Abstract
Background
The overall prognosis of glioblastoma (GBM) remains dismal, particularly for patients with unmethylated O6-methylguanine-DNA-methyltransferase (MGMT) promoter. In this phase II trial, we tested the combination of the antiangiogenic agent sunitinib with radiotherapy and temozolomide (TMZ) for newly diagnosed unmethylated MGMT GBM patients.
Methods
We enrolled 37 patients with unmethylated MGMT promoter GBM, age 18–70, and KPS ≥70. Patients received 12.5 mg of daily sunitinib for 7 days, followed by concurrent chemoradiation plus 12.5 mg sunitinib, then adjuvant TMZ. The primary endpoint was progression-free survival (PFS), and secondary endpoints were overall survival (OS), safety, and neutrophil-to-lymphocyte ratio (NLR) biomarker.
Results
At a median follow-up time of 15.3 months (range: 3.1–71.3 months), the median PFS was 7.15 months (95% CI: 5.4–10.5) and the 6-month PFS was 54.0%. Median OS was 15.0 months (95% CI: 13.8–19.4) and 2-year OS rate was 17.1%. Patients receiving >3 cycles of adjuvant TMZ, undergoing surgery at progression, and presenting a post-concurrent NLR ≤6 experienced a significant improved OS with hazard ratios of 0.197 (P = .001), 0.46 (P = .049), and 0.38 (P = .021), respectively, on multivariable analysis. Age >65 years predicted for worse OS with hazard ratio of 3.92 (P = .037). Grade ≥3 thrombocytopenia occurred in 22.9%, grade ≥3 neutropenia in 20%, and grade ≥3 thromboembolic events in 14.3% of patients. There were no grade 5 events.
Conclusion
Our findings suggest a potential benefit of combining sunitinib with chemoradiation in newly diagnosed GBM patients with unmethylated MGMT status and provide a strong rationale to test this combination in future studies.
Keywords: glioblastoma, radiation therapy, sunitinib, temozolomide, unmethylated MGMT
Key Points.
Our data suggest a potential benefit of combining sunitinib with chemoRT in MGMT unmethylated GBM.
Sunitinib potentially sensitizes MGMT unmethylated GBM to adjuvant TMZ.
NLR is a potential biomarker to identify responders to Sunitinib and chemoRT.
Importance of the Study.
The overall prognosis of GBM remains dismal, particularly for patients with unmethylated MGMT promoter. Thus, new treatment strategies are warranted for this population. This study is the first phase II clinical trial to combine the antiangiogenic drug sunitinib with standard-of-care chemoradiation in the treatment of newly diagnosed GBM patients with unmethylated MGMT promoter. This is a hypothesis-generating analysis that suggests a potential benefit of combining sunitinib with chemoradiation in this population. Importantly, the results of the study suggest that sunitinib potentially sensitizes patients with unmethylated MGMT to adjuvant temozolomide and that the neutrophil-to-lymphocyte ratio may prospectively identify patients who would better respond to the addition of sunitinib to standard chemoradiation. We believe that these results provide the basis for further studies to define the role of sunitinib in the treatment of newly diagnosed GBM patients with unmethylated MGMT promoter.
Glioblastoma (GBM) is the most common and aggressive primary malignant brain tumor in adults. The current standard of care for newly diagnosed GBM consists of maximal safe surgical resection followed by concurrent radiotherapy (RT) and temozolomide (TMZ), followed by adjuvant TMZ chemotherapy.1,2 However, even with advances in treatment modalities, the overall prognosis of GBM remains dismal. Indeed, GBM patients have a median survival time of 15 months when treated with the standard of care1,2 and only 12 months when treated with surgery and RT alone.3,4 Hegi et al.,5 in a subset of 203 patients from the EORTC/NCIC phase III trial, showed that patients with unmethylated MGMT promoter did not derive a significant benefit from combined TMZ plus RT (median OS, 12.7 months) compared with those with methylated MGMT promoter (median OS, 21.7 months).5 Thus, alternative strategies are warranted to improve the poor outcome of patients with unmethylated MGMT promoter tumors who do not derive benefit from TMZ-based therapy.
GBM, one of the most vascularized cancers, supports tumor growth through angiogenesis6–8 and overexpression of many angiogenic growth factors and their receptors,9 most notably the vascular endothelial growth factor (VEGF) and its receptors VEGFR-1/FLT-1 and VEGFR-2/KDR/FLK-1.10,11 Sunitinib (Sutent, SU11248) is an oral small molecule multitargeted receptor tyrosine kinase (RTK) inhibitor with antiangiogenic and antitumor activities. It targets multiple RTKs, including VEGFR-1/-2/-3, PDGFRα/β, stem cell factor receptor, FLT3, RET, and CSF1-R.12 Sunitinib has been FDA approved for metastatic renal cell carcinoma,13,14 gastrointestinal stromal tumors,15 and pancreatic neuroendocrine tumors. The complex molecular heterogeneity of GBM tumors and their highly angiogenic profile instigated the rationale to design preclinical studies aiming to exploit the multitargeted RTK antiangiogenic and antitumor activities of sunitinib either alone8,16 or in combination with RT17 in human GBM. The role of sunitinib was investigated in a phase II study of recurrent GBM18 and there are several clinical trials investigating its use in the recurrent setting.19 However, thus far, the potential benefit of sunitinib in the upfront treatment of newly diagnosed GBM patient in combination with TMZ and RT has not been investigated.
In a preclinical study, our group showed that GBM cells overexpressing MGMT displayed a reduced angiogenic phenotype, which was associated with a greater in vitro response to sunitinib in combination with RT + TMZ compared with MGMT(-) cells.20 Moreover, MGMT expression was associated with decreased ability to induce endothelial tube formation in vitro and low tumorigenicity in vivo compared with MGMT(-) cells, suggesting a role for MGMT in a shift toward a decreased angiogenic profile.20 Based on these findings, we hypothesized that MGMT might be an upstream modulator of genes involved in angiogenesis. We also hypothesized that addition of the antiangiogenic agent sunitinib to chemoradiation could lead to improved disease outcome for GBM patients with unmethylated MGMT promoter. Thus, in this phase II trial, we have tested for the first time the combination of sunitinib with RT and TMZ in newly diagnosed GBM patients displaying tumors with unmethylated MGMT promoter. We are reporting here, the progression-free survival (PFS) as a primary endpoint, as well as overall survival (OS) and treatment-related toxicities as secondary objectives. We also investigated the association between survival outcomes and the neutrophil-to-lymphocyte ratio (NLR), a noninvasive prognostic systemic inflammatory biomarker in GBM21–23 with a predictive value for response to sunitinib in other solid tumors.24,25
Methodology
Patient Eligibility
Patients 18–70 years of age with newly diagnosed and histologically confirmed GBM and an unmethylated MGMT promoter status verified by methylation-specific polymerase chain reaction (MS-PCR) were eligible for this study. Patient eligibility also included Karnofsky performance status (KPS) of 70 or higher, no prior history of brain tumors, RT to the brain, chemotherapy or antiangiogenic therapy, and a life expectancy of at least 6 months. Patients must have had the capacity to understand the informed consent form and be willing to sign the written informed consent document prior to registration. Normal baseline organ and marrow functions were required. For patients who have undergone tumor resection, a minimum of 14–28 days must have elapsed from the date of the surgery until the first day of the preconcurrent phase. For patients who had a stereotactic biopsy, a minimum of 14 days must have elapsed from the date of the biopsy to the first day of the preconcurrent phase.
This phase II study number A01-M121-11A (McG1132) was reviewed and approved by McGill Faculty of Medicine Institutional Review Board. All patients gave written informed consent before participation in the study (ClinicalTrials.gov Identifier: NCT02928575).
Study Design and Treatment Response Evaluation
This was a single arm, open label, phase II trial exploring the combination of sunitinib with standard-of-care chemoradiation in newly diagnosed GBM patients with unmethylated MGMT promoter. Patients were enrolled at 2 centers, the McGill University Health Centre (MUHC, Montreal, Canada) and the Tom Baker Cancer Centre (TBCC, Calgary, Canada) between 2012 and 2017. The experimental design is shown in Supplementary Figure 1. Patients received 12.5 mg of Sunitinib daily for 1 week prior to (preconcurrent phase) and during chemoradiation (concurrent phase) consisting of 60 Gy in 30 fractions and daily 75 mg/m2 TMZ. This was followed after a 4 week break by adjuvant TMZ 150–200 mg/m2 daily for 5 days every 28 days for 6 months (adjuvant phase). The first 7 recruited patients received 25 mg of sunitinib daily, however, given concerns for grade 3–4 toxicities, the protocol was amended to give 12.5 mg of sunitinib daily. Assessments were made throughout the study via MRI imaging, blood tests, and physical examinations as shown in Supplementary Figure 1. Additional blood samples were taken for biomarker analysis, notably the NLR. Adverse events (AEs) were recorded as per the Common Terminology Criteria for Adverse Events v3.0 (CTCAE v3.0). In the case of grades 3–4 AEs, sunitinib was discontinued. Patients removed from study due to AEs were continuously followed for outcome and long-term toxicities. Treatment continued until disease progression, unacceptable AE, patient withdrawal from the clinical trial, or changes in patient’s condition not allowing for further treatment. After treatment completion, patients were followed monthly for the next 6 months and quarterly thereafter.
Study Endpoints
The primary endpoint of this study was PFS, defined as the length of time between the date of diagnosis and the date of disease progression as determined based on the Response Assessment in Neuro-Oncology (RANO) criteria.26 Secondary objectives included OS, treatment-related AEs, and NLR as a biomarker/predictor for survival. OS was defined as the length of time between the date of diagnosis and the date of death. NLR was calculated as neutrophil count divided by lymphocyte count for blood samples taken at different time points Supplementary Figure 1): “pre-treatment” (T1), defined as the baseline NLR prior to the preconcurrent phase; “concurrent” (T3), 1 week after start of concurrent treatment; and “post-concurrent” (T4) following the end of concurrent treatment phase.
Data and Statistical Analysis
A power analysis was conducted to determine the minimum sample size required to test the study hypothesis. The required sample size to achieve 90% power for detecting a 20% difference in PFS from 54% as reported in the Stupp trial1 to 74%, using a 10% significance level, was N = 45. However, recruitment was challenging,27 and only 37 patients could be enrolled from the 2 centers between 2012 and 2017. The cancer-specific survival outcomes (PFS and OS) were calculated using the Kaplan–Meier method and a P value < .05 was considered as statistically significant.28 Cox proportional hazard regression model (univariable and multivariable) was used to derive hazard ratio (HR) with 95% confidence interval,29 adjusted for age, extent of surgery, number of adjuvant TMZ cycles, surgery at time of progression, and whether sunitinib was discontinued or not during the treatment. The optimal NLR cutoffs to best predict survival outcomes in our cohort was determined using a “landmark approach” statistical analysis which fits a separate Cox regression model for each landmark time point assuming that the effects of covariates on clinical outcome remain the same over time, as previously described.30
Results
Study Population and Patient Characteristics
Forty-one patients newly diagnosed with GBM were screened between 2012 and 2017, 16 patients were from the MUHC, and 25 patients were from the TBCC. Thirty-seven of these patients were enrolled and 4 patients were not eligible. Results are reported on a total of 35 patients as 2 patients stopped sunitinib during the first week of treatment (preconcurrent phase) due to toxicities. Median follow-up time is 15.3 months (range: 3.1–71.3 months). The patient and treatment characteristics are summarized in Table 1. Thirty-two/35 patients (91.4%) were ≤65 years old and 28/35 patients (80.0%) had a KPS of ≥90%. Gross tumor resection (GTR) as defined on postoperative MRI, was achieved in 19/35 patients (54.3%), 16/35 patients (45.7%) had a subtotal resection (STR) or biopsy. MGMT promoter methylation status was confirmed in all patients by MS-PCR. IDH1 R132H mutation was only present in 2/35 patients (5.7%). 33/35 patients (94.3%) received the full dose of RT (60 Gy/30 fractions) and 17/35 patients (48.6%) completed >3 cycles of adjuvant TMZ. Finally, reoperation was performed at first time of tumor progression for 25/35 patients (71.4%).
Table 1.
Patient Characteristics
| Patient Cohort (N = 35) | ||
|---|---|---|
| Characteristics | N | % |
| Age, years | ||
| Median | 52 | |
| Range | 30–76 | |
| Sex | ||
| Male | 24 | 68.6% |
| Female | 11 | 31.4% |
| Karnofsky performance status | ||
| 90%–100% | 28 | 80.0% |
| 70%–80% | 7 | 20.0% |
| Extent of surgery | ||
| GTR | 19 | 54.3% |
| STR/Biopsy | 16 | 45.7% |
| RT dose | ||
| 60 Gy/30 | 33 | 94.3% |
| <60 Gy | 2 | 5.7% |
| Number of adjuvant TMZ cycle | ||
| ≤3 cycles | 18 | 51.4% |
| >3 cycles | 17 | 48.6% |
| Surgery at time of progression | ||
| No | 10 | 28.6% |
| Yes | 25 | 71.4% |
| Treatment interruptions/delays | ||
| Pre-concomitant phase | ||
| Sunitinib | 0 | 0.0% |
| Concomitant phase | ||
| Sunitinib | 11 | 31.4% |
| RT | 7 | 20.0% |
| TMZ | 1 | 2.9% |
Treatment Efficacy of Sunitinib Combined with RT and TMZ in Newly Diagnosed Unmethylated MGMT GBM Patients
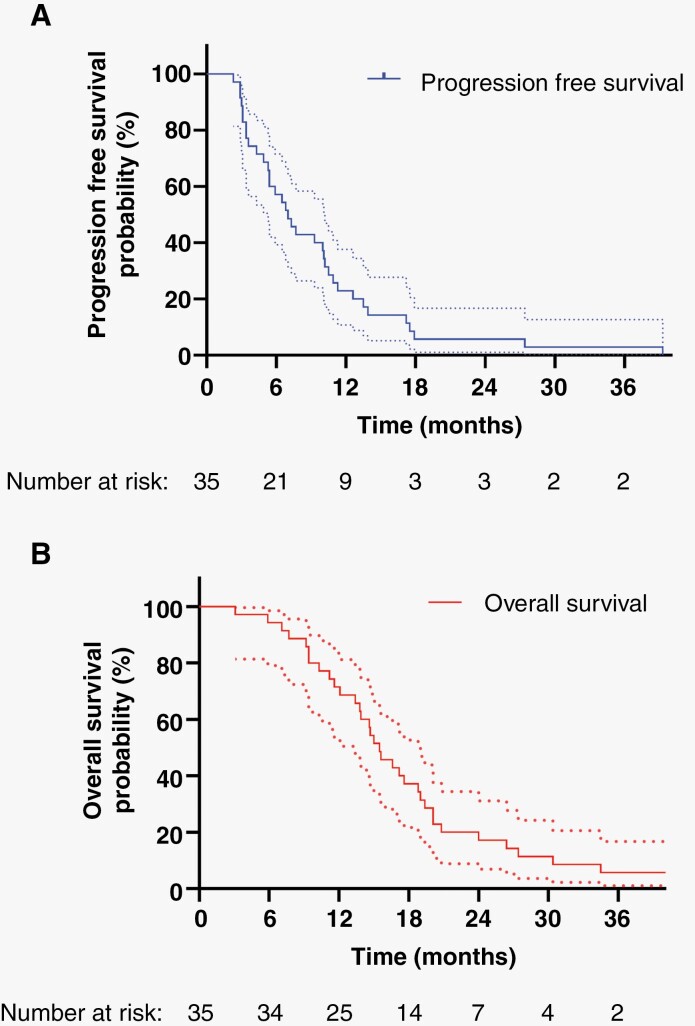
PFS and OS results are presented in Table 2 and Figure 1. Given that the study was underpowered, our analysis changed to a hypothesis generating rather than a hypothesis-confirming approach. Median PFS was 7.15 months (95% CI: 5.4–10.5 months). 6-month PFS was 54.0% (95% CI: 40.0%–73.6%). Median OS was 15.0 months (95% CI: 13.8–19.4 months). Two-year OS rate was 17.1% (8.2%–35.5%) and 3-year OS rate was 6.1% (1.5%–22.9%). Importantly, there were 2 patients that survived longer with 1 patient living up to 55 months post-initial surgery (IDH-wildtype) and the last patient in the cohort living up to 72 months (IDH1 R132H mutated).
Table 2.
Progression-free Survival and Overall Survival in Patient Newly Diagnosed Glioblastoma Patients with Unmethylated MGMT Promoter-treated with Combined Sunitinib, Temozolomide, and Radiotherapy
| Variable | Value (95% CI) |
|---|---|
| Progression-free survival (PFS) | |
| Median PFS (months) | 7.15 (5.4–10.5) |
| 6-month PFS (%) | 54.0% (40%–73.6%) |
| Overall survival (OS) | |
| Median OS (months) | 15 (13.8–19.4) |
| 2-year OS (%) | 17.1% (8.2%–35.5%) |
| 3-year OS (%) | 6.1% (1.5%–22.9%) |
Figure 1.
Kaplan–Meier estimates of progression-free survival (A) and overall survival (B) in newly diagnosed unmethylated MGMT GBM patients treated with combined Sunitinib, Temozolomide and Radiotherapy. Patients were assessed from time of diagnosis to time of tumor progression clinically or by MR imaging as per RANO criteria.
Factors Associated with Improved Survival Outcomes
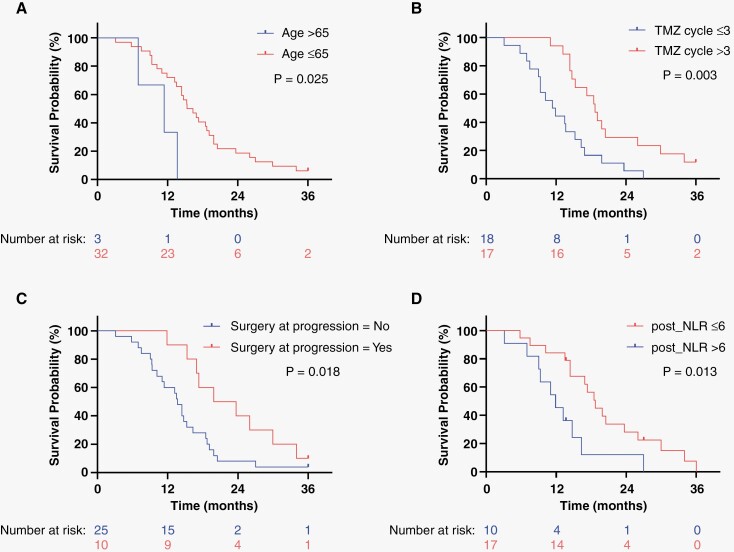
Univariable and multivariable Cox regression were performed to identify independent prognostic factors that may be associated with improved survival outcomes. There were no independent factors significantly associated with improved PFS (Table 3A). Age >65 was associated with worse OS with a 3.9-fold increase in risk of death (HR 3.92, 95% CI: 1.09–14.13, P = .037) on multivariable analysis when adjusted for extent of surgery, number of adjuvant TMZ cycles, surgery at time of progression, and whether sunitinib was discontinued or not during the treatment (Table 3B, Figure 2A). Interestingly, having received >3 cycles of adjuvant TMZ was significantly associated with improved OS on multivariable analysis when compared with having received ≤3 TMZ cycles (Table 3B, Figure 2B), with a HR of 0.197 for death (95% CI: 0.07–0.53, P = .001). Having received >3 cycles of adjuvant TMZ was also associated with improved PFS with a HR of 0.228 (95% CI: 0.08–0.63, P = .001, Table 3B). Having a STR/biopsy was not significantly associated with worse OS in this cohort compared with patients that had GTR (HR 1.74, 95% CI: 0.84–3.62, P = .136). However, additional tumor resection at the time of tumor progression was significantly associated with improved OS when compared with no additional surgery, with a HR of 0.46 (95% CI: 0.21–0.99, P = .049; Figure 2C). Delays or interruptions in sunitinib regimen during the concurrent treatment phase were not associated with worse OS (HR 1.55, 95% CI: 0.714–3.36, P = .268) and no other variables were associated with significantly improved PFS (Table 3B).
Table 3.
Clinical Variables Associated with Progression-free Survival and Overall Survival
| (A) Progression-free survival | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Univariable | Multivariable | ||||||
| Hazard Ratio | 95% CI | P-value | Hazard Ratio | 95% CI | P-value | |||
| Age >65 | 0.825 | 0.250 | 2.726 | 0.752 | 0.821 | 0.248 | 2.715 | 0.747 |
| Extent of surgery | ||||||||
| STR/BIOPSY | 1.297 | 0.649 | 2.594 | 0.462 | 1.389 | 0.681 | 2.834 | 0.367 |
| Sunitinib interruptions/delays | 1.113 | 0.524 | 0.524 | 0.781 | 0.801 | 0.357 | 1.798 | 0.590 |
| Number of adjuvant TMZ cycles | ||||||||
| TMZ.cycle ≤3 | 1.00 | 1.00 | ||||||
| TMZ cycle >3 | 0.483 | 0.240 | 0.960 | 0.038 | 0.228 | 0.080 | 0.63 | 0.004 |
| Surgery at time of progression | 0.893 | 0.420 | 1.902 | 0.770 | 1.074 | 0.503 | 2.294 | 0.853 |
| Neutrophil-to-lymphocyte ratio | ||||||||
| Pretreatment NLR ≤6 | 1.221 | 0.561 | 2.655 | 0.615 | 1.241 | 0.569 | 2.708 | 0.587 |
| Concurrent NLR ≤6 | 0.684 | 0.285 | 1.645 | 0.397 | 0.686 | 0.285 | 1.650 | 0.400 |
| Post-concurrent NLR ≤6 | 0.683 | 0.313 | 1.490 | 0.338 | 0.640 | 0.283 | 1.449 | 0.285 |
| (B) Overall survival | ||||||||
| Characteristics | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P-value | Hazard Ratio | 95% CI | P-value | |||
| Age >65 years | 3.919 | 1.084 | 14.170 | 0.037 | 3.920 | 1.087 | 14.128 | 0.037 |
| Extent of surgery | ||||||||
| STR/BIOPSY | 1.792 | 0.901 | 3.564 | 0.096 | 1.743 | 0.840 | 3.621 | 0.136 |
| Sunitinib interruptions/delays | 1.363 | 0.644 | 2.886 | 0.419 | 1.550 | 0.714 | 3.364 | 0.268 |
| Number of adjuvant TMZ cycles | ||||||||
| TMZ cycle ≤3 | 1.00 | 1.00 | ||||||
| TMZ cycle >3 | 0.347 | 0.170 | 0.720 | 0.004 | 0.197 | 0.070 | 0.530 | 0.001 |
| Surgery at time of progression | 0.424 | 0.199 | 0.902 | 0.026 | 0.456 | 0.208 | 0.997 | 0.049 |
| Neutrophil-to-lymphocyte ratio | ||||||||
| Pretreatment NLR ≤6 | 1.073 | 0.494 | 2.333 | 0.859 | 1.073 | 0.492 | 2.341 | 0.859 |
| Concurrent NLR ≤6 | 0.480 | 0.198 | 1.164 | 0.104 | 0.510 | 0.210 | 1.240 | 0.137 |
| Post-concurrent NLR ≤6 | 0.371 | 0.162 | 0.850 | 0.019 | 0.379 | 0.166 | 0.864 | 0.021 |
Univariable and multivariable Cox regression analyses showing factors associated with improved progression-free survival (Table 3A) and overall survival (Table 3B). Multivariable analysis was adjusted to age, extent of surgery, number of adjuvant TMZ cycles, surgery at time of progression, sunitinib discontinuation, and neutrophil-to-lymphocyte ratio ≤6. Significant P values are highlighted in bold. STR, subtotal resection; TMZ, temozolomide.
Figure 2.
Clinical variables significantly associated with overall survival in newly diagnosed GBM patients with unmethylated MGMT promoter treated with combined sunitinib, Temozolomide, and Radiotherapy. Kaplan–Meier curves for overall survival showing clinical variables that are significantly associated with overall survival on Cox regression and their P values: age (A), number of TMZ cycles (B), surgery at time of progression (C), and post-concurrent neutrophil-to-lymphocyte ratio (D).
Association between Post-concurrent NLR and Response to Sunitinib in Patients with Unmethylated MGMT GBM
Increasing evidence supports the use of NLR as a biomarker of systemic inflammation. Specifically, increased NLR has been correlated with poor prognosis in cancer, while its decrease predicts tumor response to treatment.21,23–25,31 Thus, we measured variations in NLRs values for patients treated with sunitinib and chemoradiation in this cohort during the pretreatment (baseline), concurrent, and post-concurrent phases of treatment. NLR values were not available for all patients in all phases of the treatment, thus results are reported for a total of 28/35 patients (80%). The median pretreatment NLR was 5.81 (range 1.00–16.86), median concurrent NLR was 4.08 (range 1.39–12.60), and median post-concurrent NLR was 4.0 (range 0.4–17.70), showing a gradual decrease in the NLR during treatment (Supplementary Figure 2A). In order to determine the optimal NLR cutoff that could best predict survival outcomes in our cohort, we performed a “landmark approach” statistical analysis, previously used to identify significant changes in blood markers during a longitudinal treatment and which fits a separate Cox regression model for each landmark time point assuming that the effects of covariates on clinical outcome would not remain the same over time.30 Using this approach, we showed that patients with a lower PFS (≤6 months) or OS (≤12 months) tended to have higher NLR values compared with those with longer PFS (>12 months) and OS (>2 years), respectively, and this was true in all phases of treatment (Supplementary Figure 2B–G). We determined an NLR cutoff of 6 to be the best at assessing the correlation between NLR and survival outcomes in this cohort. Based on these distributions we defined patients with a NLR ≤6 as “sunitinib responders” and those with NLR >6 as “sunitinib non responders.” Indeed, we show on univariable and multivariable Cox regression analysis that a post-concurrent NLR ≤6 is strongly associated with improved OS, with a HR of 0.38 for death (95% CI: 0.166–0.864, P = .021) on multivariable analysis (Table 3B, Figure 2D).
To validate our approach and confirm that post-concurrent NLR ≤6 is a strong predictor of better OS in our cohort, we compared the characteristics of patients with NLR ≤6 and those with NLR >6 to rule out any confounders (Supplementary Table 1). Post-concurrent NLR ≤6 predicted for better OS with a median OS of 18.5 months (14.4–23.7) compared with median OS of 11.9 (9.1–14.4) for those with NLR >6 (P = .037). Hence, this group of GBM patients with NLR ≤6 represent responders to sunitinib as opposed to those with NLR >6 that did not respond to sunitinib. There were no other patients characteristics (age, extent of surgery, RT or more than 3 cycles of TMZ received, PFS) that were statistically different based on an NLR cutoff of 6 (Supplementary Table 1), thus ruling out any confounding interactions, and validating post-concurrent NLR ≤6 as a predictor of better OS in newly diagnosed GBM patients with unmethylated MGMT.
Safety of Sunitinib in Addition to Concurrent RT and TMZ in GBM Patients with Unmethylated MGMT Promoter
Treatment-related AEs were recorded as per CTCAE v3.0 and are presented in Table 4. Due to safety concerns, the protocol was amended after 7 patients were recruited to reduce the sunitinib dose to 12.5 mg from 25 mg daily. Notably, 1 patient experienced grade 3 thrombocytopenia and another grade 4 thrombocytopenia and a pulmonary embolus. These AEs were taken into account in the safety analysis. Overall, 8/35 patients (22.9%) experienced grade 3 or 4 hematological toxicities, with grade 3–4 neutropenia recorded in 7 patients (20%), and grade 3–4 thrombocytopenia in 8 patients (22.9%). There were 5 grade 3–4 thrombotic events (14.3%), of which 3 cases of deep vein thrombosis (8.6%, grade 3) and 2 cases of pulmonary embolism (5.7%, grade 4). The most common AEs were fatigue and gastrointestinal toxicities, which were mostly of low grade, with a total of 13 (37.1%) patients experiencing grade 1–2 fatigue and 12 (34.3%) patients experiencing grade 1–2 nausea. There were 5 (14.3%) grade 3–4 gastrointestinal AEs (anorexia, nausea, hyperglycemia, and increased liver enzymes) and 4 (11.4%) grade 3–4 central nervous system-related AEs (headache, alopecia, muscle weakness, brain infection postsurgery). Finally, there were no grade 3 or 4 cardiovascular or respiratory AEs and no grade 5 events.
Table 4.
Treatment-related Adverse Effects
| Adverse Events (n = 35) | Grades 1–2 n (%) | Grades 3–4 n (%) | All Grades n (%) |
|---|---|---|---|
| Fatigue | 13 (37.1) | 2 (5.7) | 15 (42.9) |
| Hematologic | |||
| Anemia | 13 (37.1) | 13 (37.1) | |
| Leukopenia | 3 (8.6) | 4 (11.4) | 7 (20.0) |
| Lymphocytopenia | 5 (14.3) | 4 (11.4) | 9 (25.7) |
| Neutropenia | 6 (17.1) | 7 (20.0) | 13 (37.1) |
| Thrombocytopenia | 6 (17.1) | 8 (22.9) | 14 (40.0) |
| Thrombosis | |||
| Pulmonary embolism | 2 (5.7) | 2(5.7) | |
| Deep vein thrombosis | 3 (8.6) | 3 (8.6) | |
| Gastrointestinal system | |||
| Appetite loss (anorexia) | 5 (14.3) | 1 (2.9) | 6 (17.1) |
| Constipation | 4 (11.4) | 4 (11.4) | |
| Diarrhea | 1 (2.9) | 1 (2.9) | |
| Dysgeusia (taste alteration) | 4 (11.4) | 4 (11.4) | |
| Increased liver enzymes | 5 (14.3) | 2 (5.7) | 7 (20.0) |
| Increased creatinine | 4 (11.4) | 4 (11.4) | |
| Hyperglycemia | 1 (2.9) | 1 (2.9) | 2 (5.7) |
| Nausea | 12 (34.3) | 1 (2.9) | 13 (37.1) |
| Vomiting (emesis) | 9 (25.7) | 9 (25.7) | |
| Weight loss (anorexia) | 2 (5.7) | 2 (5.7) | |
| Central nervous system | |||
| Seizures | 4 (11.4) | 4 (11.4) | |
| Speech impairment | 3 (8.6) | 3 (8.6) | |
| Ataxia | 4 (11.4) | 4 (11.4) | |
| Muscle atrophy/weakness | 3 (8.6) | 1 (2.9) | 4 (11.4) |
| Neuropathy | 5 (14.3) | 5 (14.3) | |
| Cognitive disturbance | 4 (11.4) | 4 (11.4) | |
| Confusion | 3 (8.6) | 3 (8.6) | |
| Mood (depression/anxiety) | 6 (17.1) | 6 (17.1) | |
| Dizziness | 2 (5.7) | 2 (5.7) | |
| Drowsiness | 2 (5.7) | 2 (5.7) | |
| Headache | 9 | 1 (2.9) | 10 (28.6) |
| Fever | 3 (8.6) | 3 (8.6) | |
| Brain infection | 1 (2.9) | 1 (2.9) | |
| Alopecia | 10 (28.6) | 1 (2.9) | 11 (31.4) |
| Cardiovascular system | |||
| Hypertension | 1 (2.9) | 1 (2.9) | |
| Tachycardia | 1 (2.9) | 1 (2.9) | |
| Respiratory system | |||
| Coughing | 1 (2.9) | 1 (2.9) | |
| Dyspnea | 3 (8.6) | 3 (8.6) | |
| Shortness of breath on exertion | 1 (2.9) | 1 (2.9) | |
Discussion
In this single-arm phase II trial, we investigated for the first time the efficacy and safety of sunitinib in combination with TMZ and RT in newly diagnosed GBM patients with unmethylated MGMT promoter. We believe that our study suggests a potential benefit of combining sunitinib with chemoradiation in newly diagnosed GBM patients with unmethylated MGMT promoter, with reported median PFS of 7.15 months and median OS of 15.0 months. It also highlights the prospect that NLR may be used as a noninvasive biomarker to identify the subgroup of GBM patients who might respond to combination of sunitinib with chemoradiation. Indeed, the addition of sunitinib modestly increased OS, particularly in those with post-concurrent NLR ≤6 (responders) who had a median OS of 18.5 months (CI: 14.4–23.7) compared with 11.9 (CI 9.1–14.4) in those with NLR >6 (P = .037). In a recent meta-analysis of outcomes in GBM patients with unmethylated MGMT promoter treated with standard chemoradiation, median PFS and OS estimates were 4.99 months (95% CI: 4.25–5.72) and 14.11 months (95% CI: 13.18–15.04), respectively.32 Thus, our results are encouraging and provide a strong clinical rationale to further test the benefits of Sunitinib addition to the standard care chemoradiation, notably in the context of a randomized phase II/III trial. A prior phase II study has investigated the role of sunitinib as a single agent in the treatment of recurrent GBM regardless of MGMT promoter methylation.18 The authors reported a minimal activity in recurrent GBM with significant toxicity.18,19 The difference between recurrent and newly diagnosed GBM with respect to pathophysiology, molecular profile, and behavior argues for the addition of sunitinib in the first-line management of GBM patients with unmethylated MGMT before the onset of tumor resistance at the time of recurrence.
Two large, randomized control trials (RCTs), RTOG 0825 and Avaglio (BO21990) trials, studied the addition of another antiangiogenic drug, Bevacizumab, to chemoradiation in newly diagnosed GBM patients independently of their MGMT methylation status.33,34 In RTOG 0825, no difference was found between arms for OS (median 16.1 vs 15.7 months, P = .11) while the PFS was extended in the Bevacizumab arm (7.3 vs 10.7 months, P = .004).33 Likewise, the Avaglio trial showed a significant difference in PFS in the Bevacizumab arm (10.6 vs 6.2 months; P < .001) but no benefit in OS (median 16.8 vs 16.7 months, P = .10).34 MGMT status did not predict selective benefit for Bevacizumab in both studies. It is important to note, however, that a direct comparison cannot be made with these larger phase 3 RCTs given the small patient population and phase II design of our study.
We also investigated clinical prognostic factors potentially associated with PFS and OS in our cohort. We identified factors that were associated with improved OS such as age <65, receiving >3 cycles of adjuvant TMZ, and reoperation at the time of tumor progression. Interestingly, having received >3 cycles of adjuvant TMZ was significantly associated with decreased risk of death with an adjusted HR of 0.197 (95% CI: 0.07–0.53, P = .001, Table 3A) and improved PFS with a HR of 0.228 (95% CI: 0.08–0.63, P = .001, Table 3B). Since the majority of patients received a full course of RT and concurrent TMZ without interruption (94.3% of patients completed 60 Gy of RT, and only 1/35 patients had interrupted TMZ during the concurrent phase), we hypothesize that the only variable that could affect outcomes is the number of adjuvant TMZ cycles they have received. This benefit from adjuvant TMZ was a surprising finding given that it is well established that GBM patients with unmethylated MGMT promoter derive limited benefit from TMZ5,35–37 and there have even been suggestions to remove TMZ from the treatment regimen for these patients.38–40 A recent meta-analysis attempted to characterize the benefit of TMZ in MGMT promoter unmethylated and methylated GBM, but unfortunately a direct comparison was not possible because of the paucity of PFS and OS data for unmethylated patients.32
Importantly, we assessed the use of NLR noninvasively as a systemic inflammation biomarker of GBM progression21,23,31 and response to sunitinib treatment in another cancer.24,25 We evaluated the association of NLR in a longitudinal analysis at different time points with survival outcomes in newly diagnosed GBM patients receiving sunitinib with concurrent chemoradiation. Indeed, post-concurrent NLR ≤6 was strongly associated with improved OS (HR = 0.38, 95% CI: 0.166–0.864, P = .021) in multivariable Cox regression analysis (Table 3B). A study by Bambury et al.21 assessing the prognostic impact of NLR in 84 GBM patients showed that patients with NLR >4 had a worse median OS (7.5 months) compared with patients with NLR ≤4 (11.2 months) independently of other factors. The median baseline NLR for GBM patients in this study was 3.1 (range 1.1–34.6),21 compared with 5.81 (range 1.00–16.86) in our population. NLR has been shown to be an independent prognostic marker for OS in GBM, even in the pretreatment setting. In a study of 152 GBM patients, Han et al., showed that pretreatment NLR is an independent predictor of OS, with patients with NLR ≥4 having a shorter median OS (10.5 months) compared with those with NLR <4 (17.9 months).23 NLR has also been shown to be a prognostic biomarker for OS in recurrent GBM patients treated with bevacizumab. For instance, in a retrospective study of 103 patients diagnosed with recurrent GBM who underwent treatment with bevacizumab-irinotecan (BEVIRI), the low pretreatment NLR group (cutoff of 3.04) was found to have a longer OS than the high pretreatment NLR group (15.8 vs 9.3 months; P = .015).41 Thus, it appears that NLR has the potential of being a biomarker for antiangiogenic drugs such as bevacizumab and sunitinib, however, more prospective studies are needed. Our results suggest the potential significance of post-concurrent NLR ≤6 as a noninvasive biomarker for sunitinib responders, while an NLR >6 selected nonresponders to sunitinib who might benefit from additional early intervention to prevent GBM progression. The biological mechanisms underlying the association between high NLR and poor survival outcome in cancer patients are poorly understood. In general, the understanding is that a high NLR, characterized by a high neutrophil count and/or low lymphocyte count, reflects both an increased neutrophil-dependent inflammatory reaction and a decreased lymphocyte-mediated antitumor immune response, resulting in a tumor microenvironment that contributes to cancer progression and poor prognosis.31,42 As such, our results provide a working hypothesis to further investigate post-concurrent NLR as a noninvasive, routinely feasible, and inexpensive biomarker to predict response to combined sunitinib with chemoradiation. Conversely, post-concurrent NLR might readily identify patients who require the implementation of an alternative treatment strategy at the end of the concurrent phase.
Finally, we assessed the safety of combining 12.5 mg of sunitinib concurrently with RT and TMZ in this cohort. It is important to note that due to safety concerns, the protocol was amended after 7 patients were recruited to reduce the sunitinib dose from 25 to 12.5 mg daily. Notably, 1 patient experienced grade 3 thrombocytopenia and another grade 4 thrombocytopenia and a pulmonary embolus. The reduced dose of 12.5 mg Sunitinib was overall better tolerated although the rate of grade 3–4 hematological toxicities of 22.9% was higher than in previous reports, for instance, compared with rates of 16% in the Stupp et al. cohort.1 We believe this increased toxicity is potentially due to the interactions between Sunitinib with TMZ and RT. 14.3% of patients in our study developed grade 3–4 thrombotic events of which 3 cases of deep vein thrombosis (8.6%, grade 3) and 2 cases of pulmonary embolism (5.7%, grade 4). In other reports, the combination of an antiangiogenic drug such as bevacizumab with chemoradiation yielded grade ≥3 venous thromboembolism rates of 7.6% in the bevacizumab arm vs 8.0% in the placebo arm, and a rate of grade ≥3 arterial thromboembolitic events of 5.0% vs 1.3%, respectively, with 1 fatal arterial thromboembolism in each group.34 Importantly, we did not record any grade ≥3 cardiovascular or respiratory AEs and no grade 5 events in our cohort.
There are several limitations to this trial, including the fact that the study was underpowered due to challenges in recruitment, potential selection bias, toxicity of Sunitinib, and the absence of a direct comparison arm. Nonetheless, these results are hypothesis generating and provide a basis for further investigations to explore the relationship between sunitinib and TMZ in unmethylated MGMT GBM patients and the role of NLR as a noninvasive biomarker to identify responders to this combination therapy.
Conclusion and Perspectives
The results of this phase II trial suggest a potential benefit of combining sunitinib with chemoradiation in newly diagnosed GBM patients with unmethylated MGMT promoter and provide a strong rationale for testing this further in a randomized study design. Interestingly, our study also suggests that sunitinib combination with chemoradiation potentially sensitizes unmethylated GBM patients to further adjuvant TMZ and paves the way to further investigate NLR as a potential noninvasive biomarker to identify responders to combination of sunitinib with chemoradiation.
Supplementary Material
Acknowledgments
We would like to thank all those who have contributed to the completion of this study, in particular all the patients and clinical research coordinators.
Contributor Information
Mame Daro Faye, Division of Radiation Oncology, Mcgill University Health Centre.
Jacob Easaw, Department of Oncology, Cross Cancer Institute.
Paula De Robles, Department of Oncology, Cross Cancer Institute.
Raman Agnihotram, Department of Oncology, McGill University Health Centre Research Institute.
Alexander Torres-Vasquez, Department of Oncology, McGill University Health Centre Research Institute.
Frederic Lamonde, Department of Oncology, McGill University Health Centre Research Institute.
Kevin Petrecca, Division of Neurosurgery, McGill University Health Centre.
Scott Owen, Department of Oncology, McGill University Health Centre Research Institute.
Valerie Panet-Raymond, Division of Radiation Oncology, Mcgill University Health Centre.
George Shenouda, Division of Radiation Oncology, Mcgill University Health Centre.
Luis Souhami, Division of Radiation Oncology, Mcgill University Health Centre.
Maryam Azam, Centre for Translational Biology, The Research Institute of McGill University Health Centre, Montreal, Quebec, Canada.
Bushra Hossain, Centre for Translational Biology, The Research Institute of McGill University Health Centre, Montreal, Quebec, Canada.
Jad Alkass, Centre for Translational Biology, The Research Institute of McGill University Health Centre, Montreal, Quebec, Canada.
Siham Sabri, Centre for Translational Biology, The Research Institute of McGill University Health Centre, Montreal, Quebec, Canada.
Bassam Abdulkarim, Division of Radiation Oncology, Mcgill University Health Centre; Centre for Translational Biology, The Research Institute of McGill University Health Centre, Montreal, Quebec, Canada.
Funding
This study was funded by an investigator-initiated research grant from Pfizer (to B.A.) and CCSRI-Innovation grant (to S.S.).
Conflict of interest statement
None declared.
Pfizer provided funding but was not involved in the design, recruitment, and analysis of data in this clinical trial.
Authorship statement
B.A., S.S., and J.E. designed and drafted the study protocol. M.D.F, M.A., B.H., J.A., B.A., and S.S. performed the data collection and analysis. B.A., J.E., P.D.R., K.P., S.O., V.P.R., G.S., and L.S. participated in patient recruitment, review, editing, and approval of the manuscript. R.A., A.T.V., and F.L. performed all statistical analysis. M.D.F., B.A., and S.S wrote the manuscript and all authors participated in the review and approval of the manuscript.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 3. Laperriere N, Zuraw L, Cairncross G; Cancer Care Ontario Practice Guidelines Initiative Neuro-Oncology Disease Site G. Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiother Oncol. 2002;64(3):259–273. [DOI] [PubMed] [Google Scholar]
- 4. Paszat L, Laperriere N, Groome P, et al. A population-based study of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;51(1):100–107. [DOI] [PubMed] [Google Scholar]
- 5. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 6. Hardee ME, Zagzag D.. Mechanisms of glioma-associated neovascularization. Am J Pathol. 2012;181(4):1126–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilbert MR. Antiangiogenic therapy for glioblastoma: complex biology and complicated results. J Clin Oncol. 2016;34(14):1567–1569. [DOI] [PubMed] [Google Scholar]
- 8. de Bouard S, Herlin P, Christensen JG, et al. Antiangiogenic and anti-invasive effects of sunitinib on experimental human glioblastoma. Neuro Oncol. 2007;9(4):412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dunn IF, Heese O, Black PM.. Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J Neurooncol. 2000;50(1–2):121–137. [DOI] [PubMed] [Google Scholar]
- 10. Byrne AM, Bouchier-Hayes DJ, Harmey JH.. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med. 2005;9(4):777–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D.. Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol. 2005;15(4):297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327–337. [PubMed] [Google Scholar]
- 13. Motzer RJ, Michaelson MD, Rosenberg J, et al. Sunitinib efficacy against advanced renal cell carcinoma. J Urol. 2007;178(5):1883–1887. [DOI] [PubMed] [Google Scholar]
- 14. Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. [DOI] [PubMed] [Google Scholar]
- 15. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–1338. [DOI] [PubMed] [Google Scholar]
- 16. Verhaak RG, Hoadley KA, Purdom E, et al. ; Cancer Genome Atlas Research Network. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schueneman AJ, Himmelfarb E, Geng L, et al. SU11248 maintenance therapy prevents tumor regrowth after fractionated irradiation of murine tumor models. Cancer Res. 2003;63(14):4009–4016. [PubMed] [Google Scholar]
- 18. Hutterer M, Nowosielski M, Haybaeck J, et al. A single-arm phase II Austrian/German multicenter trial on continuous daily sunitinib in primary glioblastoma at first recurrence (SURGE 01-07). Neuro Oncol. 2014;16(1):92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grisanti S, Ferrari VD, Buglione M, et al. ; Gruppo Neuro-Oncologico Bresciano. Second line treatment of recurrent glioblastoma with sunitinib: results of a phase II study and systematic review of literature. J Neurosurg Sci. 2016;63(4):458–467. [DOI] [PubMed] [Google Scholar]
- 20. Chahal M, Xu Y, Lesniak D, et al. MGMT modulates glioblastoma angiogenesis and response to the tyrosine kinase inhibitor sunitinib. Neuro Oncol. 2010;12(8):822–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bambury RM, Teo MY, Power DG, et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol. 2013;114(1):149–154. [DOI] [PubMed] [Google Scholar]
- 22. McNamara MG, Lwin Z, Jiang H, et al. Factors impacting survival following second surgery in patients with glioblastoma in the temozolomide treatment era, incorporating neutrophil/lymphocyte ratio and time to first progression. J Neurooncol. 2014;117(1):147–152. [DOI] [PubMed] [Google Scholar]
- 23. Han S, Liu Y, Li Q, et al. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer. 2015;15(1):617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park YH, Ku JH, Kwak C, Kim HH.. Post-treatment neutrophil-to-lymphocyte ratio in predicting prognosis in patients with metastatic clear cell renal cell carcinoma receiving sunitinib as first line therapy. Springerplus. 2014;3(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keizman D, Ish-Shalom M, Huang P, et al. The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer. 2012;48(2):202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 27. Lee EQ, Chukwueke UN, Hervey-Jumper SL, et al. Barriers to accrual and enrollment in brain tumor trials. Neuro Oncol. 2019;21(9):1100–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaplan EL, Meier P.. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 29. Cox DR. Regression models and life-tables. J R Stat Soc Ser B Methodol. 1972;34(2):187–202. [Google Scholar]
- 30. Ly KI, Vakulenko-Lagun B, Emblem KE, et al. Probing tumor microenvironment in patients with newly diagnosed glioblastoma during chemoradiation and adjuvant temozolomide with functional MRI. Sci Rep. 2018;8(1):17062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. [DOI] [PubMed] [Google Scholar]
- 32. Alnahhas I, Alsawas M, Rayi A, et al. Characterizing benefit from temozolomide in MGMT promoter unmethylated and methylated glioblastoma: a systematic review and meta-analysis. Neurooncol Adv. 2020;2(1):vdaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gilbert MR, Dignam J, Won M, et al. RTOG 0825: phase III double-blind placebo-controlled trial evaluating bevacizumab (Bev) in patients (Pts) with newly diagnosed glioblastoma (GBM). J Clin Oncol. 2013;31(18_suppl):1.23129739 [Google Scholar]
- 34. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 35. Hegi ME, Diserens AC, Godard S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10(6):1871–1874. [DOI] [PubMed] [Google Scholar]
- 36. Malmstrom A, Gronberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG). Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 37. Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 38. Hegi ME, Stupp R.. Withholding temozolomide in glioblastoma patients with unmethylated MGMT promoter--still a dilemma? Neuro Oncol. 2015;17(11):1425–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hegi ME, Genbrugge E, Gorlia T, et al. MGMT promoter methylation cutoff with safety margin for selecting glioblastoma patients into trials omitting temozolomide: a pooled analysis of four clinical trials. Clin Cancer Res. 2019;25(6):1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kamson DO, Grossman SA.. The role of temozolomide in patients with newly diagnosed wild-type IDH, unmethylated MGMTp glioblastoma during the COVID-19 pandemic. JAMA Oncol. 2021;7(5):675–676. [DOI] [PubMed] [Google Scholar]
- 41. Haksoyler V, Besen AA, Koseci T, et al. Neutrophil-to-lymphocyte ratio is prognostic in recurrent glioblastoma multiforme treated with bevacizumab plus irinotecan. Biomarkers Med. 2021;15(11):851–859. [DOI] [PubMed] [Google Scholar]
- 42. Jiang T, Qiao M, Zhao C, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with outcome of advanced-stage cancer patients treated with immunotherapy: a meta-analysis. Cancer Immunol Immunother. 2018;67(5):713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.