SUMMARY
KRAS mutated non-small cell lung cancer (NSCLC) is the most common genetically altered subtype of NSCLC, yet targeted therapies remain limited. Multiple studies have investigated combinations of MEK inhibitors with chemotherapy without success. Here we discuss these studies and novel approaches to targeting KRAS mutated NSCLC.
In this issue of Clinical Cancer Research, Gadgeel and colleagues evaluated the combination of trametinib, a MEK inhibitor, with docetaxel in patients with KRAS mutated(m) non-small cell lung cancer (NSCLC) following first line platinum containing regimens in a phase II single arm study (1). Mutations in the KRAS gene, specifically KRASG12C, are common oncogenic driver mutations identified in NSCLC, however, targeted therapies remain limited and modestly beneficial in part due to the challenge of pharmacologically inhibiting KRASm (2). There has been focus on targeting downstream effector pathways such as the MEK-ERK and PI3K/AKT pathways (Figure 1) with either single agents or combinatorial approaches. Trametinib, a MEK1/2 inhibitor, when evaluated as a single agent demonstrated similar efficacy compared to docetaxel in KRASm NSCLC following first line therapy (3). Additional evidence suggested a synergistic effect of combination trametinib with docetaxel with improved response rates in KRASm NSCLC.
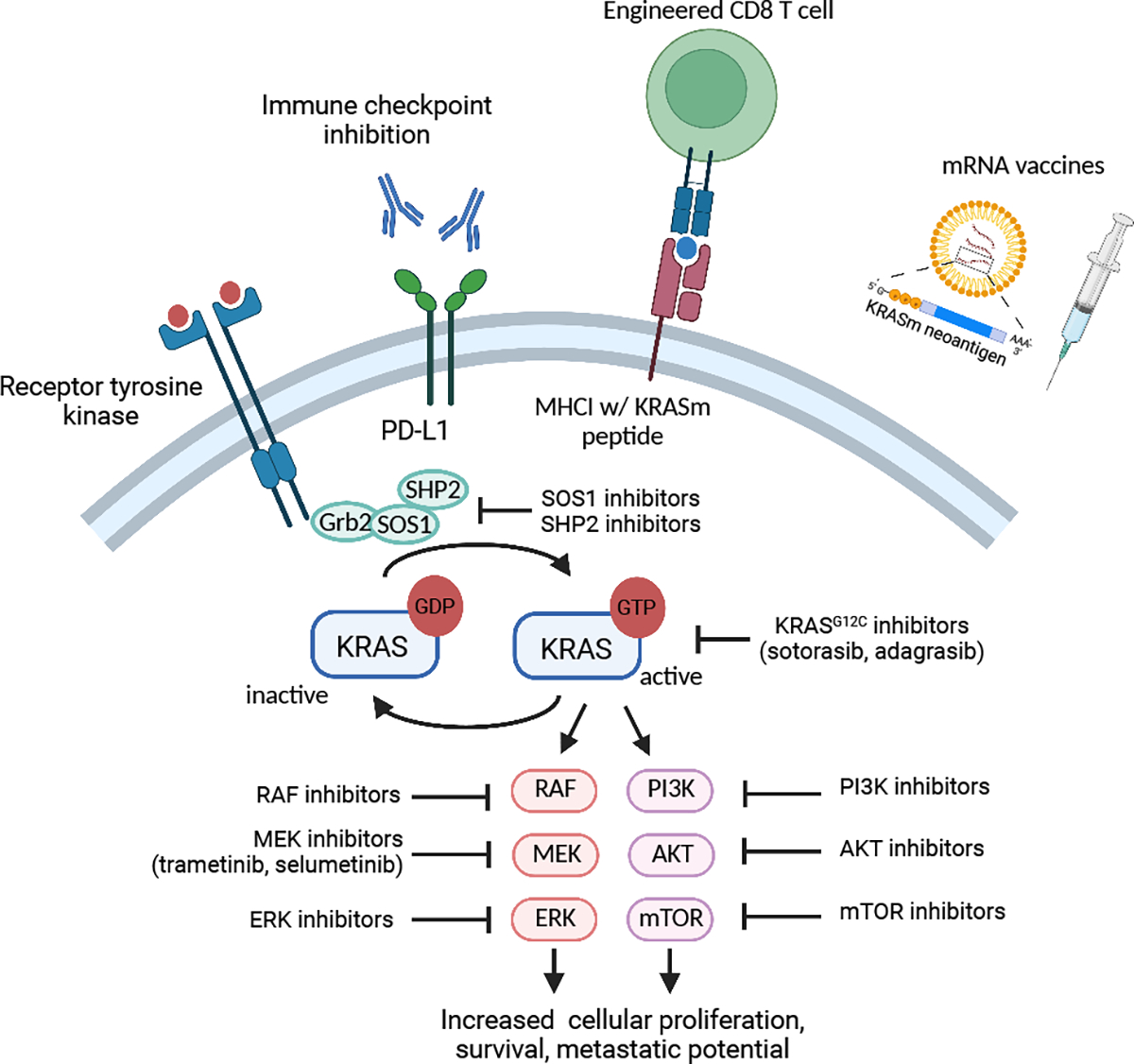
Strategies for targeting KRASm NSCLC. Represented is canonical KRAS signaling. In its inactive state, KRAS is bound to GDP. Following activation of membrane receptors, KRAS bound GDP is exchanged for GTP resulting in KRAS activation. KRAS subsequently activates downstream signaling pathways such as the MAP/ERK and PI3K/AKT pathway leading to cellular proliferation and survival. Mutant KRAS as well as upstream and downstream effectors of the KRAS pathway are targets for small molecule inhibitors. Strategies to activate the anti-tumor immune response includes immune checkpoint inhibitors, engineered T cells expressing KRASm TCRs, and mRNA vaccines expressing KRASm neoantigens.
Created with BioRender.com
Based on these early encouraging results, Gadgeel and colleagues conducted a multi-site cooperative group Phase II clinical trial to determine the efficacy of this combination in KRASm NSCLC, with a specific interest in the activity of this combination in the KRASG12C cohort (1). The combination led to an objective response rate (ORR) of 34%, with a median progression free survival (mPFS) of 4.1 months, and a median overall survival (mOS) of 10.9 months. There was no improvement in ORR amongst the KRASG12C subset as had been suggested in pre-clinical and earlier phase studies. These findings are similar to outcomes in SELECT-1, a phase III trial evaluating combination MEK inhibition with selumetinib and docetaxel versus docetaxel in a similar population, which failed to demonstrate superior outcomes with the addition of selumetinib (4). The results presented by the authors add to the existing evidence of limited clinical benefit of the addition of MEK inhibitors to chemotherapy for KRASm NSCLC.
Since the development of the discussed trial, strategies have focused on developing KRAS inhibitors based on the structure of allele specific mutations. Sotorasib and adagrasib are KRASG12C specific inhibitors that bind to an allosteric pocket and lock it into its inactive GDP-bound state (Figure 1) (5). In single arm phase II trials, both sotorasib and adagrasib demonstrated activity in relapsed KRASG12C NSCLC leading to FDA approval. Sotorasib demonstrated an ORR of 37.1% and mPFS of 6.8 months whereas adagasib demonstrated an ORR of 42.9% and a mPFS of 6.5 months (6,7). Codebreak 200, a phase III trial comparing sotorasib to docetaxel in KRASG12C NSCLC after 1st line treatment, demonstrated a clinically significant albeit modest increase in mPFS (5.6 v 4.5 months). No difference was seen in mOS although the data remain immature (8). While these efforts have established the use of sotorasib and adagrasib in the second line, ongoing clinical trials are investigating novel KRASG12C inhibitors as well as the combination KRASG12C inhibitors with inhibitors of RAS signaling pathway effectors in efforts to improve outcomes (9).
An additional area of interest is the development of agents for non-G12C KRASm NSCLC. Non-G12C KRAS subtypes have been hypothesized to have a lower GTPase activity and thus challenging to target therapeutically. Recently however, novel approaches targeting non-G12C variants, including G12D and G12V as well as pan-KRAS inhibitors, are in development (2). A KRASG12D specific inhibitor has shown promising pre-clinical activity and is currently being tested in patients with KRASG12D mutated solid cancers (NCT05737706) (10).
Beyond small molecule inhibitors, a significant effort is underway to target KRASm cancers by engaging the anti-tumor immune response (Figure 1). Unlike other subsets of oncogene driven NSCLCs such as those with alterations in EGFR and ALK, KRASm NSCLC is often associated with high PDL1 expression, high tumor mutational burden, and increased responsiveness to immune checkpoint inhibition (ICI). Several retrospective analyses have demonstrated similar outcomes with ICI monotherapy compared to chemoimmunotherapy in patients with KRASm and PDL1 >50% NSCLC which is distinct from those with KRAS wild-type tumors where chemoimmunotherapy is likely superior (11,12). Additionally, preclinical studies have suggested synergy between KRASG12C inhibitors and ICI and based on these observations, numerous clinical trials are assessing combinatorial approaches (NCT04185883, NCT04613596, NCT04449874).
Cancer vaccines have again emerged as a promising approach to enhance anti-tumor CD8 T cell responses. While older methods using peptide-based and DC-based vaccines have demonstrated KRASm specific immune responses, newer approaches utilizing mRNA vaccine technology combined with ICI have demonstrated the most promise (9), and a mRNA vaccine expressing the four most common KRAS mutations (G12C, G12V, G12D, and G13D) is currently being tested in patients with KRASm solid tumors (NCT03948763). A recent study utilizing a personalized neoantigen mRNA vaccine, autogene cevumeran, combined with atezolizumab resulted in the expansion and activation of antigen specific CD8 T cells and prolonged recurrence free intervals in patients with resected pancreatic cancer highlighting the potential of this approach across KRAS mutated cancers (13). Another emerging strategy is the generation of autologous T cells expressing KRASm specific T cell receptors (TCRs). Preclinical studies have identified numerous mutant KRAS epitopes that can be processed and presented by the MHC machinery and thus be effectively targeted with engineered TCRs against KRASm (14). Indeed, an engineered TCR has been effective in a patient with metastatic KRASG12D pancreatic cancer (15) and multiple early phase clinical trials evaluating KRASm targeted TCRs are ongoing (NCT03190941, NCT03745326, NCT04146298).
In KRASm NSCLC, another factor to consider is the presence or absence of co-mutations, such as TP53, STK11, and KEAP1. These mutations are commonly identified and influence responsiveness to both KRASG12C inhibitors and immunotherapies (2). Co-mutations in TP53 and STK11 have been associated with biologically distinct subtypes of KRASm NSCLC and impacted outcomes following MEK inhibition in mouse models of KRASm NSCLC (16,17). On this trial, in an exploratory analysis, the authors assessed whether outcomes varied among patients with these co-mutations. There was no difference in outcomes between STK11m vs STK11 wild-type (wt) tumors, however, TP53m tumors were associated with worse ORR (0% vs 56%) and mOS (5.6 vs 10.9 months)(1). While the overall number of evaluable patients with co-mutation status in this analysis is small, these findings are consistent with emerging evidence that co-mutational status provides important prognostic and predictive information and should be incorporated into clinical trial design and factor into treatment decisions in both the first- and second-line settings.
In summary, the data presented by Gadgeel and colleagues highlights the difficulty in developing therapies that improve outcomes for KRASm NSCLC. However, with the advent of KRASG12C specific small molecular inhibitors, there is increased excitement in the ability to effectively target KRAS specific mutations across tumor types. Together with efforts to develop small molecule inhibitors against effectors of the RAS pathway and strategies to enhance KRASm directed immune responses, we anticipate an increase in the treatment options for KRASm NSCLC in the coming years. Moreover, with continued increase in biomarker testing in clinical practice, treatment decisions can be further optimized based on both driver mutation and co-mutation status. Altogether this is good news for our patients.
Footnotes
Disclosure of Potential Conflicts of Interest:
Dr. Cantor has no conflicts of interest to report. Dr. Aggarwal reports receiving institutional research funding from AstraZeneca, Genentech, Incyte, Macrogenics, Medimmune, and Merck Sharp & Dohme, and receiving consultation fees from Genentech, Lilly, Celgene Merck, AstraZeneca, Blueprint Genetics, Shionogi, Daiichi Sankyo/ Astra Zeneca, Regeneron/ Sanofi, Eisai, BeiGene, Turning Point, Pfizer, Janssen, Boehringer Ingelheim.
REFERENCES
- 1.Gadgeel SM, Miao J, Riess JW, Moon J, Mack PC, Gerstner GJ, et al. Phase II study of docetaxel and trametinib in patients with KRAS mutation positive recurrent non-small cell lung cancer (NSCLC) (SWOG S1507, NCT-02642042). Clinical Cancer Research. 2023; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salgia R, Pharaon R, Mambetsariev I, Nam A, Sattler M. The improbable targeted therapy: KRAS as an emerging target in non-small cell lung cancer (NSCLC). Cell Rep Med. 2021;2:100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenschein GR, Smit EF, Planchard D, Kim D-W, Cadranel J, Pas TD, et al. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC). Ann Oncol. 2015;26:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jänne PA, Heuvel MM van den, Barlesi F, Cobo M, Mazieres, Crinò L, et al. Selumetinib Plus Docetaxel Compared With Docetaxel Alone and Progression-Free Survival in Patients With KRAS-Mutant Advanced Non–Small Cell Lung Cancer: The SELECT-1 Randomized Clinical Trial. JAMA. 2017;317:1844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janes MR, Zhang J, Li L-S, Hansen R, Peters U, Guo X, et al. Targeting KRAS Mutant Cancers with a Covalent G12 C-Specific Inhibitor. Cell. 2018;172:578–589.e17. [DOI] [PubMed] [Google Scholar]
- 6.Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou S-HI, Pacheco JM, et al. Adagrasib in Non–Small-Cell Lung Cancer Harboring a KRASG12C Mutation. New Engl J Medicine. 2022;387:120–31. [DOI] [PubMed] [Google Scholar]
- 7.Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. New Engl J Medicine. 2021;384:2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langen AJ de Johnson ML, Mazieres J Dingemans A-MC, Mountzios G, Pless M, et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS G12C mutation: a randomised, open-label, phase 3 trial. Lancet. 2023;401:733–46. [DOI] [PubMed] [Google Scholar]
- 9.Asimgil H, Ertetik U, Çevik NC, Ekizce M, Doğruöz A, Gökalp M, et al. Targeting the undruggable oncogenic KRAS: the dawn of hope. JCI Insight. 2022;7:e153688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallin J, Bowcut V, Calinisan A, Briere DM, Hargis L, Engstrom LD, et al. Anti-tumor efficacy of a potent and selective non-covalent KRASG12D inhibitor. Nat Med. 2022;28:2171–82. [DOI] [PubMed] [Google Scholar]
- 11.Sun L, Hsu M, Cohen RB, Langer CJ, Mamtani R, Aggarwal C. Association Between KRAS Variant Status and Outcomes With First-line Immune Checkpoint Inhibitor–Based Therapy in Patients With Advanced Non–Small-Cell Lung Cancer. JAMA Oncol. 2021;7:937–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakajima EC, Ren Y, Vallejo JJ, Akinboro O, Mishra-Kalyani PS, Larkins EA, et al. Outcomes of first-line immune checkpoint inhibitors with or without chemotherapy according to KRAS mutational status and PD-L1 expression in patients with advanced NSCLC: FDA pooled analysis. J Clin Oncol. 2022;40:9001–9001. [Google Scholar]
- 13.Rojas LA, Sethna Z, Soares KC, Olcese C, Pang N, Patterson E, et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature. 2023;618:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bear AS, Blanchard T, Cesare J, Ford MJ, Richman LP, Xu C, et al. Biochemical and functional characterization of mutant KRAS epitopes validates this oncoprotein for immunological targeting. Nat Commun. 2021;12:4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leidner R, Silva NS, Huang H, Sprott D, Zheng C, Shih Y-P, et al. Neoantigen T-Cell Receptor Gene Therapy in Pancreatic Cancer. N Engl J Med. 2022;386:2112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, Izzo J, et al. Co-occurring Genomic Alterations Define Major Subsets of KRAS-Mutant Lung Adenocarcinoma with Distinct Biology, Immune Profiles, and Therapeutic Vulnerabilities. Cancer Discov. 2015;5:860–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012;483:613–7 [DOI] [PMC free article] [PubMed] [Google Scholar]


