Abstract
PURPOSE
Non-V600 mutations comprise approximately 35% of all BRAF mutations in cancer. Many of these mutations have been identified as oncogenic drivers and can be classified into three classes according to molecular characteristics. Consensus treatment strategies for class 2 and 3 BRAF mutations have not yet been established.
METHODS
We performed a systematic review and meta-analysis with published reports of individual patients with cancer harboring class 2 or 3 BRAF mutations from 2010 to 2021, to assess treatment outcomes with US Food and Drug Administration–approved mitogen-activated protein kinase (MAPK) pathway targeted therapy (MAPK TT) according to BRAF class, cancer type, and MAPK TT type. Coprimary outcomes were response rate and progression-free survival.
RESULTS
A total of 18,167 studies were screened, identifying 80 studies with 238 patients who met inclusion criteria. This included 167 patients with class 2 and 71 patients with class 3 BRAF mutations. Overall, 77 patients achieved a treatment response. In both univariate and multivariable analyses, response rate and progression-free survival were higher among patients with class 2 compared with class 3 mutations, findings that remain when analyses are restricted to patients with melanoma or lung primary cancers. MEK ± BRAF inhibitors demonstrated greater clinical activity in class 2 compared with class 3 BRAF-mutant tumors than BRAF or EGFR inhibitors.
CONCLUSION
This meta-analysis suggests that MAPK TTs have clinical activity in some class 2 and 3 BRAF-mutant cancers. BRAF class may dictate responsiveness to current and emerging treatment strategies, particularly in melanoma and lung cancers. Together, this analysis provides clinical validation of predictions made on the basis of a mutation classification system established in the preclinical literature. Further evaluation with prospective clinical trials is needed for this population.
INTRODUCTION
BRAF is among the most commonly mutated genes in human cancer.1 BRAF is most frequently mutated at codon V600, resulting in enhanced activation of the downstream mitogen-activated protein kinase (MAPK) pathway.1 Clinical trials investigating MAPK targeted therapies (MAPK TTs) have yielded response rates (RRs) of > 50% and improved overall survival in patients with BRAF V600-mutant tumors.2–6 As a result, MAPK TTs are now standard of care treatments for patients with BRAF V600-mutant melanoma, lung cancer, and colorectal cancer (CRC).7–9
CONTEXT
Key Objective
Preclinical research has shown that three classes of BRAF mutations exist, and that these classes exhibit distinct sensitivities to mitogen-activated protein kinase (MAPK)–targeted therapies (MAPK TTs). However, no randomized controlled trials have been performed for this specific population to date. In this study, we synthesized the totality of clinical evidence regarding patients with non-V600 BRAF mutations treated with MAPK TT.
Knowledge Generated
We demonstrate that the postulates established in the preclinical literature largely hold true in patients. Class 2 BRAF-mutant tumors derive benefit from MEK ± BRAF inhibition, driving a significant difference in response rate and progression-free survival between class 2 and class 3 BRAF-mutant tumors treated with MAPK TT.
Relevance
This work provides the first clinical evidence supporting a role for BRAF mutation class to be used as a predictive biomarker for MAPK TT in multiple tumor types.
Approximately 35% of all BRAF mutations occur outside the V600 codon.1,10 Non-V600 BRAF mutations are composed of missense mutations, fusions, and in-frame deletions.11–13 Work by Wan et al14 demonstrated that many non-V600 mutations are oncogenic. More recently, differences in dimerization requirement and RAS dependency of non-V600 BRAF mutations have been described by Yao et al.15,16 Together, these data have led to a classification scheme for BRAF alterations.10,16 Wild-type BRAF signals as RAS-dependent dimers, and class 1 BRAF-mutants are composed of V600 mutations, which signal as constitutively active monomers in a RAS-independent manner.17,18 Class 2 BRAF mutations form kinase-activating RAS-independent dimers,15 and class 3 BRAF mutations have impaired kinase activity but signal as RAS-dependent dimers, primarily by forming heterodimers with CRAF.16
Preclinical data support the use of MEK inhibitors (MEKi) ± BRAF inhibitors (BRAFi) in tumors with class 2 or 3 mutations.19–23 Because of the dependency on RAS activation, receptor tyrosine kinase inhibitors ± MEKi have been proposed as a therapeutic strategy for class 3 BRAF-mutant tumors.16 Preclinical evidence also suggests that class 2/3 BRAF mutations may be less sensitive to BRAF + MEK inhibition than class 1 mutant tumors.15,16 Indeed, two single-arm phase II trials have reported low RRs to MEKi monotherapy of patients with non-V600 BRAF mutations.24,25 However, a multitude of case reports and case series in different cancer types have demonstrated that subsets of non–V600 BRAF-mutant tumors may indeed be sensitive to these MAPK TTs.1,19
There are currently no data from randomized controlled trials to guide targeted therapy treatment decisions in cancers with class 2/3 BRAF mutations. When standard treatment options have been exhausted, many oncologists will provide off-label MAPK TTs to these patients. Therefore, to establish a reference cohort to help guide treatment decisions and inform future clinical trial design, we synthesized all clinical evidence wherein class 2 or 3 BRAF-mutant tumors were treated with MAPK TT.
METHODS
Search Strategy
A literature search was conducted of studies published from January 2010 to September 2021 in the following databases: Medline ALL (Medline and Medline Epub Ahead of print and In-Process & Other Non-Indexed Citations), Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, all from the OvidSP platform, and Web of Science from Clarivate Analytics. Where available, both controlled vocabulary terms and text words were used (Data Supplement). There were no language or study design restrictions. Published conference abstracts were included. The AACR, ASCO, and ESMO conference proceedings were searched to identify any relevant conference abstracts. Additional publications and/or data identified outside of the search were added when applicable. The study protocol was prospectively uploaded to PROSPERO (ID: CRD42020218141) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.26
Abstracts were screened by two independent reviewers using Covidence.27 Conflicts were resolved with internal discussion between the reviewers and in the case of a lasting conflict, by a third reviewer. Response data and patient demographics were extracted by two independent reviewers. After data were extracted from all included publications, missing data were identified and requested from the original authors with up to two separate e-mails > 7 days apart.
Inclusion and Exclusion Criteria
Inclusion criteria were published reports of adult patients with cancer with individual patient data describing (1) a class 2 or class 3 BRAF mutation, (2) treatment with US Food and Drug Administration–approved MAPK TT including inhibitors of epidermal growth factor receptor (EGFR), BRAF monomers, or MEK, and (3) availability of treatment response data. Exclusion criteria were concomitant BRAF V600 E/K mutation, pediatric patients, and concurrent non-MAPK TT (such as chemotherapy, immunotherapy, and PI3K- or CDK4/6-targeted agents).
Primary and Secondary Outcomes
The coprimary outcomes were overall treatment RR and progression-free survival (PFS). When appropriate response criteria were used (Response evaluation criteria in solid tumors; RECIST), patients with partial response or complete response were considered to have had a treatment response and those with stable disease or progressive disease were considered nonresponders.28 When RECIST criteria were not used, response was recorded on the basis of the primary paper's author's assessment of response or calculated from tumor measurements on computed tomography or magnetic resonance imaging provided in the text. For PFS analysis, patients were censored if there was no indication of progression or death at the time of last follow-up.
Statistical Analyses
We performed one-stage meta-analyses of pooled individual patient-level data from all included studies. HR was used as the parameter of interest for PFS, and odds ratio (OR) was used as the parameter of interest for response. A multi-level mixed-effects logistic regression model, incorporating individual study as a random effect, was used to estimate the ORs of responses between groups and its associated 95% CI. Multivariable logistic regression models were used to estimate adjusted ORs (aOR). All study key variables that were available for all patients were incorporated into the initial multivariable model. These included cancer type, BRAF mutation class, therapy type, geographic location, study type, and response criteria. To analyze PFS, a shared frailty Cox regression model was used to account for heterogeneity across studies for all primary analyses. All study key variables were incorporated into the initial multivariable model. These included age, sex, cancer type, BRAF mutation class, therapy type, geographic location, study type, and response criteria. The final multivariable models included only those variables that were associated with P < .05. Survival curves were visualized and evaluated with the Kaplan-Meier method and the log-rank test. Statistical analyses were performed with STATA v13 (StataCorp LLC, College Station, Texas, USA).
RESULTS
Characteristics of Included Studies and Patients
We identified 18,167 articles in our search. After removing ineligible articles and adding additional studies from the author's files, a total of 80 articles were included in the review (Data Supplement), comprising a total of 238 patients with class 2 or class 3 non-V600 BRAF mutations who were treated with MAPK TT (Fig 1A). The number of studies reporting results of MAPK TT treatment outcomes in patients with tumors harboring non-V600 BRAF mutations has increased substantially over the past decade (Data Supplement). A detailed description of the different MAPK TT regimens used for patients in the study is presented in the Data Supplement. We also performed risk-of-bias (ROB) assessment for all studies included in the meta-analysis on a five-point scale (Data Supplement).
FIG 1.
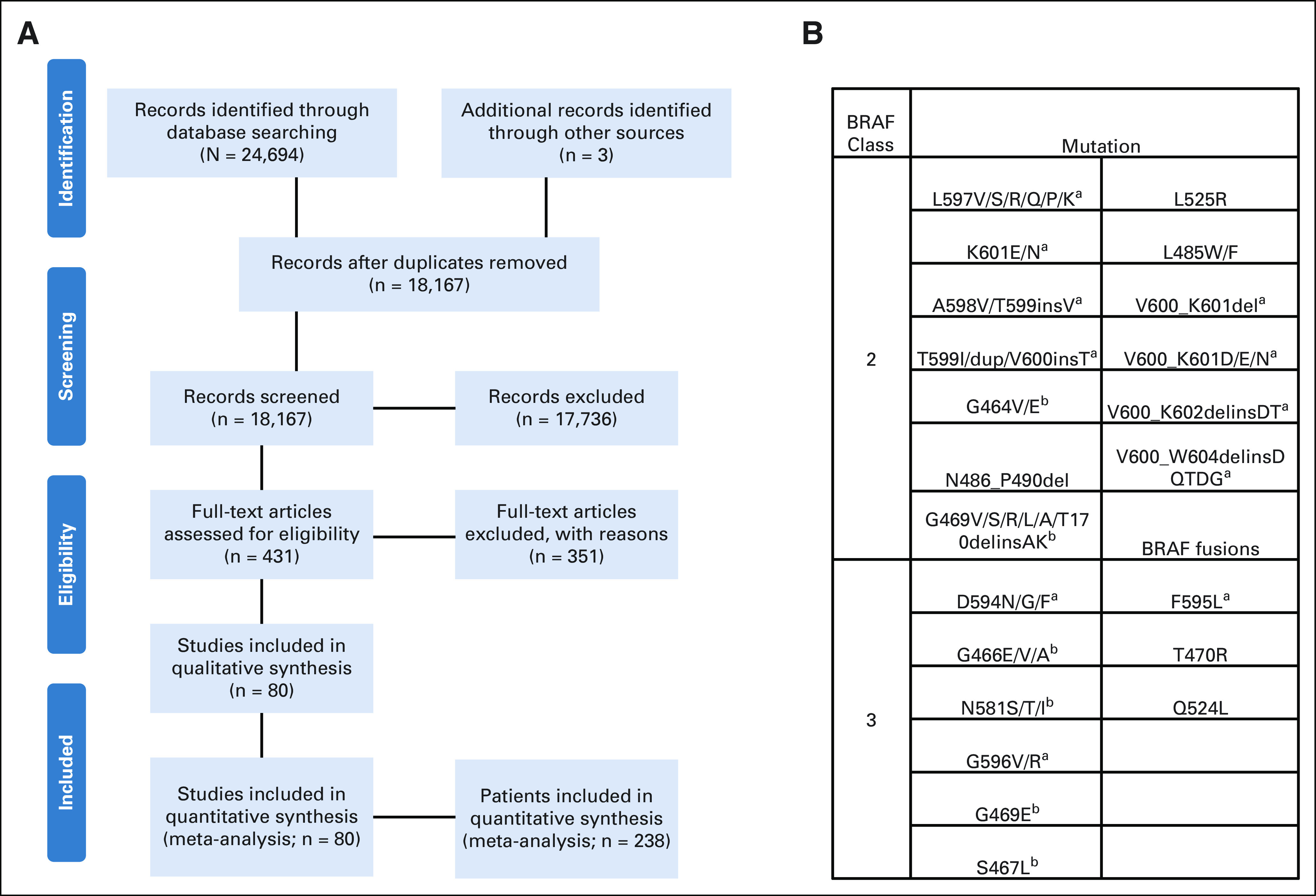
(A) Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram demonstrating search and inclusion of studies for meta-analysis. (B) List of class 2 and class 3 mutations included in the study. aIndicates exon 15. bIndicates exon 11.
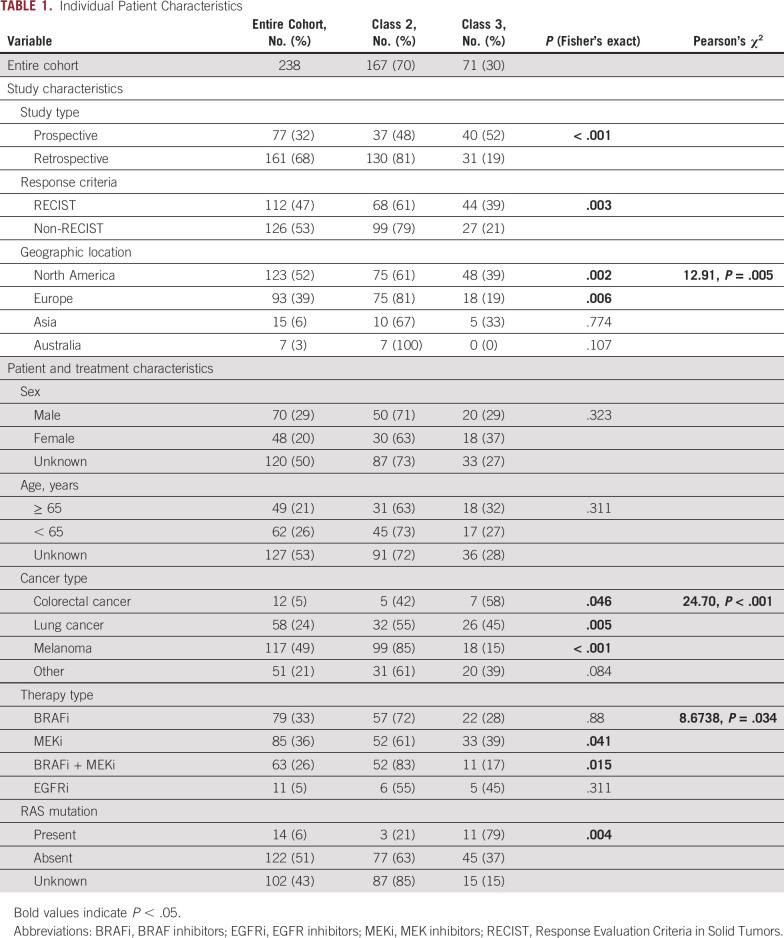
Among the 238 patients included in this study, there were 167 patients with class 2 and 71 patients with class 3 BRAF mutations (Fig 1B, Table 1).
TABLE 1.
Individual Patient Characteristics
Characteristics Associated With MAPK TT Response and PFS by BRAF Class and Primary Tumor Type
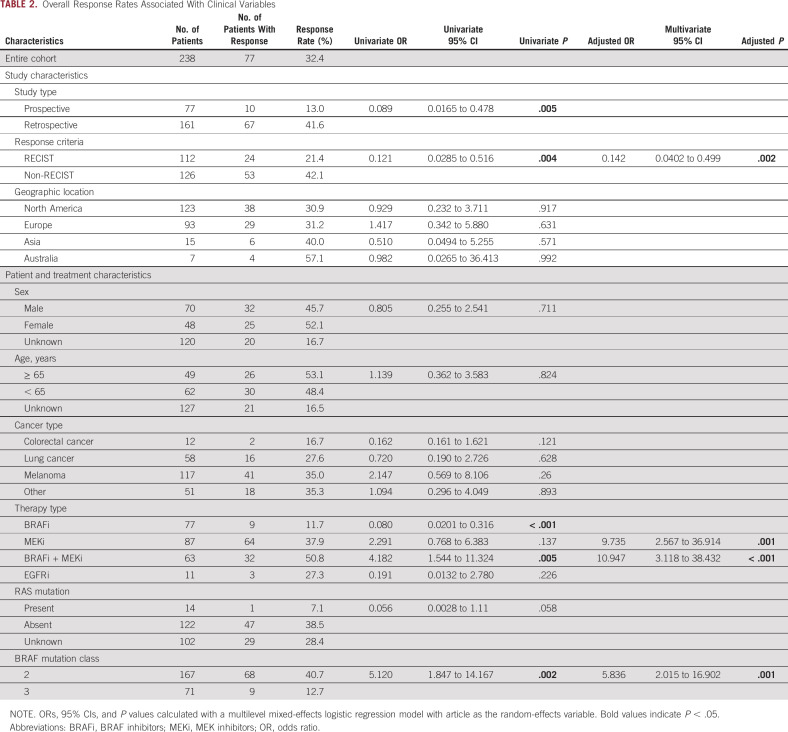
In the entire population, 77 out of 238 patients (32%) experienced a treatment response (Table 2). The RR differed according to whether tumors had a class 2 or class 3 BRAF mutation (41% v 13%, univariable OR, 5.12, P = .002; Table 2). We next compared the impact of BRAF mutation class on treatment response within each primary tumor type. Class 2 BRAF-mutant tumors demonstrated higher RRs than class 3 mutants independently in lung, melanoma, and other primaries (P = .018, .029, and .018, respectively; Fig 2A). Among those with class 2 BRAF mutations, MAPK TT RRs were highest in patients with other tumor types (48%) and lowest in CRC (20%; Fig 2A). The group of 51 patients with other tumor types had noncolorectal gastrointestinal (n = 21), genitourinary (n = 10), gynecologic (n = 5), hematopoietic (n = 4), head and neck (n = 4), breast (n = 2), spindle cell neoplasms (n = 1), low-grade glioma (n = 1), and unknown primary tumors (n = 3). Among patients whose tumors harbored class 3 mutations, RRs did not differ significantly according to primary tumor type (RR 11%-15%; Fig 2A).
TABLE 2.
Overall Response Rates Associated With Clinical Variables
FIG 2.
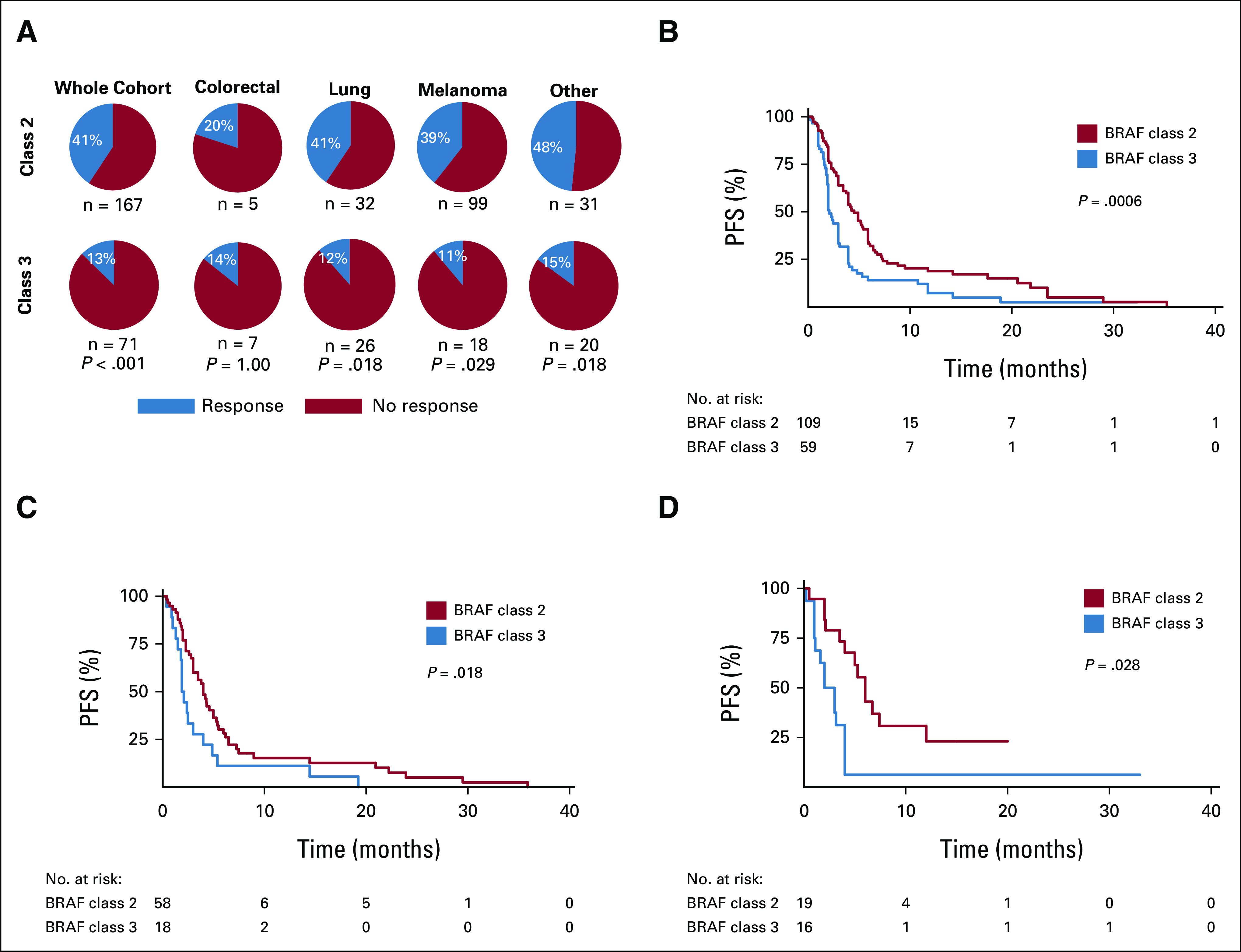
Relationship between BRAF class and tumor type in the context of MAPK-targeted therapy. (A) Response rates to MAPK-targeted therapy according to BRAF class and primary cancer type. P values calculated with Fisher's exact test. PFS according to BRAF class in the (B) entire cohort and for (C) melanoma and (D) lung primary tumors. P values calculated with log-rank test. MAPK, mitogen-activated protein kinase; PFS, progression-free survival.
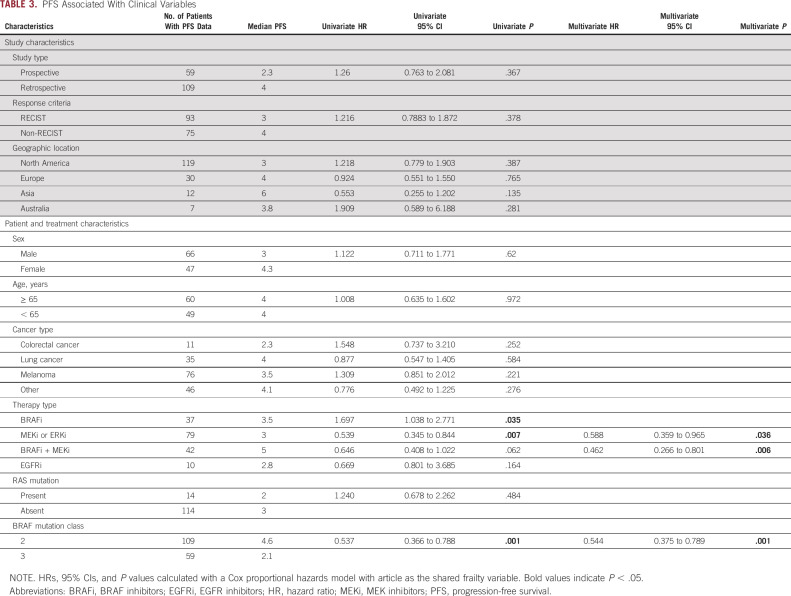
Data on PFS were available for 168 (71%) patients included in the study. Patients with class 2 BRAF mutations (median PFS [mPFS] 4.6 months, HR 0.537, P = .001) experienced longer PFS compared with patients with class 3 mutations (mPFS 2.1 months; Table 3, Fig 2B). The relationship between BRAF class and PFS remained significant when we examined specific cancer subsets, including melanoma (P = .018) or lung cancer (P = .028; Figs 2C and 2D and Data Supplement). When restricting our analyses to patients with RECIST-defined responses, from prospective data sets, or who were treated with only BRAF and/or MEKi, the differential PFS between class 2 and class 3 BRAF mutants remain (P = .024, .011, and .002, respectively; Data Supplement).
TABLE 3.
PFS Associated With Clinical Variables
Characteristics Associated With MAPK TT Response and PFS by BRAF Class and Treatment Type
In class 2 BRAF-mutant tumors, the highest RR was observed with either BRAFi + MEKi or MEKi monotherapy (RR of 56%, Fig 3A). In patients with class 3 BRAF-mutant tumors, the highest RR was observed with BRAFi + MEKi (27%), whereas MEKi monotherapy and BRAFi monotherapy were associated with the lowest RR (9%, Fig 3A). In multivariable analysis, BRAF class 2 (5.836, P = .001), MEKi (aOR 9.734, P = .001), and BRAFi + MEKi (aOR 10.947, P < .001) were independently associated with higher odds of response (Table 2). We explored whether BRAF codon or type of mutation (fusion or internal deletion) were associated with RR, but no apparent trends emerged (Data Supplement). Furthermore, when dichotomizing BRAF mutations by exon mutated (exon 11 v exon 15), no statistically significant differences were seen in RR or PFS (Data Supplement).
FIG 3.
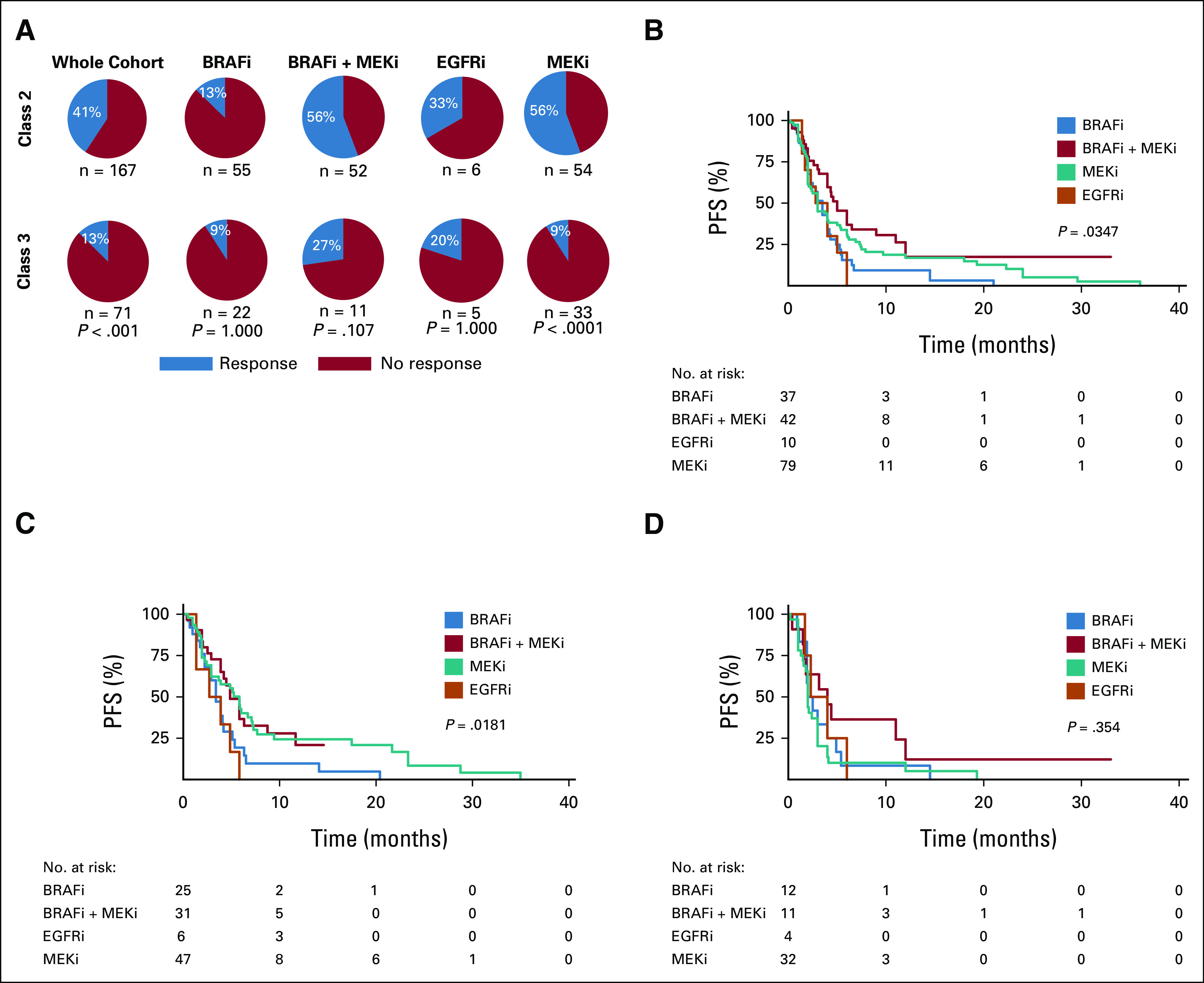
Relationship between BRAF class and treatment type in the context of MAPK-targeted therapy. (A) Response rates to MAPK-targeted therapy according to BRAF class and treatment type. P values calculated with Fisher's exact test. PFS according to treatment type in the (B) entire cohort, and when analyses are restricted to (C) class 2 and (D) class 3 BRAF-mutant tumors. P values calculated with log-rank test. BRAFi, BRAF inhibitors; EGFRi, EGFR inhibitors; MAPK, mitogen-activated protein kinase; MEKi, MEK inhibitors; PFS, progression-free survival.
In the whole cohort, patients treated with BRAFi + MEKi experienced the longest PFS (mPFS 5.0 months) and those treated with EGFR inhibitors (EGFRi) experienced the shortest PFS (mPFS 2.8 months, P = .0347, Fig 3B). In class 2 mutant tumors, BRAFi + MEKi (mPFS 5.0 months) and MEKi alone (mPFS 6.0 months) were associated with longer PFS compared with BRAFi (mPFS 3.5 months) or EGFRi (mPFS 2.8 months; P = .0181; Fig 3C). However, in class 3 mutant tumors, no specific treatment regimen was associated with significantly improved PFS (Fig 3D). In multivariable analysis, BRAFi + MEKi (HR, 0.462; 95% CI, 0.27 to 0.80; P = .006) and MEKi (HR, 0.588; 95% CI, 0.36 to 0.97; P = .036) were independently associated with longer PFS (Table 3), as was class 2 BRAF mutational status (HR, 0.544; 95% CI, 0.38 to 0.79; P = .001). We did not observe a significant association between treatment type and improved outcomes within any of the tumor types analyzed (Table 3; Data Supplement).
Depth of Response of Class 2 and 3 BRAF-Mutant Tumors to MAPK Inhibition Is Associated With PFS
To better characterize the degree of clinical benefit achieved by patients who responded to MAPK inhibitors, we assessed PFS according to response type. Patients who achieved complete response experienced longer PFS (mPFS 12 months) than patients with partial response (mPFS 6 months), stable disease (mPFS 4.2 months), or progressive disease as best response (mPFS 1.8 months; Data Supplement; P < .0001). Patients who experienced PFS > 12 months demonstrated a greater depth of tumor regression response than patients with responses lasting < 12 months (Data Supplement; P = .0082). Among responders with available data (n = 23), we observed a correlation between increased depth of response (% tumor regression of target lesions) and longer PFS (Data Supplement; R2 = 0.2153, P = .0257).
Quality Assessment
The majority of the patients included in this analysis were reported in retrospective studies. These may be more subject to bias than prospective studies. Indeed, we observed an increased RR among patients from retrospective versus prospective studies (42% v 13%, P = .005; Table 2). To better characterize ROB and its impact on our results, we performed a quality assessment of all included studies, using a validated five-point scale (Data Supplement). We analyzed whether ROB among the studies was associated with treatment response. There was a statistically significant difference in RR (44% v 21%, P < .001) between patients derived from studies with high ROB (score 0-3; n = 117) compared with those with low/moderate ROB (score 4-5; n = 121; Data Supplement); however, ROB was not associated with differences in PFS (Data Supplement). Among studies with low/moderate ROB, there was a trend toward RR being higher among patients with class 2 BRAF mutations (27% v 13%) but this difference was not statistically significant (P = .07; Data Supplement). However, the observation that patients with class 2 BRAF mutations experience longer PFS than patients with class 3 BRAF mutations was observed in patients from studies with both high and low ROB (P = .0282 and .0194, respectively; Data Supplement).
DISCUSSION
We have assembled the largest clinical cohort of patients with BRAF non-V600 mutant tumors with associated treatment response to date. This has allowed us to perform comprehensive analyses of characteristics associated with response to MAPK TT in this population. The results described herein highlight the importance of testing for the presence of non-V600 BRAF mutations in patients with many types of advanced cancer. These data will be informative for molecular tumor boards and can be used in the design of clinical trials for patients with non-V600 BRAF mutations.
We found that class 2 BRAF mutant tumors respond to MAPK TT more favorably than class 3 mutants. This finding validates preclinical studies demonstrating that class 2 BRAF-mutant tumors benefit from therapies that target downstream of mutant RAS, whereas class 3 mutant tumors require treatment upstream with receptor tyrosine kinase inhibitors.16,19,29,30
In this study, the RR to MEKi monotherapy was 38%. This compares favorably to published reports of MEKi monotherapy in RAS-mutant lung cancer31 and melanoma,32 but these comparisons are limited by our analysis of retrospective data. Two previous prospective trials examined the efficacy of trametinib for BRAF non-V600 mutant tumors. NCI-MATCH EAY131 included patients with all primary tumor types and demonstrated a 3% RR.25 Meanwhile, Nebhan et al24 included only melanomas with non-V600 BRAF mutations, and observed a 33% RR (3/9).
Although the RRs may be higher than expected in this study because of the inclusion of retrospective data, we found no difference in PFS according to whether the data were derived from retrospective versus prospective studies or high versus low ROB studies. Furthermore, we observed that, in class 2 BRAF-mutant tumors, BRAFi + MEKi and MEKi monotherapy were associated with longer PFS. This provides further evidence that a subset of patients with class 2 BRAF mutations will derive therapeutic benefit from these treatment regimens. The degree of benefit, in terms of both outcomes and tolerability, conferred by the addition of BRAF inhibition to MEKi requires further study in prospective trials. Indeed, two ongoing clinical trials are investigating binimetinib and encorafenib for the treatment of tumors with non-V600 BRAF mutations (ClinicalTrials.gov identifier: NCT03839342 and NCT03843775).33
In class 3 BRAF-mutant tumors, EGFRi-containing regimens have already been demonstrated to elicit high RR, particularly when combined with chemotherapy in the context of CRC.30 However, there is mounting evidence that class 2 and class 3 BRAF mutations can also be important drivers of resistance to EGFRi in CRC.30,34,35 Given that class 3 mutations may exhibit a degree of additional sensitivity with additional BRAF and/or MEK inhibition, triple therapy regimens such as the cetuximab, encorafenib, and binimetinib combination that proved effective in the BEACON trial for BRAF V600E mutant CRC may also be beneficial for patients with class 3 BRAF mutations.29 Currently, this is being investigated in the BIG BANG trial.36,37 Despite this possibility, it is clear from this data set that patients with class 3 BRAF-mutant tumors have only modest potential for clinical benefit when treated with currently available standard MAPK TT.
We observed a trend toward an association with decreased responsiveness to MAPK TT in tumors with co-occurring RAS mutations. RAS mutations are well-documented drivers of resistance to EGFRi in CRC, and the development of de novo RAS mutations has been reported to be a key mechanism of acquired resistance to BRAF ± MEKi in BRAF V600-mutant melanoma.38,39 Moreover, mutant RAS is capable of activating the PI3K-Akt pathway in addition to the MAPK pathway, which may contribute to RAS-mediated resistance to MAPK TT in non–V600 BRAF-mutant tumors. Although these data are hypothesis-generating, we believe that future clinical trials enrolling patients with non-V600 mutations should report RAS comutation status. Recently, KRAS G12C inhibitors have demonstrated clinical activity, and sotorasib has received US Food and Drug Administration approval for the treatment of KRAS G12C mutant lung cancer.40 Directly targeting KRAS G12C in combination with BRAFi in tumors with co-occurring non-V600 BRAF and KRAS mutations is an intriguing possibility to overcome RAS-mediated resistance. However, in our study, none of the 14 patients with co-occurring RAS mutations had KRAS G12C mutations, suggesting limited applicability of such a strategy for tumors with non-V600 BRAF mutations. Beyond RAS, it is possible that coincident genomic alterations in other common oncogenes and tumor suppressors, such as CDKN2A/p16, PTEN, or PI3K, could influence TT responsiveness in tumors with non-V600E BRAF mutations, as has been reported for BRAF V600E-mutant tumors.41,42
The rarity and variable oncogenicity of each non-V600 BRAF mutation remains a challenge for drug developers and may complicate interpretation of results from future prospective trials. To facilitate effective drug development targeted against these important driver mutations, it will be critical for the community to collaborate in multicenter trials and share data regarding patient responses, tumor types, and comutation status whenever possible. It is important to note that when examined separately, patients with class 2 BRAF mutations included in prospective studies or whose response was established with RECIST criteria still demonstrated statistically significant superior PFS compared with those with class 3 mutations.
There are several limitations of this study that are worthy of discussion. The majority of the patients included in this analysis were from retrospective studies, which reported higher RR than prospective studies. As such, the RRs we report likely over-represent the true RRs that would be observed in prospective trials and real-world settings. Another important limitation of the study is that our analyses are largely on the basis of patients receiving earlier generations of targeted therapies, such as vemurafenib. Preclinical data suggest that alternative BRAFi such as dabrafenib and encorafenib,15 as well as next-generation BRAF dimer inhibitors and pan-RAF inhibitors, which inhibit both BRAF and CRAF, hold substantial promise for non–V600 BRAF-mutant tumors.19,43–45 Finally, we are missing data on performance status, degree of tumor burden, and line of therapy, all of which may be important confounders to our results.
Taken together, the existing literature confirms many of the predictions presented by preclinical research with respect to differences between class 2 and class 3 BRAF mutants and establishes new hypotheses worthy of further investigation. Currently available MAPK TTs have demonstrated clinical activity in a subset of tumors with non-V600 BRAF mutations—especially those with class 2 BRAF mutations. However, to date, these MAPK-directed therapies appear to be associated with lower RRs than has been observed in patients with BRAF V600-mutant tumors.1,9,46–49 The efficacy of MAPK TT can be also influenced by tumor type and co-occurring mutations. More research is needed to better understand the molecular and genomic contexts in which non–V600 BRAF-mutant driver oncogenes exist. Additionally, future studies may yield more benefit if therapeutic approaches are tailored according to BRAF class and primary tumor type. These strategies may include BRAF or pan-RAF inhibitors plus MEK or ERK inhibition for class 2 mutants and EGFR inhibition (± BRAF/pan-RAF/MEK/ERK inhibition) for class 3 mutants, and in BRAF non–V600-mutated CRC.50 Finally, because of modest RRs with MAPK inhibitor monotherapies,24,25 future clinical trials should incorporate combination therapy strategies to more effectively target tumors with non-V600 BRAF mutations.
ACKNOWLEDGMENT
M.D. and Y.W. acknowledge support from Vanier Canada Graduate Scholarships. G.Z. and A.A.N.R. acknowledge support from Fonds de Recherche du Québec—Santé (FRQS) Clinical Research Scholar awards.
Benny Johnson
Consulting or Advisory Role: Gritstone Bio, Incyte, Taiho Oncology, Insmed
Research Funding: Bristol Myers Squibb (Inst), Syntrix Biosystems (Inst)
Ibiayi Dagogo-Jack
Honoraria: American Academic Health System, Total Health Conferencing, DAVA Oncology, Creative Educational Concepts, Medscape, ASCO Post, OncLive/MJH Life Sciences
Consulting or Advisory Role: Boehringer Ingelheim, AstraZeneca, Xcovery, Catalyst Pharmaceuticals, Pfizer, Syros Pharmaceuticals, Novocure, BostonGene, Bayer, Genentech, Sanofi, Janssen
Research Funding: Pfizer (Inst), Array BioPharma (Inst), Novartis (Inst), Genentech (Inst), Calithera Biosciences (Inst), Vivace Therapeutics (Inst)
Travel, Accommodations, Expenses: Pfizer, Array BioPharma
Nathaniel J. Myall
Honoraria: Patient Power
Georg Richtig
Travel, Accommodations, Expenses: Ipsen
Marco Gerlinger
Stock and Other Ownership Interests: Vertex
Research Funding: Bristol Myers Squibb, Merck KGaA, Roche/Genentech
Eiji Shinozaki
Honoraria: Yakult Honsha, Merck Serono, Chugai Pharma, Takeda, Sanofi, Lilly, Taiho Pharmaceutical, Daiichi Sankyo/UCB Japan
Takayuki Yoshino
Honoraria: Chugai Pharma, Merck, Bayer Yakuhin, Ono Pharmaceutical, MSD K.K.
Research Funding: MSD (Inst), Daiichi Sankyo Company, Limited (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Amgen (Inst), Sanofi (Inst), Pfizer (Inst), Genomedia (Inst), Sysmex (Inst), Nippon Boehringer Ingelheim (Inst), Chugai Pharma (Inst)
Daisuke Kotani
Honoraria: Takeda, Chugai Pharma, Lilly Japan, Merck Serono, Sysmex, Taiho Pharmaceutical, Ono Pharmaceutical, Bristol Myers Squibb Japan, MSD, Daiichi Sankyo/UCB Japan
Research Funding: Ono Pharmaceutical (Inst), MSD (Inst), Novartis (Inst), Janssen (Inst), IQvia (Inst), Syneos Health (Inst), CMIC (Inst)
Jason R. Fangusaro
Honoraria: AstraZeneca, Pyramid Biosciences
Consulting or Advisory Role: Celgene, AstraZeneca
Oliver Gautschi
Consulting or Advisory Role: Amgen (Inst), Lilly (Inst)
Other Relationship: Bayer (Inst), Pfizer (Inst), Roche (Inst), Merck, Lilly
Julien Mazieres
Consulting or Advisory Role: Novartis, Roche/Genentech, Pfizer, Bristol Myers Squibb, Lilly/ImClone, MSD, AstraZeneca, Pierre Fabre, Blueprint Medicines, Hengrui Therapeutics
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pierre Fabre (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, Bristol Myers Squibb
Jeffrey A. Sosman
Honoraria: Jazz Pharmaceuticals, Apexian Pharmaceuticals, Iovance Biotherapeutics
Consulting or Advisory Role: Apexigen, Jazz Pharmaceuticals, Iovance Biotherapeutics
Scott Kopetz
This author is a member of the JCO Precision Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: MolecularMatch, Lutris, Iylon, Frontier Medicines
Consulting or Advisory Role: Genentech, EMD Serono, Merck, Holy Stone Healthcare, Novartis, Lilly, Boehringer Ingelheim, Boston Biomedical, AstraZeneca/MedImmune, Bayer Health, Pierre Fabre, Redx Pharma, Ipsen, Daiichi Sankyo, Natera, HalioDx, Lutris, Jacobio, Pfizer, Repare Therapeutics, Inivata, GlaxoSmithKline, Jazz Pharmaceuticals, Iylon, Xilis, AbbVie, Amal Therapeutics, Gilead Sciences, Mirati Therapeutics, Flame Biosciences, Servier, Carina Biotech, Bicara Therapeutics, Endeavor BioMedicines, Numab, Johnson & Johnson/Janssen, Genomic Health, Frontier Medicines, Replimune, Taiho Pharmaceutical
Research Funding: Sanofi, Biocartis, Guardant Health, Array BioPharma, Genentech/Roche, EMD Serono, MedImmune, Novartis, Amgen, Lilly, Daiichi Sankyo
Vivek Subbiah
Consulting or Advisory Role: MedImmune, Helsinn Therapeutics, Loxo, R-Pharm, QED Therapeutics
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), NanoCarrier (Inst), Northwest Biotherapeutics (Inst), Genentech/Roche (Inst), Berg Pharma (Inst), Bayer (Inst), Incyte (Inst), Fujifilm (Inst), PharmaMar (Inst), D3 Oncology Solutions (Inst), Pfizer (Inst), Amgen (Inst), AbbVie (Inst), Multivir (Inst), Blueprint Medicines (Inst), LOXO (Inst), Vegenics (Inst), Takeda (Inst), Alfasigma (Inst), Agensys (Inst), Idera (Inst), Boston Biomedical (Inst), Inhibrx (Inst), Exelixis (Inst), Turning Point Therapeutics (Inst)
Travel, Accommodations, Expenses: PharmaMar, Bayer, Novartis, Helsinn Therapeutics
Other Relationship: Medscape
Michael A. Davies
Consulting or Advisory Role: Genentech/Roche, Novartis, Bristol Myers Squibb, NanoString Technologies, Array BioPharma, Apexigen, Pfizer, Eisai, ABM, Iovance Biotherapeutics
Research Funding: GlaxoSmithKline (Inst), Genentech/Roche (Inst), AstraZeneca (Inst), Merck (Inst), Oncothyreon (Inst), Myriad Genetics (Inst), Sanofi (Inst), Pfizer (Inst), ABM (Inst), LEAD (Inst)
Anna L. Groover
Employment: BioMed Valley Discoveries
Ryan J. Sullivan
Consulting or Advisory Role: Novartis, Merck, Replimune, Asana Biosciences, Alkermes, Eisai, Pfizer, Iovance Biotherapeutics, OncoSec, AstraZeneca, Bristol Myers Squibb
Research Funding: Amgen (Inst), Lilly (Inst), BioMed Valley Discoveries (Inst), Merck (Inst), Deciphera (Inst), Roche/Genentech (Inst), Moderna Therapeutics (Inst), Sanofi (Inst), Aeglea Biotherapeutics (Inst), Asana Biosciences (Inst), Viralytics (Inst), Compugen (Inst), Neon Therapeutics (Inst), Pfizer (Inst), BeiGene (Inst), Rubius Therapeutics (Inst), Strategia (Inst)
Keith T. Flaherty
Stock and Other Ownership Interests: Clovis Oncology, Loxo, X4 Pharma, Strata Oncology, PIC Therapeutics, Apricity Health, Oncoceutics, FOGPharma, Tvardi Therapeutics, Checkmate Pharmaceuticals, Kinnate Biopharma, Scorpion Therapeutics, ALX Oncology, xCures, Monopteros Therapeutics, Vibliome Therapeutics, Transcode Therapeutics, Soley Therapeutics, Nextech Invest
Consulting or Advisory Role: Novartis, Lilly, Oncoceutics, Tvardi Therapeutics, Takeda, Debiopharm Group, OmRx
Douglas B. Johnson
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Novartis, Iovance Biotherapeutics, Catalyst Pharmaceuticals, Oncosec, Pfizer, Mosaic ImmunoEngineering, Targovax, Mallinckrodt
Research Funding: Incyte, Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Intellectual property and patents pending surrounding use of MHC-II and response to immune therapy
David W. Cescon
Consulting or Advisory Role: Pfizer, AstraZeneca, Novartis, GlaxoSmithKline, Merck, Roche/Genentech, Exact Sciences, Gilead Sciences, Eisai
Research Funding: Merck (Inst), Roche/Genentech (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Inivata (Inst), AstraZeneca (Inst), Gilead Sciences (Inst)
Patents, Royalties, Other Intellectual Property: Patent (US62/675,228) for methods of treating cancers characterized by a high expression level of spindle and kinetochore-associated complex subunit 3 (ska3) gene
Uncompensated Relationships: Inivata
Anna Spreafico
Honoraria: Bristol Myers Squibb, Medison & Immunocore
Consulting or Advisory Role: Novartis, Merck, Bristol Myers Squibb, Oncorus, Medison & Immunocore
Research Funding: Bristol Myers Squibb, Novartis, Merck, Symphogen, AstraZeneca/MedImmune, Bayer, Surface Oncology, Janssen Oncology, Northern Biologics, Replimune, Roche, Alkermes, Array BioPharma, GlaxoSmithKline, Treadwell Therapeutics (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Merck, Bristol Myers Squibb, Idera, Bayer, Janssen Oncology, Roche
George Zogopoulos
Patents, Royalties, Other Intellectual Property: TC-PTP inhibitors as APC activators for immunotherapy
Travel, Accommodations, Expenses: Baxalta
April A.N. Rose
Employment: Merck (I)
Stock and Other Ownership Interests: Merck (I)
Consulting or Advisory Role: Pfizer
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented as a poster at ASCO Annual Meeting, virtual, June 4-8, 2021.
SUPPORT
This research was funded by a Conquer Cancer Foundation of ASCO Young Investigator Award and a Canadian Cancer Society Challenge Grant (Grant #707457) to AANR.
M.D. and Y.W. contributed equally to this work.
PREPRINT VERSION
Preprint version available on https://www.medrxiv.org/content/10.1101/2022.02.17.22271120v1.
AUTHOR CONTRIBUTIONS
Conception and design: Matthew Dankner, Yifan Wang, Rouhi Fazelzad, Takayuki Yoshino, George Zogopoulos, April A.N. Rose
Financial support: George Zogopoulos, April A.N. Rose
Administrative support: Matthew Dankner, Scott Kopetz, George Zogopoulos, April A.N. Rose
Provision of study materials or patients: Benny Johnson, Georg Richtig, Marco Gerlinger, Eiji Shinozaki, Oliver Gautschi, Jeffrey A. Sosman, Scott Kopetz, Vivek Subbiah, Michael A. Davies, Ryan J. Sullivan, George Zogopoulos, April A.N. Rose
Collection and assembly of data: Matthew Dankner, Yifan Wang, Benny Johnson, Caroline A. Nebhan, Ibiayi Dagogo-Jack, Nathaniel J. Myall, Georg Richtig, Jillian W.P. Bracht, Marco Gerlinger, Takayuki Yoshino, Daisuke Kotani, Oliver Gautschi, Jeffrey A. Sosman, Scott Kopetz, Vivek Subbiah, Anna L. Groover, Ryan J. Sullivan, Douglas B. Johnson, Anna Spreafico, April A.N. Rose
Data analysis and interpretation: Matthew Dankner, Yifan Wang, Benny Johnson, Eiji Shinozaki, Jason R. Fangusaro, Oliver Gautschi, Julien Mazieres, Scott Kopetz, Vivek Subbiah, Michael A. Davies, Keith T. Flaherty, Andrea Benedetti, David W. Cescon, George Zogopoulos, April A.N. Rose
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Benny Johnson
Consulting or Advisory Role: Gritstone Bio, Incyte, Taiho Oncology, Insmed
Research Funding: Bristol Myers Squibb (Inst), Syntrix Biosystems (Inst)
Ibiayi Dagogo-Jack
Honoraria: American Academic Health System, Total Health Conferencing, DAVA Oncology, Creative Educational Concepts, Medscape, ASCO Post, OncLive/MJH Life Sciences
Consulting or Advisory Role: Boehringer Ingelheim, AstraZeneca, Xcovery, Catalyst Pharmaceuticals, Pfizer, Syros Pharmaceuticals, Novocure, BostonGene, Bayer, Genentech, Sanofi, Janssen
Research Funding: Pfizer (Inst), Array BioPharma (Inst), Novartis (Inst), Genentech (Inst), Calithera Biosciences (Inst), Vivace Therapeutics (Inst)
Travel, Accommodations, Expenses: Pfizer, Array BioPharma
Nathaniel J. Myall
Honoraria: Patient Power
Georg Richtig
Travel, Accommodations, Expenses: Ipsen
Marco Gerlinger
Stock and Other Ownership Interests: Vertex
Research Funding: Bristol Myers Squibb, Merck KGaA, Roche/Genentech
Eiji Shinozaki
Honoraria: Yakult Honsha, Merck Serono, Chugai Pharma, Takeda, Sanofi, Lilly, Taiho Pharmaceutical, Daiichi Sankyo/UCB Japan
Takayuki Yoshino
Honoraria: Chugai Pharma, Merck, Bayer Yakuhin, Ono Pharmaceutical, MSD K.K.
Research Funding: MSD (Inst), Daiichi Sankyo Company, Limited (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Amgen (Inst), Sanofi (Inst), Pfizer (Inst), Genomedia (Inst), Sysmex (Inst), Nippon Boehringer Ingelheim (Inst), Chugai Pharma (Inst)
Daisuke Kotani
Honoraria: Takeda, Chugai Pharma, Lilly Japan, Merck Serono, Sysmex, Taiho Pharmaceutical, Ono Pharmaceutical, Bristol Myers Squibb Japan, MSD, Daiichi Sankyo/UCB Japan
Research Funding: Ono Pharmaceutical (Inst), MSD (Inst), Novartis (Inst), Janssen (Inst), IQvia (Inst), Syneos Health (Inst), CMIC (Inst)
Jason R. Fangusaro
Honoraria: AstraZeneca, Pyramid Biosciences
Consulting or Advisory Role: Celgene, AstraZeneca
Oliver Gautschi
Consulting or Advisory Role: Amgen (Inst), Lilly (Inst)
Other Relationship: Bayer (Inst), Pfizer (Inst), Roche (Inst), Merck, Lilly
Julien Mazieres
Consulting or Advisory Role: Novartis, Roche/Genentech, Pfizer, Bristol Myers Squibb, Lilly/ImClone, MSD, AstraZeneca, Pierre Fabre, Blueprint Medicines, Hengrui Therapeutics
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pierre Fabre (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, Bristol Myers Squibb
Jeffrey A. Sosman
Honoraria: Jazz Pharmaceuticals, Apexian Pharmaceuticals, Iovance Biotherapeutics
Consulting or Advisory Role: Apexigen, Jazz Pharmaceuticals, Iovance Biotherapeutics
Scott Kopetz
This author is a member of the JCO Precision Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: MolecularMatch, Lutris, Iylon, Frontier Medicines
Consulting or Advisory Role: Genentech, EMD Serono, Merck, Holy Stone Healthcare, Novartis, Lilly, Boehringer Ingelheim, Boston Biomedical, AstraZeneca/MedImmune, Bayer Health, Pierre Fabre, Redx Pharma, Ipsen, Daiichi Sankyo, Natera, HalioDx, Lutris, Jacobio, Pfizer, Repare Therapeutics, Inivata, GlaxoSmithKline, Jazz Pharmaceuticals, Iylon, Xilis, AbbVie, Amal Therapeutics, Gilead Sciences, Mirati Therapeutics, Flame Biosciences, Servier, Carina Biotech, Bicara Therapeutics, Endeavor BioMedicines, Numab, Johnson & Johnson/Janssen, Genomic Health, Frontier Medicines, Replimune, Taiho Pharmaceutical
Research Funding: Sanofi, Biocartis, Guardant Health, Array BioPharma, Genentech/Roche, EMD Serono, MedImmune, Novartis, Amgen, Lilly, Daiichi Sankyo
Vivek Subbiah
Consulting or Advisory Role: MedImmune, Helsinn Therapeutics, Loxo, R-Pharm, QED Therapeutics
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), NanoCarrier (Inst), Northwest Biotherapeutics (Inst), Genentech/Roche (Inst), Berg Pharma (Inst), Bayer (Inst), Incyte (Inst), Fujifilm (Inst), PharmaMar (Inst), D3 Oncology Solutions (Inst), Pfizer (Inst), Amgen (Inst), AbbVie (Inst), Multivir (Inst), Blueprint Medicines (Inst), LOXO (Inst), Vegenics (Inst), Takeda (Inst), Alfasigma (Inst), Agensys (Inst), Idera (Inst), Boston Biomedical (Inst), Inhibrx (Inst), Exelixis (Inst), Turning Point Therapeutics (Inst)
Travel, Accommodations, Expenses: PharmaMar, Bayer, Novartis, Helsinn Therapeutics
Other Relationship: Medscape
Michael A. Davies
Consulting or Advisory Role: Genentech/Roche, Novartis, Bristol Myers Squibb, NanoString Technologies, Array BioPharma, Apexigen, Pfizer, Eisai, ABM, Iovance Biotherapeutics
Research Funding: GlaxoSmithKline (Inst), Genentech/Roche (Inst), AstraZeneca (Inst), Merck (Inst), Oncothyreon (Inst), Myriad Genetics (Inst), Sanofi (Inst), Pfizer (Inst), ABM (Inst), LEAD (Inst)
Anna L. Groover
Employment: BioMed Valley Discoveries
Ryan J. Sullivan
Consulting or Advisory Role: Novartis, Merck, Replimune, Asana Biosciences, Alkermes, Eisai, Pfizer, Iovance Biotherapeutics, OncoSec, AstraZeneca, Bristol Myers Squibb
Research Funding: Amgen (Inst), Lilly (Inst), BioMed Valley Discoveries (Inst), Merck (Inst), Deciphera (Inst), Roche/Genentech (Inst), Moderna Therapeutics (Inst), Sanofi (Inst), Aeglea Biotherapeutics (Inst), Asana Biosciences (Inst), Viralytics (Inst), Compugen (Inst), Neon Therapeutics (Inst), Pfizer (Inst), BeiGene (Inst), Rubius Therapeutics (Inst), Strategia (Inst)
Keith T. Flaherty
Stock and Other Ownership Interests: Clovis Oncology, Loxo, X4 Pharma, Strata Oncology, PIC Therapeutics, Apricity Health, Oncoceutics, FOGPharma, Tvardi Therapeutics, Checkmate Pharmaceuticals, Kinnate Biopharma, Scorpion Therapeutics, ALX Oncology, xCures, Monopteros Therapeutics, Vibliome Therapeutics, Transcode Therapeutics, Soley Therapeutics, Nextech Invest
Consulting or Advisory Role: Novartis, Lilly, Oncoceutics, Tvardi Therapeutics, Takeda, Debiopharm Group, OmRx
Douglas B. Johnson
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Novartis, Iovance Biotherapeutics, Catalyst Pharmaceuticals, Oncosec, Pfizer, Mosaic ImmunoEngineering, Targovax, Mallinckrodt
Research Funding: Incyte, Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Intellectual property and patents pending surrounding use of MHC-II and response to immune therapy
David W. Cescon
Consulting or Advisory Role: Pfizer, AstraZeneca, Novartis, GlaxoSmithKline, Merck, Roche/Genentech, Exact Sciences, Gilead Sciences, Eisai
Research Funding: Merck (Inst), Roche/Genentech (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Inivata (Inst), AstraZeneca (Inst), Gilead Sciences (Inst)
Patents, Royalties, Other Intellectual Property: Patent (US62/675,228) for methods of treating cancers characterized by a high expression level of spindle and kinetochore-associated complex subunit 3 (ska3) gene
Uncompensated Relationships: Inivata
Anna Spreafico
Honoraria: Bristol Myers Squibb, Medison & Immunocore
Consulting or Advisory Role: Novartis, Merck, Bristol Myers Squibb, Oncorus, Medison & Immunocore
Research Funding: Bristol Myers Squibb, Novartis, Merck, Symphogen, AstraZeneca/MedImmune, Bayer, Surface Oncology, Janssen Oncology, Northern Biologics, Replimune, Roche, Alkermes, Array BioPharma, GlaxoSmithKline, Treadwell Therapeutics (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Merck, Bristol Myers Squibb, Idera, Bayer, Janssen Oncology, Roche
George Zogopoulos
Patents, Royalties, Other Intellectual Property: TC-PTP inhibitors as APC activators for immunotherapy
Travel, Accommodations, Expenses: Baxalta
April A.N. Rose
Employment: Merck (I)
Stock and Other Ownership Interests: Merck (I)
Consulting or Advisory Role: Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dankner M, Rose AAN, Rajkumar S, et al. : Classifying BRAF alterations in cancer: New rational therapeutic strategies for actionable mutations. Oncogene 37:3183-3199, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Ascierto PA, Dummer R, Gogas HJ, et al. : Update on tolerability and overall survival in COLUMBUS: Landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. Eur J Cancer 126:33-44, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Karaszewska B, Schachter J, et al. : Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 372:30-39, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Ascierto PA, McArthur GA, Dréno B, et al. : Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 17:1248-1260, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Flaherty KT, Robert C, Hersey P, et al. : Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367:107-114, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Kopetz S, Grothey A, Yaeger R, et al. : Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med 381:1632-1643, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Swetter SM, Thompson JA, Albertini MR, et al. : NCCN Guidelines® Insights: Melanoma: Cutaneous, Version 2.2021. J Natl Compr Canc Netw 19:364-376, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Benson AB, Venook AP, Al-Hawary MM, et al. : Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 19:329-359, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Planchard D, Smit EF, Groen HJM, et al. : Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol 18:1307-1316, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Yaeger R, Corcoran RB: Targeting alterations in the RAF-MEK pathway. Cancer Discov 9:329-341, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster SA, Whalen DM, Özen A, et al. : Activation mechanism of oncogenic deletion mutations in BRAF, EGFR, and HER2. Cancer Cell 29:477-493, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Jones DT, Kocialkowski S, Liu L, et al. : Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res 68:8673-8677, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross JS, Wang K, Chmielecki J, et al. : The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int J Cancer 138:881-890, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan PT, Garnett MJ, Roe SM, et al. : Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116:855-867, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Yao Z, Torres NM, Tao A, et al. : BRAF mutants evade ERK-dependent feedback by different mechanisms that determine their sensitivity to pharmacologic inhibition. Cancer Cell 28:370-383, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. : Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 548:234-238, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poulikakos PI, Persaud Y, Janakiraman M, et al. : RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 480:387-390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thevakumaran N, Lavoie H, Critton DA, et al. : Crystal structure of a BRAF kinase domain monomer explains basis for allosteric regulation. Nat Struct Mol Biol 22:37-43, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Dankner M, Lajoie M, Moldoveanu D, et al. : Dual MAPK inhibition is an effective therapeutic strategy for a subset of class II BRAF mutant melanomas. Clin Cancer Res 24:6483-6494, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Dahlman KB, Xia J, Hutchinson K, et al. : BRAFL597 mutations in melanoma are associated with sensitivity to MEK inhibitors. Cancer Discov 2:791-797, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahadoran P, Allegra M, Le Duff F, et al. : Major clinical response to a BRAF inhibitor in a patient with a BRAF L597R-mutated melanoma. J Clin Oncol 31:e324-e326, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Noeparast A, Teugels E, Giron P, et al. : Non-V600 BRAF mutations recurrently found in lung cancer predict sensitivity to the combination of trametinib and dabrafenib. Oncotarget 8:60094-60108, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajkumar S, Berry D, Heney KA, et al. : Melanomas with concurrent BRAF non-p.V600 and NF1 loss-of-function mutations are targetable by BRAF/MEK inhibitor combination therapy. Cell Rep 39:110634, 2022 [DOI] [PubMed] [Google Scholar]
- 24.Nebhan CA, Johnson DB, Sullivan RJ, et al. : Efficacy and safety of trametinib in non-V600 BRAF mutant melanoma: A phase II study. Oncologist 26:731-e1498, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson DB, Zhao F, Noel M, et al. : Trametinib activity in patients with solid tumors and lymphomas harboring BRAF non-V600 mutations or fusions: Results from NCI-MATCH (EAY131). Clin Cancer Res 26:1812-1819, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, et al. : Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 151:264-269, W64, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Babineau J: Product Review: Covidence (Systematic Review Software). J Can Health Libr Assoc 35:68-71, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz LH, Litière S, de Vries E, et al. : RECIST 1.1—Update and clarification: From the RECIST committee. Eur J Cancer 62:132-137, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dankner M: Targeted therapy for colorectal cancers with non-V600 BRAF mutations: Perspectives for precision oncology. JCO Precis Oncol 2:1-12, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Yaeger R, Kotani D, Mondaca S, et al. : Response to anti-EGFR therapy in patients with BRAF non-V600-mutant metastatic colorectal cancer. Clin Cancer Res 25:7089-7097, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blumenschein GR Jr, Smit EF, Planchard D, et al. : A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC). Ann Oncol 26:894-901, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dummer R, Schadendorf D, Ascierto PA, et al. : Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 18:435-445, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Rose A, Ayodele O, Genta S, et al. : 531P binimetinib and encorafenib for the treatment of advanced solid tumors with non-V600E BRAF mutations (mts): Preliminary results of the investigator initiated phase II BEAVER trial. Ann Oncol 32:S596, 2021 [Google Scholar]
- 34.Johnson B, Loree JM, Jacome AA, et al. : Atypical, non-V600 BRAF mutations as a potential mechanism of resistance to EGFR inhibition in metastatic colorectal cancer. JCO Precis Oncol 3:1-10, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woolston A, Khan K, Spain G, et al. : Genomic and transcriptomic determinants of therapy resistance and immune landscape evolution during anti-EGFR treatment in colorectal cancer. Cancer Cell 36:35-50.e9, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Cutsem E, Huijberts S, Grothey A, et al. : Binimetinib, encorafenib, and cetuximab triplet therapy for patients with BRAF V600E-mutant metastatic colorectal cancer: Safety lead-in results from the phase III BEACON colorectal cancer study. J Clin Oncol 37:1460-1469, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotani D, Bando H, Taniguchi H, et al. : BIG BANG study (EPOC1703): Multicentre, proof-of-concept, phase II study evaluating the efficacy and safety of combination therapy with binimetinib, encorafenib and cetuximab in patients with BRAF non-V600E mutated metastatic colorectal cancer. ESMO Open 5:e000624, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greger JG, Eastman SD, Zhang V, et al. : Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol Cancer Ther 11:909-920, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Van Allen EM, Wagle N, Sucker A, et al. : The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov 4:94-109, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skoulidis F, Li BT, Dy GK, et al. : Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med 384:2371-2381, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nathanson KL, Martin AM, Wubbenhorst B, et al. : Tumor genetic analyses of patients with metastatic melanoma treated with the BRAF inhibitor dabrafenib (GSK2118436). Clin Cancer Res 19:4868-4878, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paraiso KH, Xiang Y, Rebecca VW, et al. : PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res 71:2750-2760, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karoulia Z, Gavathiotis E, Poulikakos PI: New perspectives for targeting RAF kinase in human cancer. Nat Rev Cancer 17:676-691, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Posternak G, Tang X, Maisonneuve P, et al. : Functional characterization of a PROTAC directed against BRAF mutant V600E. Nat Chem Biol 16:1170-1178, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adamopoulos C, Ahmed TA, Tucker MR, et al. : Exploiting allosteric properties of RAF and MEK inhibitors to target therapy-resistant tumors driven by oncogenic BRAF signaling. Cancer Discov 11:1716-1735, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Planchard D, Besse B, Groen HJM, et al. : Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: An open-label, multicentre phase 2 trial. Lancet Oncol 17:984-993, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subbiah V, Lassen U, Élez E, et al. : Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol 21:1234-1243, 2020 [DOI] [PubMed] [Google Scholar]
- 48.Grob JJ, Amonkar MM, Karaszewska B, et al. : Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): Results of a phase 3, open-label, randomised trial. Lancet Oncol 16:1389-1398, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Long GV, Stroyakovskiy D, Gogas H, et al. : Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet 386:444-451, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Johnson B, Kopetz S: Applying precision to the management of BRAF-mutant metastatic colorectal cancer. Target Oncol 15:567-577, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]