Abstract
Objective:
To assess the impact of organ involvement on patient-reported outcomes (PROs) in light chain (AL) amyloidosis.
Methods:
PROs were evaluated using the KCCQ-12, PROMIS-29+2, and SF-36 in individuals with AL amyloidosis. The 2004 Mayo system was used to stage disease, and cardiac, neurologic, and renal involvement was considered. Global physical and mental health (MH) scores, physical function (PF), fatigue, social function (SF), pain, sleep, and MH domains were evaluated. Effect sizes between scores were measured using Cohen’s d.
Results:
Of 297 respondents, the median age at diagnosis was 60 years with 58% cardiac, 58% renal, and 30% neurologic involvement. Fatigue, PF, SF, and global physical health with PROMIS and SF-36 discriminated the most by stage. Significant discrimination in PROMIS and/or SF-36 was seen in PF, fatigue, and global physical health with cardiac involvement. For neurologic involvement, PF, fatigue, SF, pain, sleep, global physical and MH with PROMIS and role physical, vitality, pain, general health, and physical component summary with SF-36 were discriminatory. For renal amyloid, pain by SF-36 and PROMIS, and SF-36 MH and role emotional subscales were significant.
Conclusions:
Fatigue, PF, SF, and global physical health can discriminate stage, cardiac and neurologic, but not renal, AL amyloidosis involvement.
INTRODUCTION
Light chain (AL) amyloidosis and its treatment are associated with impaired health-related quality of life.1 This rare hematologic disease arises from a clonal plasma cell disorder, such as a monoclonal gammopathy of undetermined significance and multiple myeloma. The disease presents as a multisystemic disorder characterized by organ dysfunction due to deposition of insoluble fibril deposits.2, 3 The heart is the most commonly involved organ, seen in nearly 80% of patients, with development of a restrictive cardiomyopathy with diastolic dysfunction and eventually, heart failure.2 The severity of cardiac involvement determines stage and dictates the prognosis of this disease.4 Renal, neurological, gastrointestinal, and soft tissue organ involvement can also occur, though the number and pattern of organs involved by amyloidosis is often heterogeneous. The treatment of AL amyloidosis primarily involves chemotherapy to control the underlying plasma cell clone, including autologous stem cell transplantation in eligible patients along with aggressive supportive care.2 Currently, there are no approved fibril-directed treatments to remove pre-formed amyloid fibrils from organs. Thus, organ amyloidosis may never reverse fully. Patients with AL amyloidosis often experience high symptom burden associated with the type of organs affected by amyloid deposits, severity of organ dysfunction, as well as the treatment of the disease itself.5, 6
There are multiple options available to measure patient-reported outcomes (PRO) in AL amyloidosis.7, 8 The SF-36 has been the most widely studied, with documented content validity 9 and acceptable psychometric properties in AL amyloidosis patients treated at an academic center and from a community-based sample.10 Some PROMIS scales have also shown evidence of internal consistency, reliability, and construct validity in small cohorts of newly diagnosed and established AL amyloidosis patients from academic centers.11-13 The KCCQ-12 is a 12-item short form for use in patients with heart failure.14 The KCCQ-12 has been used in amyloidosis15 because cardiac involvement is common and determines prognosis. There remains a need for additional information on the performance of these measures in wider samples of AL amyloidosis patients, and no study has used all three measures in the same sample. We conducted the current study to compare the KCCQ-12, PROMIS, and SF-36 instruments in a community-based sample of AL amyloidosis patients. We evaluated known groups validity by comparing the 3 measures on how well they discriminate between patients by disease severity and pattern of organ involvement of AL amyloidosis.
METHODS
AL amyloidosis sample:
People living with AL amyloidosis who were members of the Amyloidosis Support Groups, Inc. (ASG) were invited to participate in an IRB-approved survey. The study invitation was disseminated by the President of ASG (MF) via email and shared on a closed Facebook group (AL amyloidosis ASG). Participants were provided an informational letter, and those who completed the online survey were compensated with a gift card of $50. The survey was open between 7/2/2021 to 7/28/2021.
In addition to baseline sociodemograhic information, individuals provided information on their amyloidosis, including time from diagnosis, type and number of organs involved, cardiac biomarkers at diagnosis and closest to survey completion, and treatment of their disease. The cardiac biomarkers NT-proBNP, BNP, troponin T (4th generation and 5th generation), and troponin I were considered.16 These data were reviewed (AD and AS) to assess plausibility and values that appeared erroneous were ignored. For example, a troponin T or NT proBNP value input as n/a, not measured, or inclusion of a date instead of a value were ignored. However, all other reported data from that patient was still used in the analysis. The 2004 Mayo Clinic stage4 was thus calculated in a subset of participants at diagnosis (N=106) and at time of survey (N=101). Stage at time of survey completion was analyzed.
Measure Description:
The KCCQ-12 is a 12-item questionnaire designed to measure several important aspects of heart failure.17 It includes a total index score and four subscale (physical limitation, symptoms, quality of life, and social limitation) scores. The scale is scored from 0 to 100 (higher scores = better health status). PROMIS items included Global health v1.2,18 PROMIS-29+2 v2.1 profile,19 and PROMIS Short Form v1.0 Fatigue-8a. The HealthMeasures Scoring Service was used to score each domain.20 PROMIS scores are expressed as T-scores, for which a score of 50 corresponds to the US general population with a standard deviation (SD) of 10. Higher scores correspond to more of the domain (e.g., Physical function and social roles- score >50 denotes better physical function than general population average, Fatigue- score >50 denotes greater fatigue than general population average). Because of the prevalence and impact of fatigue in AL amyloidosis patients, we administered the Fatigue-8a short form which includes the 4 items on fatigue severity that are also part of the PROMIS-29 with the addition of 4 items asking about fatigue impact. Two summary scores, global physical health summary (GPHS) and global mental health summary (GMHS), and eight domains, physical function, fatigue, ability to perform social roles and activities, anxiety, depression, pain interference, sleep disturbance, and cognitive function, were scored. The Medical Outcomes Study Short Form (SF-36 v.1) is a licensed 36-item, generic measure of health-related quality of life.21 The scores are derived using a norm-based scoring strategy that yields standardized distributions with a mean of 50 and a SD of 10 in the US general population. A higher score implies better health status. Two summary scores, physical component scale (PCS) and mental component scale (MCS), and eight subscale scores including physical function (PF), bodily pain (BP), role limitations due to personal or emotional problems (RE), general mental health (MH), social functioning (SF), energy/fatigue or vitality (VT), and general health perceptions (GH) can be calculated. Scoring was done using instructions provided by QualityMetric.22 The Physical and Mental Component Scores were computed using the oblique rotation method of Farivar et al.23
Clinical groups:
The following disease groupings were considered: 2004 AL stage I vs II vs III, cardiac organ involvement yes compared to no, neuropathic involvement yes compared to no, and renal involvement yes compared to no. We hypothesized that patients with stage I AL amyloidosis would have at least a 3-point difference in mean score with higher physical function and lower fatigue than stage III AL and that individuals with cardiac AL will have at least a 3-point difference in mean score for lower physical function and social roles, and higher fatigue compared to those with no cardiac AL. We did not have an a priori hypotheses for differences in PRO scores for renal and neuropathy known groups; these differences were considered exploratory.
Statistical analysis:
Sample size calculation:
Estimates were based on a two-tailed alpha test <0.05 and power of 80%. We sought to detect small to medium effect sizes between groups (stage and organ involvement) of equal size. With a sample size of 200 patients, with approximately 60-100 patients in each known group, we would have sufficient power to detect medium effect sizes of 0.3-0.5.
Data were summarized using means with SDs for continuous variables, frequencies with percentages for discrete variables, and mean scores with SD for PRO scores. Known clinical groups were compared by conducting analysis of variance to test for significant differences in mean scores across groups known to vary in disease severity. Cohen’s d was calculated for all pairwise comparisons. A Cohen’s d of <=0.2 was considered as a weak effect size, 0.3-0.5- medium, and >=0.8 as a large effect size.24
Missing data:
22 values of NT-proBNP and 34 values of troponin were considered erroneous (input values “?” or “unk”, date instead of value). Because the questions in KCCQ-12 specifically list heart failure, we did not compare neurologic and renal clinical groups using the KCCQ-12. Only patients with cardiac involvement were provided the KCCQ-12 survey and thus the KCCQ-12 was ‘missing’ in 126 patients without reported cardiac AL. Analysis was conducted in R 4.0.3 software (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The cohort characteristics are shown in Table 1. Median age at AL amyloidosis diagnosis was 60 years (range, 23-82) with a median time from diagnosis of 4.4 years (<0.1-21.9 years). Of these, 22% were within 2 years of diagnosis. Fifty eight percent of patients reported cardiac involvement and 39% reported involvement of 3 or more organs. Treatment included stem cell transplantation in 52% of individuals, and 50% of respondents were not on active therapy. Mean PRO scores and standard deviations are shown in Supplemental Table 1.
Table 1.
Baseline characteristics
| Characteristic | N=297 |
|---|---|
| Age at AL amyloidosis diagnosis, N = 292 | 60 (23.0-82.0) |
| Time from diagnosis to survey, years | 4.4 (<0.1-21.9) |
| ≤ 2 | 64 (22%) |
| >2 | 231 (79%) |
| Patient-reported race, N=294 | |
| White | 264 (90%) |
| Black | 10 (3.4%) |
| Other/Multiple | 16 (5.4%) |
| Prefer not to answer | 4 (1.4%) |
| Patient-reported ethnicity, N=295 | |
| Hispanic | 9 (3.1%) |
| Non-Hispanic | 278 (94%) |
| Prefer not to answer | 8 (2.7%) |
| Sex, N=296 | |
| Male | 141 (48%) |
| Female | 155 (52%) |
| AL amyloidosis subtype, N=238 | |
| Lambda | 165 (69%) |
| Kappa | 71 (30%) |
| IgM | 2 (0.8%) |
| Current 2004 AL stage, N=101 | |
| 1 | 33 (33%) |
| 2 | 37 (37%) |
| 3 | 31 (31%) |
| Median (range) number of organs involved, N=293 | 2 (1-7) |
| Number of organs involved, N=293 | |
| 1 | 126 (43%) |
| 2 | 54 (18%) |
| 3+ | 113 (39%) |
| Cardiac involvement | 171 (58%) |
| Renal involvement | 171 (58%) |
| Neurological involvement | 90 (30%) |
| Hepatic involvement | 34 (11%) |
| Gastrointestinal tract involvement | 82 (28%) |
| Tongue involvement | 47 (16%) |
| Skin/nail involvement | 45 (15%) |
| Pulmonary involvement | 13 (4.4%) |
| Muscle involvement | 20 (6.7%) |
| Other organ involvement | 28 (9.4%) |
| Treatment | |
| Prior chemotherapy | 262 (88%) |
| Prior stem cell transplant | 153 (52%) |
| Currently on active treatment | 146 (50%) |
Comparison of PROs by clinical groups:
PRO comparison by Mayo 2004 stage grouping:
Stage at the time of survey completion was available in 101 patients where cardiac biomarker information was available. Table 2 shows the differences in mean PRO scores along with the Cohen’s d values for the three stage groups. The most discriminating PRO domains included physical function, fatigue, and social roles. For physical function, PROMIS and SF-36 subscales, but not KCCQ-12, showed similar significant discrimination by stage of disease with large effect sizes showing higher physical function with >3 point difference between stages I vs II vs III. For the domains of fatigue and social roles, PROMIS showed moderate and significant discrimination with >3 point difference with higher fatigue for stage III vs II vs I, but not SF-36 or KCCQ-12. PROMIS social roles also showed a greater than 3-point difference in scores and moderate effect size with higher social roles in stage I vs II vs III. The SF-36 GH subscale additionally also showed a small (<3 point) significant discrimination by stage with stage I vs II but not stage II vs III. No discrimination was seen for the domains of pain, sleep, or mental health. For summary scores, PROMIS GPHS and SF-36 PCS showed similar discrimination between the three stage groups, but not KCCQ-12.
Table 2.
Comparison of PROs by stage of disease using Mayo 2004 stage at the time of survey completion. Significant values are shown in bold
| Domain | Measure | Stage I N=33 |
Stage II N=37 |
Stage III N=31 |
Cohen’s d III vs I |
p- value* |
|---|---|---|---|---|---|---|
| Physical Function | PROMIS Physical Function-4 | 46.7 | 43.2 | 40.4 | −0.76 (−1.26, −0.25) | 0.01 |
| SF-36 Physical Functioning | 49.3 | 46.5 | 42.2 | −0.76 (−1.28, −0.25) | 0.01 | |
| SF-36 Role Physical | 49.7 | 45.4 | 44.4 | −0.52 (−1.03, 0.00) | 0.10 | |
| KCCQ-12 Physical Limitations | 72.6 | 66.1 | 68.4 | −0.19 (−0.87, 0.50) | 0.7 | |
| Fatigue | PROMIS Fatigue-4 | 50.7 | 53.4 | 56.8 | 0.61 (0.11, 1.12) | 0.05 |
| PROMIS Fatigue-8 | 51.2 | 53.4 | 57.5 | 0.64 (0.13, 1.14) | 0.04 | |
| SF-36 Vitality | 48.8 | 46.4 | 44.4 | −0.40 (−0.90, 0.11) | 0.3 | |
| KCCQ-12 Item 3 | 4.1 | 4.4 | 3.8 | −0.16 (−0.84; 0.51) | 0.5 | |
| Social Roles | PROMIS Ability to Perform Social Roles and Activities | 51.0 | 47.2 | 44.9 | −0.68 (−1.19, −0.18) | 0.03 |
| SF-36 Social Function | 49.7 | 47.7 | 44.6 | −0.55 (−1.06, −0.04) | 0.1 | |
| KCCQ-12 Social Limitations | 76.2 | 64.4 | 62.0 | −0.54 (−1.22, 0.15) | 0.3 | |
| Pain | PROMIS Pain Interference | 47.7 | 51.5 | 51.7 | 0.42 (−0.08, 0.93) | 0.2 |
| SF-36 Bodily Pain | 54.7 | 50.4 | 52.2 | −0.27 (−0.78, 0.23) | 0.2 | |
| Sleep | PROMIS Sleep Disturbance | 50.5 | 51.3 | 49.5 | −0.10 (−0.60, 0.40) | 0.8 |
| Mental Health | PROMIS Anxiety | 50.3 | 51.6 | 51.1 | 0.09 (−0.40, 0.59) | 0.8 |
| PROMIS Depression | 46.9 | 49.9 | 48.3 | 0.18 (−0.31, 0.68) | 0.3 | |
| PROMIS Cognitive Function | 53.1 | 53.9 | 51.2 | −0.25 (−0.76, 0.25) | 0.3 | |
| SF-36 Mental Health | 54.9 | 53.4 | 53.4 | −0.21 (−0.71, 0.30) | 0.6 | |
| SF-36 Role Emotional | 52.5 | 51.4 | 48.4 | −0.42 (−0.93, 0.09) | 0.2 | |
| Summary scores and Other | PROMIS GPSS | 47.4 | 43.2 | 41.6 | −0.64 (−1.15, −0.13) | 0.04 |
| PROMIS GMSS | 51.8 | 47.3 | 46.9 | −0.51 (−1.02, −0.01) | 0.08 | |
| SF-36 PCS | 50.1 | 45.4 | 44.6 | −0.62 (−1.14, −0.11) | 0.03 | |
| SF-36 MCS | 51.8 | 49.2 | 48.2 | −0.45 (−0.96, 0.06) | 0.2 | |
| SF-36 General Health | 47.8 | 41.2 | 43.3 | −0.45 (−0.96, 0.06) | 0.03 | |
| KCCQ-12 Total | 73.3 | 67.2 | 64.6 | −0.40 (−1.08, 0.28) | 0.5 | |
| KCCQ-12 Symptoms | 73.1 | 72.2 | 68.3 | −0.21 (−0.89, 0.47) | 0.8 | |
| KCCQ-12 Quality of Life | 71.4 | 64.7 | 59.8 | −0.40 (−1.08, 0.28) | 0.5 |
One-way ANOVA
PROs by organ involvement:
For the type of organ involved, PROMIS and SF-36 were compared by presence of cardiac, renal, and neurological involvement (Table 3).
Table 3.
Comparison of PROs by amyloid organ involvement
| Domain | Measure | Cardiac AL (Yes=171, N=126) |
Neurologic AL (Yes=90, N=207) |
Renal AL (Yes=171, No=126) |
|||
|---|---|---|---|---|---|---|---|
| Cohen’s d (yes vs no) |
p- value |
Cohen’s d (yes vs no) |
p-value | Cohen’s d (yes vs no) |
p- value |
||
| Physical Function | PROMIS Physical Function-4 | −0.3 | 0.02 | −0.3 | 0.02 | 0.1 | 0.6 |
| SF-36 Physical Functioning | −0.3 | 0.02 | −0.2 | 0.1 | 0.1 | 0.5 | |
| SF-36 Role Physical | −0.2 | 0.1 | −0.3 | 0.03 | −0.2 | 0.1 | |
| Fatigue | PROMIS Fatigue-4 | 0.2 | 0.1 | 0.3 | 0.02 | 0.2 | 0.2 |
| PROMIS Fatigue-8 | 0.1 | 0.3 | 0.2 | 0.08 | 0.2 | 0.1 | |
| SF-36 Vitality | −0.3 | 0.01 | −0.3 | 0.02 | −0.1 | 0.4 | |
| Social Roles | PROMIS Ability to Perform Social Roles and Activities | −0.2 | 0.1 | −0.3 | 0.03 | −0.1 | 0.6 |
| SF-36 Social Function | −0.1 | 0.3 | −0.2 | 0.1 | −0.2 | 0.1 | |
| Pain | PROMIS Pain Interference | 0.1 | 0.5 | 0.5 | <0.001 | 0.3 | 0.01 |
| SF-36 Bodily Pain | −0.05 | 0.68 | −0.51 | <0.001 | −0.3 | 0.03 | |
| Sleep | PROMIS Sleep Disturbance | 0.2 | 0.1 | 0.3 | 0.02 | 0.05 | 0.7 |
| Mental Health | PROMIS Anxiety | 0 | 0.8 | 0 | 0.9 | 0.1 | 0.4 |
| PROMIS Depression | −0.1 | 0.5 | 0.2 | 0.1 | 0.1 | 0.3 | |
| PROMIS Cognitive Function | 0 | >0.9 | −0.2 | 0.3 | −0.1 | 0.3 | |
| SF-36 Mental Health | 0 | 0.9 | 0 | 0.9 | −0.3 | 0.04 | |
| SF-36 Role Emotional | 0.1 | 0.7 | −0.1 | 0.5 | −0.3 | 0.04 | |
| Summary scores and Other | PROMIS GPSS | −0.2 | 0.05 | −0.4 | <0.001 | 0 | 0.8 |
| PROMIS GMSS | −0.1 | 0.3 | −0.3 | 0.03 | −0.2 | 0.1 | |
| SF-36 PCS | −0.3 | 0.03 | −0.4 | 0.002 | −0.1 | 0.3 | |
| SF-36 MCS | −0.2 | 0.1 | −0.3 | 0.06 | −0.2 | 0.06 | |
| SF-36 General Health | −0.3 | 0.01 | −0.4 | 0.002 | 0.1 | 0.7 | |
KCCQ-12 was only administered to individuals reporting cardiac amyloidosis and it is not relevant for renal or neurologic conditions. Therefore, it is not shown in this table
Interpretation of Cohen’s d: 0.2- small, 0.5- medium, and 0.8- large effect sizes [22]
Cardiac involvement:
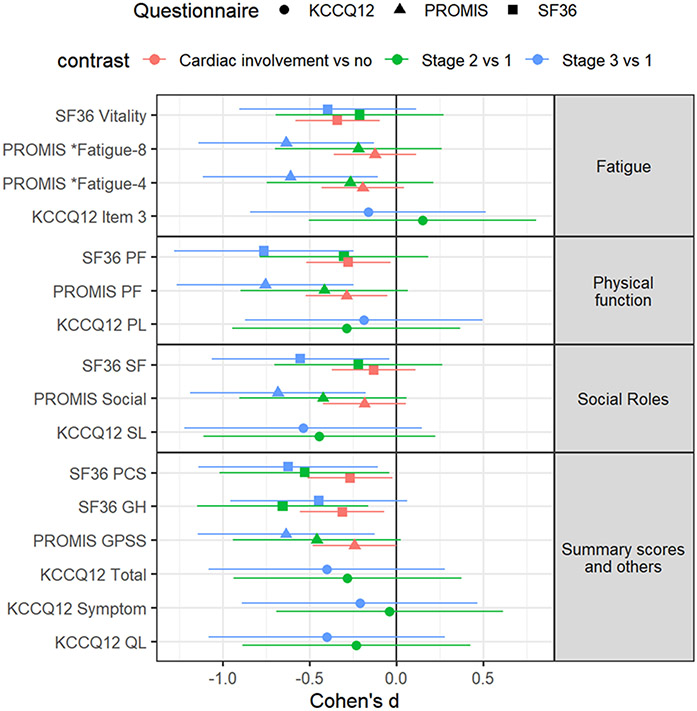
Among patients with cardiac amyloid involvement compared to those without, for the domain of physical function, PROMIS and SF-36 showed significant discrimination with a small effect size. Whereas, for fatigue, only the SF-36 vitality subscale was significant with a medium effect size. The SF-36 GH subscale also showed small and significant discrimination by stage. For summary scores, both PROMIS GPSS and SF-36 PCS showed significant and small discrimination. Table 3 and Figure 1 shows the differences for the domains of physical function, social function, and fatigue by stage and by cardiac involvement.
Figure 1.
Comparison of fatigue, physical function, social roles, and physical health summary scores by stage and cardiac involvement
Neurologic involvement:
Comparisons of patients reporting neurologic involvement with those reporting no neurologic involvement showed significant discrimination for physical function by PROMIS physical function domain and SF-36 RP but not SF-36 PF subscale. For the domain of fatigue, SF-36 Vitality and PROMIS Fatigue-4 but not Fatigue-8 showed small and significant discrimination. PROMIS social roles but not SF-36 social function was discriminatory. For pain, both PROMIS and SF-36 showed significant discrimination with a moderate effect size. PROMIS sleep disturbance also showed a significant albeit small effect size. Finally, PROMIS GPSS and SF-36 PCS summary scores in addition to PROMIS GMSS and SF-36 GH also showed significant discrimination with a small effect size.
Renal involvement:
Comparisons of PROs by renal amyloid involvement showed no significant discrimination in physical function, fatigue, social roles, or summary scores, but there was small and statistically significant discrimination by pain for both PROMIS and SF-36. Additionally, both, SF-36 MH and RE subscales also showed a small and statistically significant discrimination in this group.
DISCUSSION
The utilization of PROs to enhance the quality of care for patients with AL amyloidosis is of great significance and value. However, while clinical researchers have several options to measure PROs in in AL amyloidosis, there is no clear guidance on which measure to choose. Additionally, standardization of PRO measurement across different settings is necessary. In this analysis, we compared three PRO measures, namely the KCCQ-12, PROMIS, and SF-36, to measure health-related quality of life in individuals with AL amyloidosis. Our findings demonstrate differences in clinically significant groups of disease involvement for these three measures in the same sample. Specifically, our results indicate that for the known clinical groups of stage, cardiac and neurologic organ involvement, physical function, fatigue, social roles, and the physical health summary scores show significant discrimination, with comparable effects between PROMIS and SF-36, but not the KCCQ-12.
Previous studies have demonstrated the reliability, construct validity, and sensitivity to change of the SF-36 in patients with AL amyloidosis.10 In addition, the KCCQ-12 has been qualified by the FDA for use in heart failure,14 while the NIH PROMIS measures enable the measurement of important domains of health across chronic diseases, including cancer.25 The present study adds further support for the reliability and validity of the PROMIS and SF-36 measures as reasonable choices in the context of AL amyloidosis. In our previous work, which focused on a smaller cohort of newly diagnosed AL amyloidosis patients initiating active chemotherapy, PROMIS domains also shown evidence of responsiveness to change, further demonstrating construct validity, with changes in both hematologic response and NT-proBNP.12 The current study, which is the first to compare the three measures in AL amyloidosis, indicates that the SF-36 and PROMIS measures perform comparably in this setting.
Conceptual models of disease manifestations and PROs in AL amyloidosis consistently identify many domains, including fatigue and social roles, as being impacted.5, 26 Other studies have also highlighted physical function as being severely impaired in this disease.6, 11 Therefore, while we present results for all domains, we were particularly interested in these three domains as concepts of interest for individuals living with AL amyloidosis. Our cross-sectional sample included heterogeneity in disease natural history, with individuals early in their disease course (i.e., within 2 years of diagnosis) and long-term (>5 years) survivors, individuals on active chemotherapy, and those who had a long treatment-free interval. Given that these patients continue to experience impaired health-related quality of life,27 our approach remained relevant. Stage of disease is the strongest known predictor of outcomes in AL amyloidosis.3 When grouped by stage, PROMIS and SF-36 showed similar discrimination for physical function and summary scores, however PROMIS was better for the additional domains of fatigue and social roles. In terms of cardiac organ involvement, PROMIS and SF-36 were similar for physical function and physical health summary, but SF-36 performed better for fatigue, and neither measure showed discrimination for social roles. Among patients with neurologic amyloid involvement, in addition to physical function, fatigue, social roles, and physical summary score, pain, sleep disturbance, and mental summary scores are additionally discriminatory. Whereas, by renal amyloid involvement, physical function, social roles, fatigue, or summary scores were not discriminatory. Only pain by PROMIS and SF-36, and SF-36 MH and RE subscales show discrimination, albeit modest. This suggests that the pattern of amyloid involvement is an important factor to be considered when choosing PRO domains while designing clinical trials in this setting.
Our study has other limitations. We used the SF36v1 instead of v2 which has additional response choices for some items and improved wording. However, there is psychometric validity for both SF36v1 and SF-36v2 in AL amyloidosis.10 We were also limited by reliance of self-report of biomarkers. Though we allowed patients to upload laboratory results if available, we still had considerable missing data for cardiac biomarkers, and were thus unable to stage all patients. Furthermore, cardiac AL involvement can be a spectrum of changes which may or may not include heart failure. Organ involvement was also self-reported in this study and not confirmed with health records. Thus, the weak performance of the KCCQ-12 in this study should be interpreted with caution.
In summary, our cross-sectional community-based study provides a comparison of three PRO measures by clinical groups in AL amyloidosis. PROMIS and SF-36 measures demonstrate acceptable effect sizes to discriminate between clinical groups in AL amyloidosis patients. Our findings suggest that the domains of physical function, fatigue, social roles, and the physical health summary scores are particularly useful for discriminating between stage, cardiac and neurologic involvement, but not renal involvement. However, it is important to note that patients with AL amyloidosis can experience many other symptoms such as dyspnea, edema, and dizziness), which should also be considered in PRO measurement in this context of use. Additionally, an assessment of disease-specific symptoms along with the generic domain measures, may be helpful in providing a more comprehensive understanding of health-related quality of life in AL amyloidosis patients.
Supplementary Material
Acknowledgements:
The authors gratefully acknowledge the contribution of all participants and the Amyloidosis Support Groups, Inc. in this study.
Funding Statement:
Research reported in this publication was supported by K23 HL141445 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest:
AD reports institutional research funding from Abbvie, Caelum, Janssen, Novartis Prothena, Sanofi, Takeda and TeneoBio, Ad Board fees from BMS, Consulting fees from Prothena and Janssen. IA, AS, MF report no conflicts. KEF reports institutional research funding from Novartis, Consulting fees from Inhibikase and Pfizer. The authors confirm no competing financial interests in relation to the work in this manuscript.
Data availability statement:
Data will be shared upon reasonable request to the corresponding author
REFERENCES
- 1.Sanchorawala V, McCausland KL, White MK, Bayliss MS, Guthrie SD, Lo S et al. A longitudinal evaluation of health-related quality of life in patients with AL amyloidosis: associations with health outcomes over time. British journal of haematology 2017; 179(3): 461–470. e-pub ahead of print 2017/08/30; doi: 10.1111/bjh.14889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merlini G. AL amyloidosis: from molecular mechanisms to targeted therapies. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program 2017; 2017(1): 1–12. e-pub ahead of print 2017/12/10; doi: 10.1182/asheducation-2017.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gertz MA. Immunoglobulin light chain amyloidosis: 2022 update on diagnosis, prognosis, and treatment. American journal of hematology 2022; 97(6): 818–829. e-pub ahead of print 20220425; doi: 10.1002/ajh.26569 [DOI] [PubMed] [Google Scholar]
- 4.Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2004; 22(18): 3751–3757. doi: 10.1200/JCO.2004.03.029 [DOI] [PubMed] [Google Scholar]
- 5.D'Souza A, Myers J, Cusatis R, Dispenzieri A, Finkel M, Panepinto J et al. Development of a conceptual model of patient-reported outcomes in light chain amyloidosis: a qualitative study. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation 2022; 31(4): 1083–1092. e-pub ahead of print 20210713; doi: 10.1007/s11136-021-02943-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayliss M, McCausland KL, Guthrie SD, White MK. The burden of amyloid light chain amyloidosis on health-related quality of life. Orphanet J Rare Dis 2017; 12(1): 15. doi: 10.1186/s13023-016-0564-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurer MS, Dunnmon P, Fontana M, Quarta CC, Prasad K, Witteles RM et al. Proposed Cardiac End Points for Clinical Trials in Immunoglobulin Light Chain Amyloidosis: Report From the Amyloidosis Forum Cardiac Working Group. Circulation. Heart failure 2022; 15(6): e009038. e-pub ahead of print 20220325; doi: 10.1161/CIRCHEARTFAILURE.121.009038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizio AA, White MK, D'Souza A, Hsu K, Schmitt P, Quock TP et al. Health-Related Quality of Life Instruments for Clinical Trials in AL Amyloidosis: Report from the Amyloidosis Forum HRQOL Working Group. Patient Relat Outcome Meas 2023; 14: 153–169. e-pub ahead of print 20230518; doi: 10.2147/PROM.S399658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White MK, Bayliss MS, Guthrie SD, Raymond KP, Rizio AA, McCausland KL. Content validation of the SF-36v2(R) health survey with AL amyloidosis patients. J Patient Rep Outcomes 2017; 1(1): 13. e-pub ahead of print 2017/01/01; doi: 10.1186/s41687-017-0020-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White MK, McCausland KL, Sanchorawala V, Guthrie SD, Bayliss MS. Psychometric validation of the SF-36 Health Survey in light chain amyloidosis: results from community-based and clinic-based samples. Patient Relat Outcome Meas 2017; 8: 157–167. doi: 10.2147/PROM.S146849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Souza A, Magnus BE, Myers J, Dispenzieri A, Flynn KE. The use of PROMIS patient-reported outcomes (PROs) to inform light chain (AL) amyloid disease severity at diagnosis. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis 2020: 1–8. e-pub ahead of print 2020/01/24; doi: 10.1080/13506129.2020.1713743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Souza A, Brazauskas R, Dispenzieri A, Panepinto J, Flynn KE. Changes in patient-reported outcomes in light chain amyloidosis in the first year after diagnosis and relationship to NT-proBNP change. Blood cancer journal 2021; 11(2): 29. e-pub ahead of print 20210201; doi: 10.1038/s41408-021-00412-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty R, Rybicki L, Tomer J, Samaras CJ, Faiman BM, Valent J et al. Patient-reported outcomes in systemic AL amyloidosis with functional assessment of cancer therapy-general (FACT-G) and patient-reported outcomes measurement information system-global health (PROMIS-GH) in a real-world population. Leukemia & lymphoma 2019: 1–8. e-pub ahead of print 2019/07/06; doi: 10.1080/10428194.2019.1623885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in Clinical Trials and Clinical Care: JACC State-of-the-Art Review. Journal of the American College of Cardiology 2020; 76(20): 2379–2390. doi: 10.1016/j.jacc.2020.09.542 [DOI] [PubMed] [Google Scholar]
- 15.Lane T, Fontana M, Martinez-Naharro A, Quarta CC, Whelan CJ, Petrie A et al. Natural History, Quality of Life, and Outcome in Cardiac Transthyretin Amyloidosis. Circulation 2019; 140(1): 16–26. e-pub ahead of print 20190521; doi: 10.1161/CIRCULATIONAHA.118.038169 [DOI] [PubMed] [Google Scholar]
- 16.Ihne S, Morbach C, Obici L, Palladini G, Stork S. Amyloidosis in Heart Failure. Curr Heart Fail Rep 2019; 16(6): 285–303. doi: 10.1007/s11897-019-00446-x [DOI] [PubMed] [Google Scholar]
- 17.Spertus JA, Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes 2015; 8(5): 469–476. doi: 10.1161/CIRCOUTCOMES.115.001958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. Journal of clinical epidemiology 2010; 63(11): 1179–1194. e-pub ahead of print 2010/08/06; doi: 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewitt B, Feeny D, Fischhoff B, Cella D, Hays RD, Hess R et al. Estimation of a Preference-Based Summary Score for the Patient-Reported Outcomes Measurement Information System: The PROMIS((R))-Preference (PROPr) Scoring System. Medical decision making : an international journal of the Society for Medical Decision Making 2018; 38(6): 683–698. e-pub ahead of print 20180626; doi: 10.1177/0272989X18776637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.HealthMeasures Scoring Instructions. In.
- 21.Hays R, Sherbourne C, Mazel R. User's Manual for the Medical Outcomes Study (MOS) Core Measures of Health-Related Quality of Life, 1995. [Google Scholar]
- 22.https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/scoring.html Retreived July, 2022.
- 23.Farivar SS, Cunningham WE, Hays RD. Correlated physical and mental health summary scores for the SF-36 and SF-12 Health Survey, V.I. Health Qual Life Outcomes 2007; 5: 54. e-pub ahead of print 20070907; doi: 10.1186/1477-7525-5-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical Power Analysis for the Behavioural Sciences (2nd Edition), Lawrence Erlbaum Associates: Hillsdale, NJ, 1988. [Google Scholar]
- 25.Jensen RE, Potosky AL, Moinpour CM, Lobo T, Cella D, Hahn EA et al. United States Population-Based Estimates of Patient-Reported Outcomes Measurement Information System Symptom and Functional Status Reference Values for Individuals With Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017; 35(17): 1913–1920. e-pub ahead of print 20170420; doi: 10.1200/JCO.2016.71.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin HM, Seldin D, Hui AM, Berg D, Dietrich CN, Flood E. The patient's perspective on the symptom and everyday life impact of AL amyloidosis. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis 2015; 22(4): 244–251. doi: 10.3109/13506129.2015.1102131 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be shared upon reasonable request to the corresponding author