Abstract
Background:
Residual depressive symptoms following treatment are a burden for patients and are associated with increased risk of relapse. While this phenomenon has been explored following pharmacotherapy, there is little research into residual symptoms following electroconvulsive therapy (ECT). This study quantifies the frequency and type of residual symptoms following ECT treatment.
Methods:
This study used retrospective data from patients receiving ECT as part of routine clinical care. Depressive symptomatology was assessed using the Quick Inventory of Depressive Symptomatology – Self-Report 16 item scale (QIDS), which includes 9 symptom domains graded from 0 to 3. We quantified the frequency of mild or greater (QIDS≥1) and moderate or greater (QIDS≥2) residual symptoms following treatment among patients responding to ECT (QIDS decrease ≥50% from baseline) and non-responders (QIDS decrease <50%).
Results:
Among 1,799 patients, 1,015 (56.4%) responded to ECT and 784 (43.6%) did not. Among responders, 99.5% had at least one residual symptom of mild severity or greater (median=5, IQR=3–6) and 83.3% had at least one residual symptom of moderate severity or greater (median=1, IQR=1–2). Among non-responders, 100% had residual symptoms of mild severity or greater (median=8, IQR=7–9), and 99.2% had a residual symptom of moderate severity or greater (median=4, IQR=3–5). The most common residual symptoms among both responders and non-responders were sleep disturbances (93.1% and 98.7%, respectively) and sadness (68.9% and 96.4%, respectively).
Limitations:
Retrospective data from a single freestanding psychiatric hospital.
Conclusion:
Among patients with depression receiving ECT, there were high rates of residual symptoms even among patients responding to treatment.
Keywords: Affective disorders, Cohort studies, Electroconvulsive therapy, Residual Symptoms
Introduction:
Most clinical research on depression focuses on response to treatment, which is often defined as a fractional reduction of symptom severity by 50%from a patient’s baseline severity (Fava, 2020). As this definition does not require full remission—in other words, it does not require all depressive symptoms resolve for the patient to have “responded” to treatment—many “responsive” patients continue to experience depressive symptoms even after treatment (Fava, 2020; Nierenberg et al., 2010; Whiston et al., 2022). Residual symptoms are a burden for patients, and a greater number of residual symptoms is associated with increased risk of future relapse (Lambrichts et al., 2022; Nierenberg et al., 2010). Furthermore, low-grade residual symptoms that persist after treatment of depression have been shown to impact a patient’s functioning and quality of life (Strege et al., 2022). Recent work has shown rates of residual symptoms after treatment may be higher than previously estimated, and therefore residual symptoms represent an important target for further treatment and intervention (Strege et al., 2022).
To date there has been little research assessing residual symptoms after electroconvulsive therapy (ECT) treatment (Lambrichts et al., 2022). Prior work, primarily examining residual symptoms after pharmacological or psychotherapeutic treatment, has shown that depressed patients have a median of 3–5 residual depressive symptoms after treatment (Conradi et al., 2011; Iovieno et al., 2011; McClintock et al., 2011; Nierenberg et al., 2010; Whiston et al., 2022; Zajecka, 2013). Fatigue, sleep disturbances, anxiety, and cognitive dysfunction were the most common residual symptom domains after treatment with antidepressant medications (Conradi et al., 2011; Iovieno et al., 2011; McClintock et al., 2011; Zajecka, 2013). A higher intensity of residual symptoms has been shown to be associated with increased risk of relapse but these analyses primarily used aggregate scores on depression rating scales as opposed to individual symptom domains (Buckman et al., 2018; Lambrichts et al., 2022). However, since the mechanism of action for ECT is distinct from antidepressant treatment, we cannot assume that patients receiving ECT will have a similar constellation of residual symptoms following treatment response.
The one study examining residual symptoms after ECT treatment in 80 patients showed that residual sleep disturbances and lassitude at the end of treatment were associated with increased risk of relapse within 6 months (Lambrichts et al., 2022). However, this study was limited to a small, geriatric cohort of patients with major depressive disorder. Therefore, there is still need for additional large-scale research on residual symptoms after ECT treatment.
Thus this study quantifies the frequency of residual depressive symptoms in patients receiving ECT. We sought to determine the symptom domains with the highest rate of residual symptoms both in the cohort as a whole and stratifying patients by clinical response to treatment.
Methods:
Patient Selection:
This study used retrospective data from a large, established cohort of patients with moderate to severe symptoms of depression as assessed by the Quick Inventory of Depressive Symptomatology – Self Report 16 item scale (QIDS; moderate – severe symptoms indicated by a QIDS>10) receiving ECT as part of routine clinical care at a single freestanding psychiatric hospital in Massachusetts between May 2011 and March 2020. The QIDS was originally validated in outpatients with major depressive disorder and was designed to provide a brief assessment tool that was highly correlated with longer clinician-rated and self-reported depression scales while also providing sensitivity to assess changes in depression over time(Rush et al., 2003). The QIDS was administered prior to the first treatment and after treatment #5, #10, #15 and #20. Patients that did not reach at least treatment #5 were excluded from these analyses due to lack of follow-up QIDS assessment. To limit our analysis to patients receiving an acute course of ECT as opposed to maintenance ECT, patients were excluded from analysis if their 5th treatment within a series was more than 30 days from their baseline treatment, if their 10th treatment was more than 60 days from baseline, or if their 15th treatment was more than 150 days from baseline. If a patient received multiple courses of ECT during this period, only their initial treatment series was included in these analyses. Demographic data is from patient self-report; diagnosis was extracted from the patient’s clinical record at the time of their first treatment within the series (major depressive disorder (MDD), bipolar disorder (BPAD), or other (which includes schizophrenia, schizoaffective disorder, and catatonia)).
ECT parameters:
The decision to undergo ECT treatment was determined as part of clinical care by the treating psychiatrist in consultation with the ECT consult service. ECT was administered using a Mecta Sepctrum 5000Q (Tualatin, OR). Prior work includes additional cohort data and additional information about the treatment methodologies (Hart et al., 2022; Luccarelli et al., 2021b, 2021c). Individualized seizure threshold was determined at first treatment(Luccarelli et al., 2021a, 2020). Patients received ECT 3 times weekly, and any changes to dosing or electrode placement was determined by the treating psychiatrist’s clinical judgement.
Symptom assessment and treatment response:
The QIDS scores range from 0–27 with a higher score indicating increased depressive severity and a score >10 indicating moderate/severe depressive symptoms. Patients were stratified into ECT responders (those who had a decrease in QIDS composite score of ≥50% from baseline to at least one follow-up QIDS) and non-responders (those who did not have a ≥50% QIDS reduction at any follow-up time point), consistent with prior literature (Fava, 2020; Rush et al., 2006). Residual symptoms are defined as those present at time of response for treatment responders, or at time of last QIDS for treatment non-responders.
Due to variability in clinical care, QIDS conducted within two treatments of each timepoint were included as data for the major timepoints; for example, a QIDS conducted after treatments #3–7 were included as data for treatment #5.
The QIDS includes nine Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) depressive symptom domains: 1) sadness, 2) concentration, 3) self-criticism, 4) suicidal ideation (SI), 5) interest, 6) energy/fatigue, 7) sleep disturbance, 8) weight (increase or decrease), and 9) psychomotor (agitation or retardation). Each question is scored on a 4-point Likert scale (range 0–3). The sleep and psychomotor domains are composite scores from 4 items, and the weight domain is a composite score from 2 items. The composite score is determined by the highest individual rating on any single item. The other 6 domains are scored using 1 item.
Analyses:
We first sought to determine the frequency of residual symptoms within each domain. Since each QIDS symptom domain is scored on a 4-point Likert scale (range 0–3), we identified patients endorsing at least mild symptoms (QIDS domain score ≥1) or at least moderate symptoms (QIDS domain score ≥2) at baseline. Then we quantified the number of patients endorsing symptoms of at least the same severity at the end of treatment (either last treatment point for non-responders or time of response for treatment responders). Results were reported within the entire cohort and stratifying by response to ECT (patients responding to ECT vs. non-responders).
We also considered the change in individual QIDS domain relative to each patient’s baseline. We first calculated the difference between a patient’s QIDS domain score at baseline and at the end of treatment and identified patients that improved, stayed the same, or worsened within each domain. We then identified the domains with the largest number of patients with improvement from baseline, and the domains with the least improvement from baseline. These differences were compared between responders and non-responders.
This study was reviewed by the Mass General Brigham Institutional Review Board and approved with a waiver of informed consent (2020P001595). All analyses were completed in R (version 4.1.0).(R Core Team, 2019)
Results:
We identified 1799 patients for analysis, of which 1015 (56.4%) achieved QIDS defined response at or before treatment #20 (Figure S1). The remaining 784 patients were classified as non-responders. Demographic data is presented in Table S1. 74.7% of patients were diagnosed with MDD, 20.3% with BPAD, and 4.9% with other diagnoses. Ultrabrief pulse, unilateral ECT was the most common baseline treatment modality (ultrabrief pulse: 94.0%, unilateral: 97.1%). Patients received a median of 10 ECT treatments (IQR = 5–10; non-responders: median = 10, IQR = 5–15; responders: median = 5, IQR = 5–10). At the time of treatment initiation, patients had an average QIDS score of 18.3 (SD=3.75) indicating severe depressive symptoms. Looking across the entire cohort, 1794 patients (99.7%) had 1 or more residual symptoms of at least mild severity (Table 1; median=6, IQR=4–8). 1624 patients (90.3%) had at least 1 residual symptom of moderate severity (median=2, IQR=1–4). Figure 1A shows the percentage of patients endorsing each QIDS symptom domain score at baseline and the end of treatment.
Table 1:
Proportion of patients with at least mild or moderate residual symptoms in each domain at time of last treatment
| Responders | Non-Responders | |||||||
|---|---|---|---|---|---|---|---|---|
| Mild Thresholda | Moderate Thresholdb | Mild Thresholda | Moderate Thresholdb | |||||
| Baseline | End of Treatment | Baseline | End of Treatment | Baseline | End of Treatment | Baseline | End of Treatment | |
| N | N (%) | N | N (%) | N | N (%) | N | N (%) | |
| Sadness | 999 | 688 (68.87) | 913 | 128 (14.02) | 770 | 742 (96.36) | 720 | 502 (69.72) |
| Psychomotor | 959 | 522 (54.43) | 556 | 84 (15.11) | 738 | 684 (92.68) | 415 | 248 (59.76) |
| General Interest | 994 | 339 (34.10) | 764 | 70 (9.16) | 749 | 639 (85.31) | 557 | 282 (50.63) |
| Weight | 894 | 595 (66.55) | 631 | 172 (27.26) | 690 | 590 (85.51) | 450 | 255 (56.67) |
| Sleep | 1008 | 938 (93.06) | 934 | 658 (70.45) | 780 | 770 (98.72) | 734 | 646 (88.01) |
| View of Self | 915 | 319 (34.86) | 669 | 82 (12.26) | 725 | 615 (84.83) | 538 | 335 (62.27) |
| Suicidal Ideation | 826 | 179 (21.67) | 461 | 56 (12.15) | 647 | 489 (75.58) | 392 | 201 (51.28) |
| Concentration | 987 | 615 (62.31) | 745 | 91 (12.21) | 754 | 712 (94.43) | 595 | 397 (66.72) |
| Energy Level | 975 | 387 (39.69) | 818 | 61 (7.46) | 741 | 630 (85.02) | 618 | 332 (53.72) |
QIDS domain score ⩾1 at baseline and last treatment.
QIDS domain score ⩾2 at baseline and last treatment.
Figure 1:
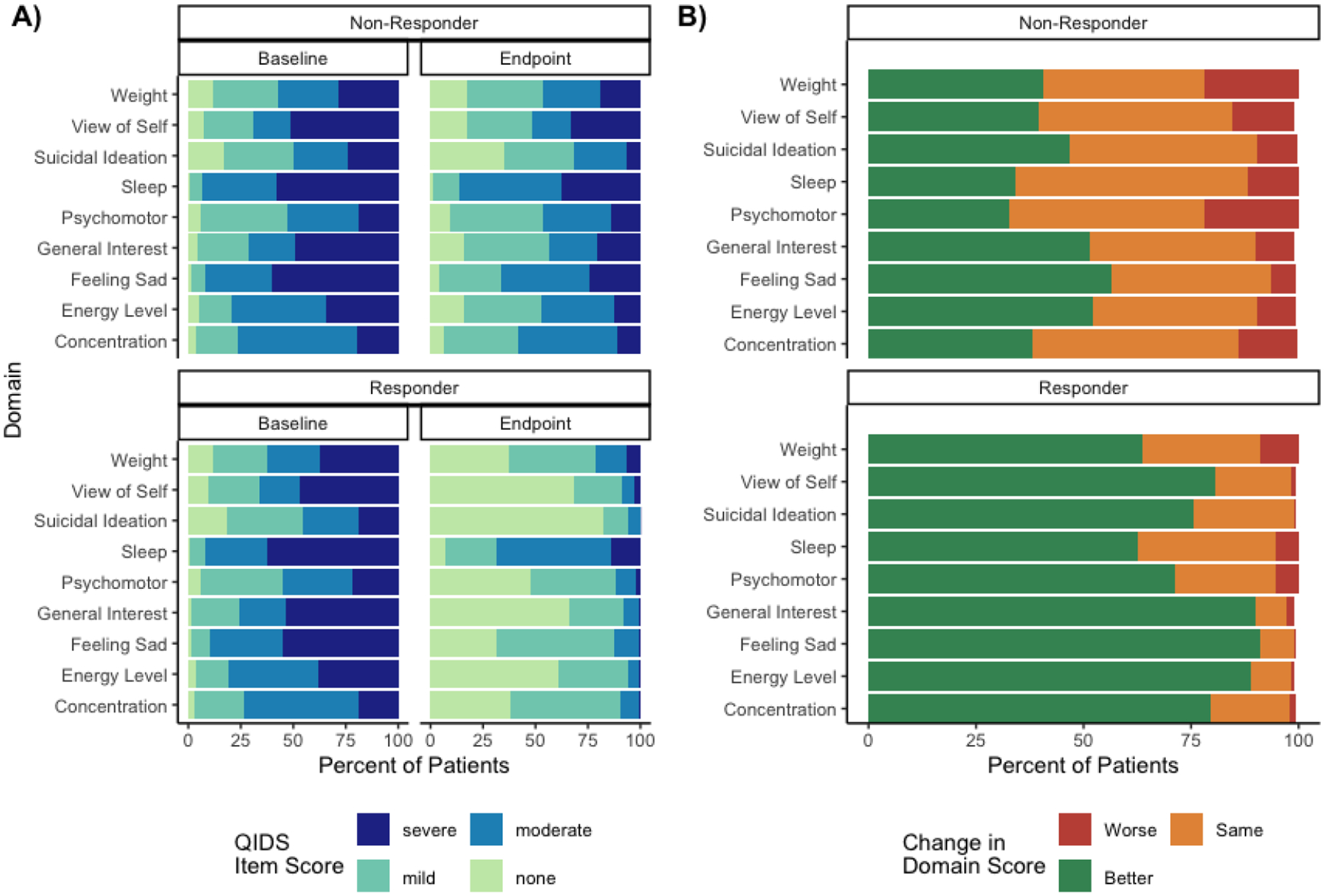
Baseline and final QIDS symptom domain scores and change in QIDS domain score from baseline to final treatment stratified by response status
A) Baseline and final QIDS symptom domain scores stratified by response status
B) Change in domain score at the end of treatment relative to baseline score stratified by response status
Among the 1015 patients responding to treatment, 1010 (99.5%) had at least one residual symptom of mild severity or greater (median=5, IQR=3–6) at the time of treatment response. The most common residual symptoms at a mild threshold among ECT responders were sleep disturbances (93.1%) and sadness (68.9%; Figure 1A). In other words, 93.1% of patients reporting at least mild sleep disturbances at baseline also reported at least mild sleep disturbance at the time of QIDS defined treatment response. 846 of the 1015 patients (83.3%) responding to treatment had at least one residual symptom of moderate severity or greater (median=1, IQR=1–2). The most common residual symptoms at a moderate threshold were sleep (70.4%) and weight changes (27.3%). SI had the lowest rate of mild residual symptoms (21.7%) and the third lowest rate of residual symptoms at the moderate threshold (12.2%). The general interest domain also had low rates of residual symptoms both at the mild (34.1%) and moderate threshold (9.16%).
Separate from these aggregate results, we also analyzed how an individuals’ QIDS domain scores changed over the course of treatment by identifying patients whose domain scores improved, worsened, or stayed the same at the end of treatment as compared to their baseline (Figure 1B). Among patients responding to treatment, the sadness and general interest domains had the highest rate of improvements from baseline at time of treatment response (91.1% and 90.1%, respectively). The weight and psychomotor domains had the highest rate of patients getting worse during treatment (8.8% and 5.4%, respectively). Data on individual change in domain scores is presented in Table S2.
Among the 784 patients that did not respond to treatment, 100% had a residual symptom of mild severity or greater at time of last treatment (median=8, IQR=7–9). The most common residual symptoms at a mild threshold among ECT non-responders were sleep disturbances (98.7%) and sadness (96.4%). 778 (99.2%) had a residual symptom of moderate severity or greater (median=4, IQR=3–5). The most common residual symptoms at a moderate threshold were also sleep disturbances (88.0%) and sadness (69.7%). The SI symptom domain had the lowest rate of mild residual symptoms (75.8%) and the second lowest rate of moderate residual symptoms (51.3%) among patients not responding to ECT treatment. Among patients not responding to treatment, the sadness domain and the energy level domain had the highest proportion of patients improving from baseline to time of last treatment (56.4% and 52.2%, respectively). The weight and psychomotor domains had the highest proportion of patients getting worse during treatment (22.1% and 21.8% respectively).
Discussion:
This study sought to determine the frequency of residual depressive symptoms after ECT treatment among both patients responding to ECT and non-responders, and to identify the most common residual symptom domains. Among patients responding to ECT treatment, 1010 (99.5%) had at least one residual symptom of mild severity or greater and 846 (83.3%) had at least one residual symptom of moderate severity or greater. The sleep disturbance and sadness domains had the highest rate of residual symptoms for both patients responding to ECT and non-responders, while SI had the lowest rate of residual symptoms. When considering how an individual’s QIDS domain score changed over the course of treatment, the sadness and general interest domains had the highest rate of improvements from baseline (91.1% and 90.1%, respectively) among treatment responders. The weight and psychomotor domains had the highest rate of patients getting worse during treatment (8.8% and 5.4%, respectively).
Prior work has shown high rates of residual symptoms after successful pharmacological, psychotherapeutic, or transcranial magnetic stimulation treatment, with greater than 90% of patients reporting at least one residual symptom of mild severity(Fava, 2020; Freeman et al., 2017; McClintock et al., 2011; Nierenberg et al., 2010; Sakurai et al., 2022). The high rates of residual symptoms among certain domains provides evidence for depression as a multidimensional construct as opposed to one homogenous disorder with a single underlying etiology(Fava, 2020; Freeman et al., 2017; Lambrichts et al., 2022; Zajecka, 2013). Therefore, given the continued evidence that our treatment modalities do not improve all symptom domains equally, we must consider that our treatments may impact specific brain circuitry that leads to improvement in individual domains. For this reason, it is crucial that we identify which residual symptoms persist after treatment and pursue other treatment modalities to prevent continued impairment within these domains. This manuscript represents a crucial first step in the identification of these residual domains after ECT treatment, as compared to other treatments of depression.
This argument is well elucidated by our finding that sleep disturbance is the most common residual symptom both for patients responding to ECT and non-responders. This observation is consistent with prior studies which showed that ECT does not improve objective or subjective sleep quality (Hoogerhoud et al., 2015; Lambrichts et al., 2022). Furthermore, residual sleep disturbances after ECT treatment were associated with higher risk of relapse(McClintock et al., 2011). Given the multifactorial etiology of sleep disturbances, and our finding that sleep disturbance was the most common residual symptoms at both a mild and moderate threshold—both among those responding to treatment and non-responders—specialized interventions for sleep disturbances may be necessary after the completion of ECT even among those with overall depressive symptom response.
We found SI to be one of the least common residual symptoms, consistent with prior work showing that ECT decreases risk of suicide and SI(Luccarelli et al., 2022). Even among patients that did not meet criteria for an overall response to ECT, there was a 48% decrease in the number of patients endorsing SI at a moderate threshold. Therefore, even when ECT does not decrease overall depressive symptoms, it may still have a substantial effect on SI.
The general interest domain also had low rates of residual symptoms as well as the largest decrease in scores during ECT treatment, both among patients responding to treatment and among patients that did not meet criteria for an overall response to ECT. Therefore, patients endorsing severe symptoms in this domain may specifically benefit from ECT treatment. It is important to note that while the sadness domain was one of the three domains with the highest percentage of patient with improvement during ECT treatment, it also had one of the highest rates of residual symptoms after treatment. While this may seem paradoxical, the sadness domain was the second most common symptom at baseline, after sleep disturbances. Therefore, even though patients showed a large decrease in sadness scores during treatment, they continued to have high rates of residual symptoms of sadness given their high baseline scores.
Limitations:
This study draws upon data collected from an existing clinical cohort in a real world, clinical setting. Therefore, the limitations of observational data—e.g., imperfections of data extracted from the medical record, patient attrition between assessments, heterogenous ECT parameters and doses, different attending psychiatrists, potential differences in patient population and treatment methods over the study period, and limited information on co-occurring psychotherapeutic or pharmacological treatment—all apply to this study. While the strength of this study is that it demonstrates residual symptomatology among a real-world, clinical cohort, future work is needed to better elucidate the relationship between these clinical variables and residual symptoms. These data are also derived from a single freestanding psychiatric hospital, which may limit the generalizability of these results.
Finally, when considering the psychometric properties of the QIDS, the 16-item scale has been validated against other commonly used depression scales and is a commonly used to assess symptom severity and treatment response as it relies on patient self-reported symptomatology. However, while the individual QIDS domains correspond to symptom domains within the DSM-IV, these individual domains have not been validated as unitary measures. Many prior studies have considered the individual QIDS domains, especially the domain assessing SI, but additional work is needed to validate this application of the QIDS and the potential concordance between QIDS items and more detailed assessments of individual domains.
Conclusions:
In this large, single center cohort study of patients with moderate to severe depression receiving ECT, there were high rates of residual symptoms even among patients responding to treatment. Sleep disturbances, sadness, and weight changes were the most common residual symptoms. These findings emphasize the high rates of residual symptoms, even after ECT treatment and the need for additional interventions to address these symptoms, especially regarding the three most common domains.
Supplementary Material
Highlights.
This study presents 1799 patients receiving acute-course ECT treatments
Among those responding to ECT (≥50% symptom reduction), 99.5% had at least one residual symptom of mild severity or greater
Among non-responders, 100% had residual symptoms of mild severity or greater
The most common residual symptoms were sleep disturbances and sadness
Funding
This work was supported by the National Institute of Mental Health (T32MH112485, JL) The sponsors had no role in study design, writing of the report, or data collection, analysis, or interpretation.
Conflicts of Interest
THM receives research funding from the Stanley Center at the Broad Institute, the Brain and Behavior Research Foundation, National Institute of Mental Health, National Human Genome Research Institute Home, and Telefonica Alfa. JL receives research funding from the National Institute of Mental Health and Harvard Medical School. He holds equity in Revival Therapeutics. The remaining authors have no disclosures to report.
References:
- Buckman JEJ, Underwood A, Clarke K, Saunders R, Hollon SD, Fearon P, Pilling S, 2018. Risk factors for relapse and recurrence of depression in adults and how they operate: A four-phase systematic review and meta-synthesis. Clin. Psychol. Rev 64, 13–38. 10.1016/j.cpr.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradi HJ, Ormel J, de Jonge P, 2011. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol. Med 41, 1165–1174. 10.1017/S0033291710001911 [DOI] [PubMed] [Google Scholar]
- Fava M, 2020. Pharmacological strategies for targeting residual symptoms in depression, in: Trivedi MH (Ed.), Depression, Primer On. Oxford University Press, New York, NY. [Google Scholar]
- Freeman MP, Fisher L, Clain A, Rabbitt R, Pooley J, Baer L, Fava M, 2017. Differentiating residual symptoms of depression from adverse events among patients initiating treatment with an antidepressant. Ann. Clin. Psychiatry Off. J. Am. Acad. Clin. Psychiatr 29, 28–34. [PubMed] [Google Scholar]
- Hart KL, Henry ME, McCoy TH, Seiner SJ, Luccarelli J, 2022. Individual response to electroconvulsive therapy is not correlated between multiple treatment courses. J. Affect. Disord 298, 256–261. 10.1016/j.jad.2021.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogerhoud A, Hazewinkel AWP, Reijntjens RHAM, van Vliet IM, van Noorden MS, Lammers GJ, van Dijk JG, Giltay EJ, 2015. Short-Term Effects of Electroconvulsive Therapy on Subjective and Actigraphy-Assessed Sleep Parameters in Severely Depressed Inpatients. Depress. Res. Treat 2015, 1–7. 10.1155/2015/764649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovieno N, van Nieuwenhuizen A, Clain A, Baer L, Nierenberg AA, 2011. Residual symptoms after remission of major depressive disorder with fluoxetine and risk of relapse. Depress. Anxiety 28, 137–144. 10.1002/da.20768 [DOI] [PubMed] [Google Scholar]
- Lambrichts S, Vansteelandt K, Hebbrecht K, Wagenmakers MJ, Oudega ML, Obbels J, van Exel E, Dols A, Bouckaert F, Schrijvers D, Verwijk E, Sienaert P, 2022. Which residual symptoms predict relapse after successful electroconvulsive therapy for late-life depression? J. Psychiatr. Res 154, 111–116. 10.1016/j.jpsychires.2022.07.056 [DOI] [PubMed] [Google Scholar]
- Luccarelli J, McCoy TH, Seiner SJ, Henry ME, 2022. Electroconvulsive therapy is associated with a reduction in self-reported suicidal ideation in adolescents. Brain Stimulat. 15, 1181–1183. 10.1016/j.brs.2022.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarelli J, McCoy TH, Seiner SJ, Henry ME, 2021a. Total Charge Required to Induce a Seizure in a Retrospective Cohort of Patients Undergoing Dose Titration of Right Unilateral Ultrabrief Pulse Electroconvulsive Therapy. J. ECT 37, 40–45. 10.1097/YCT.0000000000000714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarelli J, McCoy TH, Seiner SJ, Henry ME, 2020. Charge required to induce a seizure during initial dose titration using right unilateral brief pulse electroconvulsive therapy. Brain Stimulat. 13, 1504–1506. 10.1016/j.brs.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarelli J, McCoy TH, Shannon AP, Forester BP, Seiner SJ, Henry ME, 2021b. Rate of continuing acute course treatment using right unilateral ultrabrief pulse electroconvulsive therapy at a large academic medical center. Eur. Arch. Psychiatry Clin. Neurosci 271, 191–197. 10.1007/s00406-020-01202-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarelli J, McCoy TH, Shannon AP, Forester BP, Seiner SJ, Henry ME, 2021c. Duration of Treatment in Electroconvulsive Therapy Among Patients Beginning With Acute Course Right Unilateral Brief Pulse Stimuli. J. ECT 37, 238–242. 10.1097/YCT.0000000000000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Wisniewski SR, Nierenberg AA, Stewart JW, Trivedi MH, Cook I, Morris D, Warden D, Rush AJ, 2011. Residual Symptoms in Depressed Outpatients Who Respond by 50% But Do Not Remit to Antidepressant Medication. J. Clin. Psychopharmacol 31, 180–186. 10.1097/JCP.0b013e31820ebd2c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR, Miyahara S, Rush AJ, 2010. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol. Med 40, 41–50. 10.1017/S0033291709006011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2019. R: A Language and Environment for Statistical Computing.
- Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, Ninan PT, Thase ME, Gelenberg AJ, Kupfer DJ, Regier DA, Rosenbaum JF, Ray O, Schatzberg AF, 2006. Report by the ACNP Task Force on Response and Remission in Major Depressive Disorder. Neuropsychopharmacology 31, 1841–1853. 10.1038/sj.npp.1301131 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB, 2003. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 54, 573–83. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Uribe S, Cirillo P, Fuertes-Saiz A, Camprodon JA, Barbour T, 2022. Residual symptoms after achieving remission with repetitive transcranial magnetic stimulation in depression. J. Affect. Disord 301, 154–161. 10.1016/j.jad.2021.12.115 [DOI] [PubMed] [Google Scholar]
- Strege MV, Richey JA, Siegle GJ, 2022. What does “staying well” after depression mean? Chronic low grade symptomatology after treatment for depression is common. J. Affect. Disord 317, 228–235. 10.1016/j.jad.2022.08.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiston A, Lennon A, Brown C, Looney C, Larkin E, O’Sullivan L, Sik N, Semkovska M, 2022. A Systematic Review and Individual Patient Data Network Analysis of the Residual Symptom Structure Following Cognitive-Behavioral Therapy and Escitalopram, Mirtazapine and Venlafaxine for Depression. Front. Psychiatry 13, 746678. 10.3389/fpsyt.2022.746678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajecka JM, 2013. Residual Symptoms and Relapse: Mood, Cognitive Symptoms, and Sleep Disturbances. J. Clin. Psychiatry 74, 9–13. 10.4088/JCP.12084su1c.02 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


