Abstract
Ideally a diagnosis of infection of the central nervous system (CNS) is made by culture of the etiologic pathogen, but Borrelia burgdorferi, the causative agent of Lyme neuroborreliosis (LNB), is rarely cultured from the cerebrospinal fluid (CSF). PCR and measurement of specific antibody in the CSF also have their limitations. The role of available assays for LNB has not been studied carefully in a comparative investigation. There is a need to assess the reliability of assays and to increase the ability to document active infection in the CNS. The recent development of the nonhuman primate (NHP) model of LNB allowed us to address this need in a faithful model of human LNB. In this study we compared the abilities of PCR and culture to detect the presence of spirochetes in the CSF and brain tissue of infected NHPs and related these measures of infection to the development of anti-B. burgdorferi antibody. We also tested a bioassay, the mouse infectivity test (MIT), in this model. Fourteen of 16 CSFs from four NHPs were positive by at least one of these techniques. Detection of spirochetes in the CSF by PCR, the MIT, and culture was inversely related to the concomitant presence of anti-B. burgdorferi antibody intrathecally. The performance of any particular test was associated with the strength of the host immune response. In early CNS infection, when anti-B. burgdorferi antibody had not yet appeared, or in immunocompromised hosts, the MIT compared favorably to culture and PCR for infected NHPs; antibody in the CSF was the most useful assay for immunocompetent NHPs.
The diagnosis of Lyme neuroborreliosis (LNB) can frequently be made by characteristic clinical findings (18). Positive serologies in the serum or cerebrospinal fluid (CSF) are helpful to confirm the diagnosis. However, neurological involvement can have protean clinical manifestations (7), and the appearance of antibody in the serum or CSF may be delayed for many weeks to months after infection. Ideally, the causative organism, Borrelia burgdorferi, would be cultured from the CSF, but the spirochete is highly fastidious, and culture has poor diagnostic sensitivity (4). PCR assays have aided diagnosis but have a high incidence of false negativity (6, 10). Thus, there is a need for additional tests to improve detection of the spirochete in CSF and neurological tissue in LNB.
The recent development of the nonhuman primate (NHP) model (11, 12, 19), which is the only faithful model of LNB (1), has set the stage for answering many questions about LNB and has raised the possibility of developing diagnostic assays which may have utility in human LNB.
Transfer of infection from human CSF to experimental animals is considered to be the “gold standard” for the related spirochetal infection neurosyphilis (21, 24) and has been used to confirm human relapsing fever. This technique has not been tested for LNB. Since the two infections are closely related, we hypothesized that transfer of infection from CSF or tissue to mice, i.e., the mouse infectivity test (MIT), may be a useful adjunct in the diagnosis of neuroborreliosis. We tested this hypothesis in NHP LNB and compared the ability of the MIT to detect the presence of the spirochete in CSF and tissues to those of PCR, culture, and anti-B. burgdorferi antibody determination.
MATERIALS AND METHODS
NHPs and spirochetes.
The four adult rhesus macaques (Macaca mulatta; two male and two female) used in this study were housed, cared for, and anesthetized and underwent cisternal punctures as previously described (12). This housing and care was in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (24a) in facilities accredited by the American Association for Accreditation of Laboratory Animal Care. Prior to initiation, the study was reviewed and approved by the Georgetown University Animal Care and Use Committee.
Two NHPs, PAX219 (male) and Z1 (female), were treated orally with dexamethasone (2 mg/kg of body weight/day for 1 week, then 1 mg/kg/day) for 9.5 weeks after infection, a dosage considered low to moderate for rhesus macaques; these NHPs will be referred to as “immunocompromised.” Two NHPs, Z23 and E680, did not receive dexamethasone and will be referred to as “immunocompetent.” The immunocompromised NHPs were necropsied 9.5 weeks postinfection, after euthanasia with ketamine, xylazine, and pentobarbital. The immunocompetent NHPs were necropsied at 13 weeks postinfection. Prior to necropsy, the NHPs were perfused with 4 liters of normal salines.
The N40Br strain was used for intradermal inoculations as previously described (12). This strain has been passaged through mouse and NHP brains (12–14) and has resulted in central nervous system (CNS) invasion in all 21 NHPs tested thus far.
MIT.
CSF or tissue homogenate (0.1 ml) was injected intradermally immediately after being obtained into each of two mice per homogenate sample. The mice were given 0.25 μg of dexamethasone on the day prior to infection and daily for the 2 weeks following to delay the antibody response and allow a small inoculum to establish infection. Six weeks after injection, i.e., 4 weeks after completion of the dexamethasone treatment, the mice were bled and if the enzyme-linked immunosorbent assays (ELISAs) or immunoblots were positive, the mice were sacrificed and the hearts were cultured. Mice which were seronegative on the bleed at 6 weeks were retested at 12 weeks. The requirements for a positive MIT were all of the following: (i) positive ELISAs for anti-B. burgdorferi immunoglobulin G (IgG) antibodies in the sera of the recipient mice, (ii) positive immunoblots (IgG) of the sera of the recipient mice, and (iii) positive heart cultures of the recipient mice at sacrifice.
Culture and antibody measurement of mouse serum and NHP serum and CSF.
A sample of CSF was analyzed immediately after withdrawal for a cell count; no CSF had more than 1,000 erythrocytes/mm3. The CSF and tissues were cultured as previously described (12). Serum and CSF antibody studies were performed as previously described (9, 12, 13). In brief, the antigens used in the ELISAs and immunoblots were sonicates of the strain N40Br. Two hundred microliters of antigen coating solution was added to each well of a microtitration plate (Linbro Scientific, Hamden, Conn.) at a concentration of 5 μg/ml and incubated overnight at 4°C. The plates were washed three times with phosphate-buffered saline–0.05% Tween 20, and 200 μl of the sera was added at 1/500 dilution. The plates were incubated for 2 h at 37°C and then washed again as described above. Two hundred microliters of horseradish peroxidase-conjugated goat anti-human (or anti-mouse for mouse serum) immunoglobulin, isotypes G, A, and M (Organon Teknika-Cappel, Malvern, Pa.), was diluted 1:10,000 in phosphate-buffered saline-Tween 20 and added to each well. Incubation followed for 2 h at 37°C. The plates were washed, and 200 μl of TMB microwell peroxidase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) was added to each well, immediately after which 50 μl of 8% sulfuric acid was added to stop the reaction. The plates were read immediately on an ELISA spectrophotometer (Bio-Rad) at 450 nm. On each plate, a standard positive control was run within its linear range of dilutions, and plate-to-plate comparisons were adjusted on the basis of the readings within the linear range of the positive controls on each plate. All serum and CSF studies were performed in duplicate.
Immunoblotting of mouse and NHP sera was performed as previously described (12, 15), except that commercial B. burgdorferi nitrocellulose strips (Microbiology Reference Laboratory, Cypress, Calif.) were used instead of strips made from electrotransferred sodium dodecyl sulfate-polyacrylamide gels. The criteria for immunoblot positivity were the same as those outlined by the Centers for Disease Control and Prevention-American Society of Public Health Laboratory Directors for IgG reactivity for human Lyme disease, a modification of those published by Dressler et al. (3): for IgM, reactivity to at least two of the 23, 39, and 41 kDa proteins; for IgG, reactivity to five or more of the 18, 21, 30, 39, 41, 45, 58, 66, and 93 kDa proteins.
PCR.
Analysis of tissue and CSF samples from the NHPs for B. burgdorferi DNA was performed by PCR-ELISA, using techniques previously described (8, 11, 12). Whole-blood DNA was extracted as previously described (12). CSFs were boiled prior to the PCR to inactivate proteinases. Minced tissues were treated with proteinase K, extracted with phenol-chloroform, and precipitated with ethanol. The ratios of the optical densities at 260 nm (OD260s) and OD280s the extracted DNAs prior to PCR were required to be 1.5 or greater; if they were less than 1.5, extraction was repeated. If after reextraction the ratio of the sample was not greater than 1.5, the sample was not used and another frozen block of tissue was processed.
All reagents were from the PCR-ELISA reagents provided by Boehringer-Mannheim (Indianapolis, Ind.) unless otherwise stated. Five hundred nanograms of tissue DNA was then used as a template; digoxigenin-11-UTP (0.01 M) was one of the nucleotides added to the PCR mixture. Both OspA (20) and OspB PCR-ELISAs were run separately on all specimens, and the specimens were considered positive only if both PCRs were positive.
The PCR product was subjected to hybridization with the appropriate biotinylated probe (biotinylation was performed at Lofstrand Laboratories, Gaithersburg, Md.) and subsequently captured on a streptavidin-coated plate. The PCR product bound on the plate was detected by addition of an anti-digoxigenin antibody conjugated to alkaline phosphatase with subsequent color development with substrate. NHP brain and heart tissue from uninfected animals served as negative controls, and previously positive DNA and plasmid DNA containing the target served as positive controls. The OD reading of the ELISA plate, which served as a cutoff for positivity, was the mean plus 4 standard deviations of the negative controls.
The following probes and primers for the two B. burgdorferi genes (the OspA and OspB genes) were used in the PCR-ELISA: OspA149 (5′-TTATGAAAAAATATTTATTGGGAAT), OspA319 (5′-CTTTAAGCTCAAGCTTGTCTACTGT), and the probe OspAwt3 (5′-AGCGTTTCAGTAGATTTGCCTGCTGGTG) and OspB-1110 (5′-AAACGCTAAACAAGACCTTCCTG), OspB-1411 (5′-AGCTTTGAGAGTTTCCTCTGTTATTGA), and the probe 5′-TGAGGCTTTGAACCTTCAAGGGTTCCAGAACC.
RESULTS
CSF and serum.
The MIT, culture, PCR, and antibody studies of the CSF obtained at the time of inoculation prior to infection were negative in all four animals. A previous study (12) had documented the fact that CNS invasion did not occur prior to 3 weeks after inoculation in NHP LNB; thus, the earliest cisternal tap was done 3.5 weeks after infection. Table 1 shows the data on the CSFs at 3.5 weeks and later for the four NHPs. For CSFs obtained 3.5 weeks or more after inoculation, one or more of the four assays were positive for 14 of 16 CSF specimens.
TABLE 1.
PCR, MIT, culture, and antibody results from CSF
| NHP | Test | Results atc:
|
|||
|---|---|---|---|---|---|
| 3.5 wk | 5.5 wk | 7.5 wk | 9.5 wk | ||
| PAX219a | MIT | + | + | − | − |
| Culture | − | − | − | + | |
| PCR | − | + | − | + | |
| Antibody | − | − | − | − | |
| Z1a | MIT | − | + | + | − |
| Culture | + | + | − | + | |
| PCR | − | + | − | − | |
| Antibody | − | − | − | − | |
| E680b | MIT | − | − | − | − |
| Culture | − | − | − | − | |
| PCR | + | − | − | − | |
| Antibody | + | + | + | + | |
| Z23b | MIT | − | − | − | − |
| Culture | − | − | − | − | |
| PCR | − | − | − | − | |
| Antibody | − | + | + | + | |
Immunosuppressed with dexamethasone.
Immunocompetent.
+, positive; −, negative.
For seven of eight specimens from the immunocompromised NHPs, one or more tests of infection were positive (Table 1). For four of eight CSFs obtained after the baseline studies of the immunocompromised NHPs, the MIT was positive. This compared favorably to 3 of 8 culture positivity and 4 of 8 PCR positivity. The MIT was the only test positive in 2 of 8 CSF samples from the immunocompromised NHPs.
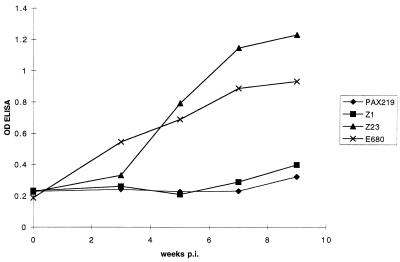
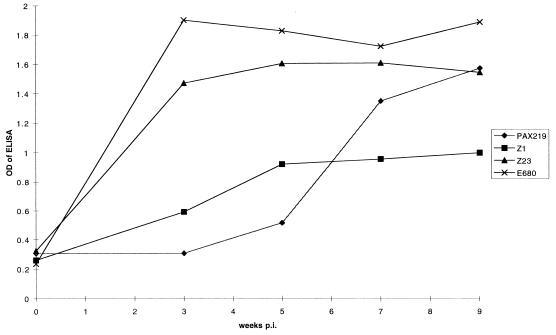
CSF antibody measured by ELISA was negative in all CSF specimens from immunocompromised NHPs (Fig. 1), despite a low level of specific antibody that appeared in the blood by week 3.5 and steadily rose by the time of necropsy at week 9.5 (Fig. 2). None of the CSFs from the immunocompromised NHPs had a lymphocytic pleocytosis. Serum immunoblot was negative at week 3.5, positive for only IgM at week 5.5, and negative thereafter. Although sera from the immunocompromised NHPs had a number of B. burgdorferi-specific and nonspecific IgG bands on immunoblots, none of the samples met the criteria for positivity, i.e., having five or more B. burgdorferi-specific bands. Since current Centers for Disease Control and Prevention criteria for positive Lyme serologies require immunoblot positivity, none of these NHPs seroconverted, according to this definition.
FIG. 1.
CSF anti-B. burgdorferi antibody levels as a function of time postinoculation (p.i.). OD is presented as raw data, e.g., the OD when saline was used as the antibody source was 0.2 to 0.25. Serum was tested at a dilution of 1:500.
FIG. 2.
Serum anti-B. burgdorferi antibody levels as a function of time postinoculation (p.i.). OD is presented as raw data, e.g., the OD when saline was used as the antibody source was 0.2 to 0.25. CSF was tested at a dilution of 1:10.
In the CSFs obtained from immunocompetent NHPs, specific antibody was detectable by 5.5 weeks after inoculation (Fig. 1). CSF pleocytosis, a leukocyte count above 5/mm3, was present in samples from week 5.5 in E680 and weeks 5.5 and 7.5 in Z23. Serum antibody levels were much higher in the two immunocompetent NHPs, as shown for 1:500 dilutions of sera in Fig. 2; this dilution of the sera was chosen to demonstrate the very slow development of serum antibody in the immunocompromised NHPs. When higher dilutions of sera were used the differences between the two groups in the ODs of the week 7.5 and 9.5 sera were even more striking. Sera were immunoblot positive for both IgM and IgG at the week 3.5 analysis. The sera from these NHPs remained IgG immunoblot positive, but the IgM response became negative in both animals after week 5.5.
Blood PCR and culture were negative in all NHPs tested except for the blood PCR from PAX219, which was positive on the week 3.5 blood draw. No animal developed any clinically evident disease, which was consistent with our previous study (12).
Tissue.
The MIT outperformed culture in samples from the CNSs of the immunocompromised NHPs in that culture of the CNS was negative for all five specimens tested while the MIT was positive for two of these specimens (Table 2). The MIT and PCR were concordant for five of the six tissue samples. The MIT was positive for one sample which was negative by both of the other studies, i.e., the pons from PAX219, and PCR was positive for one sample which was negative by both of the other studies, i.e., the medulla from PAX 219.
TABLE 2.
PCR, MIT, and culture results from tissues obtained at necropsy
| NHP | Tissue | Resultsa
|
||
|---|---|---|---|---|
| MIT | PCR | Culture | ||
| PAX219 | Pons | + | − | − |
| Medulla | − | + | − | |
| Midbrain | − | − | − | |
| Z1 | Pons | + | + | − |
| Temporal lobe | − | − | − | |
| Heart base | + | + | + | |
| E680 | Pons | − | + | − |
| Medulla | − | − | − | |
| Cervical cord | − | + | − | |
| Z23 | Pons | − | + | − |
| Midbrain | − | − | − | |
| Heart base | − | + | − | |
+, positive; −, negative.
PCR was performed on an additional 18 CNS specimens, 5 peripheral nervous system tissues, and 6 extraneural organs from the two immunocompromised NHPs—tissues not tested by the MIT. PCR with both targets in these tissues was positive in nine, five, and six of these areas, respectively. The CNS localization was primarily in subtentorial structures, as reported for previous NHPs infected with N40Br (11). Culture was positive for only 1 of 13 of the CNS tissues from the immunocompromised NHPs, a thoracic spinal cord sample from PAX 219. The only positive culture of tissue from Z1 was a heart sample. Findings on PCR with the OspA gene target were confirmed by the second PCR assay with the OspB gene target for all CSF and tissue specimens tested.
The MIT and culture were negative for all six tissues sampled from the immunocompetent NHPs. The yield of detection of B. burgdorferi in tissue at necropsy was lower for these NHPs than for the immunocompromised group, with 5 of 21 CNS tissues, 3 of 7 peripheral nervous system tissues, and 3 of 9 extraneural organs positive.
DISCUSSION
The diagnosis of human LNB can be difficult. Its major clinical manifestations—meningitis, facial palsy, radiculitis, and neuritis—are nonspecific. In this infection, as in other bacterial infections of the CNS, culture of the causative agent from the CSF would be the most useful diagnostic assay. Positive cultures from patients with Lyme neuroborreliosis have been reported (4, 17), and there were positive cultures of some CSFs in this study, but generally culture has too low a yield to be relied upon exclusively. In both human and NHP Lyme neuroborreliosis (4, 12), culture positivity of the CSF occurs relatively early after CNS invasion, prior to the development of a significant intrathecal antibody response.
PCR of the CSF has also been able to identify spirochete DNA in clinical specimens. The sensitivity of this assay is much higher than that of culture in patients with Lyme meningitis, with positive assays in up to 40% of patients with early Lyme meningitis (5, 6, 10). However, CSF PCR suffers from a number of disadvantages: it is not standardized, it is expensive, contamination can be a common cause of false positives in laboratories not ideally suited for the technique, and it detects dead as well as live spirochetes.
The presence of specific anti-B. burgdorferi antibody in the CSF is the most widely used assay for Lyme neuroborreliosis. In the immunocompetent NHPs in our study it was a very successful assay for detection of CNS invasion. However, it is frequently false negative, especially early in the course of the infection or if there is transient immunosuppression. Transient suppression of the anti-B. burgdorferi immune response in humans could occur in instances of coinfection, i.e., simultaneous transmission via the tick of pathogen other than B. burgdorferi. Coinfection of ixodid ticks has been demonstrated for a number of pathogens, including the agent of human granulocytic ehrlichiosis (16), babesiosis (25), and tick-borne encephalitis virus, as well as for a newly described virus (23) and bacterium (22). In a recent study from an endemic area of New Jersey, 18% of infected ticks were infected with more than one readily identifiable pathogen (25); this number is likely an underestimate, since many tick-borne agents have not yet been identified. Infections, even subclinical ones, with a variety of pathogens have been demonstrated to suppress the expected host immune response (2). Thus, mild immunosuppression as accomplished in this study was designed to mimic conditions in the human host which allow B. burgdorferi in the natural state to gain a firm foothold in the CNS in the 10 to 15% of B. burgdorferi-infected patients who develop clinically symptomatic nervous system disease.
This study is the first to compare the utilities of available diagnostic techniques for LNB in which necropsy proved the presence of infection in the CNS. None of the assays was ideal for all conditions, and the utility of an assay was associated with the host immune status. The differences in the responses of immunocompromised and immunocompetent NHPs in this study were striking. In immunocompetent NHPs the window of opportunity for CNS invasion prior to the development of CSF antibody was brief, and the chance of detection of spirochetes by any of the three techniques used (i.e., culture, PCR, or the MIT) was low; in this group, measurement of CSF antibody was generally diagnostic. In immunocompromised NHPs, intrathecal antibody production was delayed, and this helpful diagnostic assay was false negative; diagnosis required more labor-intensive assays, such as PCR, culture, and the MIT, during weeks 3.5 to 9.5 after infection. It is likely that had the experiment been allowed to proceed longer in the immunocompetent NHPs, antibody would have eventually been produced intrathecally.
This is the first study demonstrating that a bioassay using inoculation of mice, the MIT, has potential as a useful adjunct in the diagnosis of LNB. The MIT for LNB was modeled after the rabbit infectivity test, which is considered the “gold standard” for the diagnosis of the related CNS infection neurosyphilis and which is felt to be very sensitive and specific (21, 24). The clinical application of the MIT to human LNB is unclear. The advantages are summarized above and include direct demonstration of infection, especially in situations where other tests are negative. The disadvantages are similar to those described for the rabbit infectivity test for neurosyphilis, i.e., the requirement for special facilities, expense, and long delay in getting results after the spinal tap. Thus, it may prove to be particularly useful as an adjunctive assay in academic centers or in clinical or basic research studies of LNB.
The clinical relevance of the data on comparison of diagnostic assays is clear. The appearance of anti-B. burgdorferi antibody in the CSF may be delayed, especially when there is interference with the anti-B. burgdorferi immune response. In these circumstances, or for a short time early in CNS invasion in immunocompetent individuals, the measurement of anti-B. burgdorferi antibody in the CSF may be negative; under these circumstances the likelihood of detecting spirochetes by PCR, culture, or the MIT is highest. Conversely, detecting the presence of spirochetes by culture, PCR, or the MIT will be least likely to be successful when anti-B. burgdorferi antibody is present.
This study measured anti-B. burgdorferi antibody as both a diagnostic assay and an indicator of the host immune response toward the spirochete and its suppression by the administration of the corticosteroid dexamethasone. This drug probably also interfered with arms of the immune response other than the humoral arm. For instance, we did not include measurement of cellular immune measures or macrophage functions in our study. It is likely that these arms of the immune response are also important in the response to B. burgdorferi.
Ideally, studies such as this of an infectious disease would be performed either with humans or with subprimate models, such as rodents or rabbits. Unfortunately, performing this study with humans would be very difficult, and there are no adequate rodent or rabbit models currently in use which mimic human LNB. The use of the rhesus macaque model of LNB, which is highly faithful to the human infection (1), allowed the confirmation of CNS infection by necropsy studies, an impossible feat with humans in this infection readily treatable with antibiotics.
ACKNOWLEDGMENTS
This work was supported by the NIH (RO1-NS 34933) and by the Comparative Registry of Pathology’s support of T. O’Neill.
REFERENCES
- 1.Coyle P K. Neurological Lyme disease: is there a true animal model? Ann Neurol. 1995;38:560–562. doi: 10.1002/ana.410380403. [DOI] [PubMed] [Google Scholar]
- 2.Dempsey W L, Smith A L, Morahan P S. Effect of inapparent murine hepatitis virus infections on macrophages and host resistance. J Leukoc Biol. 1986;39:559–565. doi: 10.1002/jlb.39.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 4.Karlsson M, Hovind-Hougen K, Svenungsson B, Stiernstedt G. Cultivation and characterization of spirochetes from cerebrospinal fluid of patients with Lyme borreliosis. J Clin Microbiol. 1990;28:473–479. doi: 10.1128/jcm.28.3.473-479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebech A M, Hansen K. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in urine and cerebrospinal fluid of patients with early and late Lyme neuroborreliosis. J Clin Microbiol. 1992;30:1646–1653. doi: 10.1128/jcm.30.7.1646-1653.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nocton J J, Bloom B J, Rutledge B J, Persing D H, Logigian E L, Schmid C H, Steere A C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in cerebrospinal fluid in Lyme neuroborreliosis. J Infect Dis. 1996;174:623–627. doi: 10.1093/infdis/174.3.623. [DOI] [PubMed] [Google Scholar]
- 7.Pachner A R. Borrelia burgdorferi in the nervous system: the new “Great Imitator.”. Ann NY Acad Sci. 1988;539:56–64. doi: 10.1111/j.1749-6632.1988.tb31838.x. [DOI] [PubMed] [Google Scholar]
- 8.Pachner A R, Amemiya K, Delaney E, O’Neill T, Hughes C N, Zhang W-F. Interleukin 6 is expressed at high levels in the CNS in Lyme neuroborreliosis. Neurology. 1997;49:147–152. doi: 10.1212/wnl.49.1.147. [DOI] [PubMed] [Google Scholar]
- 9.Pachner A R, Delaney E. Murine Lyme borreliosis: route of inoculation determines immune response and infectivity. Reg Immunol. 1993;4:345–351. [PubMed] [Google Scholar]
- 10.Pachner A R, Delaney E. The polymerase chain reaction (PCR) in the diagnosis of Lyme neuroborreliosis. Ann Neurol. 1993;34:544–550. doi: 10.1002/ana.410340407. [DOI] [PubMed] [Google Scholar]
- 11.Pachner A R, Delaney E, O’Neill T. Neuroborreliosis in the non-human primates: Borrelia burgdorferi persists in the central nervous system. Ann Neurol. 1995;38:667–679. doi: 10.1002/ana.410380417. [DOI] [PubMed] [Google Scholar]
- 12.Pachner A R, Delaney E, O’Neill T, Major E. Inoculation of non-human primates with the N40 strain of Borrelia burgdorferi leads to a model of Lyme neuroborreliosis faithful to the human disease. Neurology. 1995;45:165–172. doi: 10.1212/wnl.45.1.165. [DOI] [PubMed] [Google Scholar]
- 13.Pachner A R, Itano A. Borrelia burgdorferi infection of the brain: characterization of the organism and response to antibiotics and immune sera in the mouse model. Neurology. 1990;40:1535–1540. doi: 10.1212/wnl.40.10.1535. [DOI] [PubMed] [Google Scholar]
- 14.Pachner A R, Ricalton N, Delaney E. Comparison of polymerase chain reaction with culture and serology in murine experimental Lyme borreliosis. J Clin Microbiol. 1993;31:208–214. doi: 10.1128/jcm.31.2.208-214.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pachner A R, Ricalton N S. Western blotting in evaluating Lyme seropositivity and the utility of a gel densitometric approach. Neurology. 1992;42:2185–2192. doi: 10.1212/wnl.42.11.2185. [DOI] [PubMed] [Google Scholar]
- 16.Pancholi P, Kolbert C P, Mitchell P D, Reed K D, Dumler J S, Bakken J S, Telford S R, Persing D H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 17.Pfister H W, Preac-Mursic V, Wilske B, Einhaupl K M, Weinberger K. Latent Lyme neuroborreliosis: presence of Borrelia burgdorferi in the cerebrospinal fluid without concurrent inflammatory signs. Neurology. 1989;39:1118–1120. doi: 10.1212/wnl.39.8.1118. [DOI] [PubMed] [Google Scholar]
- 18.Reik L, Steere A C, Bartenhagen N H, Shope R E, Malawista S E. Neurologic abnormalities of Lyme disease. Medicine. 1979;58:281–294. doi: 10.1097/00005792-197907000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Roberts E D, Bohm R P, Cogswell F B, Lanners H N, Lowrie R C, Povinelli L, Piesman J, Philipp M T. Chronic Lyme disease in the rhesus monkey. Lab Investig. 1995;72:146–160. [PubMed] [Google Scholar]
- 20.Rys P N. PCR detection of Borrelia burgdorferi. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C: American Society for Microbiology; 1993. pp. 203–210. [Google Scholar]
- 21.Sanchez P J, Wendel G D, Grimprel E, Goldberg M, Hall M, Arencibia-Mireles O, Norgard M V, Radolf J D. Evaluation of molecular methodologies and rabbit infectivity testing for the diagnosis of congenital syphilis and neonatal central nervous system invasion by Treponema pallidum. J Infect Dis. 1993;167:148–157. doi: 10.1093/infdis/167.1.148. [DOI] [PubMed] [Google Scholar]
- 22.Schwarzova K, Ciznar I. Spirochetal non-Borrelia microorganisms isolated from Ixodes ricinus. Folia Microbiol. 1996;41:175–180. doi: 10.1007/BF02814695. [DOI] [PubMed] [Google Scholar]
- 23.Telford S R, Armstrong P M, Katavolos P, Foppa I, Garcia A S, Wilson M L, Spielman A. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis. 1997;3:165–170. doi: 10.3201/eid0302.970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner T B, Hardy P H, Newman B. Infectivity tests in syphilis. Br J Vener Dis. 1969;45:183–195. doi: 10.1136/sti.45.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.U.S. Department of Health and Human Services. Guide for the care and use of laboratory animals. Publication no. 85-23. U.S. Washington, D.C: Department of Health and Human Services; 1985. [Google Scholar]
- 25.Varde S, Beckley J, Schwartz I. Prevalence of tick-borne pathogens in Ixodes scapularis in a rural New Jersey county. Emerg Infect Dis. 1998;4:97–99. doi: 10.3201/eid0401.980113. [DOI] [PMC free article] [PubMed] [Google Scholar]