditis had increased ROR for combination therapy compared with monotherapy [45]. It is important to note that these data are from registries, and these CV irAEs could be occurring secondary to myocarditis. There have also been case reports of ICI induced pericardial effusion, acute coronary syndrome, and takotsubo like syndrome [76][77–81].
4.8. Treatment
The first step in treatment in all suspected ICI myocarditis cases interruption of ICI treatment while diagnostic work-up is underway. This is endorsed by all society guidelines. Corticosteroids are the first line treatment for irAEs including myocarditis [57]. ESC and ESMO Guidelines recommend immediate initiation of high dose corticosteroids (methylprednisolone 500–1000 mg IV daily) for the first 3–5 days or until clinical improvement starting from when the diagnosis is considered likely. Clinical improvement is defined as troponin reduction by >50% from its peak level within 24–72 hours and resolution of ventricular dysfunction, AV block, and arrhythmias resolved. Switching to oral prednisolone is recommended after clinical improvement, starting at 1 mg/kg up to 80 mg/day and tapering it 10 mg per week under biomarker and clinical surveillance [49][62]. SITC and ASCO both recommend high-dose corticosteroids (1 mg/kg methylprednisolone IV or 2 mg/kg prednisone) in cases of confirmed myocarditis (and in those with high clinical suspicion) until resolution or significant improvement of symptoms followed by tapering for at least 4–5 weeks [82][63]. It is important to note that even though early initiation of high dose steroids has the highest recommendation (Class I) by all society guidelines, the strength of evidence beyond this recommendation is low and based on expert consensus of opinion and a case series by Zhang et al. These investigators reported a 67% decreased adjusted risk of adverse events in patients who received more than 500 mg/day methylprednisolone compared with those who had less than 60 mg/day. Moreover, patients who received corticosteroids within 24 hours had a hazard ratio of 0.3 for MACE compared with those who received steroids after 24 hours of presentation [60]. In cases where clinical and biomarker improvement with steroids is not observed, additional therapies with anecdotal evidence of efficacy could be utilized. These include tocilizumab (an IL-6 inhibitor), alemtuzumab (a CD52 antibody), anti-thymocyte immunoglobulin, and mycophenolate mofetil [83,84][85,86]. Recently abatacept, a soluble fusion protein containing the human CTLA-4 extracellular domain and Fc portion of IgG antibody was reported as an efficacious treatment option [87]. Abatacept competes with CD28 in binding to CD80/86, and thus limits costimulatory signals upstream of CTLA-4 and PD-(L)1 [88]. Salem et al. reported an impressive mortality reduction to 3.4% in Grade ≥ 3 ICI myocarditis (Table 2) patients who were treated with a combination of low dose steroids, abatacept titrated to CD86 receptor occupancy, and ruxolitinib, a JAK1/JAK2 inhibitor which shows rapid synergistic effects with abatacept by decreasing CD86 expression on APCs and impairs T-cell activation [87].
Table 2.
Common Terminology Criteria for Adverse Events Myocarditis (Version 5, 2017)
| Grade 1 Myocarditis | N/A |
| Grade 2 Myocarditis | Symptoms with moderate activity or exertion |
| Grade 3 Myocarditis | Severe with symptoms at rest or with minimal activity or exertion; intervention indicated; new onset of symptoms |
| Grade 4 Myocarditis | Life-threatening consequences: urgent intervention indicated (e.g., continuous IV therapy or mechanical hemodynamic support) |
| Grade 5 Myocarditis | Death |
4.9. Rechallenge
The decision to rechallenge requires a multidisciplinary discussion. Since each case is unique in terms of irAE presentation, alternative cancer treatment options, indication for therapy (metastatic vs. (neo)adjuvant), a treatment plan should be devised specific to the patient’s needs by the treating cardiologist and oncologist. There is sparse evidence in the literature on rechallenge. An examination of VigiBase found a 28.8% recurrence rate of the same irAE after rechallenging with the same ICI; however, myocarditis was underrepresented with three cases who received rechallenge without recurrence of myocarditis [89]. In a NSCLC cohort of 38 patients who were rechallenged after stopping treatment due to irAEs, 48% patients had no subsequent irAEs, 26% had recurrence of the initial irAE, and 26% had a new irAE; the recurrence of irAE did not differ between those who had the same combination and those switched to monotherapy [90]. All recurrent irAEs in this cohort were mild (grade 1–2) and did not include myocarditis. The data on myocarditis and ICI rechallenge is scarce. Hasson et al. reported a case series of three patients with myocarditis who were rechallenged with ICI with concomitant low dose steroids and weekly troponin follow-up. Two patients who initially had ≤ grade 2 myocarditis were successfully rechallenged without recurrence of symptoms. The third patient who had grade 3 myocarditis had reappearance of symptoms upon rechallenge and therapy was stopped after the first cycle of reintroduction [91]. Similarly, another patient with grade 3 myocarditis was rechallenged after resolution of symptoms with steroids, however his myocarditis also recurred after reintroduction [92]. The decision to restart ICI treatment in patients who developed myocarditis is difficult and depends on the availability of alternative treatments, feasibility of frequent monitoring, the overall status of the cancer, and the rapidity of onset of myocarditis after treatment initiation. The initial severity of myocarditis also plays an important role in deciding whom to rechallenge.
5. Expert Opinion
ICIs brought impressive advances in cancer survival; however, uncommon cases of cardiotoxicity come with high morbidity and mortality. While vigilance have increased amongst cardiology and oncology communities, further understanding of risk factors, surveillance methods and development of reliable diagnostic modalities are needed. We follow a strict initial risk assessment and surveillance regimen comprised of baseline ECG, BNP and troponin evaluation prior to start of therapy and then before each dose for the following three cycles and thereafter every 3 months. We also perform a baseline echocardiogram in every patient who is considered to be more than intermediate risk per the American College of Cardiology/American heart Association pooled cohort equation. Any suspected myocarditis patient gets admitted to the inpatient service immediately for expedited diagnostic work-up and monitoring. At our centers we are lucky to have the capacity for CMR with parametric mapping which we utilize as initial imaging modality. If this is not feasible due to contraindications, we default to echocardiogram and endomyocardial biopsy. We initiate steroids early at a high dose as recommended by all society guidelines. We have utilized abatacept successfully in two cases of steroid-refractory myocarditis. In selection of cases for re-challenge, we consider those with low levels of troponin elevation, no malignant arrhythmia, heart failure, LV dysfunction for rechallenge under close surveillance with troponin, serial ECGs, and mobile cardiac telemetry.
Admittedly, the field needs more evidence-based approaches to risk stratification so that therapy can be tailored towards less cardiotoxic alternatives. Data from animal models are unlikely to provide conclusive evidence since these do not fully capture the complexity of the human immune system. Multicenter registries are in place to provide clinical data and biospecimens for future -omics research. These registries would also provide us with clues on the best strategy to treat steroid resistant cases. Society guidelines recommend steroids as first line therapy based on evidence from the transplant literature, however we still lack mechanism driven treatment approaches. Moreover, we do not yet know if targeted inhibition of the culprit immune system should be preferred over the very high dose steroids in treatment of cardiotoxicities so as to not affect the overall cancer survival. There is an ongoing study (ATRIUM Trial: NCT05335928) that will provide an answer on this for abatacept. We need trials on other emerging therapies such as JAK1/JAK2 inhibitors or IL-17 inhibitors. Finally, we do not have clear evidence on long term consequences of acute cardiac irAEs which points to the need to develop well designed and funded prospective registries.
Figure 1.

Schematic of T-cell activation and exhaustion Naïve T-cells are primed through a two-signal process. The first signal requires antigen binding to the T-cell receptor (TCR) and the second signal is provided by the binding of activated antigen presenting cell (APC) ligands CD80 and 86 (also called B7–1 and B7–2) to CD28 on the T-cell. However, when the stimulation becomes chronic, the antigen-specific T-cells become exhausted and functionally unresponsive via upregulation of immune checkpoints CTLA-4 and PD-1. In the tumor tissue, cytotoxic T-cell function occurs when the TCR re-encounters the antigen for which it was primed. However, chronic stimulation induces expression of PD-1 on the exhausted T-cell. Many cancer cells express PD-L1. Engagement of PD-1 and PD-L1 results in suppression of the effector T-cell activity. Immune checkpoint inhibitors are monoclonal antibodies which attenuate these negative regulators of activation. Mφ: Macrophage; MHC: Major histocompatibility complex; exTcell: Exhausted T-cell; Ca: Cancer cell.
Article Highlights:
Although it is rare, myocarditis is the most fatal complication of ICI therapy with a mortality of 40%.
It is essential to identify high-risk patients and provide close surveillance. These patients include those with baseline CV disease, those receiving combination ICI therapy or other cardiotoxic therapies concurrently, and those with prior cancer therapy related cardiotoxicity.
If you suspect ICI myocarditis, admit the patient to a closely monitored bed. Hold ICIs. Keep in mind that the patient may have both acute coronary syndrome and myocarditis. Keep in mind that myositis, myasthenia gravis, and myocarditis can co-occur.
Initiate the diagnostic work-up with biomarkers, parametric CMR or endomyocardial biopsy. Start high-dose steroids when the diagnosis is considered likely.
In cases where clinical and biomarker improvement with steroids is not observed, consider additional immunosupressive therapies.
Funding
This paper was funded by NCI T32 CA217834 and R01HL155990.
Declaration of interests
DB Jonhson has served on advisory boards or as a consultant for BMS, Catalyst Biopharma, Iovance, Jansen, Mallinckrodt, Merck, Mosaic ImmunoEngineering, Novartis, Oncosec, Pfizer, Targovax, and Teiko, has received research funding from BMS and Incyte, and has patents pending for use of MHC-II as a biomarker for immune checkpoint inhibitor response, and abatacept as treatment for immune-related adverse events. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One reviewer’s conflicts and disclosures in the past 12 months are as follows, but do not impact my ability to perform this peer review in my judgment:
Advisory Board: EMD Serono, BMS, Merck, Astrazeneca, Seattle Genetics/Astellas, Janssen, Bicycle Therapeutics, Pfizer, Gilead, Scholar Rock, G1 Therapeutics, Eli Lilly/Loxo Oncology, Lucence Health, IMV, Vial, Tempus, Ellipses Pharma, PrecisCa, Primum
Consultant/Scientific Advisory Board (SAB): Suba Therapeutics, Syapse, Merck, Servier, Syncorp
Research Support to institution: Sanofi, Astrazeneca, Gilead, Helsinn, Lucence, EMD Serono, Jazz Therapeutics
Speaker: Seagen, Gilead, Natera, Exelixis, Janssen, Bayer, Aveo
Data safety monitoring committee (honorarium): Mereo
The remaining reviewers have no other relevant financial relationships or otherwise to disclose.
References:
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- [1].Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. 2022;21:509–528. [DOI] [PubMed] [Google Scholar]
- [2].Johnson DB, Reynolds KL, Sullivan RJ, et al. Immune checkpoint inhibitor toxicities: systems-based approaches to improve patient care and research. Lancet Oncol. 2020;21:e398–e404. [DOI] [PubMed] [Google Scholar]
- [3].Iorgulescu JB, Braun D, Oliveira G, et al. Acquired mechanisms of immune escape in cancer following immunotherapy. Genome Med. 2018;10:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Palmer E Negative selection — clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3:383–391. [DOI] [PubMed] [Google Scholar]
- [7].Xing Y, Hogquist KA. T-Cell Tolerance: Central and Peripheral. Csh Perspect Biol. 2012;4:a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schildberg FA, Klein SR, Freeman GJ, et al. Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family. Immunity. 2016;44:955–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. [DOI] [PubMed] [Google Scholar]
- [10].Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Medicine. 1995;182:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Valk E, Rudd CE, Schneider H. CTLA-4 trafficking and surface expression. Trends Immunol. 2008;29:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].JAGO CB, YATES J, CÂMARA NOS, et al. Differential expression of CTLA‐4 among T cell subsets. Clin Exp Immunol. 2004;136:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Qureshi OS, Zheng Y, Nakamura K, et al. Trans-Endocytosis of CD80 and CD86: A Molecular Basis for the Cell-Extrinsic Function of CTLA-4. Science. 2011;332:600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lo B, Zhang K, Lu W, et al. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349:436–440. [DOI] [PubMed] [Google Scholar]
- [15].Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti–CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bulliard Y, Jolicoeur R, Windman M, et al. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013;210:1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. [DOI] [PubMed] [Google Scholar]
- [18].Nishimura H, Nose M, Hiai H, et al. Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity. 1999;11:141–151. [DOI] [PubMed] [Google Scholar]
- [19].Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science. 2001;291:319–322. [DOI] [PubMed] [Google Scholar]
- [20].Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J Exp Medicine. 2000;192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- [22].Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc National Acad Sci. 2002;99:12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kamphorst AO, Pillai RN, Yang S, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1–targeted therapy in lung cancer patients. Proc National Acad Sci. 2017;114:4993–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Strauss L, Mahmoud MAA, Weaver JD, et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J Clin Oncol. 2021;39:9506–9506. [Google Scholar]
- [27].Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20:1370–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Paz-Ares L, Ciuleanu T-E, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2020;22:198–211. [DOI] [PubMed] [Google Scholar]
- [29].Overman MJ, Lonardi S, Wong KYM, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair–Deficient/Microsatellite Instability–High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36:JCO.2017.76.990. [DOI] [PubMed] [Google Scholar]
- [30].Hellmann MD, Paz-Ares L, Caro RB, et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. New Engl J Med. 2019;381:2020–2031. [DOI] [PubMed] [Google Scholar]
- [31].Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375–386. [DOI] [PubMed] [Google Scholar]
- [32].Yau T, Kang Y-K, Kim T-Y, et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib. Jama Oncol. 2020;6:e204564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Johnson DB, Nebhan CA, Moslehi JJ, et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Johnson DB, Chandra S, Sosman JA. Immune Checkpoint Inhibitor Toxicity in 2018. Jama. 2018;320:1702. [DOI] [PubMed] [Google Scholar]
- [35].Wang DY, Salem J-E, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. Jama Oncol. 2018;4:1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Turker I, Sharma A, Huang S, et al. Combination Immune Checkpoint Inhibitor Therapy is Associated With Increased Blood Pressure in Melanoma Patients. Hypertens Dallas Tex 1979. 2022;80:e43–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Moslehi J, Lichtman AH, Sharpe AH, et al. Immune checkpoint inhibitor–associated myocarditis: manifestations and mechanisms. J Clin Invest. 2021;131:e145186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Park BC, Lee AXT, Ye F, et al. Immune checkpoint inhibitors and their impact on liver enzymes and attenuation. Bmc Cancer. 2022;22:998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Turker I, Nair S, Terry J. Immune Checkpoint Inhibitors’ Effects on Calcified Aortic Plaques in Melanoma Survivors: A Retrospective Cohort Study. Jacc Cardiooncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].JD B, BJ M, CM L, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. New Engl J Med. 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chuy KL, Oikonomou EK, Postow MA, et al. Myocarditis Surveillance in Patients with Advanced Melanoma on Combination Immune Checkpoint Inhibitor Therapy: The Memorial Sloan Kettering Cancer Center Experience. Oncol. 2019;24:e196–e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Norwood TG, Westbrook BC, Johnson DB, et al. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer. 2017;5:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Meijers WC, Boer RA de. Common risk factors for heart failure and cancer. Cardiovasc Res. 2019;115:844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Salem J-E, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dolladille C, Ederhy S, Allouche S, et al. Late cardiac adverse events in patients with cancer treated with immune checkpoint inhibitors. J Immunother Cancer. 2020;8:e000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhang L, Awadalla M, Mahmood SS, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. 2020;41:1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med. 2022;386:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). European Hear J Cardiovasc Imaging. 2022;23:e333–e465. [DOI] [PubMed] [Google Scholar]
- [50].Axelrod ML, Meijers WC, Screever EM, et al. T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature. 2022;611:818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gergely TG, Kucsera D, Tóth VE, et al. Characterization of immune checkpoint inhibitor‐induced cardiotoxicity reveals interleukin‐17A as a driver of cardiac dysfunction after anti‐PD‐1 treatment. Br J Pharmacol. 2023;180:740–761. [DOI] [PubMed] [Google Scholar]
- [52].Yamaguchi S, Morimoto R, Okumura T, et al. Late-Onset Fulminant Myocarditis With Immune Checkpoint Inhibitor Nivolumab. Can J Cardiol. 2018;34:812.e1–812.e3. [DOI] [PubMed] [Google Scholar]
- [53].Naganuma K, Horita Y, Matsuo K, et al. An Autopsy Case of Late-onset Fulminant Myocarditis Induced by Nivolumab in Gastric Cancer. Internal Med. 2022;61:2867–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nguyen AT, Berry GJ, Witteles RM, et al. Late-Onset Immunotherapy-Induced Myocarditis 2 Years After Checkpoint Inhibitor Initiation. Jacc Cardiooncology. 2022;4:727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Behling J, Kaes J, Münzel T, et al. New-onset third-degree atrioventricular block because of autoimmune-induced myositis under treatment with anti-programmed cell death-1 (nivolumab) for metastatic melanoma. Melanoma Res. 2017;27:155–158. [DOI] [PubMed] [Google Scholar]
- [56].Reddy N, Moudgil R, Lopez-Mattei JC, et al. Progressive and Reversible Conduction Disease With Checkpoint Inhibitors. Can J Cardiol. 2017;33:1335.e13–1335.e15. [DOI] [PubMed] [Google Scholar]
- [57].Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Suzuki S, Ishikawa N, Konoeda F, et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology. 2017;89:1127–1134. [DOI] [PubMed] [Google Scholar]
- [59].Waliany S, Neal JW, Reddy S, et al. Myocarditis Surveillance With High-Sensitivity Troponin I During Cancer Treatment With Immune Checkpoint Inhibitors. Jacc Cardiooncology. 2021;3:137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang L, Zlotoff DA, Awadalla M, et al. Major Adverse Cardiovascular Events and the Timing and Dose of Corticosteroids in Immune Checkpoint Inhibitor–Associated Myocarditis. Circulation. 2020;141:2031–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Al-Kindi SG, Oliveira GH. Reporting of immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;392:382–383. [DOI] [PubMed] [Google Scholar]
- [62].Haanen J, Obeid M, Spain L, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up ☆. Ann Oncol. 2022;33:1217–1238. [DOI] [PubMed] [Google Scholar]
- [63].Schneider BJ, Naidoo J, Santomasso BD, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol. 2021;39:4073–4126. [DOI] [PubMed] [Google Scholar]
- [64].Awadalla M, Mahmood SS, Groarke JD, et al. Global Longitudinal Strain and Cardiac Events in Patients With Immune Checkpoint Inhibitor-Related Myocarditis. J Am Coll Cardiol. 2020;75:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Biesbroek PS, Hirsch A, Zweerink A, et al. Additional diagnostic value of CMR to the European Society of Cardiology (ESC) position statement criteria in a large clinical population of patients with suspected myocarditis. European Hear J - Cardiovasc Imaging. 2017;19:1397–1407. [DOI] [PubMed] [Google Scholar]
- [66].Caforio ALP, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. [DOI] [PubMed] [Google Scholar]
- [67].Thavendiranathan P, Zhang L, Zafar A, et al. Myocardial T1 and T2 Mapping by Magnetic Resonance in Patients With Immune Checkpoint Inhibitor–Associated Myocarditis. J Am Coll Cardiol. 2021;77:1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bonaca MP, Olenchock BA, Salem J-E, et al. Myocarditis in the Setting of Cancer Therapeutics. Circulation. 2019;140:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ederhy S, Devos P, Pinna B, et al. 18 F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography imaging for the diagnosis of immune checkpoint inhibitors associated myocarditis. 2021; [DOI] [PubMed]
- [70].Boughdad S, Latifyan S, Fenwick C, et al. 68Ga-DOTATOC PET/CT to detect immune checkpoint inhibitor-related myocarditis. J Immunother Cancer. 2021;9:e003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Seferović PM, Tsutsui H, McNamara DM, et al. Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society Position statement on endomyocardial biopsy. Eur J Heart Fail. 2021;23:854–871. [DOI] [PubMed] [Google Scholar]
- [72].Palaskas N, Lopez‐Mattei J, Durand JB, et al. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J Am Heart Assoc. 2020;9:e013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ganatra S, Neilan TG. Immune Checkpoint Inhibitor‐Associated Myocarditis. Oncol. 2018;23:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Anquetil C, Salem J-E, Lebrun-Vignes B, et al. Immune Checkpoint Inhibitor–Associated Myositis. Circulation. 2018;138:743–745. [DOI] [PubMed] [Google Scholar]
- [75].Aldrich J, Pundole X, Tummala S, et al. Inflammatory Myositis in Cancer Patients Receiving Immune Checkpoint Inhibitors. Arthritis Rheumatol. 2021;73:866–874. [DOI] [PubMed] [Google Scholar]
- [76].Yun S, Vincelette ND, Mansour I, et al. Late Onset Ipilimumab-Induced Pericarditis and Pericardial Effusion: A Rare but Life Threatening Complication. Case Reports Oncol Medicine. 2015;2015:794842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. 2016;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Cautela J, Rouby F, Salem J-E, et al. Acute Coronary Syndrome With Immune Checkpoint Inhibitors: A Proof-of-Concept Case and Pharmacovigilance Analysis of a Life-Threatening Adverse Event. Can J Cardiol. 2020;36:476–481. [DOI] [PubMed] [Google Scholar]
- [79].Nykl R, Fischer O, Vykoupil K, et al. A unique reason for coronary spasm causing temporary ST elevation myocardial infarction (inferior STEMI) – systemic inflammatory response syndrome after use of pembrolizumab. Archives Medical Sci Atheroscler Dis. 2017;2:e100–e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ederhy S, Cautela J, Ancedy Y, et al. Takotsubo-Like Syndrome in Cancer Patients Treated With Immune Checkpoint Inhibitors. Jacc Cardiovasc Imaging. 2018;11:1187–1190. [DOI] [PubMed] [Google Scholar]
- [81].Geisler BP, Raad RA, Esaian D, et al. Apical ballooning and cardiomyopathy in a melanoma patient treated with ipilimumab: a case of takotsubo-like syndrome. J Immunother Cancer. 2015;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9:e002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Doms J, Prior JO, Peters S, et al. Tocilizumab for refractory severe immune checkpoint inhibitor-associated myocarditis. Ann Oncol. 2020;31:1273–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Esfahani K, Buhlaiga N, Thébault P, et al. Alemtuzumab for Immune-Related Myocarditis Due to PD-1 Therapy. New Engl J Med. 2019;380:2375–2376. [DOI] [PubMed] [Google Scholar]
- [85].Tay RY, Blackley E, McLean C, et al. Successful use of equine anti-thymocyte globulin (ATGAM) for fulminant myocarditis secondary to nivolumab therapy. Brit J Cancer. 2017;117:921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mahmood SS, Chen CL, Shapnik N, et al. Myocarditis with tremelimumab plus durvalumab combination therapy for endometrial cancer: A case report. Gynecol Oncol Reports. 2018;25:74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Salem J-E, Bretagne M, Abbar B, et al. Abatacept/Ruxolitinib and Screening for Concomitant Respiratory Muscle Failure to Mitigate Fatality of Immune-Checkpoint Inhibitor Myocarditis. Cancer Discov. 2023;OF1–OF16. [DOI] [PubMed] [Google Scholar]
- [88].Kremer JM, Westhovens R, Leon M, et al. Treatment of Rheumatoid Arthritis by Selective Inhibition of T-Cell Activation with Fusion Protein CTLA4Ig. New Engl J Medicine. 2003;349:1907–1915. [DOI] [PubMed] [Google Scholar]
- [89].Dolladille C, Ederhy S, Sassier M, et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients With Cancer. Jama Oncol. 2020;6:865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and Efficacy of Retreating with Immunotherapy After Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol Res. 2018;6:canimm.0755.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hasson SP, Salwen B, Sivan A, et al. Re-introducing immunotherapy in patients surviving immune checkpoint inhibitors-mediated myocarditis. Clin Res Cardiol. 2021;110:50–60. [DOI] [PubMed] [Google Scholar]
- [92].Tajmir-Riahi A, Bergmann T, Schmid M, et al. Life-threatening Autoimmune Cardiomyopathy Reproducibly Induced in a Patient by Checkpoint Inhibitor Therapy. J Immunother. 2018;41:35–38. [DOI] [PubMed] [Google Scholar]


