Abstract
When the standard procedure for determining antibiotic susceptibility of bacteria is used, the results are delayed, especially for bacteria that grow slowly, such as Helicobacter pylori. Treatment for this bacterium may involve clarithromycin, a compound for which resistance has been associated with point mutations on the 23S rRNA gene. This resistance is currently found in organisms isolated from 0 to 15% of patients and jeopardizes the success of the treatment. We have designed a test involving amplification and colorimetric hybridization in the liquid phase to detect the mutation at the molecular level. First, four reference strains, including the wild type and three strains with the mutations A2143C, A2143G, and A2144G, were used to optimize the method. Amplification was carried out with primers previously published. The amplified products were added to probe-coated microtiter wells. A DNA enzyme immunoassay was used to detect the hybrids. The optimal conditions of the hybridization were defined for each probe. Nineteen H. pylori strains resistant to clarithromycin and 22 susceptible according to phenotypic data were submitted to restriction with BsaI and BbsI, and part of the 23S rRNA gene was sequenced in order to determine the mutation involved for the resistant strains. The new assay showed a complete correlation with the reference methods, except for one strain. Cross-hybridizations as well as application of the reaction to other bacteria did not lead to optical densities higher than the cutoff values chosen with the receiving operating characteristic curve. This method can be easily standardized and gives a result within a day. Its application directly to the biopsy specimens or infected gastric juice is planned in the future.
The market release of potent macrolides such as clarithromycin has stimulated the prescription of these drugs. Currently clarithromycin-based triple therapies are the treatment of choice for Helicobacter pylori infection (12). However, H. pylori can become resistant to this compound, and this resistance jeopardizes the success of these treatments (25). Resistance is due to a decrease in binding of macrolides to the ribosomes (16). This decrease in binding has been associated with point mutations on the 23S rRNA gene (24). The original work of Versalovic et al. (24) has been confirmed with clinical strains from other parts of the world, and three major point mutations have been described in which an adenine residue is replaced by a guanine or a cytosine residue: A2143G (formerly A2058G), A2143C (formerly A2058C), and A2144G (formerly A2059G) (5, 9, 16, 20, 21, 23). Currently, the determination of H. pylori resistance to macrolides is performed in clinical bacteriology laboratories by standard methods, such as disk diffusion or Etest. These methods, while efficient in discriminating between susceptible and resistant strains, can only give a result after several days and do not give insight to the type of mutation present, which could be of epidemiological value. A PCR-restriction fragment length polymorphism (PCR-RFLP) was described by Versalovic et al. (24) and applied more recently by others (5, 9, 16, 22), but this method allows the detection of only the A2143G and A2144G mutations and not the A2143C mutation.
Therefore, our goal was to develop a PCR-based hybridization method which would allow rapid detection of these point mutations. In this method, the amplified DNA is added to biotinylated probes bound in microtiter wells, and the hybrids formed are detected by an enzyme-linked immunosorbent assay (ELISA) with a labelled anti-double-stranded DNA monoclonal antibody. Forty-five strains were characterized by sequencing of the 23S rRNA gene fragment, and in 19 cases, one of the three mutations previously mentioned was present. A new DNA enzyme immunoassay (DEIA) was then applied.
MATERIALS AND METHODS
Bacterial strains and culture growth conditions.
One reference H. pylori strain, CIP 101260, and three strains (677, 683, and 825, isolated in our laboratory) were used for the establishment of the technique. Their susceptibility to clarithromycin and their nucleotide sequence at domain V of the 23S rRNA gene were determined in a previous study (16). In addition, 41 H. pylori strains isolated in our laboratory and found to be susceptible or resistant to clarithromycin, based on a routine screening test (disk diffusion or Etest), were included in this study. We used clarithromycin disks (Beckton Dickinson, Cockeysville, Md.) or Etest strips (AB Biodisk, Solna, Sweden) on Wilkins-Chalgren agar supplemented with 10% human blood. The strain was considered resistant when H. pylori grew up to the disk or when the MIC for the strain was higher than 2 μg/ml.
H. pylori strains were grown on an in-house Wilkins-Chalgren agar (Oxoid Unipath, Hampshire, England) supplemented with 10% human blood, vancomycin (10 mg/liter), cefsulodin (5 mg/liter), trimethoprim (5 mg/liter), and cycloheximide (Actidione [Upjohn]) (100 mg/liter). Plates were incubated at 37°C in a microaerobic atmosphere (GasPak in jars) for 2 to 3 days for subculture.
Fifteen other bacterial species were used to determine the specificity of the method: Escherichia coli, Campylobacter coli (three isolates), Campylobacter jejuni, Staphylococcus aureus (three isolates), Staphylococcus epidermidis, Streptococcus pneumoniae, Streptococcus viridans, Proteus mirabilis, Branhamella catarrhalis, Haemophilus influenzae, and Enterococcus faecalis. All of these strains were grown on agar supplemented with 5% sheep blood, except for H. influenzae, which was grown on chocolate agar.
Determination of MICs.
MICs of clarithromycin for the H. pylori strains were determined by an agar dilution method. Briefly, a suspension of 109 CFU/ml was applied to plates containing Wilkins-Chalgren agar enriched with 10% sheep blood and Polyvitex as well as serial dilutions of the antibiotic (ranging from 0.0035 to 128 μg/ml) by using a Steer’s apparatus. Incubation was performed in a microaerobic atmosphere for 48 h at 37°C. A strain was considered resistant if the drug MIC for it was >2 μg/ml. For other species, the susceptibility to erythromycin was determined by disk diffusion (Sanofi Diagnostics Pasteur, Marne la Coquette, France).
DNA extraction from bacterial cultures.
H. pylori DNA was extracted by a standard phenol chloroform procedure (14). Briefly, after 2 to 3 days of culture, the bacteria were harvested in 1 ml of 100 mM Tris HCl (pH 8)–100 mM EDTA (TE). They were centrifuged at 2,000 × g for 10 min, and the pellet was washed in TE and centrifuged again. The bacteria were then lysed by addition of lysozyme, sodium dodecyl sulfate, and proteinase K successively. The proteins were extracted by phenol chloroform. Finally, the DNA was precipitated by using ethanol and sodium acetate.
PCR.
The fragment Hp23S was obtained by PCR amplification of H. pylori genomic DNA with the two following primers, which we previously described: Hp23S-1 (5′CCACAGCGATGTGGTCTCAG3′) and Hp23S-2 (5′CTCCATAAGAGCCAAAGCCC3′), which are complementary to nucleotides 1820 to 1839 and 2244 to 2225, respectively (16). For PCR amplification, approximately 5 ng of genomic DNA of H. pylori was added to a 50-μl mixture containing 1× buffer, 1.5 mM MgCl2, 200 μM deoxynucleoside triphosphates (dNTPs), 1 U of Taq polymerase (Eurobio, Les Ulis, France) and 0.2 μM each primer. Amplification was carried out in a Perkin-Elmer Corporation (Norwalk, Conn.) DNA thermal cycler 480 over 39 cycles, each for 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C. These cycles were performed after a denaturation of 10 min at 94°C. The Hp23S fragment of 425 bp was visualized after electrophoresis on a 1.5% agarose gel stained with ethidium bromide. In order to test the specificity of the primers, purified DNA of non-H. pylori strains was also used as a template for PCR.
Probes and oligonucleotide sequences.
The H. pylori 23S rRNA gene sequence (GenBank accession no. U27270) was used to determine the probe sequences. Four probes were designed to be internal to the amplified fragment of the 23S rRNA gene (Hp23S) and to allow the detection of the nucleotides in positions 2143 and 2144. The probe sequences were as follows: pwt, 5′GGGGTCTTTCCGTCT3′; p43C, 5′GGTCTTGCCGTCTTG3′; p43G, 5′GGTCTTCCCGTCTTG3′; and p44G, 5′GGTCTCTCCGTCTTG3′. The probes, biotinylated at the 5′ end, were synthesized by Eurogentec (Seraing, Belgium).
Four oligonucleotides, comwt, com43C, com43G, and com44G, strictly complementary to the pwt, p43C, p43G, and p44G probes, respectively, were used to determine the hybridization conditions (i.e., hybridization temperature and probe concentration).
RFLP of amplified products.
The amplified fragment of the 23S rRNA gene was digested with the restriction enzymes BbsI and BsaI (New England Biolabs, Inc., Beverly, Mass.). The restriction enzymes BbsI and BsaI allow a discrimination between the wild-type genotype (AAAGACC, from position 2143 to position 2149) and two of the three mutant genotypes described: BbsI (restriction site, 5′GAAGAC(N)2↓3′) for A2143G and BsaI (restriction site, 5′↓ (N)5GAGACC3′) for A2144G. The restriction products were analyzed by electrophoresis on a 2% agarose gel stained with ethidium bromide (16).
Sequencing of amplified products.
In order to remove the excess dNTPs and the PCR primers, purification columns (Wizard PCR preps kit, DNA Purification System; Promega Corporation, Madison, Wis.) were used.
The sequencing reaction was performed according to the method of Sanger et al. (18) in 10 μl containing a combination of 4 μl of Terminator Ready Reaction, dNTPs, A/C/G/T-Dye terminator, and ampli-Taq polymerase FS (Perkin-Elmer, Applied Biosystems Division, Foster City, Calif.); 1.6 pmol of primer; and 1 to 4 μl of target DNA.
The amplification reaction was performed in the Perkin-Elmer 9600 thermal cycler. The amplification cycles were 10 s at 96°C, 5 s at 50°C, and 4 min at 60°C (25×). Following amplification, the sequenced DNA was purified by ethanol precipitation, and the pellet was resuspended in loaded buffer (dextran blue and formamide). After denaturation (2 min at 92°C), the reactions were analyzed by electrophoresis through a polyacrylamide (4.25%)-urea (7 M) gel in TBE buffer (89 mM Tris HCl [pH 8.3], 89 mM boric acid, 2 mM EDTA) at 51°C. The sequence analyses were resolved with the ABI PRISM collection program (Perkin-Elmer).
DEIAs.
The DEIA technique was performed according to the method of Monteiro et al. (15). All of the reagents except for the probes were provided in the GEN-ETI-K DEIA kit (Sorin Biomedica, Antony, France). Biotinylated probes (diluted in TE [pH 8]) specific for each genotype were added (50 ng/well) to the microtiter plates coated with streptavidin. The solid phase was incubated for 18 h at 2 to 8°C and washed five times with 300 μl of washing buffer (phosphate-buffered saline and Tween 20). Twenty microliters of denatured PCR products (15 min at 100°C) was added to all wells, except for the controls. The hybridization reaction was then performed with 100 μl of hybridization buffer (Denhardt’s solution, SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate] in Tris-HCl buffer, EDTA) and was carried out at 49°C under mild agitation for 1 h. After hybridization, the plates were washed, and the DNA-DNA hybrids were detected by an indirect method. First, 100 μl of anti-double-stranded DNA monoclonal antibody was added, and this mixture was then incubated for 1 h at room temperature and washed. Finally, 100 μl of horseradish peroxidase-labelled rabbit anti-mouse immunoglobulin G antibody was added, and this mixture was incubated for 1 h at room temperature. At the end of the incubation, the wells were washed. The hybridization was detected by addition of 100 μl of chromogen-substrate solution and incubation of this mixture in the dark for 30 min. After stopping the reaction with 200 μl of blocking reagent (1 N sulfuric acid solution), the A450 of each well was determined with a Titertek Multiskan Plus (Labsystems, Helsinki, Finland).
RESULTS
Determination of optimal hybridization conditions.
The optimal hybridization conditions were determined by considering the hybrids formed by the probes (pwt, p43C, p43G, and p44G) and the complementary oligonucleotides (comwt, com43C, com43G, and com44G), respectively. Two variables were taken into account.
First, the hybridization temperature was evaluated, and then the optimal concentration of the probe was specified. Based on the theoretical melting temperature of the four probes, the hybridization temperature of 48°C was first applied. A positive signal was obtained with an optical density at 450 nm (OD450) higher than 1.0. In order to increase the specificity of the reaction, the hybridization was then conducted at 49 and 50°C. Whatever the probe concentration was, saturant hybridization at 49°C was observed with an OD450 value above 2.5. When the hybridization was performed at 50°C, no signal was detected.
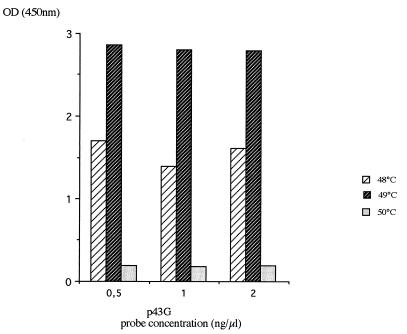
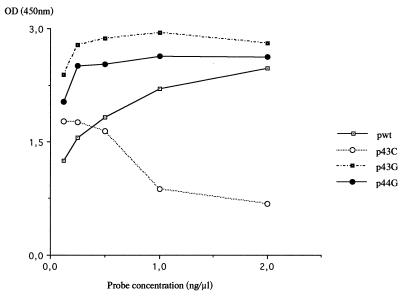
Thus, the hybridization temperature was fixed at 49°C and hybridization was performed under saturant conditions. One example is shown in Fig. 1. Different probe concentrations (ranging from 0.01 to 2 ng/μl) were mixed with a specific oligonucleotide at a given concentration (1 ng/μl). The hybridization was carried out at 49°C (Fig. 2). When the hybridization was performed with the p43G, and p44G probes, the saturation level was obtained rapidly (0.25 ng/μl). For the pwt probe, an OD450 value higher than 2.5 was obtained with a 2-ng/μl probe concentration. In contrast, a concentration-dependent inhibitor effect was observed with the p43C probe. The p43C probe was synthesized again, and these results were confirmed. To standardize the method for the four probes, a probe concentration of 0.5 ng/μl was chosen.
FIG. 1.
Effect of temperature of hybridization on the intensity of the signal detected. The p43G probe was hybridized with its complementary sequence (com43G) at various temperatures (48, 49, and 50°C) and for three probe concentrations (0.5, 1, and 2 ng/μl). The com43G concentration was 1 ng/μl.
FIG. 2.
Determination of an optimal probe concentration by using synthetic oligonucleotides. Each probe was hybridized with its complementary sequence (1 ng/μl) at various concentrations of between 0 and 2 ng/μl.
Limit of detection and specificity of the DEIA.
The minimal amount of DNA that can be detected was determined by the dilution method before amplification. Serial dilutions (50 to 5 × 10−5 ng) of reference strain DNA (CIP 101260, 677, 683, and 825) were amplified and tested for the hybridization method. The limit of detection of this assay was approximately 50 pg of specific DNA. In contrast, only 500 pg was detected by agarose gel (data not shown).
The specificity of the hybridization was tested by cross-hybridization between amplified target DNA of the reference strains with each probe. No cross-hybridization was observed. We then determined the specificity of the amplification and hybridization reactions by using species other than H. pylori as described above. Neither amplicon was revealed by electrophoresis for the different species tested, and hybridization signals were very weak compared to the values obtained with the reference strains.
Determination of phenotype and genotype by reference methods.
Among the 45 H. pylori strains tested, 23 were resistant to macrolides (drug MICs ranging between 4 and >128 μg/ml), whereas 22 were susceptible, with drug MICs ranging between 0.0035 and 2 μg/ml (Table 1).
TABLE 1.
Characteristics of the H. pylori strains tested for resistance to clarithromycin
| H. pylori isolate | Clarithromycin MIC (μg/ml) | PCR-RFLP status | Sequence of domain V of 23S rRNA genes | OD450 for probe:
|
|||
|---|---|---|---|---|---|---|---|
| pwt | p43C | p43G | p44G | ||||
| CIP101260a | 0.03 | Not restricted | Wild type | 1.380 | 0.193 | 0.089 | 0.079 |
| 594 | 0.06 | Not restricted | Wild type | 1.895 | 0.578 | 0.158 | 0.133 |
| 638 | 0.06 | Not restricted | Wild type | 1.950 | 0.472 | 0.133 | 0.071 |
| 646 | 0.06 | Not restricted | Wild type | 2.321 | 0.208 | 0.450 | 0.024 |
| 764 | 0.06 | Not restricted | Wild type | 1.071 | 0.110 | 0.027 | 0.021 |
| 803 | 0.06 | Not restricted | Wild type | 1.530 | 0.021 | 0.000 | 0.000 |
| 848 | 0.03 | Not restricted | Wild type | 1.742 | 0.076 | 0.000 | 0.000 |
| 1054 | 0.125 | Not restricted | Wild type | 1.588 | 0.136 | 0.092 | 0.412 |
| 1242 | 0.0035 | Not restricted | Wild type | 1.946 | 0.357 | 0.387 | 0.029 |
| 1309 | 0.0035 | Not restricted | Wild type | 1.738 | 0.247 | 0.053 | 0.026 |
| 1382 | 0.0035 | Not restricted | Wild type | 2.608 | 0.355 | 0.105 | 0.120 |
| 1383 | 0.0035 | Not restricted | Wild type | 2.070 | 0.319 | 0.091 | 0.113 |
| 1391 | 0.007 | Not restricted | Wild type | 2.617 | 0.555 | 0.163 | 0.128 |
| 1396 | 0.007 | Not restricted | Wild type | 2.345 | 0.331 | 0.138 | 0.120 |
| 1407 | 0.007 | Not restricted | Wild type | 2.526 | 0.368 | 0.117 | 0.105 |
| 1414 | 0.015 | Not restricted | Wild type | 1.716 | 0.084 | 0.276 | 0.030 |
| 1417 | 2 | Not restricted | Wild type | 2.422 | 0.298 | 0.083 | 0.288 |
| 1419 | 0.015 | Not restricted | Wild type | 2.773 | 0.536 | 0.098 | 0.240 |
| 1421 | 0.007 | Not restricted | Wild type | 2.652 | 0.358 | 0.081 | 0.113 |
| 1425 | 0.007 | Not restricted | Wild type | 2.957 | 0.458 | 0.104 | 0.123 |
| 1426 | 0.0035 | Not restricted | Wild type | 2.520 | 0.382 | 0.085 | 0.165 |
| 1495 | 0.0035 | Not restricted | Wild type | 1.806 | 0.229 | 0.048 | 0.022 |
| T-216 | 8 | Not restricted | Wild type | 1.768 | 0.074 | 0.133 | 0.026 |
| 825a | >128 | Not restricted | A2143C | 0.176 | 2.180 | 0.177 | 0.067 |
| 683a | 16 | BbsI | A2143G | 0.380 | 0.326 | 1.828 | 0.077 |
| 1060 | >128 | BbsI | A2143G | 0.017 | 0.023 | 0.864 | 0.009 |
| 1295 | 32 | BbsI | A2143G | 0.223 | 0.223 | 1.058 | 0.011 |
| 1307 | 32 | BbsI + NRb | A2143G/wild type | 1.467 | 0.198 | 0.524 | 0.023 |
| 1386 | 16 | BbsI + NR | A2143G | 0.515 | 0.406 | 1.116 | 0.097 |
| 1403 | >128 | BbsI + NR | A2143G | 0.426 | 0.385 | 0.893 | 0.095 |
| 1412 | 16 | BbsI | A2143G | 0.410 | 0.261 | 0.873 | 0.030 |
| 1470 | >128 | BbsI | A2143G | 0.316 | 0.215 | 1.053 | 0.016 |
| 677a | 32 | BsaI | A2144G | 0.245 | 0.142 | 0.124 | 2.051 |
| 675 | 32 | BsaI | A2144G | 0.212 | 0.065 | 0.028 | 1.347 |
| 782 | 64 | BsaI | A2144G | 0.134 | 0.000 | 0.000 | 1.560 |
| 1069 | 16 | BsaI | A2144G | 0.213 | 0.000 | 0.000 | 1.905 |
| 1190 | 16 | BsaI | A2144G | 0.170 | 0.094 | 0.050 | 1.432 |
| 1213 | 8 | BsaI | A2144G | 0.593 | 0.103 | 0.017 | 1.192 |
| 1222 | 4 | BsaI | A2144G | 0.343 | 0.098 | 0.046 | 1.130 |
| 1289 | 16 | BsaI | A2144G | 0.194 | 0.095 | 0.026 | 1.150 |
| 1377 | 16 | BsaI | A2144G | 0.254 | 0.087 | 0.050 | 1.203 |
| 1388 | 4 | BsaI | A2144G | 0.358 | 0.176 | 0.070 | 1.412 |
| 1390 | 4 | BsaI | A2144G | 0.617 | 0.170 | 0.122 | 2.040 |
| 1392 | 4 | BsaI + NR | A2144G | 0.664 | 0.148 | 0.076 | 1.996 |
| 1394 | 8 | BsaI | A2144G | 0.340 | 0.130 | 0.063 | 1.135 |
Strain used to develop the DEIA.
NR, not restricted.
Genomic DNA samples from H. pylori strains were submitted to amplification with the Hp23S-1 and Hp23S-2 primers (data not shown). A fragment of 425 bp was obtained whose size corresponded to that which could be expected. In contrast, no fragment was amplified when the DNA from other species was added.
The Hp23S fragment obtained from the 45 strains was submitted to restriction with BsaI and BbsI. Twenty-four amplicons of 45 strains were not digested. In contrast, 13 were restricted by BsaI (within one heterozygotic strain showing three bands), and 8 were restricted by BbsI (within three heterozygotic strains showing three bands) (data not shown).
All of the susceptible strains showed a wild-type genotype by sequencing of domain V or 23S rRNA genes, and among the 23 resistant strains, 13 showed the A2144G mutation, 8 showed the A2143G mutation (within 1 heterozygotic or wild-type strain), and 1 showed the A2143C mutation. Only one strain (T-216), considered resistant by MIC, showed a wild-type genotype by sequencing.
Application of the DEIA and determination of the cutoff values.
The values obtained with the DEIA are presented in Table 1. In one instance (strain 1307), a positive signal was also obtained with the wild-type probe in addition to the probes detecting the mutation A2143G.
These results are in agreement with the PCR-RFLP data, which showed the persistence of the original band in addition to the two bands obtained by restriction and with the sequencing data. In the other three cases in which PCR-RFLP showed three bands, neither the DEIA nor sequencing detected the wild type in addition to the mutant.
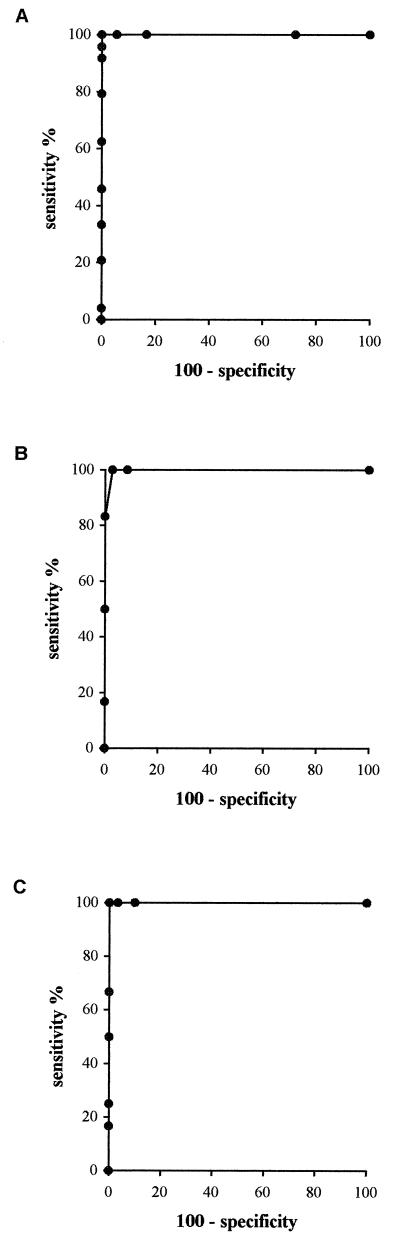
For all probes, except p43C, the determination of the cutoff values was performed by the receiving operating characteristic (ROC) curve construction method (6) (Fig. 3). Different cutoff values between 0 and 3 OD450 units were assigned, and for each cutoff value, the number of true positives, true negatives, false positives, and false negatives was determined by comparing the results of the hybridization reaction with those of the methods used as references in this study (MIC, PCR-RFLP, and sequencing). For each cutoff value, the sensitivity rate (defined as the number of true-positive strains obtained by DEIA/number of positive strains obtained by the reference method) and the specificity rate (defined as the number of false-negative strains obtained by DEIA/the number of negative strains obtained by the method used as a reference in this study) were used to draw the curve: % sensitivity = f (100% specificity). Thus, each cutoff generates a sensitivity value and a specificity value. These data are then used to choose a cutoff value which provides the best compromise between sensitivity and specificity. The pair of values generated may be represented on a graph in order to have a more complete vision of the performance of the test. The ROC curve is useful in this respect. With 100% sensitivity and 100% specificity, the cutoff values for pwt, p43G, and p44G were 0.700, 0.500, and 0.500, respectively.
FIG. 3.
ROC curves obtained for the probes corresponding to the wild-type genotype (pwt) (A), the A2143G genotype (p43G) (B), and the A2144G genotype (p44G) (C), with percent sensitivity values (true-positive rates) plotted on the y axis and the complementary percent specificity values (true-negative rates) plotted on the x axis.
For probe p43C, because of the small number of strains with the A2143C mutation, the mean of the OD450 values obtained for the strains which did not harbor the mutation, by the methods used as references in this study, was calculated, and the cutoff value was defined as 2 standard deviations above the mean. The cutoff obtained was 0.600.
DISCUSSION
In this study, we used a hybridization assay which allows the detection of point mutations associated with resistance to macrolides on the sequence amplified of the 23S rRNA genes of H. pylori. The conditions which we determined would allow discrimination of a single base change and saturant hybridization conditions (OD of ≥2.5) were rapidly obtained. However, the hybridization reaction with the p43C probe showed a concentration-dependent inhibitor effect, whereas the probe sequence was very similar to the three others (only one or two nucleotide changes). We could explain this inhibition by the existence of links between reactive groups within the probe. These bimolecular interactions would inhibit the hybridization with the target DNA (1). A way to avoid this problem could be to heat the solid phase before hybridization to break these links; and second, to increase the hybridization time to allow increased hybridization with the DNA target. We therefore assumed that the formation of bimolecular interactions was a reversible thermodynamical reaction.
The sensitivity and the specificity of the method were determined. We detected a 10-fold increase in amplicons with the hybridization reaction compared to the electrophoresis gel, as was reported in previous studies (11, 15). The limit of detection of this assay was 50 pg of specific DNA. The fragment to be amplified was also determined in a previous study based on known sequences of the H. pylori 23S rRNA gene and on the position of the expected mutations. So far the fragment has proved to be specific for H. pylori. We selected species which are potential contaminants of gastric biopsies during endoscopy. Under the same conditions of amplification and hybridization, no false-positive reaction was obtained; therefore, the method developed in this study seems to be specific for H. pylori strains.
DEIA have been used in the past to detect viruses, fungi, and bacterial genes (2–4, 8, 10). The use of a method designed to detect DNA hybrids, i.e., GEN-ETI-K DEIA, renders this technique very simple and powerful. An alternative could have been to coat the amplified products on the plate. Such a technique may facilitate hybridization by decreasing the steric interference but would have the consequence of adding a further step to the reaction.
The GEN-ETI-K DEIA has been used as a colorimetric method to detect amplicons in different situations, such as the detection of cystic fibrosis mutations (19). We applied this technique to H. pylori detection in a previous study, in comparison to other similar methods based on slightly different principles, and found it to be the most accurate (15). Since there is no “yes” or “no” response with this method, it is necessary to determine a cutoff value for a positive reaction. For this purpose, we used the ROC curve, which can be considered as the optimal method since it gives sensitivity and specificity values for all possible cutoffs. As in any determination of these parameters, a valuable reference method must be used. We used strains known to be susceptible or resistant according to their MICs and for which genetic tests had been applied to study the site of the point mutation, i.e., sequencing and restriction of amplicons. The method used for determination of MICs on agar differs slightly from the method recently recommended by the National Committee for Clinical Laboratory Standards. We used Wilkins-Chalgren agar instead of Mueller-Hinton agar, a larger inoculum (109 instead of 107 to 108 CFU/ml), and a shorter incubation time (48 h instead of 72 h). In addition to the three mutations tested in this study, two others have been recently described by Hultén et al. (9). However, because they were not present on the gene sequence of the H. pylori strains included in this study, we did not develop the corresponding probes.
The occurrence of a double signal indicates that a mixed population of strains is present. However, with a phenotypic method, only the resistant strains tend to be detected. PCR-RFLP was the only method used as reference in this work to permit the detection.
There was a good correlation with the methods used as reference. However, in one case (T-216), the method failed: resistance was detected by phenotypic methods (MIC, 8 μg/ml) but not by the DEIA. One possibility is that another resistance mechanism may be responsible. Indeed, two new mutations (2616 and 2142) were found (9), but we could verify by sequencing that they were not involved in the case of strain T-216. Another possibility is that two populations of the strain exist: one susceptible (wild type) and one resistant, the resistant population being present in much lower quantity. In such instances, MIC determination by agar dilution will only show the resistant strain. To test this hypothesis, we applied our technique directly to a biopsy specimen in this case and found both the wild type and the resistant strain. We think that during the process of subculture, the few resistant organisms present may have been lost, leading to detection of the wild type and not the resistant mutant by DEIA and also by sequencing.
We thought that a method based on hybridization and colorimetric detection would be well adapted to routine work. A similar approach has already been proposed by Stone et al. (20), namely the PCR-oligonucleotide ligation assay (PCR-OLA). This technique requires a greater number of oligonucleotides than our method for a comparable discrimination. The detection method used is also less sensitive, because it is a direct method as opposed to our indirect method. Other methods were developed in order to detect point mutations, such as the single-strand chain polymorphism (17), PCR amplification of specific alleles (1a), and denaturing gradient gel electrophoresis (7). These methods provide detection on gel and are much more difficult to implement. Furthermore, the nature of the mutated nucleotide is not determined.
Resistance to clarithromycin is the main predictor of failure of eradication treatments including this compound (13). Because of an increased use of these macrolides, not only for H. pylori eradication, but also for the treatment of respiratory tract infections, the prevalence rate of resistant strains is increasing, and the detection of resistance is becoming of major importance. To render this detection process more effective, results must be available rapidly. This method provides a result within a few hours once the strain has been isolated, compared to 2 to 3 days when a standard phenotypic method is used. Furthermore, because PCR technology and ELISA are now of general use in microbiology laboratories, it can be easily implemented. Nevertheless, the time necessary to get a positive culture is still required. Studies are currently in progress in our laboratory to apply this technique to DNA directly amplified from biopsy specimens, which would provide a result on the day of endoscopy.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the Conseil Régional d’Aquitaine and the Association pour la Recherche Médicale en Aquitaine (Université de Bordeaux 2). We thank Sorin Biomedica, Antony, France, for providing GEN-ETI-K DEIA kits.
Lurdes Monteiro was the recipient of a Junta Nacional de Investigaçâo Científica e Técnológica fellowship from Portugal.
REFERENCES
- 1. Bonnet, J. E. Personal communication.
- 1a.Bottema C D, Sommer S S. PCR amplification of specific alleles: rapid detection of known mutations and polymorphisms. Mutat Res. 1993;288:93–102. doi: 10.1016/0027-5107(93)90211-w. [DOI] [PubMed] [Google Scholar]
- 2.Buck G E. Detection of Bordetella pertussis by rapid-cycle PCR and colorimetric microwell hybridization. J Clin Microbiol. 1996;34:1355–1358. doi: 10.1128/jcm.34.6.1355-1358.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chevrier D, Popoff M Y, Dion M P, Hermant D. Rapid detection of Salmonella subspecies I by PCR combined with non-radioactive hybridisation using covalently immobilised oligonucleotide on a microplate. FEMS Immunol Med Microbiol. 1995;10:245–252. doi: 10.1111/j.1574-695X.1995.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 4.Cho S-N, van der Vliet G M E, Park S, Baik S-H, Kim S-K, Chong Y, Kolk A H J, Klatser P R, Kim J-D. Colorimetric microwell plate hybridization assay for detection of amplified Mycobacterium tuberculosis DNA from sputum samples. J Clin Microbiol. 1995;33:752–754. doi: 10.1128/jcm.33.3.752-754.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debets-Ossenkopp Y J, Sparrius M, Kusters J G, Kolkman J J, Vandenbroucke-Grauls C M J E. Mechanism of clarithromycin resistance in clinical isolates of Helicobacter pylori. FEMS Microbiol Lett. 1996;142:37–42. doi: 10.1111/j.1574-6968.1996.tb08404.x. [DOI] [PubMed] [Google Scholar]
- 6.Fauchère J L. The anti-H. pylori serum antibody response. In: Lee A, Mégraud F, editors. Helicobacter pylori: techniques for clinical diagnosis and basic research. W. B. London, United Kingdom: Saunders, Ltd.; 1996. pp. 50–73. [Google Scholar]
- 7.Fodde R, Losekoot M. Mutation detection by denaturing gradient gel electrophoresis (DGGE) Hum Mutat. 1994;3:83–94. doi: 10.1002/humu.1380030202. [DOI] [PubMed] [Google Scholar]
- 8.Fujita S-I, Lasker B A, Lott T J, Reiss E, Morrison C J. Microtitration plate enzyme immunoassay to detect PCR-amplified DNA from Candida species in blood. J Clin Microbiol. 1995;33:962–967. doi: 10.1128/jcm.33.4.962-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hultén K, Gibreel A, Sköld O, Engstrand L. Macrolide resistance in Helicobacter pylori: mechanism and stability in strains from clarithromycin-treated patients. Antimicrob Agents Chemother. 1997;41:2550–2553. doi: 10.1128/aac.41.11.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz J B, Alstad A D, Gustafson G A, Moser K M. Sensitive identification of bluetongue virus serogroup by a colorimetric dual oligonucleotide sorbent assay of amplified viral nucleic acid. J Clin Microbiol. 1993;31:3028–3030. doi: 10.1128/jcm.31.11.3028-3030.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lage A P, Fauconnier A, Burette A, Glupczynski Y, Bollen A, Godfroid E. Rapid colorimetric hybridization assay for detecting amplified Helicobacter pylori DNA in gastric biopsy specimens. J Clin Microbiol. 1996;34:530–533. doi: 10.1128/jcm.34.3.530-533.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lind T, van Zanten S V, Unge P, Spiller R, Bayerdörffer E, O’Morain C, Bardhan K D, Bradette M, Chiba N, Wrangstadh M, Cederberg C, Idstrom J P. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH1 study. Helicobacter. 1996;3:138–144. doi: 10.1111/j.1523-5378.1996.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 13.Mégraud, F., and H. P. Doermann. 1998. Clinical relevance of resistant strains of Helicobacter pylori: a review of current data. Gut 43(Suppl. 1):S61–S65. [DOI] [PMC free article] [PubMed]
- 14.Monteiro L, Birac C, Mégraud F. Detection of Helicobacter pylori in gastric biopsy by polymerase chain reaction. In: Lee A, Mégraud F, editors. Helicobacter pylori: techniques for clinical diagnosis and basic research. W. B. London, United Kingdom: Saunders, Ltd.; 1996. pp. 112–120. [Google Scholar]
- 15.Monteiro L, Cabrita J, Mégraud F. Evaluation of performances of three DNA enzyme immunoassays for detection of Helicobacter pylori PCR products from biopsy specimens. J Clin Microbiol. 1997;35:2931–2936. doi: 10.1128/jcm.35.11.2931-2936.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Occhialini A, Urdaci M, Doucet-Populaire F, Bébéar C M, Lamouliatte H, Mégraud F. Macrolide resistance in Helicobacter pylori: rapid detection of point mutations and assays of macrolide binding to ribosomes. Antimicrob Agents Chemother. 1997;41:2724–2728. doi: 10.1128/aac.41.12.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orita M, Suzuki Y, Sekiya T, Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989;5:874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- 18.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sangiuolo F, Maceratesi P, Mesoraca A, Botta A, Cavicchini A, Novelli G, Dallapiccola B. Simultaneous detection of delta F508, G542X, N1303K, G551D, and 1717-1G→A cystic fibrosis alleles by a multiplex DNA enzyme immunoassay. Int J Clin Lab Res. 1995;25:142–145. doi: 10.1007/BF02592555. [DOI] [PubMed] [Google Scholar]
- 20.Stone G G, Shortridge D, Versalovic J, Beyer J, Flamm R K, Graham D Y, Ghoneim A T, Tanaka S K. A PCR-oligonucleotide ligation assay to determine the prevalence of 23S rRNA gene mutations in clarithromycin-resistant Helicobacter pylori. Antimicrob Agents Chemother. 1997;41:712–714. doi: 10.1128/aac.41.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone G G, Shortridge D, Flamm R K, Versalovic J, Beyer J, Idler K, Zulawinski L, Tanaka S K. Identification of a 23S rRNA gene mutation in clarithromycin-resistant Helicobacter pylori. Helicobacter. 1996;4:227–228. doi: 10.1111/j.1523-5378.1996.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 22.Szczebara F, Dhaenens L, Vincent P, Husson M O. Evaluation of rapid molecular methods for detection of clarithromycin resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1997;16:162–164. doi: 10.1007/BF01709478. [DOI] [PubMed] [Google Scholar]
- 23.Taylor D E, Ge Z, Purych D, Lo T, Hiratsuka K. Cloning and sequence analysis of two copies of a 23S rRNA gene from Helicobacter pylori and association of clarithromycin resistance with 23S rRNA mutations. Antimicrob Agents Chemother. 1997;41:2621–2628. doi: 10.1128/aac.41.12.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Versalovic J, Shortridge D, Kibler K, Griffy M V, Beyer J, Flamm R K, Tanaka S K, Graham D Y, Go M F. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob Agents Chemother. 1996;40:477–480. doi: 10.1128/aac.40.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wurzer H, Rodrigo L, Stamler D, Archambault A, Rokkas T, Skandalis N, Fedorak R, Bazzoli F, Hentschel E, Mora P, Archimandritis A, Mégraud F. Short-course therapy with amoxicillin-clarithromycin triple therapy for 10 days (ACT-10) eradicates Helicobacter pylori and heals duodenal ulcer. ACT-10 Study Group. Aliment Pharmacol Ther. 1997;11:943–952. doi: 10.1046/j.1365-2036.1997.00223.x. [DOI] [PubMed] [Google Scholar]