Abstract
Background:
Ashwagandha herb is commonly used in Ayurveda and a “fad” dietary supplement for a host of indications based on low levels of evidence. Recently, ashwagandha was implicated in multiple reports of herb-induced liver injury (HILI), mainly from the United States. We present the first, and currently largest, series of ashwagandha-HILI from multiple centers in India.
Methods:
We retrospectively analyzed the respective institutional electronic medical records for ashwagandha-HILI. Patients consuming ashwagandha as part of multiherbal formulations or along with other known hepatotoxic supplements or medicines were excluded. All patients underwent a detailed diagnostic workup to exclude competing causes reasonably. Where possible, the implicated herbal formulation was retrieved and subjected to chemical analysis.
Results:
Out of 23 patients with liver injury from ashwagandha (January 2019 to December 2022), we report 8 patients with single-ingredient formulation-related HILI. Study cohort was male predominant, and cholestatic hepatitis was the commonest presentation. Five patients had underlying chronic liver disease; 3 presented with acute-on-chronic liver failure, and all 3 died on follow-up. In others, the liver injury was prolonged, nonetheless self-limiting. Liver biopsy revealed cholestatic features predominantly with hepatocellular necrosis and lymphocyte/eosinophil predominant portal-based inflammation. One patient progressed to chronic HILI. Chemical analysis revealed only natural phytochemicals without adulteration or contamination.
Conclusions:
Ashwagandha-HILI presents with cholestatic hepatitis and can lead to the syndrome of acute-on-chronic liver failure with high mortality in those with pre-existing liver disease. Educating the public on avoiding the use of potentially toxic and unrecommended herbal supplements can help mitigate the avoidable liver disease burden in the community.
INTRODUCTION
Herbal and dietary supplements (HDS) are considered food and nutritional formulations that include vitamins and minerals, whole herbs or botanical parts, amino acids, organ tissues, enzymes, and metabolites that are intended to promote health and well-being or adjunctively restore wellness associated with disease conditions.1 The HDS is not considered drugs/medications, and the responsibility for the safety of such products lies with the respective manufacturers, while good manufacturing practices approval from concerned health regulatory bodies is required before marketing. The good manufacturing practice establishes minimum standards for raw source utilization and procurement, storage, manufacturing, packaging, and labeling of HDS products to ensure purity, quality, and composition. Manufacturers of HDS formulations are not required to conduct preclinical safety and efficacy studies before product marketing.2 Hence the adverse events related to the use of such products are usually identified on postmarketing surveillance from responsible public health regulatory bodies, specific adverse-events recognition action groups or reported by physicians and patients themselves. Herbal drugs and dietary supplements are a major growing concern for liver injury, liver failure, and death or liver transplantation globally.3–6 Ayurvedic herbal supplements have been implicated in serious liver injury, acute decompensation of cirrhosis, and acute-on-chronic liver failure.7–9 Withania somnifera, popularly known as ashwagandha (also Indian Ginseng, Winter Cherry), is a commonly used and marketed herb as part of Ayurvedic herbals and dietary supplements for relieving stress and anxiety, improving sleep, increasing muscle mass and strength, improving sexual function in both males and females, increasing memory and as “immune-booster.”10 Nonetheless, conclusive evidence from rigorous, methodologically sound, well-designed, validated, and replicated trials does not exist for the aforementioned indications.11–13 The increasing use of ashwagandha for advertised but nonevidence-based indications has led to an increase in published reports associated with its liver toxicity. After the first report on ashwagandha-induced cholestatic liver injury from Japan was published, the United States—(DILI) Network (US-DILIN) and colleagues from Iceland thereafter published a series of 5 patients with ashwagandha hepatoxicity. Multiple reports have been published on ashwagandha-related liver injury, mostly from centers in the United States, along with large numbers of self-reported adverse events, such as severe itching, based on analysis of commercial websites.14 The estimated production of ashwagandha roots in India is >1500 tones and the annual requirement is about 7000 tones. As per the Charan Singh National Institute of Agricultural Marketing set up by the Government of India, the average annual Indian domestic consumption of ashwagandha within 5 large states was 39,074.592 kg.15 There are no reports of ashwagandha-related liver toxicity published from the Asian continent. We present 8 cases of severe liver injury due to the ashwagandha herb from 3 tertiary centers in India.
METHODS
Institutional electronic medical records of 3 hospitals were queried to identify 8 cases of ashwagandha-related liver injury from between January 2019 and December 2022. Patients consuming ashwagandha-only formulations were included. Those consuming ashwagandha as part of multiherbal products or ashwagandha along with other potentially toxic herbal supplements or prescription drugs were excluded from this analysis. We also excluded patients with active alcohol use and those with other competing causes of acute liver injury. A detailed diagram of the inclusion methodology of patients is shown in Supplemental Figure S1, http://links.lww.com/HC9/A532. All patients underwent a detailed diagnostic workup to exclude other well-known causes for liver injury. The evaluation included laboratory investigations, viral serologies for acute and chronic hepatotropic viruses, including HEV infection, and others such as Herpes simplex and cytomegalovirus, Ebstein-Barr virus, Dengue virus, the novel coronavirus, and other pertinent nonviral infectious agents such as malaria parasite, Rickettsia, and Leptospira. Biomarkers for autoimmune liver disease and diagnostic cross-sectional imaging were performed in all patients. In all cases, the liver injury was classified into hepatocellular, cholestatic, or mixed type based on the R-ratio, using initial (at the time of presentation to respective centers) values of alanine transaminase and alkaline phosphatase, their respective upper limits of normal. The R-ratio was calculated as alanine transaminase/upper limits of normal÷ alkaline phosphatase/upper limits of normal. When the R-ratio was <2, the liver injury was defined as cholestatic, between 2 and 5 as mixed, and >5 as hepatocellular. Thereafter, the causality assessment was performed to diagnose highly probable (score >8), probable (6-8), possible (3-5), and unlikely(1-2 or less) DILI using the 7-point Roussel Uclaf Causality Assessment Method (RUCAM).14 Percutaneous or transjugular liver biopsy was performed in all patients after informed consent. Patients were categorized into mild, moderate, or severe DILI based on bilirubin levels and liver failure. Mild DILI was defined when the liver test elevations reached criteria for DILI, but bilirubin concentration was <2.5 mg/dL, moderate when bilirubin ≥2.5 mg/dL or symptomatic hepatitis, severe when the bilirubin level was ≥2.5 mg/dL with signs of liver failure (international normalized ratio ≥1.5, presence of ascites or encephalopathy) or another organ failure directly attributed to DILI and finally, fatal, with death or liver transplantation due to DILI within 6 months of onset.16 Where possible, the implicated ashwagandha formulations were retrieved for further chemical and toxicology analysis. Heavy metal concentration was determined by an inductively coupled plasma atomic emission spectrometer (Thermo Electron, IRIS Intrepid II XSP Duo, Munich, Germany). The methodology, chemical standards, reagents, and vials were acquired per standards set by the US Environmental Protection Agency (USEPA), method 5021A, 8015, 8021, and 8260. Complete qualitative analyses were performed using triple-quadruple gas chromatography coupled to tandem mass spectrometry method (Varian Saturn 2200; Agilent Technologies, USA). The study was performed conforming to the Helsinki Declaration of 1975, as revised in 2000 and 2008, concerning human rights, and the study design and retrospective collection of patient data were approved by the respective institutional ethics and review boards. Owing to the retrospective pooled analysis but not the individualistic scrutiny of existing clinical data, the respective institutional review boards waived the requirement to obtain informed patient consent.
PATIENTS
General features and clinical details
Out of 23 patients with liver injury from herbal supplements containing ashwagandha, we included 8 patients with liver injury due to pure ashwagandha formulations. The other 15 patients were excluded because they had consumed concomitant potentially toxic herbal formulations or were on ashwagandha-based multiherbal preparations, or had other competing causes for liver injury (Supplemental Figure S1, http://links.lww.com/HC9/A532). Of the 8 patients included in the study, 6 were males and 2 were females. The median age was 49 (range 44, minimum 31 to maximum 75) years. Three patients had diabetes or hypertension and had been on medications for the same for decades without any significant prescription drug modifications pertinent to current events. Three patients were known to have pre-existing chronic liver disease, while 2 patients were diagnosed to have underlying chronic liver disease during the evaluation of herb-induced liver injury (HILI). Among the latter 2, one was diagnosed with HILI on the background of NASH on liver biopsy, while the other patient had imaging features of cirrhosis and portal hypertension during the evaluation of HILI. One patient had a history of non-Hodgkin lymphoma in remission for 4 years. Three patients presented with acute-on-chronic liver failure (ACLF) at the outset. The median duration of herb intake was 45 (interquartile range 53.5, minimum 14 to maximum 540) days, and the median time to symptom onset was 41 (interquartile range 59.5, minimum 14 to maximum 540) days. The dose of ashwagandha ranged from 10 to 15 g/d to 500 mg per day based on the type of formulation or as labeled on the product. The most common presenting symptom was jaundice (N=7/8, 87.5%), followed by pruritus (N=5/8, 62.5%). The median follow-up from diagnosis of HILI to outcome was 295 (interquartile range 164, minimum 30 to maximum 486) days. The indications for ashwagandha use were insomnia and antistress in 2, postpregnancy detox in 1, appetite stimulant in 1, anticancer prevention (who was already in long-term remission from non-Hodgkin lymphoma) in 1, fever reduction in 1, and as a “wellness and energy booster” in 2 patients. The various types of ashwagandha formulations consumed by patients included homemade powdered roots, herbal jam preparation (called lehyam), company-manufactured branded ashwagandha root formulations as powders, syrups, and tablets, nonbranded Ayurveda practitioner-prepared tablets, and herbal detox tea. All patients consumed ashwagandha per the practitioner’s advice or product labeling. A detailed representation of various ashwagandha formulations retrieved from the patients and the specific formulations that were subjected to chemical analysis and toxicology are shown in Figure 1. A registered and certified Ayurveda practitioner prescribed the ashwagandha supplement to 5 patients, while 3 reported self-use of over-the-counter supplements. The complete general features and clinical details of 8 patients are shown in Table 1.
FIGURE 1.
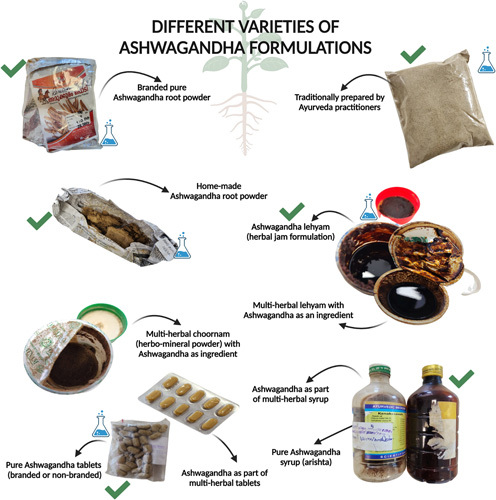
The various types of ashwagandha formulations that were retrieved from patients. The products marked with a green tick are single-ingredient ashwagandha products, the use of which was one of the inclusion criteria. This study did not include patients using multiherbal products containing ashwagandha as a predominant or additional ingredient. The products which are marked with a “blue beaker icon” were considered adequate and unspoiled for further chemical analysis.
TABLE 1.
Demography and clinical details of patients with ashwagandha-induced liver injury
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Male | Female | Male | Male | Male |
| Age (y) | 75 | 31 | 34 | 58 | 50 | 39 | 68 | 47 |
| Diabetes | Yes | No | No | Yes | No | No | Yes | No |
| Hypertension | No | No | No | No | No | No | Yes | No |
| Overweight or obese | No | No | No | No | Yes | No | No | No |
| Chronic liver disease | Yes | No | Yes | No | No | Yes | Yes | Diagnosed during event |
| Etiology of chronic liver disease | NAFLD | — | NASH | — | — | Alcohol (abstinent) | NAFLD | Cryptogenic |
| Other comorbidities | None | None | None | NHL in long remission | Bipolar disorder | None | None | None |
| Co-medications | Insulin, oral hypoglycemic agents | None | None | None | Amisulpride and lithium for over 3 y | None | Insulin, oral hypoglycemic agents | None |
| Indication for ashwagandha use | Insomnia | Postpregnancy “stress” | Appetite stimulant | “Anticancer” preventive | Stress detox and sleep aid | Fever | General wellness and energy booster | General wellness and energy booster |
| Duration of herb intake (d) | 30 | 14 | 60 | 94 | 540 | 30 | 60 | 17 |
| Dose | Approximately 20 g/d | 10–15 g/d | 10–15 g/d | 500 mg daily | ~100 mL daily | Manufacturer instruction | Manufacturer instruction | Manufacturer instruction |
| Time to symptom onset (d) | 24 | 14 | 52 | 96 | 540 | 30 | 62 | 15 |
| Onset of lab abnormalities (d) | 35 | 14 | 62 | 100 | 546 | 30 | 65 | 16 |
| Predominant symptoms | Lethargy and jaundice | Pruritus | Pruritus and jaundice | Jaundice and pruritus | Pruritus and jaundice | Jaundice and abdominal distension | Lethargy and jaundice | Jaundice and pruritus |
| Clinical presentation | ACLF | Acute hepatitis | Acute hepatitis | Acute hepatitis | Acute hepatitis | ACLF | Acute hepatitis | ACLF |
| Steroid use for treatment | No | No | Yes, 40 mg/d for 15 d | Yes, 40 mg/d for 30 d | No | No | Yes, 40 mg/d for 30 d | No |
| Resolution of HILI | No | Yes | Yes | Yes | Yes | No | Yes | No |
| Time to resolution (d) | 45 | 30 | 60 | 95 | — | 56 | — | |
| Outcome | Dead | Alive | Alive | Alive | Alive | Dead | Alive | Dead |
| Time from diagnosis to final follow-up (d) | 288 | 375 | 430 | 486 | 235 | 30 | 302 | 242 |
| Ashwagandha product consumed | Homemade powder | Lehyam (herbal jam) | Branded powder | Nonbranded tablets | Detox tea | Branded powder | Branded powder and tablets | Branded powder and syrup |
| Person who prescribed the herb | Self, OTC | Ayurveda practitioner | Self, OTC | Ayurveda practitioner | Self, OTC | Ayurveda practitioner | Ayurveda practitioner | Ayurveda practitioner |
Abbreviations: ACLF, acute-on-chronic liver failure; HILI, herb-induced liver injury; NHL, non-Hodgkin lymphoma; OTC, over-the-counter.
Investigations and outcomes
The type of liver injury according to the R-ratio was cholestatic in 4, hepatocellular in 3, and mixed in 1. The RUCAM score revealed probable ashwagandha-induced liver injury in 6 and possible liver injury in 2 patients. The HILI was severe in most patients included (N=6/8, 75%). Three patients died (mortality 37.5%; at 30, 242, and 288 d). All the patients who died on follow-up had index HILI presentation as ACLF. The complete individual investigations of 8 patients are shown in Table 2. Briefly, pertinent pooled findings included median hemoglobin 12.1 g/dL, total white cell count 8.7×109/L, platelet count 168.5×103/μL, the median total bilirubin 13.8 mg/dL, aspartate aminotransferase 171 U/L and alanine aminotransferase 173 U/L, mean serum albumin 3.51±0.93 mg/dL, and median international normalized ratio 1.85. The mean total IgG level was 16.03±2.97 (normal 6–16) g/L. Two patients were positive for antinuclear antibodies and none for anti-smooth muscle or anti-liver-kidney-microsomal antibodies. Nonetheless, autoimmune hepatitis was unlikely in all patients after exhaustive evaluation. A liver biopsy was performed in 6 patients (percutaneous in 4 and transjugular in 2). The families of 2 patients did not consent to liver biopsy in view of the critical nature of the illness, with both dying on follow-up on best supportive care. The portal area was the commonest site of inflammation, while the predominant type of inflammation was lymphocytic and eosinophilic. Interface hepatitis was not remarkable in the majority, while 50% had the presence of hepatocellular necrosis (spotty in 1, bridging in 1, and confluent in 2) (Figure 2).
TABLE 2.
Pertinent investigations of patients with ashwagandha-related liver injury
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Hemoglobin (g/L) | 12.1 | 13.4 | 12.6 | 11.8 | 12 | 11.9 | 11 | 14.2 |
| Total leukocyte count (×109/L) | 8.5 | 4.6 | 5.5 | 8.9 | 11.2 | 31 | 7.6 | 24.3 |
| Platelet counts (×103 per μL) | 148 | 234 | 180 | 215 | 192 | 80 | 112 | 157 |
| Total bilirubin (mg/dL) | 10.4 | 2 | 3.6 | 21.3 | 16.1 | 11.4 | 24.3 | 31.5 |
| Direct bilirubin (mg/dL) | 3.3 | 1 | 1.8 | 16.9 | 11.5 | 3.4 | 19.8 | 25.3 |
| Aspartate aminotransferase (IU/L) | 107 | 598 | 647 | 64 | 235 | 47 | 100 | 270 |
| Alanine aminotransferase (IU/L) | 66 | 736 | 409 | 159 | 188 | 97 | 63 | 354 |
| Alkaline phosphatase (IU/L) | 105 | 241 | 199 | 354 | 893 | 59 | 128 | 209 |
| Gamma-glutamyl transpeptidase (IU/L) | 709 | 118 | 268 | 342 | 213 | 178 | 223 | |
| Serum albumin (mg/dL) | 2.9 | 4.9 | 3.2 | 4.1 | 4.2 | 1.8 | 3.6 | 3.4 |
| Serum globulin (mg/dL) | 4.3 | 2.6 | 3.7 | 2.2 | 2.8 | 5.4 | 2.6 | 3.7 |
| International normalized ratio | 2.93 | 1 | 1.3 | 2.2 | 1 | 6.1 | 1.5 | 2.2 |
| Serum total IgG (g/L) | 14.8 | 17.2 | 20.2 | 16.4 | 11.5 | 18.6 | 17.2 | 12.4 |
| Antinuclear antibody | Negative | Negative | Positive | Negative | Negative | Positive | Negative | Positive |
| Anti-smooth muscle antibody | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Anti–liver-kidney-microsomal antibody | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| R-ratio | 1.3 | 5.6 | 5.3 | 1.3 | 0.6 | 4.9 | 1.5 | 5.1 |
| Type of liver injury | Cholestatic | Hepatocellular | Hepatocellular | Cholestatic | Cholestatic | Mixed | Cholestatic | Hepatocellular |
| RUCAM score | 8, probable | 6, probable | 8, probable | 7, probable | 5, possible | 5, possible | 8, probable | 7, probable |
| HILI severity | Severe | Mild | Moderate | Severe | Severe | Severe | Severe | Severe |
| Liver biopsy done | No | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Type of liver biopsy | — | Percutaneous | Percutaneous | Transjugular | Percutaneous | — | Percutaneous | Transjugular |
| Liver biopsy findings | ||||||||
| Main site of inflammation | — | Periportal | Portal | Portal | Portal | — | Lobular | Portal |
| Predominant type of inflammation | — | Lymphocytes, eosinophils | Lymphocytes, eosinophils | Lymphocytes, eosinophils | No or minimal inflammation | — | No or minimal inflammation | Lymphocytes, eosinophils |
| Interface hepatitis, severity | — | Yes, mild | Yes, mild | No | No | — | No | No |
| Necrosis, type | — | Yes, spotty | Yes, bridging | No | No | — | Yes, confluent | Yes, confluent |
| Fibrosis, stage (Ishak) | Known case of cirrhosis | Stage 1 | Stage 2 | No | No | Known case of cirrhosis | Stage 2 | Stage 6 |
| Steatosis, severity | — | Yes, moderate | Yes, severe | Yes, mild | Yes, mild | — | No | No |
| Eosinophilic infiltration, severity | — | Yes, moderate | Yes, severe | Yes, mild | Yes, mild | — | Yes, minimal | Yes, moderate |
| Hepatocyte rosettes | — | No | Yes | No | No | — | No | No |
| Cholestasis, site, severity | — | No | Yes, cellular and canalicular, severe | Yes, cellular and canalicular, severe | Yes, cellular and canalicular, moderate | — | Yes, cellular and canalicular, moderate | Yes, cellular and canalicular, moderate |
Abbreviations: HILI, herb-induced liver injury; RUCAM, Roussel Uclaf Causality Assessment Method.
FIGURE 2.
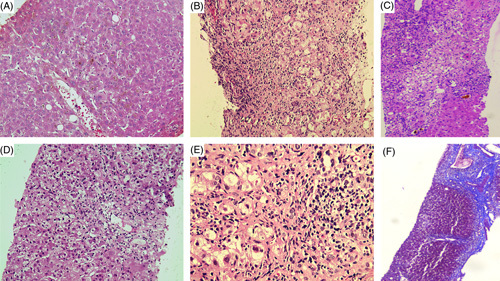
Representative liver histology of patients with ashwagandha-induced liver injury. Severe hepatocellular cholestasis (hematoxylin and eosin stain, ×40) (A); lymphocyte and eosinophil predominant portal inflammation (hematoxylin and eosin stain, ×20) (B); bridging necrosis and canalicular cholestasis in the presence of mild to moderate mixed inflammation consisting of lymphocytes, eosinophils, few plasma cells, and neutrophils (hematoxylin and eosin stain, ×20) (C); centrizonal and focal necrosis with minimal inflammation (hematoxylin and eosin stain, ×20) (D); mild to moderate interface hepatitis with lymphocyte predominant and few macrophage-based portal inflammation (E) and in the patient (shown in C) with bridging necrosis and cholestasis, repeat liver biopsy revealed progression to chronic herb-induced liver injury with the formation of vague/incomplete hepatocyte nodules and thick bridging fibrosis (Masson-trichrome stain, ×10) (F).
In 1 patient (patient 3), a second (percutaneous) liver biopsy (details not shown in Table 2) was performed 5 months after the initial HILI presentation in view of the recurrence of hepatitis (without jaundice) after prior complete resolution. The initial biopsy was consistent with HILI. Associated with severe canalicular and hepatocellular cholestasis with bridging necrosis with background NASH. Even though the histology did not favor classical autoimmune hepatitis, the antinuclear antibody was positive with an elevated serum IgG. The repeat biopsy revealed showed linear cores of liver tissue forming vague nodules separated by fibrous septa. The hepatocytes showed microvesicular steatosis in close to 45% of cells. The portal tract revealed predominantly lymphocytic and clustered macrophage-mediated inflammation, mild to moderate interface hepatitis with neutrophils and eosinophils, and minimal plasma cells. Necrotic areas (notable at baseline biopsy) were absent, cholestasis had resolved, and focal areas showed hepatocyte rosettes—these features were suggestive of chronic HILI with autoimmune-like features.
Five types of ashwagandha formulations from the patients were subjected to chemical and toxicology analysis (marked with a “beaker icon” in Figure 1). None of the formulations revealed potential hepatotoxic contaminants, adulterants, or other synthetic agents. Herb-based natural bioactive compounds were identified in all formulations, including glycosides, lactones, terpenes, saponins, and alkaloid groups. One product was additionally found to have trace amounts of lead (0.21 mg/kg), cadmium (0.038 mg/kg), and arsenic (0.089 mg/kg).
DISCUSSION
We report a series of patients with ashwagandha-related HILI from a multicenter retrospective cohort. The predominant pattern of liver injury was cholestatic, with jaundice as the notable clinical presentation. In our series, all patients underwent a thorough, exhaustive, and pragmatically detailed diagnostic evaluation to rule out other competing causes for acute liver injury. The commonest indication for ashwagandha use in our cohort was for “stress and wellness.” Three patients with ashwagandha-induced liver injury and underlying pre-existing liver disease in our cohort died with the development of ACLF, and 1 patient with chronic liver disease and acute liver injury without ACLF survived. In all other patients, ashwagandha-induced liver injury was self-limiting with supportive care. Lymphocytic and eosinophilic portal-based inflammation with cholestasis and hepatocellular necrosis were predominant in histology. Ashwagandha is marketed as clinically beneficial for many conditions, including improved exercise capacity and physical performance, increased testosterone, improved sleep, and reduced stress and anxiety. However, the highest level of clinical evidence from available studies does not clarify these findings (Supplemental Table S1, http://links.lww.com/HC9/A531), and hence the recommendation to use ashwagandha as a preventive or therapeutic modality does not exist in the current literature.
Reports of ashwagandha-induced liver injury have been notable in medical literature since 2017. An exhaustive summary of all published reports on ashwagandha-induced liver injury is shown in Table 3. The first report was from Japan, followed by multiple reports from United States, Germany, and Poland. Since the first report, a total of 14 cases (excluding the current series) of ashwagandha-induced liver injury are available.17–26 In the majority of the cases, there are many commonalities. The most common indication for ashwagandha use was stress and anxiety; young or middle-aged males predominated, and clinical presentation was jaundice with cholestasis in the majority. Most patients consumed the herb as per label instructions or as prescribed, and overdosing was seen in only 1.18 Liver biopsy revealed cholestasis with or without inflammation and sometimes necrosis. The causality assessment tool used was either RUCAM or an exhaustive diagnosis of exclusion. Except for 1 patient who required liver transplantation for survival, all other cases improved with the best supportive care and management of symptoms. In this context, our study has several commonalities and unique aspects.
TABLE 3.
Summary of published studies on liver injury due to ashwagandha
| References | Type of study (country) | Clinical indication | Comments |
|---|---|---|---|
| Inagaki et al16 | Case report (Japan) | Anxiety and stress disorder | Patient: 20-y-old male ashwagandha type: pure supplement Dose: more than twice recommended dose Duration: 1 mo Liver injury: hepatocellular type DILI: high-possibility (DDW-J 2004 criteria) Liver biopsy: canalicular cholestasis Treatment: ursodeoxycholic acid Outcome: complete resolution in 150 d |
| Björnsson et al17 | Case series (USA and Iceland) | Anxiety, stress, to increase concentration/focus, general wellness (vitality) | Patients: total of 5, 3 males and 2 females ashwagandha type: pure supplements and ashwagandha containing multiherbal products Dose: as recommended on the label, no over dosages Duration: from 14 to 110 d Liver injury: cholestatic type in 3 patients, mixed in 2 DILI: definite in 1, highly likely in 2, probable in 1, possible in 1 (DILIN expert criteria) Liver biopsy: predominantly cholestasis with portal/lobular inflammation Treatment: supportive care and ursodeoxycholic acid in few Outcome: complete resolution within 60 to 150 d |
| Weber et al19 | One case report and various self-reported adverse events on commercial websites (USA) | Not mentioned | Patient: 40-y-old male ashwagandha type: traditional formulation for 1 y, commercial formulation for 20 d Dose: as recommended on the label (450–500 mg once daily) Duration: 1 y and 20 d Liver injury: cholestatic hepatitis DILI: probable (RUCAM criteria) Liver biopsy: not done Treatment: supportive care and withdrawal of offending herb Outcome: resolution details not provided Additional points: from ashwagandha commercial website reporting of adverse events (unconfirmed)—11 with liver damage, 9 with pruritus, 6 with jaundice, 3 with choluria; 6 needed hospitalizations, 2 had acute liver failure (resolved). Additionally, 107 reviews stated severe pruritus as a side effect faced by consumers |
| Ireland et al20 | Case report (USA) | Anxiety | Patient: 39-y-old female, 3 months abstinent from alcohol ashwagandha type: an over-the-counter supplement (along with basil leaves and biotin) Dose: as recommended on the label (but on alternate days) Duration: 45 days Liver injury: cholestatic hepatitis DILI: probable (RUCAM criteria) Liver biopsy: cholestatic hepatitis with confluent necrosis Treatment: ursodeoxycholic acid Outcome: complete resolution within 1 month |
| Rattu et al21 | Case report (published on the university website as a poster presentation] (USA) | Stress relief | Patient: 44-y-old female ashwagandha type: single-ingredient supplement Dose: as recommended on the label Duration: details not provided Liver injury: cholestatic hepatitis DILI: no causality tools applied, only diagnosis of exclusion Liver biopsy: not performed Treatment: ursodeoxycholic acid Outcome: resolution details not provided |
| Patel et al22 | Case report (USA) | Details unknown | Patient: 65-y-old male ashwagandha type: single-ingredient powdered root Dose: as recommended on the label Duration: details not provided Liver injury: cholestatic hepatitis DILI: no causality tools applied, only diagnosis of exclusion Liver biopsy: severe centrilobular canalicular cholestasis Treatment: ursodeoxycholic acid Outcome: complete resolution in 70 d |
| Ali et al23 | Case report (USA) | Stress relief | Patient: 20-y-old male ashwagandha type: single-ingredient over-the-counter supplement Dose: as recommended on the label (450 mg once daily) Duration: 30 d Liver injury: cholestatic hepatitis DILI: no causality tools applied, only diagnosis of exclusion Liver biopsy: not performed Treatment: supportive care, withdrawal of agent Outcome: complete resolution within 30 d |
| Tóth et al24 | Case report (Germany) | Anxiety | Patient: 65-y-old female ashwagandha type: single-ingredient over-the-counter supplement Dose: not recorded Duration: 30 d Liver injury: cholestatic hepatitis DILI: no causality tools applied, only diagnosis of exclusion Liver biopsy: spotty necrosis, ceroid-macrophages, hepatocellular, and canalicular cholestasis Treatment: ursodeoxycholic acid Outcome: complete resolution within 60 d |
| Lubarska et al25 | Case report (Poland) | General wellness (naturopath prescribed) | Patient: 23-y-old male ashwagandha type: single-ingredient over-the-counter supplement Dose: not recorded Duration: 90 d Liver injury: hepatocellular type DILI: probable (RUCAM criteria) Liver biopsy: not performed Treatment: therapeutic plasma exchange Outcome: complete resolution within 106 d |
| Suryawanshi et al26 | Case report (USA) | — | Patient: 41-y-old female, post-thyroidectomy status 2 y ashwagandha type: single-ingredient over-the-counter supplement plus progesterone Dose: not recorded Duration: 60 d Liver injury: hepatocellular type DILI: no causality tools applied, only diagnosis of exclusion Outcome: acute liver failure Treatment: liver transplantation Explant biopsy: submassive necrosis, 20% viable hepatocytes |
| Philips et al Current series | Case series (India) | Anxiety, stress, general wellness, appetite enhancer, insomnia | Patients: a total of 8, 6 males and 2 females ashwagandha type: pure supplements in the form of powders, pills, herbal jam, detox tea, and syrups Dose: as recommended on the label or as advised by practitioners, patients reported no overdoses, but dosages could not be recorded Duration: from 14 to 540 d Liver injury: cholestatic type in 4 patients, hepatocellular in 3, and mixed in 1 DILI: 6 probable and 2 possible cases (RUCAM criteria) Liver biopsy: predominantly hepatocellular and canalicular cholestasis with portal inflammation and varying extent of necrosis Treatment: supportive care and ursodeoxycholic acid in those with cholestasis, immunosuppression in 1 patient with recurrence of hepatitis and chronic herb-induced liver injury on serial biopsy Outcome: complete resolution within 45–95 d Additional: included patients with pre-existing liver disease; 3 with acute-on-chronic liver failure died on follow-up |
Abbreviations: DILIN, drug-induced liver injury network; RUCAM, Roussel Uclaf Causality Assessment Method.
We present the current largest cohort of ashwagandha-related liver injuries from 3 participating centers that have maintained a DILI database capable of capturing such events. Our findings could only be the “tip of the iceberg” and demonstrate that the nationwide burden of liver injury due to ashwagandha could be more. The previous largest published series of ashwagandha-related liver injuries were from Iceland and the United States. DILI Network concerning 5 patients.17 Our series included only patients on pure ashwagandha-based formulations. Patients consuming ashwagandha as part of multiherbal preparations, like those included in the paper by Björnsson et al17, were systematically excluded to remove the confounding effect of complex herbal preparations causing liver injury. Our study included patients with pre-existing liver disease, a novel aspect lacking in the current published literature on ashwagandha-induced liver injury. Chronic liver disease patients developed more severe HILI, and all those who presented with the syndrome of ACLF died in our series, demonstrating the poor prognosis associated with disease severity and clinical progression noted with DILI in the cirrhosis population. In some cohorts, DILI in patients with underlying liver disease has been associated with mortality rates as high as 16%, compared to ~5% in patients without pre-existing liver disease.8,27,28 Most herbal supplements implicated in liver injury are frequently mislabeled or accurate ingredients not evidently disclosed. Many cases of DILI due to herbal supplements are caused by formulations containing multi-ingredient mixtures, and it can be difficult to prove the exact compound that is responsible for liver injury.29 In our study, all patients used marketed or prescribed ashwagandha-only formulations, and chemical and toxicology analysis did not detect other hepatotoxic substances, making our conclusions on ashwagandha causing severe liver injury and death among our patients pertinent and concerning. Although ashwagandha is generally considered safe to consume, there has been an increase in reports of DILI due to its use, driven by unscientific and nonevidence-based promotion among general people through print, visual, and social media. The underlying mechanism for liver damage due to ashwagandha could be the presence of herb-specific compounds called withanolides that cause irreversible adduction to hepatocellular DNA.30 None of the patients had a history of concomitant hepatotoxic prescription or alternative medicine use, alcohol misuse, or i.v. drug use. Even though we did not perform the PCR test for HCV and HEV infection, viral hepatitis was considered very unlikely to cause the pattern of liver injury described in our patient cohort, which was predominantly cholestatic with only mild elevation in aminotransferases. Two patients in our cohort were positive for autoantibodies with high serum IgG without other classical features of autoimmune hepatitis. Initially, in all patients except those with severe ACLF, withdrawal of offending herb and supportive care without immunosuppression resolved jaundice and hepatitis. Unique to our study, we found that in 1 patient, a young man (patient number 3), hepatitis recurred after complete resolution on best supportive care with repeat biopsy suggestive of chronic HILI without the presence of classical autoimmune hepatitis. The second hepatitis episode was well controlled by initiating low-dose immunosuppression (corticosteroids and mycophenolate), with the patient doing well on follow-up. Mild elevations of autoimmune markers are not pathognomonic for autoimmune hepatitis and are very commonly described in patients with DILI, and drug-triggered autoimmune hepatitis is not uncommon, especially in the context of HILI.7,9,17,31. Even though RUCAM is the standardized, validated tool to measure causality in DILI, it tends to underestimate the causality associated with herbal supplements because it was designed to assess the causality of a single product, whereas herbal products typically contain multiple herbal and nonherbal ingredients. Nonetheless, published studies on single-ingredient herbal supplements have used RUCAM to approximate the causality, while others have only depended on an exhaustive diagnosis of exclusion without using any causality tools. We understand that the newly developed Revised Electronic Causality Assessment Method and the gold standard DILIN consensus are better tools for causality assessment. However, due to the retrospective nature of our study, we could not gather specific data inputs (such as exact dates of transaminase peak) required for Revised Electronic Causality Assessment Method completion. The approximate days/duration (and not the date) of herb consumption and stoppage was from patient recall which may have suffered recall bias. We lack an organized, core DILI network in our country, there is a deficit of DILI coordinating centers, and paucity of centralized expert-driven electronic/online data assessment system for a rigorous and validating consensus which operates under DILIN in the United States. In our patients, the herbal supplement was a single ingredient (ashwagandha) without any other known hepatotoxic ingredients or concomitant liver toxic medication use (as such, were excluded), and hence RUCAM use was reasonably justified. We could not retrieve all implicated products for exhaustive chemical analysis. But the chemical analysis of available samples did not reveal hepatotoxic components, contamination, or adulteration that could have indirectly led to liver injury. Multiple prior studies have demonstrated a drug or herb-specific HLA association with DILI. In our study, we could not perform such testing due to a lack of resources. A summary schematic encompassing published literature and novel findings from the current study is shown in Supplemental Figure S2, http://links.lww.com/HC9/A532.
CONCLUSIONS
Ashwagandha herb is used extensively in the Indian systems of traditional medicine, but lately, its use as a dietary supplement for unproven indications has been on the rise in western countries, especially for mental health disorders and as a workout supplement among gym-goers. We present 8 cases of ashwagandha-related liver injury in which patients with pre-existing liver disease suffered high mortality without timely liver transplantation. Educating the public on avoiding unrecommended and potentially harmful herbal supplements can reduce the (avoidable) liver disease burden within the community.
Supplementary Material
Footnotes
Abbreviations: ACLF, acute-on-chronic liver failure; ALT, alanine transaminase; AST, aspartate transaminase; DILIN, drug-induced liver injury network; HCV, hepatitis C virus; HDS, herbal and dietary supplements; HILI, herb-induced liver injury; RUCAM, Roussel Uclaf Causality Assessment Method.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepcommjournal.com.
Contributor Information
Cyriac A. Philips, Email: abbyphilips@theliverinst.in.
Arun Valsan, Email: drarunvalsan@gmail.com.
Arif H. Theruvath, Email: arif.hussain@theliverinst.in.
Resmi Ravindran, Email: resmiravindran123@gmail.com.
Tharun T. Oommen, Email: tharuntom@gmail.com.
Sasidharan Rajesh, Email: rajesh387@gmail.com.
Saptarshi Bishnu, Email: saptarshibishnu@gmail.com.
Philip Augustine, Email: drphilipaugustine@yahoo.co.in.
AUTHOR CONTRIBUTIONS
Cyriac A. Philips: conceptualization, methodology, formal analysis, writing—original draft, writing—review and editing. Arun Valsan: conceptualization, data curation, writing—review and editing. Arif H. Theruvath: methodology, data curation, writing—review and editing. Resmi Raveendran: methodology, data curation, writing—review and editing. Tharun T. Oommen: methodology, supervision, writing—review and editing. Sasidharan Rajesh: supervision, writing—review and editing. Saptarshi Bishnu: conceptualization, data curation, writing—review and editing. Philip Augustine: supervision, writing—review and editing. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
The authors have no conflicts to report.
REFERENCES
- 1. Brown AC. An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food Chem Toxicol. 2017;107(Pt A):449–71. [DOI] [PubMed] [Google Scholar]
- 2. Williams CT. Herbal supplements: precautions and safe use. Nurs Clin North Am. 2021;56:1–21. [DOI] [PubMed] [Google Scholar]
- 3. Medina-Caliz I, Garcia-Cortes M, Gonzalez-Jimenez A, Cabello MR, Robles-Diaz M, Sanabria-Cabrera J, et al. Spanish DILI Registry. Herbal and dietary supplement-induced liver injuries in the Spanish DILI Registry. Clin Gastroenterol Hepatol. 2018;16:1495–502. [DOI] [PubMed] [Google Scholar]
- 4. Bessone F, García-Cortés M, Medina-Caliz I, Hernandez N, Parana R, Mendizabal M, et al. Herbal and dietary supplements-induced liver injury in Latin America: Experience from the LATINDILI Network. Clin Gastroenterol Hepatol. 2022;20:e548–63. [DOI] [PubMed] [Google Scholar]
- 5. Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M, et al. Incidence and etiology of drug-induced liver injury in Mainland China. Gastroenterology. 2019;156:2230–41.e11. [DOI] [PubMed] [Google Scholar]
- 6. Kesar V, Channen L, Masood U, Grewal P, Ahmad J, Roth NC, et al. Transplantation for acute liver injury in Asians is more likely due to herbal and dietary supplements. Liver Transpl. 2022;28:188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Philips CA, Paramaguru R, Joy AK, Antony KL, Augustine P. Clinical outcomes, histopathological patterns, and chemical analysis of Ayurveda and herbal medicine associated with severe liver injury—a single-center experience from southern India. Indian J Gastroenterol. 2018;37:9–17. [DOI] [PubMed] [Google Scholar]
- 8. Philips CA, Paramaguru R, Augustine P, Rajesh S, Ahamed R, George T, et al. A single-center experience on outcomes of complementary and alternative medicine use among patients with cirrhosis. Hepatol Commun. 2019;3:1001–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Philips CA, Theruvath AH, Raveendran R, Ahamed R, Rajesh S, Abduljaleel JK, et al. Clinical outcomes associated with complementary and alternative medicine-related “immunity-boosting” practices in patients with cirrhosis during the COVID-19 pandemic—an observational study. Medicine (Baltimore). 2023;102:e33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paul S, Chakraborty S, Anand U, Dey S, Nandy S, Ghorai M, et al. Withania somnifera (L.) Dunal (ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed Pharmacother. 2021;143:112175. [DOI] [PubMed] [Google Scholar]
- 11. D’Cruz M, Andrade C. Potential clinical applications of Ashwagandha (Withania somnifera) in medicine and neuropsychiatry. Expert Rev Clin Pharmacol. 2022;15:1067–80. [DOI] [PubMed] [Google Scholar]
- 12. Bonilla DA, Moreno Y, Gho C, Petro JL, Odriozola-Martínez A, Kreider RB. Effects of ashwagandha (Withania somnifera) on physical performance: Systematic review and Bayesian meta-analysis. J Funct Morphol Kinesiol. 2021;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akhgarjand C, Asoudeh F, Bagheri A, Kalantar Z, Vahabi Z, Shab-Bidar S, et al. Does ashwagandha supplementation have a beneficial effect on the management of anxiety and stress? A systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2022;36:4115–24. [DOI] [PubMed] [Google Scholar]
- 14. Hayashi PH. Overview of causality assessment in drug-induced liver injury. Clin Liver Dis (Hoboken). 2017;9:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh C.https://ccsniam.gov.in/images/pdfs/NMPB_Report.pdf National Institute of Agricultural Marketing report 2020, National Medicinal Plants Board, New Delhi, India. Accessed July 25, 2023.
- 16. Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806–15. [DOI] [PubMed] [Google Scholar]
- 17. Björnsson HK, Björnsson ES, Avula B, Khan IA, Jonasson JG, Ghabril M, et al. Ashwagandha-induced liver injury: A case series from Iceland and the US Drug-Induced Liver Injury Network. Liver Int. 2020;40:825–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inagaki K, Mori N, Honda Y, Takaki S, Tsuji K, Chayama K. A case of drug-induced liver injury with prolonged severe intrahepatic cholestasis induced by ashwagandha. Kanzo. 2017;58:448–54. [Google Scholar]
- 19. Weber S, Gerbes AL. Ashwagandha-induced liver injury: Self-reports on commercial websites as useful adjunct tools for causality assessment. Am J Gastroenterol. 2021;116:2151–2152. [DOI] [PubMed] [Google Scholar]
- 20. Ireland PJ, Hardy T, Burt AD, Donnelly MC. Drug-induced hepatocellular injury due to herbal supplement ashwagandha. J R Coll Physicians Edinb. 2021;51:363–5. [DOI] [PubMed] [Google Scholar]
- 21. Rattu M, Maddock E, Espinosa J, Lucerna A, Bhatnagar N. An herbal liver effect: Ashwagandha-induced hepatotoxicity. Accessed June 17, 2023. https://rdw.rowan.edu/cgi/viewcontent.cgi?article=1194&context=stratford_research_day
- 22. Patel AD, Pinsker BL, Wall A, Arbogast M, King LY, Sherzoy S. Itching to find a diagnosis. Clin Liver Dis (Hoboken). 2022;20:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ali Sajjadh MJ, Suresh Mithil G, Sanchez-Cruz J, Anand C. S2976 cry me a liver: Ashwagandha-induced liver toxicity. Am J Gastroenterol. 2022;117(10S):e1931. [Google Scholar]
- 24. Tóth M, Benedek AE, Longerich T, Seitz HK. Ashwagandha-induced acute liver injury: A case report. Clin Case Rep. 2023;11:e7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lubarska M, Hałasiński P, Hryhorowicz S, Mahadea DS, Łykowska-Szuber L, Eder P, et al. Liver dangers of herbal products: a case report of ashwagandha-induced liver injury. Int J Environ Res Public Health. 2023;20:3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suryawanshi G, Abdallah M, Thomson M, Desai N, Chauhan A, Lim N. Ashwagandha-associated acute liver failure requiring liver transplantation. Am J Ther. 2023;30:e80–3. [DOI] [PubMed] [Google Scholar]
- 27. Devarbhavi H, Choudhury AK, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, et al. APASL ACLF working party. Drug-induced acute-onchronic liver failure in Asian patients. Am J Gastroenterol. 2019;114:929–37. [DOI] [PubMed] [Google Scholar]
- 28. Chalasani NP, Maddur H, Russo MW, Wong RJ, Reddy KR. Practice Parameters Committee of the American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am J Gastroenterol. 2021;116:878–98. [DOI] [PubMed] [Google Scholar]
- 29. Björnsson ES, Navarro VJ, Chalasani N. Liver injury following Tinospora cordifolia consumption: Drug-induced AIH, or de novo AIH? J Clin Exp Hepatol. 2022;12:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Siddiqui S, Ahmed N, Goswami M, Chakrabarty A, Chowdhury G. DNA damage by Withanone as a potential cause of liver toxicity observed for herbal products of Withania somnifera (Ashwagandha). Curr Res Toxicol. 2021;2:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Navarro V, Avula B, Khan I, Verma M, Seeff L, Serrano J, et al. The contents of herbal and dietary supplements implicated in liver injury in the United States are frequently mislabeled. Hepatol Commun. 2019;3:792–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


