Abstract
PURPOSE
Anaplastic thyroid carcinoma (ATC) uniformly present with aggressive disease, but the mutational landscape of tumors varies. We aimed to determine whether tumor mutations affect survival outcomes in ATC.
MATERIALS AND METHODS
Patients who underwent mutation sequencing using targeted gene panels between 2005 and 2019 at a tertiary referral center were included. Associations between mutation status and survival outcomes were assessed using Cox proportional hazards models.
RESULTS
A total of 202 patients were included, where 122 died of ATC (60%). The median follow-up was 31 months (interquartile range, 18-45 months). The most common mutations were in TP53 (59%), BRAF (41%), TERT promoter (37%), and the RAS gene family (22%). Clinicopathologic characteristics and overall survival (OS) significantly correlated with mutations in BRAFV600E and RAS, which were mutually exclusive. The BRAFV600E mutation was associated with the presence of a papillary thyroid carcinoma precursor and significantly better OS (median OS: 24 months). RAS-mutated patients more commonly presented without cervical lymph node involvement but had the worst OS (median OS: 6 months). Tumors that were wild-type for both BRAF and RAS were enriched for NF1 mutations and harbored intermediate prognosis (median OS: 15 months). In multivariate analyses, RAS mutations were associated with a more than 2.5-fold higher risk of death (adjusted hazard ratio, 2.64; 95% CI, 1.66 to 4.20) compared with BRAFV600E. In patients treated with BRAF-directed therapy (n = 60), disease progression occurred in 48% of patients (n = 29). The median progression-free survival was 14 months. The presence of a TP53 mutation was independently associated with reduced progression-free survival in BRAFV600E-mutated patients treated with BRAF-directed therapy (adjusted hazard ratio, 2.89; 95% CI, 1.35 to 6.21).
CONCLUSION
Mutation analysis provides prognostic information in ATC and should be incorporated into routine clinical care.
INTRODUCTION
Anaplastic thyroid carcinoma (ATC) is a rare malignancy with an age-adjusted incidence of 0.11 cases per 100,000 person-years in the United States.1 It represents < 2% of all thyroid cancers but accounts for up to 50% of thyroid cancer–related mortality.2 ATC is characterized by advanced disease at presentation and rapid progression within weeks. For decades, the median survival for ATC was < 5 months.2-4 Multimodal therapy including cytotoxic chemotherapy, radiotherapy, and surgery improves outcomes, but most patients do not survive beyond 1 year.5 Until recently, tumor mutation status has not been routinely used to inform clinical management in ATC. The US Food and Drug Administration's approval of dabrafenib and trametinib for BRAFV600E-mutated ATC in 2018 has shifted the treatment paradigm toward targeted therapy and increased utilization of mutation testing in clinical practice.6 Although several studies have characterized the mutational landscape of ATC using targeted and whole-exome sequencing, the clinical significance of mutation profiles including associations with clinicopathologic characteristics, treatment outcomes, and survival has not been examined in detail.7-11 The purpose of this study is to determine whether the tumor mutation profile assessed at diagnosis is associated with clinicopathologic characteristics, overall survival (OS), and progression-free survival (PFS).
CONTEXT
Key Objective
Recent sequencing efforts have elucidated the genomic landscape of anaplastic thyroid carcinoma (ATC), demonstrating that it is genetically heterogeneous. It remains unknown whether differences in clinical manifestations and prognosis exist among subtypes of ATC determined by tumor mutation status. To our knowledge, this is the largest report assessing the impact of tumor mutation status on ATC clinical outcomes.
Knowledge Generated
ATCs can be divided into three subtypes on the basis of driver mutations in BRAF and RAS, which are mutually exclusive: (1) RAS-mutated, (2) BRAFV600E-mutated, and (3) BRAF and RAS wild-type. RAS-driven tumors appear to be the most aggressive and harbor the worst prognosis. BRAFV600E-mutated tumors have the best prognosis, largely because of benefits from BRAF-directed therapy. BRAF and RAS wild-type tumors harbor intermediate prognosis.
Relevance
These findings support inclusion of tumor mutation testing during the clinical workup of ATC and treatment decision making guided by tumor driver mutation status.
MATERIALS AND METHODS
Patients with ATC treated at the University of Texas MD Anderson Cancer Center (Houston, TX) between 2005 and 2019 were identified via retrospective chart review. Approval for the study was obtained from the MD Anderson Institutional Review Board. For this study, a waiver of informed consent was granted by the Institutional Review Board as the study was determined to be minimal risk using existing information derived from patient care. All included patients had mutation testing and confirmation of ATC diagnosis by a head and neck pathologist.
Descriptive statistics were used to summarize demographic and clinicopathologic characteristics of included patients. Survival curves were constructed using the Kaplan-Meier method and compared using the log-rank test. Hazard ratios (HRs) and 95% CIs were calculated through univariate or multivariate Cox proportional hazard regression adjusting for age at diagnosis, surgery, and overall stage.
See the Data Supplement for additional information.
RESULTS
Patient Cohort
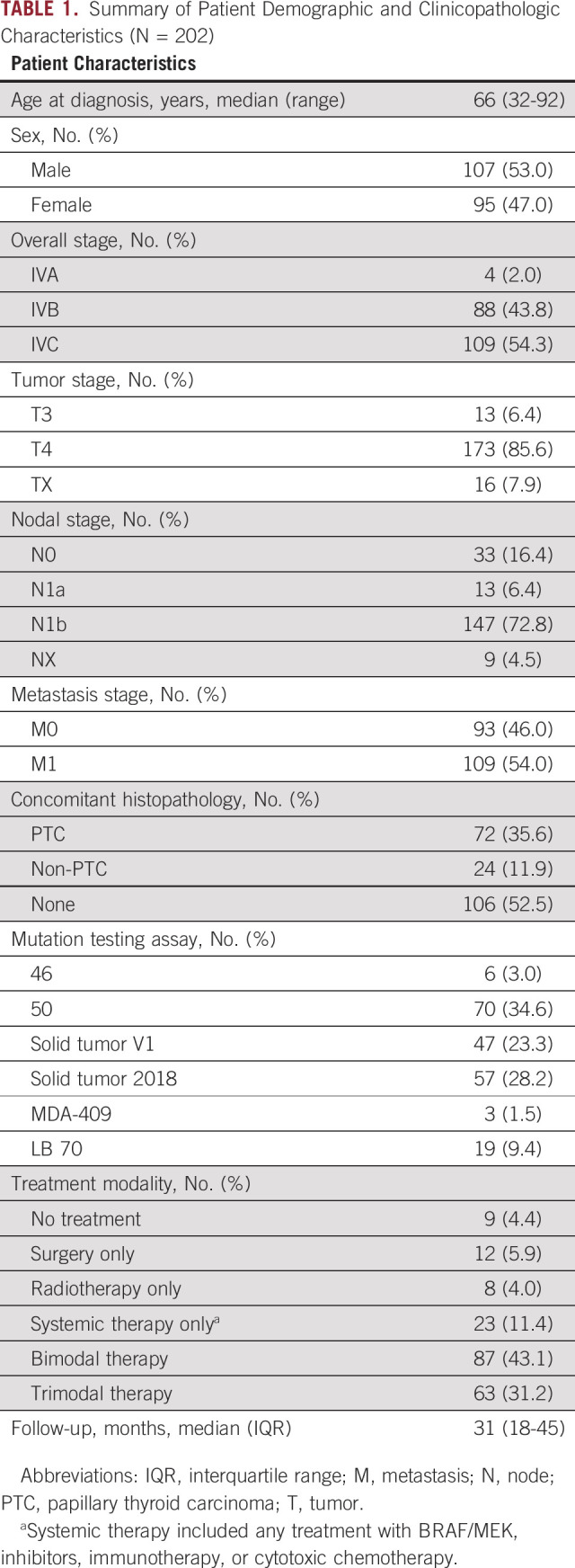
A total of 202 consecutive patients with ATC were included in the study. The clinicopathologic characteristics of the included patients are shown in Table 1. The median age at diagnosis was 66 years (range, 32-92 years). A majority of patients presented with distant metastases (stage IVC disease; n = 109, 54%). Locoregionally advanced disease was also prevalent, with 86% (n = 173) of patients presenting with T4 disease and 73% of patients (n = 147) presenting with lateral neck lymph node metastases (N1b). Ninety-six patients (47%) were noted to have a component of another coexisting thyroid carcinoma on histopathology. Papillary thyroid carcinoma (PTC) was the most common coexisting precursor and found in 72 patients. A history of well-differentiated thyroid cancer was reported in 21 patients (10%) where they had clinically documented transformation from PTC to ATC. The median duration of follow-up was 31 months. A majority of patients received multimodality treatment (Table 1). In terms of systemic therapy, 55% of patients received cytotoxic chemotherapy (n = 112), 40% received immunotherapy (n = 80), and 30% received BRAF-directed therapy (n = 60). Detailed treatment modality information is included in the Data Supplement.
TABLE 1.
Summary of Patient Demographic and Clinicopathologic Characteristics (N = 202)
Spectrum of Identified Somatic Mutations
One hundred eighty-three patients were profiled using tissue-based assays. The majority had NGS performed on thyroid tissues (n = 133), whereas cervical lymph nodes and distant metastases were used in 39 and 11 patients, respectively. In 19 patients where tumor tissues were not available, testing was performed on blood using the LB70 liquid biopsy assay. Overall, for patients who had tests of both solid tissue biopsy and liquid biopsy (n = 57), we observed an average gene-wise concordance of 96% and an average within-patient concordance of 95%.
In patients who had both liquid-based and tissue-based assays, the mutation profile from tissue-based assay was used for subsequent analyses. Identified somatic mutations are summarized in Figure 1.
FIG 1.
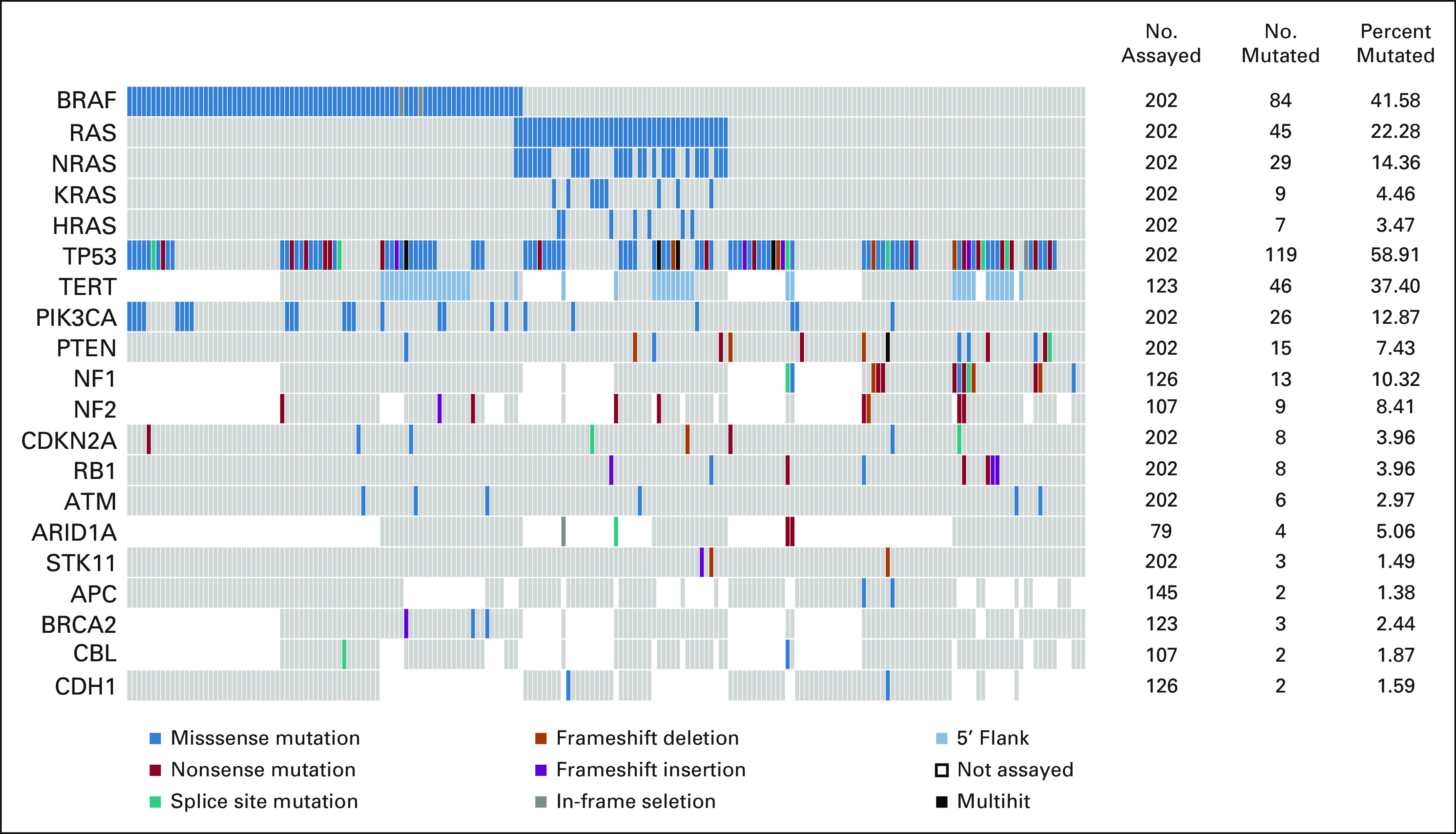
Somatic Mutations in 202 patients with ATC. OncoPrint showing recurrently mutated genes identified within the overall ATC cohort. RAS gene family (NRAS, KRAS, and HRAS) mutations are shown together and individually. Of 19 genes that are recurrently mutated, 11 were covered across panels. In the eight genes that were covered in selected panels only, the number assayed indicates the number of patients who were tested with panels covering the specific gene(s). The number and percent mutated indicate the proportion of patients who had mutation(s) detected among those who were assayed. Color key for the types of genetic alterations identified is shown on the bottom legend. ATC, anaplastic thyroid carcinoma.
No mutations were identified in 21 patients. These patients did not differ in terms of clinicopathologic characteristics from the rest of the cohort. Missense mutations in the mitogen-activated protein kinase (MAPK) pathway genes, namely, BRAF, NRAS, KRAS, and HRAS, were prevalent (Fig 1). All identified mutations in BRAF were V600E with the exception of a D594N mutation, a G469E mutation, and two in-frame deletions. In the RAS gene family, NRAS mutations (13%) were the most common followed by KRAS (4.4%) and HRAS (3.0%). The BRAFV600E and RAS mutations were mutually exclusive. Three groups of patients emerged on the basis of MAPK driver mutation status: (1) BRAFV600E-mutated and RAS wild-type, (2) RAS-mutated and BRAF wild-type, and (3) BRAF wild-type and RAS wild-type, which each accounted for 40%, 22%, and 38% of patients, respectively.
Mutations in TP53 were the most common, identified in 54% of patients (n = 110 of 202). These mutations commonly co-occurred with BRAF and RAS mutations, 46% (n = 38 of 84) in BRAF-mutated and 51% (n = 23 of 45) in RAS-mutated. A higher prevalence of TP53 mutations (64%, n = 49 of 77) was identified in patients who were BRAF and RAS wild-type. The majority of identified TP53 mutations were missense (70%). Frameshift (6%), nonsense (17%), and splice site (6%) accounted for the remaining mutations. Multiple TP53 mutations were identified in 10 patients, where five patients had mutations of different classes. Mutually exclusive PIK3CA and PTEN mutations were also identified in a significant proportion of patients, 12% and 7%, respectively. Although PIK3CA mutations were all missense, PTEN mutations included missense (33%), nonsense (33%), frameshift deletions (20%), and splice site (13%). In patients where the TERT promoter, NF1, and NF2 genes were assessed, 37% (46 of 123), 10% (13 of 126), and 8% (9 of 107) harbored mutations, respectively. TERT promoter mutations were equally distributed across driver mutation groups in tested patients (38%, 41%, and 33% in BRAF-mutated, RAS-mutated, and BRAF and RAS wild-type, respectively). NF1 mutations were only detected in patients who were BRAF wild-type and RAS wild-type. Other notable but less frequent mutations were identified in RB1, CDKN2A (n = 8 of 202, 4%), ATM (n = 6 of 202, 3%), and ARID1A (n = 4 of 79, 5%).
Clinicopathologic Characteristics and Mutation Status
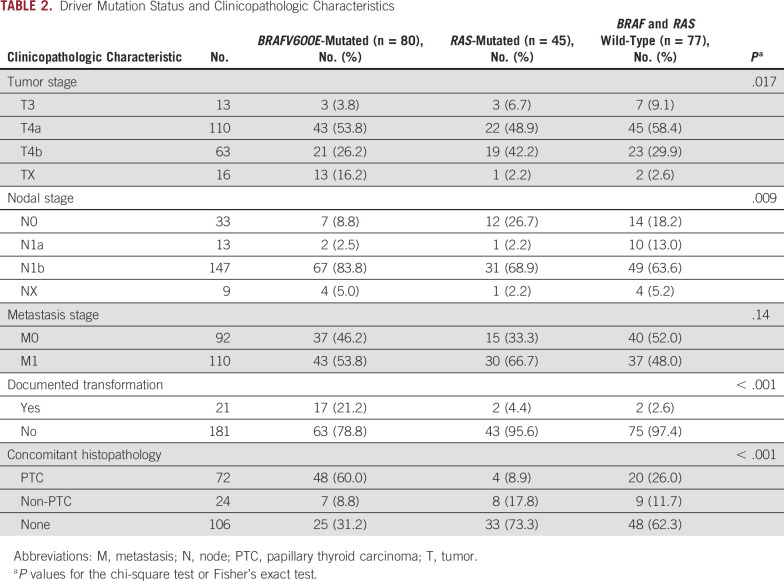
Driver mutation status was associated with histopathology and disease presentation (Table 2). ATCs with PTC precursors were enriched for the BRAFV600E mutation. Of 72 ATCs with coexisting PTC on histopathology, 48 (67%) harbored the BRAFV600E mutation. Similarly, the majority of patients (n = 17 of 21, 81%) with clinically documented transformation from PTC to ATC were BRAFV600E-mutated. RAS mutations were infrequently detected in these cases and found in four tumors with coexisting PTC on histopathology and two tumors with documented PTC to ATC transformation. BRAFV600E was less prevalent in ATCs with non-PTC precursors (n = 7, 29%). A higher prevalence of RAS mutations was identified in these patients (n = 8, 33%).
TABLE 2.
Driver Mutation Status and Clinicopathologic Characteristics
In terms of disease presentation, RAS-mutated patients more commonly presented with T4b disease and without metastases to neck lymph nodes (N0 disease) compared with patients who were BRAFV600E-mutated or BRAF and RAS wild-type. Although not statistically significant, a higher proportion of M1 disease was also observed in RAS-mutated patients (Table 2).
OS and Mutation Status
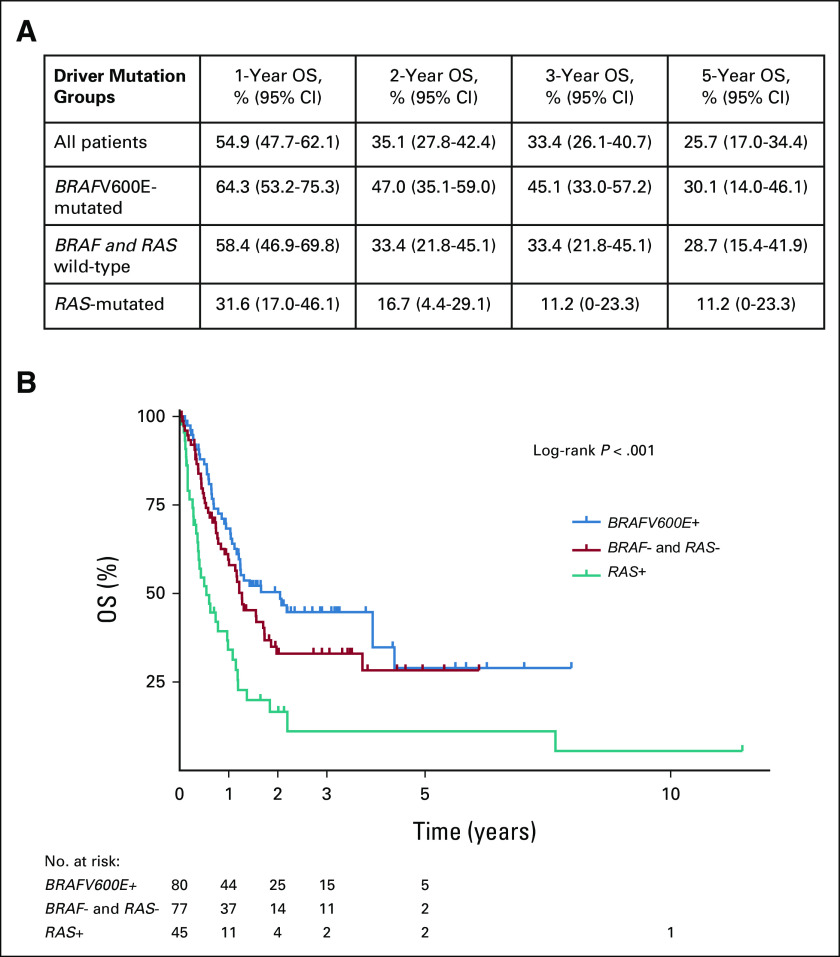
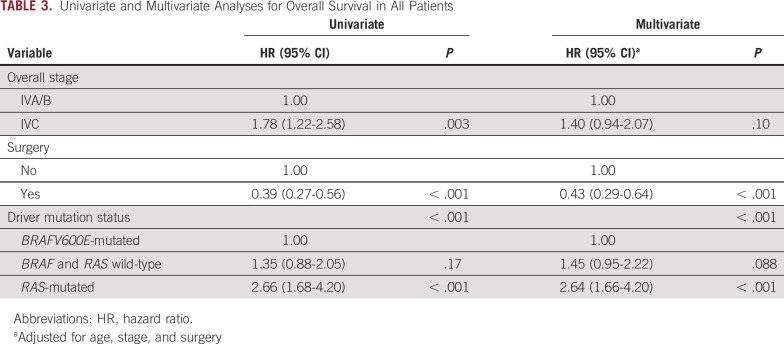
The total number of deaths within the study was 122. All deaths were due to ATC. Survival rates and estimates by driver mutation status are shown in Figure 2. The median survival time was 24 months in BRAFV600E-mutated patients, 15 months in BRAF and RAS wild-type patients, and 6 months in RAS-mutated patients. In multivariate analyses, after adjustment for age at diagnosis, overall stage, and treatment with surgery, driver mutation status was significantly associated with OS (P < .001; Table 3). Compared with BRAFV600E-mutated patients, RAS-mutated patients had a more than two-and-a-half-fold higher risk of death (adjusted HR, 2.64; 95% CI, 1.66 to 4.20; Table 3). No differences in OS were observed comparing patients with NRAS, KRAS, and HRAS mutations. Although BRAF and RAS wild-type patients had shorter OS compared with those who were BRAFV600E-mutated, this difference was not statistically significant (HR, 1.45; 95%, 0.95 to 2.22) in multivariate analyses (Table 3).
FIG 2.
OS of patients with ATC by driver mutation status: (A) OS rates at 1, 2, 3, and 5 years and (B) survival analysis. ATC, anaplastic thyroid carcinoma; OS, overall survival.
TABLE 3.
Univariate and Multivariate Analyses for Overall Survival in All Patients
We also evaluated recurrently mutated genes with identified frequencies > 5% in terms of survival outcomes. This included mutations in TP53, TERT promoter, PIK3CA, PTEN, and NF1. No significant associations were identified for these mutations. Furthermore, panel-derived tumor mutational burden (TMB) did not significantly affect survival outcomes, including in the context of immunotherapy (Data Supplement).
OS in Patients with BRAF Wild-Type ATC
Survival outcomes for RAS-mutated and BRAF and RAS wild-type patients by treatment are shown in the Data Supplement. In BRAF and RAS wild-type patients, surgery was associated with improved survival in multivariate analyses (adjusted HR, 0.49; 95% CI, 0.24 to 0.99; Data Supplement). Although the 13 patients who harbored a NF1 mutation had marginally better OS compared with wild type, this difference was not significant. Surgery in RAS-mutated patients did not significantly alter OS in multivariate analyses (Data Supplement). However, treatment with immune checkpoint blockade in this population trended towards improved survival (adjusted HR, 0.47; 95% CI, 0.22 to 1.01; Data Supplement). Co-occurrence of RAS mutations with other mutations did not significantly alter OS.
OS in Patients With BRAFV600E-Mutated ATC
Of the 80 patients who had BRAFV600E-mutated ATC, 60 (75%) received BRAF-directed therapy. All 20 patients who did not receive BRAF-directed therapy were diagnosed before the US Food and Drug Administration approval of dabrafenib/trametinib in April 2018. These patients had poorer survival outcomes similar to those who were BRAF wild-type with a median OS of 6 months (Fig 3A). Most patients treated with BRAF-directed therapy received both a BRAF inhibitor and a MEK inhibitor (n = 54 of 60, 90%). Compared with patients who did not receive BRAF-directed therapy, treated BRAFV600E-mutated patients had significantly better OS (Fig 3B). After adjusting for age, stage, and surgery, BRAF-directed therapy was associated with a 76% reduction (HR, 0.24; 95% CI, 0.11 to 0.54) in the risk of death from any cause.
FIG 3.
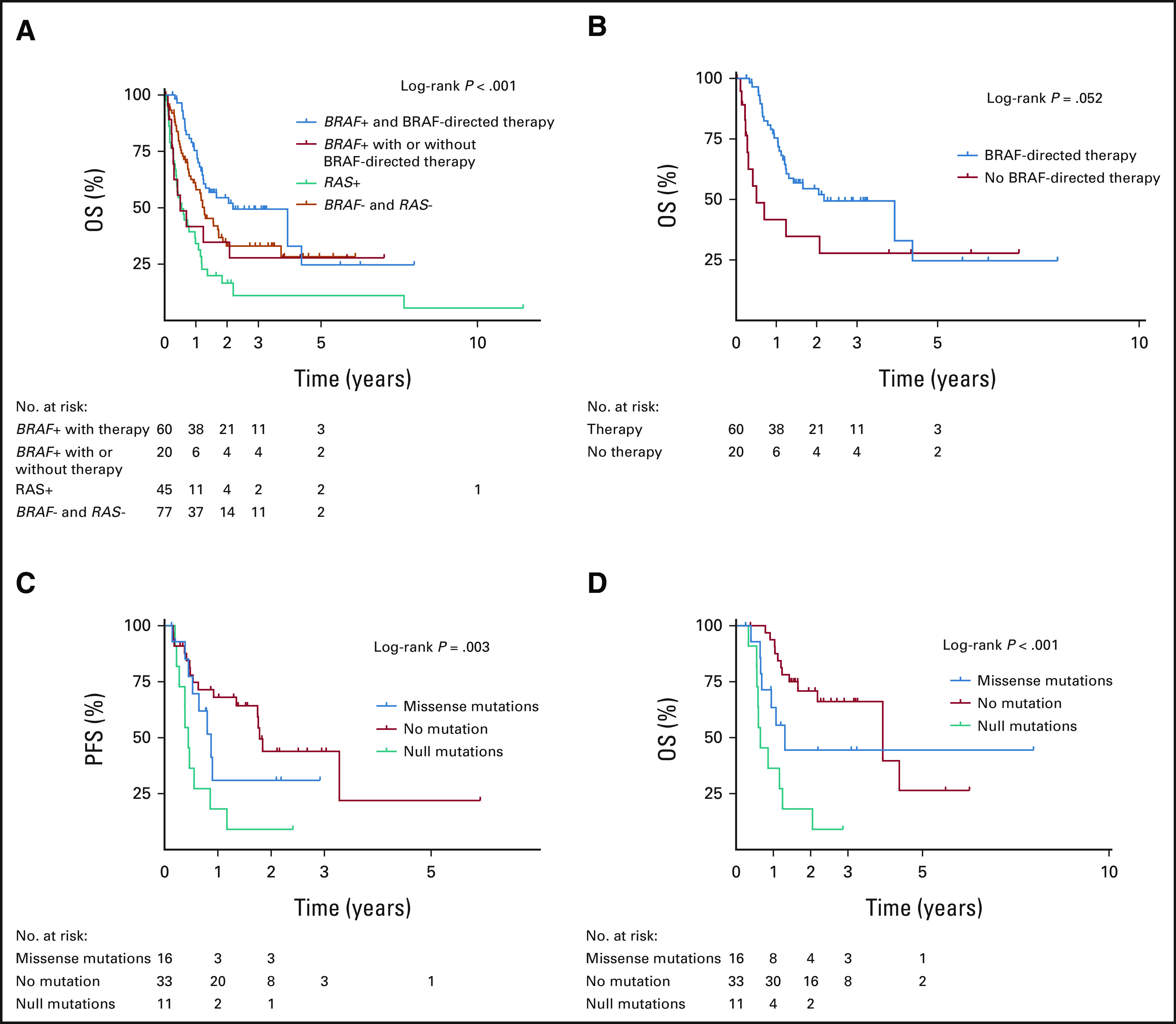
Survival outcomes analyses in BRAFV600E-mutated ATC: (A) OS in all patients by driver mutation status and BRAF-directed therapy, (B) OS in BRAFV600E-mutated patients by BRAF-directed therapy status, (C) PFS by TP53 mutation status in patients treated with BRAF-directed therapy, and (D) OS by TP53 mutation status in patients treated with BRAF-directed therapy. ATC, anaplastic thyroid carcinoma; OS, overall survival; PFS, progression-free survival.
OS and PFS in BRAFV600E-Mutated Patients Treated With BRAF-Directed Therapy
Forty-eight percent of patients who received BRAF-directed therapy (n = 29 of 60) developed disease progression. The majority progressed in their distant metastatic disease with or without locoregional progression. Distribution of mutations for progressed versus disease-free patients is shown in the Data Supplement. The median OS and PFS in patients who received BRAF-directed therapy were 26 months and 14 months, respectively. The presence of TP53 mutations was independently associated with both reduced OS and PFS after BRAF-directed treatment (Fig 3C and Data Supplement). This association was further stratified by TP53 mutation type, where protein-truncating mutations (frameshift and nonsense) were associated with worse OS compared with missense mutations (HR, 2.61; 95% CI, 1.01 to 6.73). BRAF allele frequency and co-occurrence with other mutations besides TP53 did not affect OS or PFS in patients receiving BRAF-directed therapy (Data Supplement).
Surgery significantly improved PFS and OS, whereas radiotherapy did not affect survival outcomes in patients treated with BRAF-directed therapy (Data Supplement). Moreover, 36 patients received both BRAF-directed therapy and immunotherapy. These patients had better PFS and OS compared with those who received BRAF-directed therapy alone (Data Supplement). Multivariable Cox regression analysis also demonstrated a significant benefit in survival outcomes with a combination of BRAF-directed and immune checkpoint inhibitor therapies (Data Supplement), after adjusting for age, stage, and surgery.
DISCUSSION
ATC is one of the most lethal human malignancies. For decades, the rarity of ATC, its short survival times, and the lack of sufficient tumor tissues for molecular studies impeded progress in the field. Recent characterization of the ATC mutational landscape has paved the way for more effective therapies and improved outcomes for patients with ATC, particularly for those with BRAFV600E-mutated tumors.7-11,15 As in differentiated thyroid carcinomas, mutually exclusive mutations in the BRAFV600E and RAS gene family are the main driver mutations in ATC. In this study, we found that although clinically documented transformation from PTC to ATC occurred in only 10% of patients, evidence of a differentiated thyroid carcinoma precursor was prevalent on histopathology. Confirming prior observations, the BRAFV600E mutation was associated with the presence of a PTC precursor and documented transformation from PTC to ATC.10,16-18 RAS mutations, on the other hand, were associated with other differentiated thyroid carcinoma precursors including follicular thyroid carcinoma. In patients where a precursor was not reported, fine-needle aspiration biopsy instead of a core biopsy likely contributed to lower identification rates and underestimation of precursor prevalence. These findings support the current theory that although ATC may develop de novo, the majority of ATCs arise from differentiated thyroid carcinomas.17,19,20 The reported prevalence of the BRAFV600E mutation in ATC varies, ranging from 25% to 45%.7-11,21 In this study, which examined the largest cohort of sequenced patients with ATC with clinical outcomes data reported to date, the BRAFV600E mutation was identified in 41% of patients, which aligns with previous reports.7,8,10 The rates of RAS mutations (25%) in this cohort were also similar to those in previous studies, yet we observe a lower prevalence of TP53 mutations.7,8,10 This difference may be due to a higher variant allele frequency used for filtering in our tissue-based next generation sequencing panels (≥ 5%) compared with MSK-IMPACT and Foundation Medicine.
We observed that survival outcomes in ATC were primarily determined by MAPK driver mutation status. BRAFV600E-mutated patients had the best prognosis with a median OS of 31 months followed by patients who were BRAF and RAS wild-type. The improved outcomes in BRAFV600E patients were attributed to the utilization of BRAF-directed therapy in our cohort. Without treatment with BRAF/MEK inhibitors, BRAFV600E-mutated patients had a median OS of 11 months, which was 4 months shorter than patients who were BRAF and RAS wild-type. Strikingly, the presence of RAS gene family mutations (NRAS, KRAS, and HRAS) was associated with significantly reduced survival, with a median OS of 6 months. Although RAS-mutated tumors showed less propensity for cervical lymph node involvement, there was a nonsignificant trend toward higher prevalence of distant metastatic disease at presentation, demonstrating their aggressive behavior and the usual pattern of metastasis seen in follicular and Hurthle cell thyroid cancers. Although other studies have implicated co-occurrence of TERT promoter mutations with BRAF and RAS as poor prognostic factors in ATC, the presence of RAS mutations alone as a negative prognostic factor in ATC was recently reported in a small cohort of 27 patients where 11 were RAS-mutated.7,10,22 Similar to the study by Lai et al,22 we did not identify significant associations between TERT promoter mutations and survival outcomes, including co-occurrences with BRAFV600E and RAS. Since the TERT promoter region was tested in 123 of 202 patients, missing cases might have contributed to a lower mutation prevalence in this cohort than previous reports. However, distribution of TERT promoter mutations was similar across driver mutation groups, which suggests that the poor survival outcome seen in RAS-mutated patients cannot be entirely attributed to co-occurrence of RAS with TERT promoter mutations. In BRAF and RAS wild-type patients, NF1 mutations appear to act as driver mutations but were incompletely assessed across patients in this study, which is a limitation. A larger sample size of patients with NF1 testing is needed to robustly investigate whether NF1 mutations affect survival outcomes in ATC. Moreover, additional genomic alterations, which may include mutations in genes not captured in targeted panels (ie, E1F1AX, SWI/SNF genes, and mismatch repair pathway genes), oncogenic fusions, copy number alterations, and/or epigenetic alterations, may also affect ATC phenotypes and survival outcomes, which require further investigation in future studies.
Previous studies examining the genomic landscape of ATC have not evaluated survival outcomes in the context of BRAF-directed therapy.7,10 In this large cohort of patients treated with a specialized multidisciplinary program for ATC at a tertiary referral center, we found that disease progression occurred in approximately half of treated patients. Comparable with observations in melanoma, the duration of response was limited with a PFS of approximately 1 year.23 These findings suggest that despite high initial response rates to BRAF/MEK inhibition, treatment resistance remains a challenge precluding long-term survival in BRAFV600E-mutated ATC. Our group previously showed that the emergence of a RAS mutation after BRAF/MEK inhibition leads to treatment resistance and progression.24 Here, we found that the presence of TP53 mutations was an independent predictor of disease progression. Indeed, murine models of BRAFV600E-mutated ATC with p53 loss demonstrate evidence of intrinsic resistance to BRAF inhibition.25,26 The combination of BRAFV600E and missense TP53 mutations, on the other hand, remains less studied in vivo. Additional studies are required to further investigate the impact of specific TP53 mutations on BRAF/MEK inhibition and to elucidate possible mechanisms of therapeutic resistance.
Immunotherapy with a checkpoint blockade is being actively investigated for ATC. In a recently published clinical trial evaluating the efficacy of programmed cell death protein (PD-1) blockade, the overall response rate to the checkpoint blockade was modest at 19%.27 In this trial, patients were treated with single-agent spartalizumab and BRAFV600E-mutated patients (n = 12) were found to have lower response rates than BRAF wild-type patients (8% v 23%).27 However, in this study, we found a survival benefit for 36 BRAFV600E-mutated patients treated with a checkpoint blockade and BRAF-directed therapy. These findings suggest that combination therapy with BRAF/MEK inhibition and checkpoint inhibition may be of benefit in BRAFV600E-mutated ATC. A potential benefit was also observed in RAS-mutated ATC, where effective therapies are urgently needed. Indeed, multiple ongoing clinical trials (ClinicalTrials.gov identifier: NCT03181100, NCT04675710, NCT04171622, and NCT04238624) are evaluating treatment strategies that include combination therapy with tyrosine kinase inhibitors and checkpoint inhibitors. Although TMB was not significantly associated with survival in patients treated with immunotherapy in this study, it has been demonstrated that TMB accuracy suffers when calculated from panel tests with low genome coverage.28 Given the small size of the targeted panels used in this patient cohort, the accuracy of panel TMB estimates is likely low, which limits conclusions that can be drawn from these sensitivity analyses.
In conclusion, driver mutations in ATC are associated with distinct clinicopathologic features and survival outcomes, reflecting heterogeneity in tumor biology. Although treatment with BRAF/MEK inhibitors can be initiated on the basis of knowledge of BRAFV600E status alone (ie, using immunohistochemistry), knowledge of RAS and TP53 mutation status allows for additional risk stratification and can guide further therapeutic decision making. As such, comprehensive tumor mutation profiling should be obtained for patients with ATC as a part of the routine clinical workup at diagnosis, which has been incorporated into the current American Thyroid Association treatment guidelines.29
Xiao Zhao
Patents, Royalties, Other Intellectual Property: Patent pending: Composition and Methods for Regulating Extracellular Matrix Accumulation Publication No.: 20190381133 (Inst)
Wenyi Wang
Stock and Other Ownership Interests: Genomic Health
Research Funding: Curis
Ramona Dadu
Honoraria: Bayer
Consulting or Advisory Role: Exelixis
Research Funding: AstraZeneca (Inst), Merck (Inst), Eisai (Inst), Genentech (Inst), Bristol Myers Squibb (Inst), Exelixis (Inst)
Naifa L. Busaidy
Honoraria: Eisai, Exelixis
Consulting or Advisory Role: Loxo, Eisai
Research Funding: GlaxoSmithKline/Novartis
Stephen Y. Lai
Consulting or Advisory Role: Cardinal Health
Neil D. Gross
Honoraria: Intuitive Surgical
Consulting or Advisory Role: PDS Biotechnology, Verb Surgical (Inst), Sanofi/Regeneron, Shattuck Labs
Research Funding: Regeneron, MedImmune (Inst), Genentech (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Renata Ferrarotto
Consulting or Advisory Role: Bicara Therapeutics, Prelude Therapeutics, Regeneron, IntelliSphere, Merck Serono, G1 Therapeutics, Ayala Pharmaceuticals, Guidepoint Global, Elevar Therapeutics
Research Funding: G1 Therapeutics (Inst), AstraZeneca/MedImmune (Inst), EMD Serono (Inst), Genentech/Roche (Inst), Merck Serono (Inst), Pfizer/EMD Serono (Inst), Ayala Pharmaceuticals (Inst), Prelude Therapeutics (Inst)
Michelle D. Williams
Consulting or Advisory Role: Bayer Health
Speakers' Bureau: Bayer
Mark E. Zafereo
Research Funding: Merck (Inst), Lilly (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/923970
Maria E. Cabanillas
Honoraria: Loxo/Lilly
Consulting or Advisory Role: Loxo, Ignyta, Bayer, Lilly, Exelixis
Research Funding: Kura Oncology, Eisai, Roche/Genentech, Exelixis, Merck
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented as a poster session at the ASCO Annual Meeting, Chicago, IL, June 1-5, 2018.
SUPPORT
Supported by the Mark Foundation ASPIRE award (J.R.W, W.W, and M.E.Z.), the American Thyroid Association grant (J.R.W), and the MD Anderson Cancer Center Petrick Multidisciplinary Anaplastic Thyroid Cancer Research Fund (J.R.W, R.D., N.B., S.Y.L., M.E.Z., and M.E.C.).
DATA SHARING STATEMENT
The data supporting findings from this study are available from the corresponding authors upon request.
AUTHOR CONTRIBUTIONS
Conception and design: Jennifer Rui Wang, Gilbert Cote, Naifa L. Busaidy, Mark E. Zafereo, Maria E. Cabanillas
Financial support: Jennifer Rui Wang, Wenyi Wang, Mark E. Zafereo
Administrative support: Jennifer Rui Wang, Mark E. Zafereo
Provision of study materials or patients: Jennifer Rui Wang, Gilbert Cote, Naifa L. Busaidy, Stephen Y. Lai, Renata Ferrarotto, Mark E. Zafereo, Maria E. Cabanillas
Collection and assembly of data: Jennifer Rui Wang, Li Xu, Maitrayee Goswami, Priyanka Iyer, Michelle D. Williams, Mark Routbort, Mark E. Zafereo, Maria E. Cabanillas
Data analysis and interpretation: Jennifer Rui Wang, Matthew Montierth, Li Xu, Xiao Zhao, Wenyi Wang, Ramona Dadu, Naifa L. Busaidy, Stephen Y. Lai, Neil D. Gross, Renata Ferrarotto, Charles Lu, Gary Brandon Gunn, Michelle D. Williams, Mark E. Zafereo, Maria E. Cabanillas
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Xiao Zhao
Patents, Royalties, Other Intellectual Property: Patent pending: Composition and Methods for Regulating Extracellular Matrix Accumulation Publication No.: 20190381133 (Inst)
Wenyi Wang
Stock and Other Ownership Interests: Genomic Health
Research Funding: Curis
Ramona Dadu
Honoraria: Bayer
Consulting or Advisory Role: Exelixis
Research Funding: AstraZeneca (Inst), Merck (Inst), Eisai (Inst), Genentech (Inst), Bristol Myers Squibb (Inst), Exelixis (Inst)
Naifa L. Busaidy
Honoraria: Eisai, Exelixis
Consulting or Advisory Role: Loxo, Eisai
Research Funding: GlaxoSmithKline/Novartis
Stephen Y. Lai
Consulting or Advisory Role: Cardinal Health
Neil D. Gross
Honoraria: Intuitive Surgical
Consulting or Advisory Role: PDS Biotechnology, Verb Surgical (Inst), Sanofi/Regeneron, Shattuck Labs
Research Funding: Regeneron, MedImmune (Inst), Genentech (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Renata Ferrarotto
Consulting or Advisory Role: Bicara Therapeutics, Prelude Therapeutics, Regeneron, IntelliSphere, Merck Serono, G1 Therapeutics, Ayala Pharmaceuticals, Guidepoint Global, Elevar Therapeutics
Research Funding: G1 Therapeutics (Inst), AstraZeneca/MedImmune (Inst), EMD Serono (Inst), Genentech/Roche (Inst), Merck Serono (Inst), Pfizer/EMD Serono (Inst), Ayala Pharmaceuticals (Inst), Prelude Therapeutics (Inst)
Michelle D. Williams
Consulting or Advisory Role: Bayer Health
Speakers' Bureau: Bayer
Mark E. Zafereo
Research Funding: Merck (Inst), Lilly (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/923970
Maria E. Cabanillas
Honoraria: Loxo/Lilly
Consulting or Advisory Role: Loxo, Ignyta, Bayer, Lilly, Exelixis
Research Funding: Kura Oncology, Eisai, Roche/Genentech, Exelixis, Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Lim H, Devesa SS, Sosa JA, et al. : Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA 317:1338-1348, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smallridge RC, Copland JA: Anaplastic thyroid carcinoma: Pathogenesis and emerging therapies. Clin Oncol 22:486-497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin B, Ma H, Ma M, et al. : The incidence and survival analysis for anaplastic thyroid cancer: A SEER database analysis. Am J Transl Res 11:5888-5896, 2019 [PMC free article] [PubMed] [Google Scholar]
- 4.Sugitani I, Miyauchi A, Sugino K, et al. : Prognostic factors and treatment outcomes for anaplastic thyroid carcinoma: ATC Research Consortium of Japan Cohort Study of 677 patients. World J Surg 36:1247-1254, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Prasongsook N, Kumar A, Chintakuntlawar Av, et al. : Survival in response to multimodal therapy in anaplastic thyroid cancer. J Clin Endocrinol Metab 102:4506-4514, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Maniakas A, Dadu R, Busaidy NL, et al. : Evaluation of overall survival in patients with anaplastic thyroid carcinoma, 2000-2019. JAMA Oncol 6:1397-1404, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landa I, Ibrahimpasic T, Boucai L, et al. : Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 126:1052-1066, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pozdeyev N, Gay LM, Sokol ES, et al. : Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res 24:3059, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Luthra R, Routbort MJ, et al. : Molecular profile of advanced thyroid carcinomas by next-generation sequencing: Characterizing tumors beyond diagnosis for targeted therapy. Mol Cancer Ther 17:1575, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Xu B, Fuchs T, Dogan S, et al. : Dissecting anaplastic thyroid carcinoma: A comprehensive clinical, histologic, immunophenotypic, and molecular study of 360 cases. Thyroid 30:1505-1517, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunstman JW, Juhlin CC, Goh G, et al. : Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum Mol Genet 24:2318-2329, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanagal-Shamanna R, Portier BP, Singh RR, et al. : Next-generation sequencing-based multi-gene mutation profiling of solid tumors using fine needle aspiration samples: Promises and challenges for routine clinical diagnostics. Mod Pathol 27:314-327, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Singh RR, Patel KP, Routbort MJ, et al. : Clinical validation of a next-generation sequencing screen for mutational hotspots in 46 cancer-related genes. J Mol Diagn 15:607-622, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Routbort M, Handal B, Patel KP, et al. : OncoSeek-A versatile annotation and reporting system for next generation sequencing-based clinical mutation analysis of cancer specimens. J Mol Diagn:747, 2012 [Google Scholar]
- 15.Subbiah V, Kreitman RJ, Wainberg ZA, et al. : Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol 36:7-13, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen TY, Lorch JH, Wong KS, Barletta JA: Histological features of BRAF V600E-mutant anaplastic thyroid carcinoma. Histopathology 77:314-320, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Oishi N, Kondo T, Ebina A, et al. : Molecular alterations of coexisting thyroid papillary carcinoma and anaplastic carcinoma: Identification of TERT mutation as an independent risk factor for transformation. Mod Pathol 30:1527-1537, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Takano T, Ito Y, Hirokawa M, et al. : BRAFV600E mutation in anaplastic thyroid carcinomas and their accompanying differentiated carcinomas. Br J Cancer 96:1549-1553, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong W, Nicolson NG, Choi J, et al. : Clonal evolution analysis of paired anaplastic and well-differentiated thyroid carcinomas reveals shared common ancestor. Gene Chromosomes Cancer 57:645-652, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Wiseman SM, Loree TR, Hicks WL Jr, et al. : Anaplastic thyroid cancer evolved from papillary carcinoma: Demonstration of anaplastic transformation by means of the inter–simple sequence repeat polymerase chain reaction. Arch Otolaryngol Head Neck Surg 129:96-100, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Begum S, Rosenbaum E, Henrique R, et al. : BRAF mutations in anaplastic thyroid carcinoma: Implications for tumor origin, diagnosis and treatment. Mod Pathol 17:1359-1363, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Lai W-A, Liu C-Y, Lin S-Y, et al. : Characterization of driver mutations in anaplastic thyroid carcinoma identifies RAS and PIK3CA mutations as negative survival predictors. Cancers (Basel) 12:1973, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eroglu Z, Ribas A: Combination therapy with BRAF and MEK inhibitors for melanoma: Latest evidence and place in therapy. Ther Adv Med Oncol 8:48-56, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabanillas ME, Dadu R, Iyer P, et al. : Acquired secondary RAS mutation in BRAFV600E-mutated thyroid cancer patients treated with BRAF inhibitors. Thyroid 30:1288-1296, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McFadden DG, Vernon A, Santiago PM, et al. : p53 constrains progression to anaplastic thyroid carcinoma in a Braf-mutant mouse model of papillary thyroid cancer. Proc Natl Acad Sci USA 111:E1600-E1609, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knauf JA, Luckett KA, Chen K-Y, et al. : Hgf/Met activation mediates resistance to BRAF inhibition in murine anaplastic thyroid cancers. J Clin Invest 128:4086-4097, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capdevila J, Wirth LJ, Ernst T, et al. : PD-1 blockade in anaplastic thyroid carcinoma. J Clin Oncol 38:2620-2627, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budczies J, Allgäuer M, Litchfield K, et al. : Optimizing panel-based tumor mutational burden (TMB) measurement. Ann Oncol 30:1496-1506, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Pasternak JD, Angell TE, Lorch J: Nihilism No more: The 2021 ATA anaplastic thyroid cancer guidelines. Clin Thyroidology 33:229-233, 2021 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting findings from this study are available from the corresponding authors upon request.