Abstract
PURPOSE:
Despite increasing availability of biosimilar cancer treatments, little is known about oncologists' knowledge and concerns regarding biosimilar use in the United States. We surveyed medical oncologists to examine their knowledge, attitudes, and experience with biosimilars.
METHODS:
Oncologists recruited via the ASCO Research Survey Pool completed a 29-question survey in 2020 designed with input from clinical and health care system experts and literature review.
RESULTS:
Of the 269 respondents, most treated patients with biosimilars (n = 236, 88%) and reported that biosimilars were required at their institution (n = 168, 63%). Approximately half (n = 140, 52%) of oncologists correctly responded that biosimilars were not the same as generic medicines. Commonly reported barriers to use of biosimilars included concerns regarding a perceived lack of relevant research (n = 85, 33% reporting quite a bit/very much), the potential for extrapolation (n = 83, 33%), and efficacy limitations (n = 77, 30%). More oncologists from university hospitals (n = 36, 22%) than from community/private hospitals (n = 28, 38%) or private practices (n = 13, 38%) were concerned about biosimilar efficacy. A high proportion of oncologists reported that information on safety (n = 259, 99%) and efficacy (n = 255, 99%) is important when considering whether to use biosimilars. Less than half reported that their institution provided education about biosimilars (n = 108, 40%).
CONCLUSION:
In this sample of medical oncologists, knowledge about basic features of biosimilars was limited and access to information about biosimilars was insufficient. The present study determined that educational programs on biosimilars for oncologists are needed and identified priorities for such efforts.
INTRODUCTION
To assist in cost containment across medicine, the Biologics Price Competition and Innovation Act of 2009 was enacted to create an abbreviated approval pathway for biological products that are very similar to approved biologics.1 Where patents for successful biologics are expiring, biosimilars are rising to take their place, promising significant reductions in costs without compromising the safety and efficacy of their associated reference drugs (bio-originators).
Since the first biosimilar was approved in the United States by the US Food and Drug Administration (FDA) in 2015, the FDA has approved 33 biosimilar products, and 17 of these are approved for use in cancer treatment or supportive care.2 Biosimilars have been developed and marketed as lower-cost alternatives to newer biologic treatments that have the potential to drive competitive pricing with bio-originators. Regarding oncology specifically, patents that expired on multiple biologic products in 2020 account for more than $20 billion US dollars in health care spending, and biosimilars are expected to claim larger market share.3
It is likely that many oncologists are currently, or will soon be, given the opportunity to prescribe or asked to substitute a biosimilar for a bio-originator on which they have previously relied. Despite the emergence of biosimilars, it is unclear to what extent oncologists are knowledgeable about and comfortable with this relatively new class of drugs.4 Uptake has been dampened by a number of concerns including (1) oncologist uncertainty if safety and efficacy evidence supports interchangeability with the bio-originator, (2) the complexity and dynamic nature of payer formularies and reimbursement rules, and (3) potential patient acceptance of use of a biosimilar.5,6 A 2017 survey of 1,201 international physicians in a variety of specialties (including oncology) indicated a need for knowledge-based education about biosimilars.7 This research highlighted specific gaps in knowledge about biosimilars, such as the inability to select the correct definition of the term interchangeability as related to biosimilar regulation,7 and a poor ability to articulate the difference between biosimilars and generics.8 Given growing evidence for this issue, it is critical that we better understand oncologists' knowledge and attitudes regarding biosimilars, as well as their comfort prescribing these drugs, as these factors may affect prescribing habits and, ultimately, influence patient outcomes.
In line with this need, ASCO released a statement with guidance for the oncology community around provider and patient education on biosimilars.9 To maximize these educational recommendations and develop targeted biosimilar education programs for oncologists, more evidence is needed on oncologists' specific concerns and knowledge deficits. To fill this knowledge gap, we surveyed medical oncologists to assess their knowledge and attitudes about biosimilars. More specifically, we aimed to (1) generate estimates of medical oncologists' overall levels of knowledge and characterize their attitudes about biosimilars and (2) determine if medical oncologists' knowledge and attitudes about biosimilars vary among the type of institution at which they practice. The overall objective of the study was to generate evidence to guide future educational initiatives on biosimilars for oncologists.
METHODS
Participants
We recruited a sample of oncologists to participate in a survey through the ASCO Research Survey Pool (RSP). The ASCO RSP is administered by ASCO's Center for Research and Analytics and consists of domestic and international ASCO members who have opted-in to participate in research survey projects. ASCO members complete an online consent process when they agree to be a part of the ASCO RSP; this consent applies to all surveys they receive via the RSP. Members of the research pool are invited to participate in studies through e-mail invitations. If they agree, participants use a unique link to an online survey form. Members of the ASCO RSP were eligible to participate in this study if they currently (1) practice actively in the United States, (2) have a caseload of ≥ 5 patients with cancer, and (3) treat patients with a cancer therapy that includes either oral medications or infusions. The survey was opened on October 6, 2020, and closed on November 16, 2020. Two reminder e-mails were sent to invitees on October 29, 2020, and November 10, 2020. Participants were entered into a raffle for a $500 US dollars Amazon gift card. After the survey was closed, ASCO Center for Research and Analytics delivered a deidentified data set with the survey responses to the study team at Northwestern University. The protocol for this survey study was approved by the Northwestern University institutional review board (STU00208615).
Oncologist Biosimilar Survey
A survey was designed by the study team for the purpose of this project. Topics and content for survey questions were identified from two sources: (1) published literature on biosimilars in oncology and (2) qualitative research with medical oncologists and pharmacists specializing in oncology.10 Of note, we implemented questions also administered in a study published by Cook et al,4 allowing for comparison with this study's results. This process led to draft questions on clinicians' experience using biosimilars (four questions), knowledge of biosimilars (seven questions), and attitudes toward biosimilars (14 questions). Experience questions asked for the percentage of patients the oncologist currently treated with biosimilars and which biosimilars they prescribed. Knowledge questions addressed facts about biosimilars (eg, Would you consider biosimilars to be the same as generic medicines?) and varied in response format (eg, true/false, multiple choice). Attitudes questions focused on the level of importance that oncologists ascribed to various sources of information about biosimilars, perceptions about barriers to use of biosimilars, and access to education about biosimilars (eg, How important are the following types of information in helping you decide to use biosimilar products?). In total, the survey consisted of 40 questions/items, including three questions to screen for eligibility, nine questions on the participant's demographic and practice characteristics, and three questions on whether opportunities for biosimilar education were made available. The full survey is provided in the Data Supplement (online only).
Data Analyses
All analyses were conducted in R version 4.0.0. Because this was an exploratory study, we did not calculate P values for group differences. We summarized participants' demographic and oncology practice characteristics with proportions and frequencies. We also summarized responses to each survey question on biosimilars using proportions and frequencies. For several analyses, we recoded responses or collapsed response categories. For objective knowledge questions, we recoded responses as correct versus incorrect. For questions on attitudes toward biosimilars, we dichotomized responses into very important/moderately important versus somewhat important/slightly important/not at all important or very much/quite a bit versus somewhat/a little bit/not at all, as appropriate. Primary analyses were descriptive summaries of the total sample of participants. Secondarily, we compared responses with survey questions across institution type (university hospital v community/private hospital v private practice). A single question about access to information (How motivated are you to complete trainings on biosimilars?) was rated from 1 (not motivated) to 10 (extremely motivated) by the respondents; mean scores for this question were compared among institution types.
RESULTS
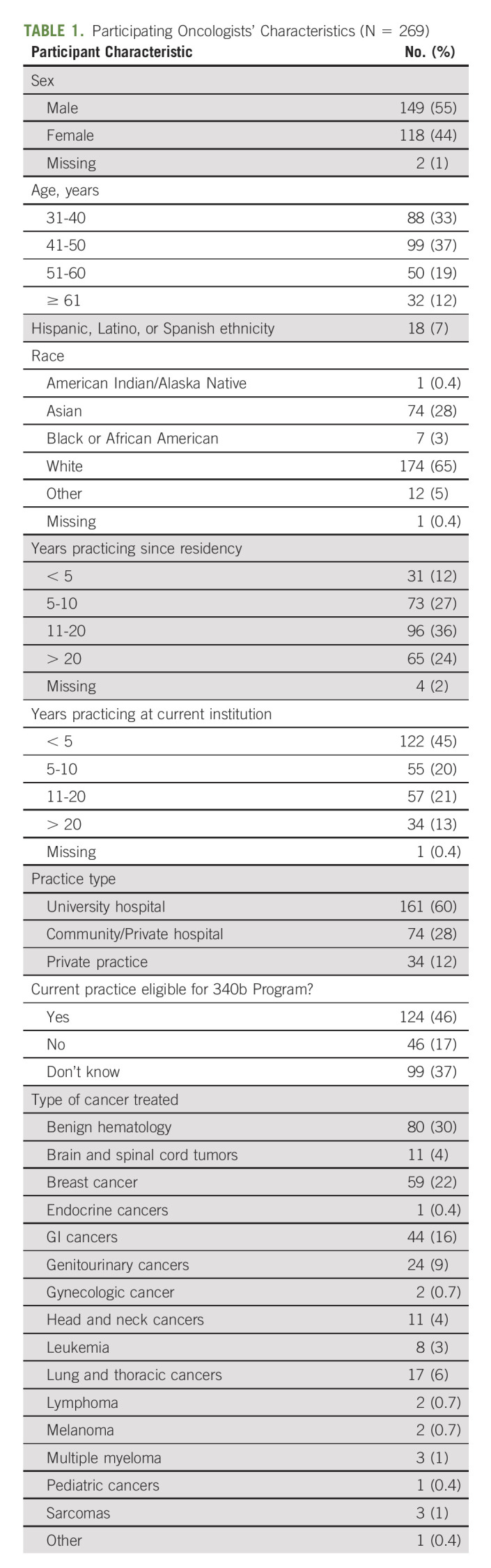
In total, 1,000 medical oncologists from the ASCO RSP were invited to participate in the survey and 283 responded to invitations. Of the 283 invitation respondents, 10 were ineligible for not being in active practice, one additional respondent was ineligible for having a caseload of less than five patients, and two additional respondents were ineligible for having no patients currently on oral or infusion cancer therapy. One eligible participant did not complete the survey. This left a sample of 269 oncologists who were eligible and completed the survey. Details on the demographic characteristics of the sample are presented in Table 1.
TABLE 1.
Participating Oncologists' Characteristics (N = 269)
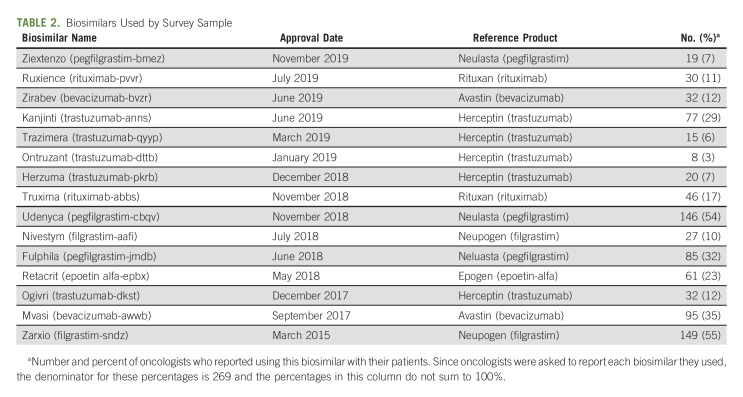
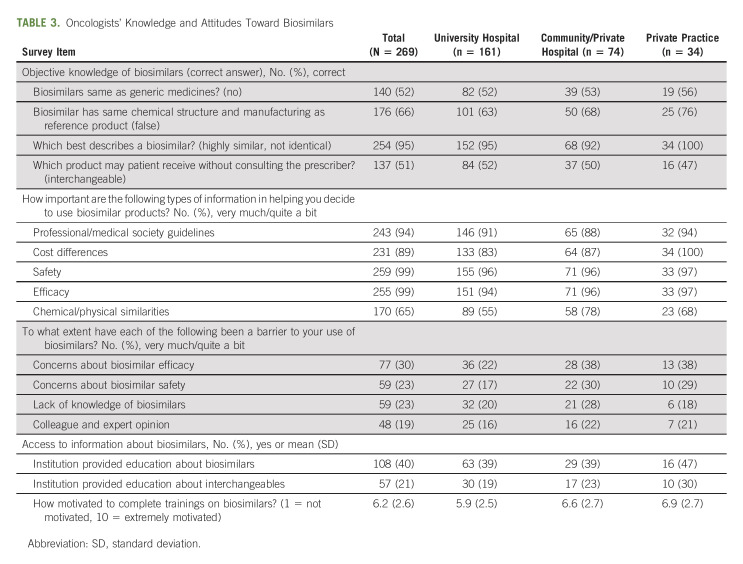
A majority of participants (n = 236, 88%) reported currently treating patients with biosimilars. The most commonly used biosimilars were Zarxio (filgrastim-sndz), Udenyca (pegfilgrastim-cbqv); Mvasi (bevacizumab-awwb); Fulphila (pegfilgrastim-jmdb); and Kanjinti (trastuzumab-anns). Each of the biosimilars reported by the survey participants is shown in Table 2, sorted in order of FDA approval date. Similar proportions of participants said that 1%-10% and 11%-25% of their patients currently received a biosimilar. The majority said that their institution required biosimilars in oncology. Regarding knowledge of biosimilars, a small minority of participants had a complete understanding, and the smallest proportion of these were from university hospitals. The largest proportion rated their overall familiarity with biosimilars as moderately familiar. Approximately half of the sample correctly responded that biosimilars were not the same as generic medicines and that interchangeables can be received without consulting the prescriber (Table 3).
TABLE 2.
Biosimilars Used by Survey Sample
TABLE 3.
Oncologists' Knowledge and Attitudes Toward Biosimilars
Regarding attitudes toward biosimilars, high proportions of oncologists reported that evidence-based research on biosimilars from professional/medical societies, cost differences between biosimilars and reference products, drug safety, and drug efficacy were very or moderately important. There were some differences in these proportions between practice type (Table 3). The most common barriers to use of biosimilars were a lack of research on biosimilars, the potential for concerns about extrapolation, and a perceived lack of drug efficacy. Comparing across institution types, fewer oncologists from university hospitals were concerned about biosimilar efficacy and safety (Table 3). Finally, a minority of oncologists reported that their institution provided education about biosimilars or interchangeables. The proportion reporting receiving education on interchangeables was higher among private practices. When asked to rate their level of motivation to complete biosimilar trainings (on a scale of 1 [not motivated] to 10 [extremely motivated]), oncologists reported a mean of 6.2, and this mean was higher among private practices than other practice types (Table 3).
DISCUSSION
Our study revealed that oncologists, especially those in private practice and in community or private hospitals, were motivated to complete trainings on biosimilars, but less than half had access to education about biosimilars from their institution. The gap between supply and demand for education indicates the need for new quality improvement and educational programs. Such programs must build their curricula to address key knowledge gaps and misperceptions among oncologists. In our study, although oncologists perceived themselves to be knowledgeable regarding biosimilars, nearly half misunderstood basic information on how this group of drugs is defined (namely, holding the false impression that biosimilars are defined in the same way as generic drugs). About the same proportion of participants were not aware that interchangeables could be dispensed without consulting the prescriber. In addition, one third to one fourth of participants were unaware that biosimilars did not necessarily have the same chemical structure and manufacturing process as their reference product. These results are consistent with a previous study by Cook et al4 in which 75% of oncologists could not provide a satisfactory definition of biosimilars, and 40% believed biosimilars were chemically identical to generic drugs.
It is evident that biosimilar educational curricula should start with basic facts about biosimilars and progress to more complex aspects (eg, interchangeability) to support a comprehensive understanding of biosimilars among oncology clinicians. In addition, our study identified several specific topics that oncologists deemed potentially useful in determining whether to prescribe biosimilars, especially information on biosimilars' safety and efficacy. Our findings revealed that oncologists who practice in private settings or private/community hospitals find lack of information about biosimilars' efficacy and safety to be a barrier, thus suggesting that educational programs used in these settings must highlight evidence on efficacy and safety. Cook et al4 found similar results regarding the need to focus on safety and efficacy in educational efforts, in which approximately two third of participants, especially those in private practices, requested information on biosimilar costs. In addition, topics that may be considered include side effects, overall impact on health care utilization, and cost effectiveness. In sum, educational resources that focus on the biological composition and approval process of biosimilars, as well as their safety, efficacy, and cost implications, will likely be sought after by oncologists, particularly those in private practice and community settings. Differences in survey responses between practice settings may be driven by differences in the economic structure of private practices versus academic sites. Each has complex pricing and compensation structures that could lead to differing perspectives on biosimilars. Future work can explore this topic further.
Another important topic for future studies will be to identify the most effective approaches to implementing biosimilar education programs. Innovative approaches to education beyond traditional lectures, peer-reviewed literature, and web-based education modules are necessary to capture the attention of clinicians who are clinically busy and often inundated with new literature. A recent systematic review of international trends in biosimilar uptake recommended academic detailing, which involves a trained educator meeting with the clinician to share the latest evidence on an emerging treatment.11 These approaches should be compared with others to determine how best to improve oncologists' knowledge of biosimilars, and these investigations should consider which approaches are likely to succeed in community settings along with academic hospitals. It is critical to consider the setting and audience for biosimilars education; clinicians of different generations and in different practice settings may respond better to different educational approaches (eg, academic detailing v continuing medical education credit courses, or in person v virtual). Although oncologists are not always directly responsible for prescribing biosimilars (eg, when biosimilars are added to the formulary), their understanding of and attitudes toward biosimilars will in part determine whether oncologists support biosimilars use in the long term.
Our study was not the first to examine oncology clinicians' knowledge and attitudes regarding biosimilars; however, it expands on previous work by confirming previous results4 using a larger sample that included community-based practitioners in private practice and community or private hospitals. Significant differences in our study between academic oncologists' attitudes and access to information about biosimilars highlight the importance of including community-based participants, whose needs for education and quality improvement initiatives may differ from those of academic oncologists. Finally, since the implementation of biosimilars has progressed quickly over the past few years and new key biosimilar approvals have occurred since the Cook et al study was published in 2019,2 an update on oncologists' use, knowledge, and attitudes toward biosimilars is warranted.
In addition to our study's strengths, there are important limitations to consider when interpreting our results. First, although oncologists throughout the United States were invited to participate in this survey, it is not clear if our sample is representative, particularly in terms of age, race, ethnicity, and subspecialty of oncology. We note that the majority of our participants were from academic institutions and specialists in benign hematology and are likely over-represented. In addition, it may be that the oncologists included in this analysis were more likely to use biosimilars for a specific purpose (eg, supportive care vs. primary treatment) that is not representative of the overall biosimilar experience in oncology in the United States. Future work could compare the acceptance of biosimilars between active treatment and supportive care. Second, we focused only on physicians while other oncological clinicians, especially pharmacists, play an important role in implementing biosimilars. Future work should target nonphysician oncological clinicians, as quality improvement initiatives may differ among this group. Finally, while our survey identified key areas for increasing knowledge of biosimilars among oncologists, the survey was relatively brief, and it is thus possible that important topics may have been missed, including whether oncologists themselves would like to learn more about biosimilars. Future research, including qualitative research, should provide additional detail in determining whether there are more specific concerns among oncologists (and other oncological clinicians) regarding biosimilar treatments, for example, concerns regarding biosimilar use among particular patient subgroups or on short- versus long-term usage.
These limitations notwithstanding, the present study provides a novel perspective on oncologists' use, knowledge of, and perspectives on biosimilars in the United States. It is critical that oncologists are familiar with and prepared to discuss biosimilars with their patients. Realizing the potential cost savings of biosimilars requires successful adoption and implementation of biosimilars, which in turn requires oncology clinicians to be fully informed of and confident about their safety and efficacy.
John Devin Peipert
Consulting or Advisory Role: FACIT.org, BMS (Inst), Pfizer (Inst), Clovis Oncology (Inst)
Research Funding: Veloxis (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Pfizer
Karen Kaiser
Stock and Other Ownership Interests: Pfizer (I)
Research Funding: EMD Serono/Merck, Alexion Pharmaceuticals, Akcea Therapeutics, Fulcrum Therapeutics
Sheetal Kircher
Stock and Other Ownership Interests: Penrose, Abbott/AbbVie
George J. Greene
Research Funding: Pfizer (Inst), Fulcrum Therapeutics (Inst), Veloxis (Inst), Bristol Myers Squibb (Inst)
David Cella
Stock and Other Ownership Interests: FACIT.org
Consulting or Advisory Role: AbbVie, GlaxoSmithKline, Pfizer, Astellas Pharma, Novartis, Bristol Myers Squibb, Ipsen, Celcuity, Immunogen, Fulcrum Therapeutics
Research Funding: Novartis (Inst), Ipsen (Inst), Pfizer (Inst), PledPharma (Inst), Bristol Myers Squibb (Inst), AbbVie (Inst), Regeneron (Inst), Clovis Oncology (Inst)
Daniel K. Mroczek
Consulting or Advisory Role: Rafael Pharmaceuticals
Research Funding: Pfizer (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented as a poster at the 2021 ASCO Quality Care Symposium, September 24-25, 2021 (abstr 35; 348033).
SUPPORT
Supported by a grant from Pfizer (41333413, PI: Mroczek).
AUTHOR CONTRIBUTIONS
Conception and design: John Devin Peipert, Karen Kaiser, David Cella, Daniel K. Mroczek
Financial support: Daniel K. Mroczek
Administrative support: Daniel K. Mroczek
Collection and assembly of data: John Devin Peipert, Karen Kaiser, Daniel K. Mroczek
Data analysis and interpretation: John Devin Peipert, Sheetal Kircher, George J. Greene, Sara Shaunfield, Katherina Hauner, David Cella, Daniel K. Mroczek
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Medical Oncologists' Knowledge and Perspectives on the Use of Biosimilars in the United States
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
John Devin Peipert
Consulting or Advisory Role: FACIT.org, BMS (Inst), Pfizer (Inst), Clovis Oncology (Inst)
Research Funding: Veloxis (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Pfizer
Karen Kaiser
Stock and Other Ownership Interests: Pfizer (I)
Research Funding: EMD Serono/Merck, Alexion Pharmaceuticals, Akcea Therapeutics, Fulcrum Therapeutics
Sheetal Kircher
Stock and Other Ownership Interests: Penrose, Abbott/AbbVie
George J. Greene
Research Funding: Pfizer (Inst), Fulcrum Therapeutics (Inst), Veloxis (Inst), Bristol Myers Squibb (Inst)
David Cella
Stock and Other Ownership Interests: FACIT.org
Consulting or Advisory Role: AbbVie, GlaxoSmithKline, Pfizer, Astellas Pharma, Novartis, Bristol Myers Squibb, Ipsen, Celcuity, Immunogen, Fulcrum Therapeutics
Research Funding: Novartis (Inst), Ipsen (Inst), Pfizer (Inst), PledPharma (Inst), Bristol Myers Squibb (Inst), AbbVie (Inst), Regeneron (Inst), Clovis Oncology (Inst)
Daniel K. Mroczek
Consulting or Advisory Role: Rafael Pharmaceuticals
Research Funding: Pfizer (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Chen B, Nagai S, Armitage JO, et al. : Regulatory and clinical experiences with biosimilar filgrastim in the U.S., the European Union, Japan, and Canada. Oncologist 24:537-548, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration : Biosimilar Product Information: FDA-Approved Biosimilar Products. 2021. https://www.fda.gov/drugs/biosimilars/biosimilar-product-information [Google Scholar]
- 3.The Statistics Portal : Total global biologics spending. 2019. https://www.statista.com/statistics/280578/global-biologics-spending/
- 4.Cook JW, McGrath MK, Dixon MD, et al. : Academic oncology clinicians' understanding of biosimilars and information needed before prescribing. Ther Adv Med Oncol 11:1758835918818335, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nabhan C, Valley A, Feinberg BA: Barriers to oncology biosimilars uptake in the United States. Oncologist 23:1261-1265, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nabhan C, Parsad S, Mato AR, et al. : Biosimilars in oncology in the United States: A review. JAMA Oncol 4:241-247, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Cohen H, Beydoun D, Chien D, et al. : Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther 33:2160-2172, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karateev D, Belokoneva N: Evaluation of physicians' knowledge and attitudes towards biosimilars in Russia and issues associated with their prescribing. Biomolecules 9:57, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyman GH, Balaban E, Diaz M, et al. : American Society of Clinical Oncology statement: Biosimilars in oncology. J Clin Oncol 36:1260-1265, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Kaiser K, Hauner K, Shaunfield S, et al. : A qualitative study of medical oncologists' knowledge and views of biosimilars in the United States. J Clin Med Res (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarnola K, Merikoski M, Jyrkkä J, et al. : Physicians' perceptions of the uptake of biosimilars: A systematic review. BMJ Open 10:e034183, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]