Abstract
Background:
Studies in adults have shown that persistent kidney dysfunction ≥7–90 days following acute kidney injury (AKI), termed acute kidney disease (AKD), increases chronic kidney disease (CKD) and mortality risk. Little is known about the factors associated with the transition of AKI to AKD and the impact of AKD on outcomes in children. The aim of this study is to evaluate risk factors for progression of AKI to AKD in hospitalized children and to determine if AKD is a risk factor for CKD.
Methods:
Retrospective cohort study of children age ≤ 18 years admitted with AKI to all pediatric units at a single tertiary-care children’s hospital between 2015–2019. Exclusion criteria included insufficient serum creatinine values to evaluate for AKD, chronic dialysis, or previous kidney transplant.
Results:
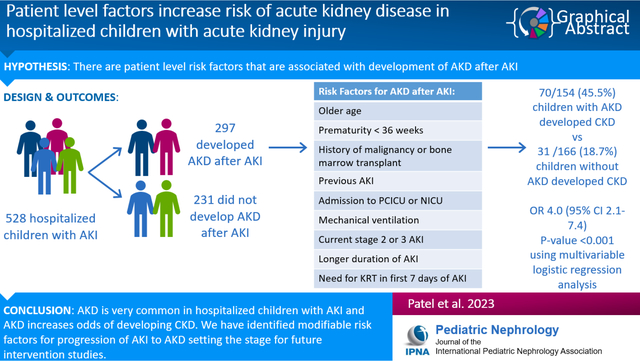
528 children with AKI were included in the study. There were 297 (56.3%) hospitalized AKI survivors who developed AKD. Among children with AKD, 45.5% developed CKD compared to 18.7% in the group without AKD (OR 4.0, 95% CI 2.1–7.4, p-value <0.001 using multivariable logistic regression analysis including other covariates). Multivariable logistic regression model identified age at AKI diagnosis, PCICU and NICU admission, prematurity, malignancy, bone marrow transplant, previous AKI, mechanical ventilation, AKI stage, duration of kidney injury, and need for kidney replacement therapy during day 1–7 as risk factors for AKD after AKI.
Conclusions:
AKD is common among hospitalized children with AKI and multiple risk factors are associated with AKD. Children that progress from AKI to AKD are at higher risk of developing CKD.
Keywords: Acute kidney injury, acute kidney disease, chronic kidney disease, epidemiology
Graphical Abstract
Introduction
Acute kidney injury (AKI) has been recognized as a risk factor for chronic kidney disease (CKD) [1–5]. AKI occurs in 5% of non-critically ill hospitalized children and 27% of hospitalized critically ill children [1, 2]. Children who develop AKI have longer hospital admission, prolonged need for mechanical ventilation, and increased mortality [2–5]. Long term complications of AKI include proteinuria, hypertension, and progression to CKD [1–4]. AKI is currently staged based on the degree of kidney dysfunction and it is thought that severe AKI is more likely to progress to CKD [6]. CKD itself causes significant multiple morbidities including anemia, metabolic bone disease, growth failure, impaired neurodevelopment, and cardiovascular disease [7–11]. These complications have devastating consequences in children as they have a significant impact on the growth and development of a child.
Previously, AKI and CKD were thought to be unique entities, however recent studies suggest that they are likely to be a continuum with the period between AKI and CKD being designated as acute kidney disease (AKD). AKD is defined as kidney dysfunction that persists beyond 7 days and up to 90 days after initial kidney injury (Figure 1) [12]. Some studies have shown that selected groups of children with AKI will progress to AKD and that severity of AKI, repeated AKI, and AKD are important risk factors for progression to CKD [13–18]. AKI due to modifiable risk factors including nephrotoxic medications has been associated with subsequent proteinuria, hypertension, and decline in eGFR when compared to non-AKI controls [19]. These findings suggest that by better understanding patient level risk factors and modifiable risk factors that are associated with development of AKD and CKD after AKI we may better be able to slow or stop AKI to CKD progression.
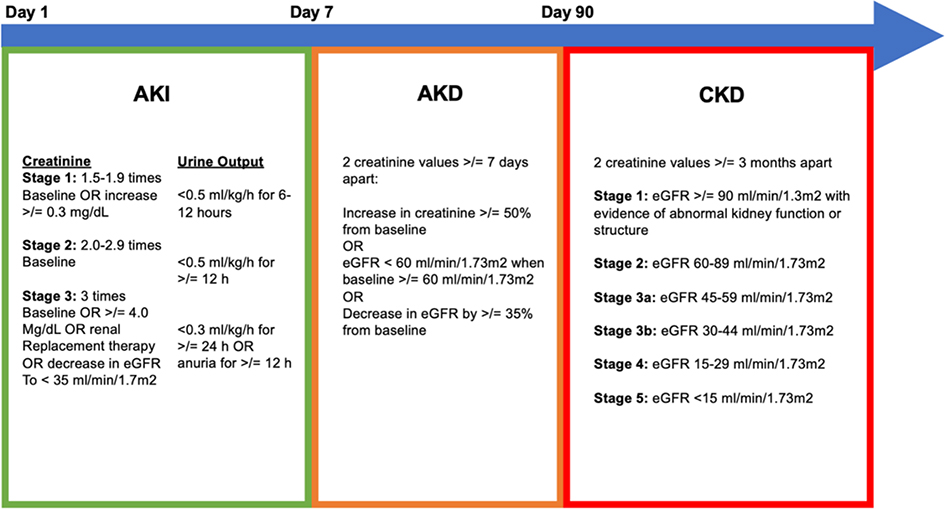
Figure 1:
Definitions of AKI, AKD, and CKD
Neonatal AKI definition- Stage 1 : SCr ≥0.3 mg/dL rise from baseline or SCr rise ≥1.5–1,9x baseline, Stage 2: SCr rise ≥2–2.9x baseline, stage 3: SCr rise ≥3x baseline or SCr ≥2.5 mg/dL or need for kidney replacement therapy
The overarching goal of the present study is to better understand the AKI to AKD to CKD transition in children. The aims of this study are to: (1) describe the incidence of AKD following an episode of AKI, (2) evaluate the risk factors for progression of AKI to AKD, and (3) evaluate the association of AKD with progression to CKD following AKI in hospitalized children. We hypothesized that AKD would occur commonly after AKI and would be associated with increased risk of CKD.
Methods
Study Design and Population:
We performed a retrospective cohort study of all children under the age of 18 years who were admitted to a tertiary care children’s hospital between 2015–2019 with a diagnosis of AKI. We included children admitted to general wards, neonatal intensive care units (NICU), pediatric cardiac intensive care units (PCICU), and pediatric intensive care units (PICU). Subjects were retrospectively identified using the electronic health record based on ICD-9 (584.5, 584.6, 584.7, 584.8, 584.9, 586) and ICD-10 (N17.0, N17.1, N17.2, N17.8, N17.9, N19) diagnosis codes. The study was approved by the Institutional Review Board at Duke University and followed the tenets of the Declaration of Helsinki.
We excluded children with less than two serum creatinine values between day 8 and 90 after AKI, those on chronic dialysis at study enrollment and previous kidney transplant recipients. Serum creatinine values were obtained from both inpatient and outpatient medical records starting from day 1 of the AKI until a minimum of 2 years and up to 7 years after the AKI or until the patient’s death. Estimated glomerular filtration rate (eGFR) was calculated using the Bedside Schwartz equation [20].
Outcomes and Definitions:
The primary outcomes of our study were new incidence of AKD and CKD after AKI. The secondary outcomes of our study were incidence of ICU stay, duration of ICU stay, hospital length of stay, discharge on dialysis, and death. Baseline serum creatinine was defined as the lowest serum creatinine within 6 months prior to the AKI. If no baseline serum creatinine was available, creatinine was calculated using an assumed eGFR of 120 ml/min/1.73m2 for children outside the neonatal age group [21]. For neonates, AKI was only diagnosed after a nadir in creatinine or if creatinine increased immediately on day of life 1 above a threshold meeting neonatal AKI criteria [22]. AKI was defined based on the maximum KDIGO staging using serum creatinine [23]. Severe AKI was defined as stage 2 or 3 AKI. Prior AKI was defined as AKI that occurred prior to the study period. AKD was defined using KDIGO criteria with at least 2 serum creatinine values >7 days apart between day 8–90 as shown in Figure 1 [12]. A new CKD diagnosis was defined using KDIGO criteria with at least 2 serum creatinine values at least 3 months apart at >90 days post initial AKI as shown in Figure 1 [24].
Covariates:
A list of covariates and their definitions is provided in Supplementary Table 1 and 2.
Statistical Analysis:
We conducted a univariable analysis of all covariates of interest using chi square testing for categorical variables and student’s t test for continuous variables. Next, we identified covariates with biological plausibility or statistical significance based on univariate analysis for inclusion in a full logistic regression model to determine factors associated with development of AKD after AKI. We then used a stepwise selection (forward inclusion p <0.05; backward elimination p >0.1) of all covariates included in the full model to build a parsimonious logistic regression model predicting probability of AKD in children with AKI. Next, we used a limited set of covariates to build a full multivariable logistic regression model to determine factors associated with development of CKD after AKI. We again used a stepwise selection (forward inclusion p <0.05; backward elimination p>0.1) of all covariates included in the full model to build a parsimonious logistic regression model predicting risk of CKD in children with AKI. We conducted standard assumption diagnostics and report model parameters in odds ratios (OR) with 95% confidence intervals (CI). Statistical significance was set at p-value ≤ 0.05. All statistical analyses were done using Stata 17.0 (Stata Corporation, College Station, TX, USA).
Results
Baseline Characteristics:
A total of 889 children with a diagnosis code for AKI were identified from the electronic medical record. 528 children were included in our final cohort (Supplementary Figure 1). Table 1 shows the baseline demographics of the cohort. The AKD and no AKD cohorts were similar in sex, age at AKI diagnosis, and race. Children with AKD were more likely to be admitted to an ICU setting (NICU, PCICU, or PICU). Of those children admitted to NICU (n=128), approximately two-thirds went on to develop AKD. 361 children who did not meet the inclusion criteria were excluded and further analysis of this cohort is shown in Supplementary Table 3 and 4. Those children had a similar prevalence of stage 2 and 3 AKI. The incidence of elevated serum creatinine (58.8%) among the excluded children with AKI non-recovery before discharge who had serum creatinine checked once (n=17) matched the AKD incidence in our study cohort.
Table 1:
Baseline demographics of the total cohort
| Whole cohort (N=528) | AKD (N=297) | No AKD (N=231) | P-value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 285 (54.0%) | 155 (52.2%) | 130 (56.3%) | 0.350 |
| Female | 243 (46.0%) | 142 (47.8%) | 101 (43.7%) | 0.287 |
| Age at AKI diagnosis (years) | 3.8 (5.0) | 3.8 (5.2) | 3.8 (4.9) | 0.840 |
| BMI (kg/m2) | 15.5 (6.3) | 15.4 (6.54) | 15.7 (6.02) | 0.825 |
| Hospital location at AKI diagnosis | ||||
| General Floor | 153 (29.0%) | 67 (22.6%) | 86 (37.2%) | <0.001 |
| ICU | 375 (71.0%) | 230 (77.4%) | 145 (62.8%) | <0.001 |
| PICU | 133 (35.5%) | 80 (34.8%) | 53 (36.6%) | 0.006 |
| PCICU | 114 (30.4%) | 67 (29.1%) | 47 (32.4%) | 0.015 |
| NICU | 128 (34.1%) | 83 (36.1%) | 45 (31.0%) | <0.001 |
| Race | ||||
| White | 207 (39.2%) | 113 (38.0%) | 94 (40.7%) | 0.537 |
| African American | 196 (37.1%) | 115 (38.7%) | 81 (35.1%) | 0.408 |
| Asian | 20 (3.8%) | 12 (4.0%) | 8 (3.5%) | 0.642 |
| Hispanic | 45 (8.5%) | 28 (9.4%) | 17 (7.4%) | 0.350 |
| Mixed | 19 (3.6%) | 6 (2.0%) | 13 (5.6%) | 0.055 |
| Other | 41 (7.8%) | 23 (7.7%) | 18 (7.8%) | 0.859 |
Categorical covariates reported as N (%), continuous variables reported as mean (standard deviation)
Incidence of AKD and outcomes by presence or absence of AKD:
A total of 297 (56%) children met criteria for AKD after AKI. Most children in our study had stage 2 or 3 AKI (n=422) compared to stage 1 AKI. Children with stage 2 and 3 AKI had higher rates of AKD compared to children with stage 1 AKI (p <0.001, Figure 2.)
Figure 2:
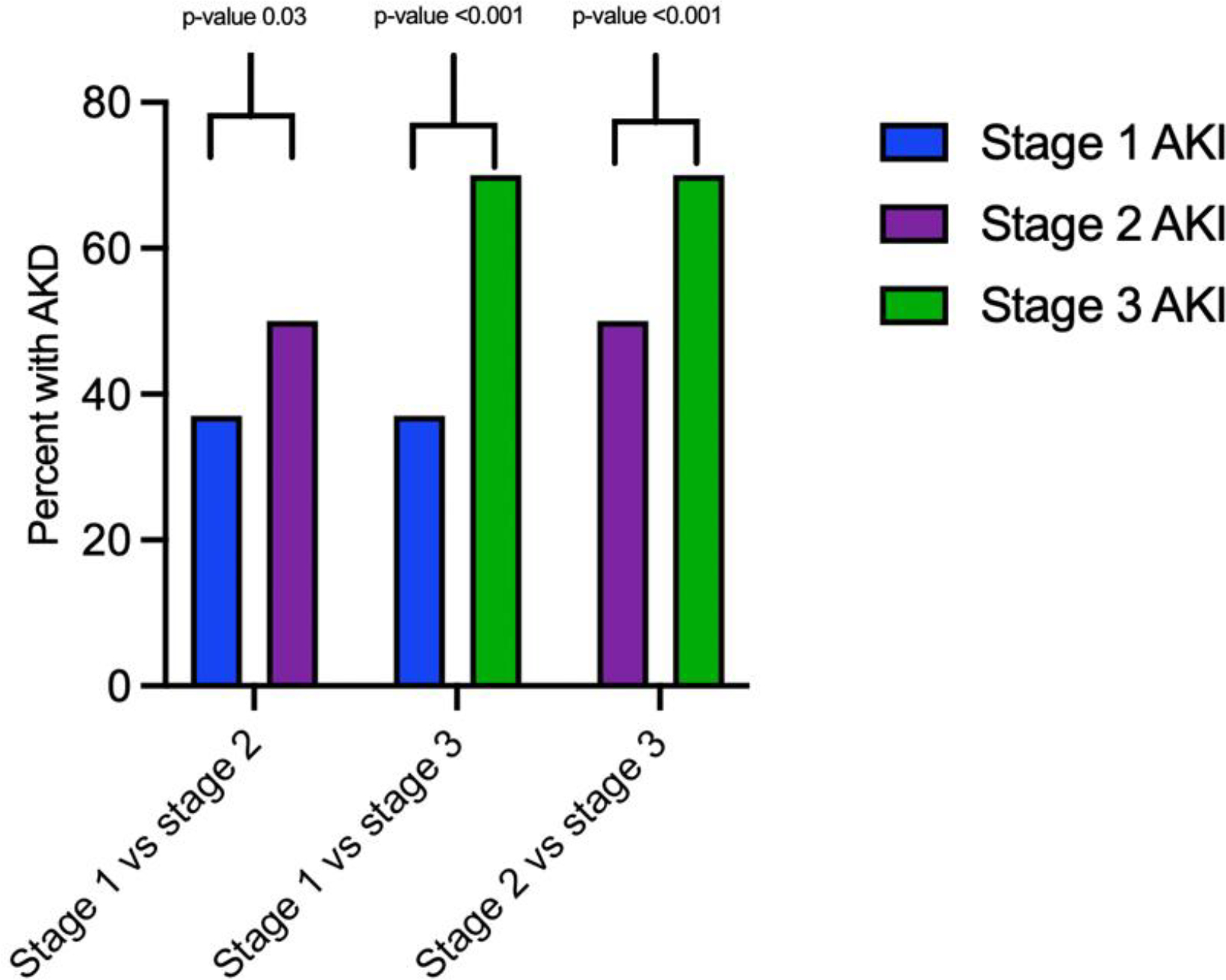
Proportion of patients with AKD by stage of AKI and pairwise comparison for odds of developing AKD by AKI stage
Results of the secondary outcomes of the study are detailed in Table 2. Children who developed AKD in general were more likely to be admitted to the ICU (85% vs 68%, OR 2.64, 95% CI 1.7–4.0, p-value <0.001), had longer mean duration of ICU stay (68 days vs 47 days, 95% CI 8.0–34.3 days, p-value 0.002), had longer mean duration of total hospital stay (82 days vs 48 days, 95% CI 22.4–46.0, p-value <0.001), and had higher mortality rates (38% vs 11%, OR 4.77, 95% CI 3.0–7.6. p-value <0.001). Two percent of children with AKD were discharged on dialysis compared to no children in the group without AKD.
Table 2:
Secondary outcomes
| Secondary Outcome | AKD (n= 297) | No AKD (n=231) | Test statistic | 95% CI | P-value |
|---|---|---|---|---|---|
| ICU stay | 84.9% | 68.0% | OR 2.6 | 1.7–4.0 | <0.001 |
| Mean duration of ICU stay (days) | 68.4 | 47.2 | Difference in means 21.2 | 8.0–34.3 | 0.002 |
| Mean total length of stay (days) | 82.2 | 48.0 | Difference in means 34.2 | 22.4–46.0 | <0.001 |
| Discharge on dialysis | 2.0% | 0.0% | |||
| Death | 37.7% | 11.3% | OR 4.8 | 3.0–7.6 | <0.001 |
Identification of AKD risk factors:
Univariate analysis of covariates of interest are shown in Table 3a and Table 3b. Multivariable logistic regression evaluating risk factors for AKD are shown in Table 4. AKI stage 2 and 3 were independently associated with development of AKD in a graded fashion with stage 2 and 3 increasing odds of AKD by 1.88 (95% confidence interval 1.03–3.44) and 3.35 (95% confidence interval 1.74–6.45), respectively. Longer duration of injury from day 1–7 also increased risk of AKD (OR 1.27, 95% confidence interval 1.13–1.42).
Table 3: Univariate analysis of risk factors for development of AKD.
Table 3a: Univariate analysis of categorical variables
| Covariate | AKD (n=297) | No AKD (n=231) | Odds Ratio | 96% CI | P-value |
|---|---|---|---|---|---|
| Female sex vs male sex | 47.5% | 43.7% | 1.2 | 0.9–1.7 | 0.287 |
| Hospital location at AKI diagnosis- PICU | 26.9% | 22.9% | 1.9 | 1.2–3.1 | 0.006 |
| Hospital location at AKI diagnosis- PCICU | 22.6% | 20.3% | 1.8 | 1.1–3.0 | 0.015 |
| Hospital location at AKI diagnosis- NICU | 27.9% | 19.5% | 2.4 | 1.5–3.8 | <0.001 |
| Family history of kidney disease | 37.0% | 36.8% | 1.0 | 0.7–1.4 | 0.955 |
| Prematurity < 36 weeks | 35.7% | 23.4% | 1.3 | 1.2–2.7 | 0.002 |
| Congenital heart disease | 31.0% | 31.2% | 1.0 | 0.7–1.4 | 0.962 |
| Chronic lung disease | 5.4% | 2.6% | 2.1 | 0.8–5.6 | 0.112 |
| Malignancy | 12.5% | 6.5% | 2.1 | 1.1–3.8 | 0.022 |
| Diabetes | 0.3% | 0.9% | 0.4 | 0.03–4.3 | 0.422 |
| Hypertension | 9.1% | 7.4% | 1.3 | 0.7–2.4 | 0.475 |
| Previous AKI | 13.1% | 6.9% | 2.0 | 1.1–3.7 | 0.021 |
| Chronic kidney disease | 5.7% | 6.9% | 0.8 | 0.4–1.7 | 0.571 |
| Severe AKI | 86.9% | 71.0% | 2.7 | 1.7–4.2 | <0.001 |
| Bone marrow transplant | 13.5% | 3.5% | 4.3 | 2.0–9.5 | <0.001 |
| Solid organ transplant | 9.1% | 10.4% | 0.9 | 0.5–1.5 | 0.616 |
| Home nephrotoxic medications | 20.9% | 21.6% | 1.0 | 0.6–1.5 | 0.830 |
| Mechanical ventilation at time of AKI | 51.2% | 32.5% | 2.2 | 1.5–3.1 | <0.001 |
| ECMO at time of AKI | 8.8% | 4.3% | 2.1 | 1.0–4.5 | 0.0454 |
| Pressor support at time of AKI | 34.3% | 31.2% | 1.2 | 0.8–1.7 | 0.441 |
| Supratherapeutic medication levels during AKI | 33.3% | 23.8% | 1.6 | 1.1–2.4 | 0.017 |
| Need for KRT day 1–7 of AKI | 19.1% | 4.3% | 5.2 | 2.6–10.5 | <0.001 |
| Hypertension at time of AKI | 25.3% | 33.8% | 0.7 | 0.5–1.0 | 0.032 |
| Hypoalbuminemia at time of AKI | 80.8% | 76.1% | 1.3 | 0.8–2.4 | 0.269 |
| Proteinuria at time of AKI | 50.5% | 49.7% | 1.0 | 0.7–1.6 | 0.881 |
| Hematuria at time of AKI | 57.8% | 55.6% | 1.1 | 0.7–1.7 | 0.671 |
| AKI Etiology- Volume depletion | 61.3% | 62.3% | 1.0 | 0.7–1.4 | 0.804 |
| AKI Etiology- Infection/sepsis | 13.5% | 7.4% | 2.0 | 1.1–3.6 | 0.025 |
| AKI Etiology- Hemorrhage | 2.7% | 1.3% | 2.1 | 0.6–8.0 | 0.267 |
| AKI Etiology- Medication induced | 25.6% | 19.5% | 1.4 | 0.9–2.2 | 0.098 |
| AKI Etiology- Urinary tract obstruction | 2.4% | 7.4% | 0.3 | 0.1–0.8 | 0.006 |
| AKI Etiology- Glomerulonephritis | 1.7% | 3.0% | 0.6 | 0.2–1.8 | 0.303 |
| AKI Etiology- Cardiac (insufficiency, arrest, bypass) | 19.9% | 19.1% | 1.1 | 0.7–1.6 | 0.814 |
| Sepsis episode during AKI | 59.0% | 43.2% | 1.9 | 1.3–2.7 | 0.004 |
Missing data includes 139 children without data regarding albumin level, 155 children without data regarding proteinuria, and 160 children without data regarding hematuria.
Table 3b:
Univariate analysis of continuous variables
| Covariate | AKD (n=297) | No AKD (n=231) | Difference in means | 95% CI | P-value |
|---|---|---|---|---|---|
| Age at AKI diagnosis (months) | 45.8 | 45.3 | −0.5 | −10.9 – 9.8 | 0.923 |
| BMI (kg/m2) | 15.4 | 15.7 | 0.3 | −0.8 – 1.4 | 0.574 |
| AKI duration (days) | 6.1 | 4.5 | −1.5 | −1.9 – −1.2 | <0.001 |
| Total number of nephrotoxic medication exposures in AKI period | 8.7 exposures | 7.2 exposures | −1.4 | −2.8 – −0.1 | 0.034 |
| Number of contrast studies in AKI period | 0.2 studies | 0.3 studies | 0.1 | −0.03 – 0.2 | 0.167 |
Table 4:
Full multivariable logistic regression model of risk factors for development AKD in children with AKI
| Covariate | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| AKI stage 2 vs stage 1 | 1.9 | 1.1–3.4 | 0.034 |
| AKI stage 3 vs stage 1 | 3.4 | 1.8–6.5 | <0.001 |
| Female sex | 1.3 | 0.8–1.9 | 0.266 |
| BMI | 1.0 | 1.0–1.1 | 0.763 |
| Age at diagnosis (months) | 1.0 | 1.0–1.01 | 0.046 |
| Hospital location at AKI diagnosis- PICU vs general floor | 1.8 | 0.9–3.5 | 0.914 |
| Hospital location at AKI diagnosis- PCICU vs general floor | 3.5 | 1.4–8.7 | 0.006 |
| Hospital location at AKI diagnosis- NICU vs general floor | 3.3 | 1.2–9.3 | 0.023 |
| Prematurity | 2.3 | 1.1–4.5 | 0.018 |
| Congenital heart disease | 1.2 | 0.6–2.2 | 0.591 |
| Malignancy | 2.7 | 1.2–6.1 | 0.018 |
| Pre-existing hypertension | 1.5 | 0.6–3.6 | 0.385 |
| Previous AKI | 2.7 | 1.2–6.2 | 0.013 |
| Pre-existing chronic kidney disease | 1.0 | 0.4–2.6 | 0.980 |
| Bone marrow transplant | 8.5 | 3.0–22.9 | <0.001 |
| Solid organ transplant | 1.0 | 0.4–2.4 | 0.931 |
| Mechanical ventilation at AKI diagnosis | 2.4 | 1.4–4.2 | 0.002 |
| ECMO at AKI diagnosis | 0.9 | 0.3–2.4 | 0.765 |
| Pressor support at AKI diagnosis | 0.5 | 0.3–0.8 | 0.011 |
| Number of home nephrotoxic medications | 1.1 | 0.7–1.7 | 0.670 |
| Hypertension at AKI diagnosis | 0.9 | 0.5–1.5 | 0.619 |
| Duration of AKI (days) | 1.3 | 1.1–1.4 | <0.001 |
| Total number of nephrotoxic medication exposures | 1.00 | 1.0–1.03 | 0.852 |
| Kidney replacement therapy during AKI period | 3.7 | 1.5–8.9 | 0.003 |
Patient level risk factors included age at diagnosis (OR 1.01, 95% confidence interval 1.00–1.01), prematurity (OR 2.34, 95% confidence interval 1.17–4.68), bone marrow transplant (OR 9.91, 95% confidence interval 3.46–28.40), malignancy (OR 2.86, 95% confidence interval 1.24–6.61), and history of previous AKI prior to the study period (OR 2.72, 95% confidence interval 1.20–6.18). Children admitted to PCICU (OR 3.63, 95% confidence interval 1.45–9.04) and NICU (OR 2.97, 95% confidence interval 1.04–8.42) as well as those who were requiring mechanical ventilation (OR 2.48, 95% confidence interval 1.39–4.42) at the time of AKI were at higher risk of developing AKD. Lastly, need for kidney replacement therapy in days 1–7 of the AKI increased the odds of developing AKD (OR 3.75, 95% confidence interval 1.52–9.23). Interestingly, patients who were requiring pressor support at the time of AKI had lower risk of developing AKD. A parsimonious multivariable logistic regression model is shown in Table 5 and confirmed the same significant risk factors as outlined in the full logistic regression model.
Table 5:
Forward and backward selection multivariable logistic regression model for development AKD in children with AKI
| Covariate | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| AKI stage 2 vs stage 1 | 2.0 | 1.1–3.5 | 0.023 |
| AKI stage 3 vs stage 1 | 3.5 | 1.9–6.5 | <0.001 |
| Age at diagnosis (months) | 1.0 | 1.0–1.01 | 0.004 |
| Hospital location at AKI diagnosis- PICU vs general floor | 1.7 | 0.9–3.3 | 0.109 |
| Hospital location at AKI diagnosis- PCICU vs general floor | 3.5 | 1.6–7.8 | 0.002 |
| Hospital location at AKI diagnosis- NICU vs general floor | 3.1 | 1.2–8.0 | 0.018 |
| Prematurity | 2.3 | 1.2–4.5 | 0.016 |
| Malignancy | 2.6 | 1.2–5.8 | 0.019 |
| Previous AKI | 3.0 | 1.4–6.4 | 0.004 |
| Bone marrow transplant | 8.2 | 3.3–20.6 | <0.001 |
| Mechanical ventilation at AKI diagnosis | 2.3 | 1.2–4.5 | 0.002 |
| Pressor support at AKI diagnosis | 0.50 | 0.3–0.90 | 0.014 |
| Duration of AKI (days) | 1.2 | 1.1–1.4 | <0.001 |
| Kidney replacement therapy during AKI period | 3.5 | 1.6–7.8 | 0.002 |
AKD as a risk factor for CKD:
101 of the children in our cohort developed CKD during the study period. The proportion of children with AKD who developed CKD was 45.5% (70/154) compared to 18.7% (31/166) of children who did not have AKD (Figure 3). 208 children did not have enough creatinine values to assess their CKD status. Multivariable logistic regression model of patient level risk factors and characteristics of the kidney injury is shown in Table 6a and demonstrated that AKD (OR 4.0, 95% CI 2.1–7.4, p-value <0.001), previous AKI (OR 2.4, 95% CI 1.1–5.2, p-value 0.032), and history of hypertension (OR 2.47, 95% CI 1.01–6.09, p-value 0.049), were associated with new diagnosis of CKD in the following period. A confirmatory forward and backward variable selection multivariable regression model (Table 6b) confirmed these findings and showed congenital heart disease as a significant risk factor for CKD after AKI.
Figure 3.
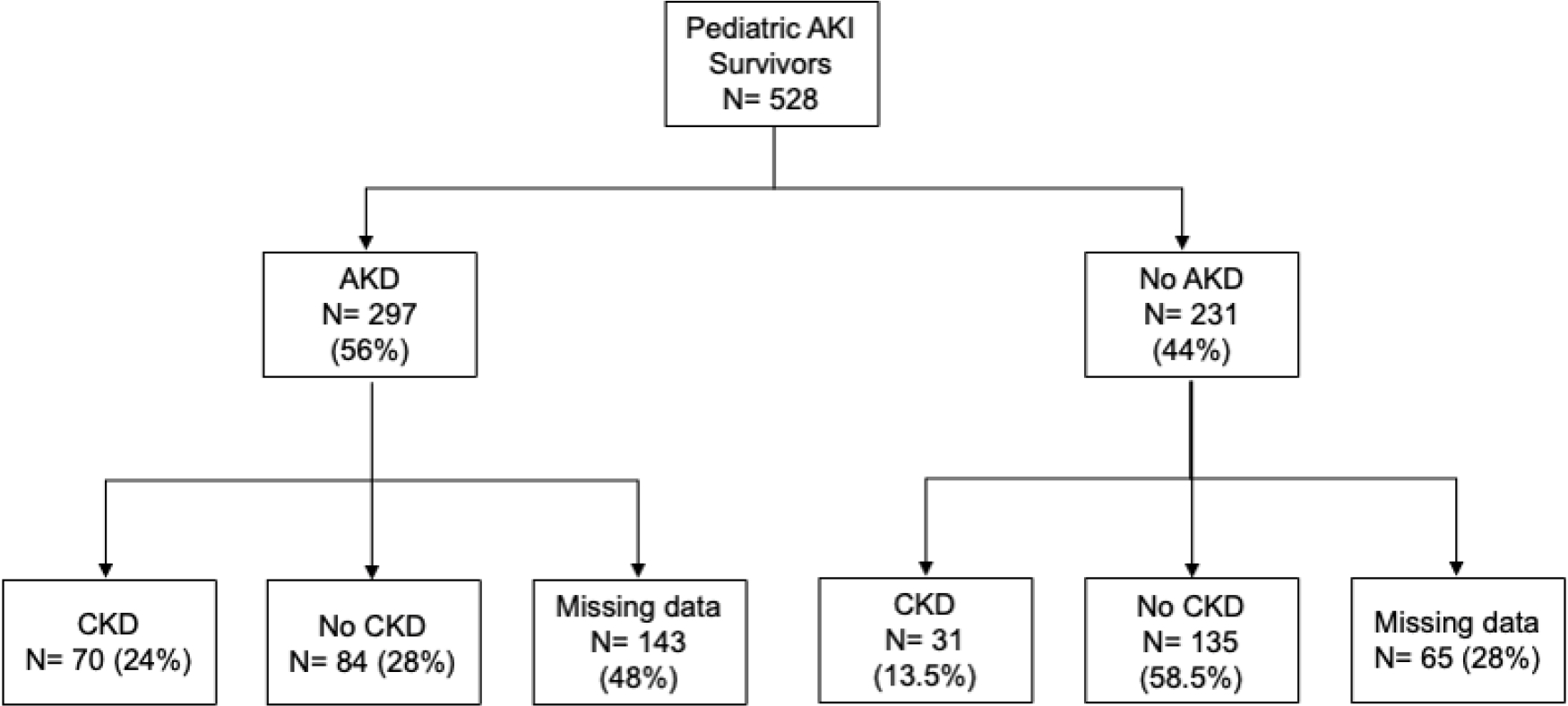
Flowchart of CKD incidence after AKD
Table 6A:
Full multivariable logistic regression model of risk factors for development CKD in children with AKI
| Covariate | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| AKD | 4.0 | 2.2–7.4 | <0.001 |
| Sex | 1.2 | 0.7–2.0 | 0.451 |
| Family history of kidney disease | 0.7 | 0.4–1.2 | 0.224 |
| Prematurity | 1.4 | 0.6–2.9 | 0.427 |
| Congenital heart disease | 1.4 | 0.7–2.6 | 0.286 |
| Malignancy | 0.8 | 0.3–2.0 | 0.624 |
| Pre-existing hypertension | 2.5 | 1.01–6.1 | 0.048 |
| Previous AKI | 2.4 | 1.1–5.2 | 0.048 |
| Solid organ transplant | 0.8 | 0.3–1.7 | 0.501 |
| AKI stage 2 | 0.6 | 0.3–1.2 | 0.153 |
| AKI stage 3 | 0.5 | 0.2–1.1 | 0.095 |
| BMI | 1.0 | 1.0–1.1 | 0.678 |
| Age at AKI diagnosis | 1.0 | 1.0–1.1 | 1.005 |
| Duration of AKI | 0.9 | 0.8–1.1 | 0.827 |
Table 6B:
Forward and backward selection multivariable logistic regression model for development CKD in children with AKI
| Covariate | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| AKD | 2.7 | 1.6–4.6 | <0.001 |
| Congenital heart disease | 1.7 | 1.0–3.0 | 0.049 |
| Pre-existing hypertension | 2.5 | 1.1–5.8 | 0.031 |
| Previous AKI | 2.3 | 1.1–4.6 | 0.025 |
Discussion
To the best of our knowledge, this study is the first to systematically evaluate the incidence of AKD in hospitalized children with AKI using the KDIGO definition of AKD. We have demonstrated that AKD is common in hospitalized AKI survivors, identified risk factors for AKD after AKI, and that AKD increases risk of CKD progression. Our study is unique in that we also demonstrate a trend towards increased ICU length of stay, hospital length of stay, and mortality in children with AKD after AKI.
In recent years there has been increasing interest in understanding the progression of AKI to CKD with a focus on better understanding AKD. The incidence of AKD was 56% and a new diagnosis of CKD occurred in 19% of children with AKI at our tertiary care pediatric center using strict application of the KDIGO definitions. Other studies have shown variable incidences of AKD. The heterogeneity in AKD definitions utilized likely contributes to the variable incidence reported in studies. A study of children admitted to a single center in China also demonstrated a high incidence of AKD of 42.3% [16]. This study, along with the study by LoBasso et al, utilized the Acute Disease Quality Initiative (ADQI) definition of AKD which only uses serum creatinine to classify AKD rather than the newer KDIGO classification of AKD which more broadly includes serum creatinine and eGFR based definitions [14, 16]. In the study by Namazzi et al. evaluating AKD in children with severe malarial infections serum creatinine was assessed at day 1, 2 and 1 month after admission. The incidence of AKD was 15.6% and was diagnosed if serum creatinine was elevated at 1 month which differs with the most recent KDIGO guidelines [15]. Daraskevicius et al. reported an incidence of AKD of 35.3% retrospective study of children undergoing hematopoietic stem cell transplant [17]. This study used the pRIFLE criteria for AKI and the ADQI definition of AKD. Lastly, he higher incidence of AKD in our study may also be due to a higher proportion of subjects who were critically ill or had stage 2 or 3 AKI. Nearly 53% of the cohort in the study by Deng et al. had stage 1 AKI compared to only 20% in our study which likely contributed to the significantly higher incidence of AKD seen in our study [16]. Overall, these differences in AKD incidences among the different pediatric studies highlight the need for applying a standard definition of AKD to future studies assessing AKD incidence.
In the current study we have begun to identify risk factors for progression of AKI to AKD and CKD. We have shown that these factors increase the risk of AKD independent of the AKI stage itself demonstrating that factors aside from AKI stage must be considered in order to fully understand a child’s risk of kidney disease progression. These factors include a history of prematurity, malignancy, bone marrow transplant, and previous AKI as being high risk for AKD development. Children who were admitted to the PCICU or NICU were at increased risk of AKD likely related to their critical illness and prematurity. Surprisingly, though PCICU admission was a risk factor for AKD, congenital heart disease was not. This is likely because all forms of congenital heart disease including non-hemodynamically significant disease were included in the cohort. A history of CKD was also not a risk factor for AKD; we speculate that a known diagnosis of kidney disease may cause providers to be more cognizant of factors that cause and worsen kidney disease.
By understanding which patients are at higher risk of developing AKD and CKD after AKI, providers may modify the care they provide to be more kidney protective, consult nephrologists earlier, and monitor post-discharge kidney function more diligently. This presents an opportunity for more widespread study and application of known AKI risk prediction tools such as the Renal Angina Index (RAI) [25]. The RAI has been shown to predict severe AKI after admission to critical care units and is now being studied in other populations. Sooner application of such a risk prediction tool, even before admission to an ICU, may help in even preventing severe AKI and its progression to AKD and CKD [25–27]. Furthermore, such models may be developed to identify those the highest risk for AKD and CKD to guide follow-up.
Our study also identified actionable risk factors associated with progression of AKI to AKD. Similar to the study done by Deng et al, we also showed that AKI severity increased risk of AKD in a graded manner with stage 2 AKI increasing risk 2-fold and stage 3 AKI increasing risk 3.5-fold when compared to stage 1 AKI [16]. This suggests that strategies to prevent severe AKI, including avoidance of nephrotoxic medications, contrast agents, early and sustained reversal of hypovolemia, may be beneficial in mitigating risk of AKD development. Early recognition of children with stage 1 AKI and prevention of AKI progression by utilizing early alert systems may also be beneficial in long term prevention of AKD and subsequent CKD. Such alert systems provide care teams with knowledge of their patient’s AKI as well as resources for optimizing their care [28]. More studies are needed to demonstrate the impact of such clinical decision support tools on the prevention of AKD and CKD in children with AKI. We demonstrated that longer duration of AKI increased risk of AKD suggesting those who have early reversal of AKI may have lower risk of progression of kidney disease. Studies in adults with AKI have also shown increased risk of AKD with nephrotoxin exposure warranting further investigation into this risk factor. Nephrotoxin exposure has long been known to be a risk factor for AKI and studies have shown that threshold values for exposures do increase risk of AKI [29]. Recent work has shown that children with nephrotoxin induced AKI are at increased risk of longer term abnormal kidney function [19]. Further study is warranted to better understand the incidence of AKD in children exposed to nephrotoxins and how these exposures affect their risk of CKD. Children with urinary obstruction as an etiology of AKI were found to be at lower risk of AKD after AKI. We suspect this is due to rapid recognition and relief of obstructive processes. Use of vasopressors at the time of AKI was a protective factor against development of AKD, but the mechanism for this remains unclear. This may in part be due to improved kidney perfusion in the setting of critical illness but further studies including closer blood pressure assessment are needed to understand this.
The limitations of our study include incomplete data inherent to retrospective studies. Selection bias was likely introduced by the initial method of cohort selection, exclusion of children with insufficient creatinine values to assess for AKD and CKD status, and exclusion of children who were deceased at < 3 months after AKI. AKD and CKD status were assessed only by serum creatinine and creatinine derived eGFR, an insensitive marker of kidney function. Most of our study population was admitted to an intensive care setting (71%) and was completed at a single, tertiary care children’s hospital in the United States potentially limiting the generalizability to children with AKI admitted to the general wards. In our analysis we treated age as a continuous variable. Age and prematurity may have some association with hospital location at admission, particularly with NICU admission. By treating these covariates as separate variables from hospital location we hoped to assess their roles as independent risk factors for AKI to AKD transition in neonates admitted to the NICU and outside of the NICU. Lastly, we were unable to complete sensitivity analysis comparing the excluded patients to the included patients due to missing data. Prospective study with standardized follow up protocols will better allow for unbiased assessment of AKD incidence after AKI and risk factors for AKI-AKD-CKD progression. Given the variable incidence of AKD and CKD after AKI we would suggest first starting with a rigorous follow up protocol for all children with AKI. Once incidence and risk factors are better understood we may categorize patients by risk level for kidney disease progression and better tailor follow up protocols.
In this retrospective cohort study of pediatric AKI survivors, we have shown that AKD is very common with greater than 50% of our cohort developing AKD. We have also identified patient level risk factors associated with AKD after AKI that will inform prospective studies. There were also many identifiable, possibly intervenable risk factors associated with AKD development. Children in our cohort who progressed from AKI to AKD were at higher risk of developing CKD. Larger prospective cohort or database studies would confirm the risk factors we have identified and in conjunction with our study would set the stage for future intervention studies.
Supplementary Material
Funding:
MP was supported by National Institutes of Health Nephrology Training Grant (5T32DK007731-24).
Footnotes
Declarations:
Competing Interests: CJD reports consultancy with UnitedHealth Group/Optum Labs. Other authors have no relevant financial or non-financial interests to disclose.
Ethics Approval: The study was approved by the Duke University Institutional Review Board.
Data availability:
All data are stored according to the Duke University Institutional Review Board approval and are available upon request.
References
- 1.McGregor TL, Jones DP, Wang L, et al. (2016) Acute kidney injury incidence in noncritically ill hospitalized children, adolescents, and young adults: A retrospective observational study. Am J Kidney Dis 67:384–390. 10.1053/j.ajkd.2015.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaddourah A, Basu RK, Bagshaw SM, et al. (2017) Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376:11–20. 10.1056/NEJMoa1611391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams DM, Sreedhar SS, Mickell JJ, Chan JCM (2002) Acute kidney failure: a pediatric experience over 20 years. Arch Pediatr Adolesc Med 156:893–900. 10.1001/archpedi.156.9.893 [DOI] [PubMed] [Google Scholar]
- 4.Sutherland SM, Ji J, Sheikhi FH, et al. (2013) AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol 8:1661–1669. 10.2215/CJN.00270113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkandari O, Eddington KA, Hyder A, et al. (2011) Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care 15:R146. 10.1186/cc10269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mammen C, Al Abbas A, Skippen P, et al. (2012) Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis 59:523–530. 10.1053/j.ajkd.2011.10.048 [DOI] [PubMed] [Google Scholar]
- 7.Atkinson MA, Warady BA (2018) Anemia in chronic kidney disease. Pediatr Nephrol 33:227–238. 10.1007/s00467-017-3663-y [DOI] [PubMed] [Google Scholar]
- 8.Hruska KA, Sugatani T, Agapova O, Fang Y (2017) The chronic kidney disease - Mineral bone disorder (CKD-MBD): Advances in pathophysiology. Bone 100:80–86. 10.1016/j.bone.2017.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drube J, Wan M, Bonthuis M, et al. (2019) Clinical practice recommendations for growth hormone treatment in children with chronic kidney disease. Nat Rev Nephrol 15:577–589. 10.1038/s41581-019-0161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen K, Didsbury M, van Zwieten A, et al. (2018) Neurocognitive and Educational Outcomes in Children and Adolescents with CKD: A Systematic Review and Meta-Analysis. Clin J Am Soc Nephrol 13:387–397. 10.2215/CJN.09650917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jankowski J, Floege J, Fliser D, et al. (2021) Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 143:1157–1172. 10.1161/CIRCULATIONAHA.120.050686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lameire NH, Levin A, Kellum JA, et al. (2021) Harmonizing acute and chronic kidney disease definition and classification: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int 100:516–526. 10.1016/j.kint.2021.06.028 [DOI] [PubMed] [Google Scholar]
- 13.Patel M, Heipertz A, Joyce E, et al. (2021) Acute kidney disease predicts chronic kidney disease in pediatric non-kidney solid organ transplant patients. Pediatr Transplant e14172. 10.1111/petr.14172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LoBasso M, Schneider J, Sanchez-Pinto LN, et al. (2022) Acute kidney injury and kidney recovery after cardiopulmonary bypass in children. Pediatr Nephrol 37:659–665. 10.1007/s00467-021-05179-5 [DOI] [PubMed] [Google Scholar]
- 15.Namazzi R, Batte A, Opoka RO, et al. (2022) Acute kidney injury, persistent kidney disease, and post-discharge morbidity and mortality in severe malaria in children: A prospective cohort study. EClinicalMedicine 44:101292. 10.1016/j.eclinm.2022.101292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng Y-H, Yan P, Zhang N-Y, et al. (2022) Acute kidney disease in hospitalized pediatric patients with acute kidney injury in china. Front Pediatr 10:885055. 10.3389/fped.2022.885055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daraskevicius J, Azukaitis K, Dziugeviciute-Tupko J, et al. (2020) Phenotypes and baseline risk factors of acute kidney injury in children after allogeneic hematopoietic stem cell transplantation. Front Pediatr 8:499. 10.3389/fped.2020.00499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigurjonsdottir VK, Chaturvedi S, Mammen C, Sutherland SM (2018) Pediatric acute kidney injury and the subsequent risk for chronic kidney disease: is there cause for alarm? Pediatr Nephrol 33:2047–2055. 10.1007/s00467017-3870-6 [DOI] [PubMed] [Google Scholar]
- 19.Menon S, Kirkendall ES, Nguyen H, Goldstein SL (2014) Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr 165:522–7.e2. 10.1016/j.jpeds.2014.04.058 [DOI] [PubMed] [Google Scholar]
- 20.Schwartz GJ, Muñoz A, Schneider MF, et al. (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. 10.1681/ASN.2008030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zappitelli M, Parikh CR, Akcan-Arikan A, et al. (2008) Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol 3:948–954. 10.2215/CJN.05431207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmody JB, Swanson JR, Rhone ET, Charlton JR (2014) Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol 9:2036–2043. 10.2215/CJN.05190514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120:c179–84. 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 24.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Workgroup (2013) KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter, Suppl 3:1–150 [Google Scholar]
- 25.Basu RK, Zappitelli M, Brunner L, et al. (2014) Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int 85:659–667. 10.1038/ki.2013.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaradilmiş RM, Öztürk B, Güngör A, et al. (2023) Success of the acute renal angina index in the early prediction of acute kidney injury in the emergency department. Acta Clin Belg 78:51–57. 10.1080/17843286.2022.2031667 [DOI] [PubMed] [Google Scholar]
- 27.Gist KM, SooHoo M, Mack E, et al. (2022) Modifying the renal angina index for predicting AKI and related adverse outcomes in pediatric heart surgery. World J Pediatr Congenit Heart Surg 13:196–202. 10.1177/21501351211073615 [DOI] [PubMed] [Google Scholar]
- 28.Menon S, Tarrago R, Carlin K, et al. (2021) Impact of integrated clinical decision support systems in the management of pediatric acute kidney injury: a pilot study. Pediatr Res 89:1164–1170. 10.1038/s41390-020-1046-8 [DOI] [PubMed] [Google Scholar]
- 29.Goldstein SL, Dahale D, Kirkendall ES, et al. (2020) A prospective multi-center quality improvement initiative (NINJA) indicates a reduction in nephrotoxic acute kidney injury in hospitalized children. Kidney Int 97:580–588. 10.1016/j.kint.2019.10.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are stored according to the Duke University Institutional Review Board approval and are available upon request.