Abstract
The circadian rhythm is a self-sustaining 24 h cycle that regulates physiological processes within the body, including cycles of alertness and sleepiness. Cells have their own intrinsic clock, which consists of several proteins that regulate the circadian rhythm of each individual cell. The core of the molecular clock in human cells consists of four main circadian proteins that work in pairs. The CLOCK-BMAL1 heterodimer and the PER-CRY heterodimer each regulate the other pair’s expression, forming a negative feedback loop. Several other proteins are involved in regulating the expression of the main circadian genes, and can therefore also influence the circadian rhythm of cells. This review focuses on the existing knowledge regarding circadian gene variants in both the main and secondary circadian genes, and their association with various diseases, such as tumors, metabolic diseases, cardiovascular diseases, and sleep disorders.
Keywords: circadian gene variants, tumors, metabolic diseases, cardiovascular diseases, sleep disorders
1. Introduction
The circadian system is a complex multioscillatory temporal network in which an ensemble of coupled neurons comprising the principal circadian pacemaker in the suprachiasmatic nucleus (SCN) of the hypothalamus is entrained to the daily light/dark cycle, and subsequently transmits synchronizing signals to peripheral clocks in all cells of the body. Peripheral clocks are a part of a cell’s physiology that regulates the cell’s circadian cycle according to the signals coming from the main clock in the SCN [1]. The importance of well-tuned mechanisms regulating circadian rhythm for health and survival is implied by their presence in a wide variety of both prokaryotic and eukaryotic species [2]. Complex organisms, including humans, have developed a central clock, consisting of several proteins, that regulates the body’s response under the influence of environmental conditions (so-called “zeitgebers”—time givers) such as light and temperature. In humans and other mammals, this center is located in the SCN of the hypothalamus, from which signals are dispensed to peripheral tissues in order to synchronize the circadian rhythm of the peripheral clocks in each individual cell with that of the main clock. While cells separated from the main clock still possess autonomic regulation of circadian cycle in vitro [3], signals from a wild-type SCN have been shown to override the lack of a functioning intrinsic clock caused by mutations in circadian genes in cells [4,5], indicating a complex network of interactions between the main clock and peripheral clocks through various pathways [1].
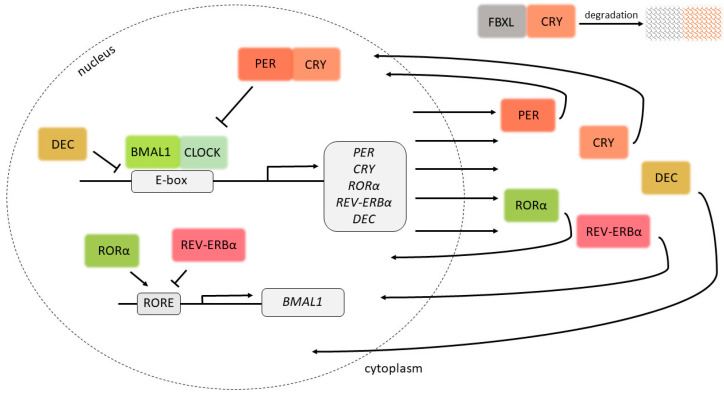
The core of the molecular clock in cells consists of four main proteins, each of which regulates the expression of the others, with these expressions fluctuating over the course of a 24 h period, as well as the expression of other downstream genes [6]. The first of the four to be described was the period gene (per) in Drosophila melanogaster in 1971 [7]. Its role in the regulation of the molecular clock was confirmed by restoring circadian rhythm in mutant flies by introducing a wild-type per allele [8,9]. Three ortholog genes, Per1, Per2 and Per3, have been described in mammals, and their mRNA and protein levels have also been shown to vary over the sleeping–wakefulness period [10,11,12,13]. The expressions of PER1, PER2 and PER3 are regulated by a heterodimeric protein consisting of the transcription factors CLOCK (circadian locomotor out-put cycles kaput) and BMAL1 (Brain and Muscle ARNT-Like 1, now denoted ARNT-Like or Arntl), whose expressions are in turn regulated by PER and CRY in a negative feedback loop (Figure 1).
Figure 1.
Circadian clock proteins and their negative feedback loop. By binding to E-boxes in DNA, the BMAL1-CLOCK heterodimer induces the expression of target genes, including PER and CRY, the protein products of which inhibit the expression of BMAL1 and CLOCK. The lack of BMAL1 and CLOCK inhibits the expression of CRY and PER, causing the cycle to start anew. DEC1 and DEC2 compete with the BMAL1-CLOCK heterodimer for the binding of E-boxes, and are therefore involved in the regulation of expression of the target genes. By binding ROR response elements (ROREs), RORα induces the expression of BMAL1, while REV-ERBα inhibits the expression of BMAL1. The activity of CRY1 is also regulated by its interaction with FBXL proteins, which regulate its degradation.
Clock was first described in mice as a gene encoding a regulator of the intrinsic circadian period and the persistence of circadian rhythmicity in conditions of constant darkness [14]. Its expression varies between different tissues and its sequence is largely conserved among species [15]. CLOCK contains a PAS dimerization domain (named for the per, ARNT and sim genes it was initially associated with), through which it binds to BMAL1 in order to form a heterodimer that binds to DNA through the basic helix–loop–helix DNA-binding domain [16]. The localization of CLOCK in the nucleus is dependent on the expression of BMAL1 and its binding to CLOCK [17]. The final component of circadian clocks corresponds to the cryptochrome genes, Cry1 and Cry2, which code for two blue-light photoreceptors. These proteins bind to PER proteins in order to form heterodimers that bind to DNA and regulate gene expression, including the expression of CLOCK and BMAL1 [18,19].
Aside from this feedback loop, there are several additional pathways that regulate the expression of circadian genes. ROR (Retinoid-related orphan receptors) transcription factors increase the expression of BMAL1, CRY1 and REV-ERBα through interactions with ROR response elements (ROREs) [20,21]. REV-ERBα, whose expression is inhibited by PER and CRYs, regulates the expression of BMAL1 [22], while NAPS2 can partially compensate for the lack of functional CLOCK protein [23]. FBXL3 and FBXL21 regulate the degradation of CRY1 [24,25]. Dec1 and Dec2 inhibit the expression of Per1 by competing with Clock for the binding of Bmal1 or by competing with Clock-Bmal1 heterodimers for the binding of E-boxes, DNA sequences that both Clock-Bmal1 heterodimers and Dec1 and Dec2 are able to bind to [26]. TIMELESS (TIM) regulates the expression–suppression activation of PER1 by regulating its entry to the nucleus [27]. Circadian proteins are also regulated on a post-translational level by reversible phosphorylation, involving several kinases/phosphatases, which affect the stability, activity and localization of these proteins [28].
Pathological changes associated with circadian genes have been described in various types of disease, including tumors [29,30,31], metabolic diseases [32], cardiac diseases [33,34], sleep disorders [35], psychiatric diseases [36] and neurodegenerative diseases [37]. This review focuses on the existing knowledge regarding circadian gene SNPs (single-nucleotide polymorphisms) and their association with these diseases. The investigated SNPs in these studies were selected on the basis of their position within the gene and their predicted function, identified according to several different databases containing data from genome-wide association studies (GWAS), such as the SNPinfo Web Server [38] and the NCBI dbSNP database [39], or by using the Tagger algorithm, implemented in the Haploview interface of HapMap’s genome browser [40,41]. The circadian gene variations in patient cohorts in the studies covered in this review were commonly determined from blood or saliva samples. Alleles whose frequency did not fit into the Hardy–Weinberg equilibrium were excluded from these studies, as it was impossible to estimate their influence on a particular disease.
2. Circadian Gene Variants in Tumors
2.1. Associations between Circadian Gene Variants and Increased Risk for Tumor Development
Associations between variants of different circadian genes and increased risk for tumor development have been observed in various tumor types (Table 1). The significance of the associations varied depending on whether they were evaluated using a dominant genetic model (DM), a recessive genetic model (RM) or an additive model (AM). DM involve comparison between homozygotes for major alleles (MM) and the combined carriers of minor alleles (Mm + mm), RMs involve comparison between combined carriers of major alleles (MM + Mm) and homozygotes for minor alleles (mm), while AMs involve comparisons among all three genotypes (MM vs. Mm vs. mm) [42].
Table 1.
Tumor types and circadian gene variants associated with increased risk for tumor development.
| Tumor Type | Gene | SNP ID | Genotype | Population | Reference |
|---|---|---|---|---|---|
| Breast cancer | PER1 | rs2735611 | AA RM | Caucasian (Polish) | [43] |
| PER2 | rs934945 | CT + TT DM | Caucasian (Polish) | [43] | |
| PER3 | rs57875989 | 4/5 + 5/5 VNTR DM | Caucasian | [44] | |
| CLOCK | rs3805151 | CT + TT DM | Chinese | [45] | |
| rs11133373 | CG + GG DM | Korean | [46] | ||
| CRY2 | rs10838524 | AG + GG DM | Caucasian (Polish) | [43] | |
| RORA | rs1482057 | French from two administrative areas | [47] | ||
| rs12914272 | French from two administrative areas | [47] | |||
| NPAS2 | rs2305160 | AG | predominantly Caucasian | [48] | |
| Prostate cancer | BMAL1 | rs7950226 | GA + AA DM | Caucasian | [49] |
| CRY2 | rs1401417 | GC + CC DM | Chinese | [50] | |
| NPAS2 | rs1369481 | GA + AA DM | Caucasian | [49] | |
| rs895521 | GA + AA DM | Caucasian | [49] | ||
| rs17024926 | TC + CC DM | Caucasian | [49] | ||
| CSNK1E | rs1534891 | TT | Caucasian | [49] | |
| Lung cancer | PER3 | rs228729 | GT + GG DM | Brazilian | [51] |
| BMAL1 | rs3816360 | CC | northeast Chinese | [52] | |
| rs2290035 | AA | northeast Chinese | [52] | ||
| Gastric cancer | NPAS2 | rs895520 | AA RM | Caucasian | [53] |
| Non-Hodgkin lymphoma/B-cell lymphoma | CRY2 | rs11038689 | GG RM | predominantly Caucasian | [54] |
| rs7123390 | AA RM | predominantly Caucasian | [54] | ||
| rs1401417 | CC RM | predominantly Caucasian | [54] |
SNP—single-nucleotide polymorphism; RM—recessive genetic model; DM—dominant genetic model.
2.2. Associations between Circadian Gene Variants and Increased Risk for Development of Different Tumor Subtypes
The risk for breast cancer (BC) development associated with circadian gene variants was also found to depend on estrogen/progesterone status, as well as on pre-/postmenopausal status. The AA genotype in the PER1 rs2735611 SNP was associated with higher BC risk based on an RM in Polish women of Caucasian origins when observing the whole cohort as well as when estrogen/progesterone-positive and negative BC were observed separately. In the same cohort, the T allele in the PER2 rs934945 SNP was generally associated with higher risk of development of breast tumors, as well as estrogen-/progesterone-positive breast tumors based on a DM. Additionally, the risk of development of estrogen-negative tumors was associated with the GG genotype of the CRY2 rs10838524 SNP based on an RM [43]. In a predominantly Caucasian cohort, the CC genotype in the CRY2 rs1401417 variant was associated with increased risk of development of BC in postmenopausal patients compared to the GG genotype [55]. In the same study, the CRY2 rs1401417 C allele, as well as three other SNPs in the same gene (rs11038689 G allele (DM), rs11605924 CC genotype (RM) and rs7123390 A allele (DM)), was found to be significantly associated with risk of estrogen-/progesterone-negative tumors [55]. The CLOCK rs3805151 T allele was associated with an increased risk of BC in postmenopausal women based on a DM in Chinese populations [45]. Zhu et al. showed that a PER3 variant containing five variable number tandem repeats (VNTR; rs57875989) was associated with higher risk of developing BC compared to a PER3 variant with four VNTRs in young Caucasian women [44] (Table 1), but this association was not observed in other studies, which included Chinese [45], Indian [56] and multiple European cohorts [57]. In a predominantly Caucasian cohort, the heterozygous genotype in the NPAS2 rs2305160 SNP was associated with greater BC risk in both pre- and postmenopausal women, as well as in the whole cohort [48]. The same locus was also associated with higher risk of BC in women of various ethnicities exposed to rotating shift work for longer periods of time [58].
Aside from the higher risk of developing BC, as described above, the C allele in the CRY2 rs1401417 SNP was also associated with higher risk of developing prostate cancer (PC) compared to the GG genotype based on a DM in a Chinese population. The risk of developing PC was even greater in patients with the C allele, who also displayed greater insulin resistance (IR) compared to patients with the GG genotype and lower IR [50]. A higher risk for the progression of both localized and advanced PC, as well as poorer survival, was also found to be associated with the T allele in the rs6542993 SNP of the NPAS2 gene compared to the AA genotype in a predominantly Taiwanese population [59]. Zhu et al. found four variants associated with risk of developing PC (Table 1), but also an additional four SNPs that were associated with the risk of a more aggressive tumor (CLOCK 11133373, NPAS2 rs895521, PER1 rs885747 and PER1 rs2289591 variants) and eight SNPs that were significantly associated with risk of less aggressive PC (PER3 rs1012477, CRY2 rs2292912, BMAL1 rs7950226, NPAS2 rs17024926 and rs1369481, CSNK1E rs1534891, CRY1 rs12315175 and PER2 rs7602358) in Caucasian men [49]. In contrast, none of the 872 analyzed SNPs in the study by Wendeu-Foyet et al. were significantly associated with risk of developing PC in a French Caucasian cohort [60]. Similarly, Markt et al. did not observe a consistent association between any of the 96 SNPs analyzed and fatal PC across three studied cohorts of European ancestry [61].
Three CRY2 SNPs, rs11038689, rs7123390 and rs1401417, were found to be associated with risk of developing non-Hodgkin lymphoma (NHL) in a predominantly Caucasian cohort consisting only of female patients. Aside from the whole NHL cohort, the same SNPs were also associated with risk for tumor development in B-cell lymphomas and follicular lymphoma (FL) subgroups (Table 1). Two of those SNPs, rs7123390 (genotype AA) and rs1401417 (genotype CC), were also significantly associated with B-cell chronic lymphocytic leukemia/prolymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). The CC genotype of the CRY2 rs1401417 SNP was also associated with the risk of developing diffuse large B-cell lymphoma (DLBCL) and T-cell lymphoma, but no CRY2 SNPs were significantly associated with risk of developing marginal zone B-cell lymphoma (MZBL) [54].
2.3. Associations between Circadian Gene Variants and Decreased Risk for Tumor Development
The CRY1 rs1056560 GT genotype was associated with a decreased risk for BC in Chinese populations [45]. In the same cohort, premenopausal carriers of the CC genotype in the CRY2 rs1401417 SNP had a significantly decreased risk of developing BC compared to carriers of the GG genotype, but this association was not observed in postmenopausal women. Decreased risk for BC was also observed for the CC genotype in the CRY2 rs1401417 SNP in estrogen-positive cases compared to in estrogen-negative cases [45]. Patients carrying the T allele in the BMAL1 rs2279287 SNP had reduced risk of developing BC [43], and the A allele in the BMAL1 rs3816358 SNP was also associated with reduced risk for BC [62]. Fu et al. observed that the C allele in the TIMELESS rs7302060 SNP was associated with reduced BC risk, as well as that the GG genotype in the TIMELESS rs2291738 SNP and the CC genotype in the TIMELESS rs7302060 SNP were associated with reduced risk of BC in patients with estrogen-/progesterone-positive BC [63]. Chu et al. observed that the A allele in the NPAS2 rs2305160 SNP was associated with decreased PC risk compared to the GG genotype [50]. Reduced risk for gastric cancer (GC) was observed in carriers of the T allele in the PER2 rs934945 SNP and carriers of the C allele in the RORA rs339972 SNP in a DM [53].
Circadian gene variants have also been associated with patients’ response to anti-tumor therapy. The results reported by Johnson et al. suggest that the toxicity of breast cancer radiotherapy can be reduced by scheduling patients for therapy based on their PER3 VNTR number and NOCT rs131116075 genotypes [64]. This finding is supported by the research by Webb et al., which showed that the time of treatment can influence the toxicity of radiotherapy in patients with breast carcinoma based on their CLOCK rs1801260, PER3 (VNTR; rs2087947) and RASD1 rs11545787 genotypes [65].
3. Circadian Gene Variants in Cardiovascular and Metabolic Diseases
Circadian gene variants have also been associated with various physiological processes and their disruptions, including obesity, different hormone levels, high sterol levels, blood pressure abnormalities, cardiovascular disease, impaired fasting glucose, and diabetes (Table 2).
Table 2.
Circadian gene variants associated with various phenotypes originating from disrupted physiological processes.
| Gene | Variant | Genotype | Phenotype | Population | Reference |
|---|---|---|---|---|---|
| PER1 | rs2585405 | GC + CC | Lower 3α-diol, higher SHBG | Chinese | [66] |
| PER2 | rs6431590 | AG + AA | Lack of overnight blood pressure decrease | Chinese | [67] |
| PER3 | rs57875989 | 4/5 + 5/5 VNTR | Increased serum IGF-I levels andIGF-I:IGFBP3 ratio | Chinese | [66] |
| CLOCK | rs4580704 | CG + GG DM | Lower blood pressure, higher serum concentrations of MCP1 and adiponectin, lower type 2 diabetes risk | Caucasian or predominantly Caucasian | [68,69,70] |
| rs1801260 (3111T/C) | GG RM | Higher fasting insulin, higher HOMA-IR | Caucasian | [68] | |
| CT + CC | Higher oddsratio for the prevalence of diabetes | Japanese | [71] | ||
| Higher risk for cardiovascular disease | multiple | [72] | |||
| rs1554483 | GC + GG | Overweight, obesity | Caucasian | [73] | |
| rs4864548 | AG + AA | Overweight, obesity | Caucasian | [73] | |
| rs13113518 | CT AM | Higher campesterol levels | Caucasian | [74] | |
| rs35115774 | C- AM | Lower campesterol and sitosterol levels | Caucasian | [74] | |
| rs6832769 | AG AM | Lower campesterol and sitosterol levels | Caucasian | [74] | |
| rs3749474 | TC + TT DM | Higher energy intake, decreased serum levels of IL-6 and MCP1 | predominantly Caucasian | [69] | |
| rs6811520 | TT RM | Higher incidence of myocardial infarction | Caucasian (Croatian) | [75] | |
| rs13124436 | GG RM | Higher incidence of myocardial infarction | Caucasian (Croatian) | [75] | |
| BMAL1 | rs6486121 | TC + CC DM | Higher campesterol levels | Caucasian | [74] |
| rs3789327 | AG + GG DM | Lower incidence of myocardial infarction | Caucasian (Croatian) | [75] | |
| rs12363415 | AG + GG DM | Lower incidence of myocardial infarction | Caucasian (Croatian) | [75] | |
| rs3816358 | TT + TG | Lack of overnight blood pressure decrease | Chinese | [67] | |
| rs7950226 | GG RM | Lower risk for MetS comorbidities | multiple | [76] | |
| CRY1 | rs2078074 | CC RM | Higher sitosterol levels | Caucasian | [74] |
| rs2287161 | CC | Higher carbohydrate intake | Spanish and Northamerican (predominatly Caucasian) | [77] | |
| RORββ | rs1410225 | TT | Presence of overnight blood pressure decrease | Chinese | [67] |
| RORα | rs10519096 | AG + AA | Lack of overnight blood pressure decrease | Chinese | [67] |
| REV-ERBα | rs2314339 | AG + AA | Lower probability of abdominal obesity, more physical activity | Spanish and Northamerican (predominatly Caucasian) | [78] |
| NPAS2 | rs3888170 | CT + CC | Lack of overnight blood pressure decrease | Chinese | [67] |
| rs2305160 | GA + AA | Decreased levels of free and bioavailable testosterone | Chinese | [66] |
3α-diol—5a-androstane-3α, 17β-diol glucuronide; SHBG—sex hormone-binding globulin; IGF-I—insulin-like growth factor I; IGFBP3—insulin-like growth factor binding protein 3; DM—dominant model; RM—recessive model; HOMA-IR—homeostasis model assessment of insulin resistance; AM—additive model; RQ—respiratory quotient; RMR—resting metabolic rate; MetS—metabolic syndrome.
Circadian gene variants are not only associated with driving factors of obesity in overall population, but also with various risk factors in obese patients.
The research by Corella et al. showed that the G allele in the CLOCK rs4580704 SNP was associated with decreased stroke risk in type 2 diabetes patients based on a DM in the Spanish population [70]. Monteleone et al. observed that overweight/obese patients with the CC genotype in the CLOCK rs1801260 SNP had significantly higher values of body mass index (BMI) [79] compared to carriers of the T allele in a Caucasian cohort, while Garaulet et al. observed that carriers of the G allele in the same SNP among obese patients were the least responsive to a weight-loss intervention in a population from southeastern Spain [80]. Among patients with essential hypertension in a Chinese population, carriers of the C allele in the CLOCK rs1801260 SNP were more susceptible to insulin resistance and were at greater risk of developing high night-time systolic blood pressure [81]. Research by Torrego-Ellacuría et al. showed that carriers of the A allele in the CLOCK rs1801260 SNP in a Caucasian cohort showed a greater degree of obesity and significantly lower weight loss and higher weight regain over time after bariatric surgery, regardless of the pre-surgery patient profile [82]. The same research also showed that the TT genotype in the CLOCK rs3749474 SNP was associated with morbid obesity in patients who underwent bariatric surgery [82]. However, it was also observed that circadian gene variants could also affect risk factors strictly in non-overweight subjects, as it was shown in a Japanese cohort that C allele in the CLOCK rs1801260 SNP was associated with the prevalence of type 2 diabetes in non-overweight subjects, but not in overweight subjects, after adjusting for potential confounding factors, including age, sex, research area, BMI, smoking habit, alcohol drinking status, leisure time exercise, energy intake and family history of diabetes [71]. Other studies have also shown associations between various CLOCK variants and obesity indicators, but they did not remain significant after correcting for multiple testing [83,84].
The GG genotype in BMAL1 rs7950226 SNP was associated with insulin resistance in patients with essential hypertension in a Chinese population [81]. In the research by Woon et al., no significant association between single BMAL1 SNPs and type 2 diabetes and hypertension were observed in British individuals of European ancestry [85].
Carriers of the CC genotype in the CRY1 rs2287161 SNP whose carbohydrate intake percentage in total energy intake was increased displayed significant increase in homeostasis model assessment of insulin resistance (HOMA-IR), fasting insulin and a decrease in quantitative insulin sensitivity check index (QUICKI) in a Mediterranean and an European origin North American population [77]. In obese patients who had undergone two years of diet intervention, the A allele of the CRY2 rs11605924 SNP was significantly associated with a greater reduction in respiratory quotient (RQ) and a greater increase in resting metabolic rate (RMR) and RMR/kg in a predominantly Caucasian cohort [86]. Kovanen et al. did not find any significant associations between specific CRY1 and CRY2 SNPs and the metabolic syndrome components after correction for multiple testing [87].
The C allele in the PER1 rs2585405 SNP was associated with extreme obesity in a Caucasian cohort in the research by Mariman et al., which is a unique finding, given that the C allele produces a functional protein, while the alternative G allele leads to a missense mutation [88]. In a Spanish population, the G allele in the PER2 rs2304672 SNP and the TT genotype in the PER2 rs4663302 SNP were associated with a greater probability of withdrawal from dietary treatment for abdominal obesity [89].
In a Mediterranean subgroup, carriers of A allele in REV-ERBα rs2314339 SNP whose total fat intake consisted of ≥55% of monounsaturated fatty acids had significantly lower BMI. These results were not observed in a North American subgroup [78].
Additionally, patients of Croatian origin with myocardial infarction were more susceptible to hypertension, type 2 diabetes and disrupted systolic blood pressure, depending on the variant in rs13124436 and rs6811520 SNPs in CLOCK and to type 2 diabetes depending on the variant in rs3789327 SNP in BMAL1 [75].
4. Circadian Gene Variants in Sleep Disorders and Psychiatric Diseases
It has been shown that circadian gene variants can determine the preference for morning or evening activity [90,91,92,93], duration of sleep [83], or sleep quality [94]. Mutations in circadian genes have also been directly linked to circadian rhythm sleep disorders (CRSDs), which can be divided into four main types, including advanced sleep-phase disorder (ASPD), delayed sleep-phase disorder (DSPD), irregular sleep–wake rhythm/free-running sleep (FRT) disorder and non-24 h sleep–wake disorder (N-24) [35] (Table 3).
Table 3.
Circadian gene variants associated with various phenotypes originating from disrupted sleeping patterns.
| Disorder | Gene | Mutation | Modification | Phenotype | Population | Reference |
|---|---|---|---|---|---|---|
| (F)DSPD | PER2 | G−>A | Val1205Met | sleep–wake phase delay, idiopathic hypersomnia | Japanese | [95] |
| PER3 | 4 VNTR | / | association with evening preference | [92] | ||
| 5 VNTR | / | association with morning preference | [92] | |||
| delayed sleep phase, association with diurnal preference | predominantly Caucasian | [93] | ||||
| CLOCK | 3111T/C | / | evening preference, significantly delayed sleep onset, shorter sleep time and greater daytime sleepiness in CC hmozygotes | Japanese | [96] | |
| CRY1 | A−>C | ∆ exon 11 | enhanced interaction with CLOCK and BMAL1, long-period behavioral and body temperature rhythms with diminished amplitudes | [97] | ||
| CKIɛ | G−>A | Ser408Asp | protective effect of A allele against DSPD | Japanese | [98] | |
| (F)ASPD | PER2 | A−>G | Ser662Gly | advance of sleep, temperature, and melatonin rhythms | A single family with ASPD | [99] |
| PER3 | C−>G | Pro415Ala | habitual early spontaneous awakening | A single family with ASPD | [100] | |
| A−>G | His417Arg | habitual early spontaneous awakening | A single family with ASPD | [100] | ||
| CRY2 | G−>A | Ala260Thr | alternation of CRY2 conformation, which results in increase in accessibility and affinity for an E3 ubiquitin ligase FBXL3 and consequently CRY2 degradation | A single family with ASPD | [101] | |
| TIMELESS | C−>T | Arg1081X | destabilization of CRY1/2 and PER1/2 heterodimer, a shortened circadian period or altered entrainment | A single family with ASPD | [102] | |
| CKIδ | A−>G | Thr44Ala | a shorter circadian period | A single family with ASPD | [103] | |
| FRT | PER3 | C−>G | Pro864Ala | G allele is more common in evening types and in FRT individuals | Japanese | [104] |
| N-24 | CKIɛ | G−>A | Ser408Asp | protective effect of A allele against N-24 | Japanese | [98] |
(F)DSDP—(familial) delayed sleep-phase disorder; (F)ASPD—(familial) advanced sleep-phase disorder; FRT—free-running type; N-24—non-24 h sleep–wake syndrome.
Contrary to the study by Mishima et al. [96], other studies have found no associations between CLOCK 3111T/C variants and diurnal preference [105,106]. However, CLOCK 3111T/C SNP has been associated with the recurrence of unfavorable sleep phenotypes in patients diagnosed with different psychiatric disorders. The CC genotype in the CLOCK 3111T/C SNP was associated with higher recurrence of initial, middle and early insomnia, with reduced need for sleep in patients diagnosed with bipolar disorder (BP) [107], as well as with a higher recurrence rate of illness (number of illness episodes/duration of illness in years) in BP patients [108]. It was also observed among depressed bipolar patients that carriers of the C allele were more active in the evening and slept less compared TT homozygotes, even though the severity of the depression was similar among all patients [109]. Patients diagnosed with major depressive disorder (MDD) who were homozygotes for the C allele in the CLOCK 3111T/C SNP had higher recurrence of initial insomnia compared to carriers of the T allele [107].
Circadian gene variants have also been associated with various psychiatric diseases independently of their effect on the sleeping patterns of patients [36]. Seasonal patterns of (hypo)manic and depressive phases, which are found in about 25% of BP patients, were associated with five SNPs in NPAS2 in a French Caucasian cohort [110]. PER3 rs228697 was associated with MDD, independently of the patients’ gender, in a Caucasian cohort. When that cohort was stratified by gender, it was found that PER3 rs228697 SNP was also associated with MDD in female patients, while CLOCK rs1801260 SNP was associated with MDD in male patients [111]. CLOCK variants have been found to be associated with a wide array of psychiatric disorders [112], but the clinical significance of these associations still remains undefined.
Among patients with MDD who had been treated with selective serotonin reuptake inhibitor (SSRI), it was found that the PER3 rs228697 CC genotype was associated with a higher sleep factor score compared with the CG genotype. Heterozygotes (TC) in the PER3 rs228729 SNP had higher risk of suffering from excitement/agitation, akathisia and weight loss compared to the CC homozygotes. Additionally, patients with the AA genotype and the GA genotype in the PER3 rs10746473 SNP were more likely to suffer from dizziness and tachycardia, respectively, when compared to patients with the GG genotype [113]. Insomnia was significantly more present in carriers of the C allele in the CLOCK 3111T/C SNP during antidepressant treatment with fluvoxamine or paroxetine [114]. These findings suggest the possibility that circadian gene genotyping could be useful for the prediction of adverse effects from psychopharmaceuticals. The study by Gyorik et al. suggested that CLOCK variants could play a role in mediating stress-induced circadian deregulation, leading to depression in a Caucasian cohort [115], thus offering a new direction for further research of a very relevant topic in modern times.
5. Circadian Gene Variants in Neurodegenerative Diseases
In the research by Bacalini et al., an association was found between PER1 rs3027178 SNP and Alzheimer’s disease (AD), with the G allele having a protective effect for AD [116]. The C allele carriers in the CLOCK 3111T/C SNP in an Italian cohort with a history of blood hypertension had a higher risk of conversion to AD than C allele carriers without hypertension [117].
The CC genotype in BMAL1 rs3789327 SNP and the CC genotype in the CLOCK rs6811520 SNP were found to be associated with higher risk for multiple sclerosis (MS) in a Caucasian cohort of Slavic origin [118], but these results were not replicated in a Spanish cohort [119].
In a Chinese population, BMAL1 rs900147 and PER1 rs2253820 SNPs were associated with Parkinson’s disease (PD). Additionally, BMAL1 rs900147 SNP was significantly associated with the tremor-dominant (TD) subtype of PD, while PER1 rs2253820 SNP was significantly associated with the postural instability and gait difficulty (PIGD) subtype of PD [120]).
6. Circadian Gene Variants in Other Diseases
The role of circadian gene variants has also been explored in some diseases that do not fall under any of the categories described in this review so far.
Mutation in NPAS2 gene causing Leu/Ser substitution at the 471 position has been suggested to be a risk factor for seasonal affective disorder (SAD), but this result was not confirmed in another group of patients [91].
The C allele in the PER1 rs2585405 SNP has been shown to be a risk factor for noise-induced hearing loss (NIHL) among Chinese noise-exposed workers [121].
7. Circadian Genes and Immune Response
The immune system also displays a circadian pattern of activation and inhibition, which manifests in different numbers of circulating immune cells and magnitudes of immune response throughout the day [122]. Circadian proteins influence both innate [123] and adaptive immunity [124], as well as the immune response to tumors [125]. The deregulation of circadian genes has also been shown to play a role in autoimmune diseases [126,127]. However, to the best of our knowledge, there have been no studies observing the associations between specific circadian gene variants and the regulation of immune response.
8. Future Research
A large amount of research on circadian genes is focused on their differential expression between healthy subjects and patients diagnosed with different diseases at both the mRNA and protein levels. However, the possible genetic contribution of circadian genes to various phenotypes cannot be overlooked, especially in sleep-related disorders. The influence of circadian gene variants on therapy efficiency and on the development of adverse side effects is a promising direction for further research.
9. Conclusions
Different circadian gene variants have been associated with various types of diseases, with the CLOCK rs1801260 (3111T/C) SNP being the most researched locus over a wide array of diseases. While it is expected that SNPs in circadian genes affect sleeping patterns, the significance of associations between circadian gene variants and other types of diseases is still unclear and requires further research. The significance of the associations varies depending on patients’ ethnicity, type of disease, and sleeping patterns, making it difficult to come to a steadfast conclusion about the roles of these SNPs. Some of the variants show the potential to be of clinical significance, for example when determining the timing of therapy in order to increase the therapy’s efficiency and predicting the adverse effects of therapy.
Author Contributions
Conceptualization, P.G. and P.K.; data curation, P.G.; writing—original draft preparation, P.G.; writing—review and editing, P.G. and P.K.; visualization, P.K.; supervision, P.K. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lowrey P.L., Takahashi J.S. Mammalian circadian biology: Elucidating genome-wide levels of temporal organisation. Annu. Rev. Genom. Hum. Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harmer S.L., Panda S., Kay S.A. Molecular bases of circadian rhythms. Annu. Rev. Cell Dev. Biol. 2001;17:215–253. doi: 10.1146/annurev.cellbio.17.1.215. [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki S., Numano R., Abe M., Hida A., Takahashi R., Ueda M., Block G.D., Sakaki Y., Menaker M., Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 4.Ralph M.R., Foster R.G., Davis F.C., Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 5.Sujino M., Masumoto K., Yamaguchi S., van der Horst G.T., Okamura H., Inouye S.I. Suprachiasmatic nucleus grafts restore circadian behavioral rhythms of genetically arrhythmic mice. Curr. Biol. 2003;13:664–668. doi: 10.1016/S0960-9822(03)00222-7. [DOI] [PubMed] [Google Scholar]
- 6.Vitaterna M.H., Shimomura K., Jiang P. Genetics of Circadian Rhythms. Neurol. Clin. 2019;37:487–504. doi: 10.1016/j.ncl.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konopka R.J., Benzer S. Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bargiello T.A., Jackson F.R., Young M.W. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature. 1984;312:752–754. doi: 10.1038/312752a0. [DOI] [PubMed] [Google Scholar]
- 9.Zehring W.A., Wheeler D.A., Reddy P., Konopka R.J., Kyriacou C.P., Rosbash M., Hall J.C. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell. 1984;39:369–376. doi: 10.1016/0092-8674(84)90015-1. [DOI] [PubMed] [Google Scholar]
- 10.Albrecht U., Sun Z.S., Eichele G., Lee C.C. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. doi: 10.1016/S0092-8674(00)80495-X. [DOI] [PubMed] [Google Scholar]
- 11.Shearman L.P., Zylka M.J., Weaver D.R., Kolakowski L.F., Jr., Reppert S.M. Two period homologs: Circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19:1261–1269. doi: 10.1016/S0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 12.Tei H., Okamura H., Shigeyoshi Y., Fukuhara C., Ozawa R., Hirose M., Sakaki Y. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997;389:512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- 13.Zylka M.J., Shearman L.P., Weaver D.R., Reppert S.M. Three period homologs in mammals: Differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110. doi: 10.1016/S0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
- 14.Vitaterna M.H., King D.P., Chang A., Kornhauser J.M., Lowrey P.L., McDonald J.D., Dove W.F., Pinto L.H., Turek F.W., Takahashi J.S. Mutagenesis and Mapping of a Mouse Gene, Clock, Essential for Circadian Behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King D.P., Zhao Y., Sangoram A.M., Wilsbacher L.D., Tanaka M., Antoch M.P., Steeves T.D.L., Vitaterna M.H., Kornhauser J.M., Lowrey P.L., et al. Positional Cloning of the Mouse Circadian Clock Gene. Cell. 1997;89:641–653. doi: 10.1016/S0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gekakis N., Staknis D., Nguyen H.B., Davis F.C., Wilsbacher L.D., King D.P., Takahashi J.S., Weitz C.J. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 17.Kondratov R.V., Chernov M.V., Kondratova A.A., Gorbacheva V.Y., Gudkov A.V., Antoch M.P. BMAL1-dependent circadian oscillation of nuclear CLOCK: Posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev. 2003;17:1921–1932. doi: 10.1101/gad.1099503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamoto Y., Sancar A. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc. Natl. Acad. Sci. USA. 1998;95:6097–6102. doi: 10.1073/pnas.95.11.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kume K., Zylka M.J., Sriram S., Shearman L.P., Weaver D.R., Jin X., Maywood E.S., Hastings M.H., Reppert S.M. CRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/S0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 20.Sato T.K., Panda S., Miraglia L.J., Reyes T.M., Rudic R.D., McNamara P., Naik K.A., FitzGerald G.A., Kay S.A., Hogenesch J.B. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–537. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Takeda Y., Jothi R., Birault V., Jetten A.M. RORγ directly regulates the circadian expression of clock genes and downstream targets in vivo. Nucleic Acids Res. 2012;40:8519–8535. doi: 10.1093/nar/gks630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preitner N., Damiola F., Lopez-Molina L., Zakany J., Duboule D., Albrecht U., Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/S0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 23.DeBruyne J.P., Weaver D.R., Reppert S.M. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat. Neurosci. 2007;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siepka S.M., Yoo S., Park J., Song W., Kumar V., Hu Y., Lee C., Takahashi J.S. Circadian Mutant Overtime Reveals F-box Protein FBXL3 Regulation of Cryptochrome and Period Gene Expression. Cell. 2007;129:1011–1023. doi: 10.1016/j.cell.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirano A., Yumimoto K., Tsunematsu R., Matsumoto M., Oyama M., Kozuka-Hata H., Nakagawa T., Lanjakornsiripan D., Nakayama K.I., Fukada Y. FBXL21 regulates oscillation of the circadian clock through ubiquitination and stabilization of cryptochromes. Cell. 2013;152:1106–1118. doi: 10.1016/j.cell.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 26.Honma S., Kawamoto T., Takagi Y., Fujimoto K., Sato F., Noshiro M., Kato Y., Honma K. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature. 2002;419:841–844. doi: 10.1038/nature01123. [DOI] [PubMed] [Google Scholar]
- 27.Gekakis N., Saez L., Delahaye-Brown A.M., Myers M.P., Sehgal A., Young M.W., Weitz C.J. Isolation of timeless by PER protein interaction: Defective interaction between timeless protein and long-period mutant PERL. Science. 1995;270:811–815. doi: 10.1126/science.270.5237.811. [DOI] [PubMed] [Google Scholar]
- 28.Andreani T.S., Itoh T.Q., Yildirim E., Hwangbo D., Allada R. Genetics of Circadian Rhythms. Sleep Med. Clin. 2015;10:413–421. doi: 10.1016/j.jsmc.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H.X. The role of circadian clock genes in tumors. Onco Targets Ther. 2019;12:3645–3660. doi: 10.2147/OTT.S203144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kettner N.M., Katchy C.A., Fu L. Circadian gene variants in cancer. Ann. Med. 2014;46:208–220. doi: 10.3109/07853890.2014.914808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morales-Santana S., Morell S., Leon J., Carazo-Gallego A., Jimenez-Lopez J.C., Morell M. An Overview of the Polymorphisms of Circadian Genes Associated with Endocrine Cancer. Front. Endocrinol. 2019;10:104. doi: 10.3389/fendo.2019.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bass J., Takahashi J.S. Circadian Integration of Metabolism and Energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durgan D.J., Young M.E. The Cardiomyocyte Circadian Clock: Emerging Roles in Health and Disease. Circ. Res. 2010;106:647–658. doi: 10.1161/CIRCRESAHA.109.209957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L., Jain M.K. Circadian regulation of cardiac metabolism. J. Clin. Investig. 2021;131:e148276. doi: 10.1172/JCI148276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C., Tang X., Gong Z., Zeng W., Hou Q., Lu R. Circadian Rhythm Sleep Disorders: Genetics, Mechanisms, and Adverse Effects on Health. Front. Genet. 2022;13:875342. doi: 10.3389/fgene.2022.875342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamont E.W., Legault-Coutu D., Cermakian N., Boivin D.B. The role of circadian clock genes in mental disorders. Dialogues Clin. Neurosci. 2007;9:333–342. doi: 10.31887/DCNS.2007.9.3/elamont. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canever J.B., Queiroz L.Y., Soares E.S., de Avelar N.C.P., Cimarosti H.I. Circadian rhythm alterations affecting the pathology of neurodegenerative diseases. J. Neurochem. 2023 doi: 10.1111/jnc.15883. [DOI] [PubMed] [Google Scholar]
- 38.SNPinfo Web Server. [(accessed on 13 August 2023)]; Available online: https://snpinfo.niehs.nih.gov/
- 39.dbSNP—NCBI—National Institutes of Health (NIH) [(accessed on 13 August 2023)]; Available online: https://www.ncbi.nlm.nih.gov/snp/
- 40.de Bakker P.I., Yelensky R., Pe’er I., Gabriel S.B., Daly M.J., Altshuler D. Efficiency and power in genetic association studies. Nat. Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- 41.Barret J.C., Fry B., Maller J., Daly M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 42.Horita N., Kaneko T. Genetic model selection for a case–control study and a meta-analysis. Meta Gene. 2015;5:1–8. doi: 10.1016/j.mgene.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lesicka M., Jabłońska E., Wieczorek E., Pepłońska B., Gromadzińska J., Seroczyńska B., Kalinowski L., Skokowski J., Reszka E. Circadian Gene Polymorphisms Associated with Breast Cancer Susceptibility. Int. J. Mol. Sci. 2019;20:5704. doi: 10.3390/ijms20225704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Y., Brown H.N., Zhang Y., Stevens R.G., Zheng T. Period3 structural variation: A circadian biomarker associated with breast cancer in young women. Cancer Epidemiol. Biomark. Prev. 2005;14:268–270. doi: 10.1158/1055-9965.268.14.1. [DOI] [PubMed] [Google Scholar]
- 45.Dai H., Zhang L., Cao M., Song F., Zheng H., Zhu X., Wei Q., Zhang W., Chen K. The role of polymorphisms in circadian pathway genes in breast tumorigenesis. Breast Cancer Res. Treat. 2011;127:531–540. doi: 10.1007/s10549-010-1231-2. [DOI] [PubMed] [Google Scholar]
- 46.Pham T.T., Lee E.S., Kong S.Y., Kim J., Kim S.Y., Joo J., Yoon K.A., Park B. Night-shift work, circadian and melatonin pathway related genes and their interaction on breast cancer risk: Evidence from a case-control study in Korean women. Sci. Rep. 2019;9:10982. doi: 10.1038/s41598-019-47480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Truong T., Liquet B., Menegaux F., Plancoulaine S., Laurent-Puig P., Mulot C., Cordina-Duverger E., Sanchez M., Arveux P., Kerbrat P., et al. Breast cancer risk, nightwork, and circadian clock gene polymorphisms. Endocr. Relat. Cancer. 2014;21:629–638. doi: 10.1530/ERC-14-0121. [DOI] [PubMed] [Google Scholar]
- 48.Zhu Y., Stevens R.G., Leaderer D., Hoffman A., Holford T., Zhang Y., Brown H.N., Zheng T. Non-synonymous polymorphisms in the circadian gene NPAS2 and breast cancer risk. Breast Cancer Res. Treat. 2008;107:421–425. doi: 10.1007/s10549-007-9565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y., Stevens R.G., Hoffman A.E., FitzGerald L.M., Kwon E.M., Ostrander E.A., Davis S., Zheng T., Stanford J.L. Testing the circadian gene hypothesis in prostate cancer: A population-based case-control study. Cancer Res. 2009;69:9315–9322. doi: 10.1158/0008-5472.CAN-09-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chu L., Zhu Y., Yu K., Zheng T., Yu H., Zhang Y., Sesterhenn I., Chokkalingam A.P., Danforth K.N., Shen M.C., et al. Variants in circadian genes and prostate cancer risk: A population-based study in China. Prostate Cancer Prostatic Dis. 2008;11:342–348. doi: 10.1038/sj.pcan.4501024. [DOI] [PubMed] [Google Scholar]
- 51.Couto P., Miranda D., Vieira R., Vilhena A., De Marco L., Bastos-Rodrigues L. Association between CLOCK, PER3 and CCRN4L with non-small cell lung cancer in Brazilian patients. Mol. Med. Rep. 2014;10:435–440. doi: 10.3892/mmr.2014.2224. [DOI] [PubMed] [Google Scholar]
- 52.Liu F., Li X., Liu P., Quan X., Zheng C., Zhou B. Association between three polymorphisms in BMAL1 genes and risk of lung cancer in a northeast Chinese population. DNA Cell Biol. 2019;38:1437–1443. doi: 10.1089/dna.2019.4853. [DOI] [PubMed] [Google Scholar]
- 53.Rajendran S., Benna C., Marchet A., Nitti D., Mocellin S. Germline polymorphisms of circadian genes and gastric cancer predisposition. Cancer Commun. 2020;40:234–238. doi: 10.1002/cac2.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffman A.E., Zheng T., Stevens R.G., Ba Y., Zhang Y., Leaderer D., Yi C., Holford T.R., Zhu Y. Clock-Cancer Connection in Non–Hodgkin’s Lymphoma: A Genetic Association Study and Pathway Analysis of the Circadian Gene Cryptochrome 2. Cancer Res. 2009;69:3605–3613. doi: 10.1158/0008-5472.CAN-08-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoffman A.E., Zheng T., Yi C.H., Stevens R.G., Ba Y., Zhang Y., Leaderer D., Holford T., Hansen J., Zhu Y. The core circadian gene cryptochrome 2 influences breast cancer risk, possibly by mediating hormone signalling. Cancer Prev. Res. 2010;3:539–548. doi: 10.1158/1940-6207.CAPR-09-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wirth M.D., Burch J.B., Hébert J.R., Kowtal P., Kapoor A., Steck S.E., Hurley T.G., Gupta P.C., Pednekar M.S., Youngstedt S.D., et al. Case-control study of breast cancer in India: Role of PERIOD3 clock gene length polymorphism and chronotype. Cancer Invest. 2014;32:321–329. doi: 10.3109/07357907.2014.919305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fores-Martos J., Cervera-Vidal R., Sierra-Roca J., Lozano-Asencio C., Fedele V., Cornelissen S., Edvarsen H., Tadeo-Cervera I., Eroles P., Lluch A., et al. Circadian PERformance in breast cancer: A germline and somatic genetic study of PER3VNTR polymorphisms and gene co-expression. NPJ Breast Cancer. 2021;7:118. doi: 10.1038/s41523-021-00329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monsees G.M., Kraft P., Hankinson S.E., Hunter D.J., Schernhammer E.S. Circadian genes and breast cancer susceptibility in rotating shift workers. Int. J. Cancer. 2012;131:2547–2552. doi: 10.1002/ijc.27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu C.C., Chen L.C., Chiou C.Y., Chang Y.J., Lin V.C., Huang C.Y., Lin I.L., Chang T.Y., Lu T.L., Lee C.H., et al. Genetic variants in the circadian rhythm pathway as indicators of prostate cancer progression. Cancer Cell Int. 2019;19:87. doi: 10.1186/s12935-019-0811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wendeu-Foyet M.G., Koudou Y., Cénée S., Trétarre B., Rébillard X., Cancel-Tassin G., Cussenot O., Boland A., Bacq D., Deleuze J.F., et al. Circadian genes and risk of prostate cancer: Findings from the EPICAP study. Int. J. Cancer. 2019;145:1745–1753. doi: 10.1002/ijc.32149. [DOI] [PubMed] [Google Scholar]
- 61.Markt S.C., Valdimarsdottir U.A., Shui I.M., Sigurdardottir L.G., Rider J.R., Tamimi R.M., Batista J.L., Haneuse S., Flynn-Evans E., Lockley S.W., et al. Circadian clock genes and risk of fatal prostate cancer. Cancer Causes Control. 2015;26:25–33. doi: 10.1007/s10552-014-0478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rabstein S., Harth V., Justenhoven C., Pesch B., Plöttner S., Heinze E., Lotz A., Baisch C., Schiffermann M., Brauch H., et al. Polymorphisms in circadian genes, night work and breast cancer: Results from the GENICA study. Chronobiol. Int. 2014;31:1115–1122. doi: 10.3109/07420528.2014.957301. [DOI] [PubMed] [Google Scholar]
- 63.Fu A., Leaderer D., Zheng T., Hoffman A.E., Stevens R.G., Zhu Y. Genetic and Epigenetic Associations of Circadian Gene TIMELESS and Breast Cancer Risk. Mol. Carcinog. 2012;51:923–929. doi: 10.1002/mc.20862. [DOI] [PubMed] [Google Scholar]
- 64.Johnson K., Chang-Claude J., Critchley A.M., Kyriacou C., Lavers S., Rattay T., Seibold P., Webb A., West C., Symonds R.P., et al. Genetic Variants Predict Optimal Timing of Radiotherapy to Reduce Side-effects in Breast Cancer Patients. Clin. Oncol. 2019;31:9–16. doi: 10.1016/j.clon.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 65.Webb A.J., Harper E., Rattay T., Aguado-Barrera M.E., Azria D., Bourgier C., Brengues M., Briers E., Bultijnck R., Chang-Claude J., et al. Treatment time and circadian genotype interact to influence radiotherapy side-effects. A prospective European validation study using the REQUITE cohort. EBioMedicine. 2022;84:104269. doi: 10.1016/j.ebiom.2022.104269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chu L.W., Zhu Y., Yu K., Zheng T., Chokkalingam A.P., Stanczyk F.Z., Gao Y.T., Hsing A.W. Correlation between Circadian Gene Variants and Serum Levels of Sex Steroids and Insulin-like Growth Factor-I. Cancer Epidemiol. Biomark. Prev. 2008;17:3268–3273. doi: 10.1158/1055-9965.EPI-08-0073. [DOI] [PubMed] [Google Scholar]
- 67.Leu H.B., Chung C.M., Lin S.J., Chiang K.M., Yang H.C., Ho H.Y., Ting C.T., Lin T.H., Sheu S.H., Tsai W.C., et al. Association of circadian genes with diurnal blood pressure changes and non-dipper essential hypertension: A genetic association with young-onset hypertension. Hypertens. Res. 2015;38:155–162. doi: 10.1038/hr.2014.152. [DOI] [PubMed] [Google Scholar]
- 68.Garaulet M., Lee Y.C., Shen J., Parnell L.D., Arnett D.K., Tsai M.Y., Lai C.Q., Ordovas J.M. CLOCK genetic variation and metabolic syndrome risk: Modulation by monounsaturated fatty acids. Am. J. Clin. Nutr. 2009;90:1466–1475. doi: 10.3945/ajcn.2009.27536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garaulet M., Lee Y.C., Shen J., Parnell L.D., Arnett D.K., Tsai M.Y., Lai C.Q., Ordovas J.M. Genetic variants in human CLOCK associate with total energy intake and cytokine sleep factors in overweight subjects (GOLDN population) Eur. J. Hum. Genet. 2010;18:364–369. doi: 10.1038/ejhg.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corella D., Asensio E.M., Coltell O., Sorlí J.V., Estruch R., Martínez-González M.A., Salas-Salvadó J., Castañer O., Arós F., Lapetra J., et al. CLOCK gene variation is associated with incidence of type-2 diabetes and cardiovascular diseases in type-2 diabetic subjects: Dietary modulation in the PREDIMED randomized trial. Cardiovasc. Diabetol. 2016;15:4. doi: 10.1186/s12933-015-0327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uemura H., Katsuura-Kamano S., Yamaguchi M., Arisawa K., Hamajima N., Hishida A., Kawai S., Oze I., Shinchi K., Takashima N., et al. Variant of the clock circadian regulator (CLOCK) gene and related haplotypes are associated with the prevalence of type 2 diabetes in the Japanese population. J. Diabetes. 2016;8:667–676. doi: 10.1111/1753-0407.12344. [DOI] [PubMed] [Google Scholar]
- 72.Škrlec I., Talapko J., Džijan S., Lepeduš H. The Association of Cardiovascular Disease with the T3111C Polymorphism in the CLOCK Gene. In Proceedings of the The 1st International Electronic Conference on Medicine, online, 20–30 June 2021. Med. Sci. Forum. 2021;6:1. doi: 10.3390/IECMD2021-10314. [DOI] [Google Scholar]
- 73.Sookoian S., Gemma C., Gianotti T.F., Burgueño A., Castaño G., Pirola C.J. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am. J. Clin. Nutr. 2008;87:1606–1615. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 74.Schroor M.M., Plat J., Mensink R.P. Relation between single nucleotide polymorphisms in circadian clock relevant genes and cholesterol metabolism. Mol. Genet. Metab. 2023;138:107561. doi: 10.1016/j.ymgme.2023.107561. [DOI] [PubMed] [Google Scholar]
- 75.Škrlec I., Milić J., Steiner R. The Impact of the Circadian Genes CLOCK and ARNTL on Myocardial Infarction. J. Clin. Med. 2020;9:484. doi: 10.3390/jcm9020484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Škrlec I., Talapko J., Džijan S., Cesar V., Lazić N., Lepeduš H. The Association between Circadian Clock Gene Polymorphisms and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Biology. 2022;11:20. doi: 10.3390/biology11010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dashti H.S., Smith C.E., Lee Y.C., Parnell L.D., Lai C.Q., Arnett D.K., Ordovás J.M., Garaulet M. CRY1 circadian gene variant interacts with carbohydrate intake for insulin resistance in two independent populations: Mediterranean and North American. Chronobiol. Int. 2014;31:660–667. doi: 10.3109/07420528.2014.886587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garaulet M., Smith C.E., Gomez-Abellán P., Ordovás-Montañés M., Lee Y.C., Parnell L.D., Arnett D.K., Ordovás J.M. REV-ERB-ALPHA circadian gene variant associates with obesity in two independent populations: Mediterranean and North American. Mol. Nutr. Food Res. 2014;58:821–829. doi: 10.1002/mnfr.201300361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Monteleone P., Tortorella A., Docimo L., Maldonato M.N., Canestrelli B., De Luca L., Maj M. Investigation of 3111T/C polymorphism of the CLOCK gene in obese individuals with or without binge eating disorder: Association with higher body mass index. Neurosci. Lett. 2008;435:30–33. doi: 10.1016/j.neulet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 80.Garaulet M., Corbalán M.D., Madrid J.A., Morales E., Baraza J.C., Lee Y.C., Ordovas J.M. CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based on the Mediterranean diet. Int. J. Obes. 2010;34:516–523. doi: 10.1038/ijo.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li G.Y., Wang H., Chen H. Association of insulin resistance with polymorphic variants of Clock and Bmal1 genes: A case-control study. Clin. Exp. Hypertens. 2020;42:371–375. doi: 10.1080/10641963.2019.1676769. [DOI] [PubMed] [Google Scholar]
- 82.Torrego-Ellacuría M., Barabash A., Matía-Martín P., Sánchez-Pernaute A., Torres A.J., Calle-Pascual A.L., Rubio-Herrera M.A. Influence of CLOCK Gene Variants on Weight Response after Bariatric Surgery. Nutrients. 2022;14:3472. doi: 10.3390/nu14173472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Riestra P., Gebreab S.Y., Xu R., Khan R.J., Gaye A., Correa A., Min N., Sims M., Davis S.K. Circadian CLOCK gene polymorphisms in relation to sleep patterns and obesity in African Americans: Findings from the Jackson heart study. BMC Genet. 2017;18:58. doi: 10.1186/s12863-017-0522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Molina-Montes E., Rodríguez-Barranco M., Ching-Lopez A., Artacho R., Huerta J.M., Amiano P., Lasheras C., Moreno-Iribas C., Jimenez-Zabala A., Chirlaque M.D., et al. Circadian clock gene variants and their link with chronotype, chrononutrition, sleeping patterns and obesity in the European prospective investigation into cancer and nutrition (EPIC) study. Clin. Nutr. 2022;41:1977–1990. doi: 10.1016/j.clnu.2022.07.027. [DOI] [PubMed] [Google Scholar]
- 85.Woon P.Y., Kaisaki P.J., Bragança J., Bihoreau M.T., Levy J.C., Farrall M., Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc. Natl. Acad. Sci. USA. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mirzaei K., Xu M., Qi Q., de Jonge L., Bray G.A., Sacks F., Qi L. Variants in glucose- and circadian rhythm–related genes affect the response of energy expenditure to weight-loss diets: The POUNDS LOST Trial. Am. J. Clin. Nutr. 2014;99:392–399. doi: 10.3945/ajcn.113.072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kovanen L., Donner K., Kaunisto M., Partonen T. CRY1, CRY2 and PRKCDBP genetic variants in metabolic syndrome. Hypertens. Res. 2015;38:186–192. doi: 10.1038/hr.2014.157. [DOI] [PubMed] [Google Scholar]
- 88.Mariman E.C.M., Bouwman F.G., Aller E.E.J.G., van Baak M.A., Wang P. Extreme obesity is associated with variation in genes related to the circadian rhythm of food intake and hypothalamic signalling. Physiol. Genom. 2015;47:225–231. doi: 10.1152/physiolgenomics.00006.2015. [DOI] [PubMed] [Google Scholar]
- 89.Garaulet M., Corbalán-Tutau M.D., Madrid J.A., Baraza J.C., Parnell L.D., Lee Y.C., Ordovas J.M. PERIOD2 Variants Are Associated with Abdominal Obesity, Psycho-Behavioral Factors, and Attrition in the Dietary Treatment of Obesity. J. Am. Diet. Assoc. 2010;110:917–921. doi: 10.1016/j.jada.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Katzenberg D., Young Y., Finn L., Lin L., King D.P., Takahashi J.S., Mignot E. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998;21:569–576. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- 91.Johansson C., Willeit M., Smedh C., Ekholm J., Paunio T., Kieseppä T., Lichtermann D., Praschak-Rieder N., Neumeister A., Nilsson L.G., et al. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology. 2003;28:734–739. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]
- 92.Archer S.N., Robilliard D.L., Skene D.J., Smits M., Williams A., Arendt J., von Schantz M. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–415. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 93.Pereira D.S., Tufik S., Louzada F.M., Benedito-Silva A.A., Lopez A.R., Lemos N.A., Korczak A.L., D’Almeida V., Pedrazzoli M. Association of the length polymorphism in the human Per3 gene with the delayed sleep-phase syndrome: Does latitude have an influence upon it? Sleep. 2005;28:29–32. doi: 10.3410/f.1024974.294734. [DOI] [PubMed] [Google Scholar]
- 94.Peng X., Li J., Han B., Zhu Y., Cheng D., Li Q., Du J. Association of occupational stress, period circadian regulator 3 (PER3) gene polymorphism and their interaction with poor sleep quality. J. Sleep Res. 2022;31:e13390. doi: 10.1111/jsr.13390. [DOI] [PubMed] [Google Scholar]
- 95.Miyagawa T., Hida A., Shimada M., Uehara C., Nishino Y., Kadotani H., Uchiyama M., Ebisawa T., Inoue Y., Kamei Y., et al. A missense variant in PER2 is associated with delayed sleep-wake phase disorder in a Japanese population. J. Hum. Genet. 2019;64:1219–1225. doi: 10.1038/s10038-019-0665-6. [DOI] [PubMed] [Google Scholar]
- 96.Mishima K., Tozawa T., Satoh K., Saitoh H., Mishima Y. The 3111T/C polymorphism of hClock is associated with evening preference and delayed sleep timing in a Japanese population sample. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005;133B:101–104. doi: 10.1002/ajmg.b.30110. [DOI] [PubMed] [Google Scholar]
- 97.Patke A., Murphy P.J., Onat O.E., Krieger A.C., Özçelik T., Campbell S.S., Young M.W. Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell. 2017;169:203–215.e13. doi: 10.1016/j.cell.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takano A., Uchiyama M., Kajimura N., Mishima K., Inoue Y., Kamei Y., Kitajima T., Shibui K., Katoh M., Watanabe T., et al. A missense variation in human casein kinase I epsilon gene that induces functional alteration and shows an inverse association with circadian rhythm sleep disorders. Neuropsychopharmacology. 2004;29:1901–1909. doi: 10.1038/sj.npp.1300503. [DOI] [PubMed] [Google Scholar]
- 99.Toh K.L., Jones C.R., He Y., Eide E.J., Hinz W.A., Virshup D.M., Ptácek L.J., Fu Y.H. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 100.Zhang L., Hirano A., Hsu P.K., Jones C.R., Sakai N., Okuro M., McMahon T., Yamazaki M., Xu Y., Saigoh N., et al. A PERIOD3 variant causes a circadian phenotype and is associated with a seasonal mood trait. Proc. Natl. Acad. Sci. USA. 2016;113:E1536–E1544. doi: 10.1073/pnas.1600039113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hirano A., Shi G., Jones C.R., Lipzen A., Pennacchio L.A., Xu Y., Hallows W.C., McMahon T., Yamazaki M., Ptáček L.J., et al. A Cryptochrome 2 mutation yields advanced sleep phase in humans. eLife. 2016;5:e16695. doi: 10.7554/eLife.16695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kurien P., Hsu P.K., Leon J., Wu D., McMahon T., Shi G., Xu Y., Lipzen A., Pennacchio L.A., Jones C.R., et al. TIMELESS mutation alters phase responsiveness and causes advanced sleep phase. Proc. Natl. Acad. Sci. USA. 2019;116:12045–12053. doi: 10.1073/pnas.1819110116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu Y., Padiath Q.S., Shapiro R.E., Jones C.R., Wu S.C., Saigoh N., Saigoh K., Ptácek L.J., Fu Y.H. Functional consequences of a CKIδ mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 104.Hida A., Kitamura S., Katayose Y., Kato M., Ono H., Kadotani H., Uchiyama M., Ebisawa T., Inoue Y., Kamei Y., et al. Screening of Clock Gene Polymorphisms Demonstrates Association of a PER3 Polymorphism with Morningness–Eveningness Preference and Circadian Rhythm Sleep Disorder. Sci. Rep. 2014;4:6309. doi: 10.1038/srep06309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robilliard D.L., Archer S.N., Arendt J., Lockley S.W., Hack L.M., English J., Leger D., Smits M.G., Williams A., Skene D.J., et al. The 3111 Clock gene polymorphism is not associated with sleep and circadian rhythmicity in phenotypically characterized human subjects. J. Sleep Res. 2002;11:305–312. doi: 10.1046/j.1365-2869.2002.00320.x. [DOI] [PubMed] [Google Scholar]
- 106.Iwase T., Kajïmura N., Uchiyama M., Ebisawa T., Yoshimura K., Kamei Y., Shibui K., Kim K., Kudo Y., Katoh M., et al. Mutation screening of the human Clock gene in circadian rhythm sleep disorders. Psychiatry Res. 2002;109:121–128. doi: 10.1016/S0165-1781(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 107.Serretti A., Benedetti F., Mandelli L., Lorenzi C., Pirovano A., Colombo C., Smeraldi E. Genetic dissection of psychopathological symptoms: Insomnia in mood disorders and CLOCK gene polymorphism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003;121B:39–43. doi: 10.1002/ajmg.b.20053. [DOI] [PubMed] [Google Scholar]
- 108.Benedetti F., Serretti A., Colombo C., Barbini B., Lorenzi C., Campori E., Smeraldi E. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003;123B:23–26. doi: 10.1002/ajmg.b.20038. [DOI] [PubMed] [Google Scholar]
- 109.Benedetti F., Dallaspezia S., Fulgosi M.C., Lorenzi C., Serretti A., Barbini B., Colombo C., Smeraldi E. Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007;144B:631–635. doi: 10.1002/ajmg.b.30475. [DOI] [PubMed] [Google Scholar]
- 110.Geoffroy P.E., Lajnef M., Bellivier F., Jamain S., Gard S., Kahn J.-P., Henry C., Leboyer M., Etain B. Genetic association study of circadian genes with seasonal pattern in bipolar disorders. Sci. Rep. 2015;5:10232. doi: 10.1038/srep10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shi S.-Q., White M.J., Borsetti H.M., Pendergast J.S., Hida A., Ciarleglio C.M., de Verteuil P.A., Cadar A.G., Cala C., McMahon D.G., et al. Molecular analyses of circadian gene variants reveal sex-dependent links between depression and clocks. Transl. Psychiatry. 2016;6:e748. doi: 10.1038/tp.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schuch J.B., Genro J.P., Bastos C.R., Ghisleni G., Tovo-Rodrigues L. The role of CLOCK gene in psychiatric disorders: Evidence from human and animal research. Am. J. Med. Genet. Part B. 2018;177B:181–198. doi: 10.1002/ajmg.b.32599. [DOI] [PubMed] [Google Scholar]
- 113.Xu Y., Ma H., Zhao t., Wen D., Wen Y., Qiao D., Liu Z. Association Between Period 3 Gene Polymorphisms and Adverse Effects of Antidepressants for Major Depressive Disorder. Genet. Test. Mol. Biomark. 2019;23:843–849. doi: 10.1089/gtmb.2019.0065. [DOI] [PubMed] [Google Scholar]
- 114.Serretti A., Cusin C., Benedetti F., Mandelli L., Pirovano A., Zanardi R., Colombo C., Smeraldi E. Insomnia improvement during antidepressant treatment and CLOCK gene polymorphism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005;137B:36–39. doi: 10.1002/ajmg.b.30130. [DOI] [PubMed] [Google Scholar]
- 115.Gyorik D., Eszlari N., Gal Z., Torok D., Baksa D., Kristof Z., Sutori S., Petschner P., Juhasz G., Bagdy G., et al. Every Night and Every Morn: Effect of Variation in CLOCK Gene on Depression Depends on Exposure to Early and Recent Stress. Front. Psychiatry. 2021;12:687487. doi: 10.3389/fpsyt.2021.687487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bacalini M.G., Palombo F., Garagnani P., Giuliani C., Fiorini C., Caporali L., Stanzani Maserati M., Capellari S., Romagnoli M., De Fanti S., et al. Association of rs3027178 polymorphism in the circadian clock gene PER1 with susceptibility to Alzheimer’s disease and longevity in an Italian population. GeroScience. 2022;44:881–896. doi: 10.1007/s11357-021-00477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bessi V., Balestrini J., Bagnoli S., Mazzeo S., Giacomucci G., Padiglioni S., Piaceri I., Carraro M., Ferrari C., Bracco L., et al. Influence of ApoE Genotype and Clock T3111C Interaction with Cardiovascular Risk Factors on the Progression to Alzheimer’s Disease in Subjective Cognitive Decline and Mild Cognitive Impairment Patients. J. Pers. Med. 2020;10:45. doi: 10.3390/jpm10020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lavtar P., Rudolf G., Maver A., Hodžić A., Starčević Čizmarević N., Živković M., Šega Jazbec S., Klemenc Ketiš Z., Kapović M., Dinčić E., et al. Association of circadian rhythm genes ARNTL/BMAL1 and CLOCK with multiple sclerosis. PLoS ONE. 2018;13:e0190601. doi: 10.1371/journal.pone.0190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Rojas I., Martin-Montero C., Fedetz M., González-Jiménez A., Matesanz F., Urcelay E., Espino-Paisán L. Polymorphisms in ARNTL/BMAL1 and CLOCK Are Not Associated with Multiple Sclerosis in Spanish Population. Biology. 2022;11:1417. doi: 10.3390/biology11101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gu Z., Wang B., Zhang Y.B., Ding H., Zhang Y., Yu J., Gu M., Chan P., Cai Y. Association of ARNTL and PER1 genes with Parkinson’s disease: A case-control study of han chinese. Sci. Rep. 2015;5:15891. doi: 10.1038/srep15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen H., Ding X., Ding E., Chen M., Wang H., Yang G., Zhu B. A missense variant rs2585405 in clock gene PER1 is associated with the increased risk of noise-induced hearing loss in a Chinese occupational population. BMC Med. Genom. 2021;14:221. doi: 10.1186/s12920-021-01075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Curtis A.M., Bellet M.M., Sassone-Corsi P., O’Neill L.A.J. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 123.Poole J., Kitchen G. Circadian regulation of innate immunity in animals and humans and implications for human disease. Semin. Immunopathol. 2022;44:183–192. doi: 10.1007/s00281-022-00921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Downton P., Early J.O., Gibbs J.E. Circadian rhythms in adaptive immunity. Immunology. 2020;161:268–277. doi: 10.1111/imm.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Z., Zeng P., Gao W., Zhou Q., Feng T., Tian X. Circadian clock: A regulator of the immunity in cancer. Cell Commun. Signal. 2021;19:37. doi: 10.1186/s12964-021-00721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Awuah W.A., Huang H., Kalmanovich J., Mehta A., Mikhailova T., Ng J.C., Abdul-Rahman T., Adebusoye F.T., Tan J.K., Kamanousa K., et al. Circadian rhythm in systemic autoimmune conditions: Potential of chrono-immunology in clinical practice: A narrative review. Medicine. 2023;102:e34614. doi: 10.1097/MD.0000000000034614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xiang H., Xu Z., Hu Y.-Q., He Y.-S., Wu G.-C., Li T.-Y., Wang X.-R., Ding L.-H., Zhang Q., Tao S.-S., et al. Circadian clock genes as promising therapeutic targets for autoimmune diseases. Autoimmun. Rev. 2021;20:102866. doi: 10.1016/j.autrev.2021.102866. [DOI] [PubMed] [Google Scholar]