Abstract
Background:
Studying youth at high risk of developing bipolar disorder may clarify neurobiological factors associated with vulnerability to this illness. We present here a baseline characterization of brain structure in youth at-risk for bipolar disorder.
Methods:
Magnetic resonance images were obtained from 115 child and adolescent offspring of bipolar disorder type I subjects and 57 healthy child and adolescent offspring of healthy parents (healthy offspring). Offspring of parents with bipolar disorder were divided into healthy bipolar offspring (n=47) or symptomatic bipolar offspring (n=68), according to presence or absence of childhood-onset psychopathology. All bipolar offspring were free of major mood and psychotic disorders. Gray (GM) and white matter (WM) volumes were compared between groups using voxel-based morphometry.
Results:
No differences in GM volumes were found across groups. Healthy bipolar offspring presented with decreased WM volumes in areas of the right frontal, temporal and parietal lobes, and in the left temporal and parietal lobes compared to healthy control offspring. Symptomatic bipolar offspring did not present with any differences in WM volumes compared to either healthy bipolar offspring or healthy control offspring.
Limitations:
Cross-sectional design and heterogeneous sample of symptomatic bipolar offspring
Conclusions:
WM volume decreases in areas of the frontal, occipital, and parietal lobes are present in bipolar offspring prior to the development of any psychiatric symptoms, and may be a correlate of familial risk to bipolar disorder. In this large cohort, we have not found evidence for regional GM volume abnormalities as an endophenotype for bipolar disorder.
Keywords: bipolar disorder, magnetic resonance imaging, at-risk, endophenotypes, cerebral cortex
1. Introduction
Bipolar disorder is highly heritable. Children of parents with bipolar disorder are at increased risk of developing mood disorders in general, and bipolar disorder in particular (DelBello & Geller, 2001; Birmaher et al., 2009; Gottesman et al., 2010; Duffy et al., 2014). When both parents have bipolar disorder, the risk of developing this illness among their offspring more than doubles when compared with the risk in offspring of one affected parent (Birmaher et al., 2009; Gottesman et al., 2010). The first symptoms of bipolar disorder often emerge during adolescence (DelBello & Geller, 2001; Birmaher et al., 2009; Gottesman et al., 2010; Duffy et al., 2014; Reichart et al., 2005). Prospective and retrospective studies suggest that early psychopathology, particularly childhood anxiety disorders, mood symptoms, and externalizing disorders, predict later development of major mood disorders in offpspring of bipolar parents (Dyffy et al., 2014; Henin et al., 2005; Goldstein et al., 2010; Nurnberger et al., 2011; Duffy et al., 2013). The neurobiological mechanisms of disease associated with the development and progression of these early symptoms – prior to a syndromic mood episode – in youth at-risk for bipolar disorder are unknown. Thus, studies of adolescents and young adults at familial risk for developing bipolar disorder may identify neurobiological correlates of familial risk and of early psychopathology, which could inform both early detection and intervention strategies (DelBello & Geller, 2001).
Bipolar disorder is characterized by dysfunction of affect regulation and attentional processes (Goodwin & Jamison, 2007). Structural, neurochemical, and functional abnormalities within emotional control networks that include the ventrolateral and ventromedial prefrontal cortices, thalamus, amygdala, and striatum might underlie the dysfunctional affect and cognitive processes seen in bipolar disorder (Strakowski et al., 2005; Strakowski et al., 2012). Prior to developing mood disorders, youth bipolar offspring present with difficulties in tasks of executive functioning, memory, and attention, suggesting that a pre-existent fronto-limbic dysfunction may contribute to the development of mood disorders in those youth at familial risk (Schneider et al., 2012; Meyer et al., 2004; Gotlib et al., 2005; Ladouceur et al., 2013).
Imaging studies of neuroanatomical substrates that could reflect these observed functional abnormalities in bipolar offspring have yielded inconclusive results (Schneider et al., 2012; Nery et al., 2013). Several studies using either region-of-interest or voxelwise approaches found no structural gray matter (GM) abnormalities in youth bipolar offspring (Hajek et al., 2008a; Hajek et al., 2008b; Singh et al., 2008; Hajek et al., 2009; Hajek et al., 2010; Takahashi et al., 2010; Sunagryes et al., 2015). Relatively few studies found increased GM volumes in right inferior frontal gyrus (Hajek et al., 2013), left parahippocampal gyrus (Ladouceur et al., 2008), and amygdala (Bauer et al., 2014) in youth bipolar offspring compared with offspring of healthy parents. Other controlled studies found decreased GM volumes in the right orbitofrontal, middle frontal, bilateral superior, and middle temporal cortices (Hanford et al., 2016a), and decreased cortical thinning in portions of the temporal and middle frontal regions (Hanford et al., 2016b) in youth bipolar offspring. In contrast, findings of WM abnormalities in either adults or youth at-risk for bipolar disorder have been more consistent (Kieseppa et al., 2003; McDonald et al., 2004; Matsuo et al., 2012; Hulshoff Pol et al., 2012; Sprooten et al., 2011; Sprooten et al., 2013; Skudlarski et al., 2013; Mahon et al., 2013; Frazier et al., 2007; Roybal et al., 2015; Teixeira et al., 2014; Versace et al., 2010). Specifically in youth, abnormal fractional anisotropy, a measure of WM integrity, in the bilateral longitudinal fasciculi (Frazier et al., 2007), cingulum, cingulate, corpus callosum, and superior and inferior fasciculi (Roybal et al., 2015) has been described in bipolar offspring compared with youth with healthy parents. To the best of our knowledge, no study has investigated WM volumes in youth bipolar offspring.
The relative preponderance of findings of WM abnormalities versus GM abnormalities in bipolar offspring is still difficult to interpret. It might reflect several different factors such as choice of imaging techniques, sample size and statistical power, variability in age and brain development between adult versus child offspring, as well as degrees of comorbid psychopathology among the study populations. An alternative hypothesis is that in bipolar disorder, WM abnormalities prior to illness onset reflect genetic risk, given the consistency of findings among bipolar offspring; in contrast, as GM changes are less frequently reported prior to illness onset, they may represent effects of emerging psychopathology, illness burden, or medication exposure (Schneider et al., 2012; Nery et al., 2013). Recent evidence suggest that even unaffected bipolar offspring present with GM abnormalities that might be markers of illness vulnerability (Bauer et al., 2014; Hanford et al., 2016b). Therefore, a study that examines brain structural differences in adolescent bipolar offspring attempting to differentiate effects of familial risk versus effects of early psychopathology might help to further investigate this assumption.
With these considerations in mind, we examined GM and WM volumes in a large sample of children and adolescents at-risk of developing bipolar disorder. We sought to investigate the effects of familial risk and psychopathology in a cohort that was large enough to facilitate within-group comparisons. We hypothesized that: 1) GM volume abnormalities in ventral prefrontal emotional control networks would be associated with early psychopathology in youth at increased familial risk for bipolar disorder; and 2) WM volume abnormalities in ventral prefrontal emotional control networks would be associated with increased familial risk for bipolar disorder.
2. Methods
This report is a baseline analysis of an ongoing prospective study to characterize neurodevelopmental changes in children and adolescents at-risk for bipolar disorder. The sample was comprised of offspring of bipolar disorder parents (“bipolar offspring,” n=115), and offspring of healthy parents (“healthy control offspring,” n=57). Participants were recruited from the local community through posted flyers and word of mouth. After study procedures were explained, participants (if > 18 years old), or parents or legal guardians gave written informed consent and youth gave written assent (if < 18 years old) to participate in the study. The University of Cincinnati Institutional Review Board approved all procedures related to this study.
Inclusion criteria for bipolar offspring were ages between 12 and 20 years, having at least one biological parent with bipolar disorder type I, and no DSM-IV-TR major mood or psychotic disorder in themselves (including bipolar disorder types I, or II, cyclothymic disorder, dysthymia, major depressive disorder, schizophrenia, schizoaffective disorder or psychotic disorder not otherwise specified). Inclusion criteria for healthy offspring were age between 12 and 20 years, no personal history of any Axis I psychiatric disorder, and no first-degree relatives with any history of mood or psychotic disorders. Exclusion criteria for both groups were any history of alcohol or drug dependence, or any alcohol or drug abuse within the previous 3 months, any medical or neurological disorder that could influence results, IQ lower than 70, any contraindication to MRI scan (e.g., braces, metallic implants), and any history of head trauma with loss of consciousness more than 10 minutes. Subjects had to be medication-free at time of participation in the study, with the exception of stimulant treatment for ADHD. Subjects on stimulants were asked to hold on taking those medications for 48 hours before the scan.
In offspring, psychiatric diagnoses were determined using the Kiddie-Schedule for Affective Disorders and Schizophrenia Lifetime Version (K-SADS-PL) (Kaufman et al., 1997) and the Mood Disorders section of the Washington University at St. Louis Kiddie-Schedule for Affective Disorders and Schizophrenia WASH-U KSADS (Geller et al., 2001). The K-SADS-PL is a semi-structured interview that integrates parent and child information to obtain current and lifetime childhood-onset psychiatric diagnoses according to DSM-IV, and has good to excellent concurrent validity and inter-rater reliability (Kaufman et al., 1997). The WASH-U KSADS Mood Disorders section is a module developed to expand the examination of onset and offset of mood episodes, and has good reliability (Geller et al., 2001). In parents, psychiatric diagnoses were determined using the Structured Clinical Interview for Diagnosis (SCID) (DSM-IV) (First et al., 2002). The SCID is a structured modular interview to diagnose current and lifetime psychiatric diagnoses according to DSM-IV criteria (First et al., 2002). A Masters-level trained clinician or board-certified psychiatrist performed these diagnostic interviews. Diagnoses were validated in best estimate meetings attended by at least three board-certified psychiatrists or child psychiatrists or psychologists. Current mood symptoms were evaluated using the Hamilton Depression Rating Scale (HAMD) 17-items for depressive symptoms (Hamilton, 1976) and the Young Mania Rating Scale (YMRS) for manic symptoms (Young et al., 1978). The HAMD-17 is a widely used scale to rate the severity of depressive symptoms occurring in the 7 days prior to the interview. It has been shown to have adequate validity and reliability in the age range of our sample (Patel et al., 2006). The YMRS is a widely used rating scale to assess presence of manic symptoms in the 7 days prior to the interview, and has been shown to valid and reliable for use in child and adolescent samples (Youngstrom et al., 2002).
We divided the bipolar offspring in two subgroups according to the presence or absence of lifetime psychopathology (hereafter called symptomatic bipolar offspring (n=68) and healthy bipolar offspring (n=47), respectively). The symptomatic bipolar offspring included youth with definite psychiatric disorders and/or with subthreshold psychiatric symptoms with obvious impairment from those symptoms, and the healthy bipolar offspring included youth with no psychiatric symptoms or with few symptoms and no impairment from those symptoms. The majority of subjects that met criteria for lifetime psychopathology also met criteria for current psychopathology, except 2 subjects with depressive disorder NOS, and 1 subject with generalized anxiety disorder.
Image acquisition
T1-weighted MRI scans were obtained using a 4-Tesla MRI Varian whole-body scanner (Varian, Inc., Palo Alto, CA). During each scan, subjects were recumbent in the bed of the scanner and a volume TEM (Transverse Electromagnetic) head coil was placed over the subject’s head. Earplugs and headphones were provided to block background noise, and precautions were taken to minimize subject motion during the MRI study. Following a threeplane gradient echo scan for alignment and localization, an automatic shim procedure was performed using FASTMAP (Fast Automatic Shimming Technique by Mapping Along Projections) to generate a homogeneous magnetic field. Using a Modified Driven Equilibrium Fourier Transform (MDEFT) pulse sequence, a high resolution T1-weighted 3-dimensional brain scan was obtained. This image was acquired in the axial orientation with repetition time (TR) 13.0 ms, echo time (TE) 5.3 ms, magnetization preparation time (TMD) 1.1 ms, field of view (FOV) 256×192×192 mm3, data matrix=256×192×96.
Image processing
Image processing was performed using statistical parametrical mapping software (SPM8, Wellcome Department of Cognitive Neurology, University College London, UK) running in MATLAB (Math Works, Natick, MA, USA). Using the VBM8 toolbox, these images were first aligned to template space and then segmented. With regard to the segmentation procedure, residual image inhomogeneities were removed by modeling smoothly varying intensity changes (Ashburner & Friston, 2000). This procedure involves estimating an intensity non-uniformity field, which is then applied to yield a smoothed image, and has been shown to increase the reproducibility of SPM-segmentation results (Chard et al., 2002). The native-space GM and WM images were then normalized to the corresponding SPM8 tissue-probability map. Spatial normalization was achieved using an initial 12parameter affine transformation, followed by 12 nonlinear iterations using 7×8×7 discrete cosine transform basis functions (Ashburner & Friston, 1999). Images were resampled in 1.5×1.5×1.5 mm3 resolution. Images were modulated by the Jacobian determinant of the normalization matrix, resulting in images that take into account global and local volume changes during spatial normalization as previously described (Good et al., 2001). Final images were smoothed using a Gaussian kernel with a full width at half-maximum of 8 mm to create a local weighted average of the surrounding pixels. All results were reported as MNI\coordinates.
Statistical analysis
Differences in demographic and clinical characteristics among groups were analyzed using analysis of variance models, chi-square tests or Fisher’s exact tests. All statistical analysis of demographic and clinical characteristics, and bivariate correlations were performed using IBM SPSS (IBM Corp., Armony, NY) version 23, and were two-sided with a significance level of 0.05.
Imaging data analysis
We performed 2 primary analyses comparing GM and WM volumes across the 3 groups (healthy bipolar offspring, symptomatic bipolar offspring, and healthy control offspring). We used SPM8 full factorial design to perform these analyses. We set absolute threshold masking at 0.1, and the analyses were adjusted for individual brain volume during DARTEL segmentation/normalization step. Age, sex, and IQ were included as nuisance variables, as they have been associated with GM and WM changes, particularly in the age range of our sample (Good et al., 2001; Reiss et al., 1996; Ball et al., 2012). Following the 3-group contrast with significant differences across groups, we performed pairwise comparisons between groups to further explore nature of difference between groups. The statistical threshold was set at a level of p<0.05, false discovery rate (FDR) corrected, with a minimum cluster size of 35 contiguous voxels for both primary and pairwise comparisons. We used MarsBar in SPM8 ToolBox to extract raw WM volumes of points of maximal association for visual inspection of differences in the 3-group analyses. We performed post hoc analyses to investigate effect of age of onset of symptoms by dividing the sample in younger than 13 years old and equal or older than 13 years old. We chose 13 as an age cutoff given the distribution of our sample (mean age=13.8, median=13.2, mode=13 years old), and evidence from literature showing age of first mood symptoms in bipolar offspring during adolescence (DelBello & Geller, 2001; Duffy et al., 2014).
3. Results
Demographic and clinical information for the two groups of bipolar offspring and healthy control offspring are displayed in Table 1. The symptomatic bipolar offspring presented, as expected, with different forms of childhood psychopathology. Of those, fifty-six (82%) presented with at least one psychiatric diagnosis (details in Table 1), and twelve (18%) presented with symptoms that were below threshold to meet criteria for the disorder, but with significant impairment.
Table 1:
Demographic and clinical characteristics of the sample, divided by presence or absence of psychiatric disorders
| Characteristics | Healthy bipolar offspring (n=47) | Symptomatic bipolar offspring (n=68) | Healthy control offspring (n=57) | P value |
|---|---|---|---|---|
| Age, mean (SD) | 14.2 (3.0) | 13.2 (2.5) | 14.1 (3.0) | 0.09 |
| Sex, female | 30 (64%) | 32 (47%) | 31 (54%) | 0.21 |
| Race | 0.73 | |||
| Caucasian | 34 (72%) | 51 (75%) | 38 (67%) | |
| African-American | 10 (20%) | 13 (19%) | 12 (21%) | |
| Other | 3 (6%) | 4 (6%) | 7 (13%) | |
| Tanner stage, growth, mean (SD) | 3.4 (1.1) | 3.2 (1.1) | 3.3 (1.3) | 0.79 |
| Tanner stage, pubic, mean (SD) | 3.4 (1.2) | 3.2 (1.1) | 3.2 (1.3) | 0.69 |
| Handedness, right-handed | 41 (87%) | 65 (96%) | 50 (88%) | 0.20 |
| IQ, WASI, mean (SD) | 102.2 (11.5) | 102.2 (12.7) | 109.7 (13.5) | 0.002* |
| HAMD, mean (SD) | 4.1 (5.2) | 9.8 (6.2) | 1.0 (1.2) | <0.001 * |
| YMRS, mean (SD) | 4.0 (3.6) | 9.3 (6.3) | 1.7 (1.9) | <0.001 * |
| Psychiatric diagnoses | ||||
| Subthreshold symptoms | 0 | 12 (17.6) | n/a | |
| Attention-deficit/hyperactivity disorder | 0 | 35 (51.5) | n/a | |
| Depressive disorder NOS | 0 | 15 (22.1) | n/a | |
| Generalized anxiety disorder | 0 | 6 (8.8) | n/a | |
| Enuresis | 0 | 6 (8.8) | n/a | |
| Oppositional-defiant disorder | 0 | 5 (7.4) | n/a | |
| Specific phobia | 0 | 3 (4.4) | n/a | |
| Encopresis | 0 | 3 (4.4) | n/a | |
| Post-traumatic stress disorder | 0 | 3 (4.4) | n/a | |
| Separation anxiety | 0 | 3 (4.4) | n/a | |
| Conduct disorder | 0 | 2 (2.9) | n/a | |
| Cannabis use disorder (past) | 0 | 2 (2.9) | n/a | |
| Tourette syndrome | 0 | 1 (1.5) | n/a | |
| Bipolar disorder NOS | 0 | 1 (1.5) | n/a |
Mean (SD) or n (%) shown
ANOVA with post hoc t-tests for continuous variables
Pairwise comparisons:
Healthy bipolar offspring vs Symptomatic bipolar offspring: IQ: p=1.0; HAMD: p<0.001; YMRS: p<0.001
Healthy bipolar offspring vs Healthy control offspring: IQ: p=0.009; HAMD: p=0.004; YMRS: p=0.04
Symptomatic bipolar offspring vs Healthy control offspring: IQ: p=0.004; HAMD: p<0.001; YMRS: p<0.001
Twenty (29.4%) of symptomatic bipolar offspring were on stimulants prior to entering the study. Those were offspring with diagnoses of attention-deficit/hyperactivity disorder.
Twenty (29.4%) reported having been previously on some psychiatric medication before study entry (13 on stimulants, 5 on antidepressants, 2 atypical antipsychotics).
Abbreviations: IQ (intelligence quotient), HAMD (Hamilton Depression Rating Scale), YMRS (Young Mania Rating Scale)
Gray matter volumes across bipolar offspring with and without psychopathology and healthy control offspring
There was no group effect on GM volumes contrasted across healthy bipolar offspring, symptomatic bipolar offspring, and healthy control offspring.
White matter volumes across bipolar offspring with and without psychopathology and healthy control offspring
Regional WM volumes contrasted across healthy bipolar offspring, symptomatic bipolar offspring, and the healthy control offspring revealed a between-group effect in the right posterior frontal lobe (F=11.2, df=2,166, p=0.05, FDR-corrected, k=101, x=14, y=−27, z=55) (Figure 1). Raw volumes extracted from this cluster using MarsBar showed that healthy bipolar offspring presented with the smallest raw volumes, symptomatic bipolar offspring presented with intermediate mean raw volumes and healthy control offspring presented with the largest WM volumes (Figure 2).
Figure 1: White matter volume differences across healthy bipolar offspring, symptomatic bipolar offspring, and healthy control offspring.
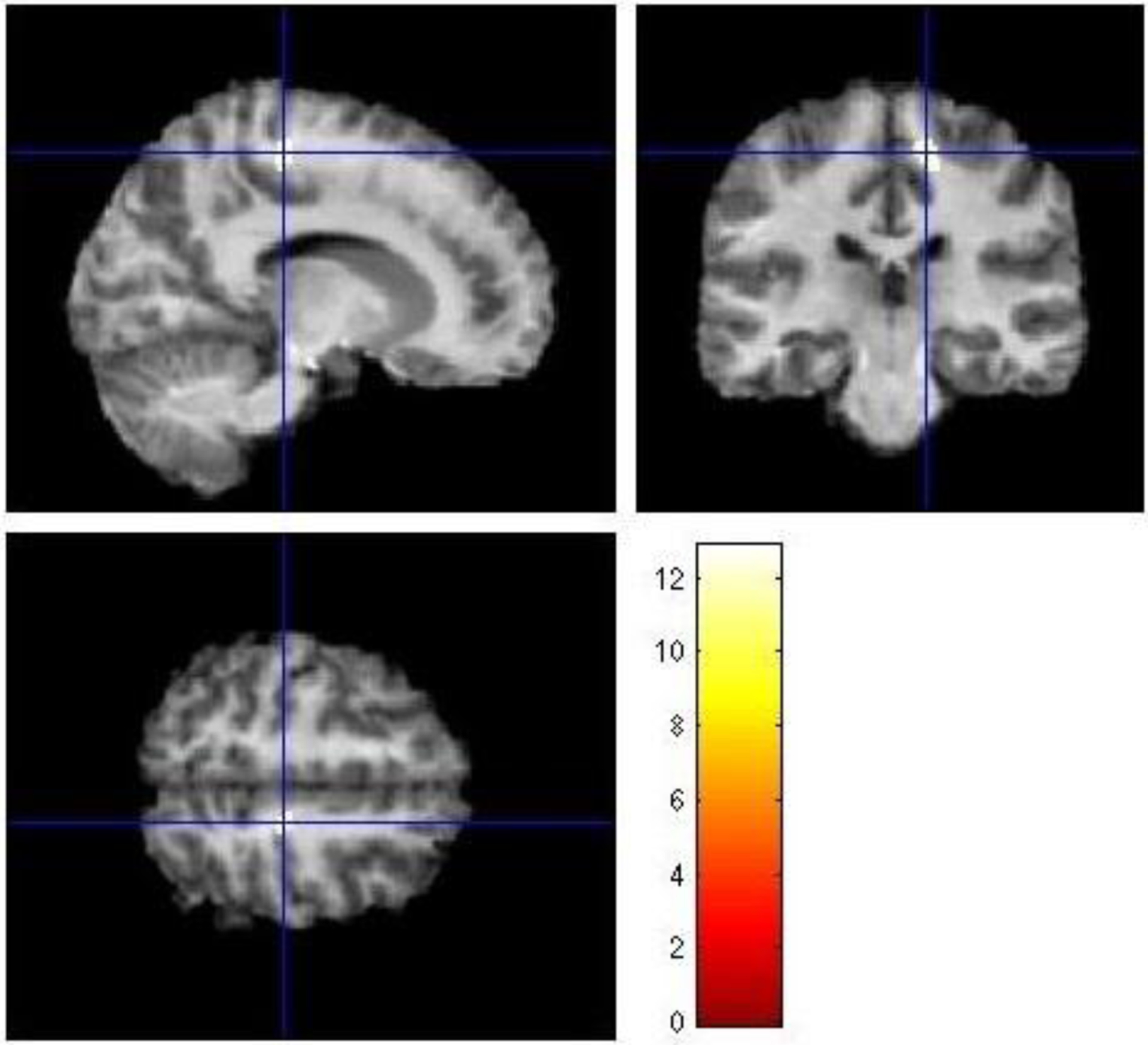
The figure depicts axial, coronal, and sagittal views of significant differences in white matter volumes across groups of healthy bipolar offspring, symptomatic bipolar offspring, and healthy control offspring. The figure depicts a cluster of significant difference in the posterior right frontal lobe (F=11.2, df=2,166, p=0.05, FDR-corrected, k=101, x=14, y=−27, z=55, right paracentral lobule).
Figure 2: While matter volumes in right posterior frontal lobe (paracentral lobule) for healthy bipolar offspring, symptomatic bipolar offspring, and healthy control offspring.
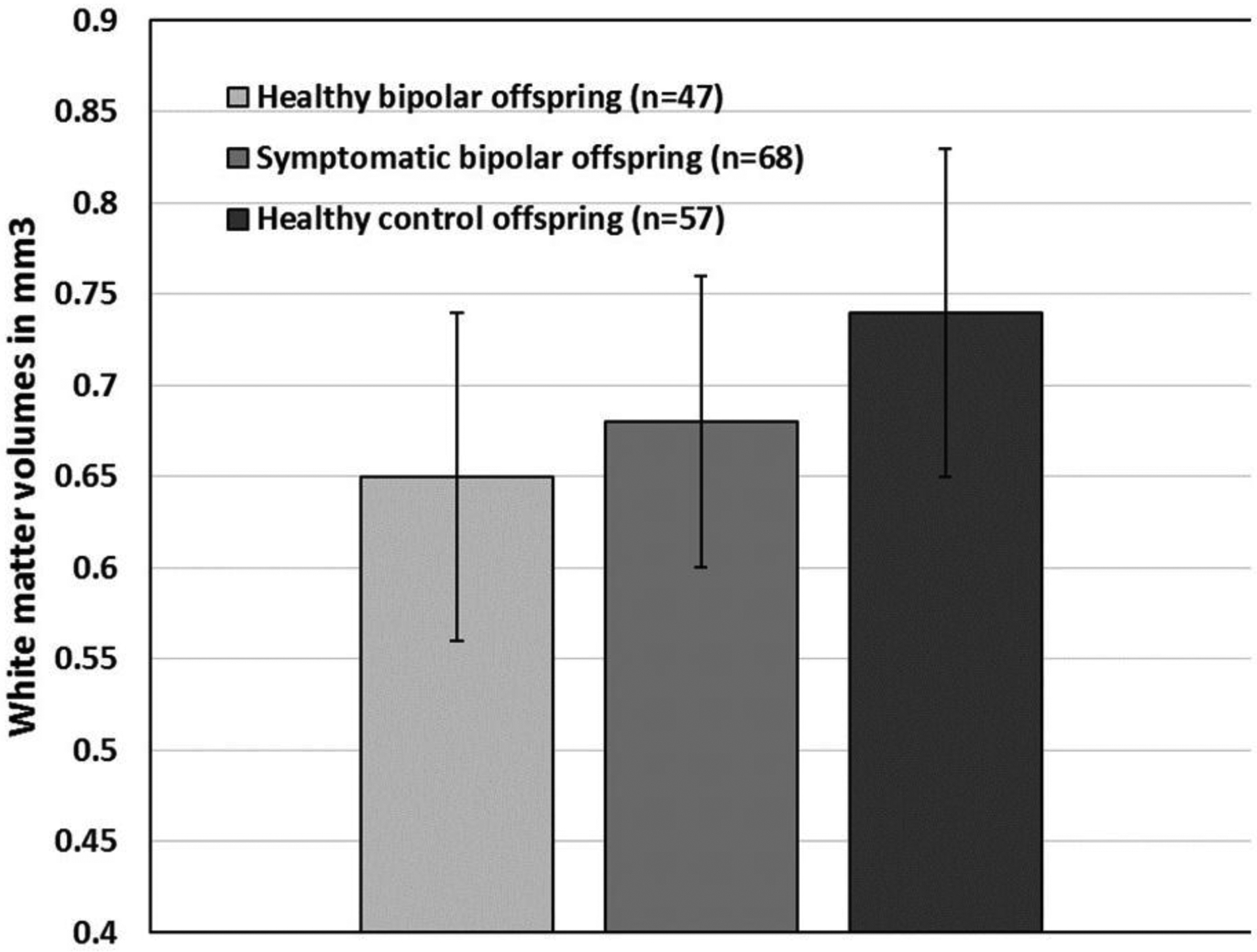
Mean raw white matter volumes were extracted by MarsBar using SPM8 ToolBox from the peak of maximum association in the 3-group analysis comparing white matter volumes across healthy bipolar offspring, symptomatic bipolar offspring, and healthy control offspring.
Pairwise comparisons showed that the healthy bipolar offspring presented with decreased WM volumes in the posterior right frontal lobe (paracentral lobule, cluster size=4244, Z=4.85, p=0.007, FDR-corrected, x=14, y=−27, z=55), left precuneus (cluster size=1106, Z=4.4, p=0.008, FDR-corrected, x=−11, y=−34, z=51), left occipital lobe (lingual gyrus, cluster size=94, Z=3.8, p=0.02, FDR-corrected, x=−14, y=−51, z=1), left parietal lobe (supramarginal gyrus, cluster size=39, Z=3.7, p=0.02, FDR-corrected, x=−48, y=−48, z=40), right posterior cingulate (cluster size=124, Z=3.7, p=0.03, FDR-corrected, x=20, y=−64, z=18), right temporal lobe (subgyral, cluster size=51, Z=3.5, p=0.03, FDR-corrected, x=41, y=−76, z=15), and right parietal lobe (precuneus, cluster size=42, Z=3.4, p=0.04, FDR-corrected, x=21, y=−64, z=39) compared with the healthy control offspring (Figure 3). Symptomatic bipolar offspring did not present with any differences in WM volumes compared with either healthy bipolar offspring or with healthy control offspring. There was no effect of increased WM volumes between groups.
Figure 3: White matter volume differences in healthy bipolar offspring compared with healthy control offspring.
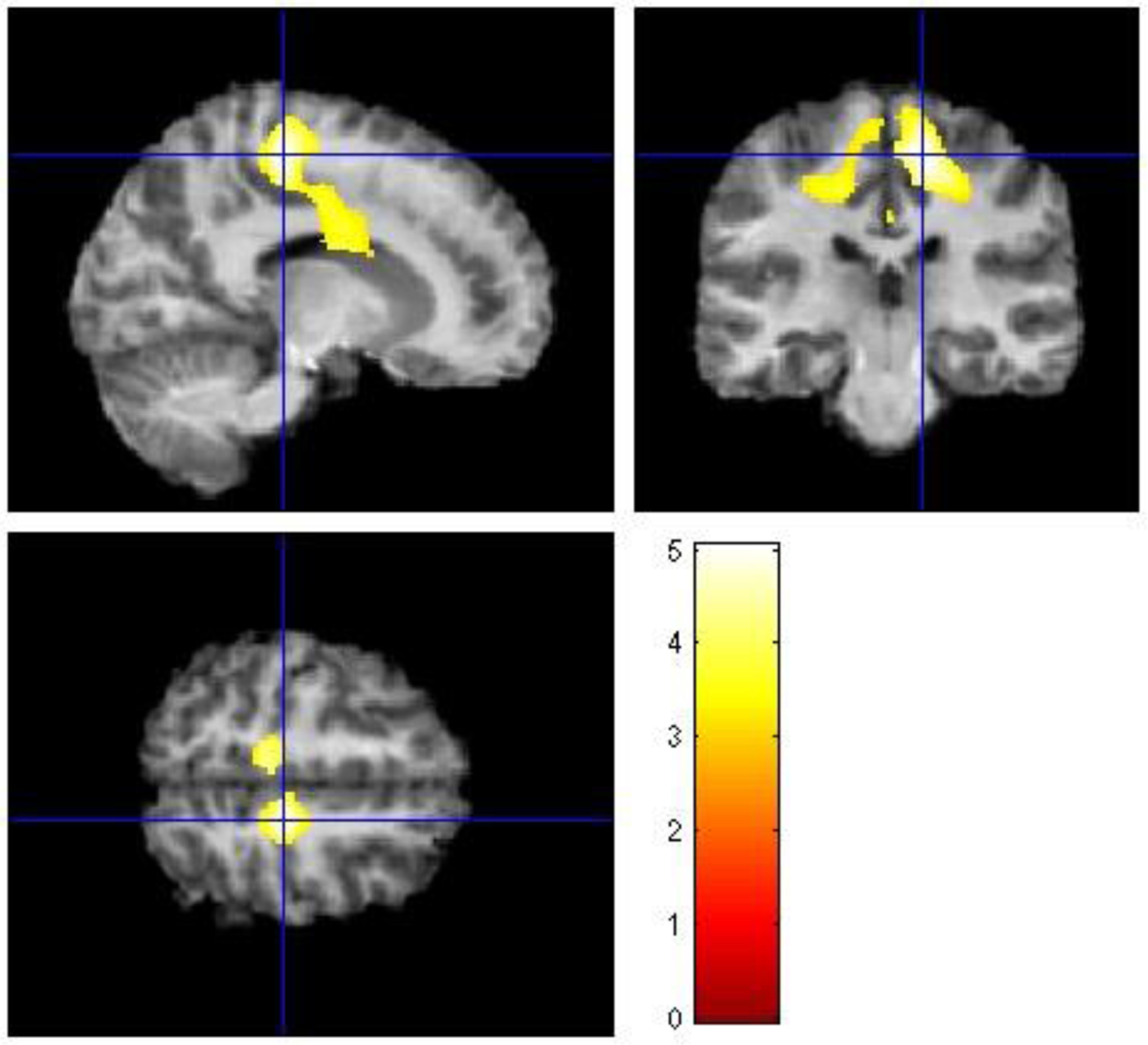
The figure depicts axial, coronal, and sagittal views of significant differences in white matter volumes between healthy bipolar offspring and healthy control offspring encompassing right frontal lobe, and right and left parietal lobes. Healthy bipolar offspring presented with decreased white matter volumes than healthy control offspring. The level of statistically significant differences in white matter volumes between groups was defined as p<0.05, FDR-corrected, with a cluster size of >35.
Post hoc analyses to explore neurodevelopmental effects on WM volumes showed that for the subjects younger than 13 years old (n=68), which included healthy bipolar offspring (n=15), symptomatic bipolar offspring (n=30), and healthy control offspring (n=23) there was no differences in WM volumes across the 3 groups. For the subjects with or older than 13 years old (n=104), which included healthy bipolar offspring (n=32), symptomatic bipolar offspring (n=38), and healthy control offspring (n=34), there were no differences in WM volumes across the 3 groups. There were also no differences in GM volumes across groups for both younger and older than 13 years old subgroups.
Post hoc analyses to investigate confounding effects of comorbid ADHD in WM volumes showed that, after excluding subjects with ADHD from the symptomatic bipolar offspring group, there was no difference in WM volumes across the 3 groups in the 3-group factorial design. A pairwise comparison using that design matrix showed similar findings of decreased WM volumes in healthy bipolar offspring compared with healthy control offspring, and no differences between symptomatic bipolar offspring and healthy control offspring.
4. Discussion
In this study, we partially confirmed our hypotheses that WM volume abnormalities are associated with increased familial risk for bipolar disorder by finding decreased WM volumes in posterior areas of the right frontal lobe and cingulate cortex, and right temporal lobe, and also in left temporal, parietal, and occipital lobes in healthy bipolar offspring compared to healthy control offspring). Again, partially in contrast to our expectations, we did not find evidence for abnormal regional GM volumes in bipolar offspring, either as an effect of familial risk or as an effect of combined familial risk and early psychopathology.
Our finding of decreased WM volumes in adolescent bipolar offspring compared with healthy control offspring are partially consistent with prior findings of decreased WM volumes in adults at-risk for bipolar disorder compared with healthy subjects (Kieseppa et al., 2003; McDonald et al., 2004; Matsuo et al., 2012; Hulshoff Pol et al., 2012). For instance, increased genetic liability for bipolar disorder was associated with decreased global (Hulshoff Pol et al., 2012) or regional WM volumes in corpus callosum, bilateral frontal, left temporal - parietal regions, and right parietal regions (McDonald et al., 2004) of adult relatives of patients with bipolar disorder. Decreased WM volumes have been reported in the right medial frontal lobe of unaffected relatives (Matsuo et al., 2012) and in the left hemisphere of unaffected twins of bipolar disorder patients (Hulshoff Pol et al., 2012). Abnormal WM integrity has also been reported by several diffusion tensor imaging (DTI) studies in adults (Sprooten et al., 2011; Sprooten et al., 2013; Skudlarski et al., 2013; Mahon et al., 2013; Chaddock et al., 2009) or in children at-risk for bipolar disorder (Frazier et al., 2007; Roybal et al., 2015; Versace et al., 2010). Specifically, in children at-risk, DTI studies showed decreased fractional anisotropy in posterior areas of the superior longitudinal fasciculi (Frazier et al., 2007), but also increased fractional anisotropy in bilateral cingulum and superior longitudinal fasciculi when compared with healthy children (Roybal et al., 2015).
Contrary to our hypothesis, we did not find structural abnormalities in ventral areas of the prefrontal cortex that pertain to emotional control networks (Strakowski et al., 2012). In contrast, we found WM volume decreases mostly in posterior areas of the right frontal lobe, in the dorsal region of the right anterior cingulate, and in the right and left parietal lobe. The cortical areas adjacent to the WM decreases are involved in assessment of emotional valence to internal and external stimuli, motivation, emotion processing, regulation of autonomic function (Devinsky et al., 1995; Martinez-Aran et al., 2000), self-awareness (Lou et al., 2004), and initiation and suppression of motor responses associated with motivation and will (Paus, 2001). These brain areas have bidirectional projections with many key areas of ventral emotional control networks, particularly the amygdala and ventral striatum, with cognitive networks, particularly dorsolateral prefrontal cortex and parietal cortex (Devinsky et al., 1995; Lou et al., 2004; Paus, 2001), and with premotor and motor cortex (Paus, 2001).
In patients with established bipolar disorder, imaging studies have found WM pathology mainly in prefrontal regions or in tracts connecting prefrontal regions to subcortical and temporal subregions (Adler et al., 2006; Brambilla et al., 2009; Vederine et al., 2011; Wise et al., 2016), as opposed to our findings mostly in frontal posterior, or posterior/lateral brain areas in bipolar offspring. From a developmental perspective, brain maturation in the same age range of our sample follows a caudal-rostral direction, with lower-order brain areas maturating before higher-order (such as prefrontal cortex) areas (Gogtay et al., 2004). Thus, if a delay in WM development is present as an effect of familial risk to bipolar disorder, it might be detected first in posterior brain areas (prior to disease installment) and later in anterior/frontal areas (at or after disease installment). Consistent with this, DTI studies in youth bipolar offspring showed aberrant WM in posterior areas of the superior longitudinal fasciculi (Frazier et al., 2007), in WM tracts that extended from frontal to occipital areas (Roybal et al., 2015), and a detrimental effect of age on WM integrity in tracts connecting occipital to temporal lobes (Versace et al., 2008). It should be noted that VBM detection of differences in WM lacks the spatial resolution that DTI has, so VBM and DTI studies cannot be directly compared. Hence, a prospective study using DTI to study WM development in bipolar offspring would be necessary to examine this hypothesis.
The WM volume reductions were present in the contrast between healthy bipolar offspring and healthy control offspring, but were not present in the contrast between symptomatic bipolar offspring and healthy control offspring. Because the healthy bipolar offspring subgroup was medication naïve and free of lifetime psychopathology, these findings suggest that those WM volume reductions are a correlate of familial risk to bipolar disorder rather than correlates of psychopathology or medication effects. It is noteworthy that the symptomatic bipolar offspring, which also are at familial risk for bipolar disorder, did not present with any differences in WM volumes compared with the healthy control offspring. A visual inspection of raw mean volumes from the significant cluster in the 3-group contrast showed that symptomatic bipolar offspring also presented with decreased WM volumes compared to healthy control offspring, and those values were intermediate between healthy bipolar offspring and healthy control offspring (Figure 2). It is possible that insufficient statistical power in the differences between symptomatic bipolar offspring and healthy control offspring explain these negative findings.
We did not find that familial risk for bipolar disorder per se is associated with regional GM volume reductions. Findings of decreased GM volumes in individuals at-risk for bipolar disorder have been inconsistent and difficult to replicate (Nery et al., 2013, Nery et al., 2015), and have been mostly negative in youth at-risk (Hajek et al., 2008a; Hajek et al., 2008b; Singh et al., 2008; Hajek et al., 2010; Takahashi et al., 2010; Sugranyes et al., 2015). The most important difference between our study and similar voxel-based morphometry studies in youth bipolar offspring (Ladouceur et al., 2008; Sugranyes et al., 2015; Hanford et al., 2016a) is the sample size in our study (115 bipolar offspring and 57 healthy offspring, which, to date, is the largest brain structure study in youth bipolar offspring). Two recent studies have described associations between psychopathology in youth bipolar offspring and enlarged amygdala (Bauer et al., 2014) and thinner cortex in superior and inferior temporal regions, supramarginal, and middle frontal regions (Hanford et al., 2016b). In common, both used a different software to analyze GM differences, which might be more sensitive to detect subtle differences in smaller subcortical structures, such as amygdala, or in cortical thickness (Hutton et al., 2009). Although possible, it is unlikely that the lack of differences in GM volumes between the bipolar offspring and healthy control offspring groups in our study stem from type II error. Future studies employing methods that are more sensitive to GM changes are necessary to confirm our negative findings.
While voxel-based morphometry analyses allow for investigating global differences in GM or WM abnormalities between groups, it does not offer a direct measure of brain tissue volumes. It is unknown to what extent the differences in the so-called volumes obtained in voxel-based morphometry analyses reflect actual cytoarchitectural or tissue abnormalities (including changes in number or size of neurons, neuropils, or WM fibers) or reflect several other factors that may affect the MRI signal, such as exercise, hydration, or brain perfusion, among others (Weinberger & Radulescu, 2016). In addition, voxelwise analyses are exploratory in nature, as they allow a whole survey of group differences and the emergence of findings in brain areas that were not pre-specified as a region-of-interest. Notwithstanding these limitations, our findings might serve as a departure point for future studies that investigate the existence of abnormal functional or structural connectivity between anterior cingulate cortex and posterior frontal or parietal regions and their role in the neurobiology of risk and resilience in youth at-risk for bipolar disorder.
Other limitations of this study include the cross-sectional nature of the investigation, which precludes any causal relationship between the “at-risk” condition and WM abnormalities, and consequently, the assumption that changes represent vulnerability or resilience factors. An ideal study to differentiate effects of familial risk versus early psychopathology or effects of vulnerability versus resilience in at-risk children would have to be prospective in nature. Another limitation is the lack of a control group characterized by healthy control offspring who presents with psychiatric symptoms (a symptomatic control offspring). The inclusion of such group would help to further characterize effects of symptomatology versus effects of familial vulnerability. Thus, our results should be considered preliminary. Third, the symptomatic bipolar offspring was a heterogeneous sample of psychiatric diagnoses, including different anxiety disorders, minor depressive disorder, and ADHD. It would be important to study effects of specific early psychopathology (e.g., mood or anxiety diagnoses) on GM or WM volumes of bipolar offspring. However, these diagnoses were often comorbid with each other, and subsamples of “pure” mood diagnoses or anxiety diagnoses had very small sizes, limiting secondary analyses. Future studies with larger samples could examine potential specific effects of mood or anxiety diagnoses in brain structure of bipolar offspring. Third, 29.4% of the symptomatic bipolar offspring sample was treated with stimulant medication, mostly for ADHD. A meta-analysis of age and stimulant medication effects in brain structure of patients with ADHD suggest that there is a potential normalizing effect of stimulant use on right basal ganglia volumes (Nakao et al., 2011). Although this potential normalizing effect is still debatable, as the evidence comes from cross-sectional studies comparing medicated and unmedicated ADHD patients, we cannot completely rule out that chronic stimulant medication exposure among one third of our symptomatic bipolar offspring may have contributed to some normalization of potential GM volume differences between symptomatic bipolar offspring and the other groups. However, repeated analyses excluding those offspring with ADHD (hence previously medicated with stimulants) suggested that “normalizing” medication effects on GM or WM volumes were unlikely, as the exclusion of medicated (hence, “normalized”) subjects would pull the differences away (not toward) the normal comparison sample. It should be noted that the most our most relevant findings were pertaining to WM volumes differences between healthy bipolar offspring and healthy control offspring, a comparison that was not influenced by medication effects.
In conclusion, we found that healthy bipolar offspring present with regional WM volume reductions in posterior areas of the right frontal lobe, right posterior cingulate, and right parietal and temporal lobe, as well as left parietal and occipital lobe when compared with healthy control offspring. These findings of decreased WM volumes are relevant as they might underlie the increased risk for mood disorders in this population. Finally, the presence of childhood-onset psychopathology in youth at high risk for bipolar disorder was not associated with WM or GM volume differences. A prospective examination could verify whether these abnormalities represent susceptibility factors for bipolar disorder, and whether their identification may help to develop preventive strategies for this disabling and severe condition.
Highlights.
Bipolar offspring are at increased risk to developing mood disorders.
We used VBM to characterize brain markers of familial risk in bipolar offspring.
No abnormal gray matter volumes were found in bipolar offspring.
Decrease in white matter (WM) volumes were found in healthy bipolar offspring.
Decreased WM volumes may be a correlate of familial risk to bipolar disorder.
Acknowledgements
This study was partly supported by a NIH grant # 5 P50 MH077138 (Strakowski).
Declaration of Interest
Dr. Fabiano G. Nery held a position of Associate Medical Advisor in Eli Lilly & Co. from 2012 to 2013. His spouse is currently an employee of Eli Lilly & Co. Dr. Jeffrey Strawn has received research support from the National institute of Mental Health, Edgemont Pharmaceuticals, Eli Lilly, Forest Research Laboratories, Lundbeck, Shire and receives material support from Assurex/Genesight. He receives royalties for the publication of 2 texts and has produced a clinical rating training video for Neuronetics. Dr. Caleb Adler has received research support from AstraZeneca, Amylin, Eli Lilly, GlaxoSmithKline, Lundbeck, Martek, Merck, Novartis, Otsuka, Pfizer, Takeda, Forest, Actavis, and Shire. He has been on the lecture bureau for Merck and Sunovion, for which he has received honoraria. Dr. Stephen Strakowski chairs DSMBs for Sunovion and is a consultant to Procter & Gamble. Dr. Melissa P. DelBello has received research support from Amylin, Eli Lilly, Pfizer, Otsuka, GlaxoSmithKline, Merck, Martek, Novartis, Lundbeck, Pfizer, Sunovion, and Shire. She has received Consulting/Advisory Board/Honoraria/Travel support from Pfizer, Lundbeck, Sunovian, Supernus and Otsuka. The remaining authors reported no conflicts of interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adleman NE, Fromm SJ, Razdan V, Kayser R, Dickstein DP, Brotman MA, Pine DS, Leibenluft E, 2012. Cross-sectional and longitudinal abnormalities in brain structure in children with severe mood dysregulation or bipolar disorder. J. Child Psychol. Psychiatry 53, 1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler C, Adams J, DelBello MP, Holland SK, Schmithorst V, Levine A, Jarvis K, Strakowski SM, 2006. Am. J. Psychiatry 163, 322–324. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ, 1999. Nonlinear spatial normalization using basis function. Hum. Brain Mapp 7, 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ, 2000. Voxel-based morphometry – the methods. Neuroimage. 11, 805–821. [DOI] [PubMed] [Google Scholar]
- Ball WS, Byars AW, Schapiro M, Bommer W, Carr A, German A, and the Brain Development Cooperative Group., 2012. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI Study of Normal Brain Development. Cereb. Cortex 22, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer IE, Sanches M, Suchting R, Green CE, El Fangary NM, Zunta-Soares GB, Soares JC, 2014. Amygdala enlargement in unaffected offspring of bipolar parents. J. Psychiatr. Res 59, 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, Obreja M, Ehmann M, Ivengar S, Shamseddeen W, Kupfer D, Brent D, 2009. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch. Gen. Psychiatry 66, 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P, Bellani M, Yeh PH, Soares JC, Tansella M, 2009. White matter connectivity in bipolar disorder. Int. Rev. Psychiatry 21, 380–386. [DOI] [PubMed] [Google Scholar]
- Chaddock CA, Barker GJ, Marshall N, Schuze K, Hall MH, Fern A, Walshe M, Bramon E, Chitnis XA, Murray R, McDonald C, 2009. White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br. J. Psychiatry 194, 527–534. [DOI] [PubMed] [Google Scholar]
- Chard DT, Parker GJ, Griffin CM, Thompson AJ, Miller DH, 2002. The reproducibility and sensitivity of brain tissue volume measurements derived from an SPM-based segmentation methodology. J. Magn. Reson. Imaging 15, 259–267. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Geller B, 2001. Review of studies of child and adolescent offspring of bipolar parents. Bipolar Disord. 3, 325–334 [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell M, Vogt B, 1995. Contributions of anterior cingulate to behavior. Brain. 118, 279–306. [DOI] [PubMed] [Google Scholar]
- Duffy A, Horrocks J, Doucette S, Keown-Stoneman C, McCloskey S, Grof P, 2013. Childhood anxiety: an early predictor of mood disorders in offspring of bipolar parents. J. Affect. Disord 150, 363–369. [DOI] [PubMed] [Google Scholar]
- Duffy A, Horrocks J, Doucette S, Keown-Stoneman C, McCloskey S, Grof P, 2014. The developmental trajectory of bipolar disorder. Br. J. Psychiatry 204, 122–128. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. State Psychiatric Institute, Biometrics Research Department, New York, NY. [Google Scholar]
- Frazier JA, Breeze JL, Papadimitriou G, Kennedy DN, Hodge SM, Moore CM, Howard JD, Rohan MP, Caviness VS, Makris N, 2007. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disord. 9, 799–809. [DOI] [PubMed] [Google Scholar]
- Frodl T, Carballedo A, Fagan AJ, Lisiecka D, Ferguson Y, Meaney JF, 2012. Effects of early-life adversity on white matter diffusivity changes in patients at risk for major depression. J. Psychiatr. Neurosci 37, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinowski A, Miranda R, Lemaitre H, Paillere Martinot ML, Artiges E, Vulser H, and IMAGEN Consortium., 2015. Resilience and corpus callosum microstructure in adolescence. Psychol. Med 45, 2284–2294. [DOI] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, Soutullo C, 2001. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J. Am. Acad. Child Adolesc. Psychiatry 40, 450–455. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Shamseddeen W, Axelson DA, Kalas C, Monk K, Brent DA, Kupfer DJ, Birmaher B, 2010. Clinical, demographic, and familial correlates of bipolar spectrum disorders among offspring of parents with bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry 49, 388–396. [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS, 2001. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 14, 21–36. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR, 2007. Manic-Depressive Illness: Bipolar disorders and recurrent depression, 2nd Ed. Oxford University Press. [Google Scholar]
- Gotlib IH, Traill SK, Montoya RL, Joorman J, Chang K, 2005. Attention and memory biases in the offspring of parents with bipolar disorder: indications from a pilot study. J. Child Psychol. Psychiatry 46, 84–93. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Laursen TM, Bertelsen A, Mortensen PB., 2010. Severe mental disorders in offspring with 2 psychiatrically ill parents. Arch. Gen. Psychiatry 67, 252–257. [DOI] [PubMed] [Google Scholar]
- Hajek T, Gunde E, Bernier D, Slaney C, Propper L, Macqueen G, Duffy A, Alda M, 2008a. Pituitary volumes in relatives of bipolar patients: high risk study. Eur. Arch. Psychiatry Clin. Neurosci 258, 357–362. [DOI] [PubMed] [Google Scholar]
- Hajek T, Gunde E, Bernier D, Slaney C, Propper L, Grof P, Macqueen G, Duffy A, Alda M, 2008b. Subgenual cingulate volumes in affected and unaffected offspring of bipolar parents. J. Affect. Disord 108, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek T, Gunde E, Slaney C, Propper L, Macqueen G, Duffy A, Alda M, 2009. Striatal volumes in affected and unaffected relatives of bipolar patients – high risk study. J. Psychiatr. Res 43, 724–729. [DOI] [PubMed] [Google Scholar]
- Hajek T, Novak T, Kopecek M, Gunde E, Alda M, Hoschl C, 2010. Subgenual cingulate volumes in offspring of bipolar parents and in sporadic bipolar patients. Eur. Arch. Psychiatry Clin. Neurosci 260, 297–304. [DOI] [PubMed] [Google Scholar]
- Hajek T, Cullis J, Novak T, Kopecek M, Blagdon R, Propper L, Stopkova P, Duffy A, Hoschl C, Uher R, Paus T, Young LT, Alda M, 2013. Brain structural signature of familial predisposition for bipolar disorder: replicable evidence for involvement of the right inferior frontal gyrus. Biol. Psychiatry 73, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1976. Hamilton Psychiatric Rating Scale for Depression. In: Guy W. Ed. ECDEU Assessment Manual for Psychopharmacology. Washington DC, US Department of Health, Education and Welfare, 179–192. [Google Scholar]
- Hanford LC, Hall GB, Minuzzi L, Sassi RB, 2016a. Gray matter volumes in symptomatic and asymptomatic offspring of parents diagnosed with bipolar disorder. Eur. Child Adolesc. Psychiatry 25, 959–967. [DOI] [PubMed] [Google Scholar]
- Hanford LC, Sassi RB, Minuzzi L, Hall GB, 2016b. Cortical thickness in symptomatic and asymptomatic bipolar offspring. Psychiatr. Res. Neuroimag 251, 26–33. [DOI] [PubMed] [Google Scholar]
- Henin A, Biederman J, Mick E, Sachs GS, Hirshfeld-Becker DR, Siegel RS, McMurrich S, Grandin L, Nierenberg AA, 2005. Psychopathology in the offspring of parents with bipolar disorder: a controlled study. Biol. Psychiatry 58, 554–561. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, van Baal GC, Schnack HG, Brans RG, van der Schot AC, Brouwer RM, van Haren NE, Lepage C, Collins DL, Evans AC, Boomsma DI, Nolen W, Kahn RS, 2012. Overlapping and segregating structural brain abnormalities in twins with schizophrenia or bipolar disorder. Arch. Gen. Psychiatry 69, 349–359. [DOI] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N, 2009. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 48, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiris N, Pantelis C, Nemoto T, Zaeski A, Hori M, Shimoji K, Saito J, Ito S, Dwyer DB, Fukunaga I, Morita K, Tsujino N, Yamaguchi T, Shiraga N, Aoki S, Mizuno M, 2015. A longitudinal study investigating sub-threshold symptoms and white matter changes in individuals with an ‘at risk mental state’ (ARMS). Schizophr. Res 162, 7–13. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for Affective Disorders and Schizophrenia for School-age Children – Present and Lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, van Erp TG, Haukka J, Partonen T, Cannon TD, Poutanen VP, Kaprio J, Lonnqvist J, 2003. Reduced left hemispheric white matter volume in twins with bipolar I disorder. Biol. Psychiatry 54, 896–905. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Almeida JR, Birmaher B, Axelson DA, Nau S, Kalas C, Monk K, Kupfer DJ, Phillips ML, 2008. Subcortical gray matter volume abnormalities in healthy bipolar offspring: potential neuroanatomical risk marker for bipolar disorder? J. Am. Acad. Child Adolesc. Psychiatry 47, 532–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Diwadkar VA, White R, Bass J, Birmaher B, Axelson DA, Phillips ML, 2013. Fronto-limbic function in unaffected offspring at familial risk for bipolar disorder during an emotional working memory paradigm. Dev. Cogn. Neurosci 5, 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, Sackheim HA, Lisanby SH, 2004. Parietal cortex and representation of the mental self. Proc. Natl. Acad. Sci. USA 101, 6827–6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon K, Burdick KE, Ikuta T, Braga RJ, Gruner P, Malhotra AK, Szeszko PR, 2013. Abnormal temporal lobe white matter as a biomarker for genetic risk of bipolar disorder. Biol. Psychiatry 73, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Colom F, Reinares M, Benabarre A, Gasto C, Salamero M, 2000. Cognitive dysfunctions in bipolar disorder: evidence of neuropsychological disturbances. Psychother. Psychosom 69, 2–18. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Kopecek M, Nicoletti MA, Hatch JP, Watanabe Y, Nery FG, Zunta-Soares G, Soares JC, 2012. New structural brain imaging endophenotype in bipolar disorder. Mol. Psychiatry 17, 412–420. [DOI] [PubMed] [Google Scholar]
- McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, Bramon E, Murray RM, 2004. Association of genetic risk for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch. Gen. Psychiatry 61, 974–984. [DOI] [PubMed] [Google Scholar]
- Meyer SE, Carlson GA, Wiggs EA, Martinez PE, Ronsaville DS, Klimes-Dougan B, Gold PW, Radke-Yarrow M, 2004. A prospective study of the association among impaired executive functioning, childhood attentional problems, and the development of bipolar disorder. Dev. Psychopathol 16, 461–476. [DOI] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D, 2011. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am. J. Psychiatry 168, 1154–1163. [DOI] [PubMed] [Google Scholar]
- Nery FG, Monkul ES, Lafer B, 2013. Gray matter abnormalities as brain structural vulnerabilities factors for bipolar disorder: a review of neuroimaging studies of individuals at high genetic risk for bipolar disorder. Aust. N. Z. J. Psychiatry 47, 1124–1135. [DOI] [PubMed] [Google Scholar]
- Nery FG, Gigante AD, Amaral JA, Fernandes FBF, Berutti M, Carneiro CG, Duran FL, Otaduy MG, Leite CC, Busatto G, Lafer B, 2015. Gray matter volumes in patients with bipolar disorder and their first degree relatives. Psychiatry. Res. Neuroimaging 234, 188–193. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI Jr., McInnis M, Reich W, Kasteic E, Wilcox HC, Glowinski A, Mitchell P, Fisher C, Erpe M, Gershon ES, Berrettini W, Laite G, Schweitzer R, Rhoadarmer K, Coleman VV, Cai X, Azzouz F, Liu H, Kamali M, Brucksch C, Monahan PO, 2011. A high-risk study of bipolar disorder: childhood clinical phenotypes as precursors of major mood disorders. Arch. Gen. Psychiatry 68, 1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NC, DelBello MP, Kowatch RA, Strakowski SM, 2006. Preliminary study of relationships among measures of depressive symptoms in adolescents with bipolar disorder. J. Child. Adolesc. Psychopharmacol 16: 327–335. [DOI] [PubMed] [Google Scholar]
- Paus T, 2001. Primate anterior cingulate córtex: where motor control, drive, and cognition interface. Nat. Rev 2, 417–424. [DOI] [PubMed] [Google Scholar]
- Reichart CG, van der Ende J, Wals M, Hilegers MH, Nolen WA, Ormel J, Verhulst FC, 2005. The use of the GBI as predictor of bipolar disorder in a population of adolescent offspring of parents with a bipolar disorder. J. Affect. Disord 89, 147–155. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB, 1996. Brain development, gender, and IQ in children. A volumetric imaging study. Brain 119, 1763–1774. [DOI] [PubMed] [Google Scholar]
- Roybal DJ, Barnea-Goraly N, Kelley R, Bararpour L, Howe ME, Reiss AL, Chang KD, 2015. Widespread white matter tract aberrations in youth with familial risk for bipolar disorder. Psychiatry Res. 232, 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MR, DelBello MP, McNamara RK, Strakowski SM, Adler CM, 2012. Neuroprogression in bipolar disorder. Bipolar Disord. 14, 356–374. [DOI] [PubMed] [Google Scholar]
- Singh MK, DelBello MP, Adler CM, Stanford KE, Strakowski SM, 2008. Neuroanatomical characterization of child offspring of bipolar parents. J. Am. Acad. Child Adolesc. Psychiatry 47, 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprooten E, Sussmann JE, Clugston A, Peel A, McKirdy J, Moorhead TW, Anderson S, Shand AJ, Giles S, Bastin ME, Hall J, Johnstone EC, Lawrie SM, McIntosh AM, 2011. White matter integrity in individuals at high genetic risk of bipolar disorder. Biol. Psychiatry 70, 350–356. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Schreten DJ, Thaker GK, Stevens MC, Keshavan MS, Sweeney JA, Tamminga CA, Clementz BA, O’Neil K, Pearlson GD, 2013. Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am. J. Psychiatry 170, 886–898. [DOI] [PubMed] [Google Scholar]
- Sprooten E, Brumbaugh MS, Knowles EE, McKay DR, Lewis J, Barrett J, Landau S, Cyr L, Kochunov P, Winkler AM, Pearlson GD, Glahn DC, 2013. Reduced white matter integrity in sibling pairs discordant for bipolar disorder. Am. J. Psychiatry 170, 1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Adler CM, 2005. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol. Psychiatry 10, 105–116. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang D, DelBello MP, Frangou S, McIntosh A, Phillips ML, Sussman JE, Townsend JD, 2012. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 14, 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugranyes G, de la Serna E, Romero S, Sanchez-Gistau V, Calvo A, Moreno D, Baeza I, Diaz-Caneja CM, Sanchez-Gutierrez T, Janssen J, Bargallo N, Castro-Fornielles J, 2015. Gray matter volume decrease distinguishes schizophrenia from bipolar offspring during childhood and adolescence. J. Am. Acad. Child Adolesc. Psychiatry 54, 677–684. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Walterfang M, Wood SJ, Kempton MJ, Jogia J, Lorenzetti V, Soulsby B, Suzuki M, Velakoulis D, Pantelis C, Frangou S, 2010. Pituitary volume in patients with bipolar disorder and their first-degree relatives. J. Affect. Disord 124, 256–261. [DOI] [PubMed] [Google Scholar]
- Teixeira AM, Kleinman A, Zanetti M, Jackowski M, Duran F, Pereira F, Lafer B, Busatto GF, Caetano SC, 2014. Preserved white matter in unmedicated pediatric bipolar disorder. Neurosci. Lett 579, 41–45. [DOI] [PubMed] [Google Scholar]
- Vederini E, Wessa M, Leboyer M, Houenou J, 2011. A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog. Neuropsychopharmacol. Biol Psychiatry 35, 1820–1826. [DOI] [PubMed] [Google Scholar]
- Versace A, Ladouceur CD, Romero S, Birmaher B, Axelson DA, Kupfer DJ, Phillips ML, 2010. Altered development of white matter in youth at high familial risk for bipolar disorder: a diffusion tensor imaging study. J. Am. Acad. Child Adolesc. Psychiatry 49, 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Radulescu E, 2016. Findings the elusive psychiatric “lesion” with 21st century neuroanatomy: a note of caution. Am. J. Psychiatry 173, 27–33. [DOI] [PubMed] [Google Scholar]
- Wise T, Radua J, Nortje G, Cleare AJ, Young AH, Arnone D, 2016. Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol. Psychiatry 79, 293–302. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensibility. Br. J. Psychiatry 133, 429–435. [DOI] [PubMed] [Google Scholar]
- Youngstrom EA, Danielson CK, Findling RL, Gracious BL, Calabrese JR, 2002. Factor structure of the Young Mania Rating Scale for use with youths ages 5 to 17 years. J. Clin. Child Adolesc. Psychol 31, 567–572. [DOI] [PubMed] [Google Scholar]


