Abstract
Serological diagnosis and follow-up of paracoccidioidomycosis (PCM) patients have relied mainly on the detection of antibody responses by using techniques such as complement fixation (CF) and immunodiffusion. We recently described a novel inhibition enzyme-linked immunosorbent assay (inh-ELISA) which proved to be useful in the diagnosis of PCM via the detection of an 87-kDa determinant in patient sera (B. L. Gomez, J. I. Figueroa, A. J. Hamilton, B. Ortiz, M. A. Robledo, R. J. Hay, and A. Restrepo, J. Clin. Microbiol. 35:3278–3283, 1997). This test has now been assessed as a means of following up PCM patients. A total of 24 PCM patients, classified according to their clinical presentation (6 with the acute form of the disease, of whom two had AIDS, 12 with the multifocal form of the disease, and 6 with the unifocal form of the disease), were studied. The four human immunodeficiency virus-negative patients with acute PCM showed a statistically significant decrease in circulating antigen levels after the start of antifungal therapy. Antigen levels in this group became negative by our criteria (≤2.3 μg/ml) before week 20 and remained so in three of four of these patients. In contrast, the two AIDS patients who also presented with the acute form of PCM showed no statistically significant decrease in circulating antigen levels even after 68 weeks of therapy. Taken together as a group, the patients with the multifocal form showed a statistically significant decrease in antigenemia after 28 weeks of therapy. In addition, five of six patients with the unifocal form became antigen negative by week 40. Antigen level decrease mirrored clinical cure in the majority of patients in all clinical groups; in contrast, measurement of anti-PCM antibodies via the CF test showed wide fluctuations in titers during the follow-up period. The inh-ELISA for the detection of the 87-kDa Paracoccidioides brasiliensis determinant would appear to be a valuable additional tool in the follow-up of PCM patients.
Paracoccidioidomycosis (PCM), one of the most important systemic mycoses in Central and South America, is caused by the dimorphic fungus Paracoccidioides brasiliensis (2, 27). The disease primarily involves the lungs and then disseminates to other organs and systems. Secondary lesions frequently appear in the mucous membranes, skin, lymph nodes, and adrenal glands. Both the clinical presentation and the course of the disease vary from patient to patient (2, 27), hindering prompt clinical diagnosis and effective follow-up (20). Active disease is classified in two main clinical forms, the acute or subacute form (juvenile type) and the chronic form (adult type) (11).
The definitive diagnosis of PCM is accomplished by direct visualization of the fungus in clinical and biopsy specimens and its isolation by culture (2). Serological tests are useful for rapid diagnosis (4, 6, 7, 21, 26, 30) and generally rely on the detection of antibody responses against components of P. brasiliensis (24). The most commonly used tests are complement fixation (CF) (9) and immunodiffusion (ID) (5, 25). However, both have important limitations that arise from cross-reactivity, a particular problem in CF, and from the lack of antigen standardization (24). In addition, their value in monitoring patients is problematic, which is due, at least in part, to the diversity and complexity of the humoral response in PCM patients (19).
Detection of P. brasiliensis circulating antigens in body fluids offers potential advantages over antibody detection, both for the initial diagnosis of PCM and the follow-up of patients. Several techniques for the detection of antigenemia in PCM have been utilized, with variable results (10, 12–14, 22, 24, 28). We recently described an inhibition enzyme-linked immunosorbent assay (inh-ELISA) for the initial diagnosis of PCM which relies on the detection of a circulating 87-kDa antigenic determinant by using P1B, a monoclonal antibody (MAb) (15). This test exhibited a sensitivity of 80.4% and a specificity of 81.4% (15). We now present results demonstrating that the inh-ELISA is also useful in monitoring the follow-up of PCM patients.
MATERIALS AND METHODS
Patients and serum samples.
Twenty-four PCM patients whose diagnosis was established by direct observation and/or isolation of P. brasiliensis in cultures, as well as by conventional serologic testing, were studied. Six patients, including 2 with AIDS, presented with the acute-subacute juvenile form, 12 presented with the chronic multifocal form, and 6 presented with the chronic unifocal form of the disease (11). A total of 229 serum samples taken at the time of diagnosis and subsequently at every follow-up appointment were analyzed. Samples were collected between January 1989 and March 1998 at the Mycology Laboratory, Corporación para Investigaciones Biológicas, Medellín, Colombia. The mean age of patients with the acute juvenile form was 21.6 years, whereas the mean age of patients with the chronic forms was 47.5 years. All patients but one were males. The time of follow-up as well as medication received and length of therapy is included in Table 1. Forty normal human serum (NHS) samples from healthy volunteers from areas where PCM is endemic and with no history of lung disease were included as negative controls. All sera were stored at −20°C until use.
TABLE 1.
Characteristics of the 24 PCM patients studied according to their clinical classification
| Disease group and patient no. | Age (yr) | No. of samples tested | Length of follow-up (wk) | Treatmenta | Length of therapy (mo) | Ag level at diagnosis (μg/ml)b | Ag level at first follow-up (μg/ml) | CS at first follow-upc | Ag level at midtreatment (μg/ml) | CS at midtreatmentc | Ag level at end of treatment (μg/ml) | CS at end of treatmentc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute juvenile form | ||||||||||||
| 1 | 24 | 4 | 24 | ITZ | 6d | 83.5 | 50.5 | 0.33 | 50.5 | 0.33 | 11.6d | 0.5d |
| 2 | 22 | 7 | 26 | FCZ | 11 | 141.7 | 107.2 | 0.6 | 62.5 | 0.66 | 0.0 | 0.93 |
| 3 | 14 | 11 | 164 | SPZ | 12 | 140.0 | 20.8 | 0.46 | 0.0 | 0.86 | 0.0 | 0.97 |
| 4 | 14 | 9 | 109 | SPZ | 12 | 51.3 | 12.3 | 0.5 | 0.0 | 0.96 | 0.0 | 1 |
| Acute juvenile form with AIDS | ||||||||||||
| 5 | 26 | 7 | 39 | ITZ | 9d | 6.5 | 9.4 | 0.75 | 20.0 | 0.89 | 11.6d | 0.93d |
| 6 | 30 | 14 | 68 | ITZ | 16d | 13.0 | 13.3 | 0.0 | 16.0 | 0.73 | 3.2d | 1.0d |
| Chronic multifocal form | ||||||||||||
| 7 | 40 | 11 | 124 | ITZ | 6 | 12.2 | 0.0 | 0.94 | 0.0 | 0.94 | 0.0 | 0.8 |
| 8 | 51 | 12 | 63 | ITZ | 6 | 6.0 | 3.7 | 0.3 | 2.4 | 0.85 | 0.0 | 0.92 |
| 9 | 59 | 9 | 92 | ITZ | 12 | 3.0 | 1.7 | 0.35 | 0.0 | 0.91 | 0.0 | 0.97 |
| 10 | 65 | 12 | 79 | ITZ | 7 | 9.6 | 4.7 | 0.05 | 1.6 | 0.85 | 0.0 | 0.85 |
| 11 | 31 | 14 | 104 | ITZ | 12 | 15.0 | 12.7 | 0.57 | 0.0 | 0.83 | 0.0 | 0.98 |
| 12 | 38 | 14 | 85 | ITZ | 8 | 6.2 | 9.6 | 0.59 | 0.0 | 0.82 | 0.0 | 0.91 |
| 13 | 25 | 8 | 52 | ITZ | 12 | 15.3 | 11.3 | 0.43 | 11.3 | 0.86 | 0.0 | 0.82 |
| 14 | 38 | 8 | 69 | SPZ | 12 | 30.7 | 15.6 | 0.93 | 4.1 | 0.83 | 0.0 | 0.9 |
| 15 | 65 | 13 | 98 | ITZ | 8 | 15.5 | 13.3 | 0.38 | 4.7 | 0.73 | 0.0 | 0.81 |
| 16 | 35 | 8 | 124 | ITZ | 6 | 24.8 | 2.3 | 0.6 | 2.9 | 0.92 | 0.0 | 0.83 |
| 17 | 48 | 7 | 96 | ITZ | 7 | 24.2 | 9.8 | 0.82 | 6.6 | 0.94 | 5.0 | 0.94 |
| 18 | 48 | 9 | 102 | ITZ | 8 | 26.7 | 40.4 | 0.88 | 36.0 | 0.66 | 22.0 | 0.61 |
| Chronic unifocal form | ||||||||||||
| 19 | 29 | 10 | 96 | ITZ | 7 | 14.9 | 8.9 | 0.71 | 3.2 | 0.87 | 0.0 | 0.9 |
| 20 | 38 | 6 | 69 | SPZ | 7 | 11.3 | 8.8 | 0.87 | 3.8 | 0.92 | 0.0 | 0.92 |
| 21 | 51 | 9 | 120 | ITZ | 13 | 3.1 | 1.0 | 0.72 | 0.0 | 0.88 | 0.0 | 0.94 |
| 22 | 54 | 9 | 122 | ITZ | 14 | 3.4 | 0.3 | 0.5 | 0.0 | 0.66 | 0.0 | 0.83 |
| 23 | 46 | 12 | 125 | ITZ | 6 | 6.7 | 0.0 | 0.6 | 0.0 | 0.8 | 0.0 | 0.9 |
| 24 | 44 | 6 | 52 | ITZ | 8 | 44.8 | 70.0 | 0.65 | 62.0 | 0.75 | 54.0 | 0.8 |
ITZ, itraconazole (100 to 200 mg/day); SPZ, saperconazole (100 to 300 mg/day); FCZ, fluconazole (200 mg/day).
Clinical score at time of diagnosis was 0 in all cases. Ag, antigen.
Clinical score (CS): 0, no change; 0.01 to 0.49, minor improvement; 0.50 to 0.99, major improvement; 1, resolution.
Patient still undergoing treatment.
Clinical evaluation.
Patients were evaluated clinically at the time of diagnosis and at each follow-up visit. Their response to therapy was measured by a comparative scoring system that has been reported previously (28). Briefly, this system has four categories, with values defined as follows: a value of 0 represents the clinical score at the time of diagnosis and also indicates no changes in clinical condition in subsequent visits, values of 0.01 to 0.49 indicate minor improvement, values of 0.50 to 0.99 indicate marked improvement, and a value of 1 denotes resolution. Clinical scores at the first follow-up visit, midtreatment, and at the end of follow-up are given in Table 1.
Antigen detection by inh-ELISA.
The inh-ELISA was performed as previously described (15). Briefly, standard inhibition curve was prepared by adding known concentrations of P. brasiliensis CIB 339 cytoplasmic yeast antigen (CYA) to pooled NHS (inhibition standards) (15). The inhibition reaction occurred when constant aliquots of MAb P1B were mixed with the inhibition standards, PCM patient serum samples, and NHS control samples; these samples were then plated on previously blocked microtiter plates (inhibition plates) and incubated overnight at 4°C. Reaction plates were coated with P. brasiliensis CIB 339 CYA and also incubated overnight at 4°C. The latter were then blocked, and samples from each well in the inhibition plates (containing a mixture of the MAb bound to circulating antigen complexes and free MAb) were transferred to the respective wells in the reaction plates. After the plates were washed, they were probed with goat anti-mouse immunoglobulin G–peroxidase conjugate and developed with a chromogenic substrate as previously described (15). The optical density (OD) readings at 490 nm were then plotted on a standard curve constructed from the data derived from MAb titration with the inhibition standards. The antigen concentration in the patient serum samples was calculated with a regression model constructed with the reciprocal values of fixed concentrations of P. brasiliensis CYA and the OD values. All of the standards, samples, and controls were tested in duplicate. The cutoff point was established as the upper limit of the 90% least significance difference confidence interval of the OD values obtained with the negative controls (NHS samples).
Antibody detection by CF and ID tests.
CF titers at the times of diagnosis and follow-up were determined by using the 50% hemolysis microtest described by the CDC Laboratories, Atlanta, Ga. (16). The ID test was performed at the time of diagnosis by using standard previously described methodology (17). Both tests used a culture filtrate antigen from the yeast form of P. brasiliensis CIB 339 produced as already described (25, 26).
Statistical analysis.
The standard inhibition curves were prepared in duplicate in at least four independent assays. A regression model was constructed by using the reciprocal values of antigen concentrations and the OD values obtained. Data were analyzed by analysis of variance (ANOVA-Type III) of square sum, including factors such as the clinical presentations and the time (weeks) of follow-up. Interactions above the second level were excluded. Comparisons within the factors were performed by a multiple-range test using the least significance difference test. Statistical analysis was performed by using Statgraphics plus (release 2, 1996; Statgraphics Corp., Rockville, Md.).
RESULTS
The standard inhibition curve accounted for 94.87% of the variations in antigen concentration of the OD values (r = 0.9487). This curve was used to determine the concentration of P. brasiliensis antigen in each patient sample tested. The cutoff point was fixed as the upper limit of the 90% confidence interval of the negative control values. Consequently, samples with an antigen concentration greater than 2.3 μg/ml were considered positive.
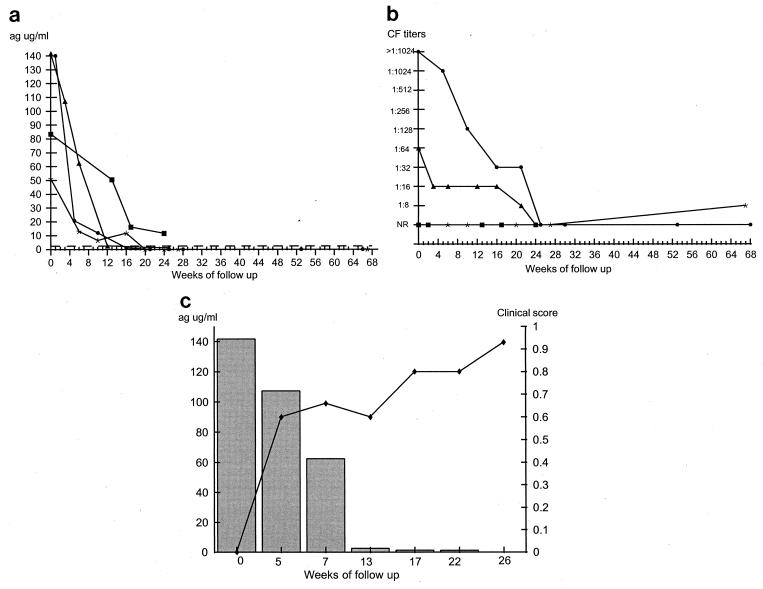
Patients were divided according to clinical presentation into four PCM groups: patients with the acute juvenile form, the acute juvenile form with AIDS, the multifocal form, or the unifocal form (Table 1). Patients in the acute juvenile group exhibited high levels of antigenemia at the time of diagnosis (50 to 141 μg/ml), although levels fell markedly in the first samples taken after the start of drug therapy (Fig. 1a). Antigenemia became negative in three of four patients by week 20 and remained so over the course of the follow-up period. These three patients showed rapid clinical improvement that appears to correlate well with the decrease in detectable circulating antigen. None of these patients relapsed, and treatment was discontinued in each individual after 12 months. The fourth patient is still undergoing treatment at 24 weeks and has already shown both a pronounced decrease in antigen levels and clinical improvement. In this group of patients, the fall of detectable circulating antigen at each follow-up point was statistically significant (P < 0.001). Figure 1b demonstrates the CF titers of these patients; two of them showed declines in antibody titers to nonreactivity at 24 weeks, while the other two patients were nonreactive at the time of diagnosis and remained so throughout the follow-up period. Figure 1c illustrates the results for a representative patient that demonstrate the correlation between diminishing antigen levels and clinical improvement seen in this group of patients.
FIG. 1.
Findings at follow-up of patients with the acute juvenile form of PCM. (a) Measurement of circulating 87-kDa antigen levels as determined by the inh-ELISA for patients 1 (■), 2 (▴), 3 (•), and 4 (∗). The dotted line represents the cutoff point of the inh-ELISA. (b) Measurement of antibody titers as determined by the CF test. Symbols represent the same patients as described for panel a. NR, nonreactive. (c) Correlation between clinical improvement (⧫) and antigen levels (histogram) in patient 2. Patient characteristics are given in Table 1. ag, antigen.
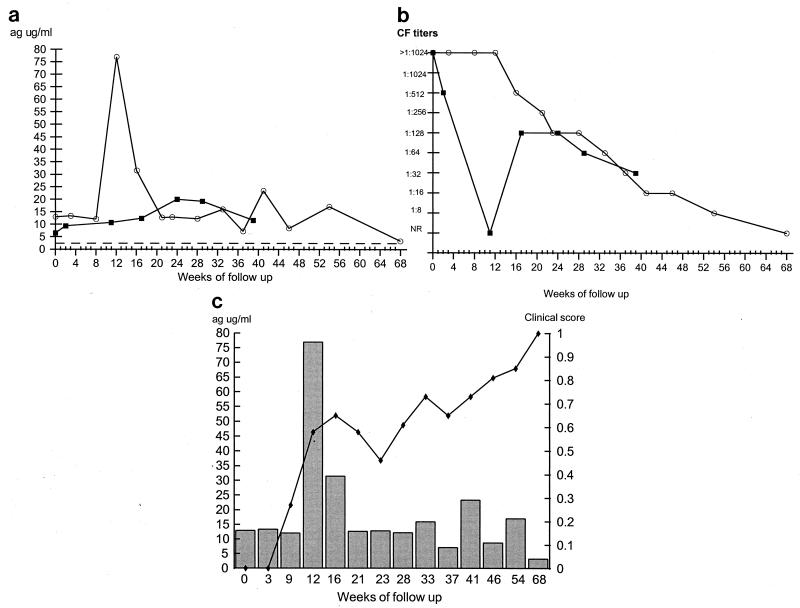
The two AIDS patients with the acute juvenile form of PCM showed persistently detectable levels of antigenemia throughout the follow-up period. No statistically significant decrease was detected during the duration of the follow-up period, even at week 68 of treatment (Fig. 2a). CF titers declined gradually over the period of study in one of the patients (Fig. 2b), while in the other one titers were erratic. Both patients showed good clinical responses to their drug regimes, although they remain on maintenance antifungal therapy because of their human immunodeficiency virus-positive status. Figure 2c represents antigen detection and clinical scores during treatment for one of the two patients.
FIG. 2.
Findings at follow-up of patients with the acute juvenile form of PCM and AIDS. (a) Measurement of circulating 87-kDa antigen levels as determined by the inh-ELISA for patients 5 (■) and 6 (○). The dotted line represents the cutoff point of the inh-ELISA. (b) Measurement of antibody titers as determined by the CF test. Symbols represent the same patients as described for panel a. NR, nonreactive. (c) Correlation between clinical improvement (⧫) and antigen levels (histogram) in patient 6. Patient characteristics are given in Table 1. ag, antigen.
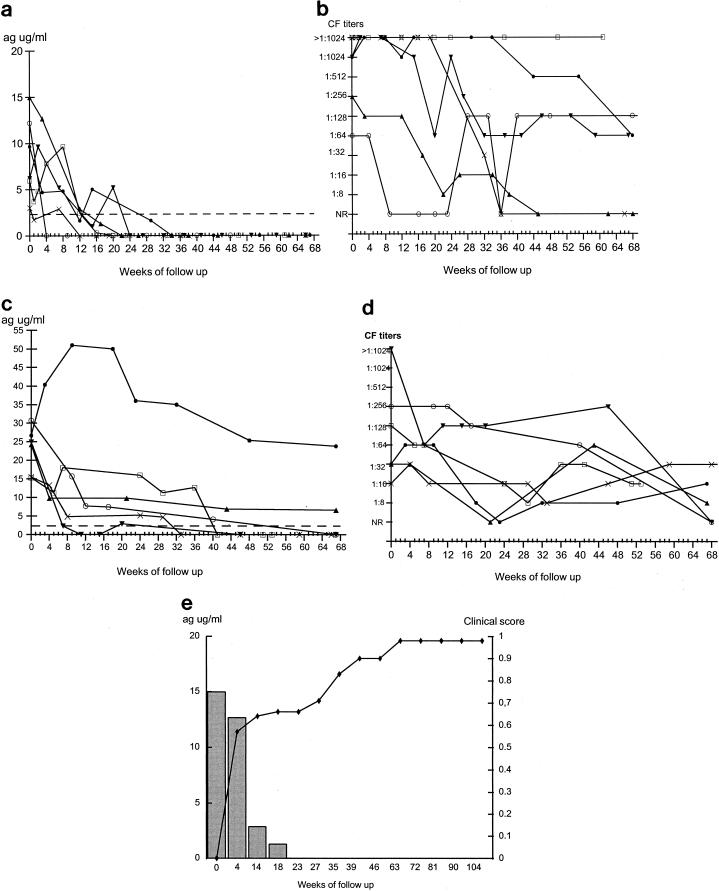
The levels of detectable circulating antigen in all patients with the multifocal form of PCM showed a tendency to decrease after initiation of treatment, but this was statistically significant only after 28 weeks of treatment (P < 0.05). These patients were divided into two groups for presentational purposes. Patients with antigen levels below 15 μg/ml at the time of diagnosis are shown in Fig. 3a, and those with initial antigen levels greater than 15 μg/ml are shown in Fig. 3c. The former group of patients showed a marked reduction in antigenemia and all serum samples became negative by week 29 of the follow-up period. These patients also showed clinical cure without relapse over the period of follow-up. In contrast, the pattern of CF titers for these patients was highly variable over the period of follow-up (Fig. 3b), and all patients exhibited medium (1:32 to 1:128) to high (1:256 to 1:1,024) antibody titers at the time of diagnosis.
FIG. 3.
Findings at follow-up of patients with the multifocal form of PCM. (a) Measurement of circulating 87-kDa antigen levels as determined by inh-ELISA for patients 7 (○), 8 (□), 9 (X), 10 (•), 11 (▴), and 12 (▾). (b) Measurement of antibody titers as determined by the CF test. Symbols represent the same patients as described for panel a. (c) Measurement of circulating 87-kDa antigen levels as determined by the inh-ELISA for patients 13 (□), 14 (○), 15 (X), 16 (▾), 17 (▴), and 18 (•). (d) Measurement of antibody titers as determined by the CF test. Symbols represent the same patients as described for panel c. (e) Correlation between clinical improvement (⧫) and antigen levels (histogram) in patient 11. Patient characteristics are given in Table 1. Dotted lines in panels a and c represent the cutoff point of the inh-ELISA. NR, nonreactive; ag, antigen.
Four of the patients (patients 13, 14, 15, and 16) with antigen levels higher than 15 μg/ml demonstrated decreases in antigenemia (Fig. 3c). Although patient 13 showed an initial fall in antigen level followed by a slight rise, he subsequently became antigen negative and remained so. Of the other two patients, one (patient 18) demonstrated a marked rise in antigenemia followed by a tendency to fall, although antigen levels never went below the initial reported value; the other patient (patient 17) showed a reduction in antigenemia but did not become antigen negative during the follow-up period. All six of these patients were clinically cured, and drug treatment was terminated for all of them at a maximum of 52 weeks. All of these patients had fluctuating medium to high levels of antibody at the time of diagnosis, as measured by CF test, with some patients becoming antibody negative and then positive again. By the end of the follow-up period, five of these patients had antibody titers below the values found at the time of diagnosis and two of them had become antibody negative. Figure 3e illustrates the results for a representative patient that demonstrates the correlation between decreasing antigen levels and clinical improvement seen in the group of patients with the multifocal form of the disease.
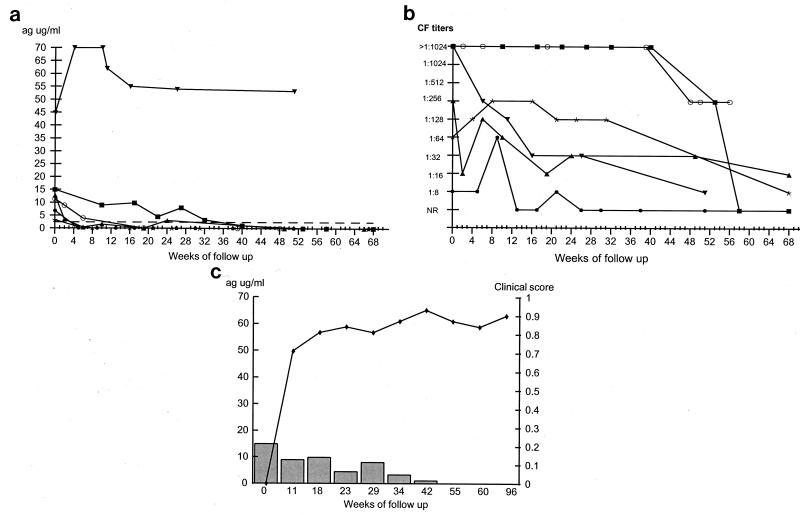
The six patients with the unifocal form of PCM had levels of circulating antigen at the time of diagnosis of between 3 and 45 μg/ml (Fig. 4a). Subsequently, five demonstrated decreases in antigenemia and four became antigen negative by week 20, whereas the other patient (patient 19) became antigen negative at week 40 (Fig. 4a). In these five patients, the decline in antigen was statistically significant (P < 0.001), which correlates well with clinical cure. The sixth patient (patient 24) demonstrated no overall decrease in antigen titers even after 52 weeks of follow-up, although antifungal therapy was stopped at 32 weeks with apparent clinical cure. Figure 4b illustrates the CF titers of the same group of patients; the pattern of responses was very variable although there was a general downward trend. This trend did not become apparent in two patients until week 40. One of the patients (patient 23) became antibody positive (titer, >1:8) only briefly at 9 weeks. Figure 4c illustrates the results for a representative patient that demonstrates the correlation between decreasing antigen levels and clinical improvement seen in this group of the patients.
FIG. 4.
Findings at follow-up of patients with the unifocal form of PCM. (a) Measurement of circulating 87-kDa antigen levels as determined by the inh-ELISA for patients 19 (■), 20 (○), 21 (∗), 22 (▴), 23 (•), and 24 (▾). The dotted line represents the cutoff point of the inh-ELISA. (b) Measurement of antibody titers as determined by the CF test. Symbols represent the same patients as described for panel a. NR, nonreactive. (c) Correlation between clinical improvement (⧫) and antigen levels (histogram) in patient 19. Patient characteristics are given in Table 1. ag, antigen.
DISCUSSION
Historically, monitoring the follow-up of patients with PCM has been accomplished by the detection of antibody responses (24) against crude antigen preparations or, more recently, against purified antigens (3, 23, 30). For many years, the CF test has been the method of choice for follow-up, although recent studies have utilized techniques such as ELISA, dot blotting, and Western blotting (3, 19), with variable results. However, the occurrence of false negatives, the variation in the sensitivity of these techniques, and the diversity and complexity of the humoral responses in PCM patients suggest that antigen detection systems may constitute a more rational approach in the monitoring of patient follow-up. There have been some reports of the use of antigen detection for the diagnosis of PCM (10, 12–14, 22, 24, 29) and thus far only two reports of the use of antigen detection for follow-up of patients (12, 22). In 1989, Mendes-Giannini et al. demonstrated a decrease in levels of circulating 43-kDa antigen in a pool of sera from PCM patients (22); more recently, in 1992, Freitas da Silva and Roque-Barreira described decreasing antigenemia in one PCM patient undergoing treatment (12). However, the effectiveness of antigen detection to monitor the clinical course of PCM patients has not been previously evaluated.
The inh-ELISA has proved useful in monitoring patients during the follow-up period and required no modification from that originally described (15); additionally, this test is suitable for the processing of large numbers of samples at one time. The correlation coefficient and the antigen cutoff point reported in this study were similar to those previously described (15). In addition, antigen levels at the time of diagnosis in PCM patients who were common to both studies were highly reproducible; samples which tested negative in the original study (15) were also negative when used in this study (data not shown). These observations provide evidence for the reproducibility of the technique.
The inh-ELISA appeared to be very useful in monitoring the course of therapy in patients with the acute juvenile form of PCM. The decrease in antigen levels closely mirrored the clinical improvement in these patients. In contrast, antibody detection by CF test appeared less useful in this context, particularly since two of the patients never developed any detectable antibody response. The generation of such false negatives when using the CF test is of obvious concern and has been noted by other authors in patients with this form of the disease (24). Indeed, this problem necessitates the use of more than one antibody detection assay, which is why CF is used in combination with ID or some other test when establishing the original diagnosis of PCM (8).
Paracoccidioidomycosis is rare in AIDS patients (2, 18, 31), and as a result we were only able to study two of such patients. In spite of intense itraconazole treatment and apparent clinical response, these patients showed no significant reduction in the level of circulating antigen. Since there is a paucity of studies dealing with the follow-up of antigenemia in AIDS patients with PCM (13), this would appear to be a novel observation. However, in AIDS patients with Histoplasma capsulatum infection, it has been shown that antigen remains persistent at a low level during follow-up (32), although perhaps not to the extent seen here. There are two possible explanations for the persistence of antigenemia in these two patients. First, it may result from the continued existence of low-level infection, partially controlled by drug treatment but not totally eradicated due to the depressed cellular immunity characteristic of AIDS patients. Second, it may result from the inability of AIDS patients to clear circulating antigen. The former would seem to be the most likely explanation given the extended time over which antigenemia was still detectable. Further studies should be performed with this patient group in order to understand this phenomenon of antigen persistence. The CF test demonstrated the presence of antibody in both of the AIDS patients, which has been previously reported (18). However, in our study, antibody titers were initially high, which is unusual in such patients, and indeed they remained so for up to 16 weeks of follow-up.
In patients with the multifocal form of PCM, there was a clear correlation between decreasing antigen levels and clinical cure. Within this group, there were three patients (patients 13, 17, and 18) whose response diverged to a certain extent from this pattern. Patient 13 was a 25-year-old male who presented with neuroparacoccidioidomycosis. Treatment was started with sodium valproate and itraconazole (ITZ) at 400 mg/day; however, the patient presented little clinical improvement with no detectable levels of ITZ in serum and an antigenemia which remained high (11.3 μg/ml) throughout this period. Subsequently, sodium valproate was replaced by clonazepam, which correlated with clinical improvement, detectable ITZ levels, and a decrease in antigenemia. The enzyme-inducing effects of sodium valproate, although less than those of other anticonvulsants, may have resulted in the reduced bioavailability of ITZ, which explains why this patient had no detectable levels of ITZ in serum, with resultant persistence of high antigen levels during concomitant treatment (1). In addition, two patients (patients 17 and 18) did not become antigen negative by the end of the follow-up period despite apparent clinical cure. Patient 18, with an initial antigen concentration of 26.6 μg/ml in serum, demonstrated a marked rise in antigenemia during the second, third, and fourth follow-up visits, followed by a slight decrease in antigen level in comparison with the initial value. This patient is a 48-year-old male with hypertension and hypercholesterolemia who presented with severe multifocal PCM with lesions in the oral mucosa, skin, and lungs. He received treatment for 8 months; however, 18 months after termination of treatment, he still presents with persistence of respiratory symptoms (cough and dyspnea) which may represent sequelae. The other patient (patient 17) showed a marked reduction of antigenemia but did not become antigen negative during the follow-up period. This patient is a 48-year-old male who also presented with lung lesions and dissemination to mucous membranes and skin but who has not exhibited sequelae a year after termination of treatment. In general, CF tests in patients with the multifocal form of PCM showed elevated antibody titers with variable and erratic responses during the follow-up period, although with a tendency towards negativity at the end of the study period.
The inh-ELISA was also useful in monitoring the follow-up of patients with the unifocal form of PCM, with a decline in antigen levels correlating with clinical cure in five of six patients. Patients with the unifocal form had lower antigen levels than those with the multifocal and acute forms, and they became antigen negative more rapidly. These observations are to be expected given the more extensive and serious nature of the disease in its multifocal or acute form. There is no obvious explanation as to why the sixth patient (patient 24) did not conform to this pattern, although it is perhaps of interest that his initial antigen levels (and those subsequent to it) were much higher than those in the other patients. This patient is a 44-year-old male with hypercholesterolemia who exhibited positive ID and counterimmunoelectrophoresis test results against Aspergillus fumigatus (aspergillin) without a confirmed diagnosis of aspergillosis. The CF test data for patients with the unifocal form of PCM showed wide variation, although eventually a general downward trend was established in all patients. However, the usefulness of CF in the follow-up and treatment of these patients is limited by titer variability combined with the fact that one patient was antibody positive for only a brief period of time.
In conclusion, we have demonstrated that the quantitation of the 87-kDa P. brasiliensis antigen is useful in the follow-up of patients with the acute, multifocal, and unifocal forms of PCM. In the vast majority of cases in all three groups, antigen levels decreased following the induction of therapy and became negative by 52 weeks. By comparison, antibody titers, as measured by CF test, appeared to be erratic and did not correlate well with the clinical status of the patients. Of particular note is the common persistence of antibodies for a considerable period of time after the cessation of an apparently successful course of treatment. Although we have now shown that the 87-kDa antigen is a useful marker of disease, relatively little is known of its chemical composition and biochemical characteristics and we are currently attempting to purify this antigen for further analysis.
ACKNOWLEDGMENTS
This work was supported by grants 2213-05-158-97 and 033-98 from Colciencias, Bogotá, Colombia, and by The Special Trustees of Guy’s Hospital, UMDS, London, United Kingdom.
REFERENCES
- 1.Brodell T T, Elewinski B E. Clinical pearl: systemic antifungal drugs and interactions. J Am Acad Dermatol. 1995;33:259–260. doi: 10.1016/0190-9622(95)90245-7. [DOI] [PubMed] [Google Scholar]
- 2.Brummer E, Castañeda E, Restrepo A. Paracoccidioidomycosis: an update. Clin Microbiol Rev. 1993;6:89–117. doi: 10.1128/cmr.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bueno J P, Mendes-Giannini M J S, Del Negro G M B, Assis C M, Takiguti C K, Shikanai-Yasuda M A. IgG, IgM and IgA antibody response for the diagnosis and follow-up of paracoccidioidomycosis: comparison of counterimmunoelectrophoresis and complement fixation. J Med Vet Mycol. 1997;35:213–217. doi: 10.1080/02681219780001161. [DOI] [PubMed] [Google Scholar]
- 4.Camargo Z P, Guesdon J L, Drouhet E, Improvisi L. Enzyme-linked immunosorbent assay (ELISA) in paracoccidioidomycosis. Comparison with counterimmunoelectrophoresis and erythroimmunoassays. Mycopathologia. 1984;88:31–37. doi: 10.1007/BF00439292. [DOI] [PubMed] [Google Scholar]
- 5.Camargo Z P, Unterkircher C, Campoy S P, Travassos L R. Production of Paracoccidioides brasiliensis exoantigens for immunodiffusion tests. J Clin Microbiol. 1988;26:2147–2151. doi: 10.1128/jcm.26.10.2147-2151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camargo Z P, Unterkircher C, Campoy S P, Travassos L R. Analysis by western blotting of the serological response in paracoccidioidomycosis. Rev Iber Micol. 1988;5:70. [Google Scholar]
- 7.Cano L E, Brummer E, Stevens D A, Restrepo A. An evaluation of the enzyme-linked immunosorbent assay (ELISA) for quantitation of antibodies to Paracoccidioides brasiliensis. J Med Vet Mycol. 1986;24:467–475. doi: 10.1080/02681218680000741. [DOI] [PubMed] [Google Scholar]
- 8.Cano L E, Restrepo A. Predictive value of serologic tests in the diagnosis and follow-up of patients with paracoccidioidomycosis. Rev Inst Med Trop Sao Paulo. 1987;29:276–283. doi: 10.1590/s0036-46651987000500003. [DOI] [PubMed] [Google Scholar]
- 9.Fava Neto C. Paracoccidioidomycosis. Proceedings of the First Pan American Symposium, Medellin, Colombia. Scientific publication no. 254. Washington, D.C: Pan American Health Organization; 1972. The serology of paracoccidioidomycosis: present and future trends; pp. 209–213. [Google Scholar]
- 10.Ferreira-da-Cruz M F, Galvao-Castro B, Daniel-Ribeiro C T. Sensitive immunoradiometric assay for the detection of Paracoccidioides brasiliensis antigens in human sera. J Clin Microbiol. 1991;29:1202–1205. doi: 10.1128/jcm.29.6.1202-1205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franco M, Montenegro M R, Mendes R P, Marquez S A, Dillon N L, Mota N G S. Paracoccidioidomycosis: a recently proposed classification on its clinical forms. Rev Soc Bras Med Trop. 1987;20:129–132. doi: 10.1590/s0037-86821987000200012. [DOI] [PubMed] [Google Scholar]
- 12.Freitas da Silva G, Roque-Barreira M C. Antigenemia in paracoccidioidomycosis. J Clin Microbiol. 1992;30:381–385. doi: 10.1128/jcm.30.2.381-385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freitas da Silva G, Martinez R, Nascimiento M M P, Bragheto I C, Roque-Barreira M C. Deteccao e identificacao de antigenos circulantes em pacientes com paracoccidioidomicose associada à Síndrome da Immunodeficiencia adquirida. Rev Argent Micol. 1992;15:47. [Google Scholar]
- 14.Garcia N, Del Negro G M, Martins H P, Lacaz C S. Detection of paracoccidioidomycosis circulating antigens by the immunoelectrophoresis-immunodiffusion technique. Preliminary report. Rev Inst Med Trop Sao Paulo. 1987;29:327–328. doi: 10.1590/s0036-46651987000500011. [DOI] [PubMed] [Google Scholar]
- 15.Gómez B L, Figueroa J I, Hamilton A J, Ortiz B, Robledo M A, Hay R J, Restrepo A. Use of monoclonal antibodies in diagnosis of paracoccidioidomycosis: new strategies for detection of circulating antigens. J Clin Microbiol. 1997;35:3278–3283. doi: 10.1128/jcm.35.12.3278-3283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman L, Huppert M, Fava-Neto C, Negroni R, Restrepo A. Manual of standardized serodiagnostic procedures for systemic mycosis. Part II. Complement fixation test. Washington, D.C: Pan American Health Organization; 1972. [Google Scholar]
- 17.Kaufman L, Huppert M, Fava-Neto C, Negroni R, Restrepo A. Manual of standardized serodiagnostic procedures for systemic mycosis. Part I. Agar gel immunodiffusion tests. Washington, D.C: Pan American Health Organization; 1972. [Google Scholar]
- 18.Marques S A, Shikanai-Yasuda M A. Paracoccidioidomycosis associated with immunosupression, AIDS, and cancer. In: Franco M, Da Silva Lacaz C, Restrepo A, del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 393–405. [Google Scholar]
- 19.Martins R, Marquez S, Alves M, Fecchio D, Franco M. Serological follow-up of patients with paracoccidioidomycosis treated with itraconazole using dot-blot, ELISA and Western-blot. Rev Inst Med Trop Sao Paulo. 1997;39:261–269. doi: 10.1590/s0036-46651997000500004. [DOI] [PubMed] [Google Scholar]
- 20.Mendes R P, Negroni R, Arechavala A. Treatment and control of cure. In: Franco M, Da Silva Lacaz C, Restrepo A, del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 373–392. [Google Scholar]
- 21.Mendes-Giannini M J S, Camargo M E, Lacaz C S, Ferreira A W. Immunoenzymatic absortpion tests for serodiagnosis of paracoccidioidomycosis. J Clin Microbiol. 1984;29:103–108. doi: 10.1128/jcm.20.1.103-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendes-Giannini M J S, Bueno J P, Shikanai-Yasuda M A, Ferreira A W, Masuda A. Detection of the 43,000-molecular-weight glycoprotein in sera of patients with paracoccidioidomycosis. J Clin Microbiol. 1989;27:2842–2845. doi: 10.1128/jcm.27.12.2842-2845.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendes-Giannini M J S, Bueno J, Shikanai-Yasuda M A, Stolf A M S, Masuda A, Amato Neto V, Ferreira A W. Antibody response to the 43 kDa glycoprotein of Paracoccidioides brasiliensis as a marker for the evaluation of patients under treatment. Am J Trop Med Hyg. 1990;43:200–206. doi: 10.4269/ajtmh.1990.43.200. [DOI] [PubMed] [Google Scholar]
- 24.Mendes-Giannini M J S, Del Negro G B, Siqueira A M. Serodiagnosis. In: Franco M, Da Silva Lacaz C, Restrepo A, del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 345–363. [Google Scholar]
- 25.Restrepo A. La prueba de immunodifusión en el diagnóstico de la paracoccidioidomicosis. Sabouraudia. 1966;4:223–230. [PubMed] [Google Scholar]
- 26.Restrepo A. Procedimientos serológicos en la paracoccidioidomicosis. Adel Microbiol Enf Infecc. 1984;3:182–211. [Google Scholar]
- 27.Restrepo A. Paracoccidioides brasiliensis. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. London, United Kingdom: Churchill Livingstone; 1995. pp. 2386–2389. [Google Scholar]
- 28.Restrepo A, Gómez I, Cano L E, Arango M D, Gutierrez F, Sanin A, Robledo M A. Post-therapy status of paracoccidioidomycosis patients treated with ketoconazole: a 3 year experience. Am J Med. 1983;74:53–57. doi: 10.1016/0002-9343(83)90514-4. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez M C, Cassaguerra C M, Lacaz C S. Probable demonstration of circulating antigen by counterimmunoelectrophoresis test. Rev Inst Trop Sao Paulo. 1984;26:285–287. doi: 10.1590/s0036-46651984000500011. [DOI] [PubMed] [Google Scholar]
- 30.Taborda C P, Camargo Z P. Diagnosis of paracoccidioidomycosis by dot immunobinding assay for antibody detection using the purified and specific antigen gp43. J Clin Microbiol. 1994;32:554–556. doi: 10.1128/jcm.32.2.554-556.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobón, A. M., B. Orozco, S. Estrada, E. Jaramillo, C. de Bedout, M. Arango, and A. Restrepo. Submitted for publication. [DOI] [PubMed]
- 32.Wheat J. Histoplasmosis in the acquired immunodeficiency syndrome. Curr Top Med Mycol. 1996;7:7–18. [PubMed] [Google Scholar]