Abstract
Aims
Mitral annular plane systolic excursion (MAPSE) is a simple and reliable index for evaluating left ventricular (LV) systolic function, particularly in patients with poor image quality; however, the lack of reference values limits its widespread use. This study aimed to establish the normal ranges for MAPSE measured using motion-mode (M-mode) and two-dimensional speckle tracking echocardiography (2D-STE) and to explore its principal determinants.
Methods and results
This multicentre, prospective, cross-sectional study included 1952 healthy participants [840 men (43%); age range, 18–80 years] from 55 centres. MAPSE was measured using M-mode echocardiography and 2D-STE. The results showed that women had a higher MAPSE than men and MAPSE decreased with age. The age- and sex-specific reference values for MAPSE were established for these two methods. Multiple linear regression analyses revealed that MAPSE on M-mode echocardiography correlated with age and MAPSE on 2D-STE with age, blood pressure (BP), heart rate, and LV volume. Moreover, MAPSE measured by 2D-STE correlated more strongly with global longitudinal strain compared with that measured using M-mode echocardiography.
Conclusion
Normal MAPSE reference values were established based on age and sex. BP, heart rate, and LV volume are potential factors that influence MAPSE and should be considered in clinical practice. Normal values are useful for evaluating LV longitudinal systolic function, especially in patients with poor image quality, and may further facilitate the use of MAPSE in routine assessments.
Keywords: mitral annulus displacement, ventricular function, normal values, speckle tracking, echocardiography
Graphical Abstract
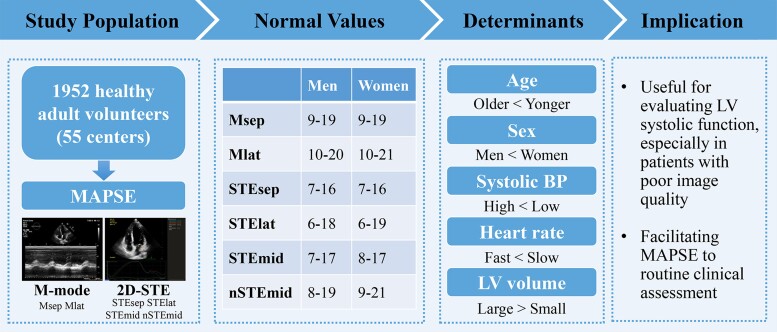
Graphical Abstract.
Normal reference values for mitral annular plane systolic excursion by motion-mode and speckle tracking echocardiography. 2D-STE, two-dimensional speckle tracking echocardiography; other abbreviations as per Tables 1 and 2.
Introduction
The assessment of left ventricular (LV) systolic function plays a key role in the diagnosis, management, and prognosis of cardiac diseases.1 Determination of LV ejection fraction (LVEF) using two-dimensional (2D) or three-dimensional methods and global longitudinal strain (GLS) using speckle tracking echocardiography (STE) is recommended for evaluating LV systolic function.2,3 However, both methods require tracing of the LV endocardium, which is highly dependent on an adequate visualization of the LV myocardium. Therefore, an approach that does not rely on image quality is desirable for assessing LV systolic function.
Mitral annular plane systolic excursion (MAPSE) quantitatively reflects LV longitudinal systolic function because the longitudinal movement of the mitral valve annulus plays a leading role in LV pumping, whereas the apex is relatively stationary.4 Moreover, MAPSE is independent of image quality since its measurement only requires visualization of the mitral annulus without delineation of the LV endocardial boundary.5 Therefore, the 2015 Chamber Quantification Guidelines from the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) recommend the use of MAPSE as a substitute parameter when LVEF or GLS cannot be detected accurately owing to suboptimal delineation or tracking in patients with poor image quality.3 Moreover, a 2020 consensus recommendation from the Heart Failure Association of the European Society of Cardiology stated that MAPSE could be used as an alternative index for evaluating LV systolic function in patients with heart failure with preserved ejection fraction.6
Currently, MAPSE is determined primarily using motion-mode (M-mode) echocardiography as this method is more readily implemented. However, M-mode echocardiography is angle dependent and has limited accuracy. Another technique, two-dimensional speckle tracking echocardiography (2D-STE), can track the mitral annulus and measure MAPSE automatically without angle dependence.7 Previous studies have verified the accuracy, convenience, and high reproducibility of 2D-STE for measuring MAPSE to assess LV systolic function.8,9 However, the lack of large, multicentre, population-based normal reference values for MAPSE using both M-mode echocardiography and 2D-STE has impeded efforts towards its wider implementation in routine clinical practice. In addition, the principal determinants of MAPSE have not yet been investigated thoroughly. Therefore, we aimed to establish the normal reference values for MAPSE measured using M-mode echocardiography and 2D-STE and to explore the primary determinants of MAPSE in a large prospective multicentre study. This may facilitate its implementation in routine clinical assessment for the detection of LV systolic dysfunction.
Methods
Study population
This was a nationwide, multicentre, prospective, observational, cross-sectional study of healthy adults in China, conducted from January 2021 to January 2022. The study was designed initially to enrol 2400 normal healthy adult volunteers with an even distribution of age and sex. Specifically, we aimed to include at least 400 participants in each age group (18–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years, with n = 200 men). The sample size was determined according to the guidelines of the Clinical and Laboratory Standards Institute,10 in which the minimum number of patients was 120 in each group. The sample size was adjusted based on an assumptive 30% exclusion rate. The principal investigators, Professors W.-D.R. and C.-Y.M., invited echocardiographic laboratories accredited by the Chinese Society of Ultrasound in Medicine from regionally representative tertiary hospitals in all provinces and municipalities throughout China to participate in the study. Ultimately, 55 echocardiographic laboratories participated in this study.
Healthy individuals were identified from check-ups at medical examination centres. The inclusion criteria were as follows: age ≥ 18 years; no history or clinical evidence of cardiac, cardiovascular, lung, or kidney disease; and no abnormal findings on physical examination, biochemical examination, electrocardiography, or echocardiography. The exclusion criteria were as follows: hypertension [blood pressure (BP) > 140/80 mmHg] or receiving medication for hypertension; body mass index > 30 kg/m2; total cholesterol ≥ 6.2 mmol/L, triglyceride ≥ 2.3 mmol/L, or high-density lipoprotein cholesterol < 1.0 mmol/L; endocrine diseases, such as diabetes mellitus (fasting blood glucose ≥ 7.0 mmol/L), thyroid disease, pheochromocytoma, adrenal insufficiency, and hyperaldosteronism; anaemia; abnormal liver function; connective tissue diseases, such as rheumatoid arthritis, vasculitis, systemic lupus erythematosus, dermatomyositis, and scleroderma; malignancy; current pregnancy or lactation; professional sport activity; and a history of alcoholism. Moreover, patients with mitral annulus calcification were excluded.
All participants provided written informed consent. This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was registered with the North American Clinical Trials Network (registration ID: NCT04399447).
Echocardiographic image acquisition
To ensure standardization and uniformity of image acquisition and clinical data records, each participating laboratory designated a supervisory physician who was intensively trained in advance at the core laboratories. The image acquisition and quantitative assessments were performed following a standardized protocol created by two core laboratories (First Hospital and Shengjing Hospital of China Medical University) as per the ASE recommendations.3,11 Each participating laboratory regularly transmitted the collected demographic information and the recorded raw digital images in Digital Imaging and Communications in Medicine using network discs to the core laboratories for central analyses. Two senior experts from the core laboratories (L.S. from Shengjing Hospital of China Medical University and S.-W.L. from First Hospital of China Medical University) performed quality control checks by reviewing each echocardiographic image. The final measurements and statistical analyses were performed at the core laboratories. All echocardiographic images were acquired and assessed by experienced cardiologists who were blinded to the clinical data.
The echocardiographic examinations were performed using an EPIQ7C imaging system (Philips Healthcare, Andover, MA, USA) equipped with an S5-1 transducer (1–5 MHz). Standard 2D images were recorded during normal respiration using optimal technical setting parameters and a frame rate of at least 50 fps. The presence of LV foreshortening in the apical view was avoided. At least three cardiac cycles were recorded for each view. LV end-diastolic volume (EDV), end-systolic volume (ESV), and EF were measured using the biplane Simpson method. LV diastolic function was assessed according to the ASE recommendations.12
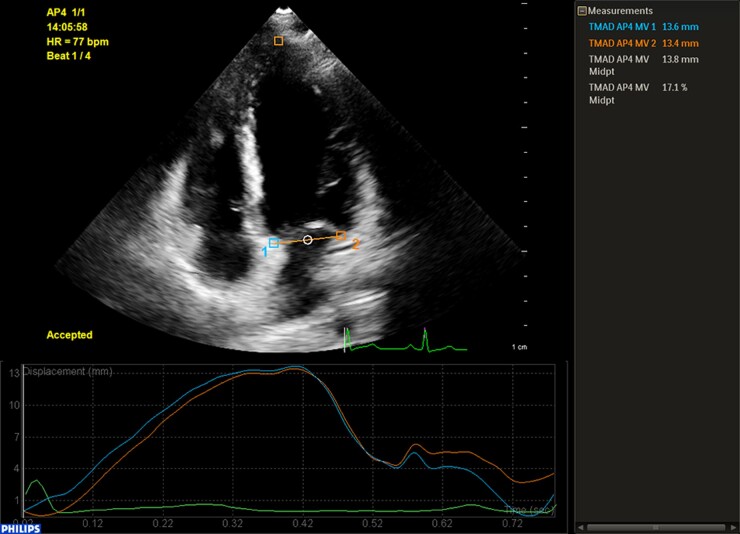
Using M-mode echocardiography in the apical four-chamber view, sampling lines were placed in the septal and lateral mitral annuli to measure the corresponding MAPSE (Msep and Mlat, respectively). MAPSE was also measured at the core laboratories using 2D-STE with the commercially available software QLAB (version 13.0; Philips Healthcare, Andover, MA, USA). After entering the tissue motion in the mitral annular displacement mode, three anatomical landmark points on the septal and lateral mitral annuli and the LV apical myocardium were selected in an automatically located diastolic frame in the apical four-chamber view. The software then tracked the three selected points frame-by-frame. The displacement of the septal and lateral mitral annuli from the LV base to the apex was calculated throughout the cardiac cycle, and the displacement–time curves were plotted (Figure 1). The maximal MAPSE in the septal and lateral mitral annuli (STEsep and STElat), and at the midpoint between the two (STEmid), was automatically displayed. STEmid was normalized by calculating the ratio of STEmid to the LV long-axis length at end-diastole (nSTEmid). The average values of the three measurements were used for further analysis.
Figure 1.
Representative image for measuring MAPSE using 2D-STE.
LV GLS was measured from the apical two-, four-, and three-chamber views using an automated method with the QLAB AutoStrain LV package. The endocardial borders were corrected manually if necessary, and LV GLS was not used for further data analysis if, even after a manual adjustment, the participant had inadequate tracking of more than one segment in at least one apical view. For simplicity, the absolute values of GLS were used for data interpretation.
Statistical analyses
Age groups were compared using an analysis of variance. A post hoc analysis (Bonferroni test) was performed to analyse intergroup differences. Bivariate correlations were presented as Pearson’s coefficients (r). Simple and multiple linear regressions were used to explore the determinants of MAPSE; parameters with P-values < 0.05 in the simple analysis were included in the multiple regression model. The degree of collinearity was reflected by the variance inflation factor, with a factor > 5 indicating severe collinearity. The normal range for MAPSE was defined as the range that included 95% of the normal population, and the values at the 2.5th and 97.5th percentiles were defined as the lower and upper limits of normality, respectively. The same observer repeated the measurements at different time points with an interval of at least 3 months to evaluate intra-observer reproducibility, and a second independent observer repeated the measurements twice to assess the inter-observer reproducibility. Intra- and inter-observer reproducibility was evaluated by calculating the coefficient of variation (CV) based on re-evaluation of the same image in a subset of 50 randomly selected participants. A P-value of <0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS software (version 26.0; IBM Corp., Armonk, NY, USA) and R version 4.1.2.
Results
Population characteristics
A total of 1952 healthy participants [age groups: 18–29 years, n = 339 (17.4%); 30–39 years, n = 458 (23.5%); 40–49 years, n = 424 (21.7%); 50–59 years, n = 366 (18.8%); 60–70 years, n = 230 (11.8%); and ≥70 years, n = 135 (6.9%)] from 55 participating institutions were included in this study. Among them were 840 men (43%) with a mean age of 45 ± 15 years (range, 18–80 years) and 1112 women (57%) with a mean age of 44 ± 14 years (range, 18–79 years).
Table 1 shows the baseline demographic characteristics of the study population. Men exhibited significantly greater heights, weights, body surface areas (BSAs), body mass indexes, systolic and diastolic BPs, and waist circumferences than those of women. However, neither age nor heart rate differed significantly between the two sexes. LV EDV, ESV, and end-diastolic and end-systolic diameters were significantly higher in men, whereas women had higher LV end-diastolic and end-systolic diameters indexed to BSAs. Women also had higher LVEF than men. Moreover, left atrial volume index, mitral E, e′, average E/e′, and E/A values were higher in women than in men (see Supplementary data online, Table S1).
Table 1.
Baseline demographic characteristics of the study population
| Parameters | Total (n = 1952) | Men (n = 840) | Women (n = 1112) | P-value (men vs. women) |
|---|---|---|---|---|
| Age (years) | 45 ± 15 | 45 ± 15 | 44 ± 14 | 0.30 |
| Height (cm) | 166 ± 8 | 172 ± 6 | 161 ± 5 | <0.001 |
| Weight (kg) | 62 ± 10 | 69 ± 9 | 57 ± 7 | <0.001 |
| BSA (m²) | 1.69 ± 0.17 | 1.82 ± 0.14 | 1.59 ± 0.11 | <0.001 |
| Body mass index (kg/m²) | 22.5 ± 2.5 | 23.2 ± 2.4 | 22.0 ± 2.4 | <0.001 |
| Systolic BP (mmHg) | 118 ± 10 | 121 ± 9 | 116 ± 10 | <0.001 |
| Diastolic BP (mmHg) | 75 ± 7 | 76 ± 7 | 74 ± 7 | <0.001 |
| Heart rate (bpm) | 73 ± 9 | 73 ± 9 | 73 ± 9 | 0.23 |
| Waistline (cm) | 79 ± 9 | 83 ± 9 | 76 ± 8 | <0.001 |
Values shown are means ± standard deviation.
BP, blood pressure; BSA, body surface area.
Establishment of normal reference values for MAPSE
The normal ranges of each MAPSE parameter stratified by sex are shown in Table 2. Compared with men, Mlat measured using M-mode echocardiography and each MAPSE parameter measured using 2D-STE were higher in women.
Table 2.
MAPSE according to sex
| Parameters | Total (n = 1952) | Men (n = 840) | Women (n = 1112) | P-value (men vs. women) |
|---|---|---|---|---|
| Msep (mm) | ||||
| Mean ± SD | 14.0 ± 2.5 | 14.0 ± 2.5 | 14.0 ± 2.5 | 0.94 |
| 95% CI | 9–19 | 9–19 | 9–19 | |
| Mlat (mm) | ||||
| Mean ± SD | 15.5 ± 2.8 | 15.2 ± 2.6 | 15.7 ± 2.8 | <0.001 |
| 95% CI | 10–21 | 10–20 | 10–21 | |
| STEsep (mm) | ||||
| Mean ± SD | 11.5 ± 2.3 | 11.3 ± 2.3 | 11.6 ± 2.3 | 0.004 |
| 95% CI | 7–16 | 7–16 | 7–16 | |
| STElat (mm) | ||||
| Mean ± SD | 12.4 ± 3.2 | 12.1 ± 3.1 | 12.6 ± 3.2 | 0.001 |
| 95% CI | 6–19 | 6–18 | 6–19 | |
| STEmid (mm) | ||||
| Mean ± SD | 12.2 ± 2.5 | 12.0 ± 2.4 | 12.4 ± 2.5 | 0.001 |
| 95% CI | 7–17 | 7–17 | 8–17 | |
| nSTEmid (%) | ||||
| Mean ± SD | 14.5 ± 3.1 | 13.8 ± 2.8 | 15.0 ± 3.2 | <0.001 |
| 95% CI | 8–21 | 8–19 | 9–21 |
CI, confidence interval; MAPSE, mitral annular plane systolic excursion; Msep and Mlat, septal and lateral MAPSE by M-mode echocardiography; STEsep, STElat, and STEmid, septal, lateral, and midpoint MAPSE measured by 2D-STE; nSTEmid, normalized STEmid by LV long-axis length at end-diastole; SD, standard deviation.
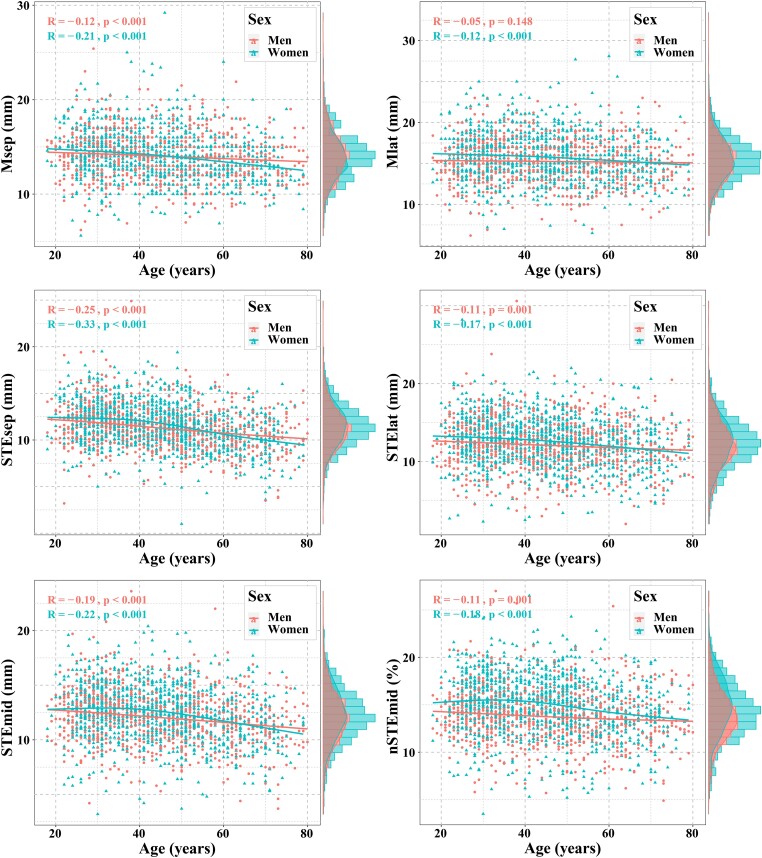
The normal ranges of each MAPSE parameter stratified by age are presented in Table 3. Msep decreased gradually with age in both sexes and was negatively correlated with age in both men (r, −0.12; P < 0.001) and women (r, −0.21; P < 0.001; Figure 2). Moreover, each MAPSE parameter measured by 2D-STE decreased gradually with age in both sexes and was correlated negatively with age in men (r, −0.11 to −0.25; all P < 0.05) and women (r, −0.17 to −0.33; all P < 0.05; Figure 2).
Table 3.
MAPSE according to age decades
| Parameters | 18–29 years (n = 339) | 30–39 years (n = 458) | 40–49 years (n = 424) | 50–59 years (n = 366) | 60–69 years (n = 230) | ≥70 years (n = 135) | P-value |
|---|---|---|---|---|---|---|---|
| Men (n) | 159 | 186 | 157 | 163 | 103 | 72 | |
| Msep (mm) | |||||||
| Mean ± SD | 14.3 ± 2.5 | 14.5 ± 2.3 | 13.9 ± 2.4 | 13.7 ± 2.7 | 13.8 ± 2.4 | 13.4 ± 2.2‡ | 0.005 |
| 95% CI | 9–19 | 10–19 | 9–19 | 8–19 | 9–19 | 9–18 | |
| Mlat (mm) | |||||||
| Mean ± SD | 15.2 ± 2.7 | 15.7 ± 2.5 | 15.3 ± 2.5 | 14.9 ± 2.7 | 15.3 ± 2.8 | 14.9 ± 2.6 | 0.09 |
| 95% CI | 10–20 | 11–21 | 10–20 | 10–20 | 10–21 | 10–20 | |
| STEsep (mm) | |||||||
| Mean ± SD | 12.0 ± 2.3 | 11.9 ± 2.3 | 11.1 ± 2.1*,‡ | 11.0 ± 2.2*,‡ | 10.6 ± 2.2*,‡ | 10.1 ± 2.4*,‡,§ | <0.001 |
| 95% CI | 7–17 | 7–16 | 7–15 | 7–15 | 6–15 | 5–15 | |
| STElat (mm) | |||||||
| Mean ± SD | 12.5 ± 3.0 | 12.7 ± 3.4 | 11.6 ± 3.1‡ | 12.1 ± 3.0 | 11.9 ± 3.0 | 11.2 ± 3.2*,‡ | 0.001 |
| 95% CI | 7–18 | 6–19 | 6–18 | 6–18 | 6–18 | 5–17 | |
| STEmid (mm) | |||||||
| Mean ± SD | 12.6 ± 2.3 | 12.6 ± 2.6 | 11.7 ± 2.2*,‡ | 12.0 ± 2.4 | 11.4 ± 2.4*,‡ | 10.9 ± 2.4*,‡,# | <0.001 |
| 95% CI | 8–17 | 8–18 | 7–16 | 7–17 | 7–16 | 6–16 | |
| nSTEmid (%) | |||||||
| Mean ± SD | 14.2 ± 2.5 | 14.1 ± 2.9 | 13.6 ± 2.6 | 13.5 ± 2.8 | 13.6 ± 3.1 | 13.2 ± 2.9 | 0.03 |
| 95% CI | 9–19 | 8–20 | 9–19 | 8–19 | 8–20 | 8–19 | |
| Women (n) | 180 | 272 | 267 | 203 | 127 | 63 | |
| Msep (mm) | |||||||
| Mean ± SD | 14.5 ± 2.4 | 14.5 ± 2.5 | 14.2 ± 2.8 | 13.6 ± 2.3*,‡ | 13.2 ± 2.3*,‡,§ | 12.8 ± 2.1*,‡,§ | <0.001 |
| 95% CI | 10–19 | 10–19 | 9–20 | 9–18 | 9–18 | 9–17 | |
| Mlat (mm) | |||||||
| Mean ± SD | 16.0 ± 2.7 | 16.1 ± 2.8 | 15.9 ± 2.9 | 15.4 ± 2.8 | 15.2 ± 2.8 | 14.9 ± 2.4 | 0.002 |
| 95% CI | 11–21 | 11–22 | 10–22 | 10–21 | 10–21 | 10–20 | |
| STEsep (mm) | |||||||
| Mean ± SD | 12.2 ± 2.2 | 12.4 ± 2.1 | 11.9 ± 2.2 | 11.0 ± 2.3*,‡,§ | 10.4 ± 2.1*,‡,§ | 9.8 ± 2.5*,‡,§,# | <0.001 |
| 95% CI | 8–17 | 8–17 | 8–16 | 6–16 | 6–15 | 5–15 | |
| STElat (mm) | |||||||
| Mean ± SD | 13.0 ± 3.3 | 13.1 ± 2.9 | 12.8 ± 3.1 | 12.1 ± 3.1‡ | 11.8 ± 3.3*,‡ | 11.1 ± 3.2*,‡,§ | <0.001 |
| 95% CI | 7–19 | 7–19 | 7–19 | 6–18 | 5–18 | 5–17 | |
| STEmid (mm) | |||||||
| Mean ± SD | 12.7 ± 2.6 | 13.0 ± 2.4 | 12.7 ± 2.5 | 12.0 ± 2.5‡ | 11.5 ± 2.5*,‡,§ | 10.7 ± 2.4*,‡,§,# | <0.001 |
| 95% CI | 8–18 | 8–18 | 8–18 | 7–17 | 7–16 | 6–15 | |
| nSTEmid (%) | |||||||
| Mean ± SD | 15.2 ± 3.0 | 15.7 ± 3.0 | 15.3 ± 3.1 | 14.4 ± 3.4‡ | 14.0 ± 3.1*,‡,§ | 13.6 ± 3.0*,‡,§ | <0.001 |
| 95% CI | 9–21 | 10–22 | 9–21 | 8–21 | 8–20 | 8–19 |
P-value, differences among age groups. *P < 0.05 vs. 18–29 years. ‡P < 0.05 vs. 30–39 years. §P < 0.05 vs. 40–49 years. #P < 0.05 vs. 50–59 years. Abbreviations as per Table 2.
Figure 2.
Scatter plot showing the correlations between MAPSE and age stratified by sex.
Determinants of MAPSE
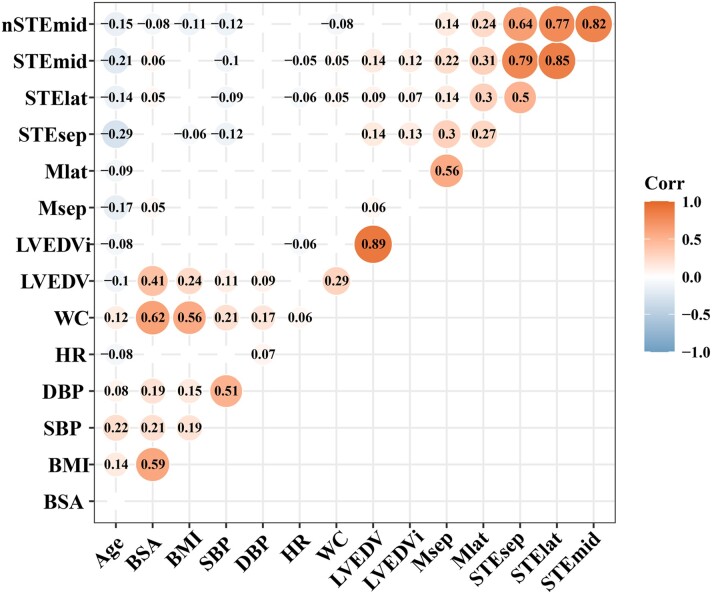
The correlation heat map showed that MAPSE measured using M-mode echocardiography may be related to age, BSA, and LV EDV (Figure 3), and the multiple linear regression analysis revealed that Msep and Mlat were independently correlated with age (Table 4).
Figure 3.
Correlation map showing the relationships between MAPSE and clinical characteristics. The red and blue bubbles, respectively, represent the positive and negative correlations, and the size and colour intensities of the bubbles represent the magnitude of correlation coefficients. Only the correlation with P < 0.05 is shown. BSA, body surface area; BMI, body mass index; DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure; WC, waist circumferences; other abbreviations as per Tables 2 and 4.
Table 4.
Multivariable analysis for determining MAPSE
| Variables | M-mode echocardiography | Speckle tracking echocardiography | ||||
|---|---|---|---|---|---|---|
| Msep | Mlat | STEsep | STElat | STEmid | nSTEmid | |
| Age | −0.03 (<0.001) | −0.02 (<0.001) | −0.04 (<0.001) | −0.03 (<0.001) | −0.03 (<0.001) | −0.03 (<0.001) |
| BSA | 0.52 (0.15) | 0.19 (0.64) | 0.22 (0.58) | 0.93 (0.10) | 0.66 (0.13) | −0.56 (0.31) |
| Body mass index | — | — | −0.05 (0.06) | −0.02 (0.53) | −0.04 (0.15) | −0.07 (0.06) |
| Systolic BP | — | — | −0.02 (0.004) | −0.03 (0.001) | −0.02 (0.001) | −0.02 (0.006) |
| Heart rate | — | — | −0.01 (0.05) | −0.02 (0.007) | −0.02 (0.01) | −0.02 (0.02) |
| LV EDV | 0.004 (0.20) | 0.004 (0.25) | 0.02 (<0.001) | 0.01 (0.007) | 0.02 (<0.001) | −0.004 (0.29) |
The correlation heat map showed that MAPSE measured using 2D-STE may be related to age, BSA, body mass index, waist circumference, systolic BP, heart rate, LV EDV, and LV EDV indexed to BSA (Figure 3). The multiple linear regression analysis revealed that STEsep, STElat, and STEmid were independently correlated with age, systolic BP, heart rate, and LV EDV, whereas nSTEmid was independently correlated with age, systolic BP, and heart rate (Table 4). Waist circumference and LV EDV indexed to BSA were excluded from multiple linear regression models because of their strong collinearity. The restricted cubic spline analysis showed that each MAPSE parameter measured using 2D-STE was negatively associated with systolic BP and heart rate after adjusting for potential confounders (see Supplementary data online, Figure S1). Participants with optimal BP (<120/80 mmHg) had a higher MAPSE measured by 2D-STE than those with non-optimal BP (≥120/80 and <140/90 mmHg; P < 0.05; see Supplementary data online, Figure S2).
Correlation of MAPSE and LV GLS
LV GLS using all three apical views with optimal image quality was obtained in 1683 participants (86.2%). LV GLS was positively correlated with Msep (r = 0.08; P = 0.002), Mlat (r = 0.15; P < 0.001), STEsep (r = 0.31; P < 0.001), STElat (r = 0.30; P < 0.001), STEmid (r = 0.35; P < 0.001), and nSTEmid (r = 0.38; P < 0.001; see Supplementary data online, Figure S3). Of these, nSTEmid showed the highest correlation with GLS.
Measurement reproducibility
Both M-mode echocardiography and 2D-STE demonstrated high intra- and inter-observer reproducibility for MAPSE measurements (see Supplementary data online, Table S2). The intra-observer CVs for Msep, Mlat, STEsep, STElat, STEmid, and nSTEmid were 4.3, 7.9, 6.8, 8.5, 5.2, and 5.6%, respectively. The inter-observer CVs for Msep, Mlat, STEsep, STElat, STEmid, and nSTEmid were 6.4, 8.5, 7.2, 9.4, 9.4, and 9.8%, respectively.
Discussion
The present study revealed three significant findings. First, the universal normal values of MAPSE measured using M-mode echocardiography and 2D-STE were established using a large, multicentre, nationwide, prospective, observational, and cross-sectional study with a wide age range. Second, women had marginally higher MAPSE values than men, and older individuals tended to have lower MAPSE values than younger individuals, underscoring the importance of contextualizing age and sex when using normal MAPSE values. Third, MAPSE on M-mode echocardiography correlated with age and sex, and MAPSE on 2D-STE correlated with age, sex, BP, heart rate, and LV volume. Normal values are useful for evaluating LV systolic function, especially in patients with poor image quality, and may further facilitate the use of MAPSE for routine clinical assessments. These principal determinants should be considered when assessing LV systolic function using MAPSE in individual patients.
MAPSE has proved to be a useful index for evaluating LV global systolic function.5 The mitral annular plane moves towards the apex in the longitudinal direction, primarily from the contraction of the (sub-endocardial and sub-epicardial) longitudinal fibres of the LV wall during systole, while the position of the apex is relatively stationary. Thus, MAPSE can reflect the capacity of the LV global longitudinal shortening deformation (contraction). LV GLS is another excellent marker of the myocardial longitudinal function, which has been proved to be a sensitive index for evaluating slight myocardial abnormalities, especially in early disease stages.3 However, similar to LVEF, optimal image quality is essential for the correct determination of GLS. In contrast, MAPSE measurement requires only visualization of the mitral annulus, which is possible in virtually all patients, without a delineation of the LV endocardial boundary. Thus, MAPSE has the inherent advantages of convenience, high reproducibility, and image quality independence. Therefore, MAPSE can be used as a simple and robust complementary index to GLS for evaluating LV subclinical systolic dysfunction, especially in patients with poor image quality. Moreover, MAPSE plays a significant role in the assessment of disease severity, differential diagnosis, risk stratification, and prognostic prediction for most cardiac diseases (heart failure, ischaemic heart disease, valvulopathy, and cardiomyopathies; see Supplementary data online, Table S3).
However, the implementation of MAPSE in routine clinical practice has been limited owing to the scarcity of direct large population-based normative data. In the HUNT study including 1266 healthy individuals, Støylen et al.13 provided normal reference values for mean MAPSE and MAPSE normalized to LV length measured using M-mode echocardiography. However, mean MAPSE was obtained by calculating the average values of MAPSE in the two, four, and six points of mitral annuli using different apical views, and MAPSE values calculated directly at the mitral annulus site were not provided. To capitalize on the simplicity and feasibility of M-mode echocardiography, the present study established direct normal ranges for MAPSE in the septal and lateral mitral annuli. Additionally, considering the advantages and disadvantages of both M-mode echocardiography and 2D-STE, this study further established the normal range of MAPSE measured using 2D-STE. This will provide a more comprehensive reference cut-off value of MAPSE for clinical applications.
MAPSE measurement techniques
Current techniques for measuring MAPSE include M-mode echocardiography, tissue Doppler imaging, and 2D-STE.14,15 Among these techniques, tissue Doppler imaging has been rarely used because of its angle dependence and relatively poor stability, which does not reflect the actual mitral annulus displacement. Although M-mode echocardiography is also subject to the angle between the ultrasound beam and the direction of motion of the mitral annulus, it is more readily implemented in daily clinical practice. In contrast, 2D-STE is angle independent and can accurately measure MAPSE.7,16 Moreover, 2D-STE can obtain MAPSE at the midpoint of the mitral annulus, which represents the mean level of MAPSE and better reflects LV global longitudinal function. Our findings also confirmed that in contrast to Msep and Mlat, MAPSE measured using 2D-STE showed a greater coefficient of correlation with GLS, indicating that 2D-STE-based MAPSE may more sensitively reflect LV longitudinal function. Therefore, MAPSE measured using M-mode echocardiography or 2D-STE has both advantages and disadvantages (Table 5), and physicians can choose different measurement techniques to evaluate LV systolic function according to the situation by selecting the corresponding normal reference values and considering the principal determinants.
Table 5.
Advantages and disadvantages of MAPSE measured by M-mode and speckle tracking echocardiography
| Technique | M-mode echocardiography | Speckle tracking echocardiography |
|---|---|---|
| Advantages | ||
| Less dependent on imaging quality | Less dependent on imaging quality | |
| Good reproducibility | Good reproducibility | |
| Easy to apply | Good correlation with GLS | |
| Less vendor or software dependent | Less angle dependent | |
| Able to measure MAPSE of the midpoint of the mitral annulus | ||
| Disadvantages | ||
| Angle dependent | Vendor and software dependent | |
| Unable to measure MAPSE of the midpoint of the mitral annulus |
GLS, global longitudinal strain.
Correlation between MAPSE and GLS
We found a significant positive correlation between MAPSE and GLS (r = 0.08–0.38), and nSTEmid had the highest correlation with GLS. This indicates that as MAPSE values increase, there is a tendency for a corresponding increase in GLS values. Our review of the relevant literature demonstrated that similar results have been reported. Chiu et al.16 reported a correlation coefficient of 0.61 in haemodialysis patients, and Wenzelburger et al.17 reported a correlation coefficient of 0.43 in patients with heart failure with preserved ejection fraction. Notably, the reported correlation coefficients varied across different studies, which may be primarily attributed to factors such as differences in study populations. In particular, the range of MAPSE and GLS is narrow in the healthy population, while the range tends to be relatively wider in pathological conditions, which may lead to higher correlation coefficients. Furthermore, the software used for measuring GLS and the analysis of different MAPSE parameters may contribute to the observed differences in correlation coefficients.
Age- and sex-related differences in MAPSE
Our results revealed a marginal yet significant effect of sex on MAPSE, with higher MAPSE values in women than in men. Conversely, Støylen et al.13 found that MAPSE measured by M-mode echocardiography was independent of sex. This discrepancy may be related to differences in race and ethnicity. Additionally, our research was based on MAPSE using M-mode echocardiography and 2D-STE, whereas their study used mean MAPSE in the four and six points of mitral annuli using M-mode echocardiography; this difference may have contributed to the inconsistency between results. Our results are also comparable with the findings of the WASE study, which demonstrated that men had relatively lower LVEF and GLS (absolute values) than women.18 The potential mechanism may be related to hormones, which generate sex differences in cardiac contractility; however, the detailed mechanism remains to be elucidated.19 Our results also showed that older individuals tend to have lower MAPSE values independent of sex, consistent with prior reports that illustrate stepwise reductions of GLS with advancing age.20 The reduction in LV function may be the result of age-related myocardial apoptosis compounded by the increased fibrosis associated with ageing.21
Primary determinants of MAPSE for 2D-STE
We found that MAPSE measured using 2D-STE was inversely correlated with BP and heart rate in addition to age and sex, suggesting that BP and heart rate should be considered when detecting LV systolic dysfunction using MAPSE. This may also reflect the sensitivity of MAPSE measured using 2D-STE for evaluating slight changes in myocardial function from another perspective. Moreover, our results showed that STEsep, STElat, and STEmid were associated significantly and independently with LV volume. In other words, healthy participants with relatively larger LV volumes may have larger STEsep, STElat, and STEmid values than those with normal LV volumes, which may interfere with the accurate assessment of LV function. Moreover, nSTEmid was independent of LV size. Therefore, nSTEmid may be a more reliable index than the other MAPSE parameters in clinical practice, given that it has a relatively better correlation with GLS and fewer determinants, and may make the comparisons between individuals more objective.
Limitations
The major limitation of this study was the unequal distribution of participants despite attempts to recruit a proportional population, with balanced sex and age groups, as per the initial study design. Unfortunately, men and older individuals had more risk factors and were, therefore, more likely to be excluded, causing the unequal distribution. This was consistent with previous studies and the real clinical setting.19
Mitral valve annulus calcification may affect the measurement value of MAPSE; therefore, we excluded these patients to ensure the accuracy of the normal reference values. Thus, our findings may not apply to patients with mitral valve calcification. Moreover, professional sport activities have been shown to be associated with morphological and metabolic changes and physiologic remodelling in the heart.22 Thus, we excluded individuals involved in professional sports to avoid any associated influences on MAPSE, which may have limited the generalizability of the results.
Moreover, while sonomicrometry is regarded as the gold standard for MAPSE measurement, it is invasive and, therefore, not applicable to this study. As a result, we could not compare our results with measurements based on the gold standard method. Additionally, because the study was based on measurements obtained using a software solution from a single vendor, the direct implementation of the reference range for MAPSE assessed using different software should be performed with caution.
Additionally, this study only included Chinese participants. Previous studies have shown subtle differences in subclinical myocardial functions across racial and ethnic groups with greater LV systolic myocardial strain among Chinese Americans.23 Therefore, further research is needed to validate whether the reference range can be extrapolated to other racial and ethnic groups.
Notably, the narrow measurement range of MAPSE may increase the likelihood of errors when applying these reference values to a wide range of cardiac diseases in routine clinical practice. Therefore, physicians should consider clinical information when using MAPSE in their daily practice, aiming to minimize the potential errors to the greatest extent possible.
Conclusion
Normal reference values of MAPSE measured using M-mode echocardiography and 2D-STE were established according to age and sex. Blood pressure, heart rate, and LV volume are potential factors that influence MAPSE and should be considered in clinical practice. The normal values are useful for evaluating LV global systolic function, especially in patients whose LV GLS or EF cannot be measured accurately because of poor image quality, and may further facilitate the use of MAPSE in routine clinical assessments.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Supplementary Material
Appendix
The following hospitals (investigators) participated in this study: The First Hospital of Dalian Medical University (Tao Cong); Suining Central Hospital (Xiao-Yong Luo); Fengning Manchu Autonomous County Hospital (Dong-Sheng Zhao); Handan First Hospital (Shao-Bo Wang); The People’s Hospital of Qinghai Province (Hai-Xia Sun); The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China (Dong-Mei Yang); Affiliated Hospital of Liaoning University of Traditional Chinese Medicine (Lin-Wei Hong); Urumqi Friendship Hospital (Bao-Ling Wang); Chengdu First People’s Hospital (Xin-Yun Chen); The Affiliated Hospital of Inner Mongolia Medical University (Xiao-Shan Zhang); The First Affiliated Hospital of Jinzhou Medical University (Yu-Hong Li); The People’s Hospital of Leshan (Ying Liu); The Second People’s Hospital of Chengdu (Yun Shi); The People’s Hospital of Chongzuo (Mei Li); The Second Hospital, Cheeloo College of Medicine, Shandong University (Yong-Mei Wang); The Affiliated Hospital of Southwest Medical University (Ji-Zhu Xia); The Affiliated Hospital of Youjiang Medical University for Nationalities (Cheng-Cai Chen); The Fourth Hospital of Hebei Medical University (Xiao-Hong Gong); The Second Affiliated Hospital of Shenyang Medical College (Wei Sun); Xingtai Third Hospital (Hong-Bing Peng); Shijiazhuang People’s Hospital (Xiao-Qing Yang); Dandong Central Hospital (Xin Jin); Baicheng Central Hospital (Fan Zhang); Nanchong Central Hospital, The Second Clinical Medical College of North Sichuan Medical College (University) (Yan-Hua Li); The Third People’s Hospital of Chengdu (Feng Xiong); Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University (Xiang-Dang Long); The Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Peng-Tao Sun); Nanyang Second General Hospital (Xu-Meng Ding); Jiujiang University Affiliated Hospital (Xue-Hua Xiao); The First Affiliated Hospital of Hainan Medical College (En-Hai Zheng); Benxi Central Hospital (Xing-Bin Wang); Guang’an People’s Hospital (Fang Yi); Guangxi International Zhuang Medicine Hospital (Min-Hua Chen); The First Affiliated Hospital of Xi’an Jiaotong University (Wen Wen); Yulin First People’s Hospital (Hong-Xia Yin); Hospital of Chengdu University of Traditional Chinese Medicine (Yan Zhang); The Second Affiliated Hospital of Xi’an Jiaotong University (Qi Zhou); and Central Hospital Affiliated to Shenyang Medical College (Tong Zang).
Contributor Information
Yong-Huai Wang, Department of Cardiovascular Ultrasound, The First Hospital of China Medical University, No. 155 Nanjingbei Street, Heping District, Shenyang 110001, China.
Lu Sun, Department of Ultrasound, Shengjing Hospital of China Medical University, No. 36 Sanhao Street, Heping District, Shenyang 110004, China.
Shi-Wen Li, Department of Cardiovascular Ultrasound, The First Hospital of China Medical University, No. 155 Nanjingbei Street, Heping District, Shenyang 110001, China.
Chun-Feng Wang, Department of Cardiovascular Ultrasound, Mineral Hospital of Liaoning Provincial Health Industry Group, Fushun, China.
Xiao-Fang Pan, Department of Ultrasonic Medicine, Central Hospital of Dalian University of Technology, Dalian, China.
Ying Liu, Department of Ultrasound, Zibo Municipal Hospital, Zibo, China.
Jun Wu, Department of Cardiovascular Ultrasound, The Second Affiliated Hospital of Dalian Medical University, Dalian, China.
Xiang-Ping Guan, Ultrasound Medical Center, ShanXi Province People’s Hospital, Xi’an, China.
Su-Li Zhang, Department of Cardiovascular Ultrasound, Chaoyang Central Hospital, Chaoyang, China.
Guo-Liang Dun, Department of Ultrasound Medicine, Baoji Central Hospital, Baoji, China.
Yi-Lin Liu, Special Inspection Section, Liaocheng People’s Hospital, Liaocheng, China.
Li-Yan Wang, Department of Ultrasound, Jilin Central General Hospital, Jilin, China.
Lei Cui, Department of Ultrasound Diagnosis, Xianyang Central Hospital, Xianyang, China.
Yan Liu, Department of Ultrasound, Dali Bai Autonomous Prefecture People’s Hospital, Dali, China.
Yu-Qiong Lai, Depatment of Cardiovascular Ultrasound, The First People’s Hospital of Foshan, Foshan, China.
Ming-Yan Ding, Department of Cardiac Function, The People’s Hospital of Liaoning Province, Shenyang, China.
Gui-Lin Lu, Department of Ultrasound Diagnosis, The First Affiliated Hospital, School of Medicine, Shihezi University, Shihezi, China.
Jing Tan, Department of Ultrasound in Medicine, Chengdu Wenjiang District People’s Hospital, Chengdu, China.
Xin-Jian Yang, Department of Ultrasound, The Second People’s Hospital of Baiyin City, Baiyin, China.
Yi-Hong Li, Department of Ultrasound, Tangshan Fengnan District Hospital, Tangshan, China.
Xin-Tong Zhang, Department of Ultrasound, Shengjing Hospital of China Medical University, No. 36 Sanhao Street, Heping District, Shenyang 110004, China.
Miao Fan, Department of Ultrasound, Shengjing Hospital of China Medical University, No. 36 Sanhao Street, Heping District, Shenyang 110004, China.
Jia-Hui Yu, Department of Ultrasound, Shengjing Hospital of China Medical University, No. 36 Sanhao Street, Heping District, Shenyang 110004, China.
Qiao-Jin Zheng, Department of Ultrasound, Shengjing Hospital of China Medical University, No. 36 Sanhao Street, Heping District, Shenyang 110004, China.
Chun-Yan Ma, Department of Cardiovascular Ultrasound, The First Hospital of China Medical University, No. 155 Nanjingbei Street, Heping District, Shenyang 110001, China.
Wei-Dong Ren, Department of Ultrasound, Shengjing Hospital of China Medical University, No. 36 Sanhao Street, Heping District, Shenyang 110004, China.
Funding
None declared.
Data availability
The data underlying this article will be shared upon reasonable request from the corresponding authors.
References
- 1. Marwick TH. Ejection fraction pros and cons. J Am Coll Cardiol 2018;72:2360–79. [DOI] [PubMed] [Google Scholar]
- 2. Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging 2018;11:260–74. [DOI] [PubMed] [Google Scholar]
- 3. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande Let al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 4. Carlsson M, Ugander M, Mosen H, Buhre T, Arheden H. Atrioventricular plane displacement is the major contributor to left ventricular pumping in healthy adults, athletes, and patients with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 2007;292:H1452–9. [DOI] [PubMed] [Google Scholar]
- 5. Matos J, Kronzon I, Panagopoulos G, Perk G. Mitral annular plane systolic excursion as a surrogate for left ventricular ejection fraction. J Am Soc Echocardiogr 2012;25:969–74. [DOI] [PubMed] [Google Scholar]
- 6. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal Eet al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020;22:391–412. [DOI] [PubMed] [Google Scholar]
- 7. Asada D, Okumura K, Ikeda K, Itoi T. Tissue motion annular displacement of the mitral valve can be a useful index for the evaluation of left ventricular systolic function by echocardiography in normal children. Pediatr Cardiol 2018;39:976–82. [DOI] [PubMed] [Google Scholar]
- 8. Buss SJ, Mereles D, Emami M, Korosoglou G, Riffel JH, Bertel Det al. Rapid assessment of longitudinal systolic left ventricular function using speckle tracking of the mitral annulus. Clin Res Cardiol 2012;101:273–80. [DOI] [PubMed] [Google Scholar]
- 9. Teraguchi I, Hozumi T, Takemoto K, Ota S, Kashiwagi M, Shimamura Ket al. Assessment of decreased left ventricular longitudinal deformation in asymptomatic patients with organic mitral regurgitation and preserved ejection fraction using tissue-tracking mitral annular displacement by speckle-tracking echocardiography. Echocardiography 2019;36:678–86. [DOI] [PubMed] [Google Scholar]
- 10. Clinical and Laboratory Standards Institute . Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. In: CLSI Document C28-A3. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
- 11. Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MCet al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019;32:1–64. [DOI] [PubMed] [Google Scholar]
- 12. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen Tet al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 13. Støylen A, Mølmen HE, Dalen H. Relation between mitral annular plane systolic excursion and global longitudinal strain in normal subjects: the HUNT study. Echocardiography 2018;35:603–10. [DOI] [PubMed] [Google Scholar]
- 14. de Knegt MC, Biering-Sorensen T, Sogaard P, Sivertsen J, Jensen JS, Mogelvang R. Concordance and reproducibility between M-mode, tissue Doppler imaging, and two-dimensional strain imaging in the assessment of mitral annular displacement and velocity in patients with various heart conditions. Eur Heart J Cardiovasc Imaging 2014;15:62–9. [DOI] [PubMed] [Google Scholar]
- 15. Black DE, Bryant J, Peebles C, Godfrey KM, Hanson M, Vettukattil JJ. Tissue motion annular displacement of the mitral valve using two-dimensional speckle tracking echocardiography predicts the left ventricular ejection fraction in normal children. Cardiol Young 2014;24:640–8. [DOI] [PubMed] [Google Scholar]
- 16. Chiu DY, Abidin N, Hughes J, Sinha S, Kalra PA, Green D. Speckle tracking determination of mitral tissue annular displacement: comparison with strain and ejection fraction, and association with outcomes in haemodialysis patients. Int J Cardiovasc Imaging 2016;32:1511–8. [DOI] [PubMed] [Google Scholar]
- 17. Wenzelburger FW, Tan YT, Choudhary FJ, Lee ES, Leyva F, Sanderson JE. Mitral annular plane systolic excursion on exercise: a simple diagnostic tool for heart failure with preserved ejection fraction. Eur J Heart Fail 2011;13:953–60. [DOI] [PubMed] [Google Scholar]
- 18. Asch FM, Miyoshi T, Addetia K, Citro R, Daimon M, Desale Set al. Similarities and differences in left ventricular size and function among races and nationalities: results of the World Alliance Societies of Echocardiography Normal Values Study. J Am Soc Echocardiogr 2019;32:1396–406 e2. [DOI] [PubMed] [Google Scholar]
- 19. Chaudhari S, Cushen SC, Osikoya O, Jaini PA, Posey R, Mathis KWet al. Mechanisms of sex disparities in cardiovascular function and remodeling. Compr Physiol 2018;9:375–411. [DOI] [PubMed] [Google Scholar]
- 20. Addetia K, Miyoshi T, Amuthan V, Citro R, Daimon M, Gutierrez Fajardo Pet al. Normal values of left ventricular size and function on three-dimensional echocardiography: results of the World Alliance Societies of Echocardiography Study. J Am Soc Echocardiogr 2021;35:449–59. [DOI] [PubMed] [Google Scholar]
- 21. Mehdizadeh M, Aguilar M, Thorin E, Ferbeyre G, Nattel S. The role of cellular senescence in cardiac disease: basic biology and clinical relevance. Nat Rev Cardiol 2022;19:250–64. [DOI] [PubMed] [Google Scholar]
- 22. Shames S, Bello NA, Schwartz A, Homma S, Patel N, Garza Jet al. Echocardiographic characterization of female professional basketball players in the US. JAMA Cardiol 2020;5:991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fernandes VR, Cheng S, Cheng YJ, Rosen B, Agarwal S, McClelland RLet al. Racial and ethnic differences in subclinical myocardial function: the Multi-Ethnic Study of Atherosclerosis. Heart 2011;97:405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request from the corresponding authors.