Abstract
Acquired resistance to ticks can develop when animals are repeatedly exposed to ticks. Recently, acquired resistance to Ixodes scapularis was induced in guinea pigs immunized with an mRNA-lipid nanoparticle vaccine (19ISP) encoding 19 I. scapularis proteins. Here, we evaluated specific mRNAs present in 19ISP to identify critical components associated with resistance to ticks. A lipid nanoparticle containing 12 mRNAs which included all the targets within 19ISP that elicited strong humoral responses in guinea pigs, was sufficient to induce robust resistance to ticks. Lipid nanoparticles containing fewer mRNAs or a single mRNA were not able to generate strong resistance to ticks. All lipid nanoparticles containing salp14 mRNA, however, were associated with increased redness at the tick bite site – which is the first manifestation of acquired resistance to ticks. This study demonstrates that more than one I. scapularis target within 19ISP is required for resistance to ticks, and that additional targets may also play a role in this process.
Keywords: Acquired resistance to ticks, Ixodes scapularis, saliva, mRNA vaccine, Lyme disease
1. Introduction
Ticks harbor a large number of viral, bacterial and protozoal pathogens, affecting animal and human health across the globe [1–3]. In North America, Ixodes scapularis transmits many infectious agents, including Borrelia, Anaplasma and Babesia. In general, transmission of most of these microbes to humans occurs accidentally, as humans are not normally considered important hosts in the I. scapularis life cycle. When I. scapularis take a blood meal, the ticks remains attached to the vertebrate host and inject saliva into the skin. Tick saliva contains a variety of bioactive molecules capable of influencing the host immune response and facilitating blood acquisition. These compounds include inhibitors of T-cell activation, modulators of the complement pathway, histamine regulators and anticoagulants [4–7]. In addition, some I. scapularis salivary proteins influence pathogen transmission from the tick to a vertebrate host, or, vice versa, pathogen acquisition by the tick from a vertebrate [8–11].
The interactions between the vertebrate host and I. scapularis range from physiological adaptations that allow the tick to digest the blood and survive [12], as well as the dependence of components of the vertebrate immune signaling pathway that aid in tick development [13]. As shown recently, the Dome1-JAK/STAT pathway that exists in most ixodid genomes is triggered by mammalian interferon gamma (IFNγ) and plays a role in arthropod development and immunity [13].
Some animals, after being repeatedly exposed to I. scapularis, develop acquired resistance against the tick, which is also known as immunity to ticks [14]. This phenomenon is often characterized by erythema at the site of the bite, interruption of tick feeding and early detachment [15–18]. In the laboratory, the guinea pig is an excellent model for studying acquired tick resistance against I. scapularis. Numerous I. scapularis targets are likely to be associated with host immune responses that lead to acquired resistance to ticks. We previously showed that a nucleoside-modified mRNA-lipid nanoparticle vaccine (named 19ISP) containing mRNAs encoding 19 I. scapularis salivary proteins, was able to induce acquired resistance to ticks in guinea pigs [19]. When I. scapularis ticks were placed on guinea pigs immunized with 19ISP, early erythema developed at the tick bite site, I. scapularis attachment and feeding were inhibited, and tick engorgement was impaired, causing early detachment of I. scapularis from the animals. In addition, 19ISP prevented the transmission of Borrelia burgdorferi, the Lyme disease agent when B. burgdorferi-infected ticks were placed on the animals and removed when erythema was first noted. This strategy was used because people are expected to rapidly remove I. scapularis when a tick bite is identified due to redness or itching [19]. Here we examine the importance of specific components of 19ISP in the genesis of acquired resistance to ticks.
2. Materials and Methods
2.1. Ethics statement
Animal care and housing was conducted according to the instructions in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, USA. The protocols for the use of guinea pigs were approved by the Yale University Institutional Animal Care and Use Committee (YUIACUC - protocol number 2020–07941). Animal experiments were conducted in a Biosafety Level 2 animal facility as required by YUIACUC.
2.2. Ticks and animals
I. scapularis nymphs were obtained from Oklahoma State University (Stillwater, OK, USA). Ticks were maintained in an incubator at 23° C and 90 % relative humidity under a 14 h light, 10 h dark photoperiod. Five-week-old female Hartley guinea pigs (Charles River laboratory, MA) were used for immunizations and tick challenge experiments.
2.3. Formulation of mRNA-lipid nanoparticles (mRNA-LNPs)
mRNA-LNPs encoding tick salivary antigens (Table1) and a control firefly luciferase (Luc) mRNA-LNP were generated, as previously described [20]. Briefly, mRNA production plasmids containing codon optimized sequences encoding tick salivary antigens or Luc were generated (GenScript). mRNAs were transcribed with a 101 nucleotide-long poly(A) tail. To generate modified nucleoside-containing mRNA, N-1-methylpseudouridine (m1Ψ−5’)-triphosphate (TriLink) instead of UTP was used. Co-transcriptional capping was performed during the in vitro transcription using the trinucleotide cap1 analog, CleanCap (TriLink). mRNAs were purified by cellulose purification, as described [21]. The mRNA was analyzed by gel electrophoresis and frozen at −20°C. The mRNA was then encapsulated using an aqueous solution of mRNA at pH 4.0 and mixed with a solution of lipids [22, 23], consisting of an ionizable cationic lipid/ phosphatidylcholine/ cholesterol/ PEG-lipid (proprietary of Acuitas, Vancouver, Canada) (50:10:38.5:1.5 mol/mol). For encapsulation, RNA was mixed with the lipids at a ratio of ~0.05 (wt./wt.). The LNP had a diameter of ~80 nm as measured by dynamic light scattering using a Zetasizer Nano ZS (Malvern Instruments Ltd, Malvern, UK) instrument, and stored at −80°C.
Table 1. List of genes tested individually and for generation of new mRNA-LNP vaccine combinations.
All genes were previously incorporated into the 19ISP mRNA-LNP vaccine [19].
| Gene | Protein accession | mRNA Combinations | |||||
|---|---|---|---|---|---|---|---|
| 12ISP | 8ISP | 7ISP | 6ISP | 4ISP | 3ISP | ||
| Salp14 | AAK97824/AF209921 | Salp14 | Salp14 | Salp14 | Salp14 | ||
| TIX5 | AEE89467/HQ605984 | TIX5 | TIX5 | TIX5 | |||
| P32 | ADO95260/HM802761 | P32 | P32 | P32 | P32 | ||
| Salp26A | AAK97822/AF209919 | Salp26A | |||||
| TSLPI | HQ605983/AEE89466 | TSLPI | TSLPI | TSLPI | TSLPI | ||
| Salp15 | AAK97817/AF209914 | Salp15 | Salp15 | Salp15 | |||
| Salp25D | AAK97814/AF209911 | Salp25D | Salp25D | Salp25D | |||
| P11 | DQ066011/AAY66648 | P11 | |||||
| SG27 | XP_002405832/XM_002405788 | SG27 | SG27 | SG27 | SG27 | ||
| SG09 | XP_002411435/XM_002411390 | SG09 | SG09 | SG09 | |||
| SG10 | XP_002411436/XM_002411391 | SG10 | SG10 | SG10 | SG10 | ||
| IsPDIA3 | XP_002406442/XM_002406398 | IsPDIA3 | IsPDIA3 | IsPDIA3 | IsPDIA3 | IsPDIA3 | IsPDIA3 |
2.4. Guinea pig immunization
Five-week-old female guinea pigs were immunized intradermally with mRNA-LNPs and received 2 booster doses at 4 and 8 weeks. The animals immunized with a single mRNA-LNP received 20 μg of each (Salp14, IsPDIA3, TIX5, P32, Salp26A, SG27, TSLPI, Salp15, Salp25D, SG10, P11, or SG09) or luciferase mRNA. Additional animals immunized with the combinations named 12ISP, 8ISP, 7ISP, 6ISP, 4ISP, 3ISP (Table1) which contained 3 μg for each mRNA present in the group. In addition, guinea pigs were immunized with 19ISP as previously described [19] for comparison. Two weeks after the last booster, 500 μl of blood from the guinea pigs was collected retro-orbitally and the sera used in enzyme linked immunosorbent assay (ELISA). For screening assays, one animal for each mRNA was used. 5 animals per group were used for the assay comparing the control, 12ISP and 19ISP vaccine.
2.5. Tick challenge
Guinea pigs were anesthetized by intramuscular injection with a mixture of 40 mg/kg ketamine/xylazine. After the backs of guinea pigs were carefully shaved, 30 I. scapularis nymphs were applied to the shaved area. The animals were housed individually in cages after the ticks attached to the skin. Animals were monitored daily for evidence of tick rejection, recovery, and erythema at the bite site.
2.6. ELISA assessment
ELISAs were performed to determine antigen-specific antibody responses in the immunized animals. 96-well plates were coated overnight at 4 °C with 250 ng of recombinant protein diluted in carbonate-bicarbonate buffer pH 9.6, washed with PBST (PBS with 0.05% Tween 20) and blocked with 3% BSA (bovine serum albumin) for 1 hour at 37° C. Sera were serially diluted (1:500, 1:5000, or 1:50,000) and incubated for 2 hours at 37° C. The wells were washed with PBST and incubated with the secondary goat anti-guinea pig IgG-HRP antibody diluted 1:2000 (Thermo Fisher, Waltham, A, USA) for 1 hour. TMB HRP substrate solution was added and incubated, followed by addition of TMB stop solution. The absorbance was read at 450 nm.
2.7. Statistical analysis
Statistical analysis was performed using Prism 9.5.1 software (GraphPad Software, CA). Data are represented as mean ± standard error of the mean (SEM). Statistical significance between control and experimental groups was determined by two-way ANOVA, or the Mann Whitney test. P ≤ 0.05 was considered statistically significant. The number (n) of animals used in each experiment is indicated in the figure legend.
3. Results
3.1. Immunization of guinea pigs with mRNA-LNPs encoding individual I. scapularis salivary proteins.
An mRNA-LNP vaccine, named 19ISP, targets tick proteins that are primarily produced in saliva [19]. This LNP contains 19 mRNAs encoding I. scapularis proteins and induce acquired resistance to ticks in guinea pigs [19]. 19ISP prevented tick-borne transmission of the Lyme disease agent, B. burgdorferi to guinea pigs [19]. Immunization of guinea pigs with 19ISP elicited substantial antibodies against 10 of the 19 targets within the LNP [19], and humoral responses have been associated with acquired resistance to ticks [24, 25]. Based on the presence of antibodies to these 10 proteins, we determined whether LNPs containing a single mRNA encoding each of these 10 targets could induce any aspect of acquired resistance to ticks, including erythema at the tick bite site, decreased tick attachment, or reduced tick engorgement. In our initial analysis, in addition to these 10 targets, we also included P11 which had a weak antibody response upon 19ISP immunization and SG09 whose antibody response was not directly tested in our previous study since SG09 and SG10 share 75% identity [19].
LNPs containing each of the following individual mRNAs - Salp14, IsPDIA3, TIX5, P32, Salp26A, SG27, TSLPI, Salp15, Salp25D, SG10, P11, or SG09 - were generated (Table1). LNPs containing Luc-encoding mRNA, which previously has been demonstrated not to impact tick feeding, was used as a negative control [26]. In this screening assay with many candidates, one guinea pig was used for immunization with each individual LNP, and 30 I. scapularis nymphs were placed on each animal, and the effect on numerous ticks was thereby examined. To evaluate acquired resistance to ticks, animals were challenge with I. scapularis 2 weeks after the final booster dose and monitored daily for evidence of tick detachment (Fig. 1 A), engorgement (Fig. 1 B) and erythema at the bite site (Fig. 1 C). Immunization using single mRNA-LNPs showed that the pattern of tick attachment was not significantly affected (Fig. 1 A). All mRNA-LNP candidates also showed similar results regarding tick weights (engorgement) and no difference was observed compared to the control (Fig.1 B). The animals were also monitored and photographed daily for the presence of erythema (Fig. 2). Redness at the site of the tick bite in the first 24 hours is an important indicator of an immune response to the tick and one of the first markers of acquired resistance to ticks [16, 27]. The guinea pig immunized with the LNPs containing salp14 mRNA developed the most robust erythema, which was also evident at an early point, 24 hours, after tick attachment (Fig. 1 C and Fig. 2). The LNPs containing SG09 also elicited some erythema at 24 hr at some of the tick bite sites (Fig. 1C), but the redness was not as robust as that induced by Salp14. The LNPs containing IsPDIA3 demonstrated modest erythema at some of the tick bite sites at 24 hr which increased at later time points (Fig. 1C and Fig. 2). The degree of erythema at each tick bite site for all the targets was much less than that induced by the salp14 mRNA-LNP, and IsPDIA3 mRNA-LNP is presented as a representative comparative example (Fig. 2). These data suggest that individual mRNAs within the 19ISP cocktail are not sufficient to induce robust acquired resistance to ticks, and that Salp14 is the main target within 19ISP that elicits profound early erythema, notable at 24 hr after a tick bite.
Figure 1. mRNA-LNP screening: Tick feeding kinetics on guinea pigs immunized with LNPs containing a single mRNA.
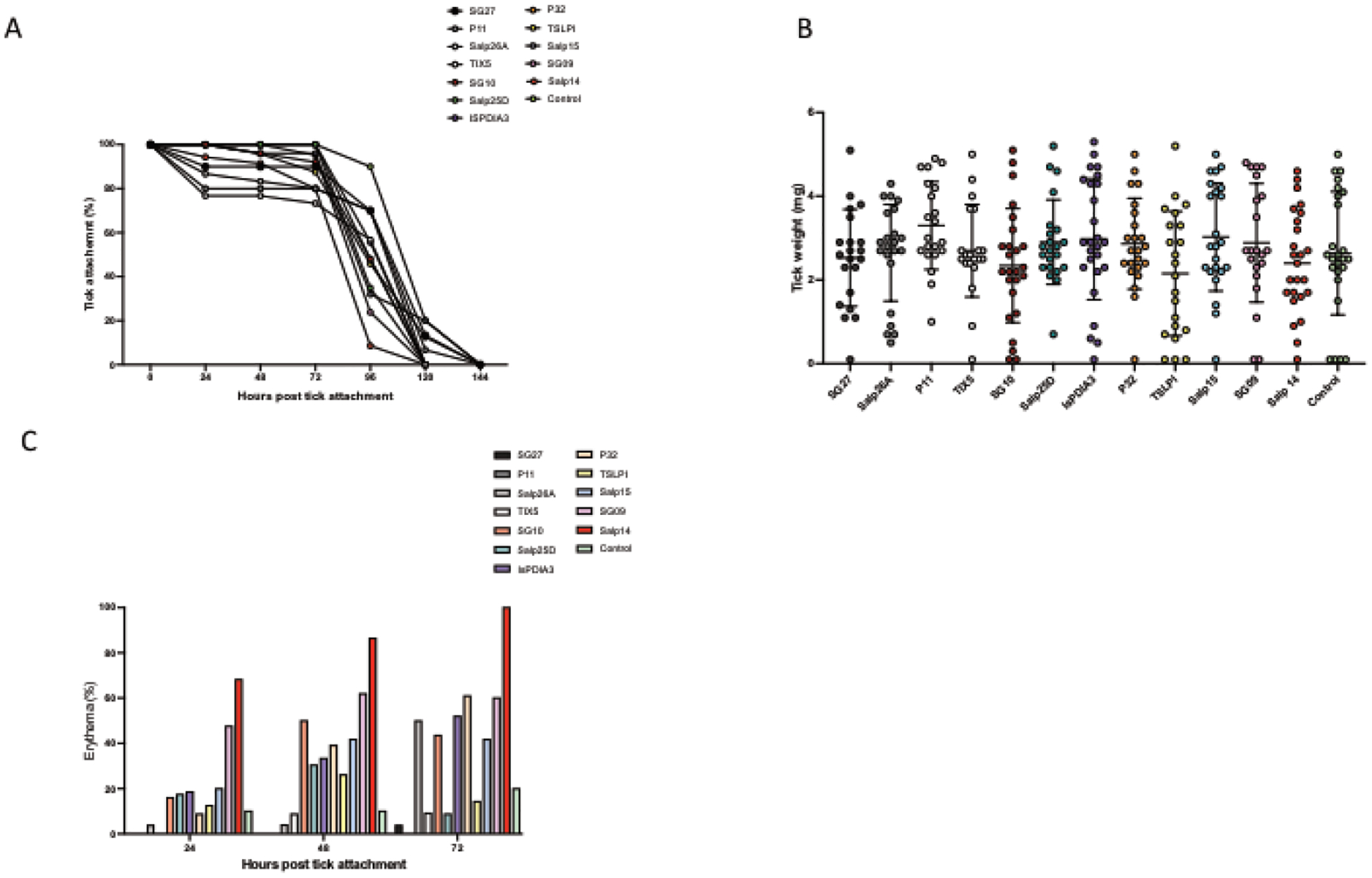
Guinea pigs were immunized three times with a single mRNA-LNP (A-C) and challenged with 30 Ixodes scapularis nymphs. Evidence of tick rejection and the tick feeding kinetics were monitored for the duration of the experiment and the graph shows the percentage of ticks that remain attached, and the detachment at a given time point (A). The success of tick feeding was determined by examining engorgement weights of the recovered ticks (B). Erythema at each tick bite site was calculated as the percent of nymphs (30 per animal as 100%) showing redness on each animal (C). One animal (n=1) was used for each mRNA, totaling 12 animals.
Figure 2. Examples of erythema induced at the bite site during tick challenge of selected mRNA LNP immunized animals.
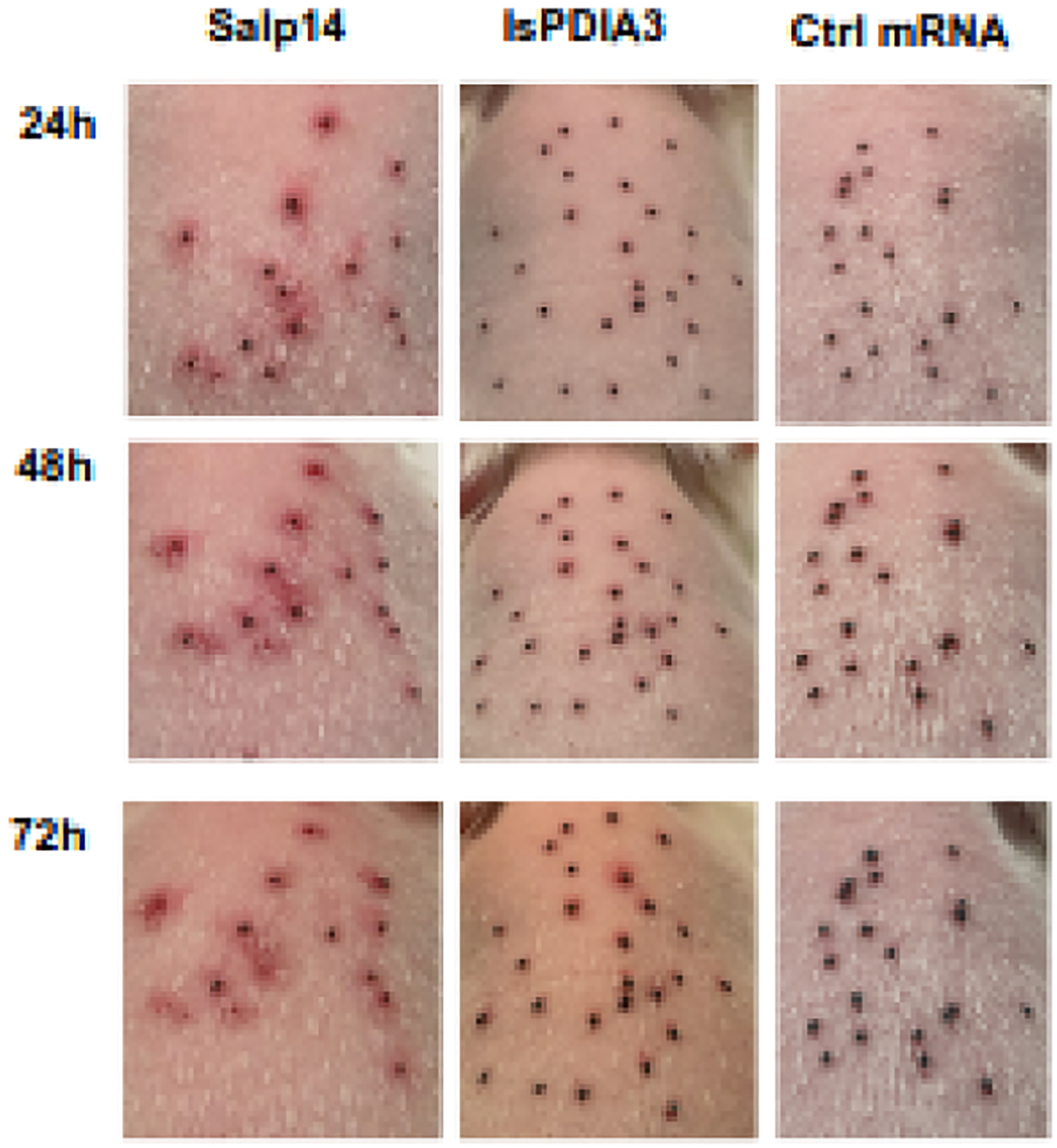
Guinea pigs were monitored following the tick challenge with 30 I. scapularis nymphs and erythema at the bite site was photographed until all ticks detached. The representative images show the backs of the animals immunized with Salp14 mRNA-LNP (severe erythema), IsPDIA3 mRNA-LNP (modest erythema) or control Luc mRNA-LNP (minimal erythema) at the indicated time points.
3.2. 12ISP mRNA-LNP induces acquired resistance to ticks.
As individual mRNA-LNPs encoding each of these 12 targets within 19ISP did not induce robust acquired resistance to ticks, we generated a cocktail that contained all 12 of these mRNAs. A new mRNA-LNP vaccine, named 12ISP, contained Salp14, IsPDI3, TIX5, P32, Salp26A, SG27, TSLPI, Salp15, Salp25D, SG10, P11, and SG09 mRNAs. Groups of 5 guinea pigs were immunized 3 times with 12ISP, or with 19ISP (positive control) or a Luc mRNA-LNP (negative control). Two weeks after the last immunization, the animals were challenged with I. scapularis and monitored to assess erythema, tick detachment, recovery, and engorgement. Our data shows that 12ISP induced tick detachment results similar to those obtained with 19ISP (Fig. 3 A). Both the 12ISP and 191SP groups showed a significant reduction in engorgement weight compared to the control (Fig. 3 B). The appearance of erythema was detected in both the 12ISP and 19ISP groups by 24 hours after tick attachment (Fig. 3 C). The animals were monitored and erythema at the bite site was photographed until all ticks detached. Strong erythema was observed in all groups except the control animals (Fig. 4).
Figure 3. 12ISP and 19ISP have similar effects on tick feeding and erythema at the tick bite side.
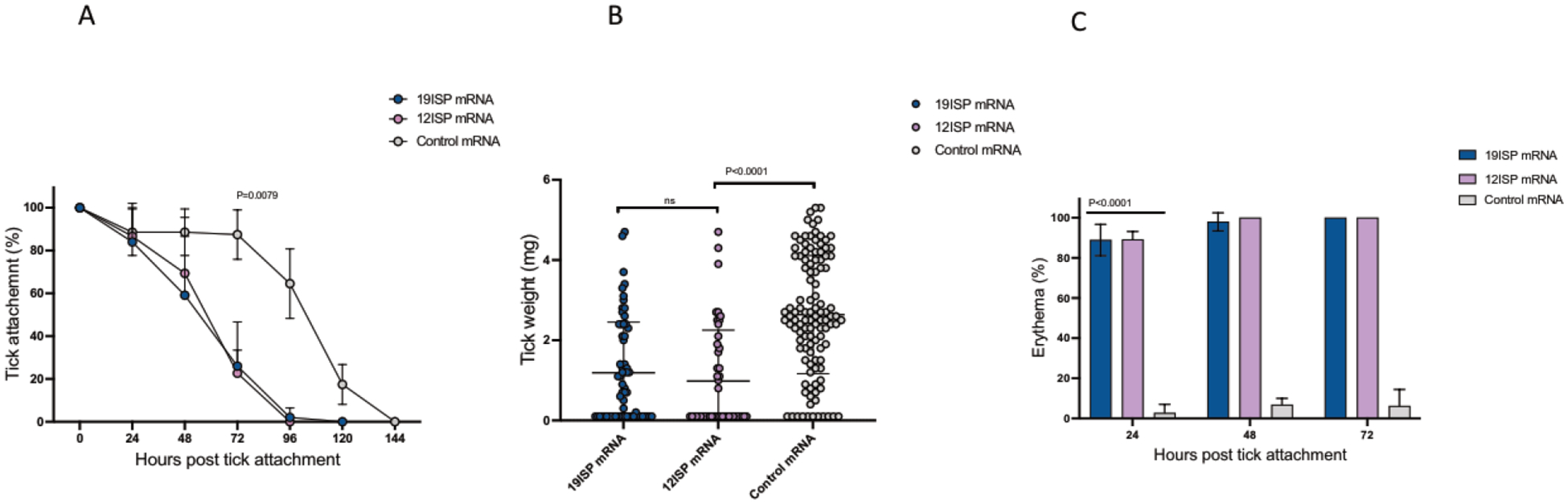
Guinea pigs were immunized three times with 12ISP (n=5), 19ISP (n=5) or control Luc mRNA-LNP (n=5) and each challenged with 30 I. scapularis nymphs two weeks after the last immunization. The animals were monitored to assess erythema, tick detachment, recovery, and engorgement. The graph shows the percentage of ticks that remain attached and the detachment at a given time point (A). The success of tick feeding was determined by examining engorgement weights of the recovered ticks (B). Erythema at each tick bite site was calculated as the percent of nymphs (30 per animal as 100%) showing redness on each animal (C). Animals immunized with 19ISP and 12ISP were compared with each other and statistical significance was determined by Mann Whitney test, P value = 0.0079 (A), P value <0.0001 (B); (C) two-way ANOVA; P value < 0.0001. The error bars represent mean with SD.
Figure 4. Comparison of erythema induced at the bite site of 12ISP and 19ISP mRNA-LNP vaccinated animals.
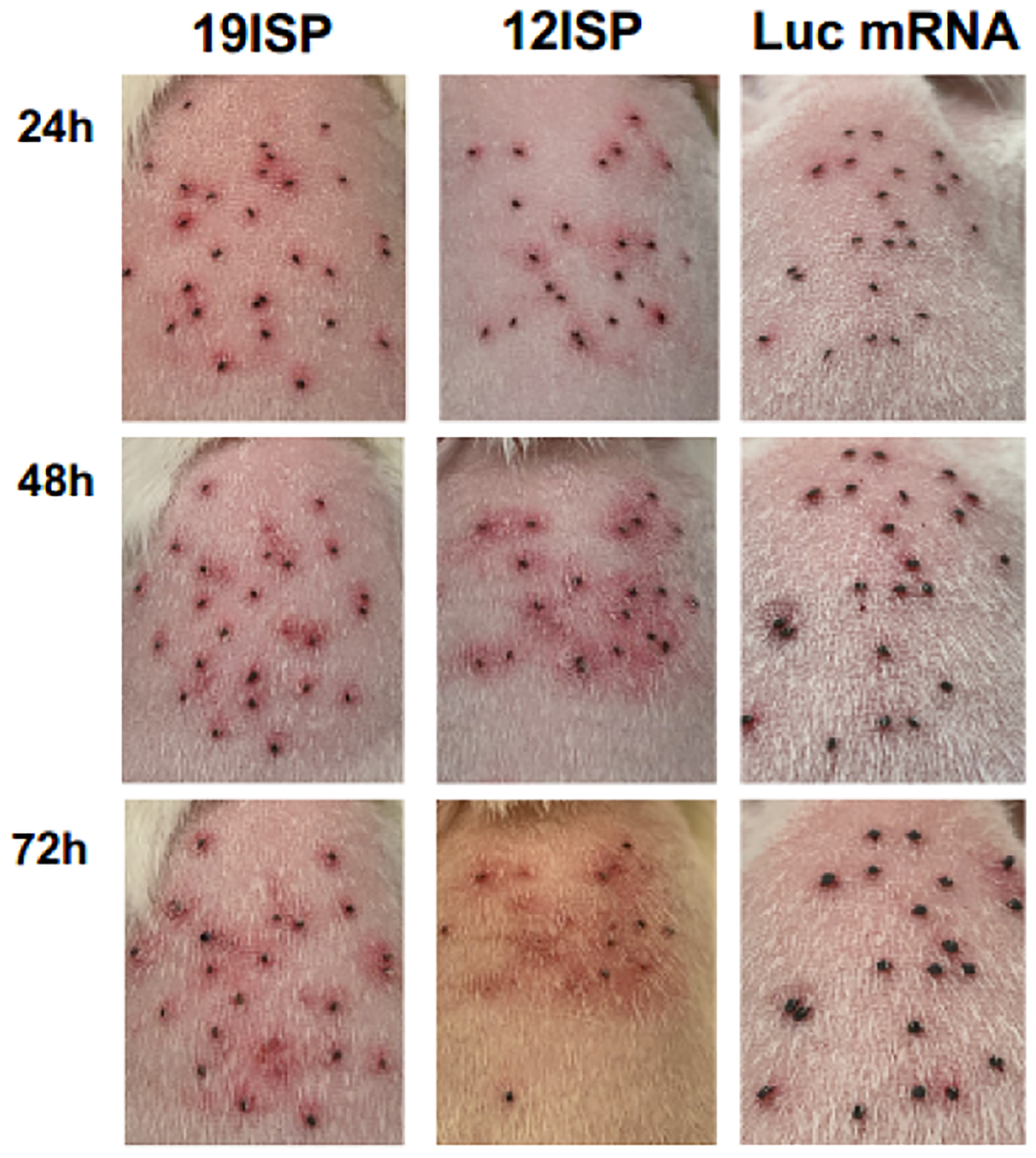
Guinea pigs were monitored following the tick challenge and erythema at the bite site was photographed until all ticks detached. The images show the backs of the animals representing 12ISP or 19ISP and control animals at the time points (24, 48 and 72 hours). 12ISP and 19ISP mRNA-LNPs showed strong early redness at 24 hours, compared with minimal erythema in the control animals.
As 12ISP elicited robust resistance to ticks, we generated smaller cocktails containing different combinations of the mRNAs incorporated into 12ISP, named 8ISP, 7ISP, 6ISP, 4ISP, 3ISP (Table1). We designed the different cocktails to potentially eliminate excessive proteins and identify critical components. Animals immunized with the various combinations (12ISP, 8ISP, 7ISP, 6ISP, 4ISP and 3ISP) received the same amount (3 μg) of each mRNA present in the group. Immunizations using different mRNA combinations demonstrated that 12ISP, 8ISP and 7ISP resulted in tick detachment within 24 hours (Fig. 5 A). The guinea pigs immunized with 12ISP showed the best rejection, with most ticks detached by 72 hours (Fig. 5 A). Additionally, 12ISP was the only mRNA-LNP combination to show a clear difference in tick engorgement weight compared to control (Fig. 5 B). Erythema at the tick bite site was noted at 24 hours in the 12ISP and 8ISP groups (Fig. 5 C). Erythema appeared in the 7ISP, 6ISP, 4ISP and 3ISP groups at later time points; however, 12ISP showed the most intense redness compared to all the other groups and control (Fig. 5 C and D).
Figure 5. Screen of candidate mRNA-LNP cocktails: Tick feeding kinetics on immunized guinea pigs with different combinations of mRNA-LNPs.
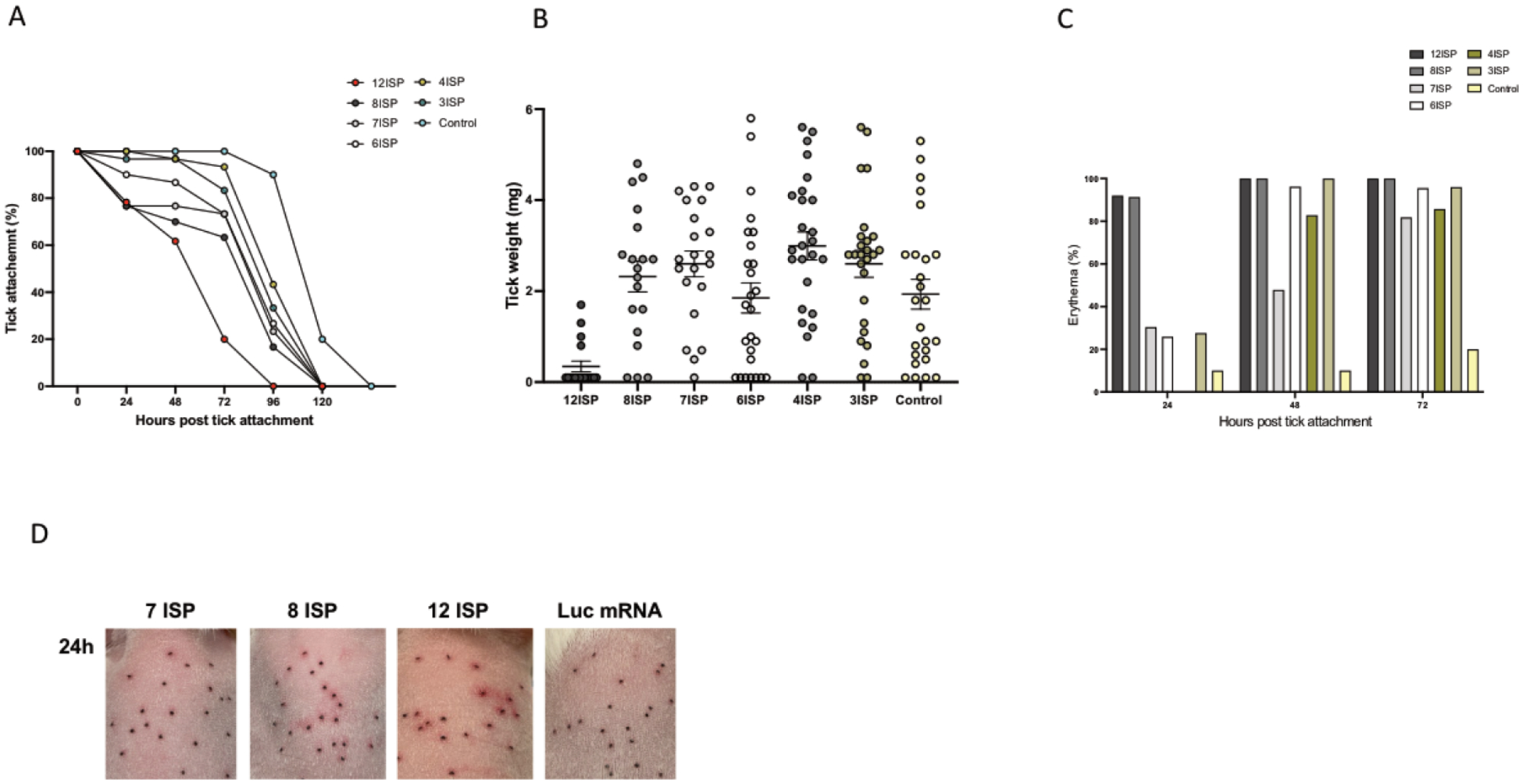
Guinea pigs were immunized three times with six different combinations of mRNA-LNPs and challenged with I. scapularis nymphs. Evidence of tick rejection and the tick feeding kinetics were monitored for the duration of the experiment and the graph shows the percentage of ticks that remain attached and the detachment at a given time point (A). The success of tick feeding was determined by examining engorgement weights of the recovered ticks (B). Erythema at each tick bite site was calculated as the percent of nymphs (30 per animal) showing redness on each animal (C). One animal (n=1) was used for each mRNA-LNP combination plus Luc control animal, totaling 7 animals. The images show erythema on the backs of animals representing 7ISP, 8ISP, 12ISP or control animals at the indicated time point (D).
3.3. Humoral immune response elicited by 12ISP mRNA-LNP immunization.
After immunizations with the cocktail 12ISP mRNA-LNP, sera were obtained from each animal before the tick challenge. Antigen-specific antibody titers were assessed by ELISA, using the serum dilutions (1:500, 1:5000 and 1:50,000). Guinea pigs immunized with the 12ISP mRNA-LNP elicited antibodies against 11 recombinant proteins tested (Salp14, Salp15, Salp15D, Salp26A, TSLPI, IsPDIA3, TIX5, P32, P11, SG10, SG27) (Fig. 6 A). Specific antibodies for SG09 were not measured in any of the animals that received the immunization since SG09 and SG10 share 75% identity. Additionally, serum from 19ISP animals was tested and antibodies were detected for Salp14, Salp15, Salp25D, Salp26A, TSLPI, IsPDIA3, TIX5, P32, SG10 and SG27 (Fig. 6 B). These results are consistent with our study published previously [19]. The 12ISP results suggest that 12ISP immunization elicited an antibody response against the same proteins detected in the 19ISP vaccine.
Figure 6. 12ISP and 19ISP mRNA-LNP immunizations elicit antibody responses to specific I. scapularis antigens.
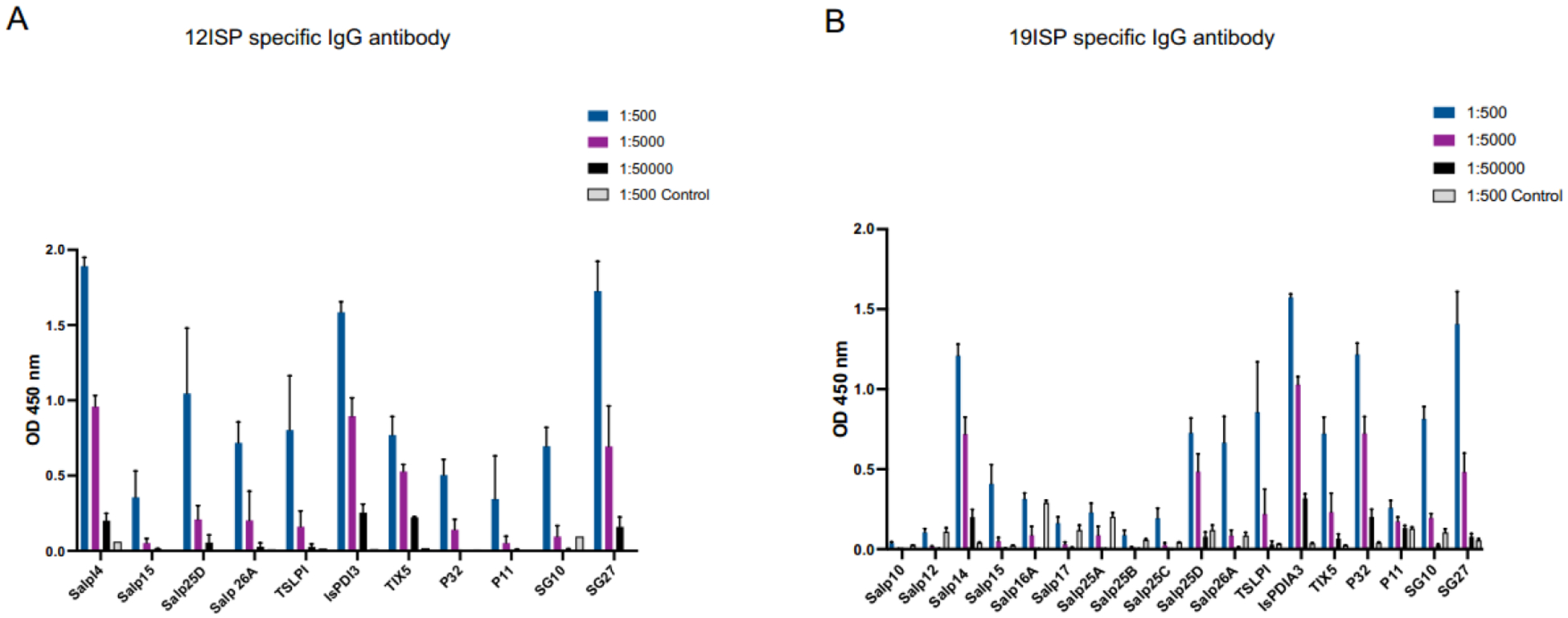
Two weeks after the last immunization, sera from 19ISP (n=5) and 12 ISP (n=5) animals were collected to assess the humoral immune response. The serum sample binding to specific recombinant proteins corresponding to the mRNAs in each group. IgG antibodies were detected by ELISA with basis of optical density (OD) 450 nm. Sera were diluted 1:500, 1:5000, 1:50,000 and control sample at 1:500. The animals immunized with 12ISP (A) had detectable antibodies against all recombinant proteins used (Salp14, IsPDI3, TIX5, P32, Salp26A, SG27, TSLPI, Salp15, Salp25D, SG10 and P11) and 19ISP (B) for 10 proteins (Salp14, Salp15, Salp25D, Salp26A, TSLPI, IsPDIA3, TIX, P32, SG10 and SG27). Data are represented as mean ± SEM.
4. Discussion
Salivary components have been associated with acquired resistance to ticks, as they are secreted into the host during a tick bite and are the prime targets for the immune response [10, 25, 28]. Determining which specific targets can be used as potential vaccine candidates has remained a major challenge since the first report of acquired resistance to ticks by Trager in the 1930s [29]. In our recent publication, we showed that a nucleoside-modified mRNA-LNP vaccine containing a combination of 19 mRNAs encoding for diverse I. scapularis proteins was able to elicit acquired resistance to ticks in guinea pigs [19]. The relative contribution of each of these 19 components in inducing resistance to ticks is not clear. A recent study by our group demonstrated that an mRNA-LNP vaccine containing salp14 is capable of inducing erythema in guinea pigs exposed to I. scapularis [26], but not other aspects of acquired resistance to ticks, including tick attachment and tick engorgement.
As humoral responses have been associated with resistance to ticks, we selected 12 targets within the original 19ISP cocktail (Table 1) for further examination as individual targets. Surprisingly, none of the 12 individual target was able to influence tick detachment or engorgement weight (Fig. 1 A and B). Consistent with our previous data [26], salp14 mRNA LNP induced early erythema at the tick bite site (Fig. 1 C and Fig. 2). None of the other 11 targets induced robust early erythema similar to Salp14. However, there was some erythema with other targets over time. The development of early erythema is very important when considering the factors that may help a human with tick removal, as many tick bites go undetected, and early tick recognition and removal can help to prevent pathogen transmission [19]. Once pathogens have migrated from the tick to the mammalian host, the removal of ticks is no longer as consequential.
A combined mRNA-LNP vaccine that comprised 12 targets, 12ISP, elicited humoral immune responses to the antigens similar to 19ISP. 12ISP also induced acquired resistance to ticks with erythema, increased tick detachment and diminished tick weights comparable to 19ISP, indicating that not all the components of 19ISP are necessary for generating the tick-resistance. Combinations smaller than 12ISP were also tested (Table 1) and none of the 5 groups (8ISP, 7ISP, 6ISP, 4ISP, 3ISP) that were examined showed better results regarding tick detachment, engorgement, and erythema than the 12ISP combination. Moreover, combined mRNA-LNP vaccines that included Salp14, including 12ISP, 8ISP, 6ISP and 3ISP, induced the most erythema. This reinforces the observation that Salp14 plays a dominant role in eliciting erythema but is not the only target that contributes to the genesis of erythema. Overall, the permutations of different combinations within 19ISP is extremely large and we have attempted one logical approach to examine the relative importance of the targets. These results suggest that the interaction between the mRNA-LNP vaccine targets that make up the 12ISP cocktail are essential to evoke the acquired resistance to ticks that was evident with 19ISP [19].
These studies demonstrate that both 12ISP and 19ISP elicit all aspects of acquired resistance to ticks, including erythema, increased tick detachment and decreased tick weight. Salp14 remains a major inducer of early robust erythema, but other antigens may contribute to this effect as well. Components of 12ISP did not elicit strong resistance to ticks. This suggests that a cocktail of antigens is required to induce robust resistance to ticks. Moreover, additional antigens in tick saliva that are not present in either 12ISP or 19ISP may also contribute to resistance to ticks and should be examined. It is also possible that hidden targets in the tick gut, which are not normally recognized by the host during tick feeding could serve as additional targets to induce a host to develop an immune response that can irritate the tick gut during blood feeding and interfere with the tick life cycle. Our study demonstrates that acquired resistance to ticks can be recapitulated by mRNA-LNP immunization, and 12ISP represents one combination that can induce robust immunity. Elucidating all the tick antigens that contribute to tick resistance, individually or collectively, remains an important and feasible goal.
Acknowledgements
This work was support by NIH grants AI165499 and AI138949, the Steven and Alexandra Cohen Foundation, and the Howard Hughes Medical Institute Emerging Pathogens Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Barbour AG, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–6. [DOI] [PubMed] [Google Scholar]
- [2].Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897–928. [DOI] [PubMed] [Google Scholar]
- [3].Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129 Suppl:S3–14. [DOI] [PubMed] [Google Scholar]
- [4].Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, et al. Salp15, an ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity. 2002;16:849–59. [DOI] [PubMed] [Google Scholar]
- [5].Schuijt TJ, Coumou J, Narasimhan S, Dai J, Deponte K, Wouters D, et al. A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent. Cell Host Microbe. 2011;10:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dai J, Narasimhan S, Zhang L, Liu L, Wang P, Fikrig E. Tick histamine release factor is critical for Ixodes scapularis engorgement and transmission of the lyme disease agent. PLoS Pathog. 2010;6:e1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schuijt TJ, Bakhtiari K, Daffre S, Deponte K, Wielders SJ, Marquart JA, et al. Factor Xa activation of factor V is of paramount importance in initiating the coagulation system: lessons from a tick salivary protein. Circulation. 2013;128:254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kurokawa C, Lynn GE, Pedra JHF, Pal U, Narasimhan S, Fikrig E. Interactions between Borrelia burgdorferi and ticks. Nat Rev Microbiol. 2020;18:587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tang X, Arora G, Matias J, Hart T, Cui Y, Fikrig E. A tick C1q protein alters infectivity of the Lyme disease agent by modulating interferon gamma. Cell Rep. 2022;41:111673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Das S, Banerjee G, DePonte K, Marcantonio N, Kantor FS, Fikrig E. Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J Infect Dis. 2001;184:1056–64. [DOI] [PubMed] [Google Scholar]
- [11].Murfin KE, Kleinbard R, Aydin M, Salazar SA, Fikrig E. Borrelia burgdorferi chemotaxis toward tick protein Salp12 contributes to acquisition. Ticks Tick Borne Dis. 2019;10:1124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kitsou C, Fikrig E, Pal U. Tick host immunity: vector immunomodulation and acquired tick resistance. Trends Immunol. 2021;42:554–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rana VS, Kitsou C, Dutta S, Ronzetti MH, Zhang M, Bernard Q, et al. Dome1-JAK-STAT signaling between parasite and host integrates vector immunity and development. Science. 2023;379:eabl3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Trager W Acquired immunity to ticks. J. Parasitol 1939; 25: 57–81. [Google Scholar]
- [15].Narasimhan S, Booth CJ, Philipp MT, Fikrig E, Embers ME. Repeated Tick Infestations Impair Borrelia burgdorferi Transmission in a Non-Human Primate Model of Tick Feeding. Pathogens. 2023;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kurokawa C, Narasimhan S, Vidyarthi A, Booth CJ, Mehta S, Meister L, et al. Repeat tick exposure elicits distinct immune responses in guinea pigs and mice. Ticks Tick Borne Dis. 2020;11:101529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gebbia JA, Bosler EM, Evans RD, Schneider EM. Acquired resistance in dogs to repeated infestation with Ixodes scapularis (Acari: Ixodidae) reduces tick viability and reproductive success. Exp Appl Acarol. 1995;19:593–605. [DOI] [PubMed] [Google Scholar]
- [18].Hewetson RW. The inheritance of resistance by cattle to cattle tick. Aust Vet J. 1972;48:299–303. [DOI] [PubMed] [Google Scholar]
- [19].Sajid A, Matias J, Arora G, Kurokawa C, DePonte K, Tang X, et al. mRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent. Sci Transl Med. 2021;13:eabj9827. [DOI] [PubMed] [Google Scholar]
- [20].Freyn AW, Ramos da Silva J, Rosado VC, Bliss CM, Pine M, Mui BL, et al. A Multi-Targeting, Nucleoside-Modified mRNA Influenza Virus Vaccine Provides Broad Protection in Mice. Mol Ther. 2020;28:1569–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Baiersdorfer M, Boros G, Muramatsu H, Mahiny A, Vlatkovic I, Sahin U, et al. A Facile Method for the Removal of dsRNA Contaminant from In Vitro-Transcribed mRNA. Mol Ther Nucleic Acids. 2019;15:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Maier MA, Jayaraman M, Matsuda S, Liu J, Barros S, Querbes W, et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol Ther. 2013;21:1570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jayaraman M, Ansell SM, Mui BL, Tam YK, Chen J, Du X, et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed Engl. 2012;51:8529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brown SJ, Askenase PW. Immune rejection of ectoparasites (ticks) by T cell and IgG1 antibody recruitment of basophils and eosinophils. Fed Proc. 1983;42:1744–9. [PubMed] [Google Scholar]
- [25].Narasimhan S, Kurokawa C, Diktas H, Strank NO, Cerny J, Murfin K, et al. Ixodes scapularis saliva components that elicit responses associated with acquired tick-resistance. Ticks Tick Borne Dis. 2020;11:101369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Matias J, Kurokawa C, Sajid A, Narasimhan S, Arora G, Diktas H, et al. Tick immunity using mRNA, DNA and protein-based Salp14 delivery strategies. Vaccine. 2021;39:7661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nazario S, Das S, De Silva A, Deponte K, Marcantonio N, Anderson JF, et al. Prevention of Borrelia burgdorferi transmission in guinea pigs by tick immunity. American Journal of Tropical Medicine and Hygiene. 1998;58:780–5. [DOI] [PubMed] [Google Scholar]
- [28].Schuijt TJ, Narasimhan S, Daffre S, DePonte K, Hovius JW, Van’t Veer C, et al. Identification and characterization of Ixodes scapularis antigens that elicit tick immunity using yeast surface display. PLoS One. 2011;6:e15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Trager W Acquired immunity to ticks. J. Parasitol 1939; 25: 57–81. [Google Scholar]


