Abstract
Biodegradable bacterial nanocellulose (BNC) is a highly in-demand but expensive polymer, and the reduction of its production cost is an important task. The present study aimed to biosynthesize BNC on biologically high-quality hydrolyzate media prepared from miscanthus and oat hulls, and to explore the properties of the resultant BNC depending on the microbial producer used. In this study, three microbial producers were utilized for the biosynthesis of BNC: individual strains Komagataeibacter xylinus B-12429 and Komagataeibacter xylinus B-12431, and symbiotic Medusomyces gisevii Sa-12. The use of symbiotic Medusomyces gisevii Sa-12 was found to have technological benefits: nutrient media require no mineral salts or growth factors, and pasteurization is sufficient for the nutrient medium instead of sterilization. The yield of BNCs produced by the symbiotic culture turned out to be 44–65% higher than that for the individual strains. The physicochemical properties of BNC, such as nanofibril width, degree of polymerization, elastic modulus, Iα allomorph content and crystallinity index, are most notably dependent on the microbial producer type rather than the nutrient medium composition. This is the first study in which we investigated the biosynthesis of BNC on hydrolyzate media prepared from miscanthus and oat hulls under the same conditions but using different microbial producers, and showed that it is advisable to use the symbiotic culture. The choice of a microbial producer is grounded on the yield, production process simplification and properties. The BNC production from technical raw materials would cover considerable demands of BNC for technical purposes without competing with food resources.
Keywords: bacterial cellulose, miscanthus, oat hulls, Komagataeibacter xylinus, Medusomyces gisevii, Kombucha, SCOBY, crystallinity index
1. Introduction
Due to the unfavorable environmental situation on the planet, there is much concern about the transition to a circular economy. Biodegradable polymers are undoubtedly a sound answer to the global ecological challenge because they are not only capable of replacing synthetic polymers, but also open up opportunities to create conceptually new materials [1,2]. One example of such materials is bacterial nanocellulose (BNC), a bacteria-synthesized polymer. The research on BNC has considerably elaborated the insights into classical materials science, owing to the nanodimension of this material and some of the properties this nanodimension provides [3,4]. The application range of BNC is immensely wide, starting from foods and biomedical devices to electronics and energy production [4,5]. This is explained by the fact that BNC, besides its native state, can also be used as a part of composites [6,7,8]. That said, BNC can act as both a matrix and a reinforcement, and it can be modified both in situ and ex situ [9,10]. It is expected that the technical field of BNC application will hold a leading position in the nearest future [3,5].
The wide adoption of BNC is impeded by its high prime cost, with the cost of a nutrient medium constituting ~30% of the total production cost of BNC [11]. Among the latest trends is the concept of the production of valuable BNC from low-cost plant cellulose. This idea has become so globally recognized that not only experimental research papers, but also numerous reviews are devoted to it [12,13,14,15,16,17,18,19,20,21,22]. At the same time, despite the economic attractiveness, the problem of BNC production from plant cellulose is very technically difficult and therefore remains unresolved so far.
In BNC production, the fermentation problem compounds the problems related to cellulosic feedstock pretreatment and substrate fermentability, which are common for the use of lignocellulose, because the composition of nutrient media required by microbial producers of BNC is very specific [13,15]. We have previously demonstrated a successful biosynthesis of BNC in nutrient media derived from oat hulls and miscanthus. Oat hulls are a feedstock with a zero prime cost, since all the expenses are already allocated to cereal production [23]. Miscanthus has been recognized as one of the leading sources of perennial biomass for the production of high-value-added products [24]. Both of the feedstocks are globally abundant.
The process stages involved the chemical pretreatment of feedstocks with dilute HNO3 and/or NaOH solutions and the enzymatic hydrolysis of the solid residue from the chemical pretreatment. The two-stage pretreatment of feedstocks with dilute HNO3 and NaOH was shown to afford a biologically good nutrient medium, in which case the Medusomyces gisevii Sa-12 culture was capable of synthesizing BNC with standard structural characteristics, irrespective of the feedstock type and its pretreatment method: a 86–93% crystallinity index and a 96–98% Iα allomorph content [25,26].
However, the issue regarding the use of the symbiotic culture caused substantial disputes among our reviewers and necessitated the continuation of our research. It is clear that the microbiota composition varies according to the culturing conditions and geographical point at which the symbiotic culture develops [27,28]; that the use of the consortium leads to an improper utilization of the substrate to form multiple metabolites of the symbiotic microorganisms; that the productivity of the consortium towards BNC is instable; and that the in-process control is extremely sophisticated and cannot be automated.
Interestingly, the symbiotic culture Kombucha is the central natural object for the isolation of pure cultures of high-productivity microbial producers of BNC, the acetobacteria of the genus Komagataeibacter [29]. Therefore, the choice between the consortium and individual strains derived therein is becoming an increasingly debated topic in actual production.
In this study, we investigated the biosynthesis of BNC on good-quality nutrient media prepared from miscanthus and oat hulls and tried out both the individual strains and the symbiotic culture as microbial producers. The present study aimed to answer the question of which microbial producer is most suitable for biosynthesizing BNC from low-cost cellulosic feedstocks to achieve a high yield of BNC, and how the choice of microbial producer affects the properties of the resulting macromolecules in this case. The answer to that question holds significant importance for designing a commercial production of high-value BNC from low-cost cellulosic feedstocks.
2. Results
2.1. Biosynthesis of BNC on Hydrolyzate Media
The hydrolyzate media were prepared from miscanthus and oat hulls under the same conditions. The first stage involved chemical pretreatment of the feedstocks, followed by enzymatic hydrolysis of the resultant pulps [25]. The media were standardized against the content of reducing sugars (RS), and the biosynthesis of BNC was conducted using three microbial producers under the same conditions.
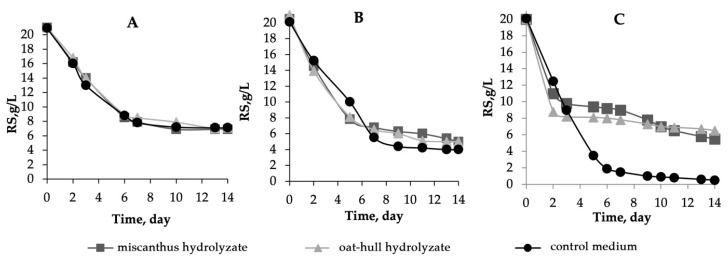
The time profiles of the RS concentration during the biosynthesis of BNC are depicted in Figure 1. The major loss of RS was observed in the first 7 days; afterwards, the curves reached a plateau. The residual RS concentrations on the hydrolyzate media were 7 g/L for the B-12429 strain, 4–5 g/L for the B-12431 strain, and 5.5–6.5 g/L for the symbiotic culture. This was the maximum concentration, while 0.5% was the minimum concentration on the control medium.
Figure 1.
The variation in the RS concentration with time during BNC biosynthesis in miscanthus hydrolyzate medium, in oat-hull hydrolyzate medium, in control medium using of microbial producer: (A)—Komagataeibacter xylinus B-12429; (B)—Komagataeibacter xylinus B-12431; and (C)—Medusomyces gisevii Sa-12. The half-width of the confidence interval for the RS concentration was ±0.2 g/L.
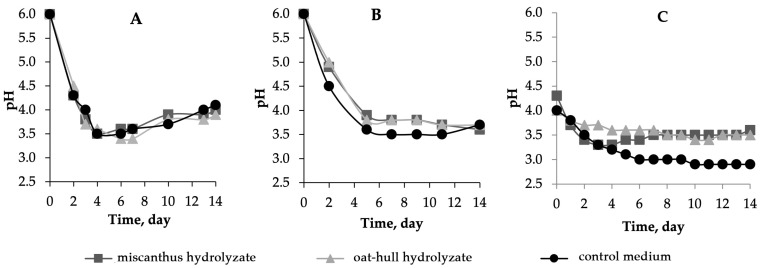
The time courses of pH are displayed in Figure 2. The peculiar feature of the B-12429 strain was the minimum pH of 3.5 observed at 4 days of biosynthesis; afterwards, the pH gradually increased to 3.9–4.3. The pH declined to 3.6–3.9 at 3–5 days of biosynthesis for the B-12431 strain and the symbiotic culture and remained unchanged until the end of the experiment. Minimum pH values were recorded for the symbiotic culture: about 3.5 on the hydrolyzate media and about 3.0 on the control nutrient medium.
Figure 2.
The variation in pH with time during BNC biosynthesis in miscanthus hydrolyzate medium, in oat-hull hydrolyzate medium, in control medium using microbial producer: (A)—Komagataeibacter xylinus B-12429; (B)—Komagataeibacter xylinus B-12431; and (C)—Medusomyces gisevii Sa-12. The half-width of the confidence interval for pH was ±0.1.
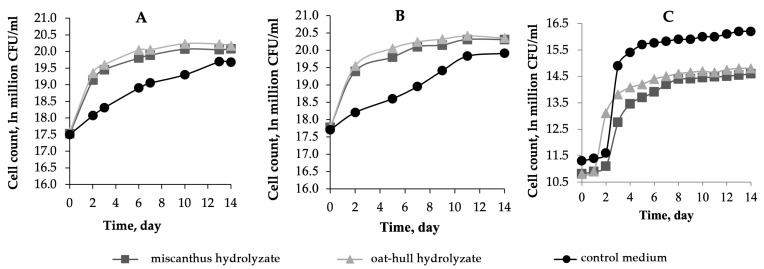
The curves of the acetobacterial count are shown in Figure 3. It is seen from the graphs that no lag phase was observed in the cultivation of the individual strains, which is likely due to pre-adaptation of the producers to the medium used. Between 2 and 14 days, a stationary phase was observed. The bacterial count at 7 days when bacteria were cultured on the hydrolyzates was 3–4 times as high as that of bacteria cultured in the control medium. The bacterial count for the B-12431 strain on all the nutrient media was 1–3% higher than that for B-12429, which is insignificant. The lag phase was observed in the cultivation of the symbiotic culture on all the media used, which is explained by the metabolic peculiarities of the consortium: the yeast proliferated first, and the metabolic products of yeast were then utilized as a substrate by the acetobacteria, which began to increase in number. The acetobacterial count is known to serve as a marker of BNC synthesis; that is, the higher the bacterial concentration, the higher the BNC yield [30]. One could predict the maximum yield of BNC on the oat hull-derived medium for the individual strains and on the control medium for the symbiotic culture.
Figure 3.
The variation in the acetobacterial count over time during BNC biosynthesis in miscanthus hydrolyzate medium, in oat-hull hydrolyzate medium, in control medium using microbial producer: (A)—Komagataeibacter xylinus B-12429; (B)—Komagataeibacter xylinus B-12431; and (C)—Medusomyces gisevii Sa-12. The half-width of the confidence interval for the cell count was ±0.1 ln million CFU/mL.
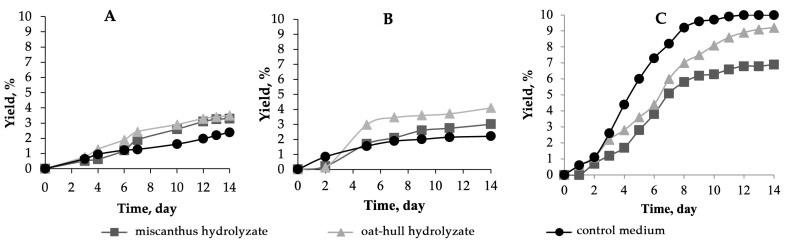
The highest yield of BNC when the individual strains were used was achieved on the oat-hull hydrolyzate medium (Figure 4): 3.5% for B-12429 and 4.1% for B-12429. Interestingly, the BNC yield in the experiment with B-12429 on the miscanthus-derived medium was close to that on the oat hull-derived medium, whereas the experiment with B-12431 showed a large gap between the yield curves. The biosynthesis of BNC in Hestrin–Schramm media took place in a different way as well: while the BNC yield kept growing for up to 14 days in the experiment with B-12429, it reached a plateau at as early as 7 days in the experiment with B-12431.
Figure 4.
The variation in the BNC yield with time during BNC biosynthesis in miscanthus hydrolyzate medium, in oat-hull hydrolyzate medium, in control medium using microbial producer: (A)—Komagataeibacter xylinus B-12429; (B)—Komagataeibacter xylinus B-12431; and (C)—Medusomyces gisevii Sa-12. The half-width of the confidence interval for the BNC yield was ±0.1%.
The lowest yields of BNC were obtained with the individual Komagataeibacter xylinus strains on control medium and were 76–78% as low as that obtained with Medusomyces gisevii Sa-12 on the control medium (2.2–2.4% vs. 10%). That said, the yields we obtained on the control medium are in complete agreement with those reported in the literature for these microbial producers, i.e., for the B-12429 strain [31] and for the B-12431 strain [32].
2.2. Physicochemical Characterization of BNC Test Samples
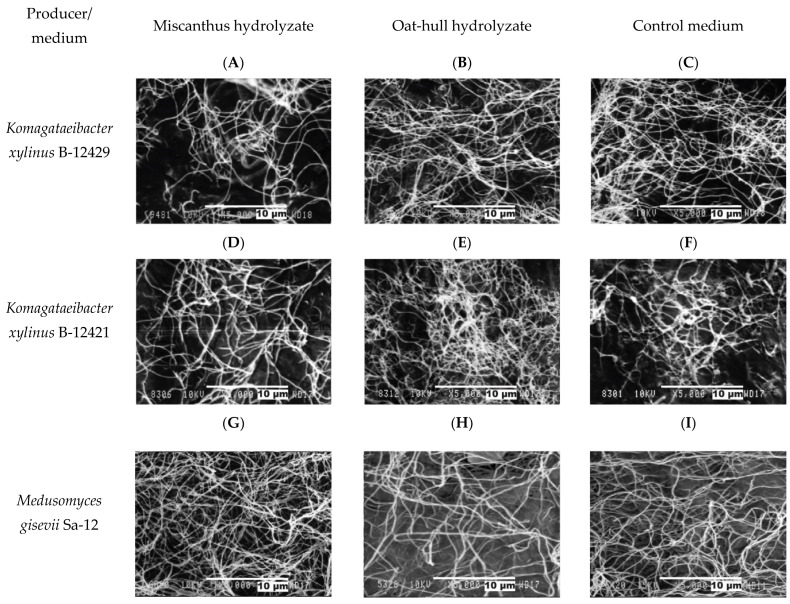
Standard analytical methods commonly used in research worldwide were employed to perform the physicochemical characterization of BNC [3,4,13]. The bacterial origin of BNC is evidenced by its nanodimension, which is confirmed via scanning electron microscopy (SEM) [3]. SEM images of the BNC samples produced by the individual strains and symbiotic culture on the hydrolyzate and control media are depicted in Figure 5. Regardless of the pretreatment method for obtaining a substrate, all the BNC samples obtained on miscanthus- and oat hull-derived hydrolyzates were found to exhibit an unordered reticulate structure, the fingerprint of BNC [3,4]. The width of microfibrils based on the SEM images (calculation was performed using the ImageJ image processing program) were measured to be from 82 nm to 91 nm; no significant difference in the microfibril width was detected.
Figure 5.
SEM images of BNC samples in 72 h of culture, ×5000 zoom: (A–C)—Komagataeibacter xylinus B-12429; (D–F)—Komagataeibacter xylinus B-12431; (G–I)—Medusomyces gisevii Sa-12; (A,D,G)—miscanthus hydrolyzate; (B,E,H)—oat-hull hydrolyzate; and (C,F,I)—control medium.
Chemical purity is the second important fingerprint of BNC that differentiates it from the other types of cellulose [3]. Microorganisms synthesize BNC devoid of hemicelluloses and lignin, and washing helps purify BNC from nutrient medium residues and microbial cells.
The absorption band assignments for functional groups of the BNC samples obtained from miscanthus and oat hulls using the Komagataeibacter xylinus B-12429 and B-12431 strains are given in Table S1 (see the Supplementary Materials for this article). All the BNC samples exhibit close values of stretching vibrations matching those of cellulose. Specifically, absorption bands at 3434 cm−1 are due to the OH-group stretching, and absorption bands at 2894 cm−1 are due to the CH2 and CH stretching. Absorption bands at 1634 cm−1 refer to the bending vibrations of OH groups tightly bound to water. Absorption bands near 1432–1369 cm−1 point to CH2 and CH bending. Absorption bands near 1318 cm−1 indicate bending vibrations of the primary alcohol OH group, those near 1280 cm−1 indicate the alcohol CH2 group bending, and those near 1163 cm−1 indicate the bending vibrations of the alcohol C-O-C and C-O groups. Absorption bands near 896 cm−1 corroborate the presence of β-1,4 glycosidic bonds between the glucose molecules. Thus, the infrared spectroscopy results confirmed that the cellulose samples had chemical functional abilities to react with radicals and cations of metals, holding them on the surface [33].
Any type of cellulose can be characterized by a universal measure such as the degree of polymerization (DP) (Table 1). The DP of BNC obtained with Komagataeibacter xylinus B-12429 was 800–900, which is 1.8–2.9 times lower than that of BNC obtained with Medusomyces gisevii Sa-12 (1450–2600). The BNC synthesized using Komagataeibacter xylinus B-12431 had a DP of 1900–2400, which is almost the same as that of BNC obtained with Medusomyces gisevii Sa-12. The lowest DP of BNC produced by Komagataeibacter xylinus B-12429 was observed when grown on the miscanthus hydrolyzate, while for Komagataeibacter xylinus B-12431, the lowest DP of BNC was observed when grown on the control medium.
Table 1.
Yield and physicochemical properties of BNC subject to the microbial producer and cellulosic feedstock used compared to the control.
| Properties | Komagataeibacter xylinus B-12429 | Komagataeibacter xylinus B-12431 | Medusomyces gisevii Sa-12 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MH | OHH | Control | MH | OHH | Control | MH | OHH | Control | |
| BNC yield in 14 days of biosynthesis, % of the RS, ±0.2% | 3.3 | 3.5 | 2.4 | 3.0 | 4.1 | 1.2 | 6.3 | 10.0 | 10.0 |
| Volumetric productivity of BNC in 14 days of biosynthesis on a dry matter basis, g/L, ±0.1 g/L | 0.59 | 0.63 | 0.43 | 0.54 | 0.74 | 0.22 | 1.13 | 1.80 | 1.80 |
| Mean width of BNC microfibrils, nm | 91.1 | 90.8 | 89.6 | 82.1 | 90.1 | 82.8 | 88.6 | 82.4 | 87.0 |
| Degree of polymerization subject to BNC biosynthesis time, ±50 | |||||||||
| 7 days | 880 | 860 | 900 | 2400 | 2600 | 1900 | 1550 | 1760 | 2600 |
| 14 days | 800 | 820 | 880 | 1920 | 1940 | 2200 | 1470 | 1450 | 2200 |
| Strength properties | |||||||||
| Breaking strength, MPa, ±0.5 MPa | 23.9 | 41.2 | 5.7 | 83.7 | 65.6 | 128.3 | 21.5 | 26.2 | 33.2 |
| Conventional yield limit, MPa, ±0.5 MPa | 3.9 | 3.0 | 1.2 | 10.9 | 5.8 | 8.4 | 3.0 | 3.7 | 5.6 |
| Relative elongation at maximum load, %, ±0.1% | 1.4 | 1.1 | 0.5 | 2.6 | 1.9 | 2.7 | 1.2 | 1.7 | 1.5 |
| Relative elongation at yield, %, ±0.1% | 0.3 | 0.3 | 0.1 | 0.5 | 0.3 | 0.4 | 0.4 | 0.7 | 0.6 |
| Sample thickness, µm, ±0.5 µm | 10.0 | 17.5 | 7.5 | 5.0 | 15.0 | 5.0 | 30.0 | 50.0 | 20.0 |
| Elastic modulus, MPa, ±10.0 MPa | 1212 | 1142 | 1033 | 2180 | 2215 | 2143 | 510 | 529 | 933 |
| Thermogravimetric data | |||||||||
| Onset temperature of decomposition, °C, ±1.0 °C | 305.8 | 294.8 | 290.7 | 301.7 | 302.1 | 300.5 | 317.6 | 329.3 | 307.1 |
| Content of cellulose allomorphs and degree of polymerization, as measured via SEM | |||||||||
| Iα allomorph, %, ±1.0% | 100.0 | 100.0 | 99.2 | 92.8 | 94.2 | 93.5 | 100.0 | 100.0 | 100.0 |
| Iβ allomorph, %, ±1.0% | 0.0 | 0.0 | 0.8 | 7.2 | 5.8 | 6.5 | 0.0 | 0.0 | 0.0 |
| Crystallinity index (in reflection geometry), %, ±5% | 74 | 87 | 76 | 90 | 91 | 94 | 94 | 93 | 86 |
| Crystallinity index (in transmission geometry), %, ±5% | 75 | 90 | 88 | 100 | 89 | 100 | 92 | 87 | 88 |
Notes: (1) MH—miscanthus hydrolyzate; OHH—oat-hull hydrolyzate; (2) controls were the Hestrin–Schramm medium for Komagataeibacter xylinus B-12429 and B-12431 and the control medium supplemented with black tea extract for Medusomyces gisevii Sa-12; (3) some data on BNCs synthesized by Medusomyces gisevii Sa-12 were reported previously [25,26].
The elastic moduli of BNCs obtained using the individual strains were higher than those of BNCs obtained with the symbiotic culture, i.e., by 1.1–2.4 times for B-12429 (1033–2112 MPa vs. 510–933 MPa) and 2.2–4.3 times for B-12431 (2215–2243 MPa vs. 510–933 MPa).
The onset temperatures of pyrolysis for all the samples were similar, irrespective of the medium in which the samples were obtained, and ranged from 290.7 °C to 329.3 °C, with this value for the symbiotic culture being slightly higher than that for the individual strains, but the difference cannot be considered significant.
The analysis of X-ray patterns is detailed in [34] for BNCs obtained using the individual strains and in [35,36] for BNCs obtained with the symbiotic culture. The estimation results for X-ray patterns via full-profile analysis showed that the structure of all the samples 92.8–100.0% matched Iα allomorph. High crystallinity indices were achieved, which depended on the strain used rather than the cellulosic feedstock type (miscanthus or oat hulls): the crystallinity index varied from 86% to 94% for Medusomyces gisevii Sa-12, from 74% to 90% for Komagataeibacter xylinus B-12429, and from 89% to 100% for Komagataeibacter xylinus B-12431.
3. Discussion
3.1. BNC Biosynthesis Process on Hydrolyzate Media
The issue of whether to use individual strains or a symbiotic culture for the biosynthesis of BNC has become particularly acute in the literature worldwide over the last five years [37,38,39]. The use of individual strains has a range of undisputed advantages: it not only offers high productivity with respect to the target product (BNC) and prospects for a further productivity enhancement via gene engineering techniques, but also the predictability of metabolism and the possibility of controlling the population count and condition [13,18,40]. At the same time, the poor stability of individual strains under production conditions (a spontaneous decline or a complete loss in the cellulose-synthesizing capability of microbial producers has been documented multiple times) impels researchers to look for new producers. One method involves stabilizing the functioning of strains using gene engineering techniques [12,15,40], and the other one involves utilizing consortia [37,41].
The “tea fungus” consortium was described in 220 B.C. [30], and is distributed over a wide geographical range under many names: it is called Manchurian mushroom, Kombucha, tea Kvass, starter tea, Japanese mushroom, and Russian mushroom in the European countries [27]; this microbial producer is called Hamza’s Khubdat in the Arabian countries and Haipao in Taiwan [42]; it is called SCOBY (symbiotic culture of bacteria and yeast) in Korea [43]. Herein, we call this symbiotic culture Medusomyces gisevii, a formal systematic name given to the consortium by Lindau [44]. These many names evidence that the consortium is very popular and highly adaptive.
Medusomyces gisevii is a natural, extremely conservative and, at the same time, extremely stable consortium. The consortia have a high adaptive potential, and the coordinated consumption of a substrate and the formation of metabolites enhance their tolerance to nutrient media of a variable composition and increase the target product (BNC) yield, which is of special importance in a real production setting [45,46]. Therefore, the whole trend of using the symbiotic culture for BNC biosynthesis has emerged in the literature worldwide in the recent times [33,38,39,41,47,48,49,50,51,52,53,54].
From a technological standpoint, it is extremely interesting that non-sterile nutrient media can be used to culture Medusomyces gisevii [43,45]. The use of black tea extract makes it unnecessary to add nutrient salts and vitamins, which are usually utilized for BNC biosynthesis [37].
Based on the present study, Table 2 compares the organization difficulties and efficiencies of BNC biosynthesis on hydrolyzate media using individual strains and the symbiotic culture and suggests that the use of the symbiotic culture has more pros than cons.
Table 2.
Comparison of BNC biosynthesis efficiency between individual strains and symbiotic culture on hydrolyzate media.
| Measure | Individual Strains | Symbiotic Culture |
|---|---|---|
| Nutrient salts added | Yes | No |
| Tea extractives added | No | Yes |
| Sterilized medium | Yes | No |
| Microbial control of BNC production | Easy and automatable | Sophisticated and cannot be automated |
| BNC yield on hydrolyzate media compared to that on synthetic one | 38–46% higher | Up to 37% lower |
| BNC yield on hydrolyzate media, % | 3.0–4.1 | 6.3–10.0 |
When the individual strains were cultured on hydrolyzate media, the BNC yield increased by 38–46% for B-12429 and by 150–242% for B-12431 compared to the synthetic medium. The phenomenon of the higher BNC yield on hydrolyzate media compared to the synthetic one has previously been for individual strains on many occasions [13,19]. It was hypothesized that it could be due to the hydrolyzates containing soluble cello-oligosaccharides that are utilizable by strains that contain endo-1,4-β-glucanase [55].
Herein, the biosynthesis of BNC using individual strains and a symbiotic culture was examined for the first time under identical conditions on identical hydrolyzate media. Despite the increased BNC yield obtained with the individual Komagataeibacter xylinus strains on hydrolyzate media compared to the control medium, this BNC yield was found to be 59–65% and 44–48% lower than that obtained with the symbiotic culture on the oat hull-derived medium and on the miscanthus-derived medium, respectively. This proves that miscanthus itself contains inhibitors of BNC biosynthesis, as the process stages were performed in the same way.
It can be inferred that the individual strains cannot compete with the symbiotic culture when cultivated on enzymatic hydrolyzates prepared from easily renewable cellulosic biomass. Apart from the critically low BNC yield, the individual strains require that (1) the medium be supplemented with nutrients without which BNC almost stops growing and that (2) nutrient media be sterilized, whereas the symbiotic culture suffices for pasteurization at 100 °C. These two factors are essential technological benefits in the commercial production of BNC [13].
The conversion of a pristine cellulosic feedstock into BNC is a very complicated process whose efficiency is influenced by multiple factors. When it comes to BNC production from cellulosic raw materials using Kombucha, our research stands alone, and we have no comparative studies to reference. Therefore, we reviewed studies in which individual strains were utilized for BNC production from cellulosic feedstock. For instance, Cheng et al. [56] used corn stalks as the feedstock, performed hydrolysis with acetic acid, and synthesized BNC for 7 days using Acetobacter xylinum ATCC 23767, with the BNC volumetric productivity being 2.93 g/L and the yield being 9.22%. Vasconcellos et al. [57] also utilized corn stalks as the feedstock subjected to hydrothermal treatment, and synthesized BNC for 7 days by Komagataeibacter hansenii ATCC 23769, with the BNC volumetric productivity being 0.71 g/L. Goelzer et al. [58] used rice husk, which was subjected to enzymatic hydrolysis, and synthesized BNC for 10 days using Acetobacter xylinum ATCC 23769, with the BNC volumetric productivity being 1.6–2.4 g/L and the yield being 4.4–6.7%. These three studies align well with the findings obtained herein.
Son et al. [24] utilized miscanthus as the feedstock, pretreated it with sulfuric acid and synthesized BNC for 4 days using Gluconacetobacter xylinus ATCC 53524, with the BNC volumetric productivity being 14.9 g/L. However, the synthesized product was not BNC, in fact, but a co-polymer of BNC and sodium alginate; therefore, these results cannot be compared. We are not aware of other examples of the deep transformation of miscanthus into BNC.
The literature reports extremely high yields of BNC resulting from the conversion of lignocellulosic biomass. Chen et al. [59] used wheat straw as the feedstock treated with ionic liquid followed by enzymatic hydrolysis, and synthesized BNC for 7 days using Gluconacetobacter xylinus ATCC 23770, with the BNC volumetric productivity being 8.3 g/L. Hong et al. [55] utilized olden uncolored T-shirts, which were treated with ionic liquid and then enzymatically hydrolyzed, and synthesized BNC for 14 days by Gluconacetobacter xylinus ATCC 23770, with the volumetric productivity being 10.8 g/L. These results are 4.6–6.0 times better than those achieved in the present study. However, the commercial processing of lignocellulosic biomass does not currently use ionic liquids. Moreover, the commercial production of BNC using individual strains is quite limited [59], whereas the commercial production of BNC using a symbiotic culture is growing steadily worldwide, including the use of BNC for technical purposes [48,59]. The result obtained in the present study is world-class.
3.2. Physicochemical Properties of BNC Test Samples
All the studied BNC samples, irrespective of the microbial producer and nutrient medium, have a 3D reticulate structure typical of BNC in particular [4], as determined by SEM, and are chemically pure celluloses, as determined by IR spectroscopy, which distinguishes BNC from the other cellulose types [33].
Herein, the elastic modulus and the degree of polymerization of the BNC samples depended mostly on the microbial producer type and slightly on the source of the glucose nutrient medium. In the literature, the BNC elastic modulus varies greatly from 15–138 GPa [15,40] to 10–17 MPa [60]. The elastic modulus of BNC obtained using Kombucha was reported to be 32 MPa [50]. Thus, the elastic moduli achieved herein are in good agreement with the literature data.
The degree of polymerization (DP) is a universal measure for any cellulose type, but BNC stands out among other celluloses for a higher DP [3,4,40]. The DPs achieved herein for BNCs are on a par with the literature values [40,50]. The literature rarely discusses the variations in the DP during the biosynthesis. All the microbial producers examined herein showed a minor decline in the DP after 14 days compared to 7 days, which is strongly consistent with the literature data. The same tendency was described in [61], where this fact was difficult to explain, and in [62], where it was explained by the action of cellulases being produced by the A. xylinum used. It could be more logical to suggest that physiology lies behind this tendency. Acetic acid bacteria are known to be strict aerobes; therefore, biosynthesis of new BNC layers occurs on the surface of the already formed ones [63]. But, as the time passes, the population condition worsens due to depleted nutrients and accumulated metabolic products. Moreover, the BNC gel-film increases in thickness and decreases in air oxygen permeability. Therefore, the length of new, later-synthesized BNC molecules becomes shorter, which is why the averaged degree of polymerization of the sample, as determined by analysis, reduces as well.
The onset temperature of decomposition of the samples ranges from 290.7 °C to 329.3 °C, which is perfectly consistent with the literature data. For instance, it was reported in the study [64] on the thermal stability of different celluloses that this range for BNC was between 184 °C and 379 °C, pointing to an excellent thermal stability.
We consider structural characteristics the most significant measures for BNCs. It is these characteristics that not only help differentiate one BNC type from the other, but also determine the pattern of the hierarchical structure of BNC and, hence, its properties [4,24].
The SEM findings were interpreted in line with [65]. The obtained findings were absolutely atypical. One of the problems that arises when replacing a synthetic nutrient medium by unconventional ones from cellulosic biomass is the reduction in the crystallinity index and Iα cellulose content of BNCs. For example, when the synthetic medium was replaced by a rice husk-derived hydrolyzate for BNC biosynthesis, the BNC index of crystallinity declined to 28% [58], while the use of grape bagasse decreased the Iα allomorph content of BNCs by 72% to 56% [66]. Such a tendency was not detected herein for the microbial producers and nutrient media used, and the transition from the synthetic to hydrolyzate media retained both the crystallinity index and the Iα allomorph content of BNCs. This is probably because the method used herein to pretreat the cellulosic feedstocks provides good-quality nutrient media [25,26].
The predominant content of Iα cellulose allomorph is characteristic of BNC [3]. However, the Iα allomorph content achieved for Medusomyces gisevii Sa-12 and Komagataeibacter xylinus B-12431 is extremely high: 99.2–100.0%. The Iα allomorph content reported in the literature ranges from 64% [67] to 90% [40].
The BNC samples produced by Komagataeibacter xylinus B-12431 and Medusomyces gisevii Sa-12 exhibited the highest index of crystallinity between 88% and 100% (as measured in reflection geometry) exceeding all the previously reported values of 46.0% to 95.6% [13,40,68].
4. Materials and Methods
4.1. Microbial Producers of BNC
Here, we used three microbial producers acquired from the Russian National Collection of Industrial Microorganisms (Scientific Center ‘Kurchatov Institute’, Research Institute for Genetics and Selection of Industrial Microorganisms, Moscow, Russia). The Komagataeibacter xylinus B-12429 strain originates from the DSMZ 2004 collection and was isolated by Brown in 1886. The Komagataeibacter xylinus B-12431 strain originates from the DSMZ 2325 collection and was isolated by Brown in 1886, with both strains designed for BNC synthesis. The symbiotic culture used herein was the microbial producer Medusomyces gisevii Sa-12, also known as Kombucha.
Medusomyces gisevii is a symbiotic culture in which acetobacteria and yeast prevail as a microflora. Medusomyces gisevii is composed of 8–10 bacterial genera such as Komagataeibacter, Gluconobacter, Lyngbya, Bifidobacterium, etc., and 15–30 yeast genera such as Candida, Lachancea, Kluyveromyces, Zygosaccharomeces, Schizosaccharomyces, etc. [43,45].
4.2. Maintenance of Cultures
The Hestrin–Schramm medium, which is classical for microbial producers of BNC, was used to maintain the vital activity of the individual Komagataeibacter xylinus strains, and consisted of 2% glucose, 0.5% peptone, 0.5% yeast extract, 0.27% Na2HPO4, and 0.115% citric acid. The pH was 6.0. To set this pH, HCl and NaOH were used. The medium was sterilized in an autoclave at 0.5 atm for 30 min. The same medium was employed for the control biosynthesis of BNC by the B-12429 and B-12431 strains.
A semisynthetic nutrient medium consisting of glucose and black tea extract was utilized to maintain Medusomyces gisevii [37]. This nutrient medium was the control and was prepared as follows: a weighed portion of dry black tea (10 g dry black tea per 1 L nutrient broth, which is equivalent to 3.2 g/L black tea extractives in the medium) was poured over with boiling-hot water, 20 g/L glucose was injected, the extraction vessel was capped, and the contents were maintained at 94–96 °C for 15 min. Afterwards, the mixture was cooled and filtered off. The nutrient medium was not sterilized.
The culture was run under static conditions at 28 ± 0.2 °C for 7 days. The pH was not adjusted during the cultivation, which is optimum for most microbial producers of BNC [13,15,40]. The culture medium was used as the inoculum and injected in the amount of 10%.
4.3. Cellulosic Feedstocks and Pretreatment
Miscanthus (Miscanthus sacchariflorus (Maxim.) Hack) var. Soranovskiy was selected and grown in the Institute of Cytology and Genetics (Novosibirsk, Russia) [67]. Miscanthus was ground by a Gardena straw grinder (Berlin, Germany) to at most 10 mm. Oat hulls were kindly provided by the ZAO Biysk Elevator (Biysk, Russia).
The feedstocks were pretreated by a two-stage method in a 250-L standard vessel under atmospheric pressure at 90–96 °C at the pilot production site of the IPCET SB RAS. The method involved prehydrolysis with a 1% HNO3 solution, treatment with a 4% HNO3 solution, water washing, treatment with a 4% NaOH solution and water washing, as described in detail in [25] and briefly in the Supplementary Materials. The chemical compositions of the feedstocks and resultant pulps are outlined in Table S2.
4.4. Enzymatic Hydrolysis of Pulps
The resultant pulps were enzymatically hydrolyzed with the commercial enzymes CelloLux-A and BrewZyme. The initial solid loading was 30 g/L. The enzymatic hydrolysis conditions and results are detailed in [69] and briefly in the Supplementary Materials (Table S3).
4.5. Preparation of Nutrient Media from Enzymatic Hydrolyzates of Pulps
The enzymatic hydrolyzates were centrifuged at 3500 rpm for 10 min; afterwards, they were standardized against glucose via the dilution method to bring the medium to a glucose content of 20 g/L.
The experiments revealed that the individual strains were not capable of producing BNC on the semisynthetic glucose medium supplemented with black tea extractives, on pristine hydrolyzate media, and on hydrolyzate media supplemented with black tea extractives. Therefore, further attempts were made to biosynthesize BNC using the individual strains on hydrolyzate media supplemented with nutrients similarly to the Hestrin–Schramm medium composition (except for glucose). Moreover, for the biosynthesis of BNC via symbiotic Medusomyces gisevii Sa-12, the nutrient media were pasteurized at 100 °C with no holding, which turned out to be inappropriate when the individual Komagataeibacter xylinus strains were used because of the observed contamination with a foreign microflora. Therefore, the nutrient media were autoclaved at 0.5 atm for 30 min.
While working with the symbiotic culture, the standardized hydrolyzates were heated to 100 °C and employed as extracting agents to recover black tea extractives. No vitamins or growth factors were added.
Prior to biosynthesis, the microbial producers were subjected to adaptation on the enzymatic hydrolyzate media under conditions set forth in Section 4.2.
4.6. Biosynthesis of BNC
During the biosynthesis of BNC on enzymatic hydrolyzates from cellulosic feedstocks, the cultivation was performed in 250 mL glass vessels, the nutrient medium volume being 100 mL. Each experiment employed 16 vessels. The inoculum comprised 10% of the nutrient medium volume. The cultivation was carried out at 27 °C for 14 days under static conditions. The pH was not adjusted during the cultivation. Each experiment was run in triplicate. BNC was further purified in line with [25] to furnish semitransparent, pearl-white BNC gel-films.
4.7. Calculation of BNC Yield
The yield of BNC was calculated by the equation:
| (1) |
where η is the BNC yield, %; mBNC is the BNC weight on an oven-dry basis, g; Cg is the glucose concentration of the medium, g/L; V is the volume of the medium, L; 0.9 is the recalculation factor attributed to the water molecule detachment upon polymerization of glucose into cellulose [70].
4.8. Analytical Techniques
The chemical composition of the cellulosic feedstocks and the resultant pulps (contents of cellulose, hemicelluloses, acid-insoluble lignin and ash) was analyzed according to the reported procedures [71,72,73,74]. The degree of polymerization was measured using a viscometer (OOO Ecroshim, Saint-Petersburg, Russia) using cadoxene (ethylenediamine, AO LenReaktiv, CAS No. 107-15-3, Saint-Petersburg, Russia; cadmium oxide, AO LenReaktiv, CAS No. 1306-19-0, Saint-Petersburg, Russia) according to the procedure described in [75]. The concentration of reducing sugars (RS) calculated as the glucose equivalent was measured using a spectrophotometer (United Products & Instruments, Dayton, NJ, USA) using 3,5-dinitrosalicylic acid (Panreac, CAS No. 609-99-4, Barcelona, Spain), as reported [76]. The content of pentosans calculated in xylose equivalent was measured using a spectrophotometer (United Products & Instruments, Dayton, NJ, USA) using the orcinol–ferric reagent (Acros Organics, a manufacturer of 99% orcinol monohydrate, CAS No. 6153-39-5, Dayton, NJ, USA), as described [77]. The moisture content of the feedstocks and substrates was measured on an Ohaus MB23 moisture analyzer (Ohaus Corporation, Parsippany, NJ, USA). The pH was measured using an I-160 MI ion meter (OOO Izmeritelnaya Tekhnika, Moscow, Russia). The microbial measures (yeast and acetobacterial counts) were monitored using an Optika B-150 microscope (Optika Microscopes, Milan, Italy). Scanning electron microscopy (SEM) of freeze-dried BNC samples was performed using a JSM-840 microscope (JEOL Ltd., Tokyo, Japan) with a Link-860 series II X-ray microanalyzer. Infrared spectroscopy (IR) was performed on an Infralum FT-801 FTIR spectrophotometer (OOO NPF Lumex Sibir, Novosibirsk city, Russia) at 4000–500 cm−1 in KBr pellets. Thermogravimetric analysis was performed on a Shimadzu DTG-60 thermogravimetric analyzer (Shimadzu, Kyoto, Japan). The test conditions were as follows: the test sample was heated at a rate of 10 °C/min to a maximum temperature of 600 °C in nitrogen environment at a flow rate of 40 mL/min. The sample weight was p = 6.0–6.5 mg. The strength of BNC was measured on a DTG-60 thermomechanical analyzer, whereby the test sample was stretched at a rate of 5.0 g/min from 0.0 g to a maximum load of 400.0 g until failure; the test temperature was 23.0 °C. X-ray diffraction was performed on a DRON-6 monochromatic diffractometer (Burevestnik, Nalchik city, Russia) with Fe-Kα radiation at scattering angles of 3° to 145°. Spectral characteristics of the X-ray micrograms were estimated using the PdWin software package v. 4.0. The crystallinity index estimation procedure and the full-profile analysis are described in detail in [35].
This work was performed with equipment provided by the Biysk Regional Center for Shared Use of Scientific Equipment of the SB RAS (IPCET SB RAS, Biysk, Russia).
5. Conclusions
Herein, we have investigated for the first time how a microbial producer (Komagataeibacter xylinus B-12429 and Komagataeibacter xylinus B-12431 individual strains and symbiotic Medusomyces gisevii Sa-12) influences the BNC yield and properties when BNC is produced from cellulosic feedstocks. The cellulosic feedstocks used were miscanthus and oat hulls that were available on a global scale. All the conversion stages were performed under the same conditions and involved pretreatment with dilute nitric acid and sodium hydroxide solutions, enzymatic hydrolysis, standardization of growth media against the contents of reducing sugars and black-tea extractives, and biosynthesis of BNC. The yield of BNC being produced by the symbiotic culture attained 1.8 g/L for oat hulls, which is 44–65% higher than that for the individual strains. For all the microbial producers, the yield of BNC derived from miscanthus was 6–37% lower than that from oat hulls, indicating the presence of inhibitors of BNC biosynthesis in miscanthus itself. The use of the symbiotic culture offers additional technological benefits, as there is no need to add mineral salts and growth factors to the media and it is enough to use pasteurization of the nutrient medium rather than sterilization. The physicochemical properties (nanofibril width, degree of polymerization, elastic modulus, Iα allomorph content, and crystallinity index) of the BNC samples obtained on hydrolyzate media depend, to a greater extent, on the microbial producer type and, to a lesser extent, on the composition of the nutrient medium used. Taken together, the criteria such as BNC yield, processability and BNC index of crystallinity have proved the expedient use of the symbiotic culture for the production of high-value BNC from low-cost cellulosic biomass.
Acknowledgments
The work was completed under the auspices of the Ministry of Science and Higher Education of the Russian Federation (project No. 121061500030-3).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241814401/s1.
Author Contributions
Conceptualization, E.A.S.; methodology E.A.S. and V.V.B.; software, N.A.S. and G.F.M.; validation, E.A.S., N.A.S. and V.V.B.; formal analysis, N.A.S.; investigation, G.F.M., M.A.S., E.A.S. and N.A.S.; data curation, E.A.S. and M.A.S.; writing—original draft preparation E.A.S., G.F.M. and M.A.S.; writing—review and editing, E.A.S., N.A.S., G.F.M. and V.V.B.; visualization, N.A.S., G.F.M. and M.A.S.; supervision, V.V.B.; project administration, V.V.B.; funding acquisition, V.V.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Taubert A., Leroux F., Rabu P., de Zea Bermudez V. Advanced Hybrid Nanomaterials. Beilstein J. Nanotechnol. 2019;10:2563–2567. doi: 10.3762/bjnano.10.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volova T., Kiselev E., Nemtsev I., Lukyanenko A., Sukovatyi A., Kuzmin A., Ryltseva G., Shishatskaya E. Properties of Degradable Polyhydroxyalkanoates with Different Monomer Compositions. Int. J. Biol. Macromol. 2021;182:98–114. doi: 10.1016/j.ijbiomac.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Klemm D., Petzold-Welcke K., Kramer F., Richter T., Raddatz V., Fried W., Nietzsche S., Bellmann T., Fischer D. Biotech Nanocellulose: A Review on Progress in Product Design and Today’s State of Technical and Medical Applications. Carbohydr. Polym. 2021;254:117313. doi: 10.1016/j.carbpol.2020.117313. [DOI] [PubMed] [Google Scholar]
- 4.Wu Z., Chen S., Li J., Wang B., Jin M., Liang Q., Zhang D., Han Z., Deng L., Qu X., et al. Insights into Hierarchical Structure–Property–Application Relationships of Advanced Bacterial Cellulose Materials. Adv. Funct. Mater. 2023;33:2214327. doi: 10.1002/adfm.202214327. [DOI] [Google Scholar]
- 5.Gregory D.A., Tripathi L., Fricker A.T.R., Asare E., Orlando I., Raghavendran V., Roy I. Bacterial Cellulose: A Smart Biomaterial with Diverse Applications. Mater. Sci. Eng. R Rep. 2021;145:100623. doi: 10.1016/j.mser.2021.100623. [DOI] [Google Scholar]
- 6.Volova T.G., Prudnikova S.V., Kiselev E.G., Nemtsev I.V., Vasiliev A.D., Kuzmin A.P., Shishatskaya E.I. Bacterial Cellulose (BC) and BC Composites: Production and Properties. Nanomaterials. 2022;12:192. doi: 10.3390/nano12020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasil’kov A., Butenko I., Naumkin A., Voronova A., Golub A., Buzin M., Shtykova E., Volkov V., Sadykova V. Hybrid Silver-Containing Materials Based on Various Forms of Bacterial Cellulose: Synthesis, Structure, and Biological Activity. Int. J. Mol. Sci. 2023;24:7667. doi: 10.3390/ijms24087667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrova V.A., Gofman I.V., Dubashynskaya N.V., Golovkin A.S., Mishanin A.I., Ivan’kova E.M., Romanov D.P., Khripunov A.K., Vlasova E.N., Migunova A.V., et al. Chitosan Composites with Bacterial Cellulose Nanofibers Doped with Nanosized Cerium Oxide: Characterization and Cytocompatibility Evaluation. Int. J. Mol. Sci. 2023;24:5415. doi: 10.3390/ijms24065415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moniri M., Boroumand Moghaddam A., Azizi S., Abdul Rahim R., Bin Ariff A., Zuhainis Saad W., Navaderi M., Mohamad R. Production and Status of Bacterial Cellulose in Biomedical Engineering. Nanomaterials. 2017;7:257. doi: 10.3390/nano7090257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stumpf T.R., Yang X., Zhang J., Cao X. In Situ and Ex Situ Modifications of Bacterial Cellulose for Applications in Tissue Engineering. Mater. Sci. Eng. C. 2018;82:372–383. doi: 10.1016/j.msec.2016.11.121. [DOI] [PubMed] [Google Scholar]
- 11.Rivas B., Moldes A.B., Domínguez J.M., Parajó J.C. Development of Culture Media Containing Spent Yeast Cells of Debaryomyces Hansenii and Corn Steep Liquor for Lactic Acid Production with Lactobacillus Rhamnosus. Int. J. Food Microbiol. 2004;97:93–98. doi: 10.1016/j.ijfoodmicro.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Cacicedo M.L., Castro M.C., Servetas I., Bosnea L., Boura K., Tsafrakidou P., Dima A., Terpou A., Koutinas A., Castro G.R. Progress in Bacterial Cellulose Matrices for Biotechnological Applications. Bioresour. Technol. 2016;213:172–180. doi: 10.1016/j.biortech.2016.02.071. [DOI] [PubMed] [Google Scholar]
- 13.Velásquez-Riaño M., Bojacá V. Production of Bacterial Cellulose from Alternative Low-Cost Substrates. Cellulose. 2017;24:2677–2698. doi: 10.1007/s10570-017-1309-7. [DOI] [Google Scholar]
- 14.Islam M.U., Ullah M.W., Khan S., Shah N., Park J.K. Strategies for Cost-Effective and Enhanced Production of Bacterial Cellulose. Int. J. Biol. Macromol. 2017;102:1166–1173. doi: 10.1016/j.ijbiomac.2017.04.110. [DOI] [PubMed] [Google Scholar]
- 15.Hussain Z., Sajjad W., Khan T., Wahid F. Production of Bacterial Cellulose from Industrial Wastes: A Review. Cellulose. 2019;26:2895–2911. doi: 10.1007/s10570-019-02307-1. [DOI] [Google Scholar]
- 16.Sharma C., Bhardwaj N.K. Bacterial Nanocellulose: Present Status, Biomedical Applications and Future Perspectives. Mater. Sci. Eng. C. 2019;104:109963. doi: 10.1016/j.msec.2019.109963. [DOI] [PubMed] [Google Scholar]
- 17.Ul-Islam M., Ullah M.W., Khan S., Park J.K. Production of Bacterial Cellulose from Alternative Cheap and Waste Resources: A Step for Cost Reduction with Positive Environmental Aspects. Korean J. Chem. Eng. 2020;37:925–937. doi: 10.1007/s11814-020-0524-3. [DOI] [Google Scholar]
- 18.Zhong C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020;8:605374. doi: 10.3389/fbioe.2020.605374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urbina L., Corcuera M.Á., Gabilondo N., Eceiza A., Retegi A. A Review of Bacterial Cellulose: Sustainable Production from Agricultural Waste and Applications in Various Fields. Cellulose. 2021;28:8229–8253. doi: 10.1007/s10570-021-04020-4. [DOI] [Google Scholar]
- 20.Lupașcu R.E., Ghica M.V., Dinu-Pîrvu C.-E., Popa L., Velescu B.Ș., Arsene A.L. An Overview Regarding Microbial Aspects of Production and Applications of Bacterial Cellulose. Materials. 2022;15:676. doi: 10.3390/ma15020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avcioglu N.H. Bacterial Cellulose: Recent Progress in Production and Industrial Applications. World J. Microbiol. Biotechnol. 2022;38:86. doi: 10.1007/s11274-022-03271-y. [DOI] [PubMed] [Google Scholar]
- 22.Andriani D., Apriyana A.Y., Karina M. The Optimization of Bacterial Cellulose Production and Its Applications: A Review. Cellulose. 2020;27:6747–6766. doi: 10.1007/s10570-020-03273-9. [DOI] [Google Scholar]
- 23.Chopda R., Ferreira J.A., Taherzadeh M.J. Biorefining Oat Husks into High-Quality Lignin and Enzymatically Digestible Cellulose with Acid-Catalyzed Ethanol Organosolv Pretreatment. Processes. 2020;8:435. doi: 10.3390/pr8040435. [DOI] [Google Scholar]
- 24.Son J., Lee K.H., Lee T., Kim H.S., Shin W.H., Oh J.-M., Koo S.-M., Yu B.J., Yoo H.Y., Park C. Enhanced Production of Bacterial Cellulose from Miscanthus as Sustainable Feedstock through Statistical Optimization of Culture Conditions. Int. J. Environ. Res. Public Health. 2022;19:866. doi: 10.3390/ijerph19020866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skiba E.A., Gladysheva E.K., Golubev D.S., Budaeva V.V., Aleshina L.A., Sakovich G.V. Self-Standardization of Quality of Bacterial Cellulose Produced by Medusomyces Gisevii in Nutrient Media Derived from Miscanthus Biomass. Carbohydr. Polym. 2021;252:117178. doi: 10.1016/j.carbpol.2020.117178. [DOI] [PubMed] [Google Scholar]
- 26.Skiba E.A., Gladysheva E.K., Budaeva V.V., Aleshina L.A., Sakovich G.V. Yield and Quality of Bacterial Cellulose from Agricultural Waste. Cellulose. 2022;29:1543–1555. doi: 10.1007/s10570-021-04372-x. [DOI] [Google Scholar]
- 27.Jayabalan R., Malbaša R.V., Lončar E.S., Vitas J.S., Sathishkumar M. A Review on Kombucha Tea-Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014;13:538–550. doi: 10.1111/1541-4337.12073. [DOI] [PubMed] [Google Scholar]
- 28.Teoh A.L., Heard G., Cox J. Yeast Ecology of Kombucha Fermentation. Int. J. Food Microbiol. 2004;95:119–126. doi: 10.1016/j.ijfoodmicro.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 29.Lin D., Liu Z., Shen R., Chen S., Yang X. Bacterial Cellulose in Food Industry: Current Research and Future Prospects. Int. J. Biol. Macromol. 2020;158:1007–1019. doi: 10.1016/j.ijbiomac.2020.04.230. [DOI] [PubMed] [Google Scholar]
- 30.Römling U., Galperin M.Y. Bacterial Cellulose Biosynthesis: Diversity of Operons, Subunits, Products, and Functions. Trends Microbiol. 2015;23:545–557. doi: 10.1016/j.tim.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casarica A., Campeanu G., Moscovici M., Ghiorghita A., Manea V. Improvement of Bacterial Cellulose Production By Aceobacter Xyilinum Dsmz-2004 on Poor Quality Horticultural Substrates Using the Taguchi Method for Media Optimization. Part I. Cellul. Chem. Technol. 2013;47:61–68. [Google Scholar]
- 32.Mangayil R., Rajala S., Pammo A., Sarlin E., Luo J., Santala V., Karp M., Tuukkanen S. Engineering and Characterization of Bacterial Nanocellulose Films as Low Cost and Flexible Sensor Material. ACS Appl. Mater. Interfaces. 2017;9:19048–19056. doi: 10.1021/acsami.7b04927. [DOI] [PubMed] [Google Scholar]
- 33.Alves A.A., Silva W.E., Belian M.F., Lins L.S.G., Galembeck A. Bacterial Cellulose Membranes for Environmental Water Remediation and Industrial Wastewater Treatment. Int. J. Environ. Sci. Technol. 2020;17:3997–4008. doi: 10.1007/s13762-020-02746-5. [DOI] [Google Scholar]
- 34.Aleshina L.A., Gladysheva E.K., Budaeva V.V., Mironova G.F., Skiba E.A., Sakovich G.V. X-ray Diffraction Data on the Bacterial Nanocellulose Synthesized by Komagataeibacter Xylinus B-12429 and B-12431 Microbial Producers in Miscanthus- and Oat Hull-Derived Enzymatic Hydrolyzates. Crystallogr. Rep. 2022;67:391–397. doi: 10.1134/S1063774522030026. [DOI] [Google Scholar]
- 35.Aleshina L.A., Gladysheva E.K., Budaeva V.V., Skiba E.A., Arkharova N.A., Sakovich G.V. X-ray Diffraction Study of Bacterial Nanocellulose Produced by the Medusomyces Gisevii Sa-12 Culture in Enzymatic Hydrolysates of Oat Hulls. Crystallogr. Rep. 2018;63:955–960. doi: 10.1134/S1063774518050024. [DOI] [Google Scholar]
- 36.Aleshina L.A., Gladysheva E.K., Budaeva V.V., Golubev D.S., Skiba E.A., Sakovich G.V. X-ray Diffraction Study of Bacterial Nanocellulose Produced by Medusomyces Gisevii Sa-12 Cultured in Enzymatic Hydrolysates of Miscanthus. Crystallogr. Rep. 2019;64:914–919. doi: 10.1134/S1063774519060026. [DOI] [Google Scholar]
- 37.Amorim L.F.A., Li L., Gomes A.P., Fangueiro R., Gouveia I.C. Sustainable Bacterial Cellulose Production by Low Cost Feedstock: Evaluation of Apple and Tea by-Products as Alternative Sources of Nutrients. Cellulose. 2023;30:5589–5606. doi: 10.1007/s10570-023-05238-0. [DOI] [Google Scholar]
- 38.Sharma C., Bhardwaj N.K., Pathak P. Static Intermittent Fed-Batch Production of Bacterial Nanocellulose from Black Tea and Its Modification Using Chitosan to Develop Antibacterial Green Packaging Material. J. Clean. Prod. 2021;279:123608. doi: 10.1016/j.jclepro.2020.123608. [DOI] [Google Scholar]
- 39.Nguyen H.T., Saha N., Ngwabebhoh F.A., Zandraa O., Saha T., Saha P. Kombucha-Derived Bacterial Cellulose from Diverse Wastes: A Prudent Leather Alternative. Cellulose. 2021;28:9335–9353. doi: 10.1007/s10570-021-04100-5. [DOI] [Google Scholar]
- 40.Campano C., Balea A., Blanco A., Negro C. Enhancement of the Fermentation Process and Properties of Bacterial Cellulose: A Review. Cellulose. 2016;23:57–91. doi: 10.1007/s10570-015-0802-0. [DOI] [Google Scholar]
- 41.Ramírez Tapias Y.A., Di Monte M.V., Peltzer M.A., Salvay A.G. Bacterial Cellulose Films Production by Kombucha Symbiotic Community Cultured on Different Herbal Infusions. Food Chem. 2022;372:131346. doi: 10.1016/j.foodchem.2021.131346. [DOI] [PubMed] [Google Scholar]
- 42.Liu C.-H., Hsu W.-H., Lee F.-L., Liao C.-C. The Isolation and Identification of Microbes from a Fermented Tea Beverage, Haipao, and Their Interactions during Haipao Fermentation. Food Microbiol. 1996;13:407–415. doi: 10.1006/fmic.1996.0047. [DOI] [Google Scholar]
- 43.Chakravorty S., Bhattacharya S., Chatzinotas A., Chakraborty W., Bhattacharya D., Gachhui R. Kombucha Tea Fermentation: Microbial and Biochemical Dynamics. Int. J. Food Microbiol. 2016;220:63–72. doi: 10.1016/j.ijfoodmicro.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 44.Hesseltine C.W. A Millennium of Fungi, Food, and Fermentation. Mycologia. 1965;57:149–197. doi: 10.1080/00275514.1965.12018201. [DOI] [PubMed] [Google Scholar]
- 45.Marsh A.J., O’Sullivan O., Hill C., Ross R.P., Cotter P.D. Sequence-Based Analysis of the Bacterial and Fungal Compositions of Multiple Kombucha (Tea Fungus) Samples. Food Microbiol. 2014;38:171–178. doi: 10.1016/j.fm.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Kutyshenko V.P., Yurkevich D.I. Influence of Heavy Water on the Metabolism of a Symbiotic Organism. Biophysics. 2003;48:648–656. [PubMed] [Google Scholar]
- 47.Zhu C., Li F., Zhou X., Lin L., Zhang T. Kombucha-Synthesized Bacterial Cellulose: Preparation, Characterization, and Biocompatibility Evaluation. J. Biomed. Mater. Res. A. 2014;102:1548–1557. doi: 10.1002/jbm.a.34796. [DOI] [PubMed] [Google Scholar]
- 48.Sharma C., Bhardwaj N.K. Biotransformation of Fermented Black Tea into Bacterial Nanocellulose via Symbiotic Interplay of Microorganisms. Int. J. Biol. Macromol. 2019;132:166–177. doi: 10.1016/j.ijbiomac.2019.03.202. [DOI] [PubMed] [Google Scholar]
- 49.Laavanya D., Shirkole S., Balasubramanian P. Current Challenges, Applications and Future Perspectives of SCOBY Cellulose of Kombucha Fermentation. J. Clean. Prod. 2021;295:126454. doi: 10.1016/j.jclepro.2021.126454. [DOI] [Google Scholar]
- 50.Pillai M.M., Tran H.N., Sathishkumar G., Manimekalai K., Yoon J., Lim D., Noh I., Bhattacharyya A. Symbiotic Culture of Nanocellulose Pellicle: A Potential Matrix for 3D Bioprinting. Mater. Sci. Eng. C. 2021;119:111552. doi: 10.1016/j.msec.2020.111552. [DOI] [PubMed] [Google Scholar]
- 51.Betlej I., Rybak K., Nowacka M., Antczak A., Borysiak S., Krochmal-Marczak B., Lipska K., Boruszewski P. Structural Properties of Bacterial Cellulose Film Obtained on a Substrate Containing Sweet Potato Waste. Crystals. 2022;12:1191. doi: 10.3390/cryst12091191. [DOI] [Google Scholar]
- 52.Ramírez Tapias Y.A., Peltzer M.A., Delgado J.F., Salvay A.G. Kombucha Tea By-Product as Source of Novel Materials: Formulation and Characterization of Films. Food Bioprocess Technol. 2020;13:1166–1180. doi: 10.1007/s11947-020-02471-4. [DOI] [Google Scholar]
- 53.Cubas A.L.V., Provin A.P., Dutra A.R.A., Mouro C., Gouveia I.C. Advances in the Production of Biomaterials through Kombucha Using Food Waste: Concepts, Challenges, and Potential. Polymers. 2023;15:1701. doi: 10.3390/polym15071701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bryszewska M.A., Tabandeh E., Jędrasik J., Czarnecka M., Dzierżanowska J., Ludwicka K. SCOBY Cellulose Modified with Apple Powder—Biomaterial with Functional Characteristics. Int. J. Mol. Sci. 2023;24:1005. doi: 10.3390/ijms24021005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong F., Guo X., Zhang S., Han S., Yang G., Jönsson L.J. Bacterial Cellulose Production from Cotton-Based Waste Textiles: Enzymatic Saccharification Enhanced by Ionic Liquid Pretreatment. Bioresour. Technol. 2012;104:503–508. doi: 10.1016/j.biortech.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 56.Cheng Z., Yang R., Liu X., Liu X., Chen H. Green Synthesis of Bacterial Cellulose via Acetic Acid Pre-Hydrolysis Liquor of Agricultural Corn Stalk Used as Carbon Source. Bioresour. Technol. 2017;234:8–14. doi: 10.1016/j.biortech.2017.02.131. [DOI] [PubMed] [Google Scholar]
- 57.Vasconcellos V.M., Farinas C.S., Ximenes E., Slininger P., Ladisch M. Adaptive Laboratory Evolution of Nanocellulose-producing Bacterium. Biotechnol. Bioeng. 2019;116:1923–1933. doi: 10.1002/bit.26997. [DOI] [PubMed] [Google Scholar]
- 58.Goelzer F.D.E., Faria-Tischer P.C.S., Vitorino J.C., Sierakowski M.-R., Tischer C.A. Production and Characterization of Nanospheres of Bacterial Cellulose from Acetobacter Xylinum from Processed Rice Bark. Mater. Sci. Eng. C. 2009;29:546–551. doi: 10.1016/j.msec.2008.10.013. [DOI] [Google Scholar]
- 59.Chen L., Hong F., Yang X., Han S. Biotransformation of Wheat Straw to Bacterial Cellulose and Its Mechanism. Bioresour. Technol. 2013;135:464–468. doi: 10.1016/j.biortech.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 60.Abol-Fotouh D., Hassan M.A., Shokry H., Roig A., Azab M.S., Kashyout A.E.-H.B. Bacterial Nanocellulose from Agro-Industrial Wastes: Low-Cost and Enhanced Production by Komagataeibacter Saccharivorans MD1. Sci. Rep. 2020;10:3491. doi: 10.1038/s41598-020-60315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi Q.S., Feng J., Li W.R., Zhou G., Chen A.M., Ouyang Y.S., Chen Y.B. Effect of Different Conditions on the Average Degree of Polymerization of Bacterial Cellulose Produced by Gluconacetobacter Inter-Medius BC-41. Cellul. Chem. Technol. 2013;47:503–508. [Google Scholar]
- 62.Bikales N.M., Segal L. Cellulose and Cellulose Derivatives. Parts IV–V. Wiley Interscience; Hoboken, NJ, USA: 1971. [Google Scholar]
- 63.Borzani W., de Souza S.J. Mechanism of the Film Thickness Increasing during the Bacterial Production of Cellulose on Non-Agitaded Liquid Media. Biotechnol. Lett. 1995;17:1271–1272. doi: 10.1007/BF00128400. [DOI] [Google Scholar]
- 64.D’Acierno F., Michal C.A., MacLachlan M.J. Thermal Stability of Cellulose Nanomaterials. Chem. Rev. 2023;123:7295–7325. doi: 10.1021/acs.chemrev.2c00816. [DOI] [PubMed] [Google Scholar]
- 65.French A.D. Increment in Evolution of Cellulose Crystallinity Analysis. Cellulose. 2020;27:5445–5448. doi: 10.1007/s10570-020-03172-z. [DOI] [Google Scholar]
- 66.Vazquez A., Foresti M.L., Cerrutti P., Galvagno M. Bacterial Cellulose from Simple and Low Cost Production Media by Gluconacetobacter xylinus. J. Polym. Environ. 2013;21:545–554. doi: 10.1007/s10924-012-0541-3. [DOI] [Google Scholar]
- 67.Kapustyanchik S.Y., Yakimenko V.N., Gismatulina Y.A., Budaeva V.V. Miscanthus—A Promising Energy Crop for Industrial Processing. Ecol. Ind. Russ. 2021;25:66–71. doi: 10.18412/1816-0395-2021-3-66-71. [DOI] [Google Scholar]
- 68.Keshk S.M. Bacterial Cellulose Production and Its Industrial Applications. J. Bioprocess. Biotech. 2014;4:150. doi: 10.4172/2155-9821.1000150. [DOI] [Google Scholar]
- 69.Kashcheyeva E.I., Gismatulina Y.A., Budaeva V.V. Pretreatments of Non-Woody Cellulosic Feedstocks for Bacterial Cellulose Synthesis. Polymers. 2019;11:1645. doi: 10.3390/polym11101645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hestrin S., Schramm M. Synthesis of Cellulose by Acetobacter xylinum. 2. Preparation of Freeze-Dried Cells Capable of Polymerizing Glucose to Cellulose. Biochem. J. 1954;58:345–352. doi: 10.1042/bj0580345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Standard Test Method for α-Cellulose. American Society for Testing and Materials; West Conshohocken, PA, USA: 1978. [Google Scholar]
- 72.Kurschner K., Hoffer A. Cellulose and Cellulose Derivative. J. Anal. Chem. 1993;92:145–154. [Google Scholar]
- 73.Om-85 Standard: Ash in Wood, Pulp, Paper, and Paperboard, Specifications of the Technical Association of the Pulp and Paper Industry. TAPPI Press; Atlanta, GA, USA: 1985. [Google Scholar]
- 74.Om-83 Standard: Acid-Insoluble Lignin in Wood and Pulp, Specifications of the Technical Association of the Pulp and Paper Industry. TAPPI Press; Atlanta, GA, USA: 1988. [Google Scholar]
- 75.Bogolitsyn K., Parshina A., Aleshina L. Structural Features of Brown Algae Cellulose. Cellulose. 2020;27:9787–9800. doi: 10.1007/s10570-020-03485-z. [DOI] [Google Scholar]
- 76.Miller G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 77.Obolenskaya A.V., Yelnitskaya Z.P., Leonovich A.A. Laboratornye Raboty po Khimii Drevesiny i Tsellyulozy (Laboratory Works on Wood and Cellulose Chemistry): Textbook for Higher Educational Institutions. Ecology Publisher; Moscow, Russia: 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.