Abstract
The MB/BacT system (MB/BacT) with a revised antibiotic supplement kit was compared with the BACTEC 460 system (BACTEC 460) in a test of 488 specimens submitted for mycobacterial culture from 302 patients. Twenty-four Mycobacterium tuberculosis isolates were detected by the BACTEC 460 versus 23 isolates by the MB/BacT. Mean time until detection of M. tuberculosis isolates identified by both systems was 11.9 days for the BACTEC 460 versus 13.7 days for the MB/BacT (P = 0.046). M. avium complex was detected in 12 specimens by the MB/BacT versus 10 specimens by the BACTEC 460. Only 8 of 14 (57%) M. avium isolates were detected by both systems, with a mean time until detection of 10.1 days for the BACTEC 460 and 14.2 days for the MB/BacT (P = 0.009). The BACTEC 460 and the MB/BacT detected M. gordonae in four specimens, but only a single specimen was positive by both systems. One M. fortuitum isolate and one of five M. kansasii isolates were recovered only by the BACTEC 460. The bacterial overgrowth rate was 7.0% for the MB/BacT versus 4.1% for the BACTEC 460. We found the MB/BacT to be comparable to the BACTEC 460 for mycobacterial detection. Even though time until detection with the MB/BacT was slightly longer (1.8 days longer for M. tuberculosis and 4.1 days for M. avium [mean values]) and the bacterial overgrowth rate was somewhat higher, the decreased labor, the availability of a computerized data management system, and the noninvasive, nonradiometric aspects of the MB/BacT offset these relative disadvantages and make it an acceptable alternative for use in the diagnostic laboratory.
About one-third of the world population is infected with Mycobacterium tuberculosis. There are about 8 million new cases of tuberculosis and 1 million deaths every year, mainly in developing nations (13, 14). New cases of tuberculosis in the United States were on a steady 5% per year decline from 1953 until 1985 (16). Subsequently, this trend reversed and there was a 20% increase in annual cases of tuberculosis from 1985 to 1992 (16). In 1993, the Centers for Disease Control and Prevention (CDC) instituted recommendations for diagnostic laboratories designed to improve detection of M. tuberculosis infection and facilitate prompt isolation and treatment of infected persons, thereby curbing the further spread of the organisms and disease (2, 16). These recommendations stated that results from concentrated acid-fast smears should be reported within 24 h of collection, culture in liquid medium should be performed in order to detect growth of M. tuberculosis within 10 to 14 days, and antimicrobial susceptibility tests should be complete within 15 to 30 days after collection of the initial diagnostic specimen. These recommendations were apparently clarified and relaxed to identification in <21 days in a 1997 commentary (15).
Laboratories striving to meet these recommendations have limited choices of diagnostic testing systems. The most widespread system currently used for detection of mycobacteria in clinical laboratories is the nonautomated, radiometric BACTEC 460 system (BACTEC 460; Becton Dickinson Co., Towson, Md.). It has several disadvantages including the need for handling radioisotopes and an increased handling of bottles, exposing them to breakage; lack of computerized data management and interface capabilities; and the need for invasive multiple sampling of cultures, which places personnel at greater risk for contracting mycobacterial infection and also increases the likelihood of cross contamination (12).
Organon Teknika Corp. (Durham, N.C.) recently developed the continuously monitored, nonradiometric MB/BacT system (MB/BacT) with a computerized database management system which was approved for diagnostic use in the United States in 1996. This system is based on colorimetric detection of CO2 and has been previously described (12). A comparison of the BACTEC 460 versus the MB/BacT with a newly revised reconstitution fluid-antibiotic supplement was performed to determine the relative sensitivities, times until detection of mycobacteria in clinical specimens, and bacterial overgrowth rates of the two systems. The original antibiotic supplement for the MB/BacT consisted of amphotericin B, polymyxin B, trimethoprim, and azlocillin with Tween 80. The revised reconstitution fluid, used in this study, contained vancomycin to suppress gram-positive bacterial overgrowth and oleic acid as a substitute for Tween 80 to enhance recovery of mycobacteria.
MATERIALS AND METHODS
Patient population and clinical specimens.
A total of 488 clinical specimens submitted for mycobacterial culture obtained from 302 patients cared for in the University of Alabama at Birmingham Medical Center between January and April 1997 were studied. There were 416 respiratory secretions and 72 samples of tissues and body fluids (except blood). The Alabama State Health Department, Division of Tuberculosis Control, was contacted to determine the start of treatment dates for all tuberculosis patients.
Specimen processing.
All cultures were inoculated within 24 h of specimen collection. Respiratory specimens were decontaminated by N-acetyl-l-cysteine-NaOH, with a final NaOH concentration of 1%. Specimens were then concentrated by centrifugation (4,000 × g for 15 min). The sediment was resuspended in 2 ml of phosphate buffer (pH 6.8). Fluids from normally sterile sites and tissues were not decontaminated. Tissues were homogenized in a tissue grinder. All procedures were carried out in accordance with current safety recommendations established by the CDC (3).
Media and inoculation.
Each specimen or concentrate (0.5 ml) was inoculated (i) into an MB/BacT bottle containing 10 ml of modified Middlebrook 7H9 broth with casein, catalase, and bovine serum albumin with a revised antibiotic supplement (amphotericin B, nalidixic acid, polymyxin B, trimethoprim, and vancomycin); (ii) into a BACTEC 460 12B bottle containing 4 ml of Middlebrook 7H12 broth with PANTA (polymyxin B, aminopterin, trimethoprim, nalidixic acid, and amphotericin B); and (iii) onto a Middlebrook 7H11/7H11 selective agar biplate (REMEL, Inc., Lenexa, Kans.). During the course of the study, the order in which the media were inoculated was varied.
Smear preparation.
Processed specimens were stained with Auramine O fluorochrome, a nonspecific stain for mycobacteria (REMEL). Specimens positive for fluorescence were confirmed with Kinyoun’s modification of the Ziehl-Neelsen acid-fast stain.
Incubation and detection of positive specimens.
Inoculated MB/BacT bottles were placed in the MB/BacT incubator/cabinet for continuous monitoring until the instrument indicated they were positive. Instrument-negative bottles were discarded after 6 weeks according to the manufacturer’s instructions. BACTEC 460 12B bottles were incubated at 35°C in an atmosphere of 5 to 10% CO2 in air. BACTEC bottles were read twice weekly for 3 weeks and once each subsequent week for a total of 6 weeks before being designated negative. Any BACTEC bottle with a growth index (GI) of >10 was considered presumptively positive and was read daily until the GI reached 100. Smears were prepared from all instrument-positive MB/BacT and BACTEC 460 12B bottles with GIs of >100. Slides were stained by Gram stain and Auramine O. Positive Auramine O smears were confirmed with Kinyoun’s stain. Positive bottles in either system were subcultured to a Middlebrook plate if there was no concomitant growth on the original Middlebrook biplate at the time the bottle was designated positive. All positive BACTEC bottles with GIs of >100, which were acid-fast stain negative, were maintained in the incubator and restained weekly for a total of 6 weeks, without additional measurements of GI. Positive MB/BacT bottles which were acid-fast stain negative, with nonmycobacteria present, were discarded according to the manufacturer’s instructions with no further work-up or incubation. The 7H11 agar plates were examined twice weekly for a total of 6 weeks before being designated negative.
Mycobacterial species identification.
The AccuProbe assay (Gen-Probe, San Diego, Calif.) was used to identify M. tuberculosis complex and M. avium complex (MAC) isolates (5). For the BACTEC 460, 3-ml aliquots from positive 12B bottles were centrifuged (15 min at 4,000 × g), and the pellet was used in the AccuProbe assay. For the MB/BacT, 1 ml was removed after mixing on a vortex mixer and tested directly with the Accuprobe assay without centrifugation due to the higher numbers of organisms in instrument-positive bottles. All acid-fast-positive cultures were batched and probed weekly for species identification. Bottles were probed on the first scheduled day after the GI was over 100 for the BACTEC 460 or the MB/BacT signaled positive. Organisms were preferentially taken from the 7H11 plate for probing. If no growth was observed on the plate, then the first of either the MB/BacT or BACTEC 12B bottles to turn positive was used for identification. The morphologies of the acid-fast bacteria from both bottles from the same specimen were compared to detect discordant cultures. Isolates not identified as M. tuberculosis or MAC were identified in the Alabama State Tuberculosis Laboratory by high-performance liquid chromatography (1).
Data analysis.
The sensitivities of mycobacterial detection for the two systems were compared by using the McNemar chi-square test for paired data. Times until detection of mycobacterial growth for the two systems were compared with the t test. P values of ≤0.05 were considered significant.
RESULTS
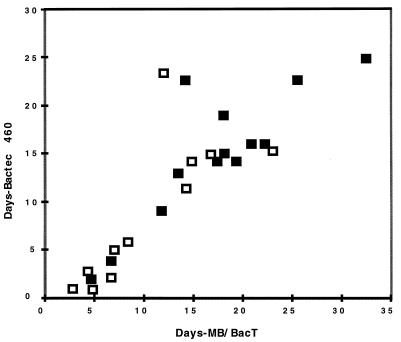
Table 1 shows the mycobacteria detected by the two systems. Table 2 shows a comparison of times until detection of mycobacterial growth as determined by acid-fast stain of fluid from the respective bottles. Days until detection for each specimen positive for M. tuberculosis by both systems are shown graphically in Fig. 1. The 23 M. tuberculosis isolates were from 13 patients. The days to detection for the first diagnostic specimen from 11 of these patients are indicated in Fig. 1. The other two patients were under treatment before specimens were received for the study.
TABLE 1.
Mycobacteria detected by BACTEC 460 versus MB/BacT
| Species | No. of positive specimens detected/total no. of positive specimens (%) by:
|
||
|---|---|---|---|
| MB/BacT | BACTEC 460 | P value | |
| M. tuberculosis | 23/24 (96) | 24/24 (100) | 0.310 |
| M. avium | 12/14 (86) | 10/14 (71) | 0.410 |
| M. kansasii | 4/5 (80) | 5/5 (100) | 0.310 |
| M. fortuitum | 0/1 (0) | 1/1 (100) | NDa |
| M. gordonaeb | 4/8 (50) | 4/8 (50) | ND |
| All clinically significant mycobacteria | 39/44 (89) | 40/44 (91) | 1.0 |
ND, not determined.
M. gordonae was not considered to be clinically significant.
TABLE 2.
Mean days until detection of a positive culture by BACTEC 460 versus MB/BacTa
| No. and type of specimen | Means (range) days until detection:
|
P value | |
|---|---|---|---|
| MB/BacT | BACTEC 460 | ||
| 23 M. tuberculosis | 13.7 (2.8–32.5) | 11.9 (0.8–24.9) | 0.046 |
| 11 initial | 10.9 (4.3–22.2) | 9.7 (0.8–23.9) | 0.427 |
| 15 smear positive | 10.3 (2.8–25.4) | 9.8 (0.8–23.3) | 0.633 |
| 8 smear negative | 20.1 (14.3–32.5) | 15.9 (11.9–24.9) | <0.001 |
| 8 M. avium | 14.2 (7–35.2) | 10.1 (2.3–26.9) | 0.009 |
| 4 M. kansasii | 9 (6.4–15) | 8.8 (3–18.7) | 0.910 |
Analysis was limited to specimens which were positive by both systems.
FIG. 1.
The times until detection for every specimen culturally positive for M. tuberculosis by both the MB/BacT and the BACTEC 460 are shown. Open squares indicate the first diagnostic specimen from each untreated patient. Solid squares indicate other specimens from these patients or treated patients.
The BACTEC 460 detected M. tuberculosis isolates in 24 specimens, whereas the MB/BacT detected M. tuberculosis isolates in 23 specimens. The single M. tuberculosis isolate missed by the MB/BacT was grown from a smear-positive specimen whose isolate was detected in the BACTEC 460 after 39 days of incubation. A few colonies grew on 7H11 agar after 30 days. Two subsequent specimens from this patient grew M. tuberculosis organisms in both systems. The difference in the mean time until detection of M. tuberculosis for BACTEC 460 versus MB/BacT was only 1.8 days (11.9 versus 13.7 days, respectively) but was statistically significant (P = 0.046). The 15 smear-positive M. tuberculosis specimens were detected significantly sooner than the 8 smear-negative specimens detected by both MB/BacT (P = 0.001) and BACTEC 460 (P = 0.028). This difference was 3.9 days for the BACTEC 460 versus 6.7 days for the MB/BacT. However, there was no significant difference in time until detection of the 11 initial diagnostic specimens for M. tuberculosis in untreated patients for either system (P = 0.427).
Only 1 of 14 (7%) specimens from which MAC was cultivated was smear positive initially, and only 8 of 14 (57%) specimens were detected by both systems. Ten of 14 (71%) were detected by the BACTEC versus 12 of 14 (86%) by the MB/BacT. Mean time until positivity for the 8 MAC isolates detected by both systems was 10.1 days for the BACTEC versus 14.2 days for the MB/BacT (P = 0.009). Two BACTEC 460 bottles with GIs of >100 were smear negative. However, on subsequent incubation and staining, both eventually stained positive and MAC was identified by the AccuProbe assay on the basis of growth on solid media. Repeat staining of contaminated MB/BacT bottles is not recommended by the manufacturer and was not performed as part of this evaluation.
The mean times until detection of the four isolates of M. kansasii that were detected by both systems were similar (BACTEC, 8.8 days; MB/BacT, 9 days). The one specimen which was positive only by the BACTEC 460 was detected after 31 days of incubation. Eight specimens grew M. gordonae in at least one system, but only one specimen grew this organism in both systems. A single isolate of M. fortuitum was identified after 6.8 days of incubation in the BACTEC 460. The MB/BacT did not detect this organism.
No additional mycobacteria-positive specimens were detected on 7H11 agar that were not otherwise identified in the broth-based systems as described above. Excluding M. gordonae, overall detection rates for all other clinically significant mycobacteria were similar, i.e., 39 of 44 (89%) for the MB/BacT and 40 of 44 (91%) for the BACTEC 460. The percentage of all specimens testing positive for any mycobacteria was 10.7%, whereas the M. tuberculosis isolation rate was 4.9%.
Bacterial overgrowth.
The nonmycobacterial overgrowth rate in decontaminated respiratory specimens was somewhat higher for the MB/BacT, with 29 contaminated specimens out of 416 (7.0%) total specimens, than for the BACTEC 12B (17 contaminated specimens of 416 [4.1%]). Gram-positive cocci with or without diphtheroids were the predominant contaminating organisms, followed by yeasts, Pseudomonas spp., other gram-negative rods, and various molds.
Direct identification of mycobacteria from MB/BacT bottles.
Although use of the AccuProbe assay directly on fluid from positive MB/BacT bottles is not specifically endorsed by the manufacturer, 3 MAC and 3 M. tuberculosis isolates were identified in this manner by using noncentrifuged specimens. Whichever culture medium was determined to be the best source of organisms for identification on the scheduled day for performing AccuProbe identification was used. Individual cultures of MAC organisms were identified on day 1, 2, or 3 after growth in the MB/BacT bottles was indicated and the bottles were smear positive. Likewise, M. tuberculosis was identified on day 1, 2, or 5 after the bottles were smear positive.
DISCUSSION
Nucleic acid amplification techniques for direct detection of mycobacteria in clinical specimens have received considerable attention in recent years because of their advantage in shortened turnaround time for diagnosis. However, they are not expected to completely supplant culture as sensitive and specific means for the definitive diagnosis of clinically significant mycobacterial infections. Obtaining cultural isolates of mycobacteria, particularly M. tuberculosis, is still important because of the need for susceptibility testing in every instance and the need for DNA fingerprinting in some cases for epidemiological purposes and for tracking laboratory contamination.
Current and projected future needs for cultural detection of mycobacteria in diagnostic laboratories and the limitations of existing liquid medium systems such as the BACTEC 460 have led to the development of the MB/BacT, a continuously monitored, nonradiometric system with computerized database and interface capabilities. This evolution is much the same as the development of the automated bacterial blood culture systems in the early 1990s (18). Preliminary studies with the MB/BacT and the BACTEC 460 have all demonstrated the comparability of these systems in recovering mycobacteria from clinical specimens (8–12, 17). In the present study, the MB/BacT with a revised reconstitution fluid-antibiotic supplement detected approximately the same number of clinically significant mycobacteria as the BACTEC 460.
Neither system correctly detected all mycobacteria, but discrepancies occurred primarily with species other than M. tuberculosis. Sensitivity as well as time until detection was quite variable for MAC isolates, suggesting that there may be small numbers of organisms present in some of the original specimens. This was also the case for an isolate of M. kansasii from a smear-negative specimen which grew only in the BACTEC 460 12B medium after 31 days and for common contaminating organisms such as M. gordonae. We limited our incubation period to 6 weeks, as is currently recommended by both manufacturers, and did not evaluate the effect that prolonged incubation might have on either system. However, Rohner et al. (12) have suggested that the incubation period for MB/BacT should be longer than 6 weeks in order to maximize organism recovery.
The time until detection of mycobacterial growth in the MB/BacT has consistently been slightly longer than that of the BACTEC 460 both in earlier studies (8, 9) and in the present one. These prior studies reported mean times until detection of M. tuberculosis in the MB/BacT ranging from 11 to 23 days. In the present evaluation, which is the first to employ the revised reconstitution fluid-antibiotic supplement formulation, the time until detection of M. tuberculosis was approximately 2 days longer for the MB/BacT compared to that for the BACTEC 460. This difference was statistically significant and is about the maximum increase that is clinically acceptable. It should be noted that while specimens positive in the MB/BacT are identified immediately since they are monitored continuously, those positive in the BACTEC 460 would be identified only when the specimens are tested according to the laboratory protocol.
The mean time to detection of 23 M. tuberculosis isolates (13.7 days) for the MB/BacT lies within the more stringent 10- to 14-day period recommended by the CDC and is more rapid than can be expected for detection of colonies on solid media. However, there were nine specimens, six of which were initially smear negative, whose time to detection exceeded 15 days before being flagged positive by the MB/BacT. This compares to seven specimens which exceeded 15 days until detection by the BACTEC 460.
The CDC guidelines were intended to apply to the first, or diagnostic, specimen from each patient. Subsequent specimens collected to monitor treatment often have delayed growth because of the administration antimycobacterial agents. Eleven of the specimens in this study were the first positive M. tuberculosis specimen from individual patients. Of these 11 specimens, 2 exceeded 15 days (though only 1 exceeded 21 days) to detection in each system, and the average times to detection were 8.9 and 10.2 days in the BACTEC 460 and the MB/BacT, respectively. These times to detection would allow laboratories to identify the species of the isolates by using the AccuProbe assay once weekly and still diagnose most new tuberculosis cases within 14 days with either system. Among the specimens collected from patients more than 20 days after initiation of antimycobacterial treatment three of six had less than 3 days’ difference in the time to detection by MB/BacT compared to BACTEC 460 and the one with the greatest difference was 7.6 days later than the time of detection by the BACTEC 460.
The bacterial overgrowth rate from respiratory specimens was somewhat higher in the MB/BacT bottle (7.0%) than in the BACTEC 460 12B bottle (4.1%). This relationship has been shown in other comparison studies, with some researchers reporting MB/BacT contamination rates of ≥ 9% (11, 12). The higher-than-desired contamination rate for the MB/BacT, coupled with the slightly longer detection time in comparison to the BACTEC 460, led the manufacturer to revise the reconstitution fluid, substituting oleic acid for Tween 80 to enhance recovery of mycobacteria following decontamination. The original antibiotic supplement for the MB/BacT consisted of amphotericin B, polymyxin B, trimethoprim, and azlocillin. In the revised supplement utilized in the present study, vancomycin was added in an attempt to reduce the growth of gram-positive cocci, particularly staphylococci. However, even with these modifications, the contamination rate for the MB/BacT was still slightly higher than that of the BACTEC 460, and gram-positive cocci predominated among contaminants. Rohner et al. (12), using the original reconstitution fluid-antibiotic supplement, reported a contamination rate of 9.4% with the MB/BacT, which was reduced to 4.3% when specimens were reprocessed. Although reprocessing is always an option when confronted with bacterial overgrowth, repeating decontamination is time-consuming and is most productive when acid-fast organisms are apparent simultaneously with the bacterial contaminants in the culture media. In some instances, adequate mycobacterial growth on solid media from the initial culture planting may eliminate the need for repeating the decontamination process. Subculture of contaminated liquid media to selective 7H11 agar may also circumvent the overgrowth problem and yield viable mycobacteria.
MAC isolates were recovered from two BACTEC 460 12B bottles that also grew nonmycobacterial organisms because of our practice of staining contaminated bottles weekly for the full 6 weeks of incubation. This practice should be considered for either system in order to prevent false-negative results.
Diagnostic laboratories which offer mycobacterial cultures now offer identification of common organisms, such as M. tuberculosis and MAC, at the species level owing to the availability of reliable commercial systems for nucleic acid hybridization such as the Accuprobe assay. Of major importance in minimizing the turnaround for organism identification is the ability to apply the probes directly to liquid media known to contain mycobacteria, since colonial growth on agar may take several days longer. BACTEC 460 12B medium can be probed directly for reliable results once the GI exceeds 500, but this may require additional days of incubation following the initial indication of a positive culture. The initial advantage of the more rapid mycobacterial growth in the BACTEC 460 may thus be offset because 12B bottles typically cannot be probed at the time the culture is first identified as being positive. This contrasts with the MB/BacT, which has been shown in previous studies (6, 9) to yield accurate species identification of M. tuberculosis and MAC isolates by Accuprobe assay on the same day as the bottle is flagged positive by the instrument and without centrifugation to concentrate the organisms. The present evaluation, using the newly revised reconstitution fluid-antibiotic supplement, also confirmed the acceptability of this practice for both M. tuberculosis and MAC isolates for a small number of specimens.
At present, only the BACTEC 460 can be used for blood cultures and susceptibility testing, but these are possibilities which should be evaluated for the MB/BacT in the future because of their potential clinical importance. Although most hospital-based laboratories do not perform on-site susceptibility testing of mycobacteria, use of liquid media for this process has been recommended (7) and is of great importance for public health laboratories.
In summary, the sensitivity of detection and time until detection of positive cultures for mycobacteria by using the MB/BacT with a revised reconstitution fluid-antibiotic supplement were similar to those that were reported by others using the original medium and supplement. This suggests that the reconstitution fluid modification and changes in antibiotics did not make a great difference. However, the many laborsaving features and other aspects that are found in the continuously monitored MB/BacT more than offset the workup required for contaminated bottles and make the MB/BacT an acceptable alternative for use in the diagnostic laboratory.
REFERENCES
- 1.Butler W R, Jost K C, Kilburn J O. Identification of mycobacteria by high-performance liquid chromatography. J Clin Microbiol. 1991;29:2468–2472. doi: 10.1128/jcm.29.11.2468-2472.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Provisional cases of selected notifiable diseases, United States, weeks ending Dec 27, 1997 and Dec 28, 1996 (52nd week) Morbid Mortal Weekly Rep. 1997;46:1261. [Google Scholar]
- 3.Centers for Disease Control and Prevention-National Institutes of Health. Biosafety in microbiological and biomedical laboratories. 3rd ed. U.S. Department of Health and Human Services publication no. (CDC) 93-8395 1993. , Atlanta, Ga. [Google Scholar]
- 4.Couchot K, Talbot R. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Direct DNA probe of MB/BacT mycobacterial culture system bottles for Mycobacterium avium complex and Mycobacterium tuberculosis complex, abstr. U-7; p. 102. [Google Scholar]
- 5.Goto M, Oka S, Okuzumi K, Kimur S, Shimada K. Evaluation of acridinium ester-labeled DNA probes for identification of Mycobacterium tuberculosis and Mycobacterium avium-Mycobacterium intracellulare complex in culture. J Clin Microbiol. 1991;29:2473–2476. doi: 10.1128/jcm.29.11.2473-2476.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna B A, Walters S B. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Evaluation of the MB/BacT mycobacteria detection system, abstr. C-18; p. 4. [Google Scholar]
- 7.Hinman A R, Hughes J M, Snider D E, Jr, Cohen M L. Meeting the challenge of multidrug-resistant tuberculosis: summary of a conference. Morbid Mortal Weekly Rep. 1992;41:51–57. [PubMed] [Google Scholar]
- 8.Kabani A, Hoban D, Laroche M, Wolfe J, Godal D, Turenne C, Karlowsky J, Zhanel G. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy 1997. Washington, D.C: American Society for Microbiology; 1997. A clinical comparison of the MB/BacT mycobacteria culture system with the BACTEC 460 and solid media for the recovery of mycobacteria, abstr. D-96; p. 100. [Google Scholar]
- 9.Lindholm-Levy P, Heifets L. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Evaluation of recovery and time to detection of mycobacterial growth in the MB/BacT system compared with three other media, abstr. U-39; p. 107. [Google Scholar]
- 10.Magee J G, Freeman R, Barrett A. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Experience with the MB/BacT automated culture system for mycobacteria, abstr. U-37; p. 107. [Google Scholar]
- 11.Postulka A, Geis G, Opferkuch W. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Clinical comparison of the MB/BacT mycobacteria detection system with the BACTEC 460 TB System, abstr. U-38; p. 107. [Google Scholar]
- 12.Rohner P, Ninet B, Metral C, Emler S, Auckenthaler R. Evaluation of the MB/BacT system and comparison to the BACTEC 460 system and solid media for isolation of mycobacteria from clinical specimens. J Clin Microbiol. 1997;35:3127–3131. doi: 10.1128/jcm.35.12.3127-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snider D E, Raviglione M, Kochi A. Global burden of tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: American Society for Microbiology; 1994. pp. 3–11. [Google Scholar]
- 14.Styblo K, Rouillon A. Estimated global incidence of smear-positive pulmonary tuberculosis. Unreliability of officially reported figures on tuberculosis. Bull Int Union Tuberc Lung Dis. 1981;56:118–126. [PubMed] [Google Scholar]
- 15.Styrt B A, Shinnick S M, Ridderhof J C, Crawford J T, Tenover F C. Turnaround times for mycobacterial cultures. J Clin Microbiol. 1997;35:1041–1042. doi: 10.1128/jcm.35.4.1041-1042.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenover F C, Crawford J T, Huebner R E, Geiter L J, Horsburgh C R, Jr, Good R C. The resurgence of tuberculosis: is your laboratory ready? J Clin Microbiol. 1993;31:767–770. doi: 10.1128/jcm.31.4.767-770.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanger A, Clark R, Bua J, Edwards A, Ho J. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Comparison of MB/BacT and conventional methods for detection of mycobacterium species, abstr. U-40; p. 107. [Google Scholar]
- 18.Wilson M L, Weinstein M P, Reller L B. Automated blood culture systems. Clin Lab Med. 1994;14:149–169. [PubMed] [Google Scholar]