Abstract
Research in African ape sanctuaries has emerged as an important context for our understanding of comparative cognition and behavior. While much of this work has focused on experimental studies of cognition, these animals semi-free-range in forest habitats and therefore can also provide important information about the behavior of primates in socioecologically-relevant naturalistic contexts. In this ‘New Approaches’ article, we describe a project where we implemented a synthetic program of observational data collection at Ngamba Island Chimpanzee Sanctuary in Uganda, directly modeled after long-term data collection protocols at the Kibale Chimpanzee Project in Uganda, a wild chimpanzee field site. The foundation for this project was a strong partnership between sanctuary staff, field site staff, and external researchers. We describe how we developed a data-collection protocol through discussion and collaboration among these groups, and trained sanctuary caregivers to collect novel observational data using these protocols. We use these data as a case study to examine: (1) how behavioral observations in sanctuaries can inform primate welfare and care practices, such as by understanding aggression within the group; (2) how matched observational protocols across sites can inform our understanding of primate behavior across different contexts, including sex differences in social relationships; and (3) how more robust collaborations between foreign researchers and local partners can support capacity-building in primate range countries, along with mentoring and training students more broadly.
Keywords: observations, behavior, sanctuaries, welfare, capacity-building
1. Introduction
In the last decade, African sanctuaries have emerged as an important context for the study of primate cognition and behavior. Primates living in African sanctuaries are typically wild-born orphans of the bushmeat and pet trade and semi-free-range in species-appropriate habitats as part of large social groups, unlike most animals in Western sanctuaries, laboratories, or zoos (Ross & Leinwand, 2020). Such sanctuaries meet or exceed recommended standards for high-quality physical and social environments for captive primates based on their wild conditions (Pruetz & McGrew, 2001), and prior work indicates that these populations have healthy patterns of cognition, behavior, and physiology compared to many other captive contexts (Cole et al., 2020; Rosati et al., 2013; van Leeuwen, Bruinstroop, & Haun, 2023; Wobber & Hare, 2011). To date, several sanctuaries accredited by the Pan African Sanctuary Alliance have supported non-invasive behavioral and health research, including cognitive experiments that would not be possible in wild populations (Ross & Leinwand, 2020; Stokes, Tully, & Rosati, 2017; Stokes, Tully, & Rosati, 2018). This combination of naturalistic ecological and social contexts, along with the possibility of controlled manipulations, means these populations are well-positioned to bridge traditional research approaches in primatology that focus either on experimental approaches with captive animals, or observational approaches in the wild.
To date, much of the research in African sanctuaries using behavioral methods has focused on cognitive experiments. For example, research at several different Pan African Sanctuary Alliance sanctuaries, including Ngamba Island Chimpanzee Sanctuary in Uganda, Tchimpounga Chimpanzee Sanctuary in Republic of Congo, Sweetwaters Chimpanzee Sanctuary in Kenya, and Lola Ya Bonobo Sanctuary in Democratic Republic of Congo, have used experimental tasks to examine how chimpanzees and bonobos think and solve problems. This has included a large variety of work spanning cooperation and prosociality (Bullinger, Melis, & Tomasello, 2011; Engelmann, Herrmann, & Tomasello, 2015; Hare, Melis, Woods, Hastings, & Wrangham, 2007; John, Duguid, Tomasello, & Melis, 2019; Koomen & Herrmann, 2018, 2019; Melis, Hare, & Tomasello, 2006a, 2006b; Rosati, DiNicola, & Buckholtz, 2018; Schneider, Melis, & Tomasello, 2012; Tan, Kwetuenda, & Hare, 2015; Warneken, Hare, Melis, Hanus, & Tomasello, 2007), social learning (Clay & Tennie, 2017; Herrmann, Call, Hernadez-Lloreda, Hare, & Tomasello, 2007; Horner & Whiten, 2005; Tennie, Call, & Tomasello, 2012), social cognition (Krupenye & Hare, 2018; MacLean & Hare, 2012), decision-making (Eckert, Call, Hermes, Herrmann, & Rakoczy, 2018; Eckert, Rakoczy, Call, Herrmann, & Hanus, 2018; Haux, Engelmann, Arslan, Hertwig, & Herrmann, 2023; Herrmann, Misch, Hernandez-Lloreda, & Tomasello, 2015; Keupp, Grueneisen, Ludvig, Warneken, & Melis, 2021; Krupenye, Rosati, & Hare, 2015; Rosati & Hare, 2011, 2012b, 2013; Sánchez-Amaro, Tan, Kaufhold, Fernández-Navarro, & Rossano, 2021; Völter et al., 2022), memory (Rosati, 2019; Rosati & Hare, 2012a), and individual variation in and the development of a variety of cognitive skills (Cantwell, Buckholtz, Atencia, & Rosati, 2022; Herrmann, Hare, Call, & Tomasello, 2010; Herrmann, Hare, Cissewski, & Tomasello, 2011; Herrmann, Hernández-Lloreda, Call, Hare, & Tomasello, 2010; Wobber, Herrmann, Hare, Wrangham, & Tomasello, 2014; Wobber, Wrangham, & Hare, 2010b). These studies typically involve experiments where animals are presented with novel stimuli or problems, such as whether they can discriminate between functional or non-functional tools, or how they may work together on an apparatus to obtain an out-of-reach treat.
There are also several instances where behavioral observations have been implemented in ape sanctuaries. For example, observational research at one chimpanzee sanctuary (Chimfunshi Wildlife Orphanage in Zambia) has used observational methods to examine several aspects of social learning and cultural transfusion within different groups (Rawlings, Davila-Ross, & Boysen, 2014; Rosati et al., 2018; van Leeuwen, Cronin, & Haun, 2014, 2018; van Leeuwen, Cronin, Haun, Mundry, & Bodamer, 2012; van Leeuwen, Mundry, Cronin, Bodamer, & Haun, 2017). Some of this work has examined if different groups exhibit different patterns of hand-clasp or high-arm grooming (Rosati et al., 2018; van Leeuwen et al., 2017) or open foods differently in extractive foraging contexts (Rawlings et al., 2014). Second, there have been observations of chimpanzee behavior at both Tchimpounga and Chimfunshi to examine behavioral indicators of these chimpanzees’ welfare, such as presence of aberrant behaviors like coprophagy (van Leeuwen et al., 2023; Wobber & Hare, 2011). Third, some work has integrated observations with experimental studies at Sweetwaters and Ngamba, such as to examine how social relationships in natural chimpanzee groups impact cooperative performance in experimental tasks (Engelmann, Haux, & Herrmann, 2019; Engelmann & Herrmann, 2016) or used keeper’s ratings of risk taking compared to experimental measures of risk assessment (Haux et al., 2023). There has also been relevant observational work with bonobos at Lola Ya Bonobo examining various aspects of behavior, including patterns of consolation and post-conflict interactions (Clay & de Waal, 2013a, 2013b), juvenile dominance (Walker & Hare, 2016), tool use (Gruber, Clay, & Zuberbuehler, 2010), and patterns of vocal communication (Clay, Pika, Griber, & Zuberbuehler, 2011; Clay & Zuberbuehler, 2012; Genty, Clay, Hobaiter, & Zuberbuehler, 2014).
Overall, this experimental work in sanctuaries has been important in elucidating the psychological mechanisms supporting behavior, and the observational work has further revealed specific aspects of their behavior. However, this has left a gap in terms of understanding the long-term patterns of day-to-day behavior and social relationships that is the foundation of much work studying wild apes. Prior research approaches in sanctuaries often involve experiments or shorter-term observations of animals, typically carried out by visiting researchers, that are driven by a particular question and therefore focus on a particular aspect of behavior or psychology. This is different from the kind of long-term focal observations that are common at wild primate sites and involve systematically collecting focal and group data across multiple behavioral contexts. The value of this kind of approach from a scientific perspective is that it allows for examination of different aspects of behavior in tandem, and provides a depth and breadth of data that can be used for a variety of future studies rather than being focused on one specific question (Boesch & Boesch-Achermann, 2000; Emery Thompson, Muller, Machanda, Otali, & Wrangam, 2020; Pusey, Pintea, Wilson, Kamenya, & Goodall, 2007; Watts, 2011). This approach also provides benefits to sanctuaries in that it allows an in-depth understanding of both individual chimpanzees’ behavioral patterns, and the overall dynamics of the group, which can inform multiple aspects of captive care to support animal well-being.
In this ‘New Approaches’ article, we describe an ongoing project collecting such systematic focal observations of sanctuary-living chimpanzees at Ngamba Island Chimpanzee Sanctuary in Uganda using methods that have been lightly modified from its use for many years in the wild at the Kibale Chimpanzee Project in Uganda. First, we describe this project’s methodological approach, and how we implemented these systematic observations via a partnership between the sanctuary, wild field site, and external researchers. Next, we discuss the benefits of this approach both to the sanctuary—which gains relevant educational and training opportunities for staff, new in-depth knowledge about the individual chimpanzees in their care which can inform best practices for their well-being, and financial support from researchers—as well as for scientists—who gain a rich behavioral dataset suitable to address various scientific questions in primatology, informed by staff with a deep knowledge of the chimpanzees. Finally, we highlight potential challenges to this approach, as well as the solutions we implemented in our project. We argue that the advantages of using this system comes from (1) its validation in the wild, providing a strong basis to implement and teach others to use it; (2) its flexibility in contexts in which individuals spend parts of day out of sight; (3) its utility in identifying both group-wide patterns and individual variations in behavior, which can inform captive care and address important scientific questions in primatology.
2. Description
This project is a collaboration between Ngamba Island Chimpanzee Sanctuary, a sanctuary in Uganda accredited by the Pan African Sanctuary Alliance; the Kibale Chimpanzee Project, a long-term project studying wild chimpanzees in Uganda composed of both local staff and foreign researchers; and the Cognitive Evolution Group, a research group based at the University of Michigan in the United States. The project adapted methods from the Kibale Chimpanzee Project to collect data at the sanctuary in a way that mirrored the collection procedures at the wild field site. Our collaborative team contributing to this paper includes personnel from the sanctuary who collect the data day-to-day; personnel from the wild field site who provided training and knowledge to develop and initiate the project; external researchers who direct the project and oversee data analyses; and university students who extract and digitize the data. Research was approved by the Uganda Wildlife Authority, the Uganda National Council for Science and Technology, and the Institutional Animal Care and Use Committee at the University of Michigan. Research practices and animal care procedures also complied with the Pan-African Sanctuary Alliance standards.
Background and development of approach
This project started because of the covid-19 pandemic, which halted routine research travel and upended many primatological research projects due to high concern about the risks to primates. While much prior behavioral research in African ape sanctuaries had been led by foreign researchers who traveled to the sanctuary and conducted or oversaw research on-site, that kind of system was not a viable research model during this time period. The pandemic also had devastating impacts on many sanctuaries that had to maintain daily operations to care for their animals, but were dependent on financial support from tourists or other visitors who could not travel during this period. In this context, our teams communicated and proposed to form a new research project where on-site animal caretakers at Ngamba Island Chimpanzee Sanctuary would collect rigorous data on chimpanzee behavior, with in-person training from Ugandan field assistants from the Kibale Chimpanzee Project, and remote training and data management by the Cognitive Evolution group and US-based Kibale Chimpanzee Project researchers. Our goal was to form a mutually beneficial partnership whereby the sanctuary benefited from new trainings and skillsets as well as resources and support stemming from the research, and foreign researchers benefited from being able to collect systematic data by working with observers with long-term expertise and knowledge about the context and history of the chimpanzee group
Our first step was the development of an ethogram and data collection procedures that would be appropriate in the sanctuary context. One likely reason that systematic behavioral observations are not as common in sanctuaries as in wild populations is because observations cannot be carried out in the same way as they can in the wild. Direct observations of wild primate populations typically involve observers following habituated animals through the forest to be able to see what they do. Chimpanzees at Ngamba Island, like apes at many other African sanctuaries, semi-free-range in large, forested habitat enclosures during the day (at Ngamba, this this forest comprises approximately 95 acres of chimpanzee-appropriate habitat).They are also quite habituated to humans. However, as they have a long history of directly interacting with caregivers, veterinarians, and other people, following animals in the forest without a barrier would pose a significant safety risk. As such, the chimpanzees’ forest is separated from human-use spaces by an electrified fence for safety purposes, as is typical at such sanctuaries. Consequently, observers could not follow the sanctuary chimpanzees in their forest enclosure as would be the case in the wild, but rather observed them while they were in the forest (see Figure 1) from observation platforms or on the ground, separated from the chimpanzees by the fence line. As such, the observations were implemented at particular times of day when the chimpanzees approached this area within a distance of about 50m of the fence (to receive food, or in the evening when they prepared to voluntarily enter a dormitory to sleep), which differs from data collection in wild contexts where observations can usually occur all day with habituated groups.
Figure 1: The chimpanzee group in the forest at Ngamba Island Chimpanzee Sanctuary.

Our collaborative project adapted focal observational methods from a wild chimpanzee field site to the sanctuary population. Photo by Innocent Ampeire.
To develop an observational approach appropriate for this context, researchers from the Cognitive Evolution Group and the Kibale Chimpanzee Project first created a modified behavioral ethogram using the primary categories of data collection used at the Kibale Chimpanzee Project, with proposed modifications about how to collect these data in the sanctuary. Researchers then discussed this proposal with the sanctuary staff, and used this feedback to refine the ethogram and ensure it appropriately captured the chimpanzee behaviors actually seen in the sanctuary context. The goal was to keep communication open and ensure that the ethogram captured the behaviors that the caretakers actually saw in the group. Next, researchers developed a series of behavior training modules using videos of wild and sanctuary-living chimpanzee behavior to illustrate how different kinds of behaviors would be recorded on paper data sheets; these training modules along with an example ethogram and datasheet from the project have now been publicly released as a broader educational tool (Sabbi, Felsche, Barnes, Rosati, & Machanda, 2021). After completing practice ‘video focals’ in these training modules, the Ngamba staff received three weeks of in-person training from an experienced Kibale Chimpanzee Project field assistant, who observed the chimpanzees simultaneously with the caretakers and completed a series of reliability focals to directly compare their data. We then implemented a program of videoconferencing meetings for caretakers and researchers to discuss data collection, refine procedures, and address any emerging questions. Once covid-19 travel restrictions were relaxed, foreign researchers also were able to provide additional in-person refresher trainings on several occasions. Finally, data records are scanned approximately every two weeks, and the research team based at the University of Michigan then tracks these datasheets and provides written feedback with questions and suggestions, as well as works to digitize these paper notes so they are in a format that can be analyzed to address various scientific questions. Overall, this data collection program has been in place for more than three years to date, since June 2020.
Data collection methods
The basic data collection protocol takes the form of ten minute focal follows that generally matches data collection procedures that have previously been used at Kibale Chimpanzee Project. During a ten-minute follow, the behavior of the focal chimpanzee is recorded every two minutes, along with the identities of all individuals involved in joint behaviors with the focal such as grooming or play, and individuals within one meter of the focal (see Figure 2a). Second, observers record detailed data on particular focal behaviors whenever they occurred during the ten-minute follow. Specifically, we recorded all instances when the focal engaged in (1) grooming (including direction of grooming, chain grooming, and bout length; see Figure 2b); (2) aggression (including forms of aggression including displays; directed aggression towards a victim such as threats, chases, or attacks; participation in coalitions; and the responses of victims to such aggression; see Figure 2c); (3) pant grunts or pant barks (given or received by the focal, to assess dominance; see Figure 2d); (4) object manipulation and tool use (including using sticks to obtain food or water; using tools in social contexts; manipulating plants or other objects; and modifying objects to produce tools; see Figure 2e). Third, we collected all-occurrence data on aberrant, species-atypical behaviors across the entire group (see Figure 2f). That is, the observer recorded if they ever saw any chimpanzee engage in these behaviors (such as coprophagy, rocking, or other more extreme behaviors; defined based on studies or laboratory or other captive chimpanzees as described in more detail below) during the focals. As of 2022, we also began recording all instances of focal social play (including type of play and bout length). Finally, staff provided ad libitum notes about additional behaviors that they observed but which did not fit any of these categories.
Figure 2: Composite example of observational data sheets.
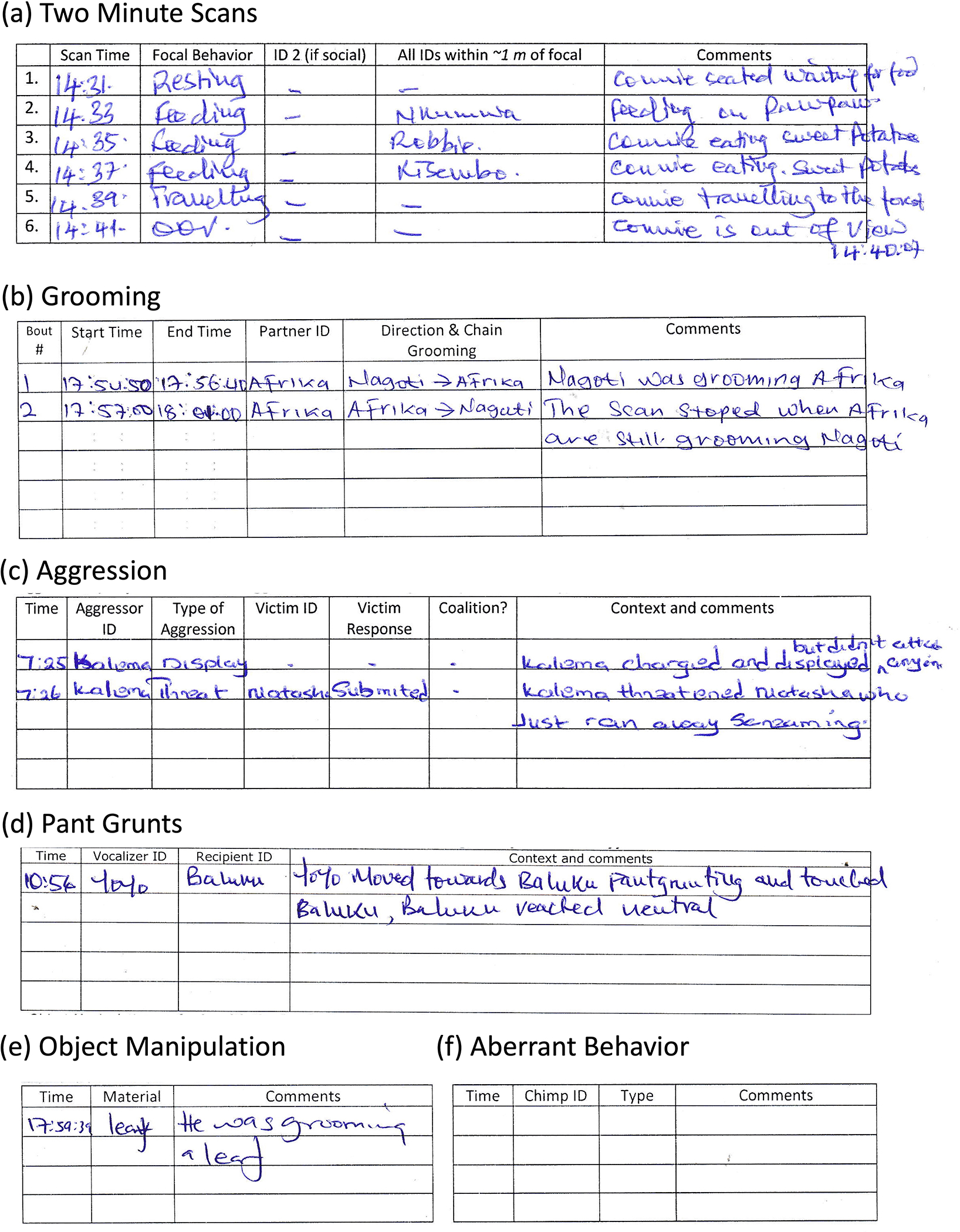
We recorded (a) the activity of the focal every two minutes, along with the identities of any individuals in 1m distance of the focal; (b) all grooming given or received by the focal, including identity of partner, direction of grooming, and duration of bout; (c) all instances of aggression given or received by the focal, including type of aggression, victim response, presence of coalition, and context; (d) all pant grunts or pant barks given or received by the focal; (e) all object manipulation or tool use by the focal; and (f) all occurrences of aberrant behavior seen for any individual in the group.
Ten-minute focal follows are collected during four timeslots: morning (~8:00 AM), midday (~11:00 AM), afternoon (~2:30 PM), and evening (~6:00 PM). The first three time slots correspond to routine feeding times for the group, when individuals tend to approach the observation platform area to receive supplemental food thrown into the enclosure by the caretakers. The final time corresponds to the period before the group voluntarily enters the dormitory for the evening, when they also naturally congregate in the area where they can be observed. After a focal is completed, a new focal individual is chosen if possible (e.g., if individuals are still present in the area where they can be seen by observers). Each observer typically can complete one or two ten-minute focals in a given timeslot. To equalize observation effort across individual chimpanzees, the project provides a check-sheet approximately every two weeks highlighting particular chimpanzees that are priorities to be observed at particular timeslots; caretakers then update the check-sheet with who they observed each day to track this. In addition, approximately once per day the two observers independently observe the same individual for reliability purposes, as detailed below.
Adapting wild site methods
There were also several adjustments from the wild data collection procedures for the sanctuary population. First, we adjusted some of the social data collection indices to make it more appropriate for the sanctuary context. For example, since it was not possible to clearly track party membership in this context (e.g., because all or the majority of the chimpanzee group always was at the observation area during these specific time points), we did not collect systematic party membership data unlike at the wild site, although we do record when chimpanzees are absent from the forest group (e.g., because they are inside the building). Similarly, we recorded chimpanzees in one meter proximity (rather than 5 meters, as is used at the Kibale Chimpanzee Project); as the density of chimpanzees in the sanctuary observation area is generally much higher than in the wild likely in part due to the active provisioning, we thought this would better capture meaningful aspects of their relationships and social choices.
One important change from the wild data collection methods is that we did not aim to collect systematic data on reproductive behavior, which is a routine aspect of Kibale Chimpanzee Project data collection. As the female sanctuary chimpanzees are typically on hormonal birth control implants, they do not experience sexual swellings in the same way as in the wild. However, we did record any copulations that were observed on two-minute scans, and for each ten-minute focal observers note any females that may exhibit sexual swellings in the group at that time. In addition, mating could be recorded as the context of aggressive interactions. Although copulations might not occur as frequently as in the wild, by recording if females had swellings, we could understand if other behaviors such as aggression were affected by reproductive contexts.
Another consideration was that some behaviors mostly or solely occur in captive contexts but are not typically present in the wild. This includes human-direct behaviors such as begging from keepers during provisioning, or using a tool to get the attention of a caretaker. Additionally, since these chimpanzees are provisioned and many of our observations occur during active provisioning, we collect routine data on food theft (e.g., one chimpanzee taking food from another in physical possession of it) as a type of interaction that is not typically seen in the wild. Conversely, certain behaviors that do occur in the wild (such as hunting monkeys) do not typically occur in the sanctuary, and thus were not symmetrically collected, although instances of predation could be recorded as ad-libitum notes. Finally, aberrant, species-atypical behaviors are an important indicator in captive groups but are typically not seen in the wild.
Finally, a key element of our project was that multiple observers were trained to collect the data, and we then implemented routine collection of reliability scans so we could systematically check whether observers recorded data in concurrent ways. This was an outgrowth of our initial training procedures for staff as they first learned the behavioral methods, where they each completed multiple reliability scans with an experienced Kibale Chimpanzee Project field assistant. After the sanctuary staff began collecting the routine data when this training period concluded, we continued to ask the pair of observers for each day to collect one reliability scan per day, allowing us to systematically track quantitative reliability as well as provide rapid feedback during every two-week data collection period. This allowed the project to be sustained for a longer period and not depend on any particular individual being present to collect the data.
3. Example
We examined one year of data collected from July 2020-June 2021. During this time, a total of 48 chimpanzees (30 females and 18 males; average age 23 years; range 1–36 years) were observed in the forest enclosure. Chimpanzees are socially housed, have semi-free-ranging access to ~40 hectares of species-appropriate tropical forest during the day, and voluntarily enter a night dormitory to sleep and receive supplemental feedings. Their diet is supplemented with species-appropriate fruits and vegetables several times a day, in addition to foods they can eat in the forest. Most sanctuary individuals were wild-born orphans who were mother-reared in the wild for approximately 1–3 years, and then integrated into species-typical social groups upon arrival at the sanctuary. One infant, two juveniles, and one adult were born at the sanctuary due to failures of birth control. Age for orphans was estimated by sanctuary veterinarians on arrival and validated by dental patterns and body weight (see Cole et al., 2020; Wobber, Wrangham, et al., 2010b).
Dataset overview
We completed an average of 74.5 ten-minute follows per individual (range: 36–83 follows) on chimpanzees in the forest enclosure, with similar observation effort for males and females (two females did not free range in the forest for part of this year for health reasons unrelated to the project, whereas the rest completed at least 66 follows). This resulted in a total of 655 hours of in-view observation over the year, with an average of 13.6 hours per individual.
Reliability of observers
Overall, 7.2% of the focal follows involved reliability scans, where two observers watched the same focal independently. We found high reliability across observers on the data. For example, in two-minute scans over the year, observers agreed on the activity state of the focal during on 97.7% of observations, agreed on the identity of social interaction partners on 99.2% of observations, and agreed on the total number of individuals in proximity to the focal on 96.1% of scans. Similarly, observers agreed on the occurrence of 99.4% of grooming bouts, and agreed on the identity of the grooming partner on 95.7% of scans, and on the direction of the grooming on 96.3% of scans. For records of aggression, observers agreed that an aggressive event occurred on 93.8% of observations, agreed on the identity of the aggressor and victim on 100% and 98.3% of events, respectively; agreed on the specific behavior of the aggressor (e.g., such as engaging in a threat or attack) on 93.3% of occurrences; and on the response of the victim on 95.0% of occurrences. This shows we were able to successfully collect reliable data using this protocol.
Aggression across the day
We next used the data to examine several aspects of the chimpanzees’ behavior to show how these data are useful both scientifically and for chimpanzee care. First, we examined patterns of aggression (threats, chases, and displays) in the 45 subadults and adults in the group ages 10 years and up (e.g., excluding the three individuals who were an infant or juveniles). We specifically assessed how aggression differed at the different observation timeslots (e.g., feeding times in the morning, midday, and afternoon, compared to the evening when the chimpanzees were not provisioned). To do so, we calculated each chimpanzee’s rate of directed aggression (e.g., threats, chases, and attacks directed towards a specific victim) as well as their rate of displays (e.g., aggressive displays without a victim; for both, this was calculated as count of aggressive events / count of in-view scans for that individual for that observation timeslot). This showed clear differences in aggression rates over the different time slots. For example, chimpanzees showed an average of 1.03 directed aggressive events per hour of observation in the morning (e.g., the first feeding when the chimpanzees are released into the forest), around 0.4 events in the midday and afternoon slots (which involve provisioning), but only 0.03 events per hour in the evening.
We then analyzed rates of aggressive behavior using linear mixed models accounting for repeated individual measurements, implemented in the lme4 package (Bates, 2010) in R version 4.2.1 (R Development Core Team, 2022). We compared model fit using likelihood ratio tests (Bolker et al., 2008) to test the importance of observation timeslot on aggression rates. Post-hoc comparisons of factors were performed with the emmeans package (Lenth, 2018) using Tukey corrections. Figures depicting model output were created using the effects package (Fox, 2003). In particular, we constructed a base model accounting for individual’s identity (as a random effect), age in years, sex (e.g., because males are generally more aggressive than females), and aggression type (display versus directed aggression). Including timeslot in a second model improved fit [χ2 = 89.65, df = 3, p < 0.0001]. Posthoc tests indicated more overall aggression in the morning compared to all other timeslots [p < 0.0001], and also more in the midday and afternoon timeslots compare to evening slot when there is no provisioning [p <0.05]. We then added the interaction between timeslot X aggression type which further improved fit [χ2 = 9.94, df = 3, p < 0.05]. Posthoc tests showed that while both directed aggression and displays were highest in the morning, only directed aggression stayed elevated in the midmorning and afternoon compared to evening levels, whereas there were similar display rates in the mid-morning, afternoon, and evening timeslots (p < 0.05 for significant comparisons; see Figure 3a). Overall, this shows that aggression was higher when animals were actively provisioned in the forest, especially when the chimpanzees are first released in the morning, providing relevant information for captive care practices.
Figure 3: Behavioral patterns in a sanctuary chimpanzee population.
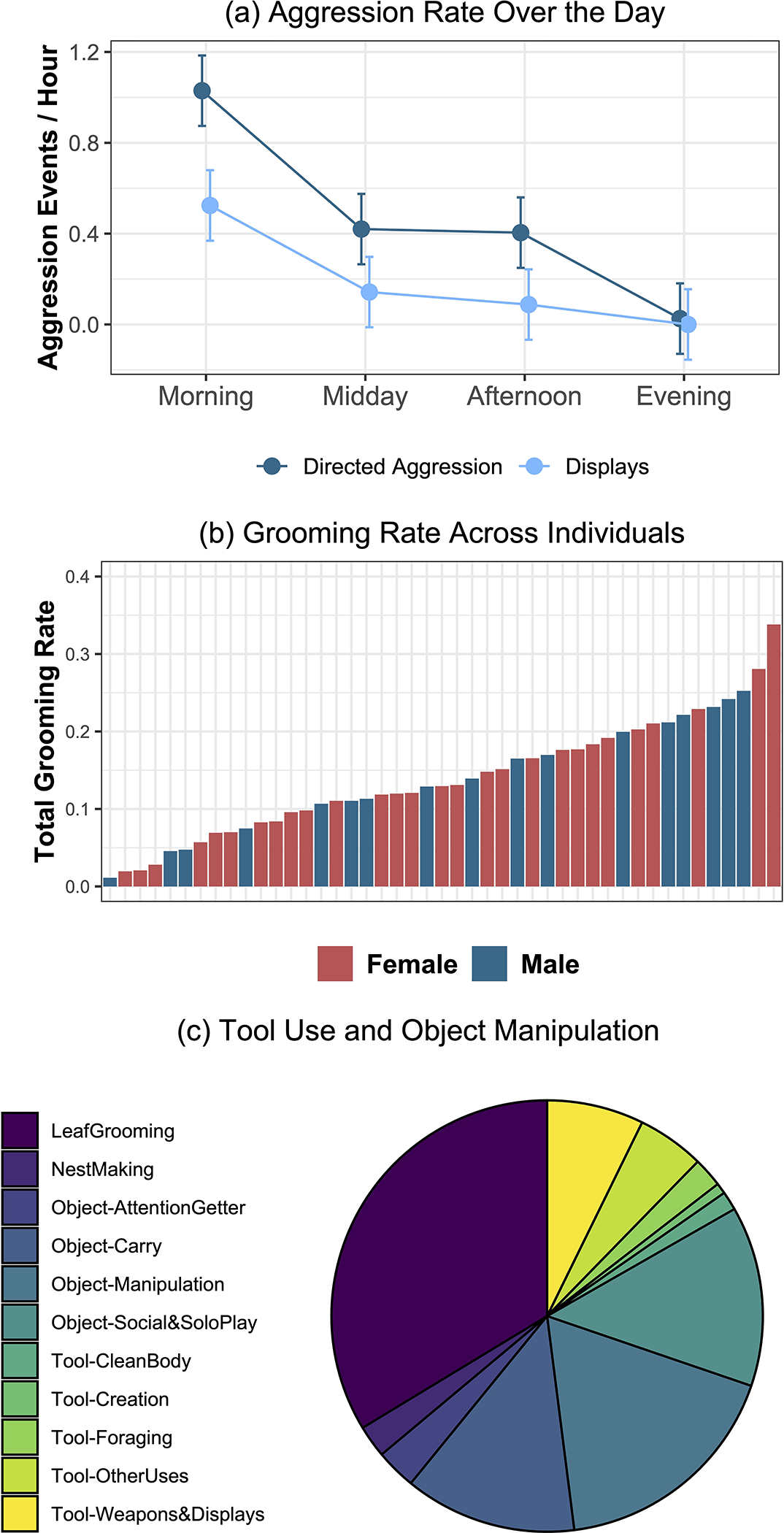
(a) Aggression rates per hour during provisioning (morning, midday, and afternoon) versus non-provisioning (evening) observation periods. Model estimates also account for individual’s identity, age, and sex. Error bars are 95% confidence estimates. (b) Individual variation in overall grooming rates between adults, by sex. (c) Distribution of tool-use and object manipulation behaviors across the group.
Grooming across individuals
We next examined grooming rates of the subadults and adults in the population using the two-minute scan data (again focusing on individuals ages 10 years and up). To do so, we calculated an overall grooming rate for each individual comprising both giving grooming and being groomed (e.g., count of grooming scans / count of in-view scans for that individual). Overall, males engaged in grooming on an average of 14.5% of scans, whereas females engaged in grooming on an average of 13.6%. We then analyzed grooming rates using the same approach described previously to test if there were differences between male and female chimpanzees’ patterns. In particular, we constructed a linear mixed model accounting for individual’s identity (as a random factor), age in years, and observation timeslot (given the influence on behavioral patterns described above). Including sex in a second model did not improve fit [χ2 = 0.179, df = 1, p > 0.67, n.s.], indicating similar participation in grooming in males and females. Indeed, the two individuals with the highest overall (adult) grooming rates in the population were females (see Figure 3b).
This provides a striking contrast to sociality patterns in wild chimpanzees, where males tend to groom and generally socialize more with adults than do females (Emery Thompson et al., 2020; Machanda, Gilby, & Wrangham, 2013). This is in line with theoretical proposals that female relationships in Pan may be constrained by competition for food, which may impact females more than males (Wrangham, 2000)—in particular, females in this group may be able to devote more time to socializing because they are provisioned. Notably, our result does align with some prior work with captive chimpanzee populations. For example, focal observations of chimpanzees living at the Arnhem Zoo and Primate Center TNO in the Netherlands revealed no sex differences in grooming rates in either group (Spijkerman, van Hooff, Dienske, & Jens, 1997). Similarly, a survey of caretakers involving more than 1000 captive chimpanzees living in different US zoos, laboratories, or sanctuaries revealed that females were more likely to have been rated as having been observed grooming than were males (Clay et al., 2023). However, this prior captive work has several possible explanations, as animals typically live in smaller social groups in zoos or labs compared to African sanctuaries, and such groups may have fewer males in terms of composition, as well as more restricted ranging space—all factors that could also shape social behavior. Our work suggests that females may exhibit robust participation in grooming even when groups consist of numerous adults and have significant space access, allowing for refinement of hypotheses about the socioecological conditions promoting female relationships. Our approach further allows for direct comparisons of social behavior in chimpanzees collected in the same manner.
Patterns of tool use and object manipulation
Next, we examined patterns of tool use and object manipulation across the entire group. Overall, in the course of the year we observed 496 examples of tool use or object manipulation (see Figure 3c). In terms of tool use, the most common form was using a weapon to hit other chimpanzees or for use in displays (36 observations). They were also observed using tools for non-foraging purposes (such as digging or raking with a stick, not directly related to accessing food or water; 25 observations), for foraging (such as using a tool to drink water or a stick to extract honey from an artificial termite mound; 11 observations), to clean their body (7 observations), and were occasionally observed making or modifying a tool (4 observations). This shows that the sanctuary population shows a wide range of tool-use behaviors, which can complement wild studies to disentangle the environmental factors that promote different kinds of material culture in animals (Koops, McGrew, & Matsuzawa, 2013; Koops, Visalberghi, & van Schaik, 2014).
They were also observed manipulating objects or plants in a variety of ways. The most common behavior in the entire dataset was leaf grooming (167 observations), but they were also observed manipulating a variety of objects (such as breaking sticks or plucking leaves with no obvious feeding or tool-use purpose; 88 observations), playing with objects (67 observations), carrying objects (64 observations), using an object to get the attention of a caregiver (15 observations), and building nests (12 observations). Interestingly, leaf grooming is also one of the most frequently observed behaviors of this nature in wild Ugandan chimpanzees (Watts, 2008). While the exact origins of sanctuary chimpanzees are not typically known due to their history, this suggests that sanctuary chimpanzees may exhibit relevant variation in tool and object use patterns paralleling those observed in wild communities.
Aberrant behaviors
Finally, we examined instances of aberrant behavior in the dataset. Unlike our other metrics, we collect all-occurrence instances of aberrant behaviors from any individuals that ever are observed performing these behaviors during observation periods (in order to collect more data on these rare events). In fact, over the course of the year of data collection there were only 9 instances of aberrant behavior ever observed: 4 instances of coprophagy (all different individuals) and 5 of hair pulling (one individual was observed pulling their hair twice, the rest one time). Other aberrant behaviors that are commonly observed in zoo and laboratory (or former laboratory) chimpanzees such as rocking, regurgitation and reingestation, feces smearing or painting, or eye poking (Fultz, Brent, & Loeser, 2010; Jacobson, Ross, & Bloomsmith, 2016; Walsh, Bramblett, & Alford, 1982) were never observed in this sanctuary chimpanzee group, in line with prior work in African sanctuaries (Wobber & Hare, 2011). Note that coprophagy, the most common behavior in in the sanctuary chimpanzees in this observational dataset, is a behavior that occurs in the wild (Bertolani & Pruetz, 2011; Krief, Wrangham, & Lestel, 2006; Payne, Webster, & Hunt, 2008) and may have adaptive value (e.g., picking undigested food out of feces). Although it is often more exaggerated in captive contexts, it also does not seem to be correlated with other, more serious welfare indicators (Hopper, Freeman, & Ross, 2016).
Overall, this suggests that such species-atypical behaviors are rare or absent while chimpanzees are in their forest enclosure, providing an important measure of the sanctuary’s high standard of welfare. This kind of data could also be used to address whether there are long-term impacts of early life experiences in these populations. Given that more Africa sanctuary apes are orphans of the bushmeat and pet trade, there is a current debate about the long-term repercussions of these experiences (e.g., capture and then later rescue) on their behavior (e.g., Clay & de Waal, 2013b; Ferdowsian et al., 2011; Leavens, Bard, & Hopkins, 2019; Rosati et al., 2013; van Leeuwen, Mulenga, & Chidester, 2013; Wobber & Hare, 2011). While our data show that aberrant behaviors are extremely rare in this sanctuary population when they free-range, systematic collection of such data at other times or from other populations could inform this point. For example, it would be informative to compare behavioral rates in the forest (where we currently observe them) to those for the same behaviors as demonstrated inside indoor sleeping dormitories to assess what contextual factors shape these responses. Moreover, all of the orphaned individuals living at the sanctuary for more than 8 years at the time of the study (and most significantly longer), but individuals might show more such behaviors upon arrival, or individuals who have lived in human environments for longer periods might show inflated rates. Finally, identical data collection procedures assessing both orphans and mother-reared individuals in the same sanctuary population can provide important clues as to the long-term consequences of maternal loss (Wobber & Hare, 2011; Wobber, Wrangham, & Hare, 2010a), which is also known to shape aspects of behavior in wild chimpanzees (Reddy & Mitani, 2019; Stanton, Lonsdrof, Murray, & Pusey, 2020).
4. Comparison and critique
In this final section, we examine the advantages and disadvantages of our approach. We specifically focus on the advantages not just for research but also for chimpanzee welfare and primatological capacity-building, as well as the unique problems (and potential solutions) that arise from this framework.
Benefits for care and welfare of chimpanzees
A key element of our approach is that animal caretakers at the sanctuary are the primary observers and data collectors. Importantly, collecting this kind of systematic observational data collection of focal individuals helps animal caretakers keep eyes on important aspects of the behavior of all the chimpanzees in the group, and thus better understand well-being of the animals under their care. As such, one aspect of the project is that the researchers managing the data provide summaries and updates of the results to the staff upon request to provide insights into the individual chimpanzees’ behavioral patterns and help inform sanctuary decisions. Moreover, because our approach focuses on training on-site staff to collect these data, this allows sanctuaries to collect such data in the long term and be self-sustaining in addressing any husbandry and care questions that becomes central to their needs.
Indeed, preliminary results from the observations have several key implications for animal husbandry and care. For example, understanding the contexts and specific individuals who impact levels of aggression in the group is an important consideration in captive care. While our preliminary analysis focused on the times of day when aggression is most likely, our data also allow us to identify specific individuals who show higher rates of aggression or direct aggression towards specific targets, information that can be used to help keepers be aware of tensions in the group, manage those dyads’ interactions more closely, and perhaps prevent injuries.
Our data can also be used to identify strong relationships between specific chimpanzees, which can inform best practices for their well-being. For example, we used grooming data to examine how the sex of partners influenced grooming rates, but we can also use the same kinds of data to identify strong bond partners (Gilby & Wrangham, 2008; Machanda et al., 2013; Rosati et al., 2020) or social networks (Thompson Gonzalez et al., 2021). Systematically assessing strong bond partners for all individuals in the group in this way can help with identifying who might provide comfort if a chimpanzee is in distress, can help inform pairings such as sleeping room locations, and can be useful information for sanctuaries planning releases as a long term goal, an increasingly urgent issue for Africans sanctuaries as they reach capacity (Andre, Kamate, Mabonzo, Morel, & Hare, 2008; Farmer, 2002; Humle, Colin, Laurans, & Raballand, 2011; Stokes et al., 2017; Stokes et al., 2018).
Our data systemically tracking aberrant behavior is also explicitly designed to contribute to our understanding of the chimpanzees’ welfare. Our preliminary data show that such behaviors are very rare or absent when chimpanzees are in the forest enclosure, aligning with prior work that these African populations are psychologically healthy (Rosati et al., 2013; Wobber & Hare, 2011). Collecting these data long-term can also provide a metric of chimpanzee welfare across contexts, including how welfare indices change in response to different care procedures, how chimpanzees fare across different contexts such as in the forest versus inside a dormitory building, or shifts over time as new arrivals are acclimated to the high-quality environment of the sanctuary.
Benefits for capacity building and training
While many research projects in African sanctuaries involve data collection performed by visiting academic researchers, an approach that prioritizes local staff pays big dividends (Emery Thompson et al., 2020). Visiting researchers to African sanctuaries provide a variety of support such as research fees or supplies, as well as the engagement of researchers with specialized training including in fields including animal behavior, genetics, endocrinology, and demography that can complement existing expertise at the sanctuary to benefit the animals’ care and primatological knowledge. Yet sanctuaries also directly benefit from an approach that engages local staff in research as this provides new opportunities for staff training, information sharing, and building a broader base of primatological knowledge.
To date, 12 animal caretakers have completed this training and contributed to the project’s data collection. Staff members’ increased knowledge about chimpanzee behavior which may have positive effects on other aspects of their jobs, including overseeing chimpanzee care and educational roles when guiding tourist groups. Also, this approach focuses more on capacity building and knowledge sharing as a mutually beneficial partnership between the sanctuary and visiting researchers where both gain, rather than the sanctuary simply hosting the research team. In our case, the project also was important in establishing a stronger link between the sanctuary and a wild chimpanzee field site in Uganda, both of which have key shared interest in chimpanzee conservation.
Finally, this approach has benefits for mentoring and outreach in education and the primatological community more generally. By developing training programs for the sanctuary staff, our project in effect created training modules that can be more broadly useful for students learning about primatological methods. In fact, we have also used our training modules to introduce these methods to more than 20 undergraduate and highschoolers to date, who have then gone on to engage in data digitization and extraction on this or other projects with deeper engagement in data, allowing for a variety of independent projects and honors theses. We have also publicly posted chimpanzee training modules on our outreach website Primate Learning in Action for wider dissemination to the community (Sabbi et al., 2021).
Benefits for basic research in primatology
Scientifically, investing in local staff knowledge and training also allows for the collection of consistent data that is not dependent on particular visiting researchers. The project produces high-quality data without foreign researchers being physically present on-site, and more individuals are well-trained to collect such data, so that the project can also collect data for longer time periods to address questions that require examining long-term patterns of behaviors rather than only responses observed in a shorter research trip. Finally, given that the caretakers are experts in these chimpanzees, they can provide crucial feedback for the data collection (e.g., refinement of our ethogram categories) and long-term contexts for and changes in chimpanzees’ behaviors that visiting researchers do not necessarily have.
The specific element of our approach that directly adapts observational methods used in research with wild chimpanzees has further benefits. Specifically, we can conduct research that explicitly compares behavioral patterns across sanctuary and wild sites. Such cross-population perspectives are crucial for primatology, yet methodological differences can be a major hindrance for harmonizing data collected at different sites by different teams. We are not aware of any other project explicitly using wild long-term focal data collection protocols to observe captive or sanctuary-living chimpanzees in multiple behavioral domains in this way. Importantly, comparisons with sanctuaries are useful for evaluating key socioecological hypotheses for primate behavior due to the differences in these populations compared to the wild. For example, chimpanzees exhibit male philopatry such that males stay in the group where they are born and thus exhibit stronger kinship ties than females. In sanctuaries, this kinship bias is absent because individuals are mostly wild-born orphans, and females do not transfer to new groups at puberty. Moreover, females do not face the same energetic constraints proposed to be important in shaping their wild behavior (Wrangham, 2000), given that individuals are provisioned. This allows for explicit tests of how these factors may impact patterns of social behavior, while accounting for socioecological features that are similar across the sanctuary and wild (such as living in large, mixed-sex groups and having access to large spaces with species-appropriate forest habitat). As noted previously, our preliminary data suggests that males and females groom at similar rates in the sanctuary, suggesting that females can in fact be quite gregarious when these constraints are removed.
Finally, observational studies of chimpanzee populations where other forms of research—such as cognitive experiments and more intense health monitoring—are possible can allow researchers to integrate across multiple kinds of data in a context that is more similar to the wild than many other captive contexts. While experimental research is uncommon with wild chimpanzees (Zuberbühler, 2014), it is fairly routine in sanctuaries as described previously, given that (for example) cognitive testing often mirrors typical enrichment activities for captive primates (Hopper, 2017; Hopper, Shender, & Ross, 2016; Ruby & Buchanan-Smith, 2015). Prior work integrating data on the chimpanzees’ real-life social relationships with their performance on cooperative tasks (Engelmann et al., 2019; Engelmann & Herrmann, 2016) shows the power of combining these approaches, something that is much more feasible in sanctuaries than in the wild. Similarly, it is also feasible to collect a variety of biological samples from sanctuary populations (Cole et al., 2020; Dunay et al., 2022; Rosati, Emery Thompson, Atencia, & Buckholtz, in press; Standley et al., 2011; Wobber, Hare, Lipson, Wrangham, & Ellison, 2013; Wobber, Hare, et al., 2010), some of which—such as saliva, or blood collected in the context of routine health checks for the animal’s own well-being—are difficult or impossible in the wild. This kind of observational data can also be linked to detailed information on individual’s heath status. Finally, these sites could further allow integration of observational, cognitive, and physiological data with monitoring from new technologies that are currently being applied to wild animals, such as trap cameras or other forms of remote sensing to understand individual and group-level behaviors (Griebling et al., 2022; Harrison & van de Waal, 2022). This would allow for integration of across data, as well as enable tests of these technologies in more controlled situations to inform their use in the wild.
Challenges and solutions
Investment in and training of local animal caretakers to collect observational data at sanctuaries involves a different mindset for research projects. One major challenge for us was thinking through a systematic program for staff trainings, as well as a way to assess performance in the context involving primarily-remote interactions in which we initiated the work. As detailed above, our project therefore (1) developed electronic training modules; (2) integrated this with in-person trainings from experienced field assistants; and (3) used a system of video conferencing and written feedback from remote researchers. We also implemented systematic reliability scans as part of the routine data collection. The constraints imposed by covid-19 lockdown necessitated that we develop several remote training procedures, but on the whole, this would have been very difficult without the crucial in-person trainings at the beginning of the collaboration from a Kibale Chimpanzee Project field assistant. Given that internet access can be difficult in some locations, and the requirement of in-person visits, this kind of program could have several barriers especially with respect to initiating the work.
Another challenge concerns the necessary equipment for such a project, which although it was minimal, still needed to be sent to the on-site team and maintained. For our project, staff needed appropriate stop watches to time the two-minute scans; paper datasheets to record observations; and clipboards and pens. There can be difficulty in transporting such equipment to the site and ensuring they continue to function. For example, since our project started during covid-19 lockdowns, we originally substituted a phone app for stopwatches until it was possible for foreign researchers to transport appropriate stop watches to the sanctuary in Uganda. Similarly, the project required the availability of a computer, a scanner, and that the sanctuary had internet access to transmit scans of the data to the research team. This final point was both because travel restrictions initially precluded that the physical paper sheets could be transported by a visiting researcher, and because this approach generally allowed for better communication and quicker feedback about the data as it was being collected. Indeed, oversight and organization of this project hinges on good communication. Our project benefited from online video-conference meetings between the sanctuary staff and external researchers, routine emails to ask questions about the information on scanned paper sheets and provide feedback on the data collection, and text messaging for more time-sensitive responses.
Finally, language obstacles and cultural differences, such as in how various chimpanzees’ behaviors are described must be kept in mind, as the American undergraduates digitizing these data sometimes do not understand the ways that Ugandan staff describe certain details in the written sheets. For example, foods the chimpanzees are eating might have different labels in Ugandan English versus American English (e.g., what the American students know as ‘eggplants’ are known as ‘garden eggs’ in Uganda). As such, ensuring that there is awareness of cross-cultural differences in language in key. Such language and communication issues would be even more important to consider when staff and foreign researchers are not all fluent in a shared language as they are in this case.
A final key challenge to this kind of research is that African sanctuaries have many roles, centered on animal welfare and providing high quality care, but also increasingly including conservation, education, and research. While research is increasingly becoming an important aspect of the many multifaceted roles that sanctuaries play (Stokes et al., 2017; Stokes et al., 2018), considering how to make such long-term research goals manageable with those other roles in mind is crucially important. In this case, keeping the data collection to short ten-minute focals allows caretakers to more easily incorporate data collection into their daily routine with its many time constraints. In addition, we believe that the fact that observational research directly and immediately benefits the sanctuary—in terms of staff training and knowledge about chimpanzee behavior and welfare—is one reason why such an approach can be especially valuable.
Conclusions
Our project aimed to collect observational data on sanctuary-living chimpanzees using data collection protocols derived from a wild chimpanzee project in order to facilitate direct comparisons across sites. Our partnership between external researchers and sanctuary staff was very successful in collecting rigorous data that can be used not only to address scientific questions but also to improve chimpanzee care and welfare. This partnership also allowed for new benefits in training and education, information sharing, and general capacity-building. We propose that such partnerships between sanctuaries and researchers can provide important joint benefits.
Acknowledgments
We thank Evelyn Amono, Joseph Kaale, and Paul Nyenje for assistance with data collection at Ngamba Island Chimpanzee Sanctuary. We also thank Arianna Mistry, Benjamin Culp, Cassandra McDaniel, Chase Braun, Kendall Mills, Lila Drasner, Noa Berman, and Ziyun Wang for assistance in data coding and entry. We thank the Uganda Wildlife Authority and the Uganda National Council for Science and Technology for supporting our research. This work was supported by NSF grant 1926653, NSF grant 1926737, and NIH grant R37AG049395.
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
Ethics Statement
Research approved by the Uganda Wildlife Authority, the Uganda National Council for Science and Technology, Chimpanzee Sanctuary and Wildlife Conservation Trust, and the Institutional Animal Care and Use Committee at the University of Michigan. Research procedures complied with Pan African Sanctuary Alliance standards and adhered to the American Society of Primatologists Principles for the Ethical Treatment of Nonhuman Primates.
Data Availability Statement
Data and example ethogram and data recording instructions are available at Dryad Digital Repository (permanent doi:10.5061/dryad.mkkwh715d; temporary link for review: https://datadryad.org/stash/share/S2POOyCZjBOOaGctIlHMRlmEziHxrs3-56AidxizKWw).
References
- Andre C, Kamate C, Mabonzo P, Morel D, & Hare B (2008). The conservation value of Lola ya Bonobo Sanctuary. In Furuichi T & Thompson J (Eds.), The bonobos: behavior, ecology and conservation (pp. 303–322). New York: Springer. [Google Scholar]
- Bates D (2010). The LME4 package: linear mixed-effects models using S4 classes. See http://www.R-project.org.
- Bertolani P, & Pruetz JD (2011). Seed reingestion in savannah chimpanzees (Pan troglodytes verus) at Fongoli, Senegal. International Journal of Primatology, 32, 1123–1132. [Google Scholar]
- Boesch C, & Boesch-Achermann H (2000). The chimpanzees of the Taï Forest: Behavioural ecology and evolution. Oxford: Oxford University Press. [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, & White JSS (2008). Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology and Evolution, 24, 127–135. [DOI] [PubMed] [Google Scholar]
- Bullinger AF, Melis AP, & Tomasello M (2011). Chimpanzees, Pan troglodytes, prefer individual over collaborative strategies towards goals. Animal Behaviour, 82, 1135–1141. [Google Scholar]
- Cantwell A, Buckholtz JW, Atencia R, & Rosati AG (2022). The origins of cognitive flexibility in chimpanzees. Developmental Science, 25, e13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay AW, Ross SR, Lambeth S, Vazquez M, Breaux S, Pietch R, . . . Bloomsmith MA. (2023). Chimpanzees (Pan troglodytes) in U.S. zoos, sanctuaries, and research facilities: A survey-based comparison of species-typical behaviors Animals, 13, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay Z, & de Waal FBM (2013a). Bonobos respond to distress in others: Consolation across the age spectrum. Plos One, 8, e55206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay Z, & de Waal FBM (2013b). Development of socio-emotional competence in bonobos. Proceedings of the National Academy of Sciences, 110, 18121–18126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay Z, Pika S, Griber T, & Zuberbuehler K (2011). Female bonobos use copulation calls as social signals. Biology Letters, 7, 513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay Z, & Tennie C (2017). Is overimitation a uniquely human phenomenon? Insights from human children as compared to bonobos. Child Development, 89, 1535–1544. [DOI] [PubMed] [Google Scholar]
- Clay Z, & Zuberbuehler K (2012). Communication during sex among female bonobos: effects of dominance, solicitation and audience. Scientific Reports, 2, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MF, Cantwell A, Rukundo J, Ajarova L, Fernandez-Navarro S, Atencia R, & Rosati AG (2020). Healthy cardiovascular biomarkers across the lifespan in wild-born chimpanzees (Pan troglodytes). Philosophical Transactions of the Royal Society B, 375, 20190609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunay E, Owens LA, Dunn CD, Rukundo J, Atencia R, Cole MF, . . . Goldberg TL. (2022). Viruses in sanctuary chimpanzees across Africa. American Journal of Primatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert J, Call J, Hermes J, Herrmann E, & Rakoczy H (2018). Intuitive statistical inferences in chimpanzees and humans follow Weber’s law. Cognition, 180, 99. [DOI] [PubMed] [Google Scholar]
- Eckert J, Rakoczy H, Call J, Herrmann E, & Hanus D (2018). Chimpanzees consider humans’ psychological states when drawing statistical inferences. Current Biology, 28, 1959–1963. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Machanda ZP, Otali E, & Wrangam RW (2020). The Kibale Chimpanzee Project: Over thirty years of research, conservation, and change. Biological Conservation, 252, 108857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Haux L, & Herrmann E (2019). Helping in young children and chimpanzees shows partiality towards friends. Evolution and Human Behavior, 40, 292–300. [Google Scholar]
- Engelmann JM, & Herrmann E (2016). Chimpanzees trust their friends. Current Biology, 26, 252–256. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Herrmann E, & Tomasello M (2015). Chimpanzees trust conspecifics to engage in low-cost reciprocity. Proceedings of the Royal Society B, 282, 20142803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer KH (2002). Pan-African Sanctuary Alliance: status and range of activities for great ape conservation. American Journal of Primatology, 58, 117–132. doi: 10.1002/ajp.10054 [DOI] [PubMed] [Google Scholar]
- Ferdowsian HR, Durham DL, Kimwele C, Kranendonk G, Otali E, Akugizibwe T, . . . Johnson CM. (2011). Sigs of mood and anxiety disrders in chimpanzees. Plos One, 6, e19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J (2003). Effect displays in R for generalised linear models. Journal of Statistical Software, 8, 1–27. [Google Scholar]
- Fultz A, Brent L, & Loeser E (2010). Abnormal behaviors in sanctuary chimpanzees (Pan troglodytes). American Journal of Primatology, 72, 28–29. [Google Scholar]
- Genty A, Clay Z, Hobaiter C, & Zuberbuehler K (2014). Multi-modal use of a socially directed call in bonobos. Plos One, 9, e84738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilby IC, & Wrangham RW (2008). Association patterns among wild chimpanzees (Pan troglodytes schweinfurthii) reflect sex differences in cooperation. Behavioral Ecology and Sociobiology, 62, 1831–1842. [Google Scholar]
- Griebling HJ, Sluka CM, Stanton LA, Barrett LP, Bastos JB, & Benson-Amram S (2022). How technology can advance the study of animal cognition in the wild. Current Opinion in Behavioral Sciences, 45, 101120. [Google Scholar]
- Gruber T, Clay Z, & Zuberbuehler K (2010). A comparison of bonobo and chimpanzee tool use: evidence for a female bias in the Pan lineage. Animal Behaviour, 80, 1023–1033. [Google Scholar]
- Hare B, Melis AP, Woods V, Hastings S, & Wrangham R (2007). Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Current Biology, 17, 619–623. [DOI] [PubMed] [Google Scholar]
- Harrison RA, & van de Waal E (2022). The unique potential of field research to understand primate social learning and cognition. Current Opinion in Behavioral Sciences, 45, 101132. [Google Scholar]
- Haux L, Engelmann JM, Arslan RC, Hertwig R, & Herrmann E (2023). Chimpanzee and human risk preferences show key similarities. Pschological Science. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Call J, Hernadez-Lloreda MV, Hare B, & Tomasello M (2007). Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science, 317, 1360–1366. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Hare B, Call J, & Tomasello M (2010). Differences in the cognitive skills of bonobos and chimpanzees. Plos One, 5, e12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann E, Hare B, Cissewski J, & Tomasello M (2011). A comparison of temperament in nonhuman apes and human infants. Develomental Science, 14, 1393–1405. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Hernández-Lloreda MV, Call J, Hare B, & Tomasello M (2010). The structure of individual differences in the cognitive abilities of children and chimpanzees. Pschological Science, 21, 102–110. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Misch A, Hernandez-Lloreda V, & Tomasello M (2015). Uniquely human self-control begins at school age. Developmental Science. [DOI] [PubMed] [Google Scholar]
- Hopper LM (2017). Cognitive research in zoos. Current Opinion in Behavioral Sciences, 16, 100–110. [Google Scholar]
- Hopper LM, Freeman HD, & Ross SR (2016). Reconsidering coprophagy as an indicator of negative welfare for captive chimpanzees. Applied Animal Behavior Science, 176, 112–119. [Google Scholar]
- Hopper LM, Shender MA, & Ross SR (2016). Behavioral research as physical enrichment for captive chimpanzees. Zoo Biology, 35, 293–297. [DOI] [PubMed] [Google Scholar]
- Horner V, & Whiten A (2005). Causal knowledge and imitation/emulation switching in chimpanzees (Pan troglodytes) and children (Homo sapiens). Animal Cognition, 8, 164–181. [DOI] [PubMed] [Google Scholar]
- Humle T, Colin C, Laurans M, & Raballand E (2011). Group release of sanctuary chimpanzees (Pan troglodytes) in the Haut Niger National Park, Guinea, West Africa: Ranging patterns and lessons so far. International Journal of Primatology, 32, 456–473. [Google Scholar]
- Jacobson LJ, Ross S, & Bloomsmith M (2016). Characterizing abnormal behavior in a large population of zoo-housed chimpanzees: prevalence and potential influencing factors. PeerJ, 4, e2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John M, Duguid S, Tomasello M, & Melis AP (2019). How chimpanzees (Pan troglodytes) share the spoils with collaborators and bystanders. Plos One, 14, e0222795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keupp S, Grueneisen S, Ludvig EA, Warneken F, & Melis AP (2021). Reduced risk-seeking in chimpanzees in a zero-outcome game. Philosophical Transactions of the Royal Society B, 376, 20190673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomen R, & Herrmann E (2018). The effects of social context and food abundance on chimpanzee feeding competition. American Journal of Primatology, 80, e22734. [DOI] [PubMed] [Google Scholar]
- Koomen R, & Herrmann E (2019). Chimpanzees overcome the tragedy of the commons with dominance. Scientific Reports, 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koops K, McGrew WC, & Matsuzawa T (2013). Ecology of culture: do environmental factors influence foraging tool use in wild chimpanzees, Pan troglodytes verus? Animal Behaviour, 85, 175–185. [Google Scholar]
- Koops K, Visalberghi E, & van Schaik CP (2014). The ecology of primate material culture. Biology Letters, 10, 20140508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krief S, Wrangham RW, & Lestel D (2006). Diversity of items of low nutritional value ingested by chimpanzees from Kanyawara, Kibale National Park, Uganda: An example of the etho-ethnology of chimpanzees. Social Science Information, 45, 227–263. [Google Scholar]
- Krupenye C, & Hare B (2018). Bonobos prefer individuals that hinder others over those that help. Current Biology, 28, 280–286. [DOI] [PubMed] [Google Scholar]
- Krupenye C, Rosati AG, & Hare B (2015). Bonobos and chimpanzees exhibit human-like framing effects. Biology Letters, 11, 20140527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Bard KA, & Hopkins WD (2019). The mismeasure of ape social cognition. Animal Cognition, 22, 487–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R (2018). emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.2.3. https://CRAN.R-project.org/package=emmeans. [Google Scholar]
- Machanda ZP, Gilby IC, & Wrangham RW (2013). Male–female association patterns among free-ranging chimpanzees (Pan troglodytes schweinfurthii). International Journal of Primatology, 34, 917–938. [Google Scholar]
- MacLean E, & Hare B (2012). Bonobos and chimpanzees infer the target of another’s attention. Animal Behaviour, 83, 345–353. [Google Scholar]
- Melis AP, Hare B, & Tomasello M (2006a). Chimpanzees recruit the best collaborator. Science, 311, 1297–1300. [DOI] [PubMed] [Google Scholar]
- Melis AP, Hare B, & Tomasello M (2006b). Engineering cooperation in chimpanzees: Tolerance constraints on cooperation. Animal Behaviour, 72, 275–286. [Google Scholar]
- Payne CLR, Webster TH, & Hunt KD (2008). Coprophagy by the semi-habituated chimpanzees of Semliki, Uganda. Pan Africa News, 15, 29–32. [Google Scholar]
- Pruetz JD, & McGrew WC (2001). What does a chimpanzee need? Using natural behavior to guide the care and management of captive populations. In Brent L (Ed.), Care and Management of Captive Chimpanzees (pp. 17–37). San Antonio: American Society of Primatologists. [Google Scholar]
- Pusey AE, Pintea L, Wilson ML, Kamenya S, & Goodall J (2007). The contribution of long-term research at Gombe National Park to chimpanzee conservation Conservation Biology, 21, 623–634. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. (2022). A language and environment for statistical computing. Vienna, Austria. Retrieved from http://www.R-project.org
- Rawlings B, Davila-Ross M, & Boysen ST (2014). Semi-wild chimpanzees open hard-shelled fruits differently across communities. Animal Cognition, 17, 891–899. [DOI] [PubMed] [Google Scholar]
- Reddy RB, & Mitani JC (2019). Social relationships and caregiving behavior between recently orphaned chimpanzee siblings. Primates, 60, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG (2019). Heterochrony in chimpanzee and bonobo spatial memory development. American Journal of Physical Anthropology, 169, 302–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, DiNicola L, & Buckholtz JW (2018). Chimpanzee cooperation is fast and independent from self-control. Psychological Science, 29, 1832–1845. [DOI] [PubMed] [Google Scholar]
- Rosati AG, Emery Thompson M, Atencia R, & Buckholtz JW (in press). Distinct developmental trajectories for risky and impulsive decision-making in chimpanzees. Journal of Experimental Psychology: General. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, Hagberg L, Enigk DK, Otali W, Emery Thomson M, Muller MN, . . . Machanda ZP. (2020). Social selectivity in aging wild chimpanzees. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, & Hare B (2011). Chimpanzees and bonobos distinguish between risk and ambiguity. Biology Letters, 7, 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, & Hare B (2012a). Chimpanzees and bonobos exhibit divergent spatial memory development. Developmental Science, 15, 840–853. [DOI] [PubMed] [Google Scholar]
- Rosati AG, & Hare B (2012b). Decision-making across social contexts: Competition increases preferences for risk in chimpanzees and bonobos. Animal Behaviour, 84, 869–879. [Google Scholar]
- Rosati AG, & Hare B (2013). Chimpanzees and bonobos exhibit emotional respones to decision outcomes. Plos One, 8, e63058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, Herrmann E, Kaminski J, Krupenye C, Melis AP, Schroepfer K, . . . Hare B. (2013). Assessing the psychological health of captive and wild apes: A response to Ferdowsian et al. (2011). Journal of Comparative Psychology, 127, 329–336. [DOI] [PubMed] [Google Scholar]
- Ross SR, & Leinwand JG (2020). A review of research in primate sanctuaries. Biology Letters, 16, 20200033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby S, & Buchanan-Smith HM (2015). The effects of individual cubicle research on the social interactions and individual behavior of brown capuchin monkeys (Sapajus apella). American Journal of Primatology, 77, 1097–1108. [DOI] [PubMed] [Google Scholar]
- Sabbi KH, Felsche E, Barnes P, Rosati AG, & Machanda ZP (2021). Chimpanzee Behavior Modules. Retrieved from https://sites.lsa.umich.edu/primatelearning/2021/06/11/chimpanzee-behavior-module-1-overview/ [Google Scholar]
- Sánchez-Amaro A, Tan J, Kaufhold SP, Fernández-Navarro S, & Rossano F (2021). How environmental unpredictability and harshness affect chimpanzees (Pan troglodytes) in risk-choice and temporal discounting tasks. Journal of Comparative Psychology. [DOI] [PubMed] [Google Scholar]
- Schneider A, Melis AP, & Tomasello M (2012). How chimpanzees solve collective action problems. Proceedings of the Royal Society of London B, 279, 4946–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijkerman RP, van Hooff JARAM, Dienske H, & Jens W (1997). Differences in subadult behaviors of chimpanzees living in peer groups and in a family group. International Journal of Primatology, 18, 439–454. [Google Scholar]
- Standley CJ, Mugisha L, Verweij JJ, Adriko M, Arinaitwe M, Rowell C, . . . Stothard JR. (2011). Confirmed infection with intestinal schistosomiasis in semi-captive wild-born chimpanzees on Ngamba Island, Uganda. Vector-Borne and Zoonotic Diseases, 11. [DOI] [PubMed] [Google Scholar]
- Stanton MA, Lonsdrof EV, Murray CM, & Pusey AE (2020). Consequences of maternal loss before and after weaning in male and female wild chimpanzees. Behavioral Ecology and Sociobiology, 74, 22. [Google Scholar]
- Stokes R, Tully G, & Rosati A (2017). Pan African Sanctuary Alliance: Primate welfare, conservation, and research. African Primatea, 12, 59–64. [Google Scholar]
- Stokes R, Tully G, & Rosati AG (2018). Pan African Sanctuary Alliance: securing a future for the African great apes. International Zoo Yearbook, 52, 1–9. [Google Scholar]
- Tan J, Kwetuenda S, & Hare B (2015). Preference or paradigm? Bonobos show no evidence of other-regard in the standard prosocial choice task. Behaviour, 152, 521–544. [Google Scholar]
- Tennie C, Call J, & Tomasello M (2012). Untrained chimpanzees (Pan troglodytes schweinfurthii) fail to imitate novel actions. Plos One, 7, e41548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson Gonzalez N, Machanda Z, Otali E, Muller MN, Enigk DK, Wrangam R, & Emery Thompson M (2021). Age-related change in adult chimpanzee social network integration. Evolution, Medicine, and Public Health, 9, 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen EJC, Bruinstroop BMC, & Haun DBM (2023). Early trauma leaves no social signature in sanctuary-housed chimpanzees (Pan troglodytes). Animals, 13, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen EJC, Cronin KA, & Haun DBM (2014). A group-specific arbitrary tradition in chimpanzees (Pan troglodytes). Animal Cognition, 17, 1421–1425. [DOI] [PubMed] [Google Scholar]
- van Leeuwen EJC, Cronin KA, & Haun DBM (2018). Population-specific social dynamics in chimpanzees. Proceedings of the National Academy of Sciences, 115, 11393–11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen EJC, Cronin KA, Haun DBM, Mundry R, & Bodamer M (2012). Neighbouring chimpanzee communities show different preferences in social grooming behaviour. Proceeding of the Royal Society of London B, 279, 4362–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen EJC, Mulenga IC, & Chidester DL (2013). Early social deprivation negatively affects social skill acquisition in chimpanzees (Pan troglodytes). Animal Cognition, 17, 407–414. [DOI] [PubMed] [Google Scholar]
- van Leeuwen EJC, Mundry R, Cronin KA, Bodamer M, & Haun DBM (2017). Chimpanzee culture extends beyond matrilineal family units. Current Biology, 27, R588–R590. [DOI] [PubMed] [Google Scholar]
- Völter CJ, Reindl E, Felsche E, Civelek Z, Whalen A, Lugosi Z, . . . Seed AM. (2022). The structure of executive functions in preschool children and chimpanzees. Scientific Reports, 12, 6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K, & Hare B (2016). Bonobo baby dominance: Did female defense of offspring lead to reduced male aggression? In Hare SYB (Ed.), Bonobos: Unique in mind, brain, and behaviour (pp. 49–64). Oxford: Oxford University Press. [Google Scholar]
- Walsh S, Bramblett CA, & Alford PL (1982). A vocabulary of abnormal behaviors in restrictively reared chimpanzees. American Journal of Primatology, 3, 315–319. [DOI] [PubMed] [Google Scholar]
- Warneken F, Hare B, Melis AP, Hanus D, & Tomasello M (2007). Spontaneous altruism by chimpanzees and young children. PLoS Biology, 5, e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DP (2008). Tool use by chimpanzees at Ngogo, Kibale National Park, Uganda. International Journal of Primatology, 29, 83–94. [Google Scholar]
- Watts DP (2011). Long-term research on chimpanzee behavioral ecology in Kibale National Park, Uganda. In Kappeler DPWPM (Ed.), Long-Term Field Studies of Primates. Heidelberg: Springer Berlin. [Google Scholar]
- Wobber V, & Hare B (2011). Psychological health of orphan bonobos and chimpanzees in African sanctuaries. Plos One, 6, e17147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobber V, Hare B, Lipson S, Wrangham R, & Ellison P (2013). Different ontogenetic patterns of testosterone production reflect divergent male reproductive strategies in chimpanzees and bonobos. Physiology & Behavior, 116–117, 44–53. [DOI] [PubMed] [Google Scholar]
- Wobber V, Hare B, Maboto J, Lipson S, Wrangham R, & Ellison PT (2010). Differential changes in steroid hormones before competition in bonobos and chimpanzees. Proceedings of the National Academy of Sciences, 107, 12457–12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobber V, Herrmann E, Hare B, Wrangham R, & Tomasello M (2014). Differences in the early cognitive development of children and great apes. Developmental Psychobiology, 56, 547–573. [DOI] [PubMed] [Google Scholar]
- Wobber V, Wrangham R, & Hare B (2010a). Application of the heterochrony framework to the study of behavior and cognition. Communicative & Integrative Biology, 3, 337–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobber V, Wrangham R, & Hare B (2010b). Bonobos exhibit delayed development of social behavior and cognition relative to chimpanzees. Current Biology, 20, 226–230. [DOI] [PubMed] [Google Scholar]
- Wrangham RW (2000). Why are male chimpanzees more gregarious than mothers? A scramble competition hypothesis. In P. M. K. (Ed.) (Ed.), Primate Males: Causes and Consequences of Variation in Group Composition (pp. 248–258). Cambridge: Cambridge University Press. [Google Scholar]
- Zuberbühler K (2014). Experimental field studies with non-human primates. Current Opinion in Neurobiology, 28, 150–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and example ethogram and data recording instructions are available at Dryad Digital Repository (permanent doi:10.5061/dryad.mkkwh715d; temporary link for review: https://datadryad.org/stash/share/S2POOyCZjBOOaGctIlHMRlmEziHxrs3-56AidxizKWw).


