Abstract
Objective:
To understand the consequences of functional cardiac stress testing among patients considering noncardiac nonophthalmologic surgery.
Design:
A retrospective cohort study of 118,552 patients who made 159,795 visits to a dedicated preoperative risk assessment and optimization clinic between 2008 and 2018.
Setting:
A large integrated health system.
Patients:
Patients who visited a dedicated preoperative risk assessment and optimization clinic before noncardiac nonophthalmologic surgery.
Measurements:
To assess changes to care delivered, we measured the probability of completing additional cardiac testing, cardiac surgery, or noncardiac surgery. To assess outcomes, we measured time-to-mortality and total one-year mortality.
Main Results:
In causal inference models, preoperative stress testing was associated with increased likelihood of coronary angiography (relative risk: 8.6, 95% CI 6.1–12.1), increased likelihood of percutaneous coronary intervention (RR: 4.1, 95% CI: 1.8–9.2), increased likelihood of cardiac surgery (RR: 6.8, 95% CI 4.9–9.4), decreased likelihood of noncardiac surgery (RR: 0.77, 95% CI 0.75–0.79), and delayed noncardiac surgery for patients completing noncardiac surgery (mean 28.3 days, 95% CI: 23.1–33.6). The base rate of downstream cardiac testing was low, and absolute risk increases were small. Stress testing was associated with higher mortality in unadjusted analysis but was not associated with mortality in causal inference analyses.
Conclusions:
Preoperative cardiac stress testing likely induces coronary angiography and cardiac interventions while decreasing use of noncardiac surgery and delaying surgery for patients who ultimately proceed to noncardiac surgery. Despite changes to processes of care, our results do not support a causal relationship between stress testing and postoperative mortality. Analyses of care cascades should consider care that is avoided or substituted in addition to care that is induced.
Keywords: Preoperative period, Perioperative care, Diagnostic techniques, cardiovascular, Outcome and Process Assessment
Introduction
The 2014 American College of Cardiology and American Heart Association (ACC/AHA) guidelines recommend preoperative stress testing for patients whose estimated risk of a major adverse cardiac event exceeds 1% and whose functional status is poor or unknown, when results from stress testing would change clinical management.[1] Precisely how clinical management changes following preoperative cardiac stress testing remains unknown.
In many settings, testing appears to promote additional downstream testing.[2–4] For example, a 12-lead electrocardiogram (ECG) before cataract surgery is associated with other preoperative testing and spending ten-fold higher than the initial ECG.[2] Similar cascades of care or other process changes may follow preoperative cardiac stress testing.
Whether preoperative stress testing changes health outcomes is also unclear. Administrative data suggest an association between preoperative stress testing and lower mortality among patients completing surgery.[5] However, because stress testing is not therapeutic, any causal impact of cardiac stress testing on mortality must be mediated by one or more downstream interventions used differently as a result of stress testing. Each potential intervention following stress testing, including surgery, angiography, and revascularization, carries different benefits and harms; changes to processes of preoperative care could change patient mortality.[1,6,7]
We therefore sought to understand how clinical management and patient outcomes might change following preoperative cardiac stress testing. In particular, we considered how preoperative stress testing was related to subsequent rates of coronary angiography, coronary revascularization, cancelled noncardiac surgery, delayed surgery, and mortality.
Methods
Patient population and characteristics
The Internal Medicine Preoperative Assessment, Consultation and Treatment (IMPACT) Center assessed patients prior to noncardiac nonophthalmologic surgery at the Cleveland Clinic. We included each visit to this clinic between 2008 and 2018 in our cohort. Descriptions of patient and visit characteristics can be found in Table 1 and previous work.[6] All analyses were approved by the Cleveland Clinic Institutional Review Board.
Table 1.
Patient and visit characteristics
| Total visits | Did not proceed to stress testing | Proceeded to stress testing | |
|---|---|---|---|
| N | 159,795 | 152,587 | 7,208 |
| Age (years) | |||
| Median | 60.4 | 60.1 | 65.6 |
| Interquartile range | 49.2–69.7 | 48.9–69.5 | 56.7–73.6 |
| Male sex (%) | 44.5 | 44.1 | 51.5 |
| Body-mass index | |||
| Median | 28.8 | 28.8 | 29.7 |
| Interquartile range | 24.8–34.0 | 24.5–33.9 | 25.7–35.0 |
| Use of tobacco (%) | |||
| Former | 40.7 | 40.3 | 49.0 |
| Current | 12.3 | 12.3 | 13.2 |
| Diabetes (%) | 23.2 | 22.5 | 37.6 |
| Congestive heart failure (%) | 8.3 | 7.8 | 17.6 |
| Systolic BP (mmHg) | |||
| Median | 129 | 128 | 131 |
| Interquartile range | 116–142 | 116–142 | 118–145 |
| Total cholesterol | |||
| Median | 176 | 176 | 168 |
| Interquartile range | 149–204 | 149–204 | 141–197 |
| HDL cholesterol | |||
| Median | 51 | 51 | 49 |
| Interquartile range | 41–63 | 41–63 | 39–61 |
| Pretest probability of obstructive CAD (%) | |||
| Median | 7.9 | 7.7 | 13.4 |
| Interquartile range | 3.0–18.3 | 2.9–17.9 | 6.0–26.3 |
| Revised cardiac risk index | |||
| Median | 0 | 0 | 1 |
| Interquartile range | 0–1 | 0–1 | 0–2 |
| Myocardial infarction or cardiac arrest risk estimate (%) | |||
| Median | 1.2 | 1.1 | 4.1 |
| Interquartile range | 0.4–3.1 | 0.4–2.8 | 2.5–5.5 |
| Subsequent testing and interventions (%) | |||
| Coronary angiography | 0.12 | 0.08 | 0.93 |
| Cardiac surgery | 0.18 | 0.14 | 1.07 |
| Major noncardiac surgery | 73.5 | 74.6 | 50.7 |
| Time to surgery (days) | |||
| Median | 7 | 7 | 11 |
| Interquartile range | 3–13 | 3–13 | 6–22 |
Process Measures
To investigate changes in care processes, we first identified the next noncardiac surgery after each visit to IMPACT. Next, we identified any cardiac stress tests, coronary angiographies, percutaneous coronary interventions (PCI), and cardiac surgeries following each visit, censoring on (1) the date of noncardiac surgery, (2) the date of any subsequent visit to IMPACT, or (3) three years after a visit to IMPACT. We identified the sequence of tests/interventions in the study period and calculated the number of days between each test/intervention. We censored each visit if more than 365 days elapsed without any of the tests or interventions considered here.
For each visit, we calculated the probability of preoperative stress testing using a multilevel multiply-imputed logistic regression model clustered by patient with an indicator variable for physician. Covariates included variables associated with referral for stress testing from this clinic, including estimated perioperative cardiac risk, patient functional status, surgical subtypes, and previous patient diagnoses. Details of this model, including our multiple imputation with chained equations, are included in the Supplemental Appendix; our modeling approach was published previously.[6] Our unit of analysis here was visits, not patients; patients who made multiple visits to IMPACT could appear multiple times.
To estimate the effect of stress testing on rates of coronary angiography, we used an inverse probability weighted model with regression adjustment to estimate the average treatment effect of preoperative stress testing, compared to no stress testing, on angiography. This “doubly-robust” approach may produce accurate estimates even if one of the two models is misspecified, and therefore reduce sensitivity of results to model specification.[8,9] Our outcome model controlled for the probability of obstructive CAD.
To estimate the effect of stress testing on rates of revascularization and cardiac surgery, we used similar models to estimate the average treatment effect of preoperative stress testing, compared to no stress testing, on PCI and cardiac surgery. Because stress testing could prompt cardiac surgeries other than revascularization, we considered any cardiac surgery in this analysis. Each model used a doubly-robust inverse probability weighted design and controlled for probability of obstructive CAD in the outcome model, similar to our analyses of angiography.
Our analysis of completed noncardiac surgery was similar: a doubly-robust inverse probability weighted model to estimate the average treatment effect of preoperative stress testing, compared to no stress testing, on surgery completion rate. To control for differential completion of different proposed surgeries, we included the CPT code of the considered surgery in the outcome model.
We then investigated the causal effect of stress testing on time to surgical completion. Because (1) probability of surgical completion is analyzed separately, (2) patients who never complete surgery would lead to inflated measures of delay, and (3) delay is only meaningful when patients complete surgery, we included only patients who completed surgery in this analysis. We fit an accelerated failure time model in this subset, selecting the parameterization that resulted in the lowest Akaike’s information criterion (AIC) from exponential, Weibull, and lognormal models. We then used an inverse-probability weighted estimator with regression adjustment, with the outcome (time to surgery) modeled as a function of considered CPT code and probability of preoperative testing, to estimate the causal effect of stress testing on time to surgery. Because this analysis is conditional on a future event (completion of surgery) its results should only be interpreted in conjunction with our model predicting likelihood of completion.
Mortality
We obtained dates of death from the state of Ohio and the Social Security death index through the 2021 calendar year. To investigate the relationship between stress testing and mortality, we used a time-to-event analysis indexed to the clinic visit and tested the association between preoperative stress testing and mortality. Upon testing Schoenfeld residuals, we found that the Cox proportional hazards assumption was violated in our data. We therefore used an accelerated failure time model, selecting the parameterization that resulted in the lowest Akaike’s information criterion (AIC) from exponential, Weibull, and lognormal models. A log-log plot of survival probability by analysis time, demonstrating failure of the proportional hazards assumption, is included in the Supplemental Appendix. We then used an inverse-probability weighted estimator with regression adjustment, with the outcome (time to mortality) modeled as a function of age, sex, and probability of preoperative testing, to estimate the causal effect of stress testing on time to mortality.
As an alternate approach to the time-to-event analysis, we used an augmented inverse probability weighted model to estimate the effect of preoperative stress testing on mortality within the 1 year following the clinic visit while controlling for the probability of preoperative stress testing, similar to the analyses of process changes described above. In this analysis, our outcome model included age, sex, and probability of stress testing.
Differential cancellation of different surgeries
We then investigated whether different surgeries that had been considered were more or less likely to be cancelled as a result of cardiac stress testing than the average surgery. To do so, we extracted the primary Current Procedural Terminology (CPT) code that had been designated by the surgeon at the time of the IMPACT visit and added the primary CPT code of the considered surgery as an indicator variable in our inverse probability weighted model predicting likelihood of noncardiac surgery. To avoid perfect predictions in less-commonly-performed surgeries, we tested only primary CPT codes that were considered at 1,000 or more visits.
To impute missing CPT codes, we used a natural language processing technique (vectorization) to compare the brief procedure description in those cases (e.g., “lap chole”) against the brief descriptions of procedures with designated CPT codes, selecting the best match as the primary CPT code for the missing observation.[10] Further detail on this step can be found in the Supplemental Appendix. We used python (version 3.7.8) and existing open software libraries (including spacy.io) for natural language processing and vectorization. All other analyses were performed in Stata (version 16.2; College Station, TX).
Results
From 2008 through 2018, 118,552 patients made 159,795 visits to IMPACT. Of those visits, 7,208 included cardiac stress testing as the next test or intervention. A total of 117,447 visits proceeded to noncardiac surgery before censoring. A flow diagram showing sequences of testing between a clinic visit and surgery is shown in Figure 1. Uncommon and retrograde sequences of testing (e.g., angiography followed by subsequent stress testing) are not diagrammed. Absolute and relative effects for stress testing on rates of four major process outcomes (angiography, PCI, cardiac surgery, and noncardiac surgery) are summarized in Table 2.
Figure 1.

Patient flow diagram.
Table 2.
Relative and absolute risks of process outcomes with cardiac stress testing in causal effect models.
| Absolute effect | Relative risk | |||
|---|---|---|---|---|
| Mean | 95% Cl | Mean | 95% Cl | |
| Coronary angiography | 0.6% | (0.4% to 0.7%) | 8.6 | (6.1 – 12.1) |
| Percutaneous coronary intervention | 0.1% | (0.0% to 0.1%) | 4.1 | (1.8 – 9.2) |
| Cardiac surgery | 0.7% | (0.5% to 0.9%) | 6.8 | (4.9 – 9.4) |
| Noncardiac surgery | −16.1% | (−17.6% to −14.6%) | 0.77 | (0.75 – 0.79) |
Preoperative stress testing increased the probability of coronary angiography, with an absolute risk increase of 0.6% (95% CI: 0.4% – 0.7%), and a relative risk of 8.6 (95% CI: 6.1 – 12.1).
Preoperative stress testing increased the probability of PCI, with an absolute risk increase of 0.1% (95% CI: 0.0% to 0.1%; p=0.043) and a relative risk of 4.1 (95% CI: 1.8 – 9.2). A total of 46 IMPACT visits were followed by PCI before censoring, all of which (necessarily) included angiography.
Preoperative stress testing increased the likelihood of cardiac surgery, with an absolute risk increase of 0.7% (95% CI: 0.5% – 0.9%), and a relative risk of 6.8 (95% CI: 4.9 – 9.4).
Preoperative stress testing decreased the probability of completing noncardiac surgery, with an absolute risk decrease of 16.1% (95% CI: 14.6% to 17.6%) and a relative risk of 0.77 (95% CI: 0.75 – 0.79).
Completion of a first preoperative stress test appeared to increase the likelihood of further stress testing. Specifically, patients who completed a first stress test had an absolute risk increase of 20.4% (95% CI: 19.3% – 21.6%) for subsequent stress testing, compared to the counterfactual of never having been tested.
When patients proceeded to surgery, stress testing incurred delay. Patients who completed surgery without preoperative stress testing had surgery a median of 7 days after their clinic visit (IQR: 3–13 days, mean 20.4 days), while patients who first completed testing had surgery a median of 11 days after their clinic visit (IQR: 6–22 days, mean 88.3 days). In our causal inference model, preoperative stress testing appeared to increase time-to-surgery by a mean of 28.3 days (95% CI: 23.1–33.6 days).
We identified 33,746 deaths over a total of 1,158,442 years at risk, for an overall mortality incidence rate of 2.9 deaths per 100 person-years. The median time at risk was 7.1 years. A seven-year survival curve is included in the Supplemental Appendix. In our time-to-mortality analyses, a lognormal model resulted in the lowest AIC. In our unadjusted analysis, preoperative stress testing was associated with time-to-mortality 0.60 years shorter (95% CI: −0.66 to −0.53) compared to visits without preoperative stress testing. In our causal inference time-to-event model, preoperative stress testing did not appear to influence time-to-mortality, with a mean time-to-mortality 0.18 years longer (95% CI: − 0.26 to 0.62, p>0.2).
We identified 6,140 deaths in the year following a visit to IMPACT (overall one-year mortality: 3.8%). Visits followed by stress testing had a higher one-year mortality rate (4.9%) than those who did not (3.8%). A survival curve for the one year after a clinic visit is shown in Figure 2, and survival curves by quintile of stress test probability, to demonstrate confounding by indication, are shown in Figure 3. In our causal effect logistic regression model, stress testing was not associated with a difference in one-year mortality, with an estimated absolute effect of −0.0% (95% CI: −0.6% to 0.4%, p>0.2).
Figure 2.
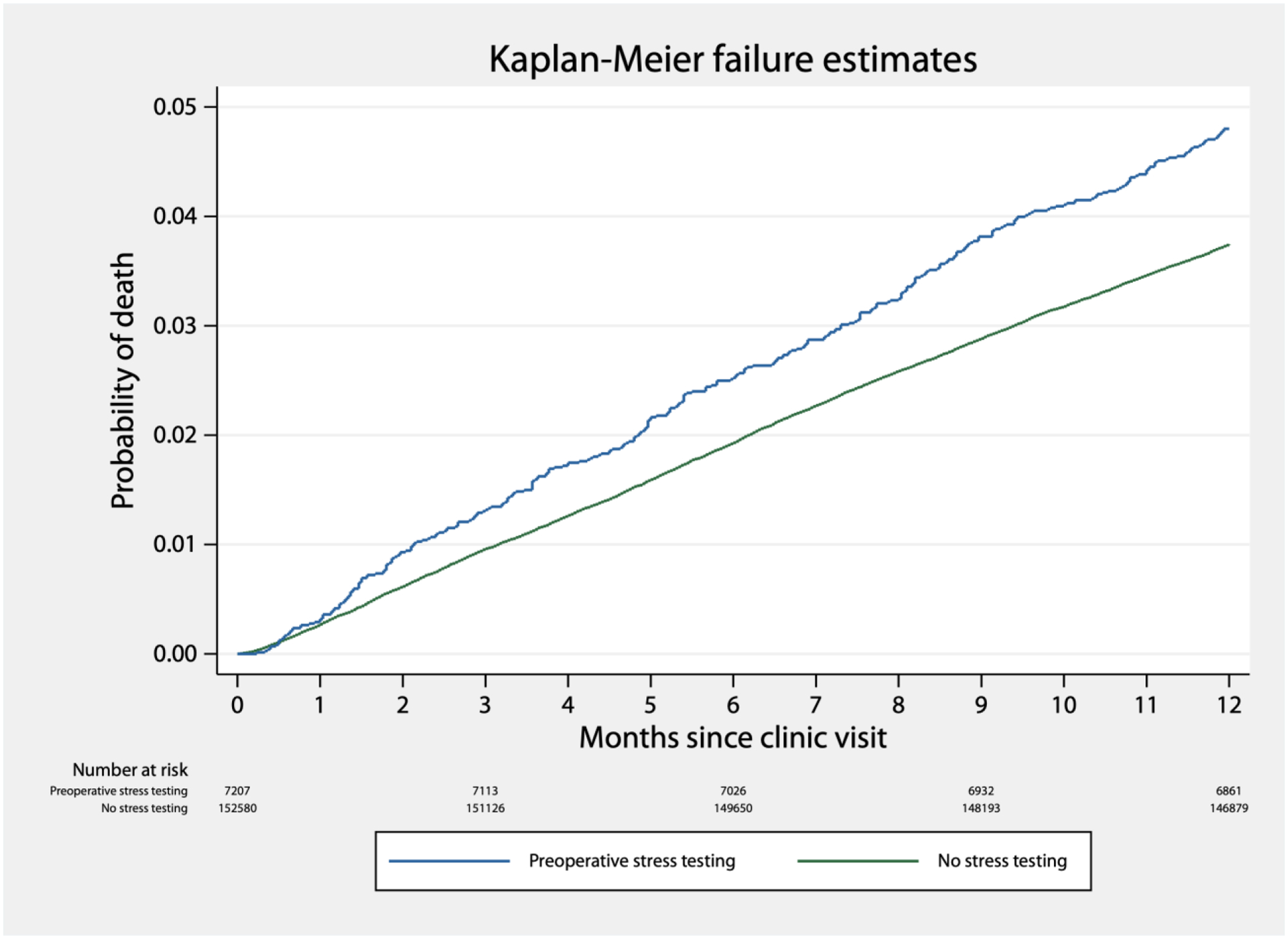
Kaplan-Meier failure estimates in the year following an IMPACT clinic visit, by completion of preoperative cardiac stress testing.
Figure 3.
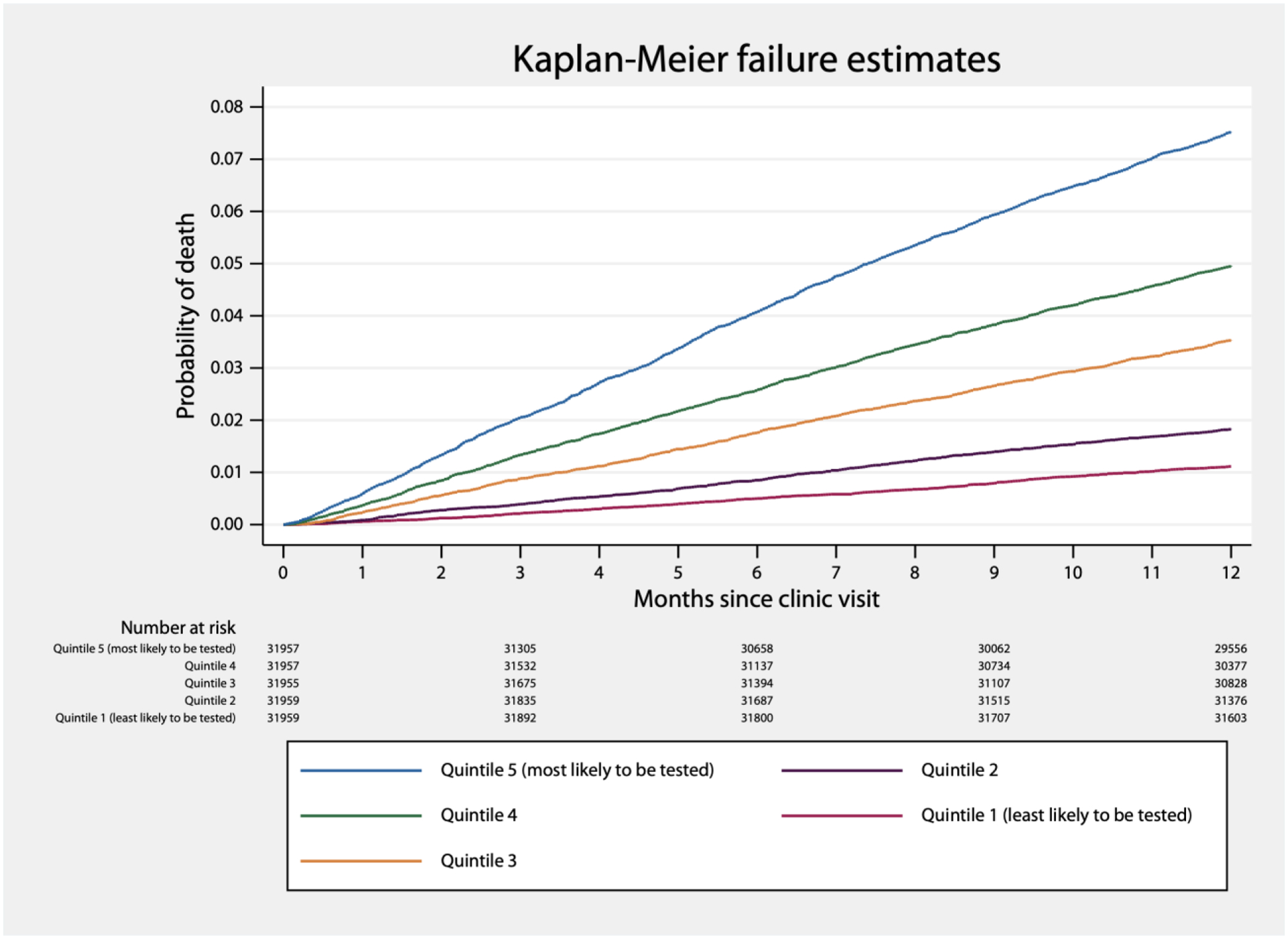
Kaplan-Meier failure estimates in the year following an IMPACT clinic visit, by binned probability of preoperative cardiac stress testing.
For 27,750 visits, no CPT code had been designated. We were able to “impute” 27,691 surgeries using vectorization. At least one surgery, partial mastectomy, was more likely than average to be cancelled as a result of stress testing. Others, including total hip arthroplasty and laparoscopic prostatectomy, were either less likely to be cancelled or demonstrated no reduction in surgery completion rate as a result of stress testing. Results from our analysis for heterogeneity of treatment effect according to considered surgery are included in the Supplemental Appendix.
Discussion
In this cohort of patients seen for preoperative risk assessment and optimization at a large integrated health system, we found that preoperative cardiac stress testing increased the likelihood of coronary angiography and cardiac surgery, decreased the likelihood of noncardiac surgery, and delayed surgical intervention for patients who completed noncardiac surgery. Initial stress testing also increased future stress testing. Despite these process changes, preoperative cardiac stress testing did not appear to change mortality.
In addition to our main findings, we demonstrated an association between preoperative stress testing and mortality, which association appears to be confounding by indication. Patients who are more likely to undergo stress testing are more likely to die; after controlling for the probability of testing in causal effect models, there did not appear to be a relationship between stress testing and mortality.
Previous work has shown that other preoperative tests are associated with downstream testing.[2,3] Our analyses extend this work in multiple ways. First, previous work has largely focused on services generally accepted to be of low value; we demonstrate a care cascade after preoperative stress testing in a population considering noncardiac nonophthalmologic surgery. Second, whereas previous work on care cascades relied on administrative databases and selected patients based on completion of surgery, we started with a visit to a clinic made as a routine part of care before noncardiac, nonophthalmologic surgery, thereby capturing patients who considered but did not complete surgery. This is a key strength of our study because identifying a cohort based on completed surgery (which necessarily comes after preoperative stress testing) conditions any such analysis on a subsequent event, introducing selection bias.[11–13] By starting with a cohort considering noncardiac surgery, we add that patients stress tested when considering surgery are less likely to complete their operation. Therefore, the care process changes we identified after stress testing may be better thought of as substitution of care than a pure cascade: stress testing generally served to replace noncardiac surgery with coronary angiography and cardiac surgery.
Current guidelines suggest that preoperative stress testing may be used among selected patients when results from stress testing would change clinical management.[1] Our study informs precisely how clinical management changed consequent to stress testing, and could inform future guidelines. To the extent that the surgeries under consideration were likely to improve patient health and function, the reduced likelihood of surgery because of stress testing represents forfeited benefit. Whether the overall net effect of stress testing is beneficial or harmful therefore depends not simply on variables included in current guidance meant to assess the risks of surgery, but on the expected benefits of surgery as well. For example, replacing breast cancer resection with cardiac testing would forfeit different health benefits than replacing cosmetic breast surgery. In our analysis of specific surgeries, care substitutions were not clearly related to near-term benefits, with some surgeries with likely near-term health benefits (e.g., parathyroidectomy, partial mastectomy/lumpectomy) cancelled more frequently than average. Although the process changes before specific procedures may not generalize from our institution to others, physicians considering preoperative stress testing should weigh the potential forfeited benefits of cancelled and delayed surgery if they decide to order stress testing.
Not all testing resulted in substitution; for example, patients considering total colectomy were equally likely to complete surgery regardless of stress testing. In such cases, stress testing adds cardiac testing and interventions to planned surgery, and can be thought of as provoking a care cascade. But our analysis finds no difference in mortality, and the largest trial of preoperative revascularization found no reduction in short- or long-term mortality.[14] It seems unlikely that adding cardiac testing and intervention to such cases will reduce mortality. In such cases, preoperative stress testing would appear to provoke more cardiac testing and interventions without altering the decision to have surgery and without improving mortality.
Moreover, when patients completed surgery, stress testing delayed that operation. Delaying surgical intervention may be appropriate for some conditions: for example, it has long been standard practice to delay surgery for patients with unstable angina or acute decompensated heart failure unless the proposed surgery offers substantial immediate benefit.[15] But stress testing would not be indicated for either acute coronary syndromes or acute decompensated heart failure. The short delay we observe is unlikely to represent substantial forfeited benefit for truly elective surgery. Still, if a considered surgery is expected to reduce morbidity or mortality, stress testing would need to improve surgical decision-making for that delay to be useful or patient-centered.
At least one large previous observational analysis reported an association between stress testing and lower post-operative mortality in patients completing surgery.[5] We found no difference, a discrepancy that may have multiple explanations. First, our cohort is smaller and from a single center; it is possible that a true difference exists that we could not detect. Second, our cohort spans a more recent time period, over which the use of stress testing appears to have declined.[6] It is possible that diminishing use has led to less effective use. Third, our use of causal inference models allowed us to separate the effect of preoperative stress testing from the many variables associated with both increased likelihood of preoperative stress testing and higher likelihood of death. Avoiding this source of confounding may have produced different results in our analysis compared to previous observational work. Fourth, because preoperative stress testing changes what care is delivered (as we demonstrate here), cohorts selected based on surgery performed cannot fully represent patients considering surgery. It is possible that this selection bias produced the apparent mortality benefit in earlier work, while no true effect exists. Alternately, if a true effect exists, our analyses demonstrate that stress testing and cancelled surgery are related to risk of death, so any effect of stress testing on mortality would likely be mediated by cancelled surgery among high-risk patients. In either case, the care process changes we describe could lead to apparent mortality benefit in administrative datasets. Even if a true benefit exists, though, if the goal stress testing is to identify unacceptably risky surgery, there are more efficient and accurate ways to achieve better predictions of surgical risk.[7]
This problem of selection bias is a limitation of other analyses of initial and subsequent testing rates (“cascades of care”) as well. Administrative datasets do not generally capture when services were considered but not completed; this is likely also a limitation of electronic health record (EHR) data outside of specific scenarios like ours, where almost all patients considering noncardiac, nonophthalmologic surgery were seen in a clinic dedicated to this purpose. Future analyses of care cascades may wish to consider what services may be avoided, in addition to what services are induced.
As with health consequences, the financial implications of substituted care are less clear than simple cascades. Future studies of care cascades should not assume increased spending; the net financial effect also depends on what care is avoided. Using comparatively inexpensive testing as a barrier to substantially more expensive surgery could in fact reduce costs. Whether such barriers are appropriate or justified is again specific to the interventions being considered, but barriers to care are generally unhelpful for patient outcomes.[16]
We wish to highlight multiple limitations. First, our data are from a single center; clinical decision-making may differ elsewhere. The specific procedures in our heterogeneity of treatment effect analysis may be especially difficult to generalize to other centers. Second, our data are observational and subject to inherent biases. While our causal effects models attempt to account for the key confounder—likelihood of preoperative stress testing—an unknown degree of bias remains. Third, we focused on the consequences of preoperative stress testing; evaluating clinical rationale would require different methods. Presumably physicians and patients reconsidered the potential benefits of surgery in light of updated cardiovascular risk assessments, but our data are not equipped to examine changes in patient or clinician perceptions.
In summary, cardiac stress testing before noncardiac surgery likely induces angiography and cardiac surgery while reducing rates of noncardiac surgery and delaying surgeries that are completed. Whether those changes in care are appropriate must depend on the anticipated benefits and harms of both the substitute and substituted care. Future analyses of care cascades should consider care that is avoided in addition to care that is provoked. Despite multiple changes to processes of care, preoperative stress testing does not appear to reduce mortality.
Supplementary Material
Highlights.
Cardiac stress testing probably leads to higher rates of cardiac testing and interventions (a “care cascade”)
However, stress testing also decreases the likelihood that patients will complete surgery
Stress testing probably does not change mortality in the short- or long-term
Administrative datasets rarely capture care that is considered but not completed, so many care cascades might be substitutions of care instead
Funding statement:
This work was funded by NHLBI K08HL141598. The authors declare no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures:
None
References
- [1].Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2215–45. 10.1161/CIR.0000000000000105. [DOI] [PubMed] [Google Scholar]
- [2].Ganguli I, Lupo C, Mainor AJ, Raymond S, Wang Q, Orav EJ, et al. Prevalence and Cost of Care Cascades after Low-Value Preoperative Electrocardiogram for Cataract Surgery in Fee-for-Service Medicare Beneficiaries. JAMA Intern Med 2019;179:1211–9. 10.1001/jamainternmed.2019.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bouck Z, Calzavara AJ, Ivers NM, Kerr EA, Chu C, Ferguson J, et al. Association of Low-Value Testing With Subsequent Health Care Use and Clinical Outcomes Among Low-risk Primary Care Outpatients Undergoing an Annual Health Examination. JAMA Intern Med 2020;180:973–83. 10.1001/jamainternmed.2020.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bhatia RS, Bouck Z, Ivers NM, Mecredy G, Singh J, Pendrith C, et al. Electrocardiograms in Low-Risk Patients Undergoing an Annual Health Examination. JAMA Intern Med 2017;177:1326–33. 10.1001/JAMAINTERNMED.2017.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wijeysundera DN, Beattie WS, Elliot RF, Austin PC, Hux JE, Laupacis A. Noninvasive cardiac stress testing before elective major non-cardiac surgery: Population based cohort study. BMJ 2010;340:252. 10.1136/bmj.b5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pappas MA, Sessler DI, Auerbach AD, Kattan MW, Milinovich A, Blackstone EH, et al. Variation in preoperative stress testing by patient, physician and surgical type: a cohort study. BMJ Open 2021;11:e048052. 10.1136/bmjopen-2020-048052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pappas MA, Auerbach AD, Kattan MW, Blackstone EH, Rothberg MB, Sessler DI. Diagnostic and prognostic value of cardiac stress testing before major noncardiac surgery. J Clin Anesth 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Howell S, Stivland TM, Stein K, Ellenbogen K, Tereshchenko LG. Response to cardiac resynchronisation therapy in men and women: a secondary analysis of the SMART-AV randomised controlled trial. BMJ Open 2021;11:3245–79. 10.1136/BMJOPEN-2021-049017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cattaneo MD. Efficient semiparametric estimation of multi-valued treatment effects under ignorability. J Econom 2010;155:138–54. 10.1016/J.JECONOM.2009.09.023. [DOI] [Google Scholar]
- [10].Mikolov T, Chen K, Corrado G, Dean J. Efficient Estimation of Word Representations in Vector Space. International Conference on Learning Representations, ICLR; 2013. [Google Scholar]
- [11].Howe CJ, Cole SR, Lau B, Napravnik S, Eron JJ. Selection Bias Due to Loss to Follow Up in Cohort Studies. Epidemiology 2016;27:91–7. 10.1097/EDE.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology 2004;15:615–25. 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- [13].Hernán MA, Robins JM. Causal Inference: What If? Boca Raton: Chapman & Hall/CRC; 2020. [Google Scholar]
- [14].McFalls EO, Ward HB, Moritz TE, Goldman S, Krupski WC, Littooy F, et al. Coronary-Artery Revascularization before Elective Major Vascular Surgery. N Engl J Med 2004;351:2795–804. 10.1056/NEJMoa041905. [DOI] [PubMed] [Google Scholar]
- [15].Goldman L, Caldera DL, Nussbaum SR, Southwick FS, Krogstad D, Murray B, et al. Multifactorial Index of Cardiac Risk in Noncardiac Surgical Procedures. N Engl J Med 1977;297:845–50. 10.1056/NEJM197710202971601. [DOI] [PubMed] [Google Scholar]
- [16].Kullgren JT, McLaughlin CG, Mitra N, Armstrong K. Nonfinancial barriers and access to care for U.S. adults. Health Serv Res 2012;47:462–85. 10.1111/J.1475-6773.2011.01308.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


