Abstract
Lipoblastoma-like tumor (LLT) is a benign soft tissue tumor demonstrating mixed morphologic features of lipoblastoma, myxoid liposarcoma and spindle cell lipoma, but lacking genetic alterations associated with those tumors. LLT was originally thought to be specific to the vulva but has since been reported in the paratesticular region. The morphologic features of LLT overlap with those of “fibrosarcoma-like lipomatous neoplasm” (FLLN), a rare, indolent adipocytic neoplasm considered by some to form part of the spectrum of atypical spindle cell and pleomorphic lipomatous tumor (ASCPLT). We compared the morphologic, immunohistochemical and genetic features of 23 tumors previously classified as LLT (n=17) and FLLN (n=6).
The 23 tumors occurred in 13 women and 10 men (mean age 42 years; range 17 to 80 years). Eighteen (78%) cases arose in the inguinogenital region, whereas 5 tumors (22%) involved non-inguinogenital soft tissue, including the flank (n=1), shoulder (n=1), foot (n=1), forearm (n=1), and chest wall (n=1). Microscopically, the tumors were lobulated and septated, with variably collagenized fibromyxoid stroma, prominent thin-walled vessels, scattered uni- or bivacuolated lipoblasts and a minor component of mature adipose tissue. By immunohistochemistry, 5 tumors (42%) showed complete RB1 loss, with partial loss in 7 cases (58%). RNA sequencing, chromosomal microarray, and DNA next generation sequencing studies were negative for significant alterations. There were no clinical, morphologic, immunohistochemical or molecular genetic differences between cases previously classified as LLT or FLLN. Clinical follow up (11 patients (48%); range 2–276 months; mean 48.2 months) showed all patients were alive without disease, and only one patient had experienced a single local recurrence.
We conclude that LLT and FLLN represent the same entity, for which “LLT” seems most appropriate. LLT may occur in either sex and any superficial soft tissue location. Careful morphologic study and appropriate ancillary testing should allow for distinction of LLT from its potential mimics.
Introduction
Lipoblastoma-like tumor (LLT), a benign neoplasm having mixed morphologic features of lipoblastoma, myxoid liposarcoma and spindle cell lipoma, was initially thought to be limited to the vulvar region1. More recently, however, cases in the paratestis have been reported, implying that LLT is not gender-specific2,3. Although the molecular pathogenesis of LLT is yet to be fully elucidated, it is known to lack PLAG1 alterations seen in lipoblastoma, DDIT3 rearrangements characteristic of myxoid liposarcoma and structural RB1 alterations that typify spindle cell lipoma4,5.
Over the past several years, we have noted considerable morphologic similarity between LLT and a small group of tumors previously described as “fibrosarcoma-like lipomatous neoplasm” (FLLN). FLLN, a very rare, indolent adipocytic tumor originally described by Deyrup and colleagues in 20136, is composed of bland, fibroblast-like spindled cells showing lipoblastic differentiation in the form of uni- and bivacuolated lipoblasts, often with a distinctive “ice cream cone” appearance, set in a variably myxoid and highly vascular background6. Genetically, FLLN is characterized by a “flat” comparative genomic hybridization (CGH) profile, without recurrent gains or losses, and lacks alterations associated with other lipomatous tumors, such as DDIT3 rearrangement, MDM2 amplification (seen in well-differentiated and dedifferentiated liposarcoma) and RB1 deletion6. Although it has been suggested that FLLN falls in the overall spectrum of what is now classified by the World Health Organization (WHO) as “atypical spindle cell and pleomorphic lipomatous tumor,”7,8 this is not universally accepted.
We studied the clinicopathologic, immunohistochemical and molecular genetic features of a well characterized series of LLT and FLLN, in order to better understand their relationship to each other and other adipocytic tumors.
Materials and Methods
Case Selection
Following approval by the Institutional Review Boards of the participating institutions, 24 tumors previously classified as “lipoblastoma-like tumor” (n=18) and “fibrosarcoma-like lipomatous neoplasm” (n=6) were retrieved from our collective consultation and institutional files, including 3 previously published FLLN6. One paratesticular tumor initially diagnosed as “LLT” was subsequently found to harbor RB1 loss, characteristic of spindle cell lipoma, and was excluded, leaving a final study group of 23 cases. Clinical histories, all available routinely stained and immunohistochemical slides, and the reports for previously performed ancillary tests were re-reviewed. Tissue blocks and/or unstained slides were obtained when available. Clinical follow up was obtained from medical records and referring pathologists.
Immunohistochemistry
Immunohistochemistry for RB1 protein (RB1, G3–245, 1:50 dilution, BD Biosciences, Santa Clara, CA, USA) was performed on formalin-fixed paraffin-embedded tissue sections using heat-induced epitope retrieval and the Dako Envision automated detection system (Dako, Tucson, AZ, USA). Positive and negative controls were utilized to confirm appropriate staining.
RNA Sequencing
Details of the RNA sequencing platform have been previously published9. In brief, the TruSeq RNA Exome assay (Illumina, San Diego, CA, USA) was used to prepare libraries according to manufacturer instructions for the TruSeq® RNA Exome Library Prep Kit to then sequence on the Illumina HiSeq 4000 instrument. Raw data were processed through the Mayo RNA seq bioinformatics pipeline, MAP-RSeq version 3.1.410. MAP-RSeq utilizes STAR to align reads to the human reference genome build hg38. The pipeline also uses clinically validated scripts for annotating gene fusions in sarcomas11. Additionally, we selected 10 random non-neoplastic adipose tissue samples from the Genotype-Tissue Expression project (GTEx) to serve as controls for differential expression analysis. The samples were also processed through the same MAP-RSeq pipeline. To adjust for batch effects between the GTEx controls and the tumor samples, ComBat-seq was used12. Differentially expressed genes were identified using bioinformatics R package edgeR 2.6.213, and reported with magnitude of change (log2 scale) and level of significance (False Discovery Rate, FDR < 5%). Cases 13–17 underwent RNA sequencing according to a previously outlined method14.
Chromosomal Microarray
Specifics of the OncoScan FFPE Assay Kit (Thermo Fisher Scientific, Santa Clara, CA, USA) have been previously described15. In brief, tumor was macrodissected for genomic DNA extraction, quantified by Qubit and processed for OncoScan according to manufacturer suggested protocol. Details of the molecular inversion probe (MIP) assay have previously been reported16, but in summary MIPs targeting a unique SNP or base pair of interest anneal to isolated DNA and circularize with the complementary nucleotide. The biotinylated oligonucleotide is hybridized overnight to a microarray. Data are then analyzed by the Affymetrix ChAS software. The genome-wide functional resolution of this array is approximately 500 kilobases for nonmosaic deletions and nonmosaic duplications. All data were analyzed and reported using the 2009 NCBI human genome build 37.1 (hg19).
DNA Next Generation Sequencing
Technical details of the targeted next generation sequencing assay have been previously published17. Briefly, a multiplex polymerase chain reaction (PCR) amplification-based next generation sequencing panel was utilized for sequencing of 50 genes of interest, including RB1. PCR products were processed with the Ion AmpliSeq Cancer Hotspot Panel v2 and Ion AmpliSeq Library Kit 2.0 (Thermo Fisher Scientific) adhering to manufacturer suggested protocol and sequenced on an Illumina MiSeq instrument using a 300-cycle MiSeq Reagent Kit v2 (Illumina). Raw data were processed using CASAVA (Illumina), and FASTQ files were aligned to human genome build hg19 using the CLC BIO Genomics Server program (Bio Genomics, Aarhus, Denmark). The alignment files were analyzed by the CLC BIO Server variant detection program within a custom bioinformatics pipeline and single nucleotide polymorphisms or insertions/deletions with an allele frequency of 5% or higher were manually reviewed and interpreted.
Results
Clinical Features and Patient Outcome
Table 1 summarizes the clinical features and immunohistochemical, molecular genetic and cytogenetic findings for the 23 studied cases. The tumors occurred in 13 women and 10 men (range 17–80 years of age; median 43 years; mean 42 years). Tumors initially diagnosed as LLT occurred in 10 women and 7 men and involved the vulva (n=8), groin (n=4), paratestis (n=1), inguinal region (n=2) and forearm (n=1), whereas those originally classified as FLLN occurred in 3 women and 3 men, and presented in the groin (n=2), paratestis (n=1), shoulder (n=1), foot (n=1) and chest wall (n=1). Of the 18 tumors involving the inguinogenital region, 13 (72%) arose in women and 5 (28%) in men. The tumors presented as soft tissue masses, swellings or nodules, occasionally with associated pain or tenderness. All were excised.
Table 1.
Clinicopathologic Features of Lipoblastoma-like Tumor and Fibrosarcoma-like Lipomatous Neoplasm
| Case | Prior Diagnosis | Site | Patient Age | Sex | Size (cm) | RB1 IHC | CMA | RNA Seq | DNA NGS | Additional Prior Testing | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LLT | Right groin | 29 | M | 5 | Mosaic | No CNA | Failed | Negative | - | NED (78 mos.) |
| 2 | LLT | Right mons pubis | 51 | F | 7.8 | NP | NP | Negative (prior testing) | NP | MDM2 RNA ISH negative | NED (18 mos.) |
| 3 | LLT | Left groin | 53 | M | 11.5 | Mosaic (prior testing) | NP | NP | NP | DDIT3 and FUS FISH negative; MDM2 RNA ISH negative; PLAG1 and CD34 IHC negative | NED (9 mos.) |
| 4 | LLT | Left forearm | 51 | M | 4 | Mosaic (prior testing) | NP | NP | NP | MDM2 RNA ISH negative; PLAG1 and CD34 IHC negative | NED (2 mos.) |
| 5 | LLT | Inguinal region | 31 | M | UNK | Deficient | cnLOH at 11p15.5p15.2 | Negative | Negative | - | UNK |
| 6 | LLT | Inguinal region | 32 | F | 3 | Deficient | Failed | Negative | NP | - | UNK |
| 7 | LLT | Mons pubis | 46 | F | 3.7 | Mosaic | No CNA | Negative | NP | - | NED (36 mos.) |
| 8 | LLT | Left labia | 28 | F | UNK | NP | NP | NP | NP | - | NED (16 mos.) |
| 9 | LLT | Left labium majus | 53 | F | UNK | NP | NP | NP | NP | - | Local recurrence (204 mos.); NED (276 mos.) |
| 10 | LLT | Right vulvar/inguinal region | 36 | F | 5.5 | NP | NP | NP | NP | MDM2 and CD34 IHC negative | UNK |
| 11 | LLT | Paratesticular | 63 | M | 5.3 | Deficient (prior testing) | NP | NP | NP | - | NED (62 mos.) |
| 12 | LLT | Vulva | 32 | F | UNK | Mosaic (prior testing) | NP | NP | NP | DDIT3, FUS, EWSR1 FISH negative; CD34 IHC negative | UNK |
| 13 | LLT | Flank | 51 | M | 6 | NP | Loss at 17q11.2q11.2; 17q12q21.2; 17q21.2q21.2 | Negative (prior testing) | Negative | CGH negative | UNK |
| 14 | LLT | Groin | 22 | F | 9 | NP | No CNA | Negative (prior testing) | Negative | CGH negative | UNK |
| 15 | LLT | Groin | 54 | M | 7 | NP | Gain of chromosomes 7 and 9 | Negative (prior testing) | Negative | DDIT3 FISH negative; CGH negative | UNK |
| 16 | LLT | Vulva | 17 | F | 8 | NP | No CNA | Negative (prior testing) | Negative | FUS-DDIT3 RT-PCR negative | UNK |
| 17 | LLT | Vulva | 52 | F | UNK | Mosaic | No CNA | Negative (prior testing) | CSF1R c.924C>G VUS | - | UNK |
| 18 | FLLN | Groin | 30 | F | 6 | Deficient | cnLOH at 12p12.3p12.1 | NP | Failed | DDIT3 FISH negative; CGH “flat”* | NED (13 mos.) |
| 19 | FLLN | Shoulder | 43 | F | 8 | Mosaic | Gain at 11q24.3q24.3 and 19p13.3p11; loss at 19p13.3p13.3 | NP | Failed | MDM2 and DDIT3 FISH negative; CGH “flat”* | NED (9 mos.) |
| 20 | FLLN | Paratesticular | 35 | M | 2.8 | Deficient | Gain of chromosome 19 | NP | Failed | MDM2 and DDIT3 FISH negative; CGH “flat”* | NED (12 mos.) |
| 21 | FLLN | Right foot | 57 | F | UNK | NP | NP | NP | NP | - | UNK |
| 22 | FLLN | Right groin | 24 | M | UNK | NP | NP | NP | NP | - | UNK |
| 23 | FLLN | Chest wall | 80 | M | UNK | NP | NP | NP | NP | - | UNK |
LLT (lipoblastoma-like tumor); FLLN (fibrosarcoma-like lipomatous neoplasm); UNK (unknown); NED (no evidence of disease); IHC (immunohistochemistry); CMA (chromosomal microarray); ISH (in situ hybridization); CNA (copy number alterations); VUS (variant of uncertain significance); cnLOH (copy neutral loss of heterozygosity); NP (not performed); CGH (comparative genomic hybridization)
Prior CGH for cases 18–20 from Deyrup, A. T. et al. Fibrosarcoma-like lipomatous neoplasm: a reappraisal of so-called spindle cell liposarcoma defining a unique lipomatous tumor unrelated to other liposarcomas. Am. J. Surg. Pathol. 37, 1373–1378 (2013).
Clinical follow up was available for 11 patients (48%) with a follow up duration of 2 to 276 months (median 16 months; mean 48.2 months). One patient whose vulvar tumor was incompletely excised developed a local recurrence at 204 months; following re-excision, she was reported to be without disease at 276 months. All patients with follow up are known to be alive without disease.
Gross and Microscopic Features
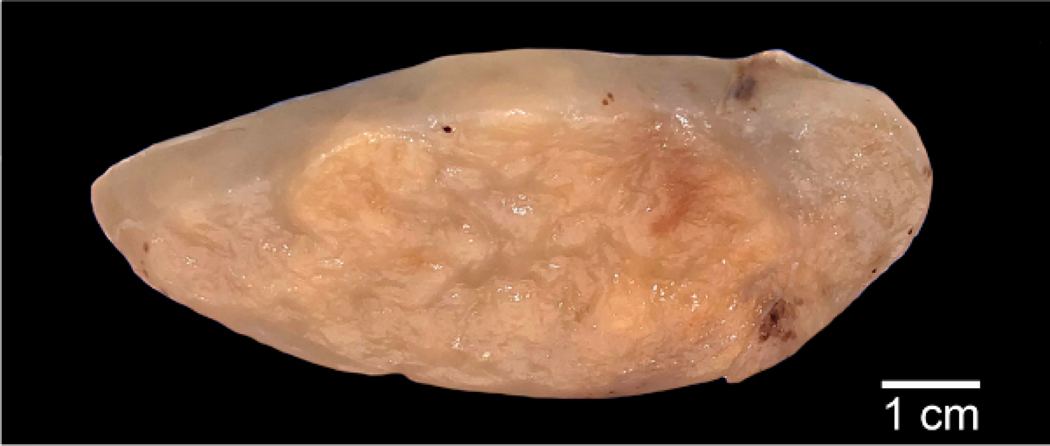
On gross examination, the tumors were 2.8 to 11.5 cm (median 6 cm; mean 6.2 cm) in greatest dimension and were described as well delineated, with a variably fibrous, myxoid or gelatinous cut surface (Fig. 1). Necrosis and hemorrhage were not noted.
Figure 1.
Macroscopic image of a paratesticular LLT demonstrating a well-defined, fibrofatty to myxo-gelatinous cut surface without necrosis.
The morphologic features of cases originally diagnosed as LLT (Fig. 2) and FLLN (Fig. 3) were essentially identical and will be described together. At low power magnification, the tumors were distinctly lobulated, with fibrous septa of varying thickness and prominent myxoid matrix (Figs. 2A, B; 3A, B). A variable amount of collagen was present within individual lobules, present either as dense aggregates or in smaller, wispy fibers (Figs. 2C; 3C). A spectrum of adipocytic differentiation was present, with some predominantly myxoid tumors containing only individual adipocytes or small clusters of adipocytes (Figs. 2D; 3D), sometimes more prominent at the periphery of the lobules. Other cases showed more extensive, lipoma-like adipocytic differentiation. A highly characteristic feature was the presence of an elaborate, arborizing, capillary vasculature reminiscent of that seen in myxoid liposarcoma (Figs. 2C; 3C). The neoplastic cells were uniform and fibroblastic in appearance, with normochromatic, ovoid to pointed nuclei, and indistinct nucleoli (Figs. 2E; 3E). Many of these spindled cells showed lipoblastic differentiation, with individual optically clear vacuoles at one or both ends of the nucleus, imparting an “ice cream cone” or “hourglass” appearance (Figs. 2F; 3F). Lipoblasts of these types varied widely in number, being easily discernible in some tumors and much more limited in extent in others. Mitotic activity was extremely low (<1 mitosis per 50 high power fields; 5.3 mm2) and necrosis was absent. Other morphologic features present in subsets of cases included extravasated red blood cells (Fig. 2F) and a patchy chronic inflammatory cell infiltrate (Fig. 3F).
Figure 2.
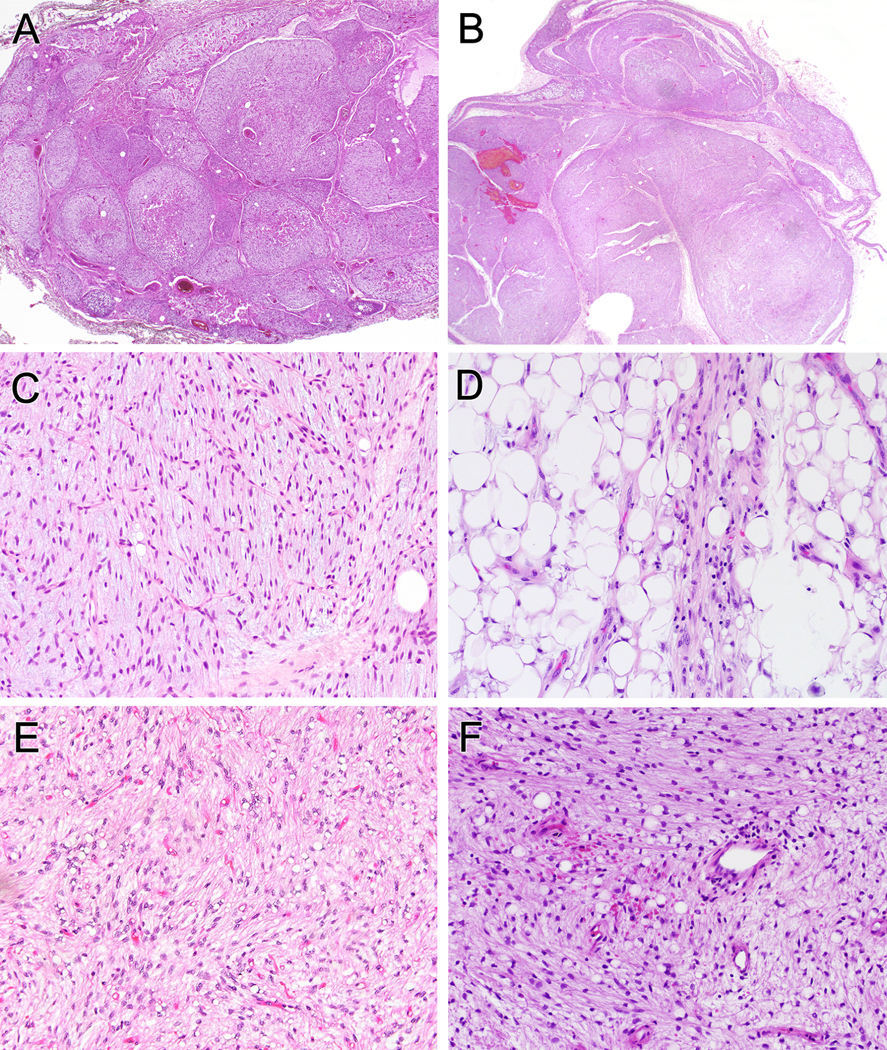
LLT often had multilobulated architecture separated by fibrous septa (A, B). A varying degree of fibromyxoid stroma was present, with accentuated delicate “chicken-wire” vasculature (C). Mature fat is often intermixed but can be more prominent at the periphery of lobules (D). All tumors were composed of bland eosinophilic spindle cells and at least focal uni- or bivacuolated lipoblasts (E, F). Erythrocyte extravasation and mild chronic inflammatory infiltrate were present in a subset (F).
Figure 3.
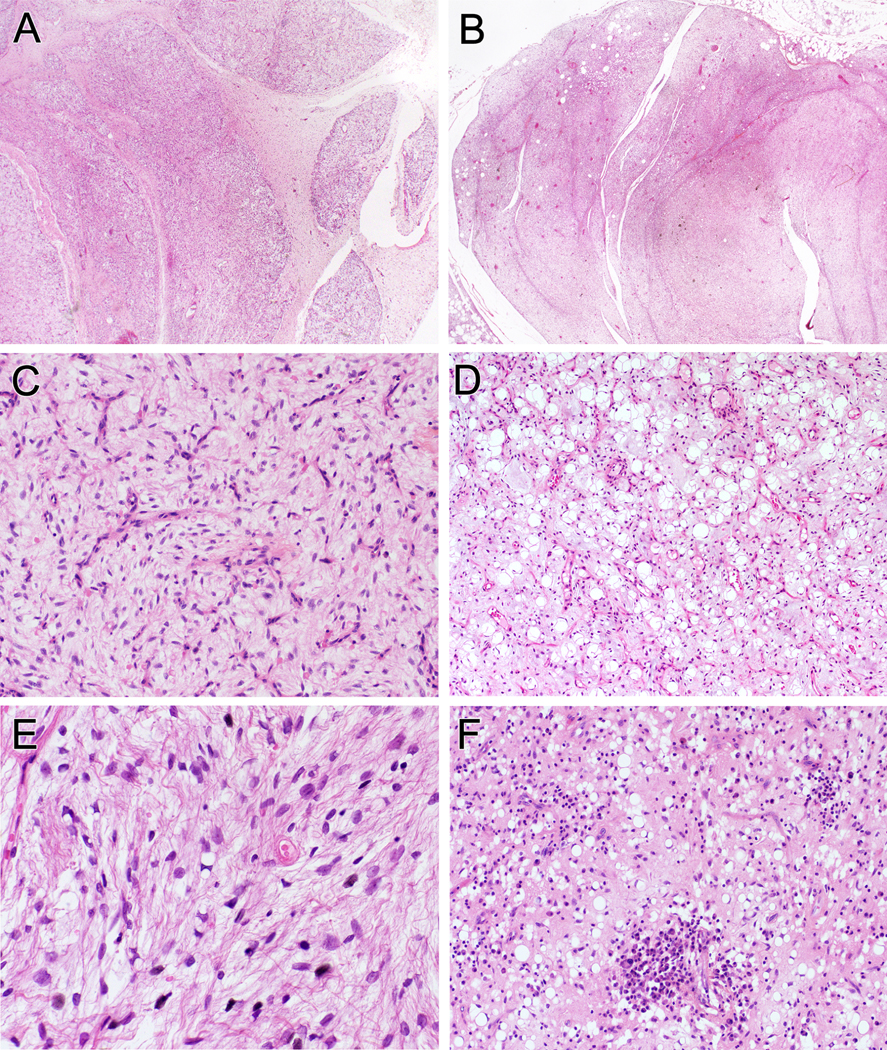
Tumors classified as FLLN were multinodular with dividing fibrous septa (A, B). Variable fibromyxoid matrix was present with prominent thin-walled capillary vessels (C). Mature adipocytes were interspersed but some tumors had clusters of mature fat concentrated at the periphery of lobules (D). All tumors were composed of bland spindle cells and uni- or bivacoulated lipoblasts (E, F). Some tumors had mild chronic inflammation (F).
Immunohistochemistry
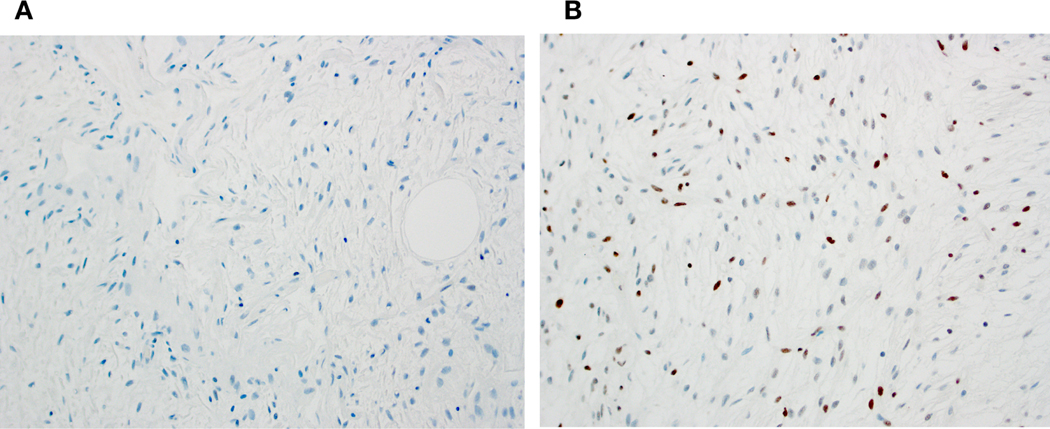
Immunohistochemical studies for RB1 protein expression were performed in 8 cases; an additional 4 cases had previously performed RB1 immunostains available for re-review. Complete loss of RB1 expression (Fig. 4A) was seen in 4 newly stained cases and in 1 previously stained case; overall 5 of 12 (42%) of tumors were RB1-deficient of which 3 had been classified as LLT and 2 as FLLN. A pattern of partial RB1 loss (“mosaic pattern”) demonstrated in Figure 4B was noted in 4 newly stained and 3 previously stained cases (7 of 12; 52%); 6 of these had been classified as LLT and 1 as FLLN.
Figure 4.
RB1 immunohistochemistry in tumors classified as LLT or FLLN showed either deficient (entirely negative) expression (A) or a mosaic pattern (heterogeneous, weak to moderate nuclear positivity) (B).
Previously performed CD34 and PLAG1 immunostains were re-reviewed for 4 and 2 tumors, respectively; all were negative.
RNA Sequencing Results
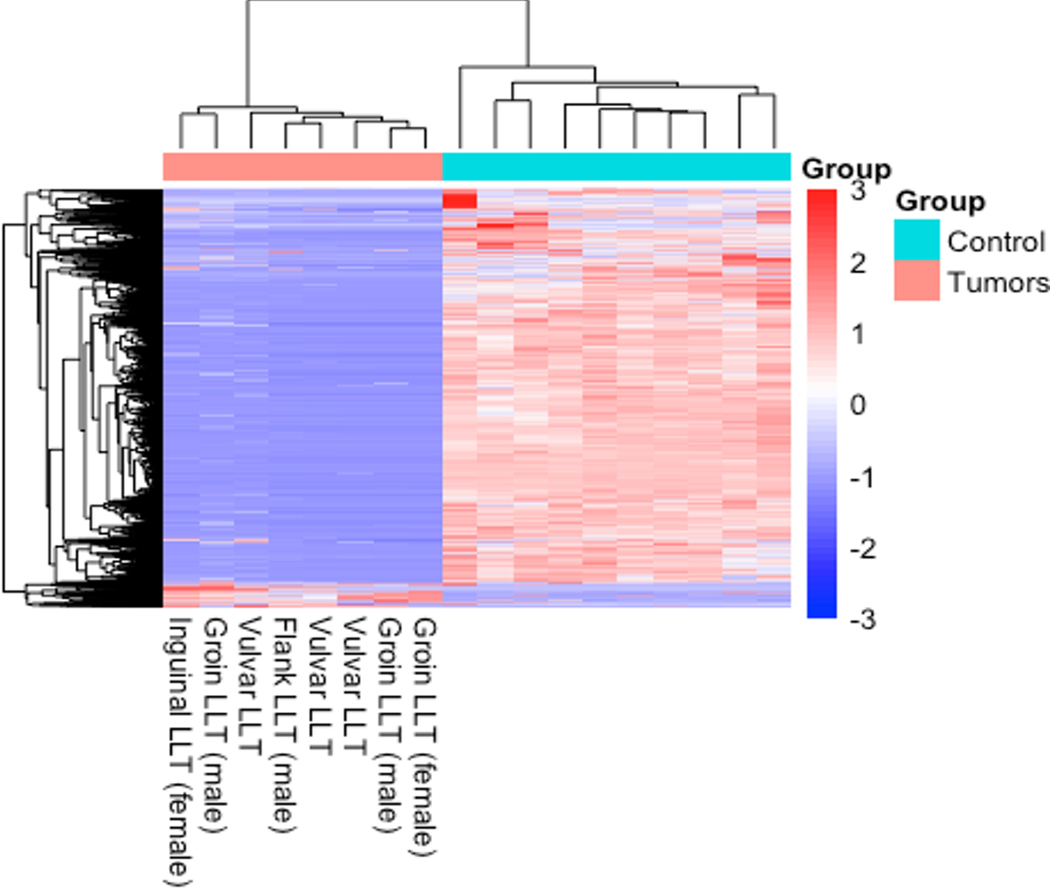
RNA sequencing, successfully performed on 9 of 10 attempted cases, was negative for gene rearrangements and/or fusion events. Unsupervised clustering of 2,813 differentially expressed genes among 8 LLTs from 5 women and 3 men demonstrated a distinct and similar expression profile compared to 10 adipose tissue controls represented as a heat map (Fig. 5). The 10 protein coding genes with the highest and lowest relative expression are listed in Supplementary Tables 1 and 2.
Figure 5.
Unsupervised clustering of 2,813 differentially expressed genes shows similar clustering among the 8 LLTs from 5 women and 3 men, and distinction from the 10 non-neoplastic adipose tissue controls, represented as a heat map.
Chromosomal Microarray Results
Twelve tumors (9 LLT, 3 FLLN) underwent copy number analysis. Five tumors (LLT) showed a “flat” profile, without significant copy number changes or regions with loss of heterozygosity (LOH). Chromosome 19 gains were seen in 2 tumors (FLLN) with regional chromosomal 19 loss also identified in 1 of these. Chromosome 11 gain/copy-neutral LOH was identified in 2 tumors (1 LLT, 1 FLLN). One case diagnosed as LLT had chromosome 12 copy-neutral LOH, 17q loss and chromosome 7/9 gain. One tumor failed processing.
DNA Next Generation Sequencing
DNA next generation sequencing was performed on 11 tumors (8 LLT, 3 FLLN). Six tumors (LLT) were negative for alterations while 3 cases (FLLN) failed processing. One tumor (LLT) had a CSF1R c.924C>G variant of uncertain significance (VUS).
ISH Results from Previous Testing
Chromogenic and fluorescence in situ hybridization for MDM2 amplification had been performed on 3 LLTs and 2 FLLNs, respectively; all were negative. DDIT3 and FUS FISH previously performed in 3 FLLNs and 2 LLTs, respectively, was negative for gene rearrangement.
Discussion
The results of the present study strongly suggest that LLT and FLLN represent the same entity and subsequently will be referred to as LLT/FLLN for the discussion. Specifically, we were unable to detect any clinical, morphologic, immunohistochemical or molecular genetic differences between cases previously classified as LLT or FLLN. In this light, a clearer picture of LLT/FLLN emerges.
Although, as might be expected, tumors previously classified as LLT occurred chiefly in the inguinogenital region of women (10 of 17 cases; 59%), this anatomic location was also involved by 3 (of 6) FLLNs, one of which occurred in a male. In agreement with this observation, 5 of 12 FLLNs originally reported by Deyrup et al occurred in the inguinogenital region, 4 in women6. Although LLT/FLLN does seem to show a predilection for the inguinogenital region, it is difficult to say whether it truly is more common in women or is alternatively less recognized in men. Certainly cases of LLT/FLLN in the male inguinogenital region were noted in our series and have been previously reported2,3,6. If LLT and FLLN are the same entity, it also follows that this tumor may occur in other somatic soft tissue locations. Five (of 23) tumors in the present series and 7 (of 12) cases reported by Deyrup et al6 involved non-inguinogenital soft tissue.
Morphologically, LLT/FLLN displays highly characteristic features, growing as a distinctly lobulated mass composed of uniform, amitotic, bland spindled cells with one or two perinuclear optically clear vacuoles. Most cases display predominantly fibromyxoid stroma, within which is found an elaborate, arborizing, capillary-sized vasculature and a variable component of mature fat. Immunohistochemical studies often demonstrate loss of RB1 protein expression, although losses at the RB1 gene locus (13q14.2) are not present by CGH or FISH5,6. PLAG1 expression is not seen. At the molecular genetic level, as shown in the present study and prior studies, LLT/FLLN is most notable for a “flat” CGH profile, without significant chromosomal losses or gains. RNA sequencing, DNA sequencing, and FISH studies have not identified fusion events, mutations or gene amplifications3–6.
Clinically, LLT/FLLN appears to behave in an almost entirely benign fashion, with a limited capacity for local recurrence, particularly when incomplete excised1,4–6. There is one report of widespread metastatic disease occurring in a 16-year-old with a purported LLT of the vulva18. However, this was a very large (13.5 cm) mass which displayed high cellularity, brisk mitotic activity and tumor cell necrosis, and had molecular genetic evidence of somatic mutations in MTOR (p.L1433S), PIK3CA (p.E545K) and a TERT promoter mutation (c.−146C>T (C250T)). While it appears that exceptional LLT/FLLN may progress to sarcoma, further study is necessary to determine whether such tumors are potentially related to other myxoid sarcomas. Apart from this one case, metastatic disease has not been reported in LLT/FLLN1,4–6.
The differential diagnosis of LLT/FLLN includes lipoblastoma, myxoid liposarcoma, spindle cell lipoma and atypical spindle cell/pleomorphic lipomatous tumor. Lipoblastoma occurs in children or young adults, develops on the extremities or trunk, and has prominent lobulated growth and mixed mature and immature adipocytic differentiation with mature adipocytes concentrated in the center of the fibrous lobules19. In contrast to LLT/FLLN, lipoblastoma is associated with PLAG1 rearrangements20. Although rearrangement of PLAG1 often correlates with overexpression of PLAG1 protein, immunohistochemical positivity for PLAG1 is not entirely specific to lipoblastoma and has been reported in a single vulvar LLT4.
Myxoid liposarcoma classically arises in deep soft tissue of extremities in young adults and shows some morphologic similarity to LLT/FLLN, in particular myxoid matrix and an elaborate capillary vasculature21. However, myxoid liposarcoma consists of small, round cells, rather than spindled cells, and usually contains multivacuolated lipoblasts, rather than the very distinctive “hourglass” lipoblasts seen in LLT/FLLN. In difficult cases, molecular genetic testing for DDIT3 rearrangements should distinguish myxoid liposarcoma from LLT/FLLN22.
Spindle cell lipoma frequently develops in the region of the neck, shoulder and back of middle aged to elderly men, but can occur in women and in unusual locations, such as the inguinogenital region23. Spindle cell lipoma consists of a uniform population of bland spindle cells distributed in small haphazard groups or bundles with wispy or ropey collagen, myxoid matrix and mature adipocytes with limited to no lipoblasts24. The absence of prominent capillary vasculature, absent (or very infrequent) lipoblasts and consistent diffuse and strong CD34 expression help to differentiate spindle cell lipoma from LLT/FLLN. Additionally, spindle cell lipomas are characterized by mono- or biallelic deletion of 13q13 which spans RB125. Loss of RB1 often results in absent expression of RB1 by immunohistochemistry26. While LLT/FLLN also can be RB1 deficient by immunohistochemistry, RB1 gene loss has not to date been detected5. As previously suggested, other mechanisms may affect immunohistochemical expression of RB1 such as alterations of other members of the RB1 pathway, epigenetic aberrations or protein degradation due to changes in the RB1 protein half-life5.
Atypical spindle cell/pleomorphic lipomatous tumor is a recently recognized entity, encompassing a group of tumors previously referred to as representing “well-differentiated spindle cell liposarcoma”27–30. In addition to morphologic features resembling those of ordinary spindle cell and pleomorphic lipomas, these lesions display infiltrative growth, higher cellularity, greater nuclear atypia, striking variation in size and shape of tumor cells, greater frequency of lipoblasts and readily identifiable mitotic activity. Furthermore, while a subset of LLT/FLLN may show patchy expression of CD34, essentially all ASCPLTs and spindle cell lipomas show robust diffuse CD34 reactivity. As in spindle cell lipoma, RB1 deletions are present in ASCPLT but typically there are other abnormalities as well, such as loss of the flanking gene RCBTB230. Although FLLN was originally thought to fall somewhere in the spectrum of “spindle cell liposarcoma,”6 it is morphologically quite different from ASCPLT. In fact, cases showing morphologic features of LLT/FLLN were specifically excluded from the currently largest study of ASCPLT28.
In summary, we have reported a series of LLT/FLLN, demonstrating that these two previously independently described tumors represent instead a single entity. Although the molecular pathogenesis of LLT/FLLN remains to be fully elucidated, all available evidence suggests that these tumors are unrelated to other myxoid adipocytic tumors, in particular lipoblastoma, myxoid liposarcoma and spindle cell lipoma. As the term “lipoblastoma-like tumor” appears to be more recognized in the literature than “fibrosarcoma-like lipomatous tumor,” we suggest that the former name be applied to these distinctive tumors in men and women at all anatomic locations.
Supplementary Material
Acknowledgement:
The authors thank Norman Barker, MS, MA, RBP for his assistance preparing figures and Patricia T. Greipp, DO and Troy Gliem of the Mayo Clinic Cytogenetics Core for their analysis.
Funding Statement:
The authors report no relevant sources of funding.
Footnotes
Ethics Approval: Approval for this study was granted by the Institutional Review Board at the participating institutions.
Conflict of Interest: The authors report no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement:
Data that support the findings of this study are available from the corresponding author on reasonable request.
References
- 1.Lae ME, Pereira PF, Keeney GL & Nascimento AG Lipoblastoma-like tumour of the vulva: report of three cases of a distinctive mesenchymal neoplasm of adipocytic differentiation. Histopathology 40, 505–509 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Droop E, Orosz Z, Michal M. & Melegh Z. A lipoblastoma-like tumour of the paratesticular region - male counterpart of lipoblastoma-like tumour of the vulva. Histopathology 76, 628–630 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Gambarotti M, Erdogan KE, Righi A. et al. Lipoblastoma-like tumor of the spermatic cord: case report and review of the literature. Virchows Arch 478, 1013–1017 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Mirkovic J. & Fletcher CD Lipoblastoma-like tumor of the vulva: further characterization in 8 new cases. Am J Surg Pathol 39, 1290–1295 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Schoolmeester JK, Michal M, Steiner P. et al. Lipoblastoma-like tumor of the vulva: a clinicopathologic, immunohistochemical, fluorescence in situ hybridization and genomic copy number profiling study of seven cases. Mod Pathol 31, 1862–1868 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Deyrup AT, Chibon F, Guillou L. et al. Fibrosarcoma-like lipomatous neoplasm: a reappraisal of so-called spindle cell liposarcoma defining a unique lipomatous tumor unrelated to other liposarcomas. Am. J. Surg. Pathol 37, 1373–1378 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Mariño-Enriquez A, Nascimento AF, Ligon AH, Liang C. & Fletcher CDM Atypical Spindle Cell Lipomatous Tumor: Clinicopathologic Characterization of 232 Cases Demonstrating a Morphologic Spectrum. Am. J. Surg. Pathol 41, 234–244 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Soft Tissue and Bone Tumours. 5th edn, (International Agency for Research on Cancer, 2020). [Google Scholar]
- 9.Martinez AP, Fritchie KJ, Weiss SW et al. Histiocyte-rich rhabdomyoblastic tumor: rhabdomyosarcoma, rhabdomyoma, or rhabdomyoblastic tumor of uncertain malignant potential? A histologically distinctive rhabdomyoblastic tumor in search of a place in the classification of skeletal muscle neoplasms. Mod Pathol 32, 446–457 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Kalari KR, Nair AA, Bhavsar JD et al. MAP-RSeq: Mayo Analysis Pipeline for RNA sequencing. BMC Bioinformatics 15, 224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winters JL, Davila JI, McDonald AM et al. Development and Verification of an RNA Sequencing (RNA-Seq) Assay for the Detection of Gene Fusions in Tumors. J Mol Diagn 20, 495–511 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Parmigiani G. & Johnson WE ComBat-seq: batch effect adjustment for RNA-seq count data. NAR Genom Bioinform 2, lqaa078 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson MD, McCarthy DJ & Smyth GK edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perret R, Tallegas M, Velasco V. et al. Recurrent YAP1::MAML2 fusions in “nodular necrotizing” variants of myxoinflammatory fibroblastic sarcoma: a comprehensive study of 7 cases. Mod Pathol 35, 1398–1404 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Freitag CE, Sukov WR, Bryce AH et al. Assessment of isochromosome 12p and 12p abnormalities in germ cell tumors using fluorescence in situ hybridization, singlenucleotide polymorphism arrays, and next-generation sequencing/mate-pair sequencing. Hum Pathol 112, 20–34 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Cottman M. & Schiffman JD Molecular inversion probes: a novel microarray technology and its application in cancer research. Cancer Genet 205, 341–355 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Gleeson FC, Kipp BR, Voss JS et al. Endoscopic ultrasound fine-needle aspiration cytology mutation profiling using targeted next-generation sequencing: personalized care for rectal cancer. Am J Clin Pathol 143, 879–888 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Kutasovic JR, Nones K, Lakis V. et al. Comprehensive histopathologic and genomic analysis of a novel case of lipoblastoma-like tumour of the vulva demonstrating malignant behaviour. Human Pathology Reports 30, 300678 (2022). [Google Scholar]
- 19.Coffin CM, Lowichik A. & Putnam A. Lipoblastoma (LPB): a clinicopathologic and immunohistochemical analysis of 59 cases. Am J Surg Pathol 33, 1705–1712 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Brandal P, Bjerkehagen B. & Heim S. Rearrangement of chromosomal region 8q11–13 in lipomatous tumours: correlation with lipoblastoma morphology. J Pathol 208, 388–394 (2006). [DOI] [PubMed] [Google Scholar]
- 21.ten Heuvel SE, Hoekstra HJ, van Ginkel RJ, Bastiaannet E. & Suurmeijer AJ Clinicopathologic prognostic factors in myxoid liposarcoma: a retrospective study of 49 patients with long-term follow-up. Ann Surg Oncol 14, 222–229 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Kuroda M, Ishida T, Horiuchi H. et al. Chimeric TLS/FUS-CHOP gene expression and the heterogeneity of its junction in human myxoid and round cell liposarcoma. Am J Pathol 147, 1221–1227 (1995). [PMC free article] [PubMed] [Google Scholar]
- 23.Ud Din N, Zhang P, Sukov WR et al. Spindle Cell Lipomas Arising at Atypical Locations. Am J Clin Pathol 146, 487–495 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Enzinger FM & Harvey DA Spindle cell lipoma. Cancer 36, 1852–1859 (1975). [DOI] [PubMed] [Google Scholar]
- 25.Dal Cin P, Sciot R, Polito P. et al. Lesions of 13q may occur independently of deletion of 16q in spindle cell/pleomorphic lipomas. Histopathology 31, 222–225 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Chen BJ, Marino-Enriquez A, Fletcher CD & Hornick JL Loss of retinoblastoma protein expression in spindle cell/pleomorphic lipomas and cytogenetically related tumors: an immunohistochemical study with diagnostic implications. Am J Surg Pathol 36, 1119–1128 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Anderson WJ, Fletcher CDM & Jo VY Atypical Pleomorphic Lipomatous Tumor: Expanding Our Current Understanding in a Clinicopathologic Analysis of 64 Cases. Am J Surg Pathol 45, 1282–1292 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Marino-Enriquez A, Nascimento AF, Ligon AH, Liang C. & Fletcher CD Atypical Spindle Cell Lipomatous Tumor: Clinicopathologic Characterization of 232 Cases Demonstrating a Morphologic Spectrum. Am J Surg Pathol 41, 234–244 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Lecoutere E. & Creytens D. Atypical spindle cell/pleomorphic lipomatous tumor. Histol Histopathol 35, 769–778 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Creytens D, Mentzel T, Ferdinande L. et al. “Atypical” Pleomorphic Lipomatous Tumor: A Clinicopathologic, Immunohistochemical and Molecular Study of 21 Cases, Emphasizing its Relationship to Atypical Spindle Cell Lipomatous Tumor and Suggesting a Morphologic Spectrum (Atypical Spindle Cell/Pleomorphic Lipomatous Tumor). Am J Surg Pathol 41, 1443–1455 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that support the findings of this study are available from the corresponding author on reasonable request.