Abstract
Background & Methods.
To determine the role of cellular senescence and the senescence associated secretory phenotype (SASP) in age-related aortic stiffening and endothelial dysfunction, we studied young (6-8 mo) and old (27-29 mo) p16-3MR mice, which allows for genetic-based clearance of senescent cells with ganciclovir (GCV). We also treated old C57BL/6N mice with the senolytic ABT-263.
Results.
In old mice, GCV reduced aortic stiffness assessed by aortic pulse wave velocity (PWV; 477±10 vs. 382±7 cm/s, P<0.05) to young levels (old-GCV vs. young-vehicle, P=0.35); ABT-263 also reduced aortic PWV in old mice (446±9 to 356±11 cm/s, P<0.05). Aortic adventitial collagen was reduced by GCV (P<0.05) and ABT-263 (P=0.12) in old mice. To show an effect of the circulating SASP, we demonstrated that plasma exposure from Old-vehicle p16-3MR mice, but not from Old-GCV mice, induced aortic stiffening assessed ex vivo (elastic modulus; P<0.05). Plasma proteomics implicated glycolysis in circulating SASP-mediated aortic stiffening. In old p16-3MR mice, GCV increased endothelial function assessed via peak carotid artery endothelium-dependent dilation (EDD; Old-GCV, 94±1% vs. Old-vehicle, 84±2%, P<0.05) to young levels (Old-GCV vs. young-vehicle, P=0.98), and EDD was higher in old C57BL/6N mice treated with ABT-263 vs. vehicle (96±1% vs. 82±3%, P<0.05). Improvements in endothelial function were mediated by increased nitric oxide (NO) bioavailability (P<0.05) and reduced oxidative stress (P<0.05). Circulating SASP factors related to NO signaling were associated with higher NO-mediated EDD following senescent cell clearance.
Conclusions.
Cellular senescence and the SASP contribute to vascular aging and senolytics hold promise for improving age-related vascular function.
Keywords: Cellular senescence, Senescence-associated secretory phenotype, Senolytics, Aging, Aortic stiffness, Endothelial function
INTRODUCTION
Cardiovascular diseases (CVD) are the leading cause of death worldwide. Advancing age is the primary non-modifiable risk factor for the development of CVD1. A key event mediating the increase in CVD risk with aging is the development of vascular dysfunction, characterized by stiffening of the large elastic arteries (primarily the aorta) and vascular endothelial dysfunction2.
Age-related aortic stiffening, as demonstrated by increased aortic pulse wave velocity (PWV), occurs mainly via changes in the extracellular matrix, featuring collagen deposition (fibrosis), elastin degradation and cross-linking of these structural proteins by advanced glycation end products (AGEs)3,4. These adverse changes to the arterial wall can be induced by excessive superoxide-related oxidative stress3. Age-associated endothelial dysfunction, as shown by reduced endothelium-dependent dilation (EDD), is mediated primarily by reduced nitric oxide (NO) bioavailability3,4. This impairment in NO-mediated endothelial function is largely due to excessive superoxide-related oxidative stress, which reacts with NO, reducing its bioavailability3,4. However, the cellular-molecular processes by which aging leads to vascular oxidative stress, and ultimately vascular dysfunction are incompletely understood.
Cellular senescence is a multi-faceted stress response that leads to a largely permanent cell cycle arrest5,6. Senescent cells alter the circulating milieu (e.g., plasma) by secreting numerous pro-inflammatory cytokines, chemokines, metabolites and growth factors termed the senescence-associated secretory phenotype (SASP), which can induce oxidative stress7. Physiological levels of cellular senescence are critical for many reasons (e.g., cancer suppression8 and optimal wound healing9); however, excessive accumulation of senescent cells occurs in multiple tissues with aging10-12. This age-related buildup of senescent cells can induce tissue dysfunction, at least in part through the SASP, and may be involved in several age-related pathologies11,12. As such, cellular senescence may contribute to vascular oxidative stress, reduced NO bioavailability, adverse changes to the aortic extracellular matrix, and vascular dysfunction with aging. However, the role of cellular senescence and the SASP in mediating aortic stiffening and vascular endothelial dysfunction with aging has not yet been systematically investigated.
Compounds that evoke the removal of senescent cells, termed senolytics, improve select indices of physiological function with advancing age13,14. The current strategy with senolytic therapy is to administer the compound in an intermittent rather than continuous fashion so as to reduce the number of senescent cells to levels that leave the basal levels which leave cellular senescence processes intact to preserve physiological homeostasis15. Late-life senolytic treatment shows promise for improving aortic stiffness and vascular endothelial function in older age, but definitive evidence is lacking.
In the present study, we first utilized the p16-3MR mouse model, which allows for genetic-based suppression of excess senescent cells9,16, to determine the role of cellular senescence and the SASP in mediating vascular dysfunction with aging. We then targeted cellular senescence with a well-established synthetic pharmacological senolytic agent (ABT-263)17 to establish proof-of-concept efficacy of senolytic therapy for improving vascular function with aging. We used innovative experimental approaches to elucidate the role of NO, oxidative stress and circulating SASP proteins in regulating cellular senescence-mediated vascular dysfunction with aging.
METHODS
All data presented in this article and in the Data Supplement will be made available upon reasonable request to the corresponding author.
Animals
All mice were housed in a conventional facility on a 12-hour light/dark cycle, given ad libitum access to an irradiated, fixed, and open standard rodent chow (Inotiv/Envigo 7917) and drinking water. In vivo testing (blood pressure and aortic pulse wave velocity [PWV]) was performed before and 3-4 weeks after the completion of the intervention periods. All mice were euthanized by cardiac exsanguination while maintained under anesthesia (inhaled isoflurane) 3-4 weeks following the completion of the intervention periods. After cardiac exsanguination, the carotid arteries were excised under a dissection microscope, dissected free of connective tissue, and cannulated to fine glass canula within a pressure myograph system (DMT, Denmark) for assessment of vascular endothelial function. The thoracic aorta was excised, dissected free of surrounding tissue, sectioned, and stored in cold physiological saline solution at −80°C (not flash frozen) for later stress-strain testing to assess aortic elastic modulus (intrinsic mechanical wall stiffness), protein abundance by WES and JES capillary electrophoresis-based immunoblotting (Protein Simple, San Jose, CA) and immunofluorescence. Investigators were blinded to treatment group for data collection and biochemical analyses. All animal protocols were approved by the University of Colorado (CU) Boulder Institutional Care and Use Committee (protocol no. 2618) and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Details on all procedures and antibodies used are provided in the Data Supplement.
p16-3MR ganciclovir (GCV) study.
p16-3MR mice (on a C57BL/6 background)9,16 were bred, weaned, and aged (to 27 and 29 months, for male and female mice, respectively) in the animal care facility at CU Boulder. In the C57BL/6 mouse strain, 27 months of age represents the median lifespan for male mice whereas 29 months of age represents the median lifespan for female mice18. Young mice were studied at 6-8 months of age, which is the human corollary to young adulthood and an age that represents sexual maturity in this mouse strain18. GCV (Sigma Aldrich, St. Louis, MO; suspended in the vehicle solution) was administered for 5 consecutive days at a dose of 25 mg/kg/day via intraperitoneal injection, a dose that has previously shown to reduce senescent cell burden back to young levels9,16. The vehicle solution (sterile saline) was administered via intraperitoneal injection and the injection volume was body weight-matched to the GCV treatment group. All mice were single-housed beginning four weeks prior to the intervention.
ABT-263 study.
Male C57BL/6N mice were obtained at 21 months of age from the aging colony at Charles River. All mice were group housed until 4 weeks prior to the onset of the study, at which time they were single housed (starting at 26 months of age). ABT-263 (Selleckchem, Houston, TX; suspended in the vehicle solution) was administered via oral gavage at a dose of 50 mg/kg/day following a one week on – two weeks off – one week on doing regimen, which is also (similar to GCV in p16-3MR mice) a dosing regimen shown to reduce senescent cell burden back to young levels13. The vehicle solution (10% ethanol; 30% PEG400; 60% Phosal 50PG) was administered via oral gavage and the gavage volume was body weight-matched to the ABT-263 treatment group.
Aortic PWV and Blood Pressure
Aortic PWV was assessed using Doppler ultrasound, as we have previously described19-21. Briefly, mice were anesthetized via inhaled isoflurane (2.5-3%) and positioned supine on a warmed platform with paws secured to electrocardiogram leads. Doppler probes were placed at the transverse aortic arch and abdominal aorta to detect pulse waves. Three consecutive 2-second recordings were made for each animal and used to determine the time delay between the electrocardiogram R-wave and the foot of the Doppler signal for each site (∆timeabdominal and ∆timetransverse). Aortic PWV was then calculated as: aortic PWV = (physical distance between the two probes)/(∆timeabdominal minus ∆timetransverse) and reported in centimeters per second. In vivo blood pressure was measured on three consecutive days using a non-invasive tail-cuff method (CODA; Kent Scientifc, Torrington, CT), as we have previously described19-21.
Vascular Endothelial Function
EDD in response to increasing doses of acetylcholine (Sigma Aldrich) and endothelium independent dilation in response to increasing concentrations of the exogenous NO donor sodium nitroprusside (Sigma Aldrich) were measured in isolated carotid arteries as previously described20-22. Further details, including all pharmacological agents used for pharmaco-dissection experiments, are provided in the Data Supplement.
Humoral Factor-Related Changes in Aortic Elastic Modulus
Aortas from young (6-8 mo) intervention naïve donor female and male p16-3MR mice were prepared for assessment of aortic intrinsic mechanical wall stiffness (elastic modulus), which is described in detail in the Data Supplement. Aortic segments from each donor animal were incubated in the following conditions in duplicate for 48 hours: 1) DMEM + 1% penicillin-streptomycin + 10% fetal calf serum (control condition); 2) DMEM + 1% penicillin-streptomycin + 10% Old vehicle plasma; and 3) DMEM + 1% penicillin-streptomycin + 10% Old GCV plasma. The plasma samples were sex-matched to that of the donor animal. Following the incubation period, aortas were immediately mounted on two wire prongs and elastic modulus was assessed exactly as described in the Data Supplement. Mouse plasma-induced changes in aortic elastic modulus were determined as a fold-change relative to the control incubation condition.
Targeted Plasma Proteomics
Approximately 150 μl of plasma per sample was used for the SomaLogic SOMAscan Assay, a novel protein-capture aptamer-based technology for detecting circulating proteins. For this study, the SOMAscan HTS Assay 7K, which includes the measurement of 7,000 protein analytes, was used and processed through SomaLogic (Boulder, CO). Final analyses were performed only on samples that passed SomaLogic quality control and in which hemolysis was not apparent (n=10 young controls, 5m/5f; n=8 old controls, 4m/4f; n=7 old GCV-treated, 4m/3f). Differential protein expression analyses and Pearson correlations among proteins and functional measurements (PWV and EDD) were conducted using SomaLogic’s StatViz platform, with Bonferroni corrections for multiple testing. Biological processes associated with proteome signatures were identified using gene names associated with proteins and the ShinyGO program23.
Statistical Analyses
Detailed descriptions of all statistical analyses performed are provided in the Data Supplement. Data are presented as mean ± SEM in text, figures, and tables unless specified otherwise. Statistical significance was set to α=0.05. All statistical analyses were performed using Prism, version 9 (GraphPad Software, Inc, La Jolla, CA).
RESULTS
Animal characteristics
p16-3MR mice
To determine the causal role of cellular senescence in vascular dysfunction with aging, we utilized the p16-3MR mouse model, which is considered the reference-standard model for assessing the role of cellular senescence in mediating physiological functions9,16,24-27. This mouse model allows for selective genetic-based suppression of excess p16INK4A-positive senescent cells with the anti-viral drug ganciclovir (GCV)9,16. Upon administration, GCV recognizes a herpes simplex thymidine kinase located on the p16-3MR transgene, which subsequently induces apoptosis of p16INK4A-positive senescent cells, as described in detail previously9,16. In the present study, young (6-8 mo) and old (27-29 mo) male and female p16-3MR mice were administered GCV (25 mg/kg/day in saline) or the respective vehicle control (vehicle; saline) for 5 days via intraperitoneal injection and were sacrificed 3-4 weeks following the final dose. At time of sacrifice, there were anticipated age-related differences (Young vehicle vs. Old vehicle) in the mass of key organs (e.g., heart, quadriceps, epididymal adipose tissue, spleen, and kidneys; all P < 0.05), which is consistent with previous studies20,28, but there was no influence of GCV on organ weights in either age group (Table 1). Old mice also had greater aortic intima media thickness relative to young (Old vehicle vs. Young vehicle, P = 0.03), which is similar to previous reports from our laboratory20,28, but there was no influence of GCV in either age group (Old vehicle vs. Old GCV, P = 0.60; Young vehicle vs. Young GCV, P = 0.37). We observed an anticipated age-related increase in the abundance of p16INK4A in our target tissue (arteries – e.g., the aorta; Young vehicle vs. Old vehicle, P = 0.004), and the age-related difference was ameliorated with GCV treatment (Old vehicle vs. Old GCV, P = 0.0003; Young vehicle vs. Old GCV, P = 0.63), and GCV treatment ing young animals did not influence aortic p16INK4A abundance (Young vehicle vs. Young GCV, P = 0.64) (Figure S1A). There were no sex differences in any outcomes, so the data were combined for all analyses.
Table 1.
Animal characteristics of young and old vehicle- and ganciclovir (GCV)-treated mice
| Young p16-3MR | Old p16-3MR | |||
|---|---|---|---|---|
| Characteristics | Vehicle | GCV | Vehicle | GCV |
| n | 17 (9 F; 8 M) | 15 (7 F; 8 M) | 21 (11 F; 10 M) | 20 (11 F; 9 M) |
| Body weight, g | 26.7 ± 1.1 | 26.5 ± 1.1 | 28.8 ± 0.7 | 28.7 ± 0.9 |
| Heart mass, mg | 144 ± 5 | 144 ± 6 | 182 ± 6* | 178 ± 5* |
| Left ventricle mass, mg | 89 ± 3 | 88 ± 4 | 117 ± 5* | 112 ± 5* |
| Quadriceps mass, mg | 287 ± 8 | 281 ± 9 | 186 ± 7* | 201 ± 15* |
| Epididymal white adipose, mg | 516 ± 10 | 550 ± 11 | 330 ± 60* | 419 ± 92* |
| Liver, g | 1.4 ± 0.7 | 1.4 ± 0.6 | 1.8 ± 0.1 | 1.6 ± 0.7 |
| Spleen, mg | 70 ± 1 | 68 ± 3 | 178 ± 37* | 171 ± 19* |
| Kidneys, mg | 349 ± 15 | 342 ± 14 | 502 ± 20* | 503 ± 17* |
| Carotid artery | ||||
| Resting diameter, μM | 421 ± 8 | 421 ± 9 | 415 ± 9 | 414 ± 9 |
| Maximal diameter, μM | 469 ± 8 | 465 ± 7 | 474 ± 8 | 479 ± 9 |
| Aorta | ||||
| Lumen Diameter, μM | 642 ± 22 | 619 ± 19 | 657 ± 49 | 697 ± 20 |
| Intima media thickness, μM | 52 ± 2 | 50 ± 1 | 60 ± 3* | 58 ± 2* |
| Systolic blood pressure, mmHg | ||||
| Pre | 96 ± 2 | 98 ± 2 | 98 ± 3 | 99 ± 2 |
| Post | 103 ± 2 | 97 ± 2 | 98 ± 2 | 97 ± 4 |
| Diastolic blood pressure, mmHg | ||||
| Pre | 69 ± 2 | 69 ± 3 | 70 ± 2 | 73 ± 3 |
| Post | 70 ± 2 | 68 ± 2 | 69 ± 1 | 71 ± 3 |
Values are means ± SEM. n, number of mice. *P < 0.05, significant effect of age within group.
ABT-263-treated mice
We also sought to determine the efficacy of targeting excess cellular senescence with a senolytic to mitigate age-related vascular dysfunction. ABT-263 is a well-established synthetic pharmacological senolytic agent that has been administered identically to the approach used in the present study, to assess the efficacy of targeting excess cellular senescence to mitigate a variety of pathophysiological processes13,26,27,29,30. However, the effects of ABT-263 on vascular dysfunction have not been determined. The mechanism-of-action of ABT-263 is to lower the activity of BCL-2 (which is upregulated in a state of cellular senescence) and selectively induce apoptosis in senescent cells17. Here, we administered ABT-263 to old (27 mo) C57BL/6N mice – an established model of vascular aging20,21,31. Because there were no sex differences in our p16-3MR/GCV study nor an influence of senescent cell clearance in young animals (young animals treated with GCV), to reduce the overall number of mice used we only studied old male mice for this study. Mice were treated intermittently (1 week on – 2 weeks off – 1 week on) with ABT-263 (50 mg/kg/day during the treatment periods), or the respective vehicle control (10% EtOH; 30% PEG 400; 60% Phosal 50 PG) by oral gavage and were sacrificed 4 weeks following the final dose. At time of sacrifice, we observed no inter-group differences in the mass of key organs or artery characteristics, which is similar to what we have observed in previous intervention studies in old mice20,28 (Supplemental Table S1). Aortic p16INK4A abundance and BCL-2 activity, assessed as BAX/BCL-2 protein abundance32,33, were ~40% and ~20% lower, respectively, in old mice that received ABT-263; (p16INK4A Old vehicle vs. Old ABT-263, P = 0.04, Figure S1B; BCL-2 activity Old vehicle vs. Old ABT-263, P = 0.16, Figure S1C).
Cellular senescence contributes to aortic stiffening with aging: Amelioration with senolytic therapy
Aortic stiffness in vivo
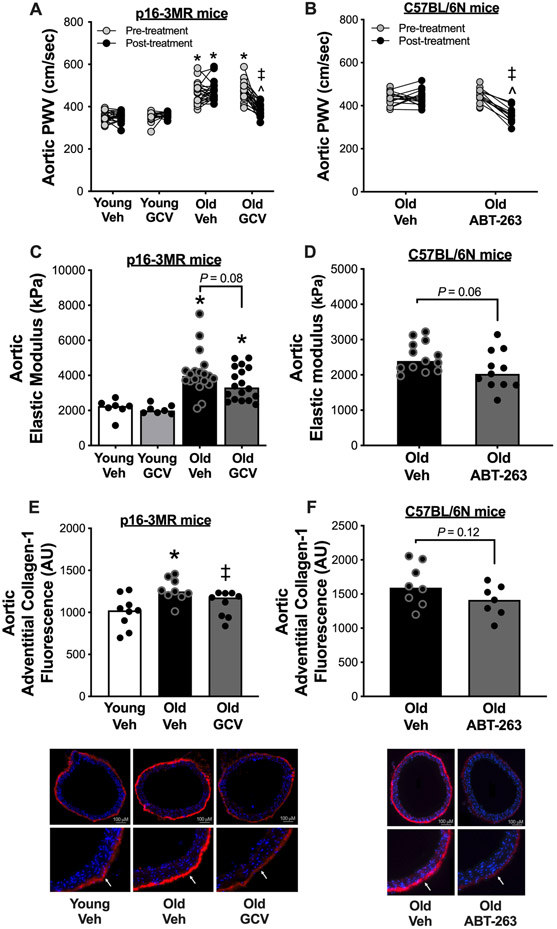
To determine whether cellular senescence contributes to aortic stiffening with aging, we noninvasively assessed aortic PWV in vivo. Aortic PWV is the mouse corollary to the non-invasive in vivo reference standard measure of aortic stiffness in people, carotid-femoral PWV34. We assessed aortic PWV at baseline and after treatment in vehicle- and GCV-treated young and old animals. Aortic PWV was ~25% greater in old versus young mice at baseline (Old vehicle, 462 ± 11 cm/sec vs. Young vehicle, 353 ± 5 cm/sec, P < 0.0001) (Figure 1A), consistent with age-related changes in aortic stiffness in humans35. GCV treatment in old mice lowered aortic PWV by ~20%, which accounted for ~95% of the age-related difference in aortic PWV (382 ± 7 cm/sec, P < 0.0001 vs. Old vehicle), whereas no significant changes were observed over time within the Young vehicle, Young GCV or Old vehicle groups (Figure 1A). These effects occurred independently of no change in systolic or diastolic blood pressure (Table 1).
Figure 1. Cellular senescence mediates aortic stiffening with aging and senolytic treatment lowers aortic stiffness in advanced age.
In young (6-8 months) and old (27-29 months) male and female (sexes combined) p16-3MR mice treated with vehicle (Veh; sterile saline) or ganciclovir (GCV, in sterile saline) at a dose of 25 mg/kg/day for five consecutive days via intraperitoneal injection: (A) Aortic pulse wave velocity (PWV) before and after treatment. (C) Aortic elastic modulus at time of sacrifice. (E) Immunofluorescence staining for Type-1 Collagen in the adventitial layer of aortic rings collected at time of sacrifice. In old male C57BL/6N mice treated with vehicle (10% ethanol; 30% PEG400; 60% Phosal 50PG) or ABT-263 (in the vehicle) at a dose of 50 mg/kg/day via oral gavage following a one week on – two weeks off – one week on dosing regimen: (B) Aortic pulse wave velocity before and after treatment. (D) Aortic intrinsic mechanical wall stiffness at time of sacrifice. (F) Immunofluorescence staining for type-1 collagen in the adventitial layer of aortic rings collected at time of sacrifice. All data are mean ± SEM. N = 16-23/group, p16-3MR study aortic PWV. N = 15-20/group, p16-3MR study aortic intrinsic mechanical wall stiffness. N = 10/group, p16-3MR study aortic adventitial type-1 collagen fluorescence. N = 14-16/group, ABT-263 study aortic PWV. N = 11-14/group, ABT-263 study aortic intrinsic mechanical wall stiffness. N = 9/group, ABT-263 study aortic adventitial type-1 collagen fluorescence. * P < 0.05, effect of aging within group; ‡ P < 0.05, effect of treatment within age group; ^ P < 0.05, effect of time.
Next, we assessed aortic PWV in old mice before and after treatment with ABT-263 to determine the efficacy of targeting cellular senescence with a senolytic to lower aortic stiffness in the setting of advanced age. We found that ABT-263 treatment reduced aortic PWV by ~20% (Pre-treatment: 446 ± 9 cm/sec vs. Post-treatment: 356 ± 11 cm/sec, P < 0.0001) in old mice, while there was no difference in aortic PWV before and after treatment (P = 0.99) in animals that received oral gavage with the vehicle control (Figure 1B). Like p16-3MR mice treated with GCV, the ABT-263-mediated reduction in aortic PWV occurred independently of changes in systolic or diastolic blood pressure (Supplemental Table S1).
Intrinsic mechanical wall stiffness
We next sought to determine whether structural changes to the arterial wall might contribute to the cellular senescence-induced increase in aortic PWV. To do so, we measured the relative difference in elastic modulus of aorta rings isolated from Young vehicle, Young GCV, Old vehicle and Old GCV mice. Elastic modulus is defined as the association between the change in stress on the arterial wall before and after a given strain (stretch) and is indicative of the intrinsic mechanical stiffness of the aortic wall36. Aortic elastic modulus was ~2-fold greater in old versus young animals (Old vehicle: 4139 ± 281 kPa vs. Young vehicle: 2152 ± 186 kPa, P = 0.0004), consistent with our previous studies20,21 (Figure 1C). Old GCV animals had ~15% lower elastic modulus relative to Old vehicle (Old GCV, 3513 ± 217 kPa), which accounted for ~30% of the age-related increase in elastic modulus (Old vehicle vs. Old GCV; P = 0.08). GCV had no effect in young mice (Young vehicle vs. Young GCV, P = 0.77), thus the effect of cellular senescence on aortic elastic modulus was specific to aging (Figure 1C).
Following, we assessed the relative difference in elastic modulus in aortic rings isolated from old mice treated with ABT-263. Similar to old p16-3MR mice treated with GCV, old mice that were administered ABT-263 had ~15% lower aortic elastic modulus relative to Old vehicle-treated mice (Old vehicle: 2514 ± 116 kPa vs. Old ABT-263: 2127 ± 166 kPa, P = 0.06); Figure 1D). Together, these results suggest that cellular senescence induces aortic stiffening with aging in part by increasing the intrinsic stiffness of the arterial wall, although other factors clearly contribute. Moreover, targeting cellular senescence with a senolytic in the setting of advanced age may improve aortic stiffness, at least in part, as result of reductions in the intrinsic stiffness of the arterial wall.
Arterial wall structural proteins
Next, we sought to determine if the cellular senescence-mediated increase in arterial stiffness with aging was associated with adverse changes in the abundance of the arterial wall structural proteins type-1 collagen (primary isoform of collagen in arteries37) and alpha-elastin and an upregulation of AGEs. Type-1 collagen is a component of the arterial adventitia that increases with aging and provides rigidity/stiffness to the arterial wall, whereas alpha-elastin is a protein that degrades with aging and confers arterial wall elasticity3,38. AGEs are signaling molecules that are elevated with aging and can increase the stiffness of the arterial wall by crosslinking structural proteins39. The aortic abundance of type-1 collagen, alpha-elastin, and AGEs were only assessed in Young vehicle, Old vehicle, and Old GCV mice in the p16-3MR mouse study, given that aortic PWV and elastic modulus in young mice were not influenced by GCV.
We found that Old vehicle mice had ~25% greater aortic adventitial type-1 collagen compared to Young vehicle mice (P = 0.005), and administration of GCV to old mice lowered aortic adventitial collagen-1 by ~15%, which accounted for ~65% of the age-related increase (Old vehicle vs. Old GCV, P = 0.02) (Figure 1E). Consistent with the findings in p16-3MR mice, we found that ABT-263 treatment in old mice tended to lower (by ~15%) aortic adventitial type-1 collagen (P=0.12) (Figure 1F). Moreover, we found that alpha-elastin was ~3-fold lower in old relative to young animals (Old vehicle vs. Young vehicle, P = 0.02); however, this did not appear to be regulated by cellular senescence as there was no difference in elastin abundance between Old GCV and Old vehicle animals (P = 0.43), and elastin abundance was ~3-fold lower in Old GCV relative to Young vehicle (P = 0.01) (Figure S2A). Aortic elastin content was also uninfluenced by ABT-263 treatment (Old vehicle vs. Old ABT-263, P = 0.56) (Figure S2B). We found AGEs were higher with aging (Old vehicle vs. Young vehicle, P = 0.04), as we have previously shown20,40, which appeared to be partially governed by cellular senescence as AGEs tended to be lower (~30%) in old animals following GCV treatment (Old GCV vs. Old vehicle, P = 0.18) (Figure S2C). Furthermore, ABT-263-treated animals tended to have lower (by ~30%) aortic AGEs (Old vehicle vs. Old ABT-263, P = 0.11) (Figure S2D). Together, these results suggest that the cellular senescence increases aortic stiffness with aging, at least in part, by increasing adventitial type-1 collagen deposition and AGEs abundance, and treatment with a senolytic may be a therapeutic strategy to mitigate these molecular processes with advanced age.
Humoral factors modulating arterial stiffness
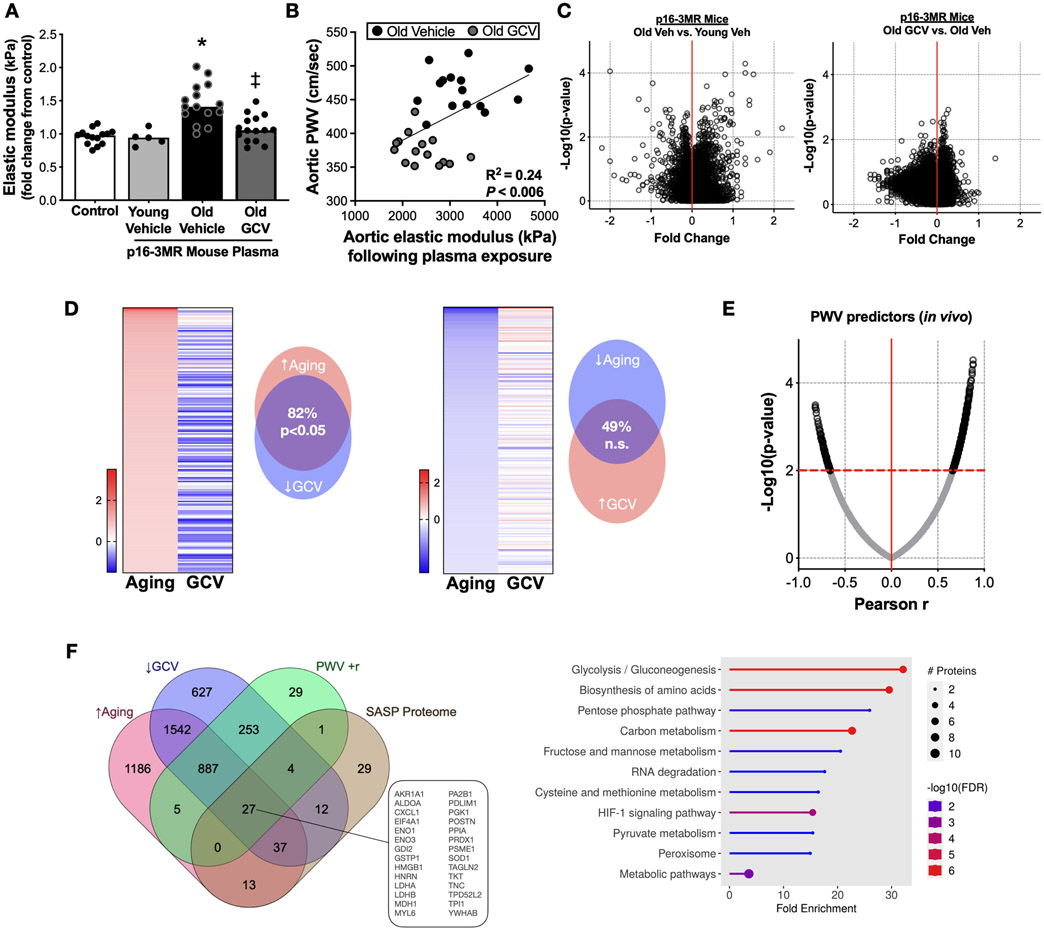
We next sought to determine the role of humoral factors in plasma as mediators of aortic stiffening, given that SASP factors are secreted into circulation (e.g., the plasma)7,41 and have both direct and indirect effects on the arterial wall. We exposed aortic rings, isolated from young (6 mo) intervention naïve p16-3MR female and male mice to standard cell culture media containing either fetal calf serum (control) or serum-free media supplemented with 10% plasma from old vehicle- and GCV-treated p16-3MR mice for 48 hours, and performed the aortic elastic modulus assay, as described above. We found that plasma from Old vehicle mice evoked a 50% increase in aortic elastic modulus (Figure 2A, P < 0.0001 vs. control media), whereas there was no effect with plasma from old mice that received GCV treatment (Old vehicle vs. Old GCV plasma, P < 0.0001; old GCV plasma vs. control media, P = 0.21), suggesting that SASP factors in plasma likely contribute to the increase in aortic stiffness with aging. In further support of this idea, linear regression analyses showed that the plasma-mediated changes in aortic elastic modulus in our ex vivo experiments were significantly (P < 0.006) related to post-intervention aortic PWV values in vivo (R2 = 0.24) (Figure 2B).
Figure 2. The age-related plasma proteome is mediated, in part, by cellular senescence and related to changes in aortic stiffness.
(A) Changes in elastic modulus of aorta rings from young intervention naïve p16-3MR mice induced by plasma from Old vehicle (Veh; sterile saline) and ganciclovir (GCV, in sterile saline) treated old (27-29 months) p16-3MR mice. (B) Aortic elastic modulus following plasma exposure relative to the post-intervention aortic pulse wave velocity (PWV) value of the plasma donor mouse. Targeted plasma proteomic analyses of young (6-8 months) male and female vehicle-treated p16-3MR mice and old (27-29 months) male and female p16-3MR mice: (C) Volcano plots of differentially expressed plasma proteins in old vehicle (sterile saline)-treated mice (Old Veh) relative to young vehicle-treated (Young Veh) mice (left-side panel); Volcano plots of differentially expressed plasma proteins in old GCV relative to Old Veh-treated animals (right-side panel). (D) Left side: Top 200 proteins that were elevated with aging (Old Veh vs. Young Veh) and their relative changes with GCV treatment (Old GCV vs. Old Veh); Right side: Top 200 proteins that were lower with aging (Old Veh vs. Young Veh) and their relative changes with GCV treatment (Old GCV vs. Old Veh). (E) Correlation among individual plasma proteins and post-intervention aortic PWV values. (F) Left side: Plasma proteins that were: i) higher with aging; ii) lower with GCV treatment; iii) positively related to post-intervention aortic PWV; and iv) accepted as senescence-associated secretory phenotype proteins; Right side: KEGG pathway analyses of the 27 proteins that met the criteria presented in Figure 2H. Data are mean ± SEM. n = 15/group (panels A-B); n = 10/group for all proteomics results. * P < 0.05 vs. control; ‡ P < 0.05 vs. Old Vehicle plasma.
Next, to identify potential molecular transducers in the circulating plasma that were changed with aging (Old vehicle vs. Young vehicle) and cellular senescence (Old GCV vs. Old vehicle), we performed targeted plasma proteomic analyses using the SOMAscan platform, as described previously41,42. Most differences in individual plasma proteins were modest, with only a few significantly altered at a false discovery rate of P < 0.01 (Figure 2C). However, we did observe broad changes in the patterns of protein abundance with aging (Old vehicle vs. Young vehicle) and with treatment (Old GCV vs. Old vehicle) (Figure 2D). Specifically, of the proteins that were most increased with aging, the majority were reduced with GCV (Old vehicle vs. old GCV, Figure 2D left side). In contrast, proteins that were lower in old mice (Old vehicle vs. Young vehicle) were largely unaltered with GCV treatment (Figure 2D right side). Taken together, these observations suggest the functional effects of GCV treatment are most related to reversal of age-associated increases in select humoral factors. Consistent with this idea, we also found that a greater number of circulating proteins were positively related to PWV in old animals following the GCV and vehicle treatment periods, rather than inversely related (Figure 2E). To determine which known SASP proteins may be involved in mediating increases in aortic PWV with aging, we identified all proteins that met the following criteria: a) increased with aging; b) decreased with GCV treatment; c) positively related to post-intervention PWV; and d) accepted as SASP proteins7 (Figure 2F, left side). Using this approach, we identified 27 putative SASP factors that influence PWV, and a pathway analysis showed that these SASP proteins were primarily related to metabolic processes (e.g., glycolysis, gluconeogenesis), as well as amino acid biosynthesis and hypoxia-related signaling, among others (Figure 2F, right side). Collectively, these results suggest that humoral SASP-related factors may directly influence aortic stiffening with aging, and that this may be due in part to cellular senescence-mediated changes in cellular metabolism associated with glycolysis and mitochondrial function.
Cellular senescence contributes to endothelial dysfunction with aging: Improvements with senolytic therapy
Endothelial function
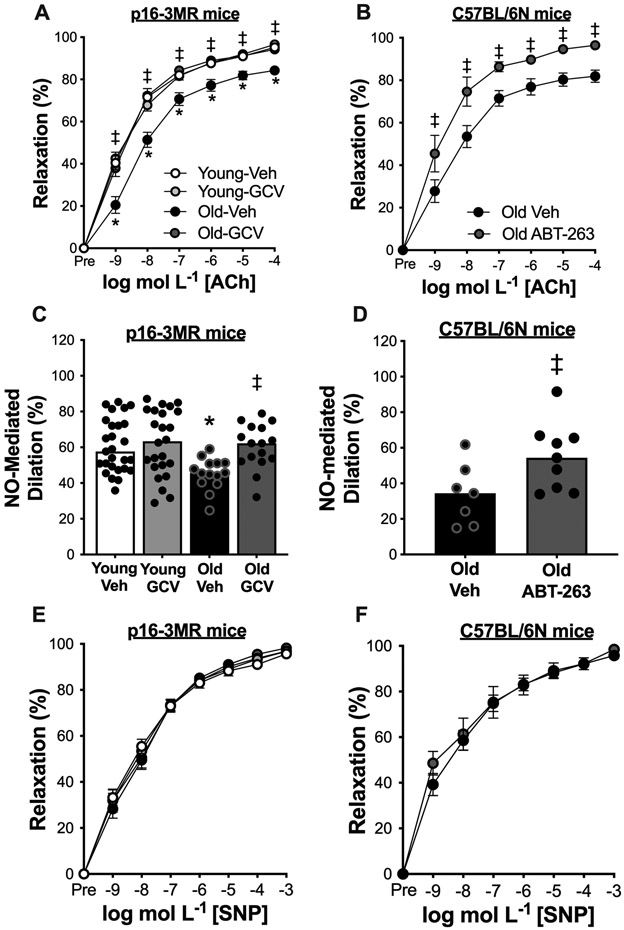
To determine whether cellular senescence contributes to endothelial dysfunction with aging, we first assessed EDD to increasing doses of acetylcholine (ACh) ex vivo in carotid arteries in Young vehicle, Young GCV, Old vehicle, and Old GCV p16-3MR mice (Figure 3A), Peak EDD was lower in old versus young animals (Old vehicle, 84 ± 2% vs. Young Veh: 96 ± 1%, P = 0.002) (Figure 3A). Administration of GCV completely restored EDD in old mice (Old GCV, 95% ± 1%; P = 0.01 vs. Old vehicle) to levels observed in young animals without affecting EDD in young mice (Young vehicle vs. Young GCV, P = 0.99). Furthermore, we found that peak EDD was higher in old C57BL/6N mice treated with ABT-263 compared vehicle control (Old ABT-263: 96 ± 1% vs. Old vehicle, 82% ± 3%; P = 0.0004) (Figure 3B). These observations indicate that cellular senescence regulates vascular endothelial dysfunction with aging and that treatment with a senolytic may be a therapeutic strategy to improve endothelial function in advanced age.
Figure 3. Cellular senescence impairs endothelial function with aging by reducing nitric oxide (NO) bioavailability not by influencing vascular smooth muscle sensitivity to NO, and senolytic treatment increases vascular endothelial function in advanced age by increasing NO bioavailability, not by altering vascular smooth muscle sensitivity to NO.
In young (6-8 months) and old (27-29 months) male and female (sexes combined) p16-3MR mice treated with vehicle (Veh; sterile saline) or ganciclovir (GCV, in sterile saline) at a dose of 25 mg/kg/day for five consecutive days via intraperitoneal injection: (A) Carotid artery endothelium-dependent dilation (EDD) to increasing doses of acetylcholine (ACh). (C) NO-mediated EDD calculated as peak EDD alone (−) peak EDD in the presence of L-NAME. (E) Carotid artery endothelium-independent dilation to increasing doses of the NO donor sodium nitroprusside (SNP). In old male C57BL/6N mice treated with vehicle (10% ethanol; 30% PEG400; 60% Phosal 50PG) or ABT-263 (in the vehicle) at a dose of 50 mg/kg/day via oral gavage following a one week on – two weeks off – one week on dosing regimen: (B) Carotid artery EDD to increasing doses of ACh. (D) NO-mediated EDD. (F) Carotid artery endothelium-independent dilation (EID) to increasing doses of SNP. All data are mean ± SEM. N = 15-20/group, p16-3MR study EDD, NO-mediated EDD, and EID. N = 10-14/group, ABT-263 study EDD, NO-mediated EDD, and EID. * P < 0.05, effect of aging within group; ‡ P < 0.05, effect of treatment within age group.
NO bioavailability
Next, we assessed the role of cellular senescence in regulating NO-mediated EDD with aging by assessing EDD with and without the presence of the NO synthase inhibitor L-NAME, as we have described previously20,21. Inhibition of NO production by L-NAME abolished group differences in peak EDD in p16-3MR mice (Figure S3A) and in Old vehicle- and ABT-263-treated mice (Figure S3B). Consistent with this idea, peak NO-mediated dilation (peak EDD to ACh alone [−] peak EDD to ACh + L-NAME) was 31% lower in old versus young animals (Old vehicle: 42 ± 4% vs. Young vehicle: 61 ± 3%, P = 0.0004), and the age-related reduction in NO-mediated EDD was fully reversed via administration of GCV (Old GCV, 60 ± 4%) (Figure 3C). NO-mediated EDD was also higher in Old ABT-263 mice relative to Old vehicle (Old ABT-263: 55 ± 6% vs. Old vehicle: 34 ± 6%, P = 0.03) (Figure 3D).
To determine if senescent cell clearance altered smooth muscle sensitivity to NO, we measured endothelium-independent dilation as the vasodilatory response to sodium nitroprusside (SNP), an NO donor. We found no group differences (P = 0.84) in p16-3MR mice administered GCV (Figure 3E) or old mice treated with ABT-263 (P = 0.29) (Figure 3F) in response to SNP. Together, these results suggest that cellular senescence contributes to endothelial dysfunction with aging by reducing NO bioavailability from the endothelium rather than by influencing vascular smooth muscle sensitivity to NO. Furthermore, treatment with a senolytic may be an effective strategy for increasing NO-mediated endothelial function with advanced age.
Superoxide-related Oxidative stress
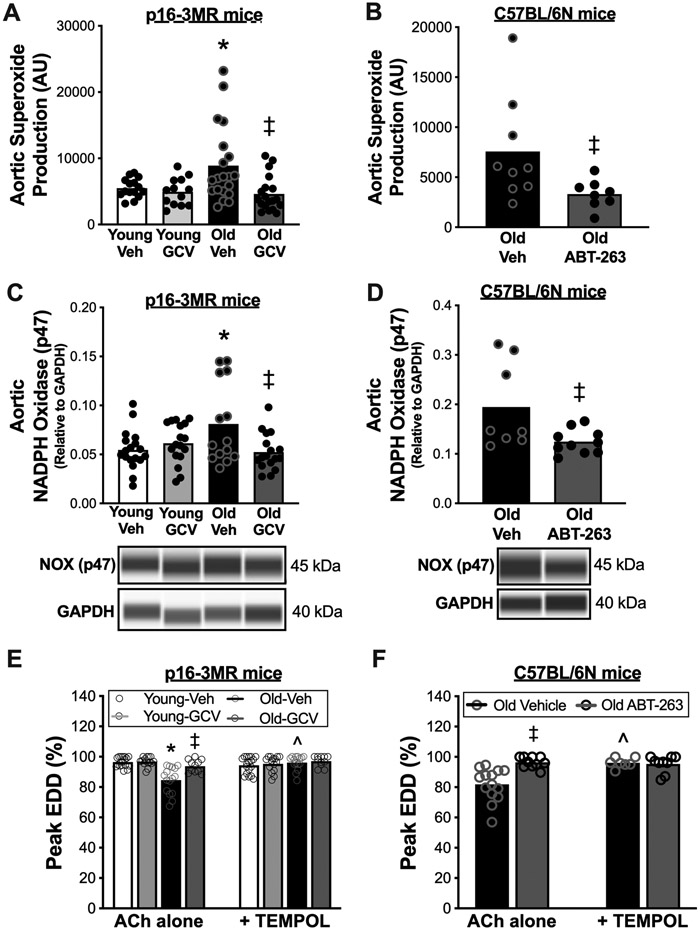
Excess superoxide-related oxidative stress can directly contribute to endothelial dysfunction with aging43, as superoxide can scavenge NO and ultimately reduce its bioavailability44. Hence, we sought to determine whether cellular senescence contributes to endothelial dysfunction with aging by increasing superoxide-related oxidative stress. To do so, we first assessed superoxide production in dissected aortic rings using the reference-standard approach for assessing superoxide production in biological samples45, electron paramagnetic resonance spectroscopy (amplitude units [AU]), as we have previously described20,21,46. Old mice had ~40% higher aortic superoxide production relative to young mice (Old vehicle: 8895 ± 1295 AU vs. Young vehicle: 5502 ± 370 AU, P = 0.03) (Figure 4A). Administration of GCV completely abolished the age-related increase in aortic superoxide production (Old GCV: 4102 ± 582 AU, P = 0.002 vs. Old vehicle) back to young levels (Old GCV vs. Young vehicle, P = 0.23) but did not affect superoxide production in young animals (Young GCV vs. Young vehicle, P = 0.41) (Figure 4A). Similarly, old mice treated with ABT-263 had 56% lower aortic superoxide production relative to vehicle-treated animals (Old ABT-263: 3313 ± 501 AU vs. Old vehicle: 7569 ± 1730 AU, P = 0.04) (Figure 4B).
Figure 4. Cellular senescence promotes vascular superoxide-related oxidative stress with aging and senolytic treatment lowers vascular superoxide-related oxidative stress in advanced age.
In young (6-8 months) and old (27-29 months) male and female (sexes combined) p16-3MR mice treated with vehicle (Veh; sterile saline) or ganciclovir (GCV, in sterile saline) at a dose of 25 mg/kg/day for five consecutive days via intraperitoneal injection: (A) Aortic superoxide production (electron paramagnetic resonance spectroscopy amplitude units [AU]). (C) Aortic abundance of nicotinamide adenine dinucleotide phosphate (NAPDH) oxidase p47 normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (E) Peak carotid artery endothelium dependent dilation (EDD) to acetylcholine (ACh) with vs. without the presence of the superoxide dismutase mimetic TEMPOL. In old male C57BL/6N mice treated with vehicle (10% ethanol; 30% PEG400; 60% Phosal 50PG) or ABT-263 (in the vehicle) at a dose of 50 mg/kg/day via oral gavage following a one week on – two weeks off – one week on dosing regimen: (B) Aortic superoxide production (electron paramagnetic resonance spectroscopy, AU). (D) Aortic abundance of NAPDH oxidase p47 normalized to GAPDH. (F) Peak carotid artery EDD to ACh with vs. without the presence of TEMPOL. All data are mean ± SEM. N = 15-20/group, p16-3MR mice aortic superoxide production, and aortic NAPDH oxidase p47 and CuZn SOD abundance. N = 10-15/group, p16-3MR mice peak EDD with TEMPOL. N = 8-9/group, ABT-263 study aortic superoxide production. N = 8-10/group, ABT-263 study aortic NADPH oxidase p47 abundance. N = 10-12/group, ABT-263 study aortic CuZn SOD abundance. N = 7-9/group, ABT-263 study peak EDD with TEMPOL. * P < 0.05, effect of aging within group; ‡ P < 0.05, effect of treatment within age group; ^ P < 0.05, ACh alone vs. ACh + TEMPOL.
Excessive superoxide-related oxidative stress with aging could occur via increased production of superoxide (e.g., via greater abundance of superoxide producing enzymes like NADPH oxidase - the primary superoxide producing enzyme in endothelial cells47), reduced superoxide scavenging capacity due to lower abundance of superoxide dismutase (SOD) enzymes48, or a combination of the two. Thus, we assessed the abundance of NADPH oxidase (NOX p47), cytosolic copper-zinc (Cu-Zn) SOD, and extracellular (ec)SOD. We found that NADPH oxidase was ~35% higher in the old versus young mice (Old vehicle vs. Young vehicle, P = 0.02), which is consistent with previous studies in our laboratory20,49,50. Greater NADPH oxidase with aging was fully ameliorated by GCV treatment (Old GCV vs. Old vehicle, P = 0.01) (Figure 4C). We also found that NADPH oxidase was lower in Old ABT-263-treated animals relative to Old vehicle (P = 0.02) (Figure 4D). Regarding SODs, we found that arterial antioxidant defense proteins, Cu-Zn SOD and ecSOD, were lower in Old vehicle compared to Young vehicle animals (Cu-Zn SOD, P = 0.04; ecSOD, P = 0.03) which was unaffected by GCV administration (Old vehicle vs. Old GCV, Cu-Zn SOD: P = 0.82; ecSOD: P = 0.42) (Cu-Zn SOD, Figure S4A; ec SOD, Figure S4B). We also observed no difference in vascular Cu-Zn SOD or ecSOD following ABT-263 treatment in old mice (Cu-Zn SOD, Old ABT-263 vs. Old vehicle, P = 0.78, Figure S4C; ecSOD, Old ABT-263 vs. Old vehicle, P = 0.92, Figure S4D). These results collectively demonstrate that cellular senescence increases superoxide production in arteries by directly upregulating pro-oxidant proteins without a compensatory increase in antioxidant enzymes, and that senolytic therapy may reduce superoxide production in arteries by downregulating pro-oxidant proteins rather than increasing antioxidant enzymes.
Lastly, we aimed to determine the causal role of the cellular senescence-mediated increase in superoxide production on vascular function. To accomplish this, we used a well-established bioassay for determining the direct role of excess superoxide production in suppressing endothelial function. Specifically, we assessed carotid artery EDD with and without prior incubation with the superoxide scavenger TEMPOL20,46. TEMPOL administration to the vessel perfusate restored peak EDD selectively in Old vehicle p16-3MR mice (P < 0.0001 with vs. without TEMPOL) to levels not different (P = 0.87) from Young-vehicle mice, without affecting EDD in the Young-vehicle animals. Thus, the impaired EDD in Old vehicle mice is mediated by excess vascular superoxide production, as we have previously shown20,28. Preincubation with TEMPOL did not further improve EDD in old p16-3MR mice that received GCV treatment (Old GCV: ACh alone vs. ACh + TEMPOL, P = 0.66) (Figure 4E). Preincubation with TEMPOL also increased peak EDD in Old-vehicle C57BL/6N mice (P = 0.003), while there was no effect of TEMPOL on peak EDD in ABT-263-treated animals (P = 0.94) (Figure 4F). Collectively, these results suggest that cellular senescence regulates excess tonic superoxide-related suppression of EDD with aging and that senolytic therapy may be a favorable therapeutic strategy to suppress excess superoxide-associated oxidative stress and ultimately improve endothelial function in advanced age.
Humoral factors related to NO-mediated EDD.
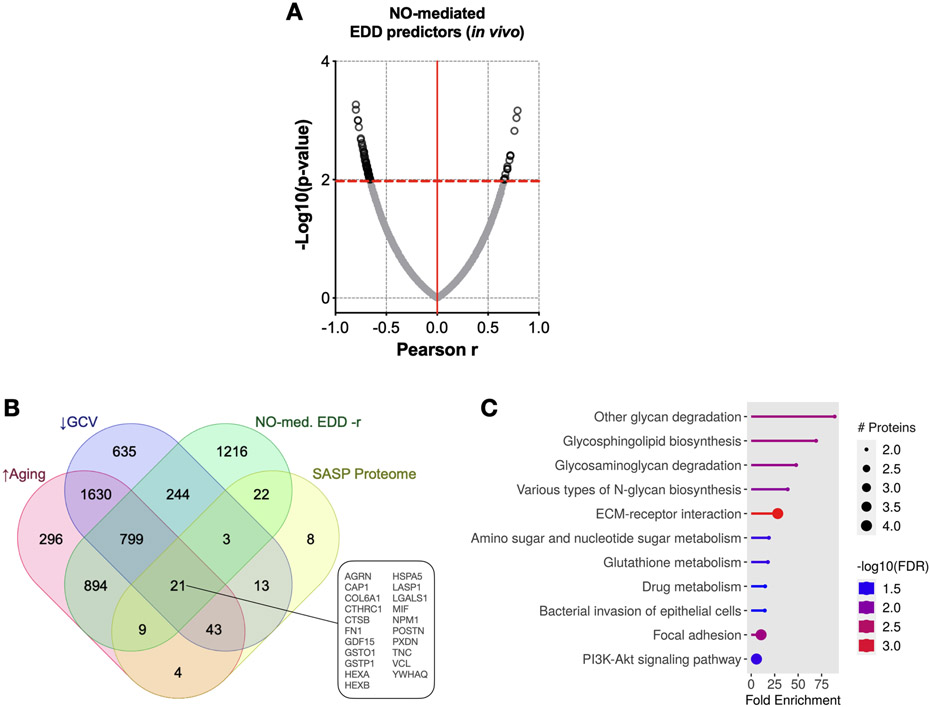
Changes in humoral factors with advanced age can directly modulate oxidative stress and NO production in endothelial cells51,52. Furthermore, alterations in the circulating SASP can directly increase oxidative stress53. Accordingly, we next aimed to identify potential SASP factors in the circulation related to cellular senescence-associated changes in NO-mediated EDD with aging. Among the proteins that predicted NO-mediated EDD (Figure 5A), we found 21 that increased with aging, decreased with GCV, and have been identified as SASP proteins7 (Figure 5B). Moreover, a pathway analysis indicated these SASP proteins associated with NO-mediated EDD were mostly related to sphingolipid biosynthesis, glycoprotein synthesis/degradation, cell adhesion, glutathione metabolism (e.g., oxidative stress), and PI3K-Akt signaling—all processes that have been implicated in altered NO production54 (Figure 5C). Although we were unable to perform plasma incubation experiments and assess EDD as we did with aortic stiffness due to the need for specialized equipment, our results suggest that, in addition to modulating PWV, circulating SASP factors may contribute to decreased NO-mediated EDD with aging.
Figure 5. The age-related plasma proteome is mediated, in part, by cellular senescence and related to changes in nitric oxide (NO)-mediated endothelium-dependent dilation (EDD).
(A) Relative plasma protein changes that were related to post-intervention NO-mediated EDD. (B) Plasma proteins that were relatively: i) higher with aging; ii) lower with GCV treatment; iii) positively related to post-intervention NO-mediated EDD; and iv) accepted as senescence-associated secretory phenotype (SASP) proteins. (C) KEGG pathway analyses of the 21 proteins that met the criteria presented in Figure 5B. Data are mean ± SEM. n = 10/group (Young Vehicle; Old Vehicle; Old GCV).
DISCUSSION
In the present study we used complementary in vivo and ex vivo models of age-related vascular dysfunction, in male and female mice, to establish the role of cellular senescence and circulating SASP factors in mediating vascular dysfunction with aging. We also present evidence supporting the efficacy for targeting cellular senescence with senolytic therapy to lower senescent cell burden in the vasculature (e.g., lower p16INK4A abundance and BCL-2 activity) and improve vascular function in advanced age.
Stiffening of the aorta occurs with advancing age, as indicated by an increase in aortic PWV55, and is a major independent risk factor for age-associated CV events, clinical CVD56, kidney dysfunction57, and cognitive impairment58. It is important to understand the mechanism(s) mediating arterial stiffening with aging, as these mechanism(s) could serve as viable therapeutic targets for treatments. To date, these mechanisms are incompletely understood. Here, we show for the first time that cellular senescence directly contributes to the age-associated increase in aortic PWV. Moreover, we demonstrate proof-of-principle efficacy for the use of senolytic treatment to lower aortic stiffness in advanced age. As such, our findings have potential clinical significance for mitigating aortic stiffening and its pathological sequelae in humans as presently there are no established pharmacological treatments for aortic stiffening in humans.
The mechanisms by which the aorta stiffens with aging are incompletely understood, but an important contributing event is increased intrinsic stiffness of the aortic wall, as indicated by an increase in the elastic modulus of segments of the proximal aorta upon stress-strain analysis34. The cellular and molecular mechanisms underlying increased intrinsic aortic wall stiffness with age largely involve remodeling of the composition of structural compounds within the extracellular matrix of the aortic wall4. Type-1 collagen is the primary load-bearing protein in the aortic wall and its abundance increases with advancing age, conferring increased mechanical stiffness20,59. In contrast, alpha-elastin, a structural protein providing elasticity to the aorta, becomes fragmented and undergoes degradation with aging, reducing its abundance20,60. Moreover, with advancing age the formation and accumulation of AGEs, which crosslink structural proteins, increase in the aortic wall, which further increases stiffness20,40. In the present study, we found that cellular senescence contributes to age-related changes in aortic intrinsic mechanical wall stiffness, collagen deposition, elastin degradation, and the increase in AGEs, and that senolytic treatment largely reverses these adverse changes.
An additional contributing factor to aortic stiffening with aging is a change in the composition of humoral factors. Specifically, senescent cells secrete SASP factors (e.g., metabolites, chemokines, and growth factors) into circulation41,61, which, in turn, directly interact with the arterial wall. In the present study, plasma from old control animals, but not plasma from old animals cleared of senescent cells, induced stiffening of aorta rings from young control mice. These observations provide novel evidence that the circulating SASP directly contributes to aortic stiffening with aging.
The composition of circulating SASP factors is adversely altered with aging41,61, but the relation of these changes to age-related arterial stiffening is unknown. Mitochondrial dysfunction is a feature of senescent cells that promotes a shift toward glycolytic metabolism and ultimately alters the SASP62,63. Consistent with these observations, here we determined that SASP factors mediating changes in aortic stiffness are associated with glucose metabolism (e.g., glycolysis). Moreover, changes in glycolytic metabolites may contribute to aortic stiffening, in part, by promoting crosslinking of collagen fibers in the arterial wall and/or increasing superoxide-related oxidative stress64,65. Taken together, our findings suggest cellular senescence-associated changes in metabolism -- towards a more glycolytic state -- may alter the circulating SASP to promote aortic stiffening with aging.
Vascular endothelial dysfunction is a major antecedent to clinical CVD with aging1. Clinical assessments of EDD in humans are independently predictive of age-related CVD morbidity and mortality66. In the present study, using carotid artery EDD, we found that cellular senescence mediates endothelial dysfunction with aging and that senolytic therapy is an effective strategy for improving endothelial function in older mice. Our findings of enhanced EDD upon reducing cellular senescence provide causal evidence to extend previous cross-sectional observations of a relation between EDD and markers of endothelial senescence with aging in humans67. Moreover, our results add to previous observations of improvements in EDD following genetic clearance of senescent cells (INK-ATTAC mouse) and senolytic (Dasatanib + Quercetin) treatment in old mice68.
Reduced bioavailability of NO and increased oxidative stress are primary mechanisms underlying endothelial dysfunction with aging3; however, the integrative mechanistic event(s) governing these responses are not completely understood. Excess oxidative stress, particularly superoxide, can reduce NO bioavailability by scavenging NO69. Here, we show that the age-related impairment in NO bioavailability is mediated by cellular senescence and that senolytic therapy can increase the bioavailability of NO in older age. Additionally, we demonstrate that cellular senescence induces vascular oxidative stress via excessive arterial superoxide production and senolytic treatment decreases vascular oxidative stress to improve endothelial function. Cellular senescence may also contribute to reductions in EDD in part through the SASP70, as SASP factors can promote NO degradation, impairment in NO production-related signaling (via the PI3K-AKT signaling pathway), and an increase in oxidative stress70. Consistent with this concept, we observed a relation between NO-mediated EDD and SASP factors linked to sphingolipid biosynthesis, which governs NO production and degradation, as well as to NO signaling pathways71. We also observed a relation between SASP factors related to oxidative stress (e.g., altered glutathione metabolism) and NO-mediated EDD, suggesting the SASP may contribute to endothelial dysfunction by promoting excessive superoxide-related scavenging of NO.
In conclusion, the results of the present study provide key proof-of-principle evidence for the role of cellular senescence and the SASP in contributing to age-related aortic stiffening and endothelial dysfunction, as well as providing novel insight into the mechanisms mediating these effects. Moreover, our findings establish the efficacy of senolytic therapy for lowering aortic stiffness and improving endothelial function in advanced age. Importantly, senolytic therapy was administered intermittently, which has broad translational implications relative to chronic treatment strategies because this approach likely allows the homeostatic cellular senescence processes to remain intact (i.e., suppresses cellular senescence back to young/basal levels), yet is more time efficient and may enhance adherence compared with sustained therapeutic approaches15.
Perspectives
Here, we demonstrate the role of cellular senescence and the circulating SASP in mediating age-related large elastic artery stiffness, as assessed via aortic PWV, and endothelial dysfunction, determined by carotid artery EDD. Moreover, we show efficacy for targeting cellular senescence with senolytic therapy to improve vascular function in old age. Lifestyle strategies or targeted pharmacological therapies (synthetic or natural) that suppress cellular senescence and/or the SASP in arteries may be effective strategies for treating age-related vascular dysfunction, which could ultimately decrease the risk for CVD, kidney dysfunction and cognitive impairment in mid-life/older adults.
Several research gaps remain to be addressed. Future studies should attempt to determine the causal role of individual SASP factors in mediate vascular aging and how senolytic therapy alters individual SASP factors to induce favorable changes in vascular function. Moreover, ongoing72 and completed73-76 clinical trials with senolytic compounds in humans generally have been conducted in patients with severe clinical conditions and these studies are not assessing or have not assessed vascular outcomes. Importantly, the broad translatability of current synthetic pharmacological senolytic compounds is limited due to potential safety concerns and off-target toxic effects on non-senescent cells15. As such, natural food-derived senolytic compounds (e.g., fisetin77 and grapeseed extract78) and/or lifestyle strategies shown to reduce senescent cell burden (e.g., aerobic exercise79) represent viable avenues for overcoming current barriers to senolytic therapy for healthy vascular aging.
Supplementary Material
Novelty and Relevance.
What is New?
Using complementary in vivo and ex vivo translational models, we systematically demonstrated that cellular senescence and senescence associated secretory phenotype (SASP) factors in circulation contribute to aortic stiffening and endothelial dysfunction with aging.
We established efficacy of senolytic therapy for reducing aortic stiffness and improving endothelial function in advanced age.
What is Relevant?
Vascular dysfunction (e.g., aortic stiffening and endothelial dysfunction) is a major antecedent and key initiating step in the development of cardiovascular diseases.
Cellular senescence has been implicated in age-related vascular dysfunction, but the direct role of cellular senescence and circulating SASP factors in mediating aortic stiffening and endothelial dysfunction with aging has not been established.
Our findings show that cellular senescence and circulating SASP factors are key mechanisms involved in vascular aging and that senolytic therapy may improve vascular health and reduce cardiovascular disease risk with aging.
Clinical/Pathophysiological Implications
We show that cellular senescence and circulating SASP factors contribute to age-related aortic stiffening and endothelial dysfunction and establish proof-of-principal efficacy for the use of senolytic therapy to improve vascular function with aging. Together, these findings suggest that increased cellular senescence is a viable therapeutic target to prevent and/or treat age-related vascular dysfunction.
Acknowledgements:
Authors thank Jill Miyamoto-Ditmon, Zachary Cook and Anthony Sun for assistance with data collection. We acknowledge SomaLogic Operating Co., Inc. as the provider of the proteomic data measured using the modified aptamer-based SomaScan® Assay. SomaScan® and SOMAmer® are registered trademarks of SomaLogic Operating Co., Inc. and are used under license.
Sources of Funding:
T32 DK007135 (Z.S.C.); F32 HL151022 (Z.S.C.); K99 HL159241 (Z.S.C.); K01 DK115524 (M.J.R.); and R01 AG055822 (D.R.S.; J.C.; and S.M.)
Non-standard abbreviations and acronyms:
- AGE
advanced glycation end products
- CVD
cardiovascular disease
- EDD
endothelium-dependent dilation
- PWV
pulse wave velocity
- SASP
senescence associated secretory phenotype
Footnotes
Disclosures: none.
REFERENCES
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. Jan 2003;107(1):139–46. [DOI] [PubMed] [Google Scholar]
- 2.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. Sep 2005;46(3):454–62. doi: 10.1161/01.HYP.0000177474.06749.98 [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. Jan 2003;107(3):490–7. doi: 10.1161/01.cir.0000048894.99865.02 [DOI] [PubMed] [Google Scholar]
- 4.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of Vascular Aging. Circ Res. Sep 2018;123(7):849–867. doi: 10.1161/CIRCRESAHA.118.311378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. Sep 2007;8(9):729–40. doi: 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- 6.Kuehnemann C, Hughes JB, Desprez PY, Melov S, Wiley CD, Campisi J. Antiretroviral protease inhibitors induce features of cellular senescence that are reversible upon drug removal. Aging Cell. Jan 2023;22(1):e13750. doi: 10.1111/acel.13750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basisty N, Kale A, Jeon OH, et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. Jan 2020;18(1):e3000599. doi: 10.1371/journal.pbio.3000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demaria M, Ohtani N, Youssef SA, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. Dec 2014;31(6):722–33. doi: 10.1016/j.devcel.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erusalimsky JD, Kurz DJ. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp Gerontol. 2005 Aug-Sep 2005;40(8-9):634–42. doi: 10.1016/j.exger.2005.04.010 [DOI] [PubMed] [Google Scholar]
- 11.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. Dec 2015;21(12):1424–35. doi: 10.1038/nm.4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam MT, Tuday E, Allen S, et al. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell. Feb 2023;22(2):e13767. doi: 10.1111/acel.13767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang J, Wang Y, Shao L, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. Jan 2016;22(1):78–83. doi: 10.1038/nm.4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkland JL, Tchkonia T. Cellular Senescence: A Translational Perspective. EBioMedicine. Jul 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaib S, Tchkonia T, Kirkland JL. Cellular senescence and senolytics: the path to the clinic. Nat Med. Aug 2022;28(8):1556–1568. doi: 10.1038/s41591-022-01923-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demaria M, O'Leary MN, Chang J, et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 02 2017;7(2):165–176. doi: 10.1158/2159-8290.CD-16-0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. May 01 2008;68(9):3421–8. doi: 10.1158/0008-5472.CAN-07-5836 [DOI] [PubMed] [Google Scholar]
- 18.Hagan Catherine. When are mice considered old? June 22, 2020, 2017. [Google Scholar]
- 19.Clayton ZS, Brunt VE, Hutton DA, et al. Tumor Necrosis Factor Alpha-Mediated Inflammation and Remodeling of the Extracellular Matrix Underlies Aortic Stiffening Induced by the Common Chemotherapeutic Agent Doxorubicin. Hypertension. Mar 2021:HYPERTENSIONAHA12016759. doi: 10.1161/HYPERTENSIONAHA.120.16759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clayton ZS, Hutton DA, Brunt VE, et al. Apigenin restores endothelial function by ameliorating oxidative stress, reverses aortic stiffening, and mitigates vascular inflammation with aging. Am J Physiol Heart Circ Physiol. Jun 2021;doi: 10.1152/ajpheart.00118.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gioscia-Ryan RA, Clayton ZS, Zigler MC, et al. Lifelong voluntary aerobic exercise prevents age- and Western diet- induced vascular dysfunction, mitochondrial oxidative stress and inflammation in mice. J Physiol. 02 2021;599(3):911–925. doi: 10.1113/JP280607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casso AG, VanDongen NS, Ziemba BP, et al. Initiation of the Gut Microbiome Targeted Compound 3, 3-Dimethyl-1-butanol at Mid-life Prevents Age-related Vascular Dysfunction. presented at: FASEB J; 2020; [Google Scholar]
- 23.Ge SX, Jung D, Yao R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics. Apr 15 2020;36(8):2628–2629. doi: 10.1093/bioinformatics/btz931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HN, Chang J, Iyer S, et al. Elimination of senescent osteoclast progenitors has no effect on the age-associated loss of bone mass in mice. Aging Cell. 06 2019;18(3):e12923. doi: 10.1111/acel.12923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon OH, Kim C, Laberge RM, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. Jun 2017;23(6):775–781. doi: 10.1038/nm.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yabluchanskiy A, Tarantini S, Balasubramanian P, et al. Pharmacological or genetic depletion of senescent astrocytes prevents whole brain irradiation-induced impairment of neurovascular coupling responses protecting cognitive function in mice. Geroscience. 04 2020;42(2):409–428. doi: 10.1007/s11357-020-00154-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acklin S, Zhang M, Du W, et al. Depletion of senescent-like neuronal cells alleviates cisplatin-induced peripheral neuropathy in mice. Sci Rep. Aug 2020;10(1):14170. doi: 10.1038/s41598-020-71042-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunt VE, Gioscia-Ryan RA, Richey JJ, et al. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J Physiol. Feb 2019;doi: 10.1113/JP277336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Limbad C, Doi R, McGirr J, et al. Senolysis induced by 25-hydroxycholesterol targets CRYAB in multiple cell types. iScience. Feb 18 2022;25(2):103848. doi: 10.1016/j.isci.2022.103848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.López-Domínguez JA, Rodríguez-López S, Ahumada-Castro U, et al. transcript variant 2 is a marker of aging and cellular senescence. Aging (Albany NY). May 25 2021;13(10):13380–13392. doi: 10.18632/aging.203110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gioscia-Ryan RA, Clayton ZS, Fleenor BS, et al. Late-life voluntary wheel running reverses age-related aortic stiffness in mice: a translational model for studying mechanisms of exercise-mediated arterial de-stiffening. Geroscience. 02 2021;43(1):423–432. doi: 10.1007/s11357-020-00212-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishiuchi S, Yagi K, Saito H, et al. Investigation of drugs for the prevention of doxorubicin-induced cardiac events using big data analysis. Eur J Pharmacol. Aug 05 2022;928:175083. doi: 10.1016/j.ejphar.2022.175083 [DOI] [PubMed] [Google Scholar]
- 33.Raisova M, Hossini AM, Eberle J, et al. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol. Aug 2001;117(2):333–40. doi: 10.1046/j.0022-202x.2001.01409.x [DOI] [PubMed] [Google Scholar]
- 34.Chirinos JA, Segers P, Hughes T, Townsend R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 09 2019;74(9):1237–1263. doi: 10.1016/j.jacc.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seals DR. Edward F. Adolph Distinguished Lecture: The remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol (1985). Sep 2014;117(5):425–39. doi: 10.1152/japplphysiol.00362.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butlin M, Tan I, Spronck B, Avolio AP. Measuring Arterial Stiffness in Animal Experimental Studies. Arterioscler Thromb Vasc Biol. 05 2020;40(5):1068–1077. doi: 10.1161/ATVBAHA.119.313861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Shi GP. Vascular wall extracellular matrix proteins and vascular diseases. Biochim Biophys Acta. Nov 2014;1842(11):2106–2119. doi: 10.1016/j.bbadis.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleenor BS. Large elastic artery stiffness with aging: novel translational mechanisms and interventions. Aging Dis. Apr 2013;4(2):76–83. [PMC free article] [PubMed] [Google Scholar]
- 39.Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation - a mini-review. Gerontology. 2012;58(3):227–37. doi: 10.1159/000334668 [DOI] [PubMed] [Google Scholar]
- 40.Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB, Seals DR. Sodium nitrite de-stiffening of large elastic arteries with aging: role of normalization of advanced glycation end-products. Exp Gerontol. Aug 2012;47(8):588–94. doi: 10.1016/j.exger.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka T, Basisty N, Fantoni G, et al. Plasma proteomic biomarker signature of age predicts health and life span. Elife. 11 2020;9doi: 10.7554/eLife.61073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sproull M, Shankavaram U, Camphausen K. Novel Murine Biomarkers of Radiation Exposure Using An Aptamer-Based Proteomic Technology. Front Pharmacol. 2021;12:633131. doi: 10.3389/fphar.2021.633131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond). May 2011;120(9):357–75. doi: 10.1042/CS20100476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. Nov 1996;271(5 Pt 1):C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424 [DOI] [PubMed] [Google Scholar]
- 45.Murphy MP, Bayir H, Belousov V, et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat Metab. Jun 2022;4(6):651–662. doi: 10.1038/s42255-022-00591-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clayton ZS, Brunt VE, Hutton DA, et al. Doxorubicin-Induced Oxidative Stress and Endothelial Dysfunction in Conduit Arteries Is Prevented by Mitochondrial-Specific Antioxidant Treatment. JACC CardioOncol. Sep 2020;2(3):475–488. doi: 10.1016/j.jaccao.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal. Apr 2009;11(4):791–810. doi: 10.1089/ars.2008.2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. Sep 15 2011;15(6):1583–606. doi: 10.1089/ars.2011.3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead Box O phosphorylation. J Gerontol A Biol Sci Med Sci. Apr 2011;66(4):409–18. doi: 10.1093/gerona/glq233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durrant JR, Seals DR, Connell ML, et al. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. Jul 2009;587(Pt 13):3271–85. doi: 10.1113/jphysiol.2009.169771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Craighead DH, Heinbockel TC, Freeberg KA, et al. Time-Efficient Inspiratory Muscle Strength Training Lowers Blood Pressure and Improves Endothelial Function, NO Bioavailability, and Oxidative Stress in Midlife/Older Adults With Above-Normal Blood Pressure. J Am Heart Assoc. July 06 2021;10(13):e020980. doi: 10.1161/JAHA.121.020980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossman MJ, Gioscia-Ryan RA, Santos-Parker JR, et al. Inorganic Nitrite Supplementation Improves Endothelial Function With Aging: Translational Evidence for Suppression of Mitochondria-Derived Oxidative Stress. Hypertension. Apr 2021;77(4):1212–1222. doi: 10.1161/HYPERTENSIONAHA.120.16175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xin MG, Zhang J, Block ER, Patel JM. Senescence-enhanced oxidative stress is associated with deficiency of mitochondrial cytochrome c oxidase in vascular endothelial cells. Mech Ageing Dev. 2003 Aug-Sep 2003;124(8-9):911–9. [DOI] [PubMed] [Google Scholar]
- 54.Luiking YC, Engelen MP, Deutz NE. Regulation of nitric oxide production in health and disease. Curr Opin Clin Nutr Metab Care. Jan 2010;13(1):97–104. doi: 10.1097/MCO.0b013e328332f99d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rey-García J, Townsend RR. Large Artery Stiffness: A Companion to the 2015 AHA Science Statement on Arterial Stiffness. Pulse (Basel). Sep 2021;9(1-2):1–10. doi: 10.1159/000518613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. Feb 2010;121(4):505–11. doi: 10.1161/CIRCULATIONAHA.109.886655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Townsend RR. Arterial Stiffness in CKD: A Review. Am J Kidney Dis. 02 2019;73(2):240–247. doi: 10.1053/j.ajkd.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Q, Fang J, Cui C, et al. Association of Aortic Stiffness and Cognitive Decline: A Systematic Review and Meta-Analysis. Front Aging Neurosci. 2021;13:680205. doi: 10.3389/fnagi.2021.680205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. May 2005;25(5):932–43. doi: 10.1161/01.ATV.0000160548.78317.29 [DOI] [PubMed] [Google Scholar]
- 60.Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol (1985). May 2018;124(5):1194–1202. doi: 10.1152/japplphysiol.00670.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanaka T, Biancotto A, Moaddel R, et al. Plasma proteomic signature of age in healthy humans. Aging Cell. Oct 2018;17(5):e12799. doi: 10.1111/acel.12799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wiley CD, Campisi J. From Ancient Pathways to Aging Cells-Connecting Metabolism and Cellular Senescence. Cell Metab. Jun 14 2016;23(6):1013–1021. doi: 10.1016/j.cmet.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wiley CD, Velarde MC, Lecot P, et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. Feb 2016;23(2):303–14. doi: 10.1016/j.cmet.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zagura M, Kals J, Kilk K, et al. Metabolomic signature of arterial stiffness in male patients with peripheral arterial disease. Hypertens Res. Dec 2015;38(12):840–6. doi: 10.1038/hr.2015.71 [DOI] [PubMed] [Google Scholar]
- 65.van der Bruggen MM, Spronck B, Delhaas T, Reesink KD, Schalkwijk CG. The Putative Role of Methylglyoxal in Arterial Stiffening: A Review. Heart Lung Circ. Nov 2021;30(11):1681–1693. doi: 10.1016/j.hlc.2021.06.527 [DOI] [PubMed] [Google Scholar]
- 66.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. May 2007;115(18):2390–7. doi: 10.1161/CIRCULATIONAHA.106.678276 [DOI] [PubMed] [Google Scholar]
- 67.Rossman MJ, Kaplon RE, Hill SD, et al. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol. Nov 2017;313(5):H890–H895. doi: 10.1152/ajpheart.00416.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roos CM, Zhang B, Palmer AK, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 10 2016;15(5):973–7. doi: 10.1111/acel.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bachschmid MM, Schildknecht S, Matsui R, et al. Vascular aging: chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann Med. Feb 2013;45(1):17–36. doi: 10.3109/07853890.2011.645498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhayadia R, Schmidt BM, Melk A, Hömme M. Senescence-Induced Oxidative Stress Causes Endothelial Dysfunction. J Gerontol A Biol Sci Med Sci. Feb 2016;71(2):161–9. doi: 10.1093/gerona/glv008 [DOI] [PubMed] [Google Scholar]
- 71.Perrotta C, De Palma C, Clementi E. Nitric oxide and sphingolipids: mechanisms of interaction and role in cellular pathophysiology. Biol Chem. Nov 2008;389(11):1391–7. doi: 10.1515/BC.2008.155 [DOI] [PubMed] [Google Scholar]
- 72.Gonzales MM, Garbarino VR, Marques Zilli E, et al. Senolytic Therapy to Modulate the Progression of Alzheimer's Disease (SToMP-AD): A Pilot Clinical Trial. J Prev Alzheimers Dis. 2022;9(1):22–29. doi: 10.14283/jpad.2021.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hickson LJ, Langhi Prata LGP, Bobart SA, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. Sep 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nambiar A, Kellogg D, Justice J, et al. Senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis: results of a phase I, single-blind, single-center, randomized, placebo-controlled pilot trial on feasibility and tolerability. EBioMedicine. Feb 27 2023;90:104481. doi: 10.1016/j.ebiom.2023.104481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. Feb 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu Y, Prata LGPL, Gerdes EOW, et al. Orally-active, clinically-translatable senolytics restore α-Klotho in mice and humans. EBioMedicine. Mar 2022;77:103912. doi: 10.1016/j.ebiom.2022.103912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. Oct 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu Q, Fu Q, Li Z, et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat Metab. 12 2021;3(12):1706–1726. doi: 10.1038/s42255-021-00491-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang X, Englund DA, Aversa Z, Jachim SK, White TA, LeBrasseur NK. Exercise Counters the Age-Related Accumulation of Senescent Cells. Exerc Sport Sci Rev. Jul 01 2022;doi: 10.1249/JES.0000000000000302 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.