Supplemental Digital Content is Available in the Text.
Self-reported allodynia, a surrogate marker of central sensitization, significantly related to all sleep elements, hinting to a possible influence of central sensitization on sleep disruption.
Keywords: Neuropathic pain, Sleep disturbance, Central sensitization, Allodynia, Questionnaires
Abstract
Introduction:
Patients with neuropathic pain (NP) report a higher impairment of quality of life and sleep than patients with chronic pain without neuropathic characteristics. These include somatosensory peculiarities like allodynia, a surrogate marker for central sensitization.
Objectives:
This study aimed to investigate the relation between symptoms of central sensitization and sleep disturbances in patients with NP.
Methods:
Within this cross-sectional study, data sets of 3339 patients with chronic NP syndromes (painful diabetic polyneuropathy, n = 543; postherpetic neuralgia, n = 1480) or complex regional pain syndromes (CRPS, n = 1316) were analyzed. Neuropathic pain symptoms were assessed with the painDETECT questionnaire (PD-Q), depression with the Patient Health Questionnaire-9, and sleep impairment with items of the Medical Outcomes Study Sleep Scale in 4 subscales. The association of demographic/clinical data, somatosensory phenotype, depression, and pain intensity with sleep impairment was assessed by unadjusted Spearman correlation analyses and multivariable regression analyses.
Results:
Sleep impairment was observed in all pain aetiologies although with some significant differences in the single sleep items. The intensity of the individual PD-Q items differed to some extent between the 3 pain entities, whereas the PD-Q sum score was similar. Thermal hyperalgesia and burning assessed by the PD-Q were significantly associated with sleep disturbance, adequacy, and quantity but not with sleep somnolence. Only depression and self-reported allodynia had a significant relation to all 4 sleep elements.
Conclusion:
Beside depression, allodynia as a surrogate marker hints to a possible impact of central sensitization on the sleep disruption of patients with NP.
1. Introduction
Patients with neuropathic pain display a range of somatosensory abnormalities with gain and loss of function. These may vary from being troublesome (ie, paresthesias like ant crawling, prickling) to being painful (eg, spontaneous superficial burning pain or electric-shock-like pain attacks). Central mechanisms are known to play an important role in the development and maintenance of neuropathic pain, including central disinhibition and central sensitization.5 The latter is defined as an increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input,46 which is mirrored clinically by evoked types of pain induced by certain stimuli implicating hypersensitivity.3 Animal research suggests that this fundamental secondary modification is induced by a peripheral nerve lesion.6,19,26 Both dynamic mechanical allodynia and mechanical hyperalgesia (punctate, deep somatic) are supposed to be characteristic sensory features, ie, surrogate markers, of central sensitization.5,8,46 Previous studies using quantitative sensory testing (QST) found that these signs of central sensitization are present in a relevant proportion of patients with neuropathic pain, eg, in 23% of patients with polyneuropathy, in 44% of patients with postherpetic neuralgia (PHN), and in 38% of patients with complex regional pain syndromes (CRPS).30
However, it might not be sensory signs alone that are altered in neuropathic pain conditions. Sleep disturbance is a highly prevalent complaint of patients with chronic pain.39 Patients with neuropathic pain report an even higher impairment of quality of life and sleep than those without neuropathic characteristics.4,12,25 Although a bidirectional and multidimensional relationship is assumed, the exact origins of their mutual interference are still a matter of ongoing debate21,40 and the majority of neuropathic pain studies so far have only assessed the impact of pain intensity on sleep disturbances,2,36,38 whereas the entire range of neuropathic somatosensory abnormalities has not been investigated yet in this regard. The mutual interference of vigilance and sensory perception has been examined by Wang et al.44 through somatosensory evoked potentials in healthy volunteers, where consciousness has been shown to have an effect on pain-specific components of A-delta fibres. It is possible that this physiological inhibition of pain signals during sleep will cease in chronic pain conditions. In patients with migraine, for instance, it has been shown that sleep interference increased with the presence of mechanical allodynia,29 possibly pointing to an association of altered endogeneous pain modulation and sleep disturbances (Fig. 1A). In patients with neuropathic pain, however, the association of sleep disturbances and central amplifying mechanisms of pain processing, ie, central sensitization, is yet to be clarified (Fig. 1B).
Figure 1.
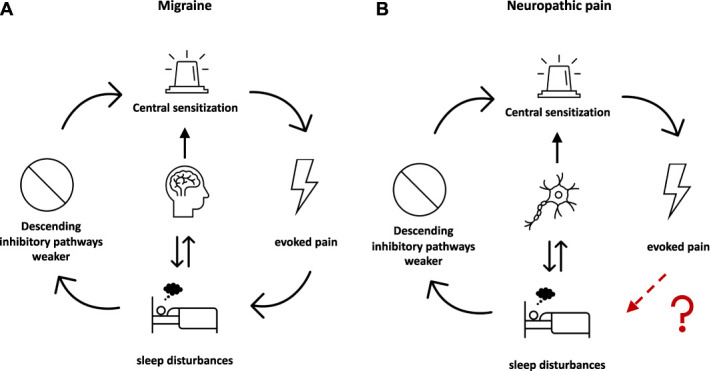
Connection between chronic pain, central sensitization, and sleep disturbance. (A) Displayed are the so-far examined interrelations between migraine, evoked pain, sleep disturbances, and inhibitory pathways. (B) Displayed is the formation of the hypothesis for the present article. Although the interrelations between neuropathic pain and sleep disturbances and neuropathic pain and inhibitory pathways/central sensitization have been investigated, the effect of evoked pain on sleep disturbances remains unclear.
This study therefore aimed to
(1) assess the co-occurrence of sensory signs and various aspects of sleep quality;
(2) highlight hallmark symptoms of central sensitization such as mechanical allodynia and hyperalgesia5,8,46 with sleep disruption in patients with neuropathic pain. Neuropathic pain symptoms and sleep quality were examined with validated patient-reported outcome measures (PROMs), given their unparalleled strength of time and resource efficiency.
2. Methods
2.1. Study population
This was a cross-sectional study of patient data collected from the painDETECT software as part of usual care (between 2006 and 2013). The painDETECT software is an open pain registry in Germany originally developed to validate the painDETECT questionnaire (PD-Q).12 The software contains validated questionnaires (eg, the PD-Q, the Funktionsfragebogen Hannover (FFbH), the Patient Health Questionnaire [PHQ-9]) and is used in outpatient primary care centres for the clinical and epidemiological survey of patients with chronic pain. During regular consultation hours, patients were asked to complete several electronic questionnaires on a hand-held computer (personal digital assistants, PDAs; Palm Tungsten E operating on the platform OS 5.4). Questionnaire results are used in daily practice for treatment purposes and, if the patient agrees, for research purposes, ie, to gain knowledge about pain characteristics and comorbidities in the German population. For the latter, PDAs were collected at regular intervals, and data were transferred anonymously and encrypted to a central database for analysis. Each recruiting study site (general practitioners, rheumatologists, orthopaedists, diabetologists, neurologists, and pain specialists in Germany) was responsible for the correct implementation of the study procedures and the correct pain diagnosis. To focus on clinical relevance, we have chosen frequent neuropathic pain conditions that are reported to be associated with sleep disturbances.10,20,27 All data of patients with the diagnosis “painful diabetic polyneuropathy” (PDNP; n = 1340), “postherpetic neuralgia” (PHN, n = 2593), or “complex regional pain syndrome” (CRPS; n = 1316) exported to the central database between the implementation of the painDETECT software (April 2006) and July 2013 were analyzed. Even though recently redefined as a nociplastic pain syndrome, CRPS type 1 was here included as pain syndrome with neuropathic features because it has been shown to display similar sensory signs and symptoms like CRPS type 2.13
The study was performed in accordance with the Declaration of Helsinki and approved by the ethical committee of the University of Düsseldorf. All subjects gave their written informed consent for participating in the study.
2.2. Questionnaires
In addition to demographic (age, sex, and body mass index) and clinical data (pain duration, pain medication [yes/no], pension request, and hospitalization because of the pain disease), validated questionnaires were used to assess the pain intensity, pain quality (PD-Q12), sleep quality (Medical Outcomes Study [MOS] Sleep Scale15,42), and co-morbidities.
2.2.1. Pain intensity
Pain intensity was rated on a visual analogue scale (VAS) ranging from 0 (no pain) to 100 (maximum imaginable pain). Patients were asked to rate their average and maximum pain intensity during the preceding 4 weeks.
2.2.2. painDETECT questionnaire
The PD-Q serves as a screening tool for the detection of patients with neuropathic pain with a high sensitivity and specificity.12 In a previous study by Baron et al.,7 different questionnaire descriptors were shown to enable the identification and characterization of neuropathic pain subgroups. Furthermore, although it has not been specifically validated to evaluate central sensitization, it has also been shown to actually be able to identify aspects of central sensitization in selected patient cohorts11,14,16 and to be associated to the Central Sensitization Inventory (CSI) score.41
The questionnaire consists of 3 components: general pain intensity (current pain, average pain during the past 4 weeks, and maximum pain during the past 4 weeks), pain course pattern, and 7 questions addressing typical neuropathic pain symptoms (burning, prickling, dynamic mechanical allodynia, pain attacks, “thermal hyperalgesia,” numbness, and pressure-evoked pain). Thermal hyperalgesia is used in quotation marks throughout the article as the corresponding symptom has been assessed through the PD-Q. The latter are rated on a 6-point Likert scale (0 = never, 5 = very strongly). The PD-Q total score is the sum of the rating of the single questions ranging from −1 to 38. Total PD-Q scores of ≤12 indicate that a neuropathic pain component is unlikely (negative), scores of ≥19 mean that a neuropathic pain component is likely (positive), and scores in between are uncertain.
2.2.3. Patient Health Questionnaire-9
The PHQ-9 consists of 9 items and is an established questionnaire to discover the presence and to quantify the severity of depression.22 The frequency of each item within the last 2 weeks is rated on a scale of 0 to 3 (0, not at all; 1, several days; 2, more than half the days; 3, nearly every day). The degree of depression can be interpreted by determining the total score of all items (0–4 minimal depression; 5–9 mild depression; 10–14 moderate depression; 15–19 moderately severe depression; and 20–27 severe depression).
2.2.4. Medical Outcomes Study Sleep Scale
The MOS Sleep Scale is a self-report measure of sleep quality and contains 12 items to assess 6 sleep scores describing the dimension “sleep disturbance” (items: have trouble falling asleep, how long to fall asleep, sleep was not quiet, awaken during your sleep time, and have trouble falling asleep again), “somnolence” (items: drowsy during day, have trouble staying awake during the day, take naps), “sleep adequacy” (items: get enough sleep to feel rested upon waking in the morning, get amount of sleep needed), “snoring,” “sleep awakening short of breath or with headache,” and “sleep quantity.”15 Each item refers to the previous 4 weeks of assessment. Sleep quantity is the average sleep duration in hours per night with optimal sleep duration defined as 7 to 8 hours of sleep.42 For score of the other dimensions, a 0 to 100 scale is used, with higher scores for sleep disturbance, somnolence, snoring, and shortness of breath/headache and lower scores for adequacy reflecting worse sleep.42 It is also possible to calculate a sleep problems index summarizing information of 9 items of the MOS-Sleep. It has earlier been shown that the MOS Sleep Scale is a valid mean to investigate sleep and its numerous aspects.15 For our analyses, we adhered to the MOS Sleep scores of the single dimensions (sleep disturbance, somnolence, adequacy, and quantity), which is similar to the assessment of sleep disturbances during medical history retrieval. The single dimension sleep scores allow a more differentiated analysis of sleep disorders or sleep disturbances.
2.3. Statistical analysis
Statistical analysis was performed only for cases including complete data set on demographics, anamnesis, MOS Sleep questionnaire, PHQ-9, and PD-Q values, leading to a total number of 3339 patients (1293 male and 2046 female patients).
First, demographic and clinical data of the entire patient cohort were analyzed using descriptive analysis (arithmetic mean + SD). Differences in parameters concerning pain history (ongoing use of analgesics, pain duration, pension request, and hospitalisations), pain intensity (average and maximum pain assessed with the VAS), PD-Q score, depression score (assessed with PHQ-9), and MOS Sleep Score were calculated for the whole study cohort addressing the 3 different pain entities (PDNP, PHN, and CRPS). Unadjusted analyses of continuous variables for pain entities were realized using ANOVA including post hoc analyses for pairwise comparisons of pain entities. P-values were adjusted for multiple testing according to the Bonferroni test (factor 3 because of 3 pairwise comparisons). Categorical variables were analyzed by χ2 tests.
Second, the association of the somatosensory phenotype (PD-Q), especially of symptoms reflecting central sensitization, depression (PHQ-9), and pain intensity (VAS) with patterns of sleep (MOS Sleep Scale), was investigated first without accounting for confounders by use of an unadjusted Spearman rank–order correlation analysis, resulting in nonparametric correlation coefficients. Because the aim of the study was to analyze the relationship of the single sensory symptoms, indicative of different pain mechanisms, to distinct patterns of sleep, single PD-Q and MOS Sleep Scale dimensions were used instead of sum scores. Unadjusted correlation analysis was performed in the entire neuropathic patient cohort and afterwards in subgroups of diagnosis. P-values were adjusted for multiple testing according to the Bonferroni principle (factor 49 because of 49 pairwise tests).
For each sleep subscale (sleep disturbance, sleep adequacy, sleep somnolence, and sleep quantity), a multivariable regression analysis was applied to investigate the parallel relationship of various independent variables.
All the data collected (ie, all variables recorded, including pain etiology, demographic and clinical variables, pain intensity, PD-Q items, and PHQ-9 score) were defined as independent variables, without a preselection and independent of the results of the univariate analysis. (Adjusted) P-values were considered to be statistically significant below 0.05.
Statistical analysis was performed with SAS 9.4 (SAS Institute Inc, Cary, NC).
3. Results
3.1. Descriptive analysis of demographic and clinical data
In total, 3339 patients with chronic pain with neuropathic features (PDNP, n = 543; PHN, n = 1480; CRPS, n = 1316) were included in the analysis. Demographic and clinical data are shown in Table 1. There were significant differences in age, sex distribution, and pain duration between all investigated pain syndromes (P < 0.001). For example, patients with CRPS were significantly younger than patients with PDNP and PHN (50.3 ± 13.7 vs 62.6 ± 12.1 vs 65.8 ± 13.1 years). Patients with PDNP exhibited the longest pain duration compared with patients with CRPS and PHN (2.9 ± 2.0 vs 1.2 ± 1.7 vs 0.9 ± 1.6 years). In contrast, there were no significant differences in average and maximum pain intensity during the last 4 weeks between the investigated pain entities. In total, 72.9% of patients with PDNP, 84.4% of patients with PHN, and 75.5% of patients with CRPS were on pain medication.
Table 1.
Demographic and clinical data of patients with respect to the underlying pain syndrome.
| PDNP n = 543 |
PHN n = 1480 |
CRPS n = 1316 |
P | |
|---|---|---|---|---|
| Male, n (%) | 322 (59.3) | 596 (40.3) | 375 (28.5) | <0.001* |
| Age, mean ± SD (range) | 62.6 ± 12.1 (18–89) | 65.8 ± 13.1 (18–97) | 50.3 ± 13.7 (18–90) | <0.001* |
| BMI [kg/m2], mean ± SD (range) | 30.9 ± 6.5 (16–64) | 26.7 ± 4.7 (14–62) | 26.6 ± 5.2. (15–61) | <0.001 PDNP vs CRPS <0.001 PDNP vs PHN |
| Pain duration [y], mean ± SD (range) | 2.9 ± 2.0 (0–8) | 0.9 ± 1.6 (0–6) | 1.2 ± 1.7 (0–6) | <0.001* |
| ≤3 y, n (%) | 266 (49.0) | 1304 (88.1) | 1102 (83.7) | |
| >3 y, n (%) | 277 (51.0) | 176 (11.9) | 214 (16.3) | |
| Pain medication, yes, n (%) | 396 (72.9) | 1248 (84.3) | 993 (75.5) | <0.001 PDNP vs PHN <0.001 PHN vs CRPS |
| Pension request, yes, n (%) | 356 (65.6) | 931 (62.9) | 422 (32.1) | <0.001 PDNP vs CRPS <0.001 PHN vs CRPS |
| Hospitalisation, yes, n (%) | 215 (39.6) | 343 (23.2) | 516 (39.2) | <0.001 PDNP vs PHN <0.001 PHN vs CRPS |
| Average pain intensity, mean ± SD (range) | 6.1 ± 2.1 (0–10) | 6.0 ± 2.1 (0–10) | 5.9 ± 2.0 (0–10) | n.s. |
| Maximum pain intensity, mean ± SD (range) | 7.8 ± 2.0 (0–10) | 7.7 ± 2.0 (0–10) | 7.8 ± 2.0 (0–10) | n.s. |
| PD-Q sum, mean ± SD | 18.9 ± 7.8 | 17.6 ± 7.2 | 18.6 ± 7.7 | |
| Negative, n (%) | 127 (23.4) | 373 (25.2) | 309 (23.5) | |
| Unclear, n (%) | 128 (23.6) | 435 (29.4) | 306 (23.3) | |
| Positive, n (%) | 288 (53.0) | 672 (45.4) | 701 (53.3) | |
| PD-Q items, mean ± SD | ||||
| Burning (0–5) | 3.0 ± 1.7 | 3.1 ± 1.6 | 2.6 ± 1.7 | <0.001 PDNP vs CRPS <0.001 PHN vs CRPS |
| Prickling (0–5) | 3.1 ± 1.6 | 2.5 ± 1.7 | 2.5 ± 1.6 | <0.001 PDNP vs CRPS <0.001 PDNP vs PHN |
| Allodynia (0–5) | 2.2 ± 1.7 | 2.9 ± 1.6 | 2.5 ± 1.6 | 0.003 PDNP vs CRPS <0.001 PHN vs CRPS <0.001 PDNP vs PHN |
| Attacks (0–5) | 2.6 ± 1.8 | 2.5 ± 1.8 | 2.7 ± 1.7 | n.s. |
| Thermal (0–5) | 1.7 ± 1.6 | 1.8 ± 1.5 | 2.2 ± 1.7 | <0.001 PDNP vs CRPS <0.001 PHN vs CRPS |
| Numbness (0–5) | 2.9 ± 1.7 | 1.5 ± 1.6 | 2.2 ± 1.6 | <0.001* |
| Pressure (0–5) | 2.5 ± 1.6 | 2.8 ± 1.6 | 3.2 ± 1.4 | <0.001* |
| PHQ-9 score (0–27), mean ± SD | 10.3 ± 5.8 | 9.9 ± 5.6 | 10.1 ± 5.8 | n.s. |
| MOSS Scale mean ± SD | ||||
| Sleep disturbance (0–100) | 49.3 ± 26.7 | 46.2 ± 25.2 | 49.7 ± 25.9 | <0.001 PHN vs CRPS |
| Sleep adequacy (0–100) | 44.9 ± 29.4 | 48.7 ± 28.4 | 43.2 ± 28.7 | <0.001 PHN vs CRPS 0.028 PDNP vs PHN |
| Somnolence (0–100) | 48.9 ± 22.7 | 47.0 ± 21.8 | 41.3 ± 22.6 | <0.001 PDNP vs CRPS <0.001 PHN vs CRPS |
| Sleep quantity (0–12) | 5.8 ± 1.7 | 6.1 ± 1.7 | 6.9 ± 1.6 | 0.013 PDNP vs PHN |
Significant differences between all 3 disease entities.
BMI, body mass index; average and maximum pain of the last 4 weeks assessed with VAS, numeric rating scale; CRPS, complex regional pain syndrome; MOSS Scale, Medical Outcome Study Sleep Scale; n.s., not significant; PDNP, painful diabetic polyneuropathy; PD-Q, painDETECT questionnaire; PHN, postherpetic neuralgia; PHQ-9, Patient Health Questionnaire-9.
The analysis of the PD-Q sum score revealed the presence of neuropathic pain features in most of the patients, ie, only one quarter within each pain etiology scored below 12. Comparison of the single PD-Q items revealed some differences between the pain entities. For example, patients with PHN exhibited the highest score for allodynia compared with patients with PDNP and CRPS (2.9 ± 1.6 vs 2.2 ± 1.7 vs 2.5 ± 1.6; P < 0.001), whereas patients with CRPS showed the highest score for pressure pain hyperalgesia (3.2 ± 1.4 vs 2.5 ± 1.6 vs 2.8 ± 1.6; P < 0.001) and patients with PDNP the highest score for numbness (2.9 ± 1.7 vs 1.5 ± 1.6 vs 2.2 ± 1.6; P < 0.001) and prickling (3.1 ± 1.6 vs 2.5 ± 1.7 vs 2.5 ± 1.6; P < 0.001). Depression scores were similar between patients with PDNP, PHN, and CRPS, ie, indicating mild-to-moderate depression (mean PHQ-9 score: 10.3 ± 5.8 vs 9.9 ± 5.6, 10.1 ± 5.8, P = 0.307). The MOS Sleep Questionnaire was applied to detect differences in sleep profiles between the 3 different pain entities. Sleep impairment was observed in all pain entities regardless of the investigated sleep item (disturbance, adequacy, somnolence, and quantity), although with slight differences between the single patient groups. Sleep disturbance was significantly higher, and sleep adequacy was significantly lower in patients with CRPS compared with those with PHN (disturbance: 49.7 ± 25.9 vs 46.2 ± 25.2, P < 0.001; adequacy: 43.2 ± 28.7 vs 48.7 ± 28.4, P < 0.001). Sleep somnolence differed significantly between patients with CRPS and PDNP (41.3 ± 22.6 vs 48.9 ± 22.7, P < 0.001) and between patients with CRPS and PHN (47.0 ± 21.8, P < 0.001). Concerning sleep quantity, each pain etiology reached a mean of 6 to 7 hours of sleep per night.
3.2. Relationship between pain intensity, neuropathic pain symptoms, depression, and sleep impairment
The correlation analysis resulted in statistically significant pairwise correlations of all single PD-Q symptoms, average/maximum pain ratings (VAS), and PHQ-9 score with all patterns of sleep assessed with MOS Sleep Scale for the entire patient cohort (Fig. 2). The stronger the respective symptom, the more distinct was the sleep impairment (greater sleep disturbance and somnolence, less sleep adequacy and quantity). However, only the depression score showed a moderate correlation of r > 0.5 with sleep disturbance, adequacy, and somnolence and of r > 0.3 with sleep quantity and average/maximum pain ratings. Concerning the single PD-Q symptoms, correlation with patterns of sleep was low overall. Stratification according to the single pain syndromes (PDNP, PHN, and CRPS) led to similar results in each pain condition, ie, only the PHQ-9 score showed a moderate correlation with the sleep items. For some PD-Q symptoms, patients with PDNP and CRPS had a slightly stronger correlation with sleep items than patients with PHN (eg, allodynia showed a correlation of r > 0.3 with sleep disturbance in PDNP and CRPS vs r = 0.17 in PHN), nevertheless these were still lower than for the depression score (see Supplement Tables 1–3, available at http://links.lww.com/PR9/A205).
Figure 2.
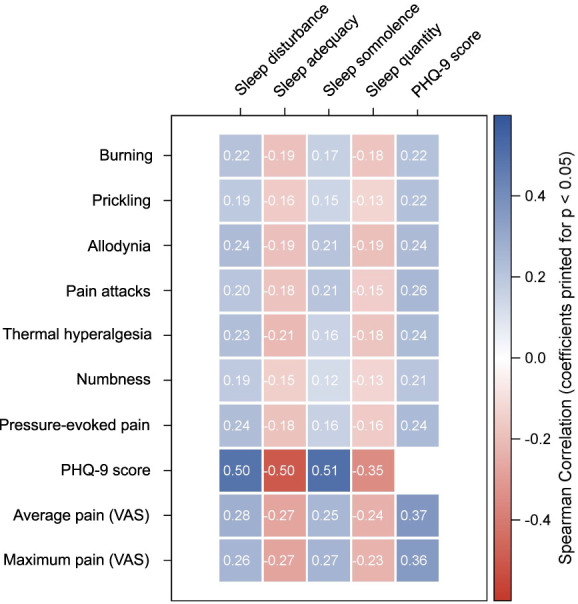
Heatmap of correlations of the somatosensory phenotype (PD-Q), depression (PHQ-9), and pain intensity (VAS) on patterns of sleep (MOS Sleep Scale) in patients with neuropathic pain symptoms. The correlation analysis resulted in statistically significant correlations of all single PD-Q symptoms, average/maximum pain ratings (VAS), and PHQ-9 score with all patterns of sleep (sleep disturbance, sleep adequacy, sleep somnolence, and sleep quantity) and PHQ-9 score for the entire patient cohort with neuropathic features (diabetic polyneuropathy, postherpetic neuralgia, complex regional pain syndrome). Only the depression score (PHQ-9) showed a correlation coefficient of >0.5. MOS, Medical Outcomes Study; PD-Q, painDETECT questionnaire; PHQ-9, Patient Health Questionnaire-9; VAS, visual analogue scale.
The multivariable analyses also revealed several significant associations of the investigated variables (demographic parameters, pain history, and 7 PD-Q symptoms) with the 4 sleep items of the MOS Sleep Scale (sleep disturbance, sleep adequacy, somnolence, and sleep quantity; Table 2). Comparing men and women revealed a 1.93 points higher sleep disturbance, a 2.46 points lower adequacy, and a 1.54 lower sleep somnolence for female patients with pain. As it has already been shown in the correlation analysis, depression assessed with the PHQ-9 showed the strongest significant relationship to all sleep items (P < 0.001). An increase of depression of 1 point resulted in a 1.85-point higher sleep disturbance, a 2.11-point lower sleep adequacy, a 1.76-point higher sleep somnolence, and a 4.2-minute shortened sleep duration per night. Concerning the PD-Q symptoms, allodynia was the only symptom with a significant association with all sleep parameters, ie, a 0.99-point higher sleep disturbance, a 0.98-point lower adequacy, a 0.88-point higher somnolence, and a 4.8-minute shortened sleep duration were found when allodynia was elevated by 1 point.
Table 2.
Multivariable analysis (estimates and P-values in brackets) for the entire patient cohort.
| Parameters of sleep | Sleep disturbance (R2 = 0.29) | Sleep adequacy (R2 = 0.29) | Sleep somnolence (R2 = 0.32) | Sleep quantity (R2 = 0.14) | DF |
|---|---|---|---|---|---|
| Diagnosis | |||||
| CRPS vs PHN | 1.33 (0.180) | −0.69 (0.531) | −3.45 (<0.001) | −0.12 (0.084) | 2 |
| PDNP vs PHN | 1.85 (0.150) | −1.14 (0.426) | 0.43 (0.695) | −0.13 (0.150) | 2 |
| CRPS vs PDNP | −0.52 (0.690) | 0.45 (0.759) | −3.88 (<0.001) | 0.01 (0.914) | 2 |
| Demographic/clinical data | |||||
| Sex (female vs male) | 1.93 (0.017) | −2.46 (0.006) | −1.54 (0.024) | −0.01 (0.867) | 1 |
| Age 10-y estimate | −0.78 (0.027) | 2.78 (<0.001) | 1.29 (<0.001) | −0.02 (0.389) | 1 |
| BMI 5-points estimate | −0.28 (0.439) | −0.53 (0.193) | 1.67 (<0.001) | −0.06 (0.023) | 1 |
| Pain medication (no vs yes) | −0.21 (0.828) | 1.69 (0.118) | −2.48 (0.003) | 0.05 (0.468) | 1 |
| Pain duration (≤3 vs > 3 y) | −2.04 (0.050) | 4.26 (<0.001) | 0.22 (0.804) | 0.24 (0.002) | 1 |
| Pension request (yes vs no) | −0.69 (0.478) | 1.97 (0.069) | 1.87 (0.024) | 0.16 (0.021) | 1 |
| Hospitalisation (no vs yes) | −0.11 (0.901) | 0.08 (0.932) | −1.85 (0.013) | −0.01 (0.911) | 1 |
| PD-Q symptoms | |||||
| Burning | 0.85 (0.003) | −0.94 (0.003) | −0.34 (0.157) | −0.05 (0.019) | 1 |
| Prickling | −0.13 (0.649) | 0.30 (0.356) | −0.33 (0.177) | 0.03 (0.124) | 1 |
| Allodynia | 0.99 (0.001) | −0.98 (0.004) | 0.88 (<0.001) | −0.08 (<0.001) | 1 |
| Pain attacks | 0.14 (0.569) | −0.03 (0.928) | 0.77 (<0.001) | −0.01 (0.771) | 1 |
| Thermal hyperalgesia | 0.66 (0.019) | −0.76 (0.015) | 0.23 (0.338) | −0.06 (0.004) | 1 |
| Numbness | 0.59 (0.023) | −0.03 (0.912) | 0.11 (0.630) | −0.02 (0.376) | 1 |
| Pressure-evoked pain | 0.52 (0.097) | 0.60 (0.085) | −0.12 (0.658) | 0.03 (0.193) | 1 |
| VAS | |||||
| Average pain | 1.01 (<0.001) | −1.08 (<0.001) | −0.27 (0.267) | −0.07 (<0.001) | 1 |
| Maximum pain | 0.10 (0.740) | −0.18 (0.581) | 1.06 (<0.001) | −0.02 (0.346) | 1 |
| PHQ-9 | |||||
| Depression | 1.85 (<0.001) | −2.11 (<0.001) | 1.76 (<0.001) | −0.07 (<0.001) | 1 |
P-values in brackets. Significant values are shown as bold entries.
Parameters of sleep were assessed with Medical Outcome Study (MOS) Sleep Scale.
BMI, body mass index; average and maximum pain of the last 4 weeks assessed with VAS, visual analogue scale; CRPS, complex regional pain syndrome; PDNP, painful diabetic polyneuropathy; PD-Q, painDETECT questionnaire; PHN, postherpetic neuralgia; PHQ-9, Patient Health Questionnaire-9.
“Thermal hyperalgesia” and burning were significantly associated with sleep disturbance, adequacy, and quantity but not with sleep somnolence. In contrast, pressure-evoked pain and prickling showed no significant effect on any sleep parameter.
4. Discussion
The present study evaluated self-reported sensory symptoms and sleep quality, especially focusing on whether markers of central sensitization were related with sleep disturbances in patients with neuropathic pain. For this purpose, sensory abnormalities, the sleep profile, and depression score of patients with chronic pain syndromes with neuropathic pain features (PDNP, PHN and CRPS) were assessed through PROMs.
There was a significant correlation of the intensity of neuropathic pain symptoms (assessed by the PD-Q: burning, prickling, dynamic mechanical allodynia, pain attacks, “thermal hyperalgesia,” numbness, and pressure-evoked pain) with troubles in sleep behaviour (assessed by the MOS Sleep Scale: sleep quantity, sleep adequacy, sleep disturbance, and somnolence). This is in line with previous studies reporting greater sleep impairment for patients with neuropathic features compared with those without neuropathic characteristics.4,25 A study of Yamada et al.48 even suggested a reciprocal effect: Sleep shortage was associated with a higher risk to develop a neuropathic pain syndrome (postherpetic neuralgia) mediated by hyperesthesia and severe acute pain (although correlation coefficients of neuropathic symptoms and sleep patterns were low overall).
Our data suggest that the different mechanisms of pain processing, ie, deep sensitivity (pressure-evoked pain) and pain because of stimuli that do not normally provoke pain (allodynia),31,43 affect sleep parameters distinctly. For instance, the multivariable analysis revealed allodynia, a physiologically absent sensory sign30 shown to be indicative of central sensitization,5,8,46 to be the sole symptom in the entire patient cohort to have a significant association to all 4 sleep items of the MOS Sleep Scale. Pressure-evoked pain, however, another possible sign for central sensitization (mechanical deep somatic hyperalgesia), had no significant association for any sleep item. So, while we can report a possible involvement of central sensitization processes in sleep impairment, this seems to be especially valid concerning the hypersensitivity of the skin as mirrored by the aforementioned profound association of allodynia with all MOS Sleep items.
“Burning” and “thermal hyperalgesia” were further signs associated with higher sleep disturbance, lower sleep adequacy, and lower sleep quantity. This is coherent with frequent reports on burning pain, typically described to be worse at night and sleep-impairing in patients with polyneuropathy (“burning feet syndrome”).5 A sharp burning pain is also described by patients with postherpetic neuralgia, both in the primary affected area and in the surrounding areas when stroking the skin with a nonpainful, light stimulus (eg, a cotton swab).5 These observations suggest a close interaction of allodynia and evoked burning pain, possibly explaining the present association of burning pain with sleep impairment. Sleep deprivation has been shown to decrease pain thresholds.23 More specifically, a significant decrease in heat pain thresholds and a tendency for a decrease in cold pain thresholds was reported without an alteration for warmth and cold detection thresholds.24 Again, this is in line with our findings concerning the correlation of “thermal hyperalgesia” with lower sleep quality.
Unsurprisingly, the strongest association with sleep quality in all analyses was detected for the item “depression” assessed with the PHQ-9. On the one hand, there is an intrinsic correlation with the MOS Sleep items (PHQ-9 item 3 asks about trouble falling asleep, staying asleep or sleeping to much), which is why this significance was to be expected. But on the other hand, we must acknowledge “sleep disturbance” as a characteristic clinical symptom within the DSM-V criteria of depressive disorders.1 According to the biopsychosocial approach to pain, there is a multidimensional, dynamic integration between psychological, physiological, and social factors that mutually influence each other.32 As a matter of fact, the bidirectional influence of pain and mental illness is suggested to be of similar magnitude.9 As for neuropathic pain in specific, animal models found a connection between neuropathic pain and mood disorders47 and a high prevalence of depression and sleep comorbidities for patients with diabetic polyneuropathy is reported.34 Consequently, the association of depression and chronic pain to sleep cannot be examined independently from each other. Yet, our results did show that both depression and allodynia have an independent, significant relationship to sleep in each multivariable model.
The connection between allodynia and depression has been well-investigated in patients with migraine. Here, allodynia was associated with higher prevalence of depression,28 consistent with repots about anxious and depressive symptoms in patients with migraine with allodynia.33
Our findings suggest that mechanisms of both peripheral and central sensitization, shown here through self-reported surrogate markers, have a negative impact on sleep quality. Because of the strongest effect for “allodynia” (central sensitization) and “thermal hyperalgesia”—which includes cold hyperalgesia as a surrogate of both peripheral and central sensitization18,45—we presume a stronger role of central sensitization may be possible.
4.1. Limitations
Unfortunately, an influence of patient (pain and sleep) medication on sleep quality and depression cannot be fully excluded as the noninterventional painDETECT database of actual patient care did not capture the exact drugs the patients were using, which is an important limitation of the study. The observed significant pairwise correlation of all pain parameters with patterns of sleep and depression might partly be because of the high number of cases. However, Spearman rank–order correlation analysis according to single neuropathic pain entities, which are much smaller in number, revealed similar significant correlations (Supplement Tables 1–3, available at http://links.lww.com/PR9/A205).
Furthermore, although signs of central sensitization as assessed with QST were shown to be more likely in patients with higher PD-Q scores,16 the PD-Q alone cannot entirely provide the necessary findings to prove central sensitization. Another limitation is that the question whether the pathophysiological process of central sensitization has an impact on sleep quality or whether sleep deprivation induces signs of central sensitization cannot be answered. Hence, to investigate the influence of central sensitization on sleep in patients with neuropathic pain, further studies including questionnaires specifically validated to assess symptoms of central sensitization (eg, the CSI35), thorough sensory phenotyping through sensory testing or specific electrophysiological paradigms,17 are needed. In a study by Schuh-Hofer et al.,37 a single night of total sleep deprivation induced signs of generalized hyperalgesia for thermal (heat and cold) and mechanical stimuli. As proposed by the authors, this could be a model for further examinations on the mutual influence of pain and sleep.
5. Conclusion
According to our results, the self-reported surrogate markers “allodynia” and partly “thermal hyperalgesia” hint to a possible impact of central sensitization on the sleep disruption of patients with neuropathic pain. Although no conclusion can be drawn concerning the direction of the effects, our findings also support the current state of knowledge suggesting a triangle of components interplaying (ie, allodynia, depression, and sleep disruption) with each other. Further studies with an extensive diagnostic approach are necessary to clarify the mechanisms, causal directions, and particularly the extent of this mutual influence. The results of additional investigations might also have an impact on pharmacotherapeutic choices.
Disclosures
J.S. reports travel support from Alnylam Pharmaceuticals Inc. and Pfizer, consultant fees from Pfizer Pharma GmbH and speaker fees from Grünenthal GmbH and Alnylam Germany GmbH outside the submitted work. D.K. reports a grant from Grünenthal GmbH, a personal fee for a podcast episode, and nonfinancial support from Grünenthal GmbH outside the submitted work. P.H. reports grants from Zambon and German Federal Ministry of Education and Research (BMBF): Verbundprojekt: Frühdetektion von Schmerzchronifizierung (NoChro) (13GW0338C). D.A. and M.K. are employers of StatConsult and receive remunerations. T.K. worked as a statistical consultant for StatConsult GmbH and received remunerations. R.F. reports speaker and consultant fees from AOP Orphan, Augustine Therapeutics, Grünenthal, Hikma, Lilly, Merck, Mitsubishi Tanabe, P&G, Pfizer, Roche, and Scilex outside the submitted work. A.B. reports honoraria from Astellas, Allergan, Bayer, Boehringer-Ingelheim, Grünenthal, and Pfizer, and he participated in advisory boards of Astellas, Boehringer-Ingelheim, Genzyme, and Grünenthal. R.B. reports grants/research support from EU Projects: “Europain” (115007), DOLORisk (633491), and IMI Paincare (777500). German Federal Ministry of Education and Research (BMBF): Verbundprojekt: Frühdetektion von Schmerzchronifizierung (NoChro) (13GW0338C). German Research Network on Neuropathic Pain (01EM0903). Pfizer Pharma GmbH, Genzyme GmbH, Grünenthal GmbH, Mundipharma Research GmbH und Co. KG., Novartis Pharma GmbH, Alnylam Pharmaceuticals Inc, Zambon GmbH and Sanofi-Aventis Deutschland GmbH, speaker fees from Pfizer Pharma GmbH, Genzyme GmbH, Grünenthal GmbH, Mundipharma, Sanofi Pasteur, Medtronic Inc Neuromodulation, Eisai Co. Ltd., Lilly GmbH, Boehringer Ingelheim Pharma GmbH & Co. KG, Astellas Pharma GmbH, Desitin Arzneimittel GmbH, Teva GmbH, Bayer-Schering, MSD GmbH, Seqirus Australia Pty. Ltd, Novartis Pharma GmbH, TAD Pharma GmbH, Grünenthal SA Portugal, Sanofi-Aventis Deutschland GmbH, Agentur Brigitte Süss, Grünenthal Pharma AG Schweiz, Grünenthal B.V. Niederlande, Evapharma, Takeda Pharmaceuticals International AG Schweiz, Ology Medical Education Netherlands, Ever Pharma GmbH, Amicus Therapeutics GmbH, Novo Nordisk Pharma GmbH, Chiesi GmbH, Stada Mena DWC LLC Dubai, and Helxal AG and consultant fees from Pfizer Pharma GmbH, Genzyme GmbH, Grünenthal GmbH, Mundipharma Research GmbH und Co. KG, Allergan, Sanofi Pasteur, Medtronic, Eisai, Lilly GmbH, Boehringer Ingelheim Pharma GmbH&Co.KG, Astellas Pharma GmbH, Novartis Pharma GmbH, Bristol-Myers Squibb, Biogenidec, AstraZeneca GmbH, Merck, Abbvie, Daiichi Sankyo, Glenmark Pharmaceuticals S.A., Seqirus Australia Pty. Ltd, Teva Pharmaceuticals Europe Niederlande, Teva GmbH, Genentech, Mundipharma International Ltd. UK, Astellas Pharma Ltd. UK, Galapagos NV, Kyowa Kirin GmbH, Vertex Pharmaceuticals Inc, Biotest AG, Celgene GmbH, Desitin Arzneimittel GmbH, Regeneron Pharmaceuticals Inc USA, Theranexus DSV CEA Frankreich, Abbott Products Operations AG Schweiz, Bayer AG, Grünenthal Pharma AG Schweiz, Mundipharma Research Ltd. UK, Akcea Therapeutics Germany GmbH, Asahi Kasei Pharma Corporation, AbbVie Deutschland GmbH & Co. KG, Air Liquide Sante International Frankreich, Alnylam Germany GmbH, Lateral Pharma Pty Ltd, Hexal AG, Angelini, Janssen, SIMR Biotech Pty Ltd Australien, Confo Therapeutics N. V. Belgium, Merz Pharmaceuticals GmbH, Neumentum Inc, F. Hoffmann-La Roche Ltd. Switzerland, AlgoTherapeutix SAS France, Nanobiotix SA France, and AmaceaThera Inc Canada outside the submitted work. T.R.T. reports no conflicts of interest.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A205.
Supplementary Material
Acknowledgements
Supported by PFIZER Pharma GmbH.
Data availability: The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
J. Sachau and D. Kersebaum contributed equally and share first authorship.
Contributor Information
Juliane Sachau, Email: juliane.Sachau@uksh.de.
Philipp Hüllemann, Email: p.huellemann@neurologie.uni-kiel.de.
Daniela Adolf, Email: daniela.adolf@statconsult.de.
Maria Kabelitz, Email: maria.kabelitz@statconsult.de.
Thomas Keller, Email: t.keller.statconsult@acomed.de.
Rainer Freynhagen, Email: R.Freynhagen@Krankenhaus-tutzing.de.
Thomas R. Tölle, Email: thomas.toelle@tum.de.
Andreas Binder, Email: abinder@klinikum-saarbruecken.de.
Ralf Baron, Email: r.baron@neurologie.uni-kiel.de.
References
- [1].American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5). Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
- [2].Anastassiou E, Iatrou CA, Vlaikidis N, Vafiadou M, Stamatiou G, Plesia E, Lyras L, Vadalouca A; ATLAS Investigators. Impact of pregabalin treatment on pain, pain-related sleep interference and general well-being in patients with neuropathic pain: a non-interventional, multicentre, post-marketing study. Clin Drug Investig 2011;31:417–26. [DOI] [PubMed] [Google Scholar]
- [3].Arning K, Baron R. Evaluation of symptom heterogeneity in neuropathic pain using assessments of sensory functions. Neurotherapeutics 2009;6:738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D. The specific disease burden of neuropathic pain: results of a French nationwide survey. PAIN 2011;152:2836–43. [DOI] [PubMed] [Google Scholar]
- [5].Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807–19. [DOI] [PubMed] [Google Scholar]
- [6].Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpää M, Hansson P, Hüllemann P, Jensen TS, Freynhagen R, Kennedy JD, Magerl W, Mainka T, Reimer M, Rice ASC, Segerdahl M, Serra J, Sindrup S, Sommer C, Tölle T, Vollert J, Treede R-D. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. PAIN 2017;158:261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baron R, Tölle TR, Gockel U, Brosz M, Freynhagen R. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: differences in demographic data and sensory symptoms. PAIN 2009;146:34–40. [DOI] [PubMed] [Google Scholar]
- [8].den Boer C, Dries L, Terluin B, van der Wouden JC, Blankenstein AH, van Wilgen CP, Lucassen P, van der Horst HE. Central sensitization in chronic pain and medically unexplained symptom research: a systematic review of definitions, operationalizations and measurement instruments. J Psychosom Res 2019;117:32–40. [DOI] [PubMed] [Google Scholar]
- [9].Bondesson E, Larrosa Pardo F, Stigmar K, Ringqvist Å, Petersson IF, Jöud A, Schelin MEC. Comorbidity between pain and mental illness—evidence of a bidirectional relationship. Eur J Pain 2018;22:1304–11. [DOI] [PubMed] [Google Scholar]
- [10].Drolet M, Brisson M, Schmader KE, Levin MJ, Johnson R, Oxman MN, Patrick D, Blanchette C, Mansi JA. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ 2010;182:1731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fernández-de-Las-Peñas C, Valera-Calero JA, Herrero-Montes M, Del-Valle-Loarte P, Rodríguez-Rosado R, Ferrer-Pargada D, Arendt-Nielsen L, Parás-Bravo P. The self-reported Leeds assessment of neuropathic symptoms and signs (S-LANSS) and PainDETECT questionnaires in COVID-19 survivors with post-COVID pain. Viruses 2022;14:1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–20. [DOI] [PubMed] [Google Scholar]
- [13].Gierthmühlen J, Binder A, Baron R. Mechanism-based treatment in complex regional pain syndromes. Nat Rev Neurol 2014;10:518–28. [DOI] [PubMed] [Google Scholar]
- [14].Gierthmühlen J, Binder A, Förster M, Baron R. Do we measure what patients feel?: an analysis of correspondence between somatosensory modalities upon quantitative sensory testing and self-reported pain experience. Clin J Pain 2018;34:610–7. [DOI] [PubMed] [Google Scholar]
- [15].Hays RD, Martin SA, Sesti AM, Spritzer KL. Psychometric properties of the medical outcomes study sleep measure. Sleep Med 2005;6:41–4. [DOI] [PubMed] [Google Scholar]
- [16].Hochman JR, Davis AM, Elkayam J, Gagliese L, Hawker GA. Neuropathic pain symptoms on the modified painDETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthritis Cartilage 2013;21:1236–42. [DOI] [PubMed] [Google Scholar]
- [17].Hüllemann P, von der Brelie C, Manthey G, Düsterhöft J, Helmers AK, Synowitz M, Baron R. Reduced laser-evoked potential habituation detects abnormal central pain processing in painful radiculopathy patients. Eur J Pain 2017;21:918–26. [DOI] [PubMed] [Google Scholar]
- [18].Jørum E, Warncke T, Stubhaug A. Cold allodynia and hyperalgesia in neuropathic pain: the effect of N-methyl-D-aspartate (NMDA) receptor antagonist ketamine—a double-blind, cross-over comparison with alfentanil and placebo. PAIN 2003;101:229–35. [DOI] [PubMed] [Google Scholar]
- [19].Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 2001;413:203–10. [DOI] [PubMed] [Google Scholar]
- [20].Kioskli K, Scott W, Winkley K, Kylakos S, McCracken LM. Psychosocial factors in painful diabetic neuropathy: a systematic review of treatment trials and survey studies. Pain Med 2019;20:1756–73. [DOI] [PubMed] [Google Scholar]
- [21].Koffel E, Kats AM, Kroenke K, Bair MJ, Gravely A, DeRonne B, Donaldson MT, Goldsmith ES, Noorbaloochi S, Krebs EE. Sleep disturbance predicts less improvement in pain outcomes: secondary analysis of the SPACE randomized clinical trial. Pain Med 2020;21:1162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kundermann B, Krieg J-C, Schreiber W, Lautenbacher S. The effect of sleep deprivation on pain. Pain Res Manag 2004;9:25–32. [DOI] [PubMed] [Google Scholar]
- [24].Kundermann B, Spernal J, Huber MT, Krieg J-C, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med 2004;66:932–7. [DOI] [PubMed] [Google Scholar]
- [25].Langley PC, Van Litsenburg C, Cappelleri JC, Carroll D. The burden associated with neuropathic pain in Western Europe. J Med Econ 2013;16:85–95. [DOI] [PubMed] [Google Scholar]
- [26].Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee J, Lim YH, Hong SJ, Jeong JH, Choi HR, Park SK, Kim JE, Park EH, Kim JH. Multicenter survey of symptoms, work life, economic status, and quality of life of complex regional pain syndrome patients. Korean J Pain 2021;34:288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Louter MA, Wardenaar KJ, Veen G, van Oosterhout WPJ, Zitman FG, Ferrari MD, Terwindt GM. Allodynia is associated with a higher prevalence of depression in migraine patients. Cephalalgia 2014;34:1187–92. [DOI] [PubMed] [Google Scholar]
- [29].Lovati C, D'Amico D, Bertora P, Raimondi E, Rosa S, Zardoni M, Bussone G, Mariani C. Correlation between presence of allodynia and sleep quality in migraineurs. Neurol Sci 2010;31(suppl 1):S155–8. [DOI] [PubMed] [Google Scholar]
- [30].Maier C, Baron R, Tölle TR, Binder A, Birbaumer N, Birklein F, Gierthmühlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihöfner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uçeyler N, Valet M, Wasner G, Treede R-D. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. PAIN 2010;150:439–50. [DOI] [PubMed] [Google Scholar]
- [31].McMahon SB, Wall PD. Changes in spinal cord reflexes after cross-anastomosis of cutaneous and muscle nerves in the adult rat. Nature 1989;342:272–4. [DOI] [PubMed] [Google Scholar]
- [32].Meints SM, Edwards RR. Evaluating psychosocial contributions to chronic pain outcomes. Prog Neuropsychopharmacol Biol Psychiatry 2018;87:168–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mendonça MD, Caetano A, Viana-Baptista M; CHLO Headache Study Group. Association of depressive symptoms with allodynia in patients with migraine: a cross-sectional study. Cephalalgia 2016;36:1077–81. [DOI] [PubMed] [Google Scholar]
- [34].Naranjo C, Del Reguero L, Moratalla G, Hercberg M, Valenzuela M, Failde I. Anxiety, depression and sleep disorders in patients with diabetic neuropathic pain: a systematic review. Expert Rev Neurother 2019;19:1201–9. [DOI] [PubMed] [Google Scholar]
- [35].Neblett R, Cohen H, Choi Y, Hartzell MM, Williams M, Mayer TG, Gatchel RJ. The Central Sensitization Inventory (CSI): establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J Pain 2013;14:438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Roth T, van Seventer R, Murphy TK. The effect of pregabalin on pain-related sleep interference in diabetic peripheral neuropathy or postherpetic neuralgia: a review of nine clinical trials. Curr Med Res Opin 2010;26:2411–9. [DOI] [PubMed] [Google Scholar]
- [37].Schuh-Hofer S, Wodarski R, Pfau DB, Caspani O, Magerl W, Kennedy JD, Treede R-D. One night of total sleep deprivation promotes a state of generalized hyperalgesia: a surrogate pain model to study the relationship of insomnia and pain. PAIN 2013;154:1613–21. [DOI] [PubMed] [Google Scholar]
- [38].van Seventer R, Serpell M, Bach FW, Morlion B, Zlateva G, Bushmakin AG, Cappelleri JC, Nimour M. Relationships between changes in pain severity and other patient-reported outcomes: an analysis in patients with posttraumatic peripheral neuropathic pain. Health Qual Life Outcomes 2011;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev 2004;8:119–32. [DOI] [PubMed] [Google Scholar]
- [40].Tang NKY. Insomnia Co-occurring with chronic pain: clinical features, interaction, assessments and possible interventions. Rev Pain 2008;2:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Úbeda-D’Ocasar E, Valera-Calero JA, Gallego-Sendarrubias GM, Fernández-de-Las-Peñas C, Arias-Buría JL, Morales-Cabezas M, Arendt-Nielsen L, Cigarán-Méndez M. Association of neuropathic pain symptoms with sensitization related symptomatology in women with fibromyalgia. Biomedicines 2022;10:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Viala-Danten M, Martin S, Guillemin I, Hays RD. Evaluation of the reliability and validity of the Medical Outcomes Study sleep scale in patients with painful diabetic peripheral neuropathy during an international clinical trial. Health Qual Life Outcomes 2008;6:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wall PD, Woolf CJ. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol (Lond) 1984;356:443–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang X, Inui K, Qiu Y, Hoshiyama M, Tran TD, Kakigi R. Effects of sleep on pain-related somatosensory evoked potentials in humans. Neurosci Res 2003;45:53–7. [DOI] [PubMed] [Google Scholar]
- [45].Wasner G, Schattschneider J, Binder A, Baron R. Topical menthol—a human model for cold pain by activation and sensitization of C nociceptors. Brain 2004;127:1159–71. [DOI] [PubMed] [Google Scholar]
- [46].Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. PAIN 2011;152:S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yalcin I, Bohren Y, Waltisperger E, Sage-Ciocca D, Yin JC, Freund-Mercier M-J, Barrot M. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol Psychiatry 2011;70:946–53. [DOI] [PubMed] [Google Scholar]
- [48].Yamada K, Kubota Y, Shimizu Y, Cui R, Mori Y, Okuno Y, Asada H, Yamanishi K, Iso H; SHEZ Study Group. Sleep shortage is associated with postherpetic neuralgia development through hyperesthesia and acute pain intensity: a community-based prospective cohort study. Pain Pract 2019;19:476–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A205.


