Abstract
Molecular and chemical characteristics often provide complementary information in the differentiation of closely related organisms. The genus Brucella consists of a highly conserved group of organisms. Identification of the four species pathogenic in humans (Brucella melitensis, Brucella abortus, Brucella suis, and Brucella canis) is problematic for many clinical laboratories that depend primarily on serology and phenotypic characteristics to differentiate species. PCR amplification of the 16S-23S ribosomal DNA interspace region was evaluated for species-specific polymorphism. B. abortus, B. melitensis, B. suis, and B. canis produced identical PCR interspace profiles. However, these PCR products were unique to brucellae, allowing them to be readily distinguished from other gram-negative bacteria (including Bartonella spp. and Agrobacterium spp.). Carbohydrate profiles differentiated B. canis from the other three Brucella species due to the absence of the rare amino sugar quinovosamine in the three other species. PCR of the rRNA interspace region is useful in identification of the genus Brucella, while carbohydrate profiling is capable of differentiating B. canis from the other Brucella species.
The bacterial genus Brucella is a genetically homologous group containing six species designated primarily on the basis of host specificity. Brucellae are gram-negative, facultative, intracellular pathogens. Brucella melitensis is highly infectious in humans. Three additional species (Brucella abortus, Brucella suis, and Brucella canis) are also pathogenic in humans. B. abortus is of major economic consideration to the cattle industry. Brucella ovis and Brucella neotomae are not generally isolated from human sources (8) and are phenotypically distinct from the other species (10).
Differentiation of the species of Brucella that infect humans is difficult by conventional physiological and serological means. Misidentification of Brucella species with commercial identification kits can be a particular problem (1, 24, 25). Many isolates of B. melitensis and B. suis display a morphologically smooth colony form and are readily distinguished from B. canis, which exists only in rough form. However, rough strains of B. melitensis, B. suis, and B. abortus are encountered in clinical specimens and are difficult to differentiate from B. canis even with monoclonal antibodies (33). Slow hydrolysis of urea and growth in dilute basic fuchsin allows B. abortus and B. melitensis to be differentiated from B. canis. Unfortunately, none of the conventional physiological tests allow differentiation of B. suis from B. canis (8).
The six species of Brucella are sufficiently related by DNA-DNA hybridization that a monospecies genus has been suggested (32). The 16S rRNA sequences of B. abortus and the other five species are also 98.5 to 99.7% similar, and PCR generates products of the same molecular weights (9, 22, 27, 28). The 16S rRNA sequence indicates that this genus is a member of the alpha-2 subdivision of the class Proteobacteria and that it is closely related to Bartonella and Agrobacterium (22).
The use of conserved sequences such as that of 16S rRNA often does not distinguish closely related species. The spacer region in the rRNA operon between the 16S and 23S loci is not subject to the same selective pressure as the rRNA structural genes and has been used to compare both closely and distantly related organisms (16). In this instance, primers for conserved regions of the 16S and 23S RNAs are employed to generate PCR products from the intervening spacer region. The spacer region often varies not only in sequence but also in length among species (16, 26, 29, 34). Thus, simple visual observation of the sizes of PCR products is sufficient for species differentiation. The rRNA spacer regions for single strains of B. abortus, B. melitensis, and B. suis have been studied and are essentially identical, but the sequence for B. canis remains to be assessed (26).
Carbohydrate analysis of lipopolysaccharide (LPS) isolated from a single strain of B. canis demonstrated that it lacks the rare sugar quinovosamine (2). Quinovosamine (2-amino-2,6-dideoxyglucose) has been found in the LPSs of B. melitensis, B. abortus, and B. suis (2, 21). It remains to be determined whether the absence of quinovosamine is a general characteristic of B. canis. The LPSs of B. melitensis and B. abortus have been reported to contain mannose and glucose (17, 21). Whether the presence of mannose and glucose is also a general characteristic of B. canis and B. suis remains to be determined. There is clearly a need for further study of the carbohydrate contents of brucellae. Whole-cell carbohydrate profiling by gas chromatography-mass spectrometry (GC-MS) has proven successful in differentiating other closely related groups of organisms, including legionellae and bacilli (12, 14, 15, 34).
In summary, the brucellae are a group of closely related organisms that present a challenge in terms of finding distinguishing features for species discrimination. There is a real need for further studies using modern analytical chemical and molecular biology techniques. This work was concerned with evaluating the 16S-23S interspace region (ISR), by PCR, and determining the total cellular carbohydrate profiles, by GC-MS, of the four Brucella species pathogenic in humans.
MATERIALS AND METHODS
Bacterial cells were cultured and sterilized before being sent to the University of South Carolina (USC) for molecular and chemical analysis. Cultures of the various strains were processed at the Armed Forces Institute of Pathology, Washington, D.C., the Department of Health and Environmental Control, Columbia, S.C., and the U.S. Army Medical Research Institute for Infectious Diseases. Initially, Brucella spp. were grown in Brucella medium at 37°C, harvested by centrifugation, washed three times in water, and subsequently sterilized by gamma irradiation followed by freeze-drying (protocol 1). Subsequently, Brucella spp. were grown on Brucella agar plates, harvested by rinsing the plates with a few milliliters of water, and autoclaved before being washed and freeze-dried (protocol 2). The latter protocol decreased the possibility of human exposure to the pathogen. On chemical analysis, cells prepared by protocol 1 often contained higher levels of glucose than those prepared by protocol 2 but were otherwise identical. PCR results were identical with DNA extracted from cells prepared by either protocol. Brucella strains originated from the American Type Culture Collection (designated with ATCC plus a number), from George Stewart at the University of Kansas Veterinary College (designated with the number 30 followed by three other numbers), and from Ted Hadfield at the Army Institute of Pathology and included S19, 16M, 38, 53, and A5402. The Brucella strains studied were B. abortus ATCC 23448, S19, 30101, 30102, 30104, 30105, 30106, and 30155; B. canis ATCC 23365, 30201, 30202, 30203, 30204, 51630, and A5402; B. melitensis ATCC 23456 (16M), ATCC 23457, ATCC 31242, 30401, and 38; and B. suis 30301, ATCC 23444, ATCC 23445, ATCC 23447, ATCC 4312, and 53.
The Bartonella strains studied were Bartonella elizabethae ATCC 49927 and Bartonella henselae ATCC 49793. Bartonella strains were grown on 5% sheep blood agar at 37°C in 5% CO2. Agrobacterium strains, provided by Mihaly Czako, Department of Biological Science, USC, were Agrobacterium tumefaciens C58 and Agrobacterium vitis SV2. The Agrobacterium strains were grown on 5% sheep blood agar at 26°C. The Escherichia coli strain used, K-12, was also grown on sheep blood agar (at 37°C).
Chromosomal DNA was prepared from previously sterilized cells. The cells were resuspended in 10 ml of water and lysed by either lysozyme treatment at 37°C for 30 min or by freezing followed by boiling. The cleared samples were treated with RNase (40 μg/ml) for 25 min at 37°C and then protease K (100 μg/ml) for 25 min. An equal volume of buffer-saturated phenol-chloroform was added and emulsified. The aqueous layer was removed after centrifugation. The DNA was precipitated at −70°C. The preparations were washed with 70% ethanol and resuspended in water.
For amplification of the 16S-23S rRNA spacer regions, we used primers synthesized at the Oligonucleotide Synthesis Facility at USC. Primer KF5 (5′-GAAGTCGTAACAAGG-3′) corresponds to a conserved region of the 3′ end of the 16S sequence, and primer KF6 (5′-CAAGCATCCATCGT-3′) corresponds to a conserved region of the 5′ ends of 23S sequences (16). The amplifications were carried out on a Rapidcycler (Idaho Technology, Idaho Falls, Idaho) with the following steps: initial denaturation at 96°C for 30 s, cycling at 96°C for 30 s and 50°C for 1 min, with a ramp to 72°C at 2°C/s, and then extension at 72°C for 1 min for 40 cycles, with a final extension at 72°C for 5 min. The amplification reaction mixtures contained 200 μmol of each deoxynucleoside triphosphate, 0.5 pmol of each primer, 20× Tfl PCR buffer and 1 U of Tfl polymerase (Epicenter Technologies, Madison, Wis.), and 1 μg of template DNA to a final volume of 50 μl. Brucella reaction mixtures contained 3 mM MgCl2 and 4× MasterAmp enhancer (Epicenter Technologies). The PCR products were visualized on 3%, 3:1 agarose gels (Ameresco, Solon, Ohio) run in TAE (40 mM Tris-acetate, 1 mM EDTA [pH 8.0]) and stained with ethidium bromide. Molecular weight estimations were calculated based on Promega (Madison, Wis.) pGEM low-molecular-weight standards.
Carbohydrate profiles were determined by the alditol acetate method as described previously (11, 13). Alditol acetates of whole-cell carbohydrates were prepared by hydrolysis of the bacteria for 3 h at 100°C in 2 N sulfuric acid, neutralization with 50% N,N-dioctylmethylamine in chloroform, and extraction on C18 columns (hydrophobic cleanup). The aqueous effluent was reduced with sodium borodeuteride at 4°C. Borodeuteride was removed by multiple methanol-acetic acid (200:1) evaporations under nitrogen. Samples were dried under vacuum for 3 h at 60°C. After drying, the samples were acetylated for 15 h at 100°C. Hydrophilic postderivatization cleanup included acid and alkaline extractions. GC-MS analyses were carried out with a mass selective detector (model 5970; Hewlett-Packard Co., Palo Alto, Calif.) interfaced to a GC (model 5890; Hewlett-Packard) equipped with an automated sample injector. Chromatography was accomplished on a DB5-MS fused silica capillary column (J & W Scientific, Folsom, Calif.). Electron ionization was performed at 70 eV for both total-spectrum scanning and selected ion monitoring. Sugar standards were purchased from Sigma Chemical Company (St. Louis, Mo.).
RESULTS
The 16S-23S rRNA spacer region was characterized by PCR, and whole-cell carbohydrate profiles were determined by GC-MS. Twenty-five strains of brucellae (B. abortus [eight strains], B. melitensis [five strains], B. suis [five strains], and B. canis [seven strains]) were studied.
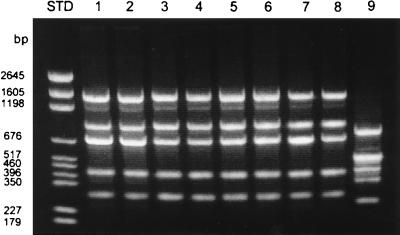
Amplification of the rRNA 16S-23S spacer region consistently generated five major bands characteristic of the genus Brucella. The bands’ estimated sizes were 1,530, 916, 701, 399, and 316 bp (Fig. 1). This banding pattern was reproducibly observed for all strains of B. abortus, B. melitensis, B. suis, and B. canis analyzed. No significant differences were seen among the four species and 25 strains. A primer was designed based on the published ISR sequence (26) containing two tRNAs; this primer, when used in conjunction with the 16S-specific primer, amplified a band equivalent to the 1,530-bp band. Only one band was amplified when this primer pair was used (data not shown).
FIG. 1.
PCR of the 16S-23S rRNA ISR. Lanes contain B. abortus (lane 1, 30101; lane 2, 30102), B. canis (lane 3, 30201; lane 4, 30202), B. melitensis (lane 5, 30401; lane 6, ATCC 31242), B. suis (lane 7, 30301; lane 8, 4312), and E. coli (lane 9, K-12). Note that the four species of Brucella have identical profiles which are distinct from that of E. coli. Numbers to the left indicate standard (STD) pGEM base pair markers.
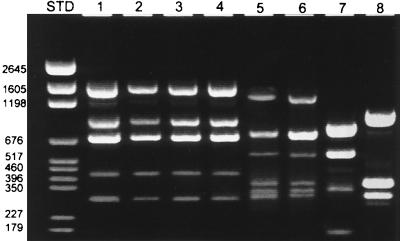
Closely related organisms (including Agrobacterium and Bartonella species as well as the more distantly related E. coli) were evaluated with the 16S-23S rRNA primer pair (Fig. 2). The PCR pattern for brucellae was readily distinguished from those of these other gram-negative species. This suggests that PCR of the ISR is useful as a marker for Brucella spp. pathogenic in humans.
FIG. 2.
PCR of the 16S-23S rRNA ISR. Lane 1, B. abortus (30104); lane 2, B. canis (ATCC 23765); lane 3, B. melitensis (ATCC 23457); lane 4, B. suis (ATCC 23444); lane 5, Bartonella elizabethae (ATCC 49927); lane 6, Bartonella henselae (ATCC 49793); lane 7, A. vitis (SV2); lane 8, A. tumefaciens (C58). Note that the four species of Brucella have identical profiles which are distinct from those of Bartonella and Agrobacterium. Numbers to the left indicate standard (STD) pGEM base pair markers.
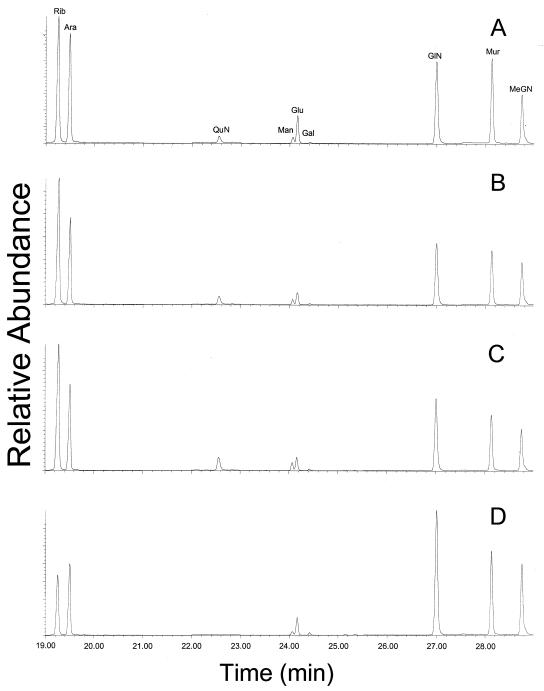
All four species of Brucella (25 strains) contained ribose, mannose, glucose, muramic acid, and glucosamine as the major sugar constituents. Additionally, galactose was detected in 12 of 25 strains. Ribose was presumably derived from RNA. Muramic acid and glucosamine were derived from peptidoglycan. Mannose and some of the glucose were derived from LPS. Heptoses, which are often found in gram-negative bacterial LPSs, were not detected in any Brucella species. The profiles for B. abortus, B. melitensis, and B. suis were indistinguishable (Table 1 and Fig. 3).
TABLE 1.
Carbohydrate profiles of Brucella species determined by GC-MS
| Species and strain | % Dry weight of the sugar componenta:
|
||||||
|---|---|---|---|---|---|---|---|
| Rib | QuN | Man | Glu | Gal | GlN | Mur | |
| B. abortus | |||||||
| ATCC 23448 | 0.3 | 0.3 | 0.05 | 0.1 | 0.6 | 0.9 | |
| 30101 | 0.5 | 0.08 | 0.06 | 0.4 | 0.01 | 0.7 | 0.8 |
| 30102 | 0.6 | 0.1 | 0.06 | 0.4 | 0.01 | 0.6 | 0.7 |
| 30104 | 0.5 | 0.2 | 0.1 | 0.4 | 0.02 | 0.6 | 0.7 |
| 30105 | 0.4 | 0.1 | 0.08 | 0.3 | 0.5 | 0.6 | |
| 30106 | 0.4 | 0.1 | 0.1 | 0.2 | 0.5 | 0.7 | |
| 30155 | 0.6 | 0.1 | 0.07 | 0.2 | 0.4 | 0.5 | |
| S19 | 0.5 | 0.2 | 0.1 | 0.2 | 0.7 | 0.8 | |
| B. melitensis | |||||||
| ATCC 23456 | 1.3 | 0.2 | 0.1 | 2.4 | 0.3 | 0.3 | |
| ATCC 23457 | 0.6 | 0.06 | 0.04 | 0.2 | 0.04 | 0.7 | 0.7 |
| 30401 | 0.5 | 0.1 | 0.09 | 1.6 | 0.5 | 0.6 | |
| ATCC 31242 | 0.7 | 0.09 | 0.06 | 0.2 | 0.02 | 0.5 | 0.6 |
| 38 | 1.6 | 0.2 | 0.1 | 2.6 | 0.3 | 0.3 | |
| B. suis | |||||||
| ATCC 23444 | 0.8 | 0.1 | 0.07 | 0.2 | 0.02 | 0.6 | 0.6 |
| ATCC 23445 | 0.6 | 0.03 | 0.02 | 0.07 | 0.02 | 0.4 | 0.4 |
| ATCC 23447 | 0.7 | 0.2 | 0.1 | 0.2 | 0.6 | 0.6 | |
| ATCC 4312 | 0.5 | 0.1 | 0.03 | 0.5 | 0.4 | ||
| 53 | 1.1 | 0.2 | 0.2 | 1.7 | 0.03 | 0.5 | 0.5 |
| B. canis | |||||||
| ATCC 23365 | 0.6 | 0.01 | 0.09 | 0.5 | 0.5 | ||
| 30201 | 0.4 | 0.03 | 0.2 | 0.02 | 0.6 | 0.5 | |
| 30202 | 1.2 | 0.02 | 2.6 | 0.4 | 0.3 | ||
| 30203 | 1.5 | 0.02 | 2.6 | 0.5 | 0.5 | ||
| 30204 | 1.5 | 0.02 | 2.4 | 0.01 | 0.4 | 0.4 | |
| 51630 | 1.3 | 1.6 | 0.4 | 0.3 | |||
| A5402 | 1.4 | 0.04 | 3.3 | 0.02 | 0.5 | 0.4 | |
Rib, ribose; QuN, quinovosamine; Man, mannose; Glu, glucose; Gal, galactose; GlN, glucosamine; Mur, muramic acid.
FIG. 3.
Carbohydrate profiles of B. abortus 30101 (A) B. melitensis 31242 (B) B. suis 23444 (C), and B. canis 30201 (D). Rib, ribose; Ara, arabinose (internal standard); QuN, quinovosamine; Man, mannose; Glu, glucose; Gal, galactose; GlN, glucosamine; Mur, muramic acid; MeGN, methylglucamine (internal standard). Note the presence of quinovosamine in B. canis and its absence in the other three species.
With the exception of one strain of B. melitensis, all eight B. abortus strains, five B. melitensis strains, and five B. suis strains contained quinovosamine. However, B. canis strains (n = 7) were unique in that all lacked quinovosamine. Quinovosamine is a constituent of the O-antigen side chain of LPS. The quinovosamine-negative B. melitensis strain, like B. canis, presumably synthesizes a rough form of LPS which lacks this side chain (2). Quinovosamine is not commercially available and was identified by GC-MS analysis. It displayed a gas chromatographic retention time and mass spectrum of quinovosamine identical to those previously identified for Legionella pneumophila (14).
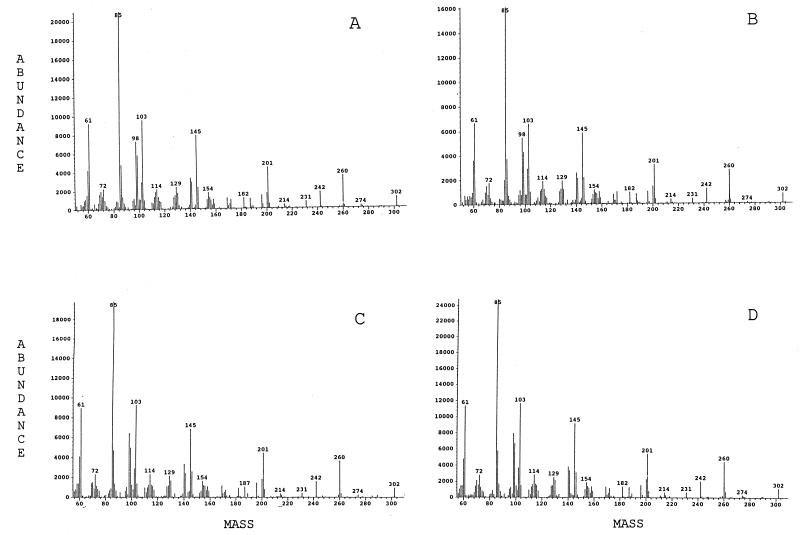
Mass spectra of quinovosamine, isolated from B. abortus, B. melitensis, B. suis, and L. pneumophila are shown in Fig. 4. The peaks represent fragments of the alditol acetate of quinovosamine (2H labeled on C-1). The molecular weight of the alditol acetate of quinovosamine is 376. Loss of carbon (C) 1 (74 Da) generates the molecular ion 302. Breakage of the bond between C-2 and C-3 generates the other primary fragment, 145. The dominant peak, 85, is generated from mass 145 by loss of acetic acid (60 Da). Secondary fragments from 302 include prominent peaks of mass 201 (loss of 59, the acetylinium ion) and 260 (loss of 42, ketene).
FIG. 4.
Mass spectra of alditol acetates of quinovosamine from B. melitensis (A), B. abortus (B), B. suis (C), and L. pneumophila (D).
DISCUSSION
The high degree of relatedness of the brucellae by DNA-DNA hybridization has led to suggestions that they may represent a monospecies genus (32). The 16S rRNA is also highly conserved in Brucella and the species cannot be differentiated by sequence (22, 27, 28). The spacer regions between 16S and 23S rRNAs in B. abortus, B. melitensis, and B. suis are also essentially identical (26). The ISR for B. canis has not yet been sequenced. The ISR of the genome is not subject to the same selective pressure as the 16S or 23S rRNA structural gene and has been demonstrated to exhibit variability which may be used to distinguish closely related organisms (29, 34). The base sequences and overall lengths of the spacer regions display a great deal of polymorphism among bacterial species. For example, Bacillus cereus and Bacillus subtilis (92 to 94% related by 16S rRNA sequence) display only 75.5 to 86.5% similarity in their ISRs (34). ISR amplification for Bacillus subtilis produces bands of 270, 400, and 430 nucleotides in length, while that for the Bacillus cereus group produces bands of 250, 430, and 480 nucleotides.
In the present study, amplification of the rRNA 16S-23S spacer region consistently generated five bands characteristic of the genus Brucella. The bands were found in all 25 strains examined. These five bands were generated with a primer pair that hybridized to conserved sequences within the 16S and 23S rRNAs flanking the ISR. In order to determine which band corresponded to the sequence described by Rijpens et al. (26), we used a primer pair that included the 16S primer and a new primer hybridizing to a unique sequence within the reported ISR. This primer pair gave only one band corresponding to the 1,530-bp band. The other four bands clearly did not contain a sequence identical to this ISR primer.
There are often multiple operons of the rRNA region within a single genome. Multiple operons may contain one or more tRNA genes or none. Among the best characterized are those of Bacillus subtilis, whose genome has been entirely sequenced and for which 10 rRNA operons have been identified. Two of the 10 16S-23S ISRs contain the sequences for isoleucine and alanine tRNA. The remaining eight ISRs lack tRNA sequences. With the same primers as in the present study, three PCR products are generated for Bacillus subtilis. The smallest PCR product has been sequenced and corresponds to the spacer regions lacking tRNA genes. The upper band represents the rRNA operon with both tRNA genes present. The third band has not been identified but does not correspond in size to any ISR. All the rRNA operons in Brucella spp. have not yet been identified, and the PCR products have not been sequenced. However, the 1,530-bp band corresponds to the tRNA containing the ISR identified by Rijpens et al. (26). As noted above, the remaining four bands are likely to represent multiple operons (without tRNA). It is possible that one or more of the products may result from nonspecific amplification. However, regardless of the source of the multiple bands, the banding patterns are reproducible and useful in identifying Brucella (23).
The similarity of the PCR products for the brucellae provides further evidence for their high degree of genetic relatedness. Others have noted the usefulness of PCR of conserved genes for differentiation of the genus Brucella from other bacterial genera (6). PCR is important since there is only a small battery of physiological tests available for characterization of brucellae. These tests do not readily differentiate brucellae from certain other gram-negative bacterial species. For example, in two recent studies using the API 20NE rapid identification system, B. melitensis was misidentified as Moraxella phenylpyruvica (1, 24).
There has been considerable effort expended in an attempt to use molecular approaches to provide species-specific markers for identification of the brucellae. A multiplex primer set recognizing an insertion sequence (IS711) and six other primers to genes observed in other Brucella species identifies specific biovars within a species. However, the primer set does not generate species-specific PCR products (3, 4). Use of another set of primers for six other gene sequences (a 31-kDa Brucella protein, four heat shock proteins, and 16S rRNA) detected no differences between Brucella biovars or species, even after restriction analysis of amplified fragments (6).
Analysis of outer membrane proteins (Omp) of brucellae has provided some species-specific information. Omp-10 and -19 are common to all Brucella species. However, Omp-19 in B. ovis is of higher molecular weight than in the other species (31). Sequence analysis of the Omp-2 locus suggested that B. ovis and B. neotomae are distinct from the other Brucella species. Based on Omp-2 sequence, B. suis and B. canis are closely related and only recently diverged from B. melitensis and B. abortus (10). This finding has been confirmed with restriction maps of the entire chromosomes of these organisms (19). PCR for the Omp-2 gene with primers recognizing the B. abortus sequence detected all strains of B. melitensis and B. abortus but only 50% of strains of B. suis. B. canis and B. ovis did not generate PCR products (18). By PCR of the Omp-2 locus, it has been reported that representatives of the six species can be differentiated. However, it was unclear whether differences observed were at the strain or species level (30).
Despite the difficulty encountered with attempts to differentiate brucellae by molecular means, there have been some encouraging reports with analytical chemical approaches. The fatty acid profiles of B. melitensis, B. abortus, and B. suis, including 16:0, 17:0, 17 cyclopropane, 18:0, 18:1, and 19:0 cyclopropane, are extremely similar (5, 7, 8). B. canis can be readily distinguished from these three species by a lack of 19:0 cyclopropane. B. ovis does contain 19:0 cyclopropane; however, it can be differentiated from B. abortus by the presence of C15 and higher amounts of 17:0 and 18:1 (5).
Earlier reports on the carbohydrate compositions of the purified LPSs from a few strains of brucellae suggested their potential in species identification (2). The present study demonstrated that all four species of Brucella contained ribose, mannose, glucose, muramic acid, and glucosamine as their major carbohydrate constituents. Mannose and glucose have been reported previously to be components of the LPSs of B. abortus and B. melitensis (17, 21). Ribose was presumably derived from RNA. Muramic acid and glucosamine were derived from peptidoglycan. Heptoses, which are often found in gram-negative bacterial LPSs, were not detected in any Brucella species, results in agreement with those of others (20).
B. canis could readily be distinguished from B. suis, B. melitensis, and B. abortus by the absence of the amino sugar quinovosamine in whole-cell hydrolysates. The absence of quinovosamine in LPS isolated from a single strain of B. canis has been previously noted. However, quinovosamine has been found in the LPSs of a limited number of strains of B. melitensis, B. abortus, and B. suis. The present report confirms this observation, noting that this unusual amino sugar is absent in all 7 strains of B. canis studied but is present in all but one strain of the other 3 Brucella species (18 strains in total). GC-MS analysis, to determine carbohydrate profiles, has an advantage over other methods, since analysis can be performed directly without purification of cellular constituents (e.g., LPS). The uniqueness of the carbohydrate profiles of B. canis compared with those of the other brucellae is in agreement with the results of earlier studies noting that the fatty acid profiles are also distinct for this organism (7).
In conclusion, molecular analysis of the 16S-23S rRNA operon (including both 16S rRNA and the less genetically conserved ISR) lumps together the four species of brucellae pathogenic in humans but differentiates them from other gram-negative species (including Bartonella and Agrobacterium spp.). PCR profiles may now be used as a confirmatory test for isolates that have been presumptively identified by conventional physiological tests. It is possible that, with more extensive evaluation in the clinical laboratory, PCR profiling might become a primary method for the designation of isolates as Brucella. However, chemical methods, including profiling of cellular sugars (as presented here) and fatty acids (5, 7, 8), are capable of differentiating B. canis from the other Brucella species. This finding is consistent with similar observations we have made for other genetically closely related bacterial species (15, 34). This study clearly demonstrates the utility of using a combination of both chemical and molecular approaches in taxonomic characterization of closely related bacterial species.
ACKNOWLEDGMENTS
This research was supported by Army Research Office grant DAAH04-95-1-0359 and Office of Naval Research grant N00014-97-1-0806.
Kelly Kim assisted in some of the preliminary molecular and chemical studies. We thank Arthur Wozniak for the use of the isolation facility at the Department of Health and Environmental Control, Columbia, S.C.
REFERENCES
- 1.Barham W, Church P, Brown J, Paparello S. Misidentification of Brucella species with use of rapid bacterial identification systems. Clin Infect Dis. 1993;17:1068–1069. doi: 10.1093/clinids/17.6.1068. [DOI] [PubMed] [Google Scholar]
- 2.Bowser D, Wheat R, Foster J, Leong D. Occurrence of quinovosamine in lipopolysaccharides of Brucella species. Infect Immun. 1974;9:772–774. doi: 10.1128/iai.9.4.772-774.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bricker B J, Halling S M. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, B. ovis, and Brucella suis bv. 1 by PCR. J Clin Microbiol. 1994;32:2660–2666. doi: 10.1128/jcm.32.11.2660-2666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bricker B J, Halling S M. Enhancement of the Brucella AMOS PCR assay for differentiation of Brucella abortus vaccine strains S19 and RB51. J Clin Microbiol. 1995;33:1640–1642. doi: 10.1128/jcm.33.6.1640-1642.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coloe P, Sinclair A, Slattery J, Burke D. Differentiation of Brucella ovis from Brucella abortus by gas liquid chromatographic analysis of cellular fatty acids. J Clin Microbiol. 1984;19:896–898. doi: 10.1128/jcm.19.6.896-898.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Da Costa M, Guillou J-P, Garin-Bastuji B, Thiébaud M, Dubray G. Specificity of six gene sequences for the detection of the genus Brucella by DNA amplification. J Appl Bacteriol. 1996;81:267–275. doi: 10.1111/j.1365-2672.1996.tb04328.x. [DOI] [PubMed] [Google Scholar]
- 7.Dees S, Hollis D, Weaver R, Moss W. Cellular fatty acids of Brucella canis and Brucella suis. J Clin Microbiol. 1981;14:111–112. doi: 10.1128/jcm.14.1.111-112.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dees S, Thanabalasundrum S, Moss C, Hollis D, Weaver R. Cellular fatty acid composition of group Ive, a nonsaccharolytic organism from clinical sources. J Clin Microbiol. 1980;11:664–668. doi: 10.1128/jcm.11.6.664-668.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorsch M, Moreno E, Stackebrandt E. Nucleotide sequences of the 16S rRNA from Brucella abortus. Nucleic Acids Res. 1989;17:1765. doi: 10.1093/nar/17.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ficht T A, Husseinen H S, Derr J, Bearden S W. Species-specific sequences at the omp2 locus of Brucella type strains. Int J Syst Bacteriol. 1996;46:329–331. doi: 10.1099/00207713-46-1-329. [DOI] [PubMed] [Google Scholar]
- 11.Fox A, Black G. Identification and detection of carbohydrate markers for bacteria: derivatization and gas chromatography-mass spectrometry. In: Fenselau C, editor. Mass spectrometry for the characterization of microorganisms. Washington, D.C: American Chemical Society; 1994. pp. 107–131. [Google Scholar]
- 12.Fox A, Black G E, Fox K, Rostovtseva S. Determination of carbohydrate profiles of Bacillus anthracis and Bacillus cereus including identification of O-methyl methylpentoses by using gas chromatography-mass spectrometry. J Clin Microbiol. 1993;31:887–894. doi: 10.1128/jcm.31.4.887-894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox A, Morgan S L, Gilbart J. Preparation of alditol acetates and their analysis by gas chromatography and mass spectrometry. In: Bierman J, McGinnis G, editors. Analysis of carbohydrates by GLC and MS. Boca Raton, Fla: CRC Press; 1989. pp. 87–117. [Google Scholar]
- 14.Fox A, Rogers J C, Fox K F, Schnitzer G, Morgan S L, Brown A, Aono R. Differentiation of legionellae by detection and characterization of aminodideoxyhexoses and other unique sugars using gas chromatography-mass spectrometry. J Clin Microbiol. 1990;28:546–552. doi: 10.1128/jcm.28.3.546-552.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox K, Brown A. Properties of the genus Tatlockia. Differentiation of Tatlockia (Legionella) maceachernii and micdadei from each other and from other Legionella. Can J Microbiol. 1993;39:486–491. doi: 10.1139/m93-069. [DOI] [PubMed] [Google Scholar]
- 16.Jenson M A, Webster J A, Straus N. Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphisms. Appl Environ Microbiol. 1993;59:945–952. doi: 10.1128/aem.59.4.945-952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreutzer D, Buller C, Robertson D. Chemical characterization and biological properties of lipopolysaccharides isolated from smooth and rough strains of Brucella abortus. Infect Immun. 1979;23:811–818. doi: 10.1128/iai.23.3.811-818.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leal-Klevezas D S, Martínez-Vázquez I O, López-Merino A, Martínez-Soriano J P. Single-step PCR for detection of Brucella spp. from blood and milk of infected animals. J Clin Microbiol. 1995;33:3087–3090. doi: 10.1128/jcm.33.12.3087-3090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaux-Charachon S, Bourg G, Jumas-Bilak E, Guigue-Talet P, Allardet-Servent A, O’Callaghan D, Ramuz M. Genome structure and phylogeny in the genus Brucella. J Bacteriol. 1997;179:3244–3249. doi: 10.1128/jb.179.10.3244-3249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno E, Pitt M, Jones L, Schurig G, Berman D. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. Infect Immun. 1979;138:361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno E, Speth S L, Jones L M, Berman D T. Immunochemical characterization of Brucella lipopolysaccharides and polysaccharides. Infect Immun. 1981;31:214–222. doi: 10.1128/iai.31.1.214-222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno E, Stackebrandt E, Dorsch M, Wolters J, Busch M, Mayer H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J Bacteriol. 1990;172:3569–3576. doi: 10.1128/jb.172.7.3569-3576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagpal, M. L., K. F. Fox, and A. Fox. Utility of 16S-23S rRNA spacer region methodology: how similar are interspace regions within a genome and between strains for closely related organisms? J. Microbiol. Methods, in press.
- 24.Peiras V, Fraser S, Fairhurst M, Weston D, Kaczmarski E. Laboratory diagnosis of Brucella: some pitfalls. Lancet. 1992;339:1415–1416. doi: 10.1016/0140-6736(92)91234-y. [DOI] [PubMed] [Google Scholar]
- 25.Picket M. Identification of Brucella species with a procedure for detecting acidification of glucose. Clin Infect Dis. 1994;19:976–977. doi: 10.1093/clinids/19.5.976. [DOI] [PubMed] [Google Scholar]
- 26.Rijpens N P, Jannes G, Van Asbroeck M, Rossau R, Herman L M F. Direct detection of Brucella spp. in raw milk by reverse hybridization with 16S-23S rRNA spacer probes. Appl Environ Microbiol. 1996;62:1683–1688. doi: 10.1128/aem.62.5.1683-1688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero C, Gamazo C, Pardo M, López-Goñi I. Specific detection of Brucella DNA by PCR. J Clin Microbiol. 1995;33:615–617. doi: 10.1128/jcm.33.3.615-617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero C, Pardo M, Grillo M, Diaz R, Blasko J, Lopez-Goni I. Evaluation of PCR and indirect enzyme immunoassay on milk samples for diagnosis of brucellosis in dairy cattle. J Clin Microbiol. 1995;33:3198–3200. doi: 10.1128/jcm.33.12.3198-3200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheinert P, Krausse R, Ullman U, Söller R, Krupp G. Molecular differentiation of bacteria by PCR amplification of the 16S-23S rRNA spacer region. J Microbiol Methods. 1996;26:103–117. [Google Scholar]
- 30.Sifuentes-Rincón A, Revol A, Barrera-Saldañav A. Detection and differentiation of the six Brucella species by polymerase chain reaction. Mol Med. 1997;3:734–739. [PMC free article] [PubMed] [Google Scholar]
- 31.Tibor A, Saman E, de Wergifosse P, Cloeckaert A, Limet J, Letesson J. Molecular characterization, occurrence, and immunogenicity in infected sheep and cattle of two minor outer membrane proteins of Brucella abortus. Infect Immun. 1996;64:100–107. doi: 10.1128/iai.64.1.100-107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verger J-M, Grimont F, Grimont P A D, Grayon P. Brucella, a monospecific genus as shown by deoxyribonucleic acid hybridization. Int J Syst Bacteriol. 1985;35:292–295. [Google Scholar]
- 33.Vizcaíno N, Fernández-Lago L. A rapid and sensitive method for the identification of Brucella species with a monoclonal antibody. Res Microbiol. 1992;143:513–518. doi: 10.1016/0923-2508(92)90098-9. [DOI] [PubMed] [Google Scholar]
- 34.Wunschel D, Fox K, Black G, Fox A. Discrimination among the B. cereus group, in comparison to B. subtilis, by structural carbohydrate profiles and ribosomal RNA spacer region PCR. Syst Appl Microbiol. 1994;17:625–635. [Google Scholar]