Abstract
Vitamin D is a pro-hormone characterized by an intricate metabolism and regulation. It is well known for its role in calcium and phosphate metabolism, and in bone health. However, several studies have assessed a huge number of extra-skeletal functions, ranging from cell proliferation in some oncogenic pathways to antioxidant and immunomodulatory functions. Vitamin D exerts its role by binding to VDRs (vitamin D receptors), which are located in many different tissues. Moreover, VDRs are able to bind hundreds of genomic loci, modulating the expression of various primary target genes. Interestingly, plenty of gene polymorphisms regarding VDRs are described, each one carrying a potential influence against gene expression, with relapses in several chronic diseases and metabolic complications. In this review, we provide an overview of the genetic aspects of vitamin D and VDR, emphasizing the gene regulation of vitamin D, and the genetic modulation of VDR target genes. In addition, we briefly summarize the rare genetic disease linked to vitamin D metabolism.
Keywords: vitamin D, VDR, vitamin D target gene, RXR
1. Introduction
Vitamin D is a fat-soluble vitamin that has been historically known as a molecule. A deficiency in vitamin D might lead to bone diseases, primarily rickets [1]. The discovery of vitamin D dates back to the first half of the 20th century and, despite it still being named as a vitamin, it is well known that it is truly a pro-hormone, with complex endocrine regulation [2]. Indeed, it binds to cytosolic receptors, located mainly in intestinal cells, and osteocytes, but also in several other tissues, such as muscle cells, hematopoietic cells, and the brain. Vitamin D is consequently transported to the cell nucleus, where is able to interact with DNA and modulate the expression of more than 900 genes [3].
The most important effects of vitamin D are on calcium metabolism and bone mineralization; however, it is involved in several physiological and pathological processes, such as cancer, immune modulation, cardiovascular diseases, and metabolic syndrome [4]. Most vitamin D effects are mediated by vitamin D receptors, which are able to regulate a large number of target genes, influencing, consequently, many cellular pathways. Interestingly, VDRs are actually expressed in almost every type of human cell, and they have been found to modulate the transcription of about 3% of human genes [5].
Moreover, there is increasing evidence of the potential role of several VDR polymorphisms in a huge number of diseases [6], such as hypertension, non-alcoholic fatty liver disease, cancer, obesity, and many more [7].
The aim of this review is to provide an overview of the genetic aspects related to vitamin D and VDRs, emphasizing the gene regulation of vitamin D, and the genetic modulation of VDR target genes. In addition, we examine the pathogenic role of the most-known VDR polymorphisms, and report a brief summary of the rare genetic diseases linked to vitamin D metabolism.
2. Vitamin D Metabolism and Homeostasis
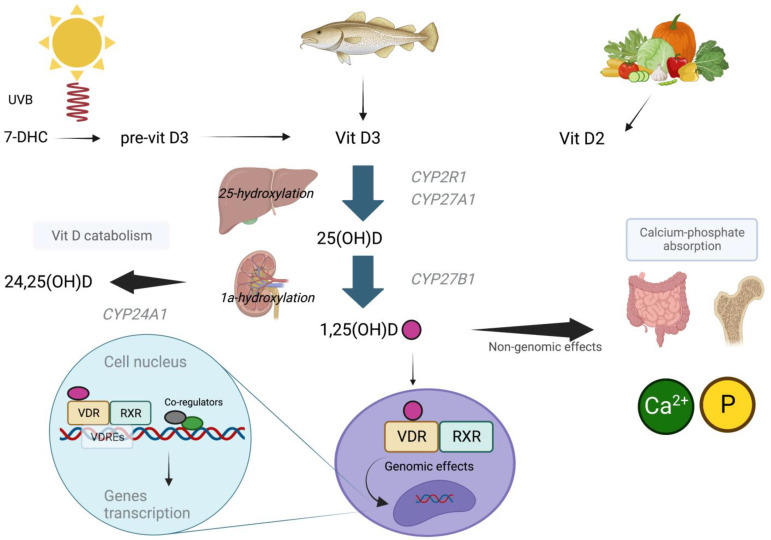
The two main chemical structures of vitamin D are cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2). There are several exogenous ways to obtain vitamin D, including dietary sources, such as oily fish (vitamin D3), mushrooms (vitamin D2), or enriched foods (vitamin D2 and vitamin D3) [3,8]. However, the dietary vitamin D assumption provides only a minor portion of the total daily human intake [9]. The main source of vitamin D is the production in the skin layers, through exposure to the sun’s ultraviolet B rays, especially in the spectral range of 290–320 nm. This is an example of a photochemical process, which does not require any enzymatic involvement, and that leads to the conversion of 7-dehydrocholesterol to pre-vitamin D. Afterwards, pre-vitamin D undergoes an isomerization to vitamin D, through a thermosensitive non-catalytic process [10]. However, vitamin D is biologically inactive, as it requires further hydroxylation steps to turn into its active form, which is able to activate vitamin D receptors (VDRs) [11]. Hence, vitamin D is transported to the liver, carried in the bloodstream by vitamin-D-binding protein (VDBP), where it is hydroxylated to 25-hydroxylated vitamin D (25(OH)D) [12]. The responsible enzyme is CYP2R1, located in the liver endoplasmic reticulum, which can 25-hydroxylate either vitamin D2 or vitamin D3 [13]. Interestingly, CYP27A1 displays a similar enzymatic activity, but is distributed throughout the whole body, and is not able to 25-hydroxylate vitamin D2 [14]. Other enzymes exerting a 25-hydroxylase action, especially in terms of extrahepatic vitamin D production, are CYP3A4, CYP2J3, and CYP2J2. Anyway, CYP2R1 is undoubtfully the major player [15]. The measurement of the circulating levels of 25 (OH)D is considered the best marker for assessing vitamin D status [14].
The hormonally active form of vitamin D is derived from the additional hydroxylation of a C1-carbon atom, in the proximal renal tubule, leading to the production of 1,25-hydroxylated vitamin D (1,25(OH)D). CYP27B1 is responsible for this metabolic step; although the major expression is predominant in the kidney, it has also been found in other sites, including the placenta, monocytes, and macrophages [16,17]. Interestingly, the extra-renal production of 1,25(OH)D is not dependent on parathyroid hormone (PTH) action; thus, the serum availability and sufficiency of 25(OH)D are the limiting factors for the extrarenal synthesis of calcitriol [18].
The importance of this metabolic step was demonstrated by Kitanaka et al., who reported that patients carrying inactivating mutations of the CYP27B1 gene were characterized by vitamin D-dependent type-1 rickets [19].
Interestingly, the kidney is also the main site at which vitamin D catabolism takes place. Indeed, CYP24A1 is a mitochondrial enzyme that can produce 24,25-hydroxylated vitamin D (24,25(OH)D), an inactive metabolite. Thus, CYP24A1 limits the total amount of 1,25(OH)D in tissues, by accelerating its catabolism, and reducing the pool of 25(OH)D available for 1-hydroxylation.
These enzymes belong to the cytochrome P450 class, a superfamily of monooxygenase-containing heme groups. Their nomenclature derives from their specific spectral properties. Moreover, they are detectable in a large number of organisms, from bacteria to humans, configuring themselves as a wide and heterogeneous enzyme family. The main feature of p450s is that they catalyze the selective oxidation of many molecules, ranging from the biosynthesis of natural products to the degradation of xenobiotic compounds [20].
The production of 1,25(OH)D is finely regulated through an intriguing series of negative and positive feedback. 1-hydroxylation is primarily enhanced by PTH, via the stimulation of CYP27B1 transcription. Therefore, a low calcium and phosphate intake, and hypocalcemia, with the consequent rise of PTH, result in active vitamin D production [21]. Conversely, the increased levels of 1,25(OH)D suppress both PTH secretion and CYP27B1 activity. Vitamin D catabolism is, instead, mutually regulated, as 24,25(OH)D production is stimulated by 1,25(OH)D itself, and is inhibited by hypocalcemia and PTH [22]. This negative feedback results in a protective mechanism against hypercalcemia.
Fibroblast growth factor 23 (FGF23) is a significant regulator of vitamin D homeostasis, too. Indeed, 1,25(OH)D enhances FGF23 production in bones, which, vice versa, suppresses the expression of CYP27B1, and increases 24,25(OH) production in the kidneys. As a result, the final effect of FGF23 is to reduce 1,25(OH)D secretion, further leading to a consequent decrease in FGF23, too [23,24].
Notably, there are some genetic issues influencing vitamin D homeostasis, as well. For example, Thacher et al. described a group of mutations affecting the expression, or the function, of CYP2R1 that were found with a higher prevalence in patients with rickets [25]. Other mutations were, instead, associated with lower circulation 25(OH)D levels, and a decreased sensitivity to vitamin D supplementation [26].
Interestingly, many single-nucleotide variations (SNVs) in CYP2R1were found to be related to some chronic diseases, such as obesity and asthma, but also to cancer and all-cause mortality [27,28]. However, further investigations are necessary to assess whether the connection between CYP2R1 activity and these chronic diseases is significant.
3. Genomic and Non-Genomic Effects of Vitamin D
Vitamin D actions might be considered in two distinct ways: genomic and non-genomic effects. The most important non-genomic effect is probably the enhanced calcium and phosphate uptake from the small intestine [29]. This action is the effector mode of PTH calcium reabsorption, which also occurs through the induction of the synthesis of calbindin, a protein that binds calcium ions and transports them from the lumen to the cytoplasm of gut cells. Interestingly, 1,25(OH)D is able to facilitate the passive absorption of calcium, by increasing the permeability of intercellular tight junctions [30]. In addition, vitamin D can also promote phosphate reabsorption in renal tubules [31]. Calcium and phosphorus are essential to hydroxyapatite formation and, by increasing their intake, vitamin D acts indirectly on the bone [32].
The discussion about the genomic aspect is strictly linked to the vitamin D receptor, as it plays a key role. The VDR is a member of the superfamily of nuclear hormone receptors, which might be considered to be ligand-induced transcription factors. VDRs are expressed in the skin, parathyroid glands, adipocytes, small intestine, colon, and other tissues [33,34]. After binding to 1,25(OH)D, the VDR forms a heterodimer with the retinoid acid receptor (RXR) that translocates to the cell nucleus, joining the vitamin D response element (VDRE). VDR/RXR complex is considered the major active transcription unit in regulating the vitamin D target genes’ transcription [35]. Moreover, the VDRE depends specifically on the cell type, differing from cell to cell. This might be one mechanism of the action specificity of vitamin D [36]. Surprisingly, the complex 1,25(OH)D/VDR can directly interact the with cAMP-response element-binding protein (CREB), hampering its binding to CRE (the cAMP-response element). These activities seem not to require the presence of liganded VDR heterodimerization with RXR [37]. The main steps of vitamin D metabolism, and its genomic and non-genomic effects, are depicted in Figure 1.
Figure 1.
Metabolic pathways regarding the production, activation, and effects of vitamin D. The highest amount of vitamin D is produced in the skin, via the conversion of 7-DHC to vitamin D3. Vitamin D2 is mostly assumed through foods. Vitamin D2 and Vitamin D3 are further hydroxylated in carbonium 25 and 1. 1,25(OH)D exerts its genomic effects by binding to VDR and RXR, and translocating to the nucleus, where it interacts with VDRE. Non-genomic effects are mainly involved in calcium–phosphate homeostasis. 7-DHC: 7dehydrocolesterol, vitamin D3: vitamin D3, vitamin D2: vitamin D2, 25(OH)D: 25-hydroxylated vitamin D, 1,25(OH)D: 1,25-hydroxylated vitamin D, VDR: vitamin D receptor, RXR: retinoic acid receptor, VDRE: vitamin D response element.
4. Genetic Factors Influencing Vitamin D Status
An individual’s vitamin D status depends strongly on environmental factors related to geographical region and lifestyle. However, there are also genetic factors which could influence individual serum vitamin D levels and, consequently, be linked to the pathogenesis of many chronic diseases, such as osteoporosis, cancers, and autoimmune diseases [38,39,40]. Interestingly, an association between epigenetic modifications and vitamin D levels has been identified, as well.
Many studies involving twins and close relatives evaluated the inheritance of hypovitaminosis D, which is estimated to be between 23 and 80%, depending also on the study design and environmental variables [41,42].
Numerous candidate gene studies and genome-wide association studies have been carried out over the years, identifying a series of genetic mutations and polymorphisms affecting the genes encoding the molecules involved in the production and activation of vitamin D, transport proteins, VDRs, and coactivating proteins, and alterations affecting proteins secondarily involved in the regulation of vitamin D expression (e.g., mechanisms related to calcium or PTH concentrations). The most investigated genes are DHCR7, CYP2R1, CYP27B1, GC, VDR, CYP24A1, and RXR [43].
4.1. DHCR7 (7-Dehydrocholesterol Reductase)
The DHCR7 gene is mapped on chromosome 11, and encodes for a reductase that is responsible for the epidermal conversion of 7-DHC into cholesterol. Mutations in this gene result in an accumulation of 7-DHC, which leads to Smith–Lemli–Opitz syndrome [43].
Some studies identified the following SNVs, which are localized in the 5’ edge region, and are associated with vitamin D deficiency: rs11234027, rs1790349, and rs12785878 [27]. On the other hand, Zhang et al. found that the SNVs rs1790349, rs7122671, rs1790329, rs11606033, rs2276360, rs1629220, and rs2282618 would be genetic protective factors against hypovitaminosis D [44].
4.2. CYP2R1 (Vitamin D 25-Hydroxylase)
The CYP2R1 gene is located on chromosome 11, and encodes for the main 25-hydroxylase that converts cholecalciferol to 25(OH)D [15].
The rs10741657 polymorphism, which is found in the 5′ edge region, is associated with reduced levels of 25(OH)D. Other SNVs that potentially influence an individual’s vitamin D status are rs12794714, rs10766197 [28], rs1562902, rs7116978 [45], rs2060793, rs1993116 [27], rs11023332, and rs1007392 [46].
4.3. CYP27B1 (25(OH)D-1-α Hydroxylase)
The CYP27B1 gene is located on chromosome 12, and encodes for the most important 1α-hydroxylase, which converts 25(OH)D to the active form 1,25(OH)D [43]. Many genetic variants related to vitamin D expression are described. The SNV rs10877012, situated in the promoter region of the CYP27B1 gene, is associated with reduced serum levels in 25(OH)D [47]. Two other SNVs that might be associated with hypovitaminosis D are rs4646536, located at the 5′ edge region, and the intronic SNV rs703842 [28].
Hence, the inactivating mutations in the CYP27B1 gene could lead to a deficient conversion of calcidiol to calcitriol, and are better known as vitamin-D-dependent rickets type 1A (VDDR1A) [48]. Less commonly, some variants affect the CYP2R1 (VDDR1B) result in a deficient 25-hydroxylation process, hampering the conversion of cholecalciferol to calcidiol. This variant is also known as vitamin-D-dependent rickets type 1B (VDDR1B). These disorders cause impaired intestinal absorption of calcium and phosphate, further leading to hypocalcemia and abnormal bone mineralization [49].
The clinical features vary, depending on the severity of the disease. Patients present soon after birth with rickets, and signs of hypocalcemia, tetany, or convulsions [50].
4.4. CYP24A1 (Vitamin D 24-Hydroxylase)
The CYP24A1 gene is located on chromosome 20, and is a mitochondrial enzyme expressed in several target cells containing VDRs, which catalyzes 25(OH)D and 1,25(OH)D catabolism, as aforementioned. This enzyme prevents the accumulation of toxic levels of these molecules; on the other hand, it prolongs the half-life of 25(OH)D when vitamin D levels are reduced [51]. Interestingly, an intronic SNV (rs17219315) was found to be associated with serum 25(OH)D concentrations [52]. Moreover, Barry et al. described that SNVs such as rs2209314, rs2762939, and rs6013897 might potentially modify the efficacy of cholecalciferol supplementation in increasing 25(OH)D serum levels [45].
4.5. GC (Vitamin D Binding Protein)
GC is mapped on chromosome 4, and encodes for VDBP, which carries vitamin D to various sites of action, facilitating its activity, as well [53]. The DNA sequence analysis of this gene showed two SNVs at exon 11, causing, respectively, a Glu/Asp amino acid change (rs7041) and a Thr/Lys amino acid change (rs4588), associated with a reduction in vitamin D [54]. Some studies identified many polymorphisms associated with a reduced expression of vitamin D and, also, a higher affinity of VDPB to vitamin D [39]: these included some intronic SNVs, such as rs222020 [28], rs2282679, rs1155563 [27], rs2298849, and rs222035 [55]. Other examples of polymorphisms related to vitamin D status are rs16846876, rs17467825, rs842999, and rs12512631, mapped on the 3′ edge region of the GC gene [56].
4.6. VDR (Vitamin D Receptor)
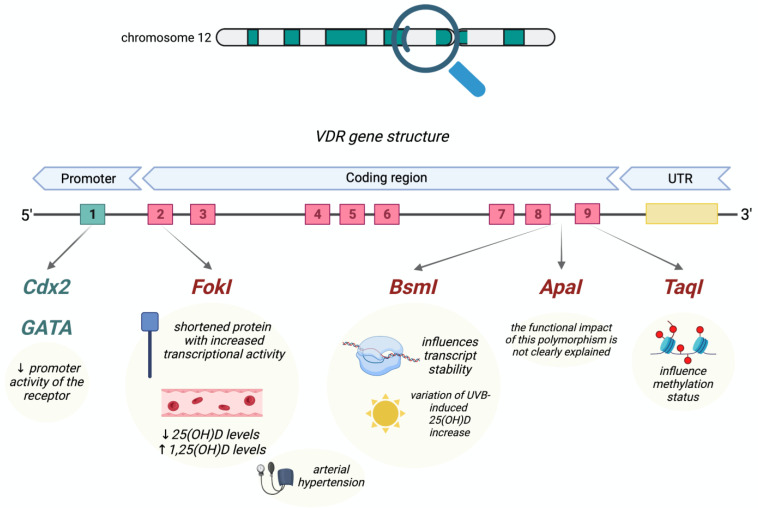
The VDR gene encodes for the vitamin D receptor, located on chromosome 12, and contains six promoter regions, and eight exons 2 to 9. The DNA-binding domain (exons 2–4) interacts with the VDRE in target genes, whereas the ligand-binding domain (exons 6–9) binds 1,25(OH)D [39]. Several studies have identified hundreds of polymorphisms in the vitamin D receptor gene, but the functional implication is still largely unknown. The most characterized polymorphic sites in the VDR gene are recognized by the restriction endonuclease enzymes TaqI, BsmI, ApaI, and FokI, after which they are named. These polymorphisms are strictly correlated with various diseases, but also with homeostatic processes, such as bone mineralization and calcium imbalance [57]. FokI (rs10735810, also known as rs2228570) is located in exon 2, and consists of a C > T nucleotide substitution: the T nucleotide is also referred to as allele “f”, while the C nucleotide is defined as allele “F”. The presence of site “F” results in a three-amino-acid-shortened protein, which is characterized by increased transcriptional activity. FokI, in particular, the F-allele, as well as having functional consequences on the structure of the vitamin D receptor, is associated with lower serum 25(OH)D levels [28,58]. TaqI (rs731236) is located in exon 9 of the VDR gene, and consists of T > C substitution. The T nucleotide is defined as allele “T”, while the C nucleotide corresponds to allele “t”. This polymorphism occurs in a CpG island, resulting in an influence on the methylation status. BsmI (rs1544410) is located in intron 8 of the gene, and consists of an A > G nucleotide substitution; the A nucleotide corresponds to allele B, and the G nucleotide corresponds to allele b. This polymorphism influences transcript stability; moreover, it was found to influence the variation in the UVB-induced 25(OH)D increase, interfering in the interaction with RXRA and CYP24A1, as well [59]. ApaI (rs7975232) is also located in intron 8, and consists of a C > A substitution (the C nucleotide is referred to as allele “A”, while the A nucleotide is defined as allele “a”). The functional impact of this polymorphism is not clearly explained. There are two lesser-known polymorphisms in the promoter region of the VDR gene: Cdx2 (rs11568820) [60] and GATA (rs4516035) [61], which are located upstream and downstream of exon 1, respectively, causing a decreased promoter activity in the receptor [6,7].
Even if, currently, the functional significance and the clinical implications of these polymorphisms need to be clarified, every mutation that leads to a decrease in VDR functionality thereby prevents calcitriol’s action. This leads to an impaired intestinal absorption of calcium and phosphate, and is classified as vitamin-D-dependent rickets type II, or hereditary vitamin-D-resistant rickets. The main clinical features comprise progressive rickets disease, which starts to manifest during the first years of life. Total body alopecia is present in severe forms of the disease. In some cases, skin lesions or epidermal cysts can be observed, along with alopecia. The disease presents a broad clinical picture that largely depends on the genotype [50,62].
Interestingly, some studies in humans have demonstrated that low circulating levels of 25(OH)D seem to be associated with a higher plasma renin activity, and higher angiotensin II concentrations [63,64]. In addition, a supplementation therapy with cholecalciferol might reduce the increased renin–angiotensin–aldosterone system (RAAS) activity secondary to vitamin D deficiency [65].
The complex vitamin D/VDR, indeed, is able to bind CRE, consequently preventing the binding of cAMP. The fact that cAMP is one of the main stimulating factors for renin production in renal juxtaglomerular cells makes it easy to understand how vitamin D might play a potential role in hampering the development of arterial hypertension [66]. Notably, in patients affected by arterial hypertension, renin activity was found to be inversely related to 1,25(OH)D levels [67].
Several studies have investigated the FokI polymorphism of VDRs as a possible condition linked with a higher risk of arterial hypertension. As aforementioned, FokI results in the formation of a truncated protein, which is thought to be associated with an increased production of renin and angiotensin II, thereby promoting the development of hypertension [68,69,70]. Other evidence suggests a further association between BsmI and arterial hypertension, as well, especially characterizing male patients [71,72,73].
Interestingly, a possible influence of BsmI and FokI on the development of, and BsmI, TaqI, and ApaI on the progression of, non-alcoholic fatty liver disease (NAFLD) has been hypothesized, as recently reported [74]. The main polymorphisms affecting VDR are resumed in Figure 2.
Figure 2.
The main polymorphisms characterizing a vitamin D receptor. Each one is pictured below its corresponding exon (indicated by progressive numbers from 1 to 9) matched via black arrows, and with its known clinical implications. VDR: vitamin D receptor, 25(OH)D: 25-hydroxylated vitamin D, 1,25(OH)D: 1,25-hydroxylated vitamin D.
Other genes indirectly involved in the control of vitamin D homeostasis and expression have also been studied, despite the evidence being less overt. For example, intracellular domain polymorphisms of the calcium-sensing receptor (CaSR), and polymorphisms of cubilin were studied; however, no associations with vitamin D homeostasis were observed [56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75]. Interestingly, a study investigated the impact of FGF23 gene variation on phosphate homeostasis and bone health, detecting nine FGF23 polymorphisms, three of which were quite common: rs3832879, rs7955866, and rs11063112 [76]. A study showed significant correlations between the RXR SNVs rs3132299 or rs9409929 and 1,25(OH)D, as well as between rs877954 and 25(OH)D levels [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77].
4.7. Epigenetic Factors Influencing Vitamin D Status
Vitamin D might exert an epigenetic effect in the transcription of several target genes; similarly, vitamin D levels and bioavailability are influenced by epigenetic factors. This is, undoubtfully, a developing field of research. Many studies have suggested the role either of the epigenetic modulation of genes involved in vitamin D metabolism and several pathologies, or the association between the epigenetic modifications of genes involved in vitamin D metabolism and vitamin D status [78,79]. The most common epigenetic mechanisms are the acetylation, methylation, and phosphorylation of histone proteins [18]. Among them, the main one comprises methylation via CpG islands located at a gene’s promoter region, resulting in a lower gene expression. These mechanisms would be responsible for nearly 18% of the vitamin D level variance among the population, as well as being a contributing factor to vitamin D deficiency.
For example, a few studies have shown that the CYP2R1 methylation status regulates the effect of calcium and vitamin D intake or radiance on vitamin D serum levels: subjects presenting sufficient vitamin D levels, or taking vitamin D supplementation, show lower methylation at the CpG site of the CYP2R1 gene [80,81]. Similarly, several studies have shown a correlation between vitamin D status and the methylation levels of the CYP24A1, CYP27B1, GC, RXRA and VDR, and DHCR7 genes [82,83,84]. Moreover, some studies have reported a potential role of serum B12 and folate in the regulation of the methylation of CYP27B1 and VDR, respectively [85].
Interestingly, many groups of people might be characterized by different effects of vitamin D supplementation on biochemical vitamin D parameters, epigenetic modifications, and the response of transcriptome-wide vitamin D target genes. Moreover, groups of low, medium, and high responders to vitamin D supplementation have been identified, with different molecular responses in 25(OH)D serum levels [85]. These findings have paved the way for the concept of various responses to vitamin D supplementation that lead to a personalized need for daily intake of vitamin D.
5. Genetic Effects of Vitamin D
It is now a well-established opinion that vitamin D exerts numerous extra-skeletal effects. Indeed, vitamin D deficiency is associated with an increased risk for many diseases, thus suggesting its crucial role [4].
These effects range from the modulation of cell growth and differentiation, potentially promoting the carcinogenesis process, to the regulation of immune and muscle function. Moreover, vascular and metabolic actions are described, as well [86,87,88,89].
5.1. Genomic Action
The vitamin D receptor is one of the nuclear receptors for steroid hormones that functions as a ligand-activated transcription factor, thereby regulating gene expression. It plays an essential role in the genomic mechanism of action of vitamin D [90,91]. Notably, 1,25(OH)D is one of the most potent regulators of its function [92]. Interestingly, the inner surface of the VDR is characterized by a ligand-binding pocket that is able to enclose and bind 1,25(OH)D with an affinity of 0.1 nM, which is a very high affinity, especially in comparison with other nuclear receptors [93].
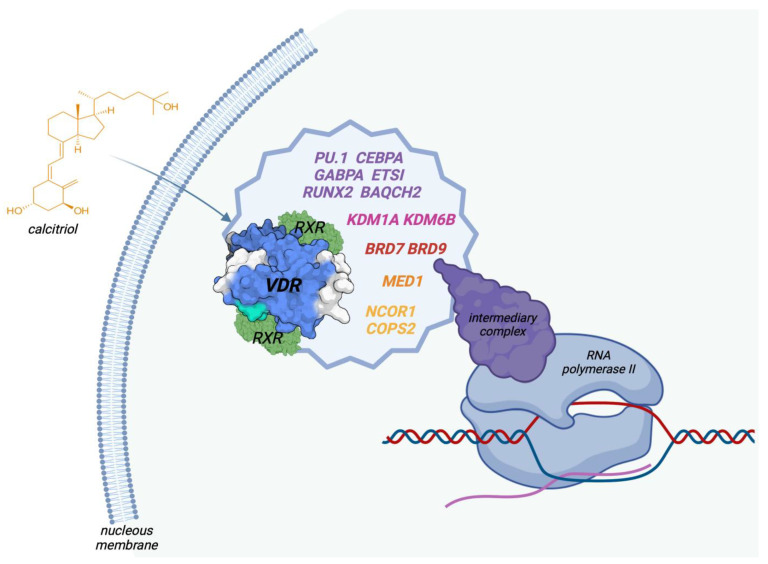
Through its activation of the VDR, 1,25(OH)D has direct effects on the epigenome, and versus the expression of more than 1000 genes in several human tissues and cell types, resulting in changes in the transcriptome and proteome, as well. Interestingly, VDR is the unique target of 1,25(OH)D in the cell nucleus. There is still no general descriptive model of the regulatory mechanism of vitamin D target genes. After the binding of 1,25(OH)D, VDR interacts with many other nuclear receptors, forming a multi-protein complex that attaches preferentially to DR3-type binding sites within enhancer regions. This VDR multi-complex contains co-receptors, such as RXR, pioneer factors (PU.1, CEBPα, GABPα, ETS1, RUNX2, BACH2), chromatin modifiers (KDM1A, KDM6B), chromatin remodelers (BRD7, BRD9), co-activators (MED1), and co-repressors (NCOR1, COPS2). A mediator complex connects the activated VDR complex with the RNA polymerase II located on a specific gene transcription start site (TSS) [35,93,94,95,96], as shown in Figure 3.
Figure 3.
Describing the multi-complex of the VDR, comprising the receptor, co-receptors, co-repressors, co-activators, and pioneer factors that finally exert genomic effects on gene transcription. VDR: vitamin D receptor, RXR: retinoid acid receptor, PU.1, CEBPα, GABPα, ETS1, RUNX2, BACH2: pioneer factors, KDM1A, KDM6B: chromatin modifiers, BRD7, BRD9: chromatin remodelers, MED1: co-activator, NCOR2, COPS2: co-repressors.
However, these VDR complexes, necessarily, need to interact with their respective genomic-binding sites; hence, they must have access to the open chromatin, named euchromatin [97].
Thus, 1,25(OH)D modulates the epigenome in its target tissue in different ways, involving the chromatin status. Essentially, it could act through direct interaction with chromatin-modifying enzymes, as well as through up- or down-regulating the genes encoding for chromatin modifiers [98]. For example, 1,25(OH)D affects histone markers for active chromatin, such asH3K27ac (acetylated histone H3 at lysine 27), and for TSS regions, such as H3K4me3 (tri-methylated histone H3 at lysine 4) [99]. Moreover, the binding of chromatin-organizing protein to several genes is modulated by 1,25(OH)D, and the organization of various genomic loops of DNAs is vitamin-D-dependent. Thus, 1,25(OH)D is able to affect the three-dimensional structure of chromatin [100].
All these mediated mechanisms, in terms of net effects, are finalized, to increase or decrease the activity of RNA polymerase II, and the mRNA expression of 1,25(OH)D target genes.
Notably, the enhancers of the VDR-encoding gene are located near or far, i.e., promoter-proximally or promoter-distally, and many enhancers are also located in clusters hundreds of kilobases away from their target genes, meaning that the intervening genomic DNA forms a regulatory loop. In this way, the expression of the vitamin D target genes is either increased or decreased [35].
To downregulate a gene, the mechanism most often employed is to block one or more of its upregulatory factors. This implies that most of the downregulated target genes must be classified as indirect targets, meaning that vitamin D does not directly downregulate them but, rather, counteracts the upregulation of their expression [101,102].
5.2. Immune System Regulation
Vitamin D is able to modulate the expression of the genes involved in innate and adaptative immune functions.
In this contest, both 25(OH)D and 1,25(OH)D act in multiple ways, and in several immune cells, such as macrophages, monocytes, and B- and T-type lymphocytes [103].
In macrophages, the expression of CYP27B1 is induced via immune-specific inputs, leading to the local production of hormonal 1,25(OH)D at the sites of infection, which, in turn, directly induces the expression of genes encoding antimicrobial peptides.
In this scenario, numerous inflammatory or proinflammatory cytokines are modulated via vitamin D, with the aim of limiting inflammation. Indeed, 1,25(OH)D, through autocrine mechanisms, increases the expression of cathelicidin, which, in turn, exerts antiviral and antibacterial effects. Moreover, 1,25(OH)D acts in a paracrine manner, stimulating adjacent macrophages. Therefore, 1,25(OH)D is able to maintain immune tolerance in APC cells, and finely manage the surface expression of MHC class II molecules, immunogenic cytokines, and co-stimulation molecules [104,105].
IL-10 expression, which is characterized by anti-inflammatory activity, is increased; contrarywise, IL-6 and IL-17, which provide pro-inflammatory and atherogenic effects, are reduced [106].
In addition, 1,25(OH)D seems able to activate and modulate natural killer cells, interfering with their metabolism and immunogenic activity [107].
5.3. Focus on Clinical Outcomes
It might be speculated that vitamin D could carry antitumor effects, both directly, by controlling the differentiation, proliferation, and apoptosis of neoplastic cells, and indirectly, by regulating the immune cells that belong to the microenvironment of malignant tumors [108].
Data, mainly obtained from observational studies, show that the levels of circulating 25(OH)D concentration are inversely correlated with the risk of breast, prostate, and colorectal cancers, but not the overall cancer risk [109,110,111,112]. Epidemiologic studies are still inconclusive in determining the true effect of vitamin D on reducing cancer risk, and improving patient outcomes.
Interestingly, supplementation with vitamin D did not result in a lower incidence of invasive cancer [7,113,114].
Moving to another topic, considering the role of vitamin D in the downregulation of the adaptive immune system, it is conceivable that observational studies would find an association between vitamin D deficiency and multiple sclerosis, inflammatory bowel disease, and type 1 diabetes [115,116,117].
The evidence from randomized controlled trials (RCTs) of the effect of vitamin D supplementation on clinical outcomes is inconclusive. Hence, the observation that treatment with Vitamin D might reduce the risk of these diseases is still lacking.
5.4. Viral Infections and COVID-19
More recently, a role of vitamin D in the course of the COVID-19 pandemic has been postulated.
Unfortunately, in this context, the data obtained from observational and interventional studies are discordant, too.
Circulating low serum vitamin D levels seem to correlate with the risk and the severity of COVID-19 infection, as well as with high mortality and morbidity. However, the results are unreliable, due to the evidence of many confounding factors, also depending on individual patients’ situations [118,119,120,121].
Currently, definitive conclusions about the utility of vitamin D treatment in the context of the prevention of the infection are conflicting. In addition, reliable interventional data on vitamin D supplementation in hospitalized COVID-19 patients are still lacking [122,123,124].
6. Conclusions
Despite vitamin D originally having been discovered through its fundamental role in calcium homeostasis and bone formation, nowadays, vitamin D metabolism and signaling are extensively being studied for also having a critical role in extra-skeletal terms. In this context, genetic alterations affecting vitamin D metabolism might be crucial. Indeed, as reported in this review, there are several genes that, if altered, might lead to dramatic variations in 25(OH)D status, with clinical consequences that may become cumbersome, as shown in vitamin D-dependent rickets. An important aspect to keep in mind is the potential role of vitamin D as a modulator in the fields of carcinogenesis, the inflammatory response, and autoimmune diseases. However, a direct link between a potential target therapy via vitamin D supplementation is still unavailable.
Notably, in the future, new research into the role of gene polymorphism and epigenetic modifications in vitamin D status might open up new methods for the clinical application of a personalized approach. Genetic alterations, indeed, might allow physicians to identify patients who are low, medium, or high responders to vitamin D and, consequently, those who most need vitamin D supplementation.
Author Contributions
Conceptualization, G.V. and V.C.; methodology, G.V. and F.C.; validation, F.C. and V.C.; writing—original draft preparation, G.V., M.C. and M.F.; writing—review and editing, F.C. and V.C. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data presented are included in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Reijven P., Soeters P. Vitamin D: A magic bullet or a myth? Clin. Nutr. 2020;39:2663–2674. doi: 10.1016/j.clnu.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 2.DeLuca H.F. Vitamin D. Vitam. Horm. 2016;100:1–20. doi: 10.1016/bs.vh.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Battistini C., Ballan R., Herkenhoff M.E., Saad S.M.I., Sun J. Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2020;22:362. doi: 10.3390/ijms22010362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouillon R., Marcocci C., Carmeliet G., Bikle D., White J.H., Dawson-Hughes B., Lips P., Munns C.F., Lazaretti-Castro M., Giustina A., et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019;40:1109–1151. doi: 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouillon R., Carmeliet G., Verlinden L., van Etten E., Verstuyf A., Luderer H.F., Lieben L., Mathieu C., Demay M. Vitamin D and Human Health: Lessons from Vitamin D Receptor Null Mice. Endocr. Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uitterlinden A.G., Fang Y., van Meurs J.B., Pols H.A., van Leeuwen J.P. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Berretta M., Quagliariello V., Bignucolo A., Facchini S., Maurea N., Di Francia R., Fiorica F., Sharifi S., Bressan S., Richter S.N., et al. The Multiple Effects of Vitamin D against Chronic Diseases: From Reduction of Lipid Peroxidation to Updated Evidence from Clinical Studies. Antioxidants. 2022;11:1090. doi: 10.3390/antiox11061090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holick M.F., Uskokovic M., Henley J.W., MacLaughlin J., Holick S.A., Potts J.T. The Photoproduction of 1α,25–Dihydroxyvitamin D3in Skin. N. Engl. J. Med. 1980;303:349–354. doi: 10.1056/NEJM198008143030701. [DOI] [PubMed] [Google Scholar]
- 9.Lamberg-Allardt C. Vitamin D in foods and as supplements. Prog. Biophys. Mol. Biol. 2006;92:33–38. doi: 10.1016/j.pbiomolbio.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Klein G.L., Chen T.C., Holick M.F., Langman C.B., Price H., Celis M.M., Herndon D.N. Synthesis of vitamin D in skin after burns. Lancet. 2004;363:291–292. doi: 10.1016/S0140-6736(03)15388-3. [DOI] [PubMed] [Google Scholar]
- 11.Hossein-Nezhad A., Holick M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013;88:720–755. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holick M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J.G., Ochalek J.T., Kaufmann M., Jones G., DeLuca H.F. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc. Natl. Acad. Sci. USA. 2013;110:15650–15655. doi: 10.1073/pnas.1315006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollis B.W. Circulating 25-Hydroxyvitamin D Levels Indicative of Vitamin D Sufficiency: Implications for Establishing a New Effective Dietary Intake Recommendation for Vitamin D. J. Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 15.Mazahery H., Von Hurst P.R. Factors Affecting 25-Hydroxyvitamin D Concentration in Response to Vitamin D Supplementation. Nutrients. 2015;7:5111–5142. doi: 10.3390/nu7075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoffels K., Overbergh L., Bouillon R., Mathieu C. Immune regulation of 1α-hydroxylase in murine peritoneal macrophages: Unravelling the IFNγ pathway. J. Steroid Biochem. Mol. Biol. 2007;103:567–571. doi: 10.1016/j.jsbmb.2006.12.091. [DOI] [PubMed] [Google Scholar]
- 17.Christakos S., Ajibade D.V., Dhawan P., Fechner A.J., Mady L.J. Vitamin D: Metabolism. Endocrinol. Metab. Clin. N. Am. 2010;39:243–253. doi: 10.1016/j.ecl.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross H.S. Extrarenal Vitamin D Hydroxylase Expression and Activity in Normal and Malignant Cells: Modification of Expression by Epigenetic Mechanisms and Dietary Substances. Nutr. Rev. 2007;65:S108–S112. doi: 10.1301/nr.2007.aug.S108-S112. [DOI] [PubMed] [Google Scholar]
- 19.Kitanaka S., Takeyama K.-I., Murayama A., Kato S. The Molecular Basis of Vitamin D-Dependent Rickets Type I. Endocr. J. 2001;48:427–432. doi: 10.1507/endocrj.48.427. [DOI] [PubMed] [Google Scholar]
- 20.Permana D., Kitaoka T., Ichinose H. Conversion and synthesis of chemicals catalyzed by fungal cytochrome P450 monooxygenases: A review. Biotechnol. Bioeng. 2023;120:1725–1745. doi: 10.1002/bit.28411. [DOI] [PubMed] [Google Scholar]
- 21.Murayama A., Takeyama K.-I., Kitanaka S., Kodera Y., Kawaguchi Y., Hosoya T., Kato S. Positive and Negative Regulations of the Renal 25-Hydroxyvitamin D3 1α-Hydroxylase Gene by Parathyroid Hormone, Calcitonin, and 1α,25(OH)2D3 in Intact Animals. Endocrinology. 1999;140:2224–2231. doi: 10.1210/endo.140.5.6691. [DOI] [PubMed] [Google Scholar]
- 22.Omdahl J.L., Morris H.A., May B.K. Hydroxylaseenzymes of thevitamind pathway: Expression, Function, and Regulation. Annu. Rev. Nutr. 2002;22:139–166. doi: 10.1146/annurev.nutr.22.120501.150216. [DOI] [PubMed] [Google Scholar]
- 23.Liu S., Tang W., Zhou J., Stubbs J.R., Luo Q., Pi M., Quarles L.D. Fibroblast Growth Factor 23 Is a Counter-Regulatory Phosphaturic Hormone for Vitamin D. J. Am. Soc. Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 24.Shimada T., Kakitani M., Yamazaki Y., Hasegawa H., Takeuchi Y., Fujita T., Fukumoto S., Tomizuka K., Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Investig. 2004;113:561–568. doi: 10.1172/JCI200419081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thacher T.D., Fischer P.R., Singh R.J., Roizen J., Levine M.A. CYP2R1 Mutations Impair Generation of 25-hydroxyvitamin D and Cause an Atypical Form of Vitamin D Deficiency. J. Clin. Endocrinol. Metab. 2015;100:E1005–E1013. doi: 10.1210/jc.2015-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng J.B., Levine M.A., Bell N.H., Mangelsdorf D.J., Russell D.W. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc. Natl. Acad. Sci. USA. 2004;101:7711–7715. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn J., Yu K., Stolzenberg-Solomon R., Simon K.C., McCullough M.L., Gallicchio L., Jacobs E.J., Ascherio A., Helzlsouer K., Jacobs K.B., et al. Genome-wide association study of circulating vitamin D levels. Hum. Mol. Genet. 2010;19:2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bu F.-X., Armas L., Lappe J., Zhou Y., Gao G., Wang H.-W., Recker R., Zhao L.-J. Comprehensive association analysis of nine candidate genes with serum 25-hydroxy vitamin D levels among healthy Caucasian subjects. Hum. Genet. 2010;128:549–556. doi: 10.1007/s00439-010-0881-9. [DOI] [PubMed] [Google Scholar]
- 29.Goltzman D. Functions of vitamin D in bone. Histochem. Cell Biol. 2018;149:305–312. doi: 10.1007/s00418-018-1648-y. [DOI] [PubMed] [Google Scholar]
- 30.Goltzman D., Mannstadt M., Marcocci C. Physiology of the Calcium-Parathyroid Hormone-Vitamin D Axis. Front. Horm. Res. 2018;50:1–13. doi: 10.1159/000486060. [DOI] [PubMed] [Google Scholar]
- 31.Śledzińska K., Landowski P., Żmijewski M.A., Kamińska B., Kowalski K., Liberek A. Diet, Sun, Physical Activity and Vitamin D Status in Children with Inflammatory Bowel Disease. Nutrients. 2022;14:1029. doi: 10.3390/nu14051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLuca H.F. The Metabolism and Functions of Vitamin D. Adv. Exp. Med. Biol. 1986;196:361–375. doi: 10.1007/978-1-4684-5101-6_24. [DOI] [PubMed] [Google Scholar]
- 33.Sirajudeen S., Shah I., Menhali A. A Narrative Role of Vitamin D and Its Receptor: With Current Evidence on the Gastric Tissues. Int. J. Mol. Sci. 2019;20:3832. doi: 10.3390/ijms20153832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charoenngam N., Shirvani A., Kalajian T.A., Song A., Holick M.F. The Effect of Various Doses of Oral Vitamin D3 Supplementation on Gut Microbiota in Healthy Adults: A Randomized, Double-blinded, Dose-response Study. Anticancer Res. 2020;40:551–556. doi: 10.21873/anticanres.13984. [DOI] [PubMed] [Google Scholar]
- 35.Zella L.A., Meyer M.B., Nerenz R.D., Lee S.M., Martowicz M.L., Pike J.W. Multifunctional Enhancers Regulate Mouse and Human Vitamin D Receptor Gene Transcription. Mol. Endocrinol. 2010;24:128–147. doi: 10.1210/me.2009-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norman A.W., Okamura W.H., Hammond M.W., Bishop J.E., Dormanen M.C., Bouillon R., van Baelen H., Ridall A.L., Daane E., Khoury R., et al. Comparison of 6-s-cis- and 6-s-trans-Locked Analogs of 1α,25-Dihydroxyvitamin D3 Indicates That the 6-s-cis Conformation Is Preferred for Rapid Nongenomic Biological Responses and That Neither 6-s-cis- nor 6-s-trans-Locked Analogs Are Preferred for Genomic Biological Responses. Mol. Endocrinol. 1997;11:1518–1531. doi: 10.1210/mend.11.10.9993. [DOI] [PubMed] [Google Scholar]
- 37.Legarth C., Grimm D., Wehland M., Bauer J., Krüger M. The Impact of Vitamin D in the Treatment of Essential Hypertension. Int. J. Mol. Sci. 2018;19:455. doi: 10.3390/ijms19020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahrami A., Mazloum S.R., Maghsoudi S., Soleimani D., Khayyatzadeh S.S., Arekhi S., Arya A., Mirmoosavi S.J., Ferns G.A., Bahrami-Taghanaki H., et al. High Dose Vitamin D Supplementation Is Associated with a Reduction in Depression Score Among Adolescent Girls: A Nine-Week Follow-Up Study. J. Diet. Suppl. 2018;15:173–182. doi: 10.1080/19390211.2017.1334736. [DOI] [PubMed] [Google Scholar]
- 39.McCullough M.L., Bostick R.M., Mayo T.L. Vitamin D Gene Pathway Polymorphisms and Risk of Colorectal, Breast, and Prostate Cancer. Annu. Rev. Nutr. 2009;29:111–132. doi: 10.1146/annurev-nutr-080508-141248. [DOI] [PubMed] [Google Scholar]
- 40.Tabatabaeizadeh S., Avan A., Bahrami A., Khodashenas E., Esmaeili H., Ferns G.A., Abdizadeh M.F., Ghayour-Mobarhan M. High Dose Supplementation of Vitamin D Affects Measures of Systemic Inflammation: Reductions in High Sensitivity C-Reactive Protein Level and Neutrophil to Lymphocyte Ratio (NLR) Distribution. J. Cell. Biochem. 2017;118:4317–4322. doi: 10.1002/jcb.26084. [DOI] [PubMed] [Google Scholar]
- 41.Shea M.K., Benjamin E.J., Dupuis J., Massaro J.M., Jacques P.F., D’Agostino R.B., Ordovas J.M., O’Donnell C.J., Dawson-Hughes B., Vasan R.S., et al. Genetic and non-genetic correlates of vitamins K and D. Eur. J. Clin. Nutr. 2009;63:458–464. doi: 10.1038/sj.ejcn.1602959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orton S.-M., Morris A.P., Herrera B.M., Ramagopalan S.V., Lincoln M.R., Chao M.J., Vieth R., Sadovnick A.D., Ebers G.C. Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis. Am. J. Clin. Nutr. 2008;88:441–447. doi: 10.1093/ajcn/88.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bahrami A., Sadeghnia H.R., Tabatabaeizadeh S., Bahrami-Taghanaki H., Behboodi N., Esmaeili H., Ferns G.A., Mobarhan M.G., Avan A. Genetic and epigenetic factors influencing vitamin D status. J. Cell. Physiol. 2018;233:4033–4043. doi: 10.1002/jcp.26216. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z., He J.-W., Fu W.-Z., Zhang C.-Q., Zhang Z.-L. An analysis of the association between the vitamin D pathway and serum 25-hydroxyvitamin D levels in a healthy Chinese population. J. Bone Miner. Res. 2013;28:1784–1792. doi: 10.1002/jbmr.1926. [DOI] [PubMed] [Google Scholar]
- 45.Barry E.L., Rees J.R., Peacock J.L., Mott L.A., Amos C.I., Bostick R.M., Figueiredo J.C., Ahnen D.J., Bresalier R.S., Burke C.A., et al. Genetic Variants in CYP2R1, CYP24A1, and VDR Modify the Efficacy of Vitamin D3Supplementation for Increasing Serum 25-Hydroxyvitamin D Levels in a Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2014;99:E2133–E2137. doi: 10.1210/jc.2014-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson D., Holt B.J., Pennell C.E., Holt P.G., Hart P.H., Blackwell J.M. Genome-wide association study of vitamin D levels in children: Replication in the Western Australian Pregnancy Cohort (Raine) study. Genes Immun. 2014;15:578–583. doi: 10.1038/gene.2014.52. [DOI] [PubMed] [Google Scholar]
- 47.Hyppönen E., Berry D.J., Wjst M., Power C. Serum 25-hydroxyvitamin D and IgE—A significant but nonlinear relationship. Allergy. 2009;64:613–620. doi: 10.1111/j.1398-9995.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- 48.Lips P. Relative Value of 25(OH)D and 1,25(OH)2D Measurements. J. Bone Miner. Res. 2007;22:1668–1671. doi: 10.1359/jbmr.070716. [DOI] [PubMed] [Google Scholar]
- 49.Demir K., Kattan W.E., Zou M., Durmaz E., BinEssa H., Nalbantoğlu Ö., Al-Rijjal R.A., Meyer B., Özkan B., Shi Y. Novel CYP27B1 Gene Mutations in Patients with Vitamin D-Dependent Rickets Type 1A. PLoS ONE. 2015;10:e0131376. doi: 10.1371/journal.pone.0131376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malloy P.J., Hochberg Z., Tiosano D., Pike J.W., Hughes M.R., Feldman D. The molecular basis of hereditary 1,25-dihydroxyvitamin D3 resistant rickets in seven related families. J. Clin. Investig. 1990;86:2071–2079. doi: 10.1172/JCI114944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Datta P., Philipsen P.A., Olsen P., Bogh M.K., Johansen P., Schmedes A.V., Morling N., Wulf H.C. The half-life of 25(OH)D after UVB exposure depends on gender and vitamin D receptor polymorphism but mainly on the start level. Photochem. Photobiol. Sci. 2017;16:985–995. doi: 10.1039/c6pp00258g. [DOI] [PubMed] [Google Scholar]
- 52.Wjst M., Altmüller J., Faus-Kessler T., Braig C., Bahnweg M., André E. Asthma families show transmission disequilibrium of gene variants in the vitamin D metabolism and signalling pathway. Respir. Res. 2006;7:60. doi: 10.1186/1465-9921-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurylowicz A., Ramos-Lopez E., Bednarczuk T., Badenhoop K. Vitamin D-Binding Protein (DBP) Gene Polymorphism is Associated with Graves’ Disease and the Vitamin D Status in a Polish Population Study. Exp. Clin. Endocrinol. Diabetes. 2006;114:329–335. doi: 10.1055/s-2006-924256. [DOI] [PubMed] [Google Scholar]
- 54.Engelman C.D., Fingerlin T.E., Langefeld C.D., Hicks P.J., Rich S.S., Wagenknecht L.E., Bowden D.W., Norris J.M. Genetic and Environmental Determinants of 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D Levels in Hispanic and African Americans. J. Clin. Endocrinol. Metab. 2008;93:3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nissen J., Rasmussen L.B., Ravn-Haren G., Andersen E.W., Hansen B., Andersen R., Mejborn H., Madsen K.H., Vogel U. Common Variants in CYP2R1 and GC Genes Predict Vitamin D Concentrations in Healthy Danish Children and Adults. PLoS ONE. 2014;9:e89907. doi: 10.1371/journal.pone.0089907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hibler E.A., Hu C., Jurutka P.W., Martinez M.E., Jacobs E.T. Polymorphic Variation in the GC and CASR Genes and Associations with Vitamin D Metabolite Concentration and Metachronous Colorectal Neoplasia. Cancer Epidemiol. Biomark. Prev. 2012;21:368–375. doi: 10.1158/1055-9965.EPI-11-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Usategui-Martín R., De Luis-Román D.-A., Fernández-Gómez J.M., Ruiz-Mambrilla M., Pérez-Castrillón J.-L. Vitamin D Receptor (VDR) Gene Polymorphisms Modify the Response to Vitamin D Supplementation: A Systematic Review and Meta-Analysis. Nutrients. 2022;14:360. doi: 10.3390/nu14020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shree T., Banerjee P., Senapati S. A meta-analysis suggests the association of reduced serum level of vitamin D and T-allele of Fok1 (rs2228570) polymorphism in the vitamin D receptor gene with celiac disease. Front. Nutr. 2023;9:996450. doi: 10.3389/fnut.2022.996450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levin G.P., Robinson-Cohen C., de Boer I.H., Houston D.K., Lohman K., Liu Y., Kritchevsky S.B., Cauley J.A., Tanaka T., Ferrucci L., et al. Genetic Variants and Associations of 25-Hydroxyvitamin D Concentrations with Major Clinical Outcomes. JAMA. 2012;308:1898–1905. doi: 10.1001/jama.2012.17304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arai H., Miyamoto K.-I., Yoshida M., Yamamoto H., Taketani Y., Morita K., Kubota M., Yoshida S., Ikeda M., Watabe F., et al. The Polymorphism in the Caudal-Related Homeodomain Protein Cdx-2 Binding Element in the Human Vitamin D Receptor Gene. J. Bone Miner. Res. 2001;16:1256–1264. doi: 10.1359/jbmr.2001.16.7.1256. [DOI] [PubMed] [Google Scholar]
- 61.D’Alésio A., Garabédian M., Sabatier J.P., Guaydier-Souquières G., Marcelli C., Lemaçon A., Walrant-Debray O., Jehan F. Two single-nucleotide polymorphisms in the human vitamin D receptor promoter change protein–DNA complex formation and are associated with height and vitamin D status in adolescent girls. Hum. Mol. Genet. 2005;14:3539–3548. doi: 10.1093/hmg/ddi382. [DOI] [PubMed] [Google Scholar]
- 62.Glorieux F.H. Calcitriol treatment in vitamin D-dependent and vitamin D-resistant rickets. Metabolism. 1990;39:10–12. doi: 10.1016/0026-0495(90)90264-D. [DOI] [PubMed] [Google Scholar]
- 63.Forman J.P., Williams J.S., Fisher N.D. Plasma 25-Hydroxyvitamin D and Regulation of the Renin-Angiotensin System in Humans. Hypertension. 2010;55:1283–1288. doi: 10.1161/HYPERTENSIONAHA.109.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaidya A., Forman J.P., Hopkins P.N., Seely E.W., Williams J.S. 25-Hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. J. Renin-Angiotensin-Aldosterone Syst. 2011;12:311–319. doi: 10.1177/1470320310391922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carrara D., Bernini M., Bacca A., Rugani I., Duranti E., Virdis A., Ghiadoni L., Taddei S., Bernini G. Cholecalciferol administration blunts the systemic renin–angiotensin system in essential hypertensives with hypovitaminosis D. J. Renin-Angiotensin-Aldosterone Syst. 2014;15:82–87. doi: 10.1177/1470320312471149. [DOI] [PubMed] [Google Scholar]
- 66.Yuan W., Pan W., Kong J., Zheng W., Szeto F.L., Wong K.E., Cohen R., Klopot A., Zhang Z., Li Y.C. 1,25-Dihydroxyvitamin D3 Suppresses Renin Gene Transcription by Blocking the Activity of the Cyclic AMP Response Element in the Renin Gene Promoter. J. Biol. Chem. 2007;282:29821–29830. doi: 10.1074/jbc.M705495200. [DOI] [PubMed] [Google Scholar]
- 67.Burgess E.D., Hawkins R.G., Watanabe M. Interaction of 1,25-Dihydroxyvitamin D and Plasma Renin Activity in High Renin Essential Hypertension. Am. J. Hypertens. 1990;3:903–905. doi: 10.1093/ajh/3.12.903. [DOI] [PubMed] [Google Scholar]
- 68.Nunes I.F.O.C., Cavalcante A.A.C.M., Alencar M.V.O.B., Carvalho M.D.F., Sarmento J.L.R., Teixeira N.S.C.C.A., Paiva A.A., Carvalho L.R., Nascimento L.F.M., Cruz M.S.P., et al. Meta-Analysis of the Association Between the rs228570 Vitamin D Receptor Gene Polymorphism and Arterial Hypertension Risk. Adv. Nutr. Int. Rev. J. 2020;11:1211–1220. doi: 10.1093/advances/nmaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jurutka P.W., Remus L.S., Whitfield G.K., Thompson P.D., Hsieh J.-C., Zitzer H., Tavakkoli P., Galligan M.A., Dang H.T.L., Haussler C.A., et al. The Polymorphic N Terminus in Human Vitamin D Receptor Isoforms Influences Transcriptional Activity by Modulating Interaction with Transcription Factor IIB. Mol. Endocrinol. 2000;14:401–420. doi: 10.1210/mend.14.3.0435. [DOI] [PubMed] [Google Scholar]
- 70.Padma T., Vamsi U., Swapna N., Usha G. Risk conferred by FokI polymorphism of vitamin D receptor (VDR) gene for essential hypertension. Indian J. Hum. Genet. 2011;17:201–206. doi: 10.4103/0971-6866.92104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muray S., Parisi E., Cardús A., Craver L., Fernández E. Influence of vitamin D receptor gene polymorphisms and 25-hydroxyvitamin D on blood pressure in apparently healthy subjects. J. Hypertens. 2003;21:2069–2075. doi: 10.1097/00004872-200311000-00016. [DOI] [PubMed] [Google Scholar]
- 72.Lee B.K., Lee G.S., Stewart W.F., Ahn K.D., Simon D., Kelsey K.T., Todd A.C., Schwartz B.S. Associations of blood pressure and hypertension with lead dose measures and polymorphisms in the vitamin D receptor and delta-aminolevulinic acid dehydratase genes. Environ. Health Perspect. 2001;109:383–389. doi: 10.1289/ehp.01109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang L., Ma J., Manson J.E., Buring J.E., Gaziano J.M., Sesso H.D. A prospective study of plasma vitamin D metabolites, vitamin D receptor gene polymorphisms, and risk of hypertension in men. Eur. J. Nutr. 2013;52:1771–1779. doi: 10.1007/s00394-012-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tourkochristou E., Mouzaki A., Triantos C. Gene Polymorphisms and Biological Effects of Vitamin D Receptor on Nonalcoholic Fatty Liver Disease Development and Progression. Int. J. Mol. Sci. 2023;24:8288. doi: 10.3390/ijms24098288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harding B., Curley A.J., Hannan F.M., Christie P.T., Bowl M.R., Turner J.J.O., Barber M., Gillham-Nasenya I., Hampson G., Spector T.D., et al. Functional characterization of calcium sensing receptor polymorphisms and absence of association with indices of calcium homeostasis and bone mineral density. Clin. Endocrinol. 2006;65:598–605. doi: 10.1111/j.1365-2265.2006.02634.x. [DOI] [PubMed] [Google Scholar]
- 76.Pekkinen M., Laine C.M., Mäkitie R., Leinonen E., Lamberg-Allardt C., Viljakainen H., Mäkitie O. FGF23 gene variation and its association with phosphate homeostasis and bone mineral density in Finnish children and adolescents. Bone. 2015;71:124–130. doi: 10.1016/j.bone.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 77.Hibler E., Jurutka P., Egan J., Hu C., LeRoy E., Martinez M., Thompson P., Jacobs E. Association between polymorphic variation in VDR and RXRA and circulating levels of vitamin D metabolites. J. Steroid Biochem. Mol. Biol. 2010;121:438–441. doi: 10.1016/j.jsbmb.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henikoff S., Greally J.M. Epigenetics, cellular memory and gene regulation. Curr. Biol. 2016;26:R644–R648. doi: 10.1016/j.cub.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 79.Carlberg C. Nutrigenomics of Vitamin D. Nutrients. 2019;11:676. doi: 10.3390/nu11030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou Y., Zhao L.-J., Xu X., Ye A., Travers-Gustafson D., Zhou B., Wang H.-W., Zhang W., Hamm L.L., Deng H.-W., et al. DNA methylation levels of CYP2R1 and CYP24A1 predict vitamin D response variation. J. Steroid Biochem. Mol. Biol. 2014;144:207–214. doi: 10.1016/j.jsbmb.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu H., Wang X., Shi H., Su S., Harshfield G.A., Gutin B., Snieder H., Dong Y. A Genome-Wide Methylation Study of Severe Vitamin D Deficiency in African American Adolescents. J. Pediatr. 2013;162:1004–1009.e1. doi: 10.1016/j.jpeds.2012.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meyer M.B., Zella L.A., Nerenz R.D., Pike J.W. Characterizing Early Events Associated with the Activation of Target Genes by 1,25-Dihydroxyvitamin D3 in Mouse Kidney and Intestine In Vivo. J. Biol. Chem. 2007;282:22344–22352. doi: 10.1074/jbc.M703475200. [DOI] [PubMed] [Google Scholar]
- 83.Wang M., Kong W., He B., Li Z., Song H., Shi P., Wang J. Vitamin D and the promoter methylation of its metabolic pathway genes in association with the risk and prognosis of tuberculosis. Clin. Epigenetics. 2018;10:118. doi: 10.1186/s13148-018-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wjst M., Heimbeck I., Kutschke D., Pukelsheim K. Epigenetic regulation of vitamin D converting enzymes. J. Steroid Biochem. Mol. Biol. 2010;121:80–83. doi: 10.1016/j.jsbmb.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 85.Beckett E.L., Duesing K., Martin C., Jones P., Furst J., King K., Niblett S., Yates Z., Veysey M., Lucock M. Relationship between methylation status of vitamin D-related genes, vitamin D levels, and methyl-donor biochemistry. J. Nutr. Intermed. Metab. 2016;6:8–15. doi: 10.1016/j.jnim.2016.04.010. [DOI] [Google Scholar]
- 86.Holick M.F. Vitamin D: A millenium perspective. J. Cell. Biochem. 2002;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 87.Holick M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2003;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 88.Tangpricha V., Flanagan J.N., Whitlatch L.W., Tseng C.C., Chen T.C., Holt P.R., Lipkin M.S., Holick M.F. 25-hydroxyvitamin D-1α-hydroxylase in normal and malignant colon tissue. Lancet. 2001;357:1673–1674. doi: 10.1016/S0140-6736(00)04831-5. [DOI] [PubMed] [Google Scholar]
- 89.Gombart A.F., Borregaard N., Koeffler H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 90.Haussler M.R., Jurutka P.W., Mizwicki M., Norman A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011;25:543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 91.Pike J.W., Meyer M.B. The Vitamin D Receptor: New Paradigms for the Regulation of Gene Expression by 1,25-Dihydroxyvitamin D3. Endocrinol. Metab. Clin. N. Am. 2010;39:255–269. doi: 10.1016/j.ecl.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tanaka H., Abe E., Miyaura C., Kuribayashi T., Konno K., Nishii Y., Suda T. 1α,25-Dihydroxycholecalciferol and a human myeloid leukaemia cell line (HL-60) Biochem. J. 1982;204:713–719. doi: 10.1042/bj2040713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molnár F., Peräkylä M., Carlberg C. Vitamin D Receptor Agonists Specifically Modulate the Volume of the Ligand-binding Pocket. J. Biol. Chem. 2006;281:10516–10526. doi: 10.1074/jbc.M513609200. [DOI] [PubMed] [Google Scholar]
- 94.Carlberg C. Genome-wide (over)view on the actions of vitamin D. Front. Physiol. 2014;5:167. doi: 10.3389/fphys.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carlberg C. Vitamin D and Its Target Genes. Nutrients. 2022;14:1354. doi: 10.3390/nu14071354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vaquerizas J.M., Kummerfeld S.K., Teichmann S.A., Luscombe N.M. A census of human transcription factors: Function, expression and evolution. Nat. Rev. Genet. 2009;10:252–263. doi: 10.1038/nrg2538. [DOI] [PubMed] [Google Scholar]
- 97.Tuoresmäki P., Väisänen S., Neme A., Heikkinen S., Carlberg C. Patterns of Genome-Wide VDR Locations. PLoS ONE. 2014;9:e96105. doi: 10.1371/journal.pone.0096105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nurminen V., Neme A., Seuter S., Carlberg C. The impact of the vitamin D-modulated epigenome on VDR target gene regulation. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2018;1861:697–705. doi: 10.1016/j.bbagrm.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 99.Nurminen V., Neme A., Seuter S., Carlberg C. Modulation of vitamin D signaling by the pioneer factor CEBPA. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2019;1862:96–106. doi: 10.1016/j.bbagrm.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 100.Neme A., Seuter S., Carlberg C. Vitamin D-dependent chromatin association of CTCF in human monocytes. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2016;1859:1380–1388. doi: 10.1016/j.bbagrm.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 101.Cross H.S., Bareis P., Hofer H., Bischof M.G., Bajna E., Kriwanek S., Bonner E., Peterlik M. 25-Hydroxyvitamin D3-1α-hydroxylase and vitamin D receptor gene expression in human colonic mucosa is elevated during early cancerogenesis. Steroids. 2001;66:287–292. doi: 10.1016/S0039-128X(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 102.Hanel A., Carlberg C. Time-Resolved Gene Expression Analysis Monitors the Regulation of Inflammatory Mediators and Attenuation of Adaptive Immune Response by Vitamin D. Int. J. Mol. Sci. 2022;23:911. doi: 10.3390/ijms23020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arora J., Wang J., Weaver V., Zhang Y., Cantorna M.T. Novel insight into the role of the vitamin D receptor in the development and function of the immune system. J. Steroid Biochem. Mol. Biol. 2022;219:106084. doi: 10.1016/j.jsbmb.2022.106084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bikle D.D. Vitamin D regulation of immune function during COVID-19. Rev. Endocr. Metab. Disord. 2022;23:279–285. doi: 10.1007/s11154-021-09707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pérez-Ferro M., Romero-Bueno F.I., del Castillo C.S., Mahillo I., Alvear A., Largo R., Herrero-Beaumont G., Sánchez-Pernaute O. A subgroup of lupus patients with nephritis, innate T cell activation and low vitamin D is identified by the enhancement of circulating MHC class I-related chain A. Clin. Exp. Immunol. 2019;196:336–344. doi: 10.1111/cei.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Komisarenko Y.I., Bobryk M.I. Vitamin D Deficiency and Immune Disorders in Combined Endocrine Pathology. Front. Endocrinol. 2018;9:600. doi: 10.3389/fendo.2018.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oh S., Chun S., Hwang S., Kim J., Cho Y., Lee J., Kwack K., Choi S.-W. Vitamin D and Exercise Are Major Determinants of Natural Killer Cell Activity, Which Is Age- and Gender-Specific. Front. Immunol. 2021;12:594356. doi: 10.3389/fimmu.2021.594356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carlberg C., Velleuer E. Vitamin D and the risk for cancer: A molecular analysis. Biochem. Pharmacol. 2022;196:114735. doi: 10.1016/j.bcp.2021.114735. [DOI] [PubMed] [Google Scholar]
- 109.Gandini S., Boniol M., Haukka J., Byrnes G., Cox B., Sneyd M.J., Mullie P., Autier P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int. J. Cancer. 2011;128:1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 110.Heath A.K., Hodge A.M., Ebeling P.R., Eyles D.W., Kvaskoff D., Buchanan D.D., Giles G.G., Williamson E.J., English D.R. Circulating 25-Hydroxyvitamin D Concentration and Risk of Breast, Prostate, and Colorectal Cancers: The Melbourne Collaborative Cohort Study. Cancer Epidemiol. Biomark. Prev. 2019;28:900–908. doi: 10.1158/1055-9965.EPI-18-1155. [DOI] [PubMed] [Google Scholar]
- 111.Dimitrakopoulou V.I., Tsilidis K.K., Haycock P.C., Dimou N.L., Al-Dabhani K., Martin R., Lewis S.J., Gunter M.J., Mondul A., Shui I.M., et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ. 2017;359:j4761. doi: 10.1136/bmj.j4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu K., Knuiman M., Divitini M., Hung J., Lim E.M., Cooke B.R., Walsh J.P. Lower serum 25-hydroxyvitamin D is associated with colorectal and breast cancer, but not overall cancer risk: A 20-year cohort study. Nutr. Res. 2019;67:100–107. doi: 10.1016/j.nutres.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 113.Manson J.E., Cook N.R., Lee I.M., Christen W., Bassuk S.S., Mora S., Gibson H., Gordon D., Copeland T., D’Agostino D., et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Manson J.E., Bassuk S.S., Buring J.E. Principal results of the VITamin D and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant Vitamin D trials. J. Steroid Biochem. Mol. Biol. 2020;198:105522. doi: 10.1016/j.jsbmb.2019.105522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feige J., Moser T., Bieler L., Schwenker K., Hauer L., Sellner J. Vitamin D Supplementation in Multiple Sclerosis: A Critical Analysis of Potentials and Threats. Nutrients. 2020;12:783. doi: 10.3390/nu12030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gubatan J., Moss A.C. Vitamin D in inflammatory bowel disease: More than just a supplement. Curr. Opin. Gastroenterol. 2018;34:217–225. doi: 10.1097/MOG.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 117.Manousaki D., Harroud A., Mitchell R.E., Ross S., Forgetta V., Timpson N.J., Smith G.D., Polychronakos C., Richards J.B. Vitamin D levels and risk of type 1 diabetes: A Mendelian randomization study. PLOS Med. 2021;18:e1003536. doi: 10.1371/journal.pmed.1003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Radujkovic A., Hippchen T., Tiwari-Heckler S., Dreher S., Boxberger M., Merle U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients. 2020;12:2757. doi: 10.3390/nu12092757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ferrari D., Locatelli M., Faraldi M., Lombardi G. Changes in 25-(OH) Vitamin D Levels during the SARS-CoV-2 Outbreak: Lockdown-Related Effects and First-to-Second Wave Difference—An Observational Study from Northern Italy. Biology. 2021;10:237. doi: 10.3390/biology10030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murai I.H., Fernandes A.L., Antonangelo L., Gualano B., Pereira R.M.R. Effect of a Single High-Dose Vitamin D3 on the Length of Hospital Stay of Severely 25-Hydroxyvitamin D-Deficient Patients with COVID-19. Clinics. 2021;76:e3549. doi: 10.6061/clinics/2021/e3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Petrelli F., Oldani S., Borgonovo K., Cabiddu M., Dognini G., Ghilardi M., Parati M.C., Petro’ D., Dottorini L., Rea C., et al. Vitamin D3 and COVID-19 Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Antioxidants. 2023;12:247. doi: 10.3390/antiox12020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ulivieri F.M., Banfi G., Camozzi V., Colao A., Formenti A.M., Frara S., Lombardi G., Napoli N., Giustina A. Vitamin D in the COVID-19 era: A review with recommendations from a G.I.O.S.E.G. expert panel. Endocrine. 2021;72:597–603. doi: 10.1007/s12020-021-02749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented are included in the manuscript.