Abstract
Nonbacterial thrombotic endocarditis (NBTE) is a form of endocarditis that occurs in patients with predisposing conditions, including malignancies, autoimmune diseases (particularly antiphospholipid antibody syndrome, which accounts for the majority of lupus-associated cases), and coagulation disturbances for which the correlation with classical determinants is unclear. The condition is commonly referred to as “marantic”, “verrucous”, or Libman–Sacks endocarditis, although these are not synonymous, representing clinical–pathological nuances. The clinical presentation of NBTE involves embolic events, while local valvular complications, generally regurgitation, are typically less frequent and milder compared to infective forms of endocarditis. In the past, the diagnosis of NBTE relied on post mortem examinations, while at present, the diagnosis is primarily based on echocardiography, with the priority of excluding infective endocarditis through comprehensive microbiological and serological tests. As in other forms of endocarditis, besides pathology, transesophageal echocardiography remains the diagnostic standard, while other imaging techniques hold promise as adjunctive tools for early diagnosis and differentiation from infective vegetations. These include cardiac MRI and 18FDG-PET/CT, which already represents a major diagnostic criterion of infective endocarditis in specific settings. We will herein provide a comprehensive review of the current knowledge on the clinics and therapeutics of NBTE, with a specific focus on the diagnostic tools.
Keywords: non-infective endocarditis, marantic, Libman–Sacks, echocardiography, immunology, cardiac imaging, coagulopathy, antiphospholipid antibodies, anticoagulants
1. Introduction, Methodology and Aims
1.1. Terminology
The term “nonbacterial thrombotic endocarditis” (NBTE) was first introduced by Gross and Friedberg in 1936, inspired by Ziegler’s initial description of sterile endocardial vegetations in 1888 [1,2]. Libman and Sacks had reported similar findings in patients with systemic lupus erythematosus (SLE) in the early 1920s [3]. From a pathogenic perspective, a key distinction is made from infective endocarditis, which occasionally leads to the use of the term “non-infective endocarditis”. However, non-infective forms encompass other conditions, such as rheumatic carditis and hyper-eosinophilic endocarditis [4]. These conditions exhibit distinct pathology, pathophysiology, and clinical manifestations, and require different treatment approaches compared to NBTE. Underlying disorders include cancer, autoimmune diseases, and thrombophilia [5], and as a consequence, various terms have been used in recent decades to describe NBTE based on these underlying features. As an example, marantic endocarditis, derived from the Greek word marantikos meaning “wasting away”, is used to describe NBTE specifically in patients with advanced malignancy or chronic end-stage diseases. Other variations, such as “thrombotic” and “verrucous” endocarditis, highlight specific pathological characteristics of NBTE vegetations. Additionally, “Libman-Sacks endocarditis” conventionally refers to the presence of NBTE in association with SLE and/or antiphospholipid antibody syndrome (APS) [5,6].
1.2. Epidemiology
Data on the epidemiology of NBTE are limited and are primarily derived from post mortem series. One of the largest autoptic studies reported an overall prevalence of 3.7%, three times higher than that of infective endocarditis [7]. The prevalence significantly increases in high-risk populations such as patients with cancer or predisposing autoimmune conditions, reaching 15% in a large echocardiographic study [8]. While the occurrence of NBTE has traditionally been considered equal between sexes [5], a slight female predominance has been observed in several reports, and is primarily attributed to the association with autoimmune diseases [9]. In a cohort of patients with antiphospholipid syndrome (APS), the prevalence of NBTE was higher in females compared to males [8], and SLE was found to be responsible for a threefold increase in thrombotic endocarditis compared to unaffected subjects [10].
The reported age at NBTE diagnosis varies, ranging from 40 to 80 years, partly reflecting differences in epidemiology, detection methods [7,9], and underlying conditions [6,7,9]. For instance, the median age at diagnosis tends to be lower in patients with NBTE associated with autoimmune disorders compared to paraneoplastic forms.
1.3. Methodology and Scope of the Review
The aim of this work is to provide a comprehensive narrative review of the existing data on NBTE to emphasize the significance of early suspicion, differential diagnosis, and key diagnostic and therapeutic insights. A methodical search was conducted on PubMed utilizing keywords such as “NBTE”, “nonbacterial thrombotic endocarditis”, and “non-infective endocarditis”. The selection of articles from the published literature was carried out on 20 August 2023. Given the relatively slow advancement within this field, this review transcended a mere synthesis of the current state of the art in NBTE. Instead, our objective is to underscore the multitude of unmet requirements and address the fundamental research inquiries that necessitate further exploration.
2. Pathogenesis and Associated Conditions
The intricate pathogenesis of NBTE involves inflammatory and thrombotic disturbances, ultimately leading to the formation of sterile thrombi composed of platelets and fibrin on the endocardial surface, without evidence of microorganisms and inflammatory cells [5]. Autoimmune diseases, malignancy, and coagulopathy represent the clinical archetypes of NBTE-associated conditions and recapitulate the underlying pathogenic mechanisms. Importantly, these three etiological categories should always be thoroughly investigated and excluded when approaching a patient with suspected NBTE [11]. Non-malignant conditions associated with systemic inflammation and wasting, such as end-stage heart failure, advanced chronic kidney disease, chronic infections (e.g., tuberculosis, HIV; particularly reflecting immunosuppression), burns, and severe sepsis, have also been observed in association with NBTE [12].
2.1. Neoplasm
The association between malignancy and thromboembolic manifestations is well-established, with certain histotypes carrying a higher risk, such as mucinous adenocarcinomas (e.g., lung, pancreas, breast) and lymphoproliferative syndromes [13,14,15,16]. Malignant neoplasms were the most commonly observed clinical condition in patients diagnosed with NBTE in a large observational study [9], with a notable predilection for lung, pancreatic, gynecological, and breast cancers [9,17]. Further analyses revealed that the risk is significantly higher with adenocarcinoma histology, accounting for 65% of the paraneoplastic forms of NBTE, especially in advanced stages, and metastatic cancer [17]. Despite the less clear correlation with hematologic malignancies, cases of NBTE have been described in association with acute promyelocytic leukemia [18] and T- and B-cell lymphoproliferative syndromes [19,20,21], in analogy with the other coagulation alterations commonly observed with such conditions. A thorough assessment for occult malignancy, with a specific focus on adenocarcinomas and myeloproliferative and lymphoproliferative syndromes, is thus essential in suspected or confirmed cases of NBTE.
The hyper-coagulant state associated with neoplasm may represent the primum movens leading to the deposition of sterile thrombotic vegetations on the endothelial surface of the cardiac valves [22], and this phenomenon was primarily observed with cardiac myxomas [23]. Malignant cancers release pro-coagulant molecules, mostly tissue factors, contributing to the hyper-coagulant state; as a paradigm, acute promyelocytic leukemia often presents with features of disseminated intravascular coagulation (DIC) [24], and the anticoagulant activity of recombinant soluble thrombomodulin has shown promising results in the treatment of such condition [25]. Additionally, the chronic subclinical inflammatory response towards the tumor, primarily driven by cancer-associated macrophages, leads to an increase in pro-inflammatory cytokines and pro-thrombotic molecules, including fibrinogen, factor VIII, and von Willebrand factor [26]. These factors drive the altered hemostasis leading to NBTE in cancer patients.
2.2. Autoimmune Diseases
The well-established association between NBTE and autoimmune diseases is represented by Libman–Sacks endocarditis as the cardiac involvement in patients with SLE [27]. Although valvular heart disease is not currently considered a major classification criterion for APS [28], evidence suggests a significantly higher prevalence of endocardial involvement compared to the general population [8]. From a pathogenic point of view, the deposition of antibodies in the sub-endothelial layer of the affected valves has been reported in cases of NBTE associated with APS, while the presence of immune complexes at the interface between the endocardium and vegetations has been described in Libman-Sacks endocarditis associated to SLE [29,30,31,32].
In patients with APS, there is an association between the risk of developing NBTE, the presence of lupus anticoagulant (LAC), anti-cardiolipin, and anti-beta-2-glycoprotein I antibodies of the IgG isotype, as well as the presence of two or more antiphospholipid antibodies [8]. NBTE is more frequent in patients with APS and a history of cerebral arterial events or obstetric syndrome, while an inverse association has been described in individuals with previous venous thromboembolism [8]; such correlations require further evidence but point towards the high heterogeneity of clinical APS manifestations [33]. Further research is required in order to establish whether NBTE should represent a “novel” classification criterion for APS. Moreover, the role of “non-criteria” antibodies, such as anti-phosphatidylserine/prothrombin (aPS/PT), remains to be determined, especially in the case of otherwise seronegative APS [34].
Not surprisingly, Libman–Sacks endocarditis is more frequent in patients with SLE with positive antiphospholipid antibodies, particularly triple-positive cases, whereas a negative correlation with lupus-specific autoantibodies has been demonstrated [35]. In addition, there is no established association between SLE disease activity and the risk of NBTE [35], while a correlation with neuropsychiatric lupus manifestations has been described [36,37]. These considerations highlight the need for diligent diagnostic efforts to exclude NBTE in patients with SLE and APS, especially in those with the highest serological risk profiles (two or more antiphospholipid antibodies positive) [28], as well as in the case of neurological involvement associated with SLE. It remains to be established whether new-onset NBTE may be sufficient to escalate immunosuppressive therapies in patients with SLE.
In the case of other autoimmune diseases, sterile thrombotic endocardial vegetations have been described also in patients with rheumatoid arthritis [38,39,40], inflammatory myositis [41], systemic sclerosis [42,43], Behçet’s disease [44], Crohn’s disease [45,46,47], and vasculitis, particularly with anti-neutrophil cytoplasmic antibodies (ANCA) [48,49,50,51] and giant-cell arteritis [52,53] (Table 1).
Table 1.
Autoimmune diseases that have been associated with an increased risk of NBTE.
| Established Associations |
| Systemic lupus erythematosus |
| Antiphospholipid antibodies syndrome |
| Reported cases |
| Other connective tissue diseases |
| Systemic sclerosis |
| Inflammatory myositis—antisynthetase syndrome |
| Rheumatoid arthritis |
| Systemic vasculitis |
| Giant-cell arteritis |
| ANCA-associated vasculitis (granulomatosis with polyangiitis, microscopic polyangiitis, eosinophilic granulomatosis with polyangiitis) |
| Behçet’s disease |
| Inflammatory bowel disease |
| Crohn’s disease |
Patients diagnosed with NBTE without a definite diagnosis of an autoimmune condition are more likely to test positive for rheumatoid factors [5]. Nevertheless, the presence of serum rheumatoid factors has limited specificity, as it is frequently detected in patients with infective endocarditis as well [54]. Therefore, rheumatoid factors’ positivity cannot be relied upon as a diagnostic criterion to distinguish between infective and NBTE. Also, serum antiphospholipid antibodies have been reported in up to 14% of the patients with infective endocarditis in an observational cohort [55], and could be linked to an increased risk of embolic events in such a population [55].
2.3. Coagulopathy
The role of hypercoagulability in the pathogenesis of NBTE has been postulated since the first observation of DIC in up to 50% of autopsy cases [56]. Animal models have provided further support to this hypothesis, demonstrating that consumption coagulopathy is associated with NBTE in rats, likely due to abnormal production of tissue factor by sub-endocardial macrophages [57,58]. Additionally, alterations in the serum levels of coagulation factors, hypo- and hyperfibrinogenemia, and increased platelet turnover have been variably reported in patients with NBTE, especially when associated with malignancy or DIC [59]. In a large autoptic case analysis comparing infective endocarditis with NBTE, concomitant deep venous thrombosis and acute pulmonary embolism were significantly more common in the latter group, along with a higher prevalence of malignancy [60]. These findings raise the question of whether coagulopathy itself can be implicated in the formation of endocardial thrombi or whether it only represents the epiphenomenon of conditions that are likely to carry a pro-thrombotic risk (e.g., malignancy, autoimmune disorders, APS).
Currently, there are no data in the literature regarding the possible association between NBTE and classical risk factors of thrombophilia, including protein C, protein S, and anti-thrombin deficiencies; factor V Leiden G1691A and factor II G20210A mutations [61]; as well as emerging candidates like the Janus kinase 2 (JAK2) V617F mutation [62], the driver mutation of polycythemia vera-like myeloproliferative neoplasms [63]. Considering the negative correlation between NBTE and deep vein thrombosis in APS patients, it is debatable whether traditional risk factors for venous thromboembolism should be routinely screened in all patients presenting with NBTE. On the other hand, given the association between DIC and marantic endocarditis, coagulation studies should be performed in all patients with suspected or proven NBTE.
3. Clinical Presentation and Diagnosis
Due to the clinical heterogeneity of the condition, often presenting with established complications, the absence of systemic symptoms is directly attributable to thrombotic vegetations, as well as the usually mild valvular involvement. Post mortem examinations have historically been relied upon for the diagnosis of NBTE. When suspicion arises, the poor prognosis of NBTE and the potential fatality or disability associated with its complications make it necessary to pursue adequate tests. The diagnostic efforts should prioritize the following objectives: (i) identification and characterization of endocardial vegetations; (ii) rigorous exclusion of infective endocarditis (including cases of culture-negative infective endocarditis); (iii) screening and detection of peripheral embolic complications; and (iv) determination of the underlying condition that predisposes one to NBTE, particularly if it has not been previously identified [5,6].
3.1. Clinical Presentation
The diagnosis of NBTE can be challenging, as a portion of patients remains asymptomatic [64], while in over 50% of cases, clinical symptoms manifest following peripheral embolization from endocardial vegetations [65,66]. Signs and symptoms deriving from embolism represent the most common clinical presentation [10], and are significantly more frequent in NBTE compared to infective endocarditis, as non-infective vegetations are generally more friable than their infective counterparts [5]. As proof, embolic stroke manifests in approximately 20% of patients diagnosed with infective endocarditis, while it complicates a significantly higher proportion, up to 75%, of cases associated with NBTE [59]. The cerebral district is the primary site of embolization, although this observation may be influenced by the clinical significance of ischemic stroke presentation compared to other areas [67,68]. The distribution of cerebral ischemic lesions, evaluated using diffusion-weighted imaging on brain magnetic resonance, can aid in differentiating embolization from infective and non-infective sources, the last being associated with disseminated brain lesions which vary in size, whereas infective endocarditis can lead to single, cortical, territorial, or multiple strokes [69].
Additional sites of embolization include the abdominal area, i.e., the mesenteric, splenic, and renal arteries, and the peripheral limbs. In rare cases, emboli may reach the coronary arteries, leading to acute coronary syndrome [26,70]. However, despite this heterogeneity, the clinical presentation is similar irrespective of the different underlying etiologies [17,71]. Therefore, NBTE should be excluded in all patients with definite or suspected associated conditions (i.e.,: malignancy, cachexia, autoimmune disorders, or DIC), particularly when cryptogenic cerebral or splanchnic embolism is diagnosed [59]. On the other hand, whole-body assessment for peripheral embolization is mandatory in patients with non-infective endocardial vegetations.
Unlike infective endocarditis, NBTE does not cause significant damage to the valvular apparatus and is not associated with local complications such as abscesses, fistulae, or disruption of the leaflets and/or chordae [5,72]. Clinically significant valvular dysfunction is uncommon, with regurgitation being more frequent than stenosis. Left-sided heart valves are typically involved, with the mitral valve being the most common site, whereas right-sided vegetations are rare [72,73]. In general terms, the presence of a new-onset murmur or qualitative changes in pre-existing murmurs is a rare finding during physical examinations; however, when present, they should raise concerns for NBTE in the appropriate clinical context [5].
Opposite to NBTE, infective endocarditis usually manifests with fever and chills, with possible septic signs. Also, (subacute) infective endocarditis can be associated with the presence of micro-embolic phenomena such as retinal Roth spots; splinter and conjunctival hemorrhages; and immunologic manifestations presenting with vasculitic cutaneous lesions, namely, the Osler’s nodules and Janeway’s lesions [74]. Metastatic infection is also common in cases of infective endocarditis, particularly with peripheral abscesses, mycotic aneurysms, and spondylodiscitis [75,76].
Clinical features suggestive of associated underlying conditions should instead be investigated whenever a diagnosis of NBTE is suspected or confirmed. These features include general symptoms of wasting, weight loss, low-grade fever, specific organ involvement, or concomitant paraneoplastic syndromes, especially with thrombophilia or coagulopathy. Clinical hints of autoimmune diseases should also be explored in the appropriate contexts, such as the evidence of skin rashes, oral and genital aphthae, arthralgia and arthritis, Raynaud’s phenomenon, pleural or pericardial effusions, and a history of recurrent miscarriages [77,78].
3.2. Pathology
Post mortem histologic examination still represents the gold standard for NBTE detection. Numerous studies [12,59,60] have provided evidence that the mitral valve is particularly prone to NBTE, making it the most commonly involved site, followed by the aortic valve. Thrombotic vegetations can vary in size from less than 1 mm to 2.5 cm, can involve both the leaflets and the commissures, and are typically located on the side of low-pressure chambers, i.e.: the atrial side of the mitral valve and the ventricular side of the aortic valve [6,12,59,60,79]. Although a higher prevalence of NBTE has been reported on structurally normal valves compared to infective vegetations [60], autopsies have revealed that underlying valvular heart disease, particularly calcific or rheumatic degeneration, is also commonly present in case of thrombotic vegetations [12,59].
Sterile thrombotic vegetations represent the histological characteristics of NBTE, indicating abnormal activation of the hemostatic cascade within the affected area. Notably, such masses form in the absence of microorganisms or inflammatory cells, highlighting the noninfectious nature of the condition. Thrombotic vegetations consist of platelets enclosed and organized within a fibrin mesh, with the occasional inclusion of small amounts of red blood cells. Early signs of organization can be observed, such as the formation of granulation tissue [6,59,79]. Notably, there have been no reported histological distinctions based on the underlying comorbidity, specifically in cases of SLE/APS and cancer [79]. However, an autopsy series revealed that thrombotic vegetations associated with SLE or APS are more likely to demonstrate histological organization with granulation tissue and recruitment of fibroblastic foci, while marantic vegetations tend to undergo degenerative processes without organization [79]. Such pathological distinctions should reflect different embolic risk and require further characterization. Additionally, it remains to be determined whether signs of histological and immune-histochemical endothelitis, such as complement and immunoglobulin deposition, play a role in triggering thrombotic phenomena on the endocardium of patients with SLE and/or APS.
3.3. Laboratory and Microbiological Investigations
Laboratory investigations in patients with suspected NBTE primarily aim to exclude infective endocarditis, while in cases of autoimmune disorders, they contribute to defining the underlying condition. Additionally, coagulation studies should be performed in all patients with NBTE due to the high prevalence of coagulopathy and DIC.
To establish a diagnosis of NBTE, all infective etiologies must be ruled out, as blood culture-negative endocarditis is defined when three or more sets of blood cultures collected over 48 h remain negative despite prolonged (>1 week) incubation time [80]; collecting more than three sets does not improve the diagnostic yield [4]. Early antibiotic therapy, right-sided or device-associated endocarditis, and fastidious microorganisms are the most common causes of culture-negative infective endocarditis [4]. Blood culture-negative endocarditis may be associated with intracellular organisms (e.g., Coxiella burnetii, Mycoplasma spp., Legionella spp., Tropheryma whipplei), fastidious bacteria (e.g., Bartonella spp., Brucella spp., Francisella spp., Neisseria spp.), and HACEK group agents (Haemophilus spp., Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella and Kingella spp.). Fastidious and HACEK group pathogens require specific culture media and incubation times, while cell cultures are needed for intracellular bacteria. When available, serology is a valid surrogate for diagnosis, as in the case of Coxiella burnetii infection [4,11]. Table 2 summarizes the most common microorganisms causing culture-negative infective endocarditis and the associated diagnostic strategies [81].
Table 2.
Principal microorganisms causing blood-culture-negative infective endocarditis.
| Microorganism | Frequency | Proposed Diagnostic Tests |
|---|---|---|
| Coxiella burnetii | 37% | Serology Surgical sample: histology, immunohistochemistry, PCR |
| Bartonella spp. | 12% | Blood cultures Serology Surgical sample: culture, PCR |
| HACEK group | 2–3% | Blood cultures Surgical sample: culture, PCR |
| Tropheryma whipplei | 2–3% | Surgical sample: histology, immunohistochemistry, PCR |
| Fungi | 1–2% | Blood cultures (Serum galactomannan) Surgical sample: culture, PCR (pan-fungal and Aspergillus spp.) |
| Legionella spp. | <1% | Blood cultures Serology Surgical sample: immunohistochemistry, PCR |
| Mycoplasma spp. | <1% | Serology Surgical sample: immunohistochemistry, PCR |
| Chlamydia spp. | <1% | Serology Surgical sample: immunohistochemistry, PCR |
| Mycobacterium spp. | <1% | Blood cultures in mycobacterial media, PCR Surgical sample: histology, PCR |
| No cause diagnosed | 37% | Blood cultures Serologies Broad range and specific PCR on blood and tissues Surgical sample: cultures, histology, immunohistochemistry, PCR (broad range and specific) |
List of abbreviations—HACEK: Haemophilus spp., Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella and Kingella spp.; PCR: polymerase chain reaction.
Coagulation screening, including prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, and D-dimer, can reveal coagulopathy and DIC. However, it is unclear whether screening all patients with NBTE for conventional risk factors for inherited thrombophilia is an appropriate strategy [5,6]. Detection of antiphospholipid antibodies is strongly recommended in patients with NBTE, and, as per recommendations, includes LAC testing and solid-phase assays for anti-cardiolipin and anti-beta-2-glycoprotein I antibodies of both the IgG and IgM isotypes [28]. Interpretation of antiphospholipid serum assays falls outside the scope of this review and should follow the established guidelines [28].
When there is suspicion of an underlying autoimmune disease, the laboratory diagnostic workup should focus on serum autoantibodies, including antinuclear antibodies [82] at immunofluorescent assay on HEp-2 cells (ANA HEp-2 IFA), anti-double-stranded DNA antibodies [83], antibodies directed towards extractable nuclear antigens (ENA), ANCA [84], rheumatoid factors, and antibodies directed towards citrullinated peptides (ACPA). The performance quality of these diagnostic tools depends on the pre-test probability. It is important to note that patients with infective endocarditis have reported a prevalence of 24–50% RF positivity [54,85]. Antiphospholipid antibodies have also been detected in a significant proportion of patients, with the IgM isotypes possibly being associated with an increased risk of embolic events [86,87]. ANCA positivity has been reported in a small cohort of subjects with infective endocarditis, particularly directed towards proteinase 3 (PR3-ANCA) [88]. These observations reflect the prominent immunological activation that characterizes patients with infective endocarditis, as suggested by the frequent evidence of complement consumption [89]. Therefore, it is crucial to carefully exclude the presence of an infective etiology in a patient with endocardial vegetations, even in the presence of suggestive autoimmune serology. No single diagnostic test is currently available to distinguish between infective and NBTE; also, apart from antiphospholipid antibodies, no autoantibody can predict the risk of NBTE during the disease history of a patient with SLE and/or APS.
3.4. Echocardiography
Echocardiography remains the diagnostic standard for suspected endocarditis, with transthoracic echocardiogram (TTE) recommended as the primary imaging modality in cases of clinical concern. Transesophageal echocardiography (TEE) is recommended as a secondary investigation when TTE results are inconclusive but clinical suspicion remains elevated, and as an initial tool in the presence of prosthetic valve or intra-cardiac device infection [11]. A meta-analysis on infective endocarditis demonstrated that TEE exhibits a higher sensitivity than TTE, leading the authors to conclude that TEE should always be performed to rule out endocarditis [90]. In patients with NBTE, TEE is significantly more sensitive than TTE in detecting vegetations, particularly when small (<5 mm) [6], with a large observational study reporting a sensitivity of 97% for TEE compared to 47% for TTE [9]. As a result of these differences and despite the absence of specific guidelines, it is strongly recommended to perform TEE to exclude NBTE in high-suspicion clinical contexts, such as cases involving disseminated cerebral infarctions or peripheral arterial embolization in patients with a history of cancer or autoimmune disease.
During the echocardiographic evaluation, sterile thrombotic vegetations may manifest as either (a) small (typically < 1 cm), broad-based, hyperechoic, verrucoid nodules, usually located at the level of the valve commissure or on the low-pressure side of the leaflets [6], or (b) diffuse valve thickening. NBTE is characterized by a low-grade or lack of compromise on valve integrity [72], and patients with NBTE experience valvular dysfunction less frequently compared to those with infective endocarditis [6]. Regurgitation is more prevalent than stenosis, occurring in 54% of patients [10], and is typically of mild to moderate severity [72]. Multi-valvular involvement can occur in up to one-third of patients, commonly involving the mitral and aortic leaflets, whereas right-sided valves are generally spared [10,72,91].
3.5. Computed Tomography, Magnetic Resonance Imaging, and Multimodality Imaging
Data regarding non-ultrasonography imaging techniques in the diagnosis and management of NBTE are limited and are mainly represented by case reports and small case series, while most of the evidence is instead derived from infective endocarditis. These techniques include computed tomography (CT), magnetic resonance imaging (MRI), and nuclear imaging.
Cardiac CT scanning offers the highest spatial resolution compared to ultrasonography and MRI and demonstrates good sensitivity and specificity in detecting valvular lesions in patients with infective endocarditis compared to TEE [6,11], while also assessing valve thickness, mobility, and the degree of calcifications, thus overcoming one of the most important limitations of TEE, represented by calcific valve degeneration [92]. However, the temporal resolution of cardiac CT may limit the detection of small and mobile vegetations during certain phases of the cardiac cycle [92,93]. These vegetations are frequently associated with NBTE, as is the case of Libman–Sacks verrucae. Nevertheless, one of the significant advantages of a CT scan is the ability to extend the examination into a panoramic full-body view, which can simultaneously detect the presence of endocardial vegetations and peripheral, cerebral, and coronary embolization, as well as screen for malignant lesions [92].
Cardiac MRI has gained importance in recent years as a technique for evaluating cardiac morphology and function, particularly in non-ischemic and inflammatory myocardial diseases [94]. Moreover, MRI now represents the gold standard technique for the evaluation of heart valves, especially in the case of regurgitation, as it can quantify their function and degree of fibrosis [95]. Despite not being mentioned in the latest guidelines of the European Society of Cardiology for the management of infective endocarditis [11], promising data have been reported in recent years. Cardiac MRI is capable of detecting and characterizing endocardial vegetations, as well as the presence of endothelial delayed contrast enhancement, suggesting endocardial inflammation even in the absence of obvious masses [96]. Such diagnostic ability could help in differentiating “purely” thrombotic forms of NBTE, such as, for example, malignancy-associated cases, from mild-inflammatory forms, as in the case of Libman–Sacks endocarditis. Cardiac MRI can also assess the presence of local complications of infective endocarditis, such as valvular lesions and endo-myocardial abscesses [92], and has demonstrated high sensitivity in detecting NBTE [97]. However, it is questionable whether the cost-effectiveness of cardiac MRI can surpass that of TEE in cases of suspected NBTE, as this condition is typically not associated with local complications, except for the possibility of mild valvular dysfunction. On the other hand, due to its high sensitivity in detecting cerebral embolisms, MRI offers the possibility to study the endo-, myo-, and pericardium (which is often inflamed in patients with SLE), as well as the central nervous system, with a single examination [92,98].
Multimodality imaging with 18-fluorodeoxyglucose positron emission tomography/CT scan (18FDG-PET/CT) and 99Technetium-labeled leukocyte single photon emission computed tomography/CT scan (SPECT/CT) represents a major diagnostic criterion for infective endocarditis, according to the most recent European and American guidelines, particularly in cases of prosthetic or intra-cardiac device infections [11,99]. Moreover, the panoramic full-body representation of nuclear imaging techniques is useful in detecting systemic embolization and complications. 18FDG-PET/CT and 99Tc-SPECT/CT can identify areas of endothelial inflammation before morphological alterations are established, thus increasing diagnostic sensitivity [100]. Limitations include lower spatial resolution (limited to >5 mm) compared to TEE or CT scans, as well as poor specificity. Identifying areas of increased glucose metabolism (18FDG) or leukocyte accumulation (99Tc-labeled leukocytes) can indeed lead to false positive results in the case of large vessel vasculitis, atherosclerotic lesions, and recent surgical procedures [11]. Additionally, the diagnostic power of 18FDG-PET/CT is low in tissues with physiologically high baseline metabolisms, such as the brain, kidneys, and myocardium. SPECT/CT has been shown to be effective in differentiating infective from thrombotic endocarditis in a small cohort of patients with endocardial vegetations, as it does not detect labeled inflammatory cells in sterile vegetations [101]. However, there are limited and conflicting data regarding the role of 18FDG-PET/CT. A case of Libman–Sacks endocarditis with increased 18FDG uptake was reported in a patient with SLE [102]. On the other hand, retrospective data on cancer patients indicate that sterile thrombotic vegetations should lack metabolic activity [103], suggesting that 18FDG-PET/CT may assist in distinguishing between NBTE and infective endocarditis in cases of confirmed endocardial vegetations. Most importantly, 18FDG-PET/CT improves patient management by revealing occult cancers in over 50% of cases [103]. Efforts are required in order to elucidate whether different underlying etiologies lead to distinct findings in nuclear imaging, thus reflecting a more complex and intricate pathogenesis.
3.6. Proposed Diagnostic Algorithm
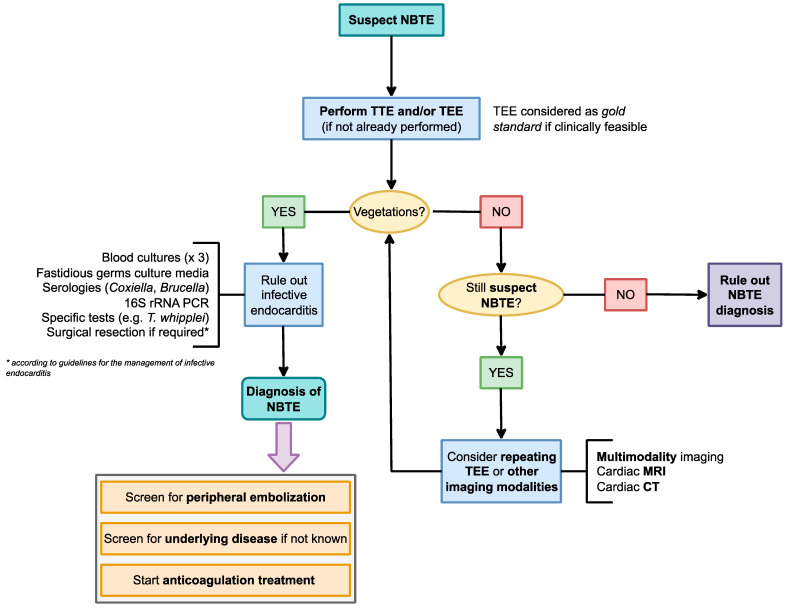
Despite representing a neglected clinical entity, prompt suspicion and diagnosis are required when managing NBTE, particularly to rule out infective vegetations and proceed to the diagnosis of the underlying disorder, and assessment of peripheral embolization should proceed concurrently during the diagnostic work-up. A high level of suspicion remains the most important diagnostic element, and should take into account all of the aforementioned factors. A proposed algorithm outlining the key steps for suspected NBTE is presented in Figure 1. Clinical suspicion of NBTE should arise in patients with known or suspected risk factors presenting with peripheral embolization. For example, the occurrence of an acute neurological event in a patient with malignancy or SLE/APS should prompt consideration of NBTE. These patients should undergo comprehensive clinical, laboratory, and radiological evaluations to identify signs of embolization. Moreover, although infective endocarditis is more commonly linked to valve dysfunction, local complications, and hemodynamic disturbances, NBTE can be associated with valvular damage.
Figure 1.
Proposed diagnostic algorithm for suspect NBTE. List of abbreviations—CT: computed tomography; MRI: magnetic resonance imaging; NBTE: nonbacterial thrombotic endocarditis; PCR: polymerase chain reaction; TTE/TEE: transthoracic/transesophageal echocardiogram.
Endocardial vegetations may be incidentally detected during an echocardiographic evaluation performed for other clinical reasons [5]. The differential diagnosis should consider other causes of endocardial masses, such as primary valvular tumors (e.g., papillary fibroelastoma, cardiac myxoma), infective endocarditis, and Lambl’s excrescences. Clinical and echocardiographic criteria for these conditions have been proposed [104,105]. Once an endocardial mass has been identified as vegetation, the exclusion of infective endocarditis is crucial, particularly in cases of culture-negative endocarditis where ancillary laboratory and microbiological tests play a vital role [105]. No specific blood test can sufficiently and specifically detect NBTE or confirm its diagnosis without thorough exclusion of an infectious process. As previously mentioned, the results of coagulation studies, autoimmune serologies, and immune-function tests (e.g., assessment of complement fraction) are often elusive, as they rarely yield false positives in patients with infective endocarditis and are thus insufficient to identify a specific subset of patients with autoimmune-related NBTE.
When associated with cancer, NBTE can potentially offer an opportunity for earlier diagnosis [103] and better prognosis with timely treatment options. In a comprehensive meta-analysis, cancer emerged as an independent predictor of all-cause in-hospital mortality and the occurrence of embolic complications in NBTE patients, with no significant distinction between metastatic and non-metastatic neoplasms [106]. Attention should be given to the patient’s overall performance status and general conditions, which may raise suspicion of an underlying occult malignancy. These factors include weight loss, unexplained cachexia, concurrent paraneoplastic phenomena, and abnormalities in routine chemistries (such as chronic disease anemia [107], or persistent low-grade elevation of acute phase reactants not attributable to other causes [108]). The question of whether every NBTE patient should undergo an exhaustive diagnostic work-up for occult malignancy remains a topic of debate. Nonetheless, it has been suggested that patients should undergo neoplasm screening in a sex- and age-dependent manner [105]. No single diagnostic test can definitively indicate the presence of occult neoplasm in a subject with NBTE.
3.7. Diagnostic Unmet Needs
To identify a biomarker capable of discerning infective from NBTE, particularly among patients at high risk of thrombotic endocarditis, would be of major clinical relevance. Such biomarker could encompass tests for blood clotting times, lupus anticoagulant, solid-phase antiphospholipid antibodies, autoantibodies and assays associated with SLE or other connective tissue diseases, and, potentially, tumoral markers. However, considering the limited sensitivity and specificity of all these tests, especially when conducted individually or in the absence of high clinical suspicion, careful selection of the target population becomes of utmost importance. A composite index should incorporate echocardiographic peculiarities (e.g., dimensions and position of the vegetations) along with the pertinent clinical features. This tool could further categorize patients with (suspected) endocardial vegetations according to a high, moderate, or low risk for NBTE. The proposed questions aiming to identify potential red flags in this intricate differential diagnosis are outlined in Table 3, and should serve as straightforward reminders to guide the diagnosis even in the absence of gold standard techniques. This scenario is particularly relevant in low-income countries where NBTE is prevalent. Efforts should be addressed to define classification criteria for NBTE.
Table 3.
Red flags in the differential diagnosis of NBTE.
| 1. Does the patient have a history of (a) cancer or (b) systemic autoimmune disease (especially SLE or APS)? Or does the patient’s history/physical examination suggest these conditions (e.g., unexplained weight loss, symptoms attributable to specific neoplasms, history of venous/arterial thrombosis, history of recurrent miscarriage, skin rash, etc.)? |
| 2. Has the patient presented with an episode of arterial embolization, and in particular: (a) multiple infarcts? (b) cerebral infarcts? (c) splanchnic infarcts? |
| 3. Are endocardial masses located in the left-sided heart valves (especially the mitral)? |
| 4. Is the patient afebrile? Does the patient have any other sign or symptom of infection (e.g., persistent low-back pain suggesting spondylodiscitis, meningeal or neurological signs suggestive of central nervous system dissemination of microorganisms)? |
| 5. Does the patient manifest any signs that may suggest infective endocarditis, e.g., Roth’s spots, Janeway’s lesions, Osler’s nodules, periungual microhemorrhages? |
| 6. Has the patient ever tested positive for antiphospholipid antibodies or connective tissue disease-associated autoantibodies? |
Whether the diagnosis of NBTE in a patient with an established underlying condition should prompt physicians to investigate additional predisposing factors is yet to be determined. Patients with SLE, for instance, carry a higher risk of malignancy compared to the general population [109], while a notable prevalence of antiphospholipid antibody positivity has been observed in various types of cancers and is correlated with an increased risk of malignancy-associated thrombotic events [110,111]. Further research is thus necessary to establish whether the presence of Libman–Sacks endocarditis should raise suspicion of occult malignancy in patients with SLE and/or APS, and vice versa if patients with marantic endocarditis should be tested for antiphospholipid antibodies or other coagulation defects. These observations, which extend beyond speculation, have the potential to induce significant changes in treatment strategies and prognoses.
4. Therapeutic Approaches
The treatment approach for NBTE is based on several aspects. First, it is important to address the underlying condition that contributes to NBTE. Second, efforts should be made to prevent the peripheral embolization of endocardial vegetations. Third and last, any associated valvular dysfunction should be corrected [11,99]. Given the lack of specific guidelines and limited evidence, which is mostly derived from case reports and series, the management of NBTE should involve a dedicated multidisciplinary “endocarditis team” consisting of imaging cardiologists, radiologists, nuclear medicine physicians, cardiac surgeons, infectious disease specialists, oncologists/hematologists, specialists in coagulation disorders, and internists/rheumatologists [6,112,113].
Unless contraindicated, anticoagulant therapy should be initiated in all patients promptly upon the diagnosis of NBTE [6,11,99], but special attention should be given to the risk of hemorrhagic infarction in patients presenting with embolic ischemic stroke [114]. Striking the right balance between thrombotic and hemorrhagic risks is crucial, particularly in patients with stigmata of coagulopathy [115]. In the absence of sufficient data on the use of oral anticoagulants, heparins, including both unfractionated (UFH) and low-molecular-weight heparin (LMWH), are preferred [6,113]. Vitamin K antagonists (VKAs) may be less effective than LMWH in patients with marantic endocarditis associated with malignancy; thus, heparin-based products have been the standard treatment for cancer-associated venous thromboembolism for many years [116]. However, promising evidence exists for the use of direct oral anticoagulants (DOACs) in the treatment and secondary prevention of thrombotic complications in cancer patients [117,118]. Further research is needed to evaluate the role of DOACs in paraneoplastic NBTE. Conversely, DOACs have been associated with an increased risk of thromboembolic events in patients with APS compared to VKAs (target INR 2–3), and their use is not recommended by the current guidelines [28]. Recent evidence, however, suggests that DOACs might be considered for venous thromboembolic events in APS patients without the antiphospholipid “triple positivity” profile (i.e.,: LAC, anti-cardiolipin, and anti-beta-2-glycoprotein I positivity), which is associated with the highest risk of thrombotic events [28,119]. Studies investigating different antithrombotic treatments for NBTE are warranted, and the role of DOACs should be clarified for both cancer-associated and autoimmune-related forms, particularly APS and SLE, with and without associated antiphospholipid antibodies.
Determining the optimal duration of anticoagulant therapy is unclear, and lifelong anticoagulation is recommended in cases of venous thromboembolism in patients with advanced malignancy [117], but if the cancer is cured, long-term treatment appears to be unnecessary [120]. Patients with APS-associated thromboembolic events should receive indefinite antithrombotic therapy [28]. Similar approaches could be considered to define the duration of anticoagulant therapy in NBTE patients. Furthermore, more evidence is needed to determine whether the resolution of thrombotic vegetations on TEE is associated with a significantly lower risk of embolic complications, allowing for the discontinuation of anticoagulation after an appropriate treatment period. In this regard, reduced-dose DOACs (i.e.,: rivaroxaban 10 mg qd, apixaban 2.5 mg bid) have already been approved for long-term secondary prophylaxis of venous thromboembolism [121,122]; their role could be explored in cases of resolved NBTE with persistent, longstanding risk factors for relapse (e.g.: advanced, incurable cancer).
Unlike infective endocarditis, there are no established indications for surgical intervention in NBTE patients, and decisions should be made on a case-by-case basis [11,99]. Cardiac surgery may be considered in cases of acute heart failure, severe valvular dysfunction, presence of large mobile vegetations at high risk of embolization, or recurrent embolic events despite adequate anticoagulant therapy [5,113]. Diagnosing and treating the underlying condition is crucial, although it remains unclear whether these steps contribute to preventing NBTE relapse. For example, conflicting data have been reported on the association between SLE disease activity and the risk of Libman–Sacks endocarditis [35,123]. Additionally, the role of immunosuppressive treatment in preventing the formation, progression, and complications of thrombotic endocardial vegetations in patients with autoimmune diseases requires further investigation.
5. Concluding Remarks
NBTE is a rare, potentially severe condition that requires prompt diagnosis and management since it is associated with underlying malignancy and autoimmune disorders. Elucidating the pathogenesis of NBTE linked to different diseases is a major area of research. Distinguishing NBTE from infective endocarditis, particularly in culture-negative forms, remains a challenge of the utmost importance, and more accurate and sensitive diagnostic strategies are warranted. While TEE currently represents the diagnostic standard, other imaging techniques hold promise as adjunctive tools for early diagnosis and differentiation from infective vegetations, including cardiac MRI and 18FDG-PET/CT, the latter already representing a major diagnostic criterion of endocarditis in specific situations. However, NBTE is often a neglected entity; this is reflected by the lack of specific guidelines and underscores the necessity of a holistic and multidisciplinary approach to the entire management process, from clinical suspicion to vegetation detection; differential diagnosis; comprehensive work-up, including the search of underlying conditions; and treatment. Collaborative efforts among various specialists are essential in order to optimize the patient care and outcomes involved in this complex and multi-faced condition.
Author Contributions
Conceptualization, A.T. and M.D.S.; writing—original draft preparation, A.T. and I.S.; writing—review and editing, M.D.S.; supervision, C.S. and G.L.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was partially supported by “Ricerca Corrente” funding from Italian Ministry of Health to IRCCS Humanitas Research Hospital.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ziegier E. Ueber Den Bau Und Die Entstehung Der Endocaridtis Chen Efflorescenzen. Ver. Kong Inn. Med. 1888;7:339–343. [Google Scholar]
- 2.Gross L., Friedberg C.K. Nonbacterial Thrombotic Endocarditis: Classification and General Description. Arch. Intern. Med. 1936;58:620–640. doi: 10.1001/archinte.1936.00170140045004. [DOI] [Google Scholar]
- 3.Libman E., Sacks B. A Hitherto Undescribed Form of Valvular and Mural Endocarditis. Arch. Intern. Med. 1924;33:701–737. doi: 10.1001/archinte.1924.00110300044002. [DOI] [Google Scholar]
- 4.Katsouli A., Massad M.G. Current Issues in the Diagnosis and Management of Blood Culture-Negative Infective and Non-Infective Endocarditis. Ann. Thorac. Surg. 2013;95:1467–1474. doi: 10.1016/j.athoracsur.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 5.Hurrell H., Roberts-Thomson R., Prendergast B.D. Non-Infective Endocarditis. Heart. 2020;106:1023–1029. doi: 10.1136/heartjnl-2019-315204. [DOI] [PubMed] [Google Scholar]
- 6.Zmaili M., Alzubi J., Lo Presti Vega S., Ababneh E., Xu B. Non-Bacterial Thrombotic Endocarditis: A State-of-the-Art Contemporary Review. Prog. Cardiovasc. Dis. 2022;74:99–110. doi: 10.1016/j.pcad.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Bussani R., DE-Giorgio F., Pesel G., Zandonà L., Sinagra G., Grassi S., Baldi A., Abbate A., Silvestri F. Overview and Comparison of Infectious Endocarditis and Non-Infectious Endocarditis: A Review of 814 Autoptic Cases. In Vivo. 2019;33:1565–1572. doi: 10.21873/invivo.11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenz C.J., Mankad R., Klarich K., Kurmann R., McBane R.D. Antiphospholipid Syndrome and the Relationship between Laboratory Assay Positivity and Prevalence of Non-Bacterial Thrombotic Endocarditis: A Retrospective Cohort Study. J. Thromb. Haemost. 2020;18:1408–1414. doi: 10.1111/jth.14798. [DOI] [PubMed] [Google Scholar]
- 9.Zmaili M.A., Alzubi J.M., Kocyigit D., Bansal A., Samra G.S., Grimm R., Griffin B.P., Xu B. A Contemporary 20-Year Cleveland Clinic Experience of Nonbacterial Thrombotic Endocarditis: Etiology, Echocardiographic Imaging, Management, and Outcomes. Am. J. Med. 2021;134:361–369. doi: 10.1016/j.amjmed.2020.06.047. [DOI] [PubMed] [Google Scholar]
- 10.Quintero-Martinez J.A., Hindy J.-R., El Zein S., Michelena H.I., Nkomo V.T., DeSimone D.C., Baddour L.M. Contemporary Demographics, Diagnostics and Outcomes in Non-Bacterial Thrombotic Endocarditis. Heart. 2022;108:1637–1643. doi: 10.1136/heartjnl-2022-320970. [DOI] [PubMed] [Google Scholar]
- 11.Habib G., Lancellotti P., Antunes M.J., Bongiorni M.G., Casalta J.-P., Del Zotti F., Dulgheru R., El Khoury G., Erba P.A., Iung B., et al. 2015 ESC Guidelines for the Management of Infective Endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur. Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 12.Llenas-García J., Guerra-Vales J.M., Montes-Moreno S., López-Ríos F., Castelbón-Fernández F.J., Chimeno-García J. Nonbacterial thrombotic endocarditis: Clinicopathologic study of a necropsy series. Rev. Esp. Cardiol. 2007;60:493–500. doi: 10.1016/S0300-8932(07)75066-2. [DOI] [PubMed] [Google Scholar]
- 13.Corrales-Rodriguez L., Blais N. Lung Cancer Associated Venous Thromboembolic Disease: A Comprehensive Review. Lung Cancer. 2012;75:1–8. doi: 10.1016/j.lungcan.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Hisada Y., Mackman N. Cancer-Associated Pathways and Biomarkers of Venous Thrombosis. Blood. 2017;130:1499–1506. doi: 10.1182/blood-2017-03-743211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horowitz N.A., Brenner B. Thrombosis in Hematological Malignancies: Mechanisms and Implications. Thromb. Res. 2020;191((Suppl. S1)):S58–S62. doi: 10.1016/S0049-3848(20)30398-4. [DOI] [PubMed] [Google Scholar]
- 16.Gerotziafas G.T., Taher A., Abdel-Razeq H., AboElnazar E., Spyropoulos A.C., El Shemmari S., Larsen A.K., Elalamy I., COMPASS–CAT Working Group A Predictive Score for Thrombosis Associated with Breast, Colorectal, Lung, or Ovarian Cancer: The Prospective COMPASS-Cancer-Associated Thrombosis Study. Oncologist. 2017;22:1222–1231. doi: 10.1634/theoncologist.2016-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahouma M., Khairallah S., Dabsha A., Elkharbotly I.A.M.H., Baudo M., Ismail A., Korani O.M., Hossny M., Dimagli A., Girardi L.N., et al. Lung Cancer as a Leading Cause among Paraneoplastic Non-Bacterial Thrombotic Endocarditis: A Meta-Analysis of Individual Patients’ Data. Cancers. 2023;15:1848. doi: 10.3390/cancers15061848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto T., Aoki T., Kawabata Y., Owai Y., Matsuda Y., Tamura S. Nonbacterial Thrombotic Endocarditis Associated with Acute Promyelocytic Leukemia: An Autopsy Case Report. Medicina. 2021;57:1264. doi: 10.3390/medicina57111264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed S., Jani P., Yamani M.H., Ailawadhi S., Alegria V.R., Ailawadhi M. Marantic Endocarditis Associated with T-Cell Large Granular Lymphocytic Leukemia: First Report of Its Occurrence with a Lymphoproliferative Malignancy in Adults. J. Oncol. Pract. 2018;14:625–627. doi: 10.1200/JOP.18.00168. [DOI] [PubMed] [Google Scholar]
- 20.Wang C.-Y., Lee H.-C., Lin R.-J., Tsai J.-J. Spondyloarthritis and Nonbacterial Thrombotic Endocarditis as Paraneoplastic Manifestations in Treatment-Naive Burkitt Lymphoma. Int. J. Rheum. Dis. 2023;26:1608–1611. doi: 10.1111/1756-185X.14651. [DOI] [PubMed] [Google Scholar]
- 21.Wang C.-Y., Lee H.-C., Lin R.-J., Tsai J.-J. Nonbacterial Thrombotic Endocarditis as a Paraneoplastic Manifestation of Newly Diagnosed Splenic Large B Cell Lymphoma. Kaohsiung J. Med. Sci. 2023;39:644–645. doi: 10.1002/kjm2.12677. [DOI] [PubMed] [Google Scholar]
- 22.Falanga A., Marchetti M., Vignoli A. Coagulation and Cancer: Biological and Clinical Aspects. J. Thromb. Haemost. 2013;11:223–233. doi: 10.1111/jth.12075. [DOI] [PubMed] [Google Scholar]
- 23.Salyer W.R., Page D.L., Hutchins G.M. The Development of Cardiac Myxomas and Papillary Endocardial Lesions from Mural Thrombus. Am. Heart J. 1975;89:4–17. doi: 10.1016/0002-8703(75)90003-4. [DOI] [PubMed] [Google Scholar]
- 24.Ikezoe T. Pathogenesis of Disseminated Intravascular Coagulation in Patients with Acute Promyelocytic Leukemia, and Its Treatment Using Recombinant Human Soluble Thrombomodulin. Int. J. Hematol. 2014;100:27–37. doi: 10.1007/s12185-013-1463-0. [DOI] [PubMed] [Google Scholar]
- 25.Saito H., Maruyama I., Shimazaki S., Yamamoto Y., Aikawa N., Ohno R., Hirayama A., Matsuda T., Asakura H., Nakashima M., et al. Efficacy and Safety of Recombinant Human Soluble Thrombomodulin (ART-123) in Disseminated Intravascular Coagulation: Results of a Phase III, Randomized, Double-Blind Clinical Trial. J. Thromb. Haemost. 2007;5:31–41. doi: 10.1111/j.1538-7836.2006.02267.x. [DOI] [PubMed] [Google Scholar]
- 26.El-Shami K., Griffiths E., Streiff M. Nonbacterial Thrombotic Endocarditis in Cancer Patients: Pathogenesis, Diagnosis, and Treatment. Oncologist. 2007;12:518–523. doi: 10.1634/theoncologist.12-5-518. [DOI] [PubMed] [Google Scholar]
- 27.Moyssakis I., Tektonidou M.G., Vasilliou V.A., Samarkos M., Votteas V., Moutsopoulos H.M. Libman-Sacks Endocarditis in Systemic Lupus Erythematosus: Prevalence, Associations, and Evolution. Am. J. Med. 2007;120:636–642. doi: 10.1016/j.amjmed.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Tektonidou M.G., Andreoli L., Limper M., Amoura Z., Cervera R., Costedoat-Chalumeau N., Cuadrado M.J., Dörner T., Ferrer-Oliveras R., Hambly K., et al. EULAR Recommendations for the Management of Antiphospholipid Syndrome in Adults. Ann. Rheum. Dis. 2019;78:1296–1304. doi: 10.1136/annrheumdis-2019-215213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziporen L., Goldberg I., Arad M., Hojnik M., Ordi-Ros J., Afek A., Blank M., Sandbank Y., Vilardell-Tarres M., de Torres I., et al. Libman-Sacks Endocarditis in the Antiphospholipid Syndrome: Immunopathologic Findings in Deformed Heart Valves. Lupus. 1996;5:196–205. doi: 10.1177/096120339600500306. [DOI] [PubMed] [Google Scholar]
- 30.Amital H., Langevitz P., Levy Y., Afek A., Goldberg I., Pras M., Livneh A., Shoenfeld Y. Valvular Deposition of Antiphospholipid Antibodies in the Antiphospholipid Syndrome: A Clue to the Origin of the Disease. Clin. Exp. Rheumatol. 1999;17:99–102. [PubMed] [Google Scholar]
- 31.Bidani A.K., Roberts J.L., Schwartz M.M., Lewis E.J. Immunopathology of Cardiac Lesions in Fatal Systemic Lupus Erythematosus. Am. J. Med. 1980;69:849–858. doi: 10.1016/S0002-9343(80)80010-6. [DOI] [PubMed] [Google Scholar]
- 32.Ansari A., Larson P.H., Bates H.D. Cardiovascular Manifestations of Systemic Lupus Erythematosus: Current Perspective. Prog. Cardiovasc. Dis. 1985;27:421–434. doi: 10.1016/0033-0620(85)90003-9. [DOI] [PubMed] [Google Scholar]
- 33.Knight J.S., Branch D.W., Ortel T.L. Antiphospholipid Syndrome: Advances in Diagnosis, Pathogenesis, and Management. BMJ. 2023;380:e069717. doi: 10.1136/bmj-2021-069717. [DOI] [PubMed] [Google Scholar]
- 34.Liu X., Zhu L., Liu H., Cai Q., Yun Z., Sun F., Jia Y., Guo J., Li C. Non-Criteria Antiphospholipid Antibodies in Antiphospholipid Syndrome: Diagnostic Value Added. Front. Immunol. 2022;13:972012. doi: 10.3389/fimmu.2022.972012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoo B.-W., Lee S.-W., Song J.J., Park Y.-B., Jung S.M. Clinical Characteristics and Long-Term Outcomes of Libman-Sacks Endocarditis in Patients with Systemic Lupus Erythematosus. Lupus. 2020;29:1115–1120. doi: 10.1177/0961203320930097. [DOI] [PubMed] [Google Scholar]
- 36.Devinsky O., Petito C.K., Alonso D.R. Clinical and Neuropathological Findings in Systemic Lupus Erythematosus: The Role of Vasculitis, Heart Emboli, and Thrombotic Thrombocytopenic Purpura. Ann. Neurol. 1988;23:380–384. doi: 10.1002/ana.410230411. [DOI] [PubMed] [Google Scholar]
- 37.Ramirez G.A., Canti V., Del Rosso S., Erra R., Moiola L., Magnoni M., Bozzolo E.P., Manfredi A.A., Rovere-Querini P. Diagnostic Performance of APS/PT Antibodies in Neuropsychiatric Lupus and Cardiovascular Complications of Systemic Lupus Erythematosus. Autoimmunity. 2020;53:21–27. doi: 10.1080/08916934.2019.1696778. [DOI] [PubMed] [Google Scholar]
- 38.Choi J.-H., Park J.-E., Kim J.-Y., Kang T. Non-Bacterial Thrombotic Endocarditis in a Patient with Rheumatoid Arthritis. Korean Circ. J. 2016;46:425–428. doi: 10.4070/kcj.2016.46.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durães Campos I., Marques A.R., Sousa L., Fonseca S., Oliveira C., Vieira C., Apolinário I., Brandão I. Nonbacterial Thrombotic Endocarditis. Rev. Port. Cardiol. (Engl. Ed.) 2019;38:511–514. doi: 10.1016/j.repc.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 40.Garcia R.A., Guragai N., Vasudev R., Randhawa P., Habib M.G. Rare Association of Non-Bacterial Thrombotic Endocarditis, Myocardial Infarction, and Acute Limb Ischemia Secondary to Rheumatoid Arthritis: Comprehensive Case Series with Literature Review. Cureus. 2021;13:e13319. doi: 10.7759/cureus.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vege A., Beery J., Kara A. Antisynthetase Syndrome in a Patient with Pulmonary Embolism and Nonbacterial Thrombotic Endocarditis. Case Rep. Rheumatol. 2023;2023:9068597. doi: 10.1155/2023/9068597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Langhe E., Seghers A., Demaerel P., Verschueren P., Lemmens R. Non-Infective Endocarditis with Systemic Embolization and Recurrent Stroke in Systemic Sclerosis. Rheumatology. 2016;55:589–591. doi: 10.1093/rheumatology/kev381. [DOI] [PubMed] [Google Scholar]
- 43.Karan A., Guo H.J., Omar M., Streit S. Extensive Nonbacterial Thrombotic Endocarditis with Recurrent Strokes as a Manifestation of Diffuse Scleroderma. Am. J. Med. 2023;136:e142–e143. doi: 10.1016/j.amjmed.2023.02.016. [DOI] [PubMed] [Google Scholar]
- 44.Mazzoni C., Scheggi V., Mariani T. Cardiac Involvement in Behçet Disease Presenting as Non-Bacterial Thrombotic Endocarditis: A Case Report. J. Cardiol. Cases. 2021;24:157–160. doi: 10.1016/j.jccase.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida W., Mutsuga M., Ito H., Oshima H., Usui A. Nonbacterial Thrombotic Endocarditis Associated with Crohn Disease. Ann. Thorac. Surg. 2018;105:e199–e201. doi: 10.1016/j.athoracsur.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Dammassa V., Raineri C., Aiello M., Mojoli F., Tavazzi G. Non-Infective Endocarditis in Crohn’s Disease: An Anecdotal Case of Obstructive Shock. Eur. Heart J. Cardiovasc. Imaging. 2021;22:e139. doi: 10.1093/ehjci/jeab003. [DOI] [PubMed] [Google Scholar]
- 47.Gettigan N.M., Sheehan M., Slattery E. Metastatic Crohn’s Disease of the Aortic Valve Resulting in Severe Aortic Insufficiency, Non-Infective Endocarditis, and Pericarditis. Inflamm. Bowel Dis. 2019;25:e117–e118. doi: 10.1093/ibd/izz124. [DOI] [PubMed] [Google Scholar]
- 48.Paik M.L., MacLennan G.T., Seftel A.D. Embolic Testicular Infarction Secondary to Nonbacterial Thrombotic Endocarditis in Wegener’s Granulomatosis. J. Urol. 1999;161:919–920. doi: 10.1016/S0022-5347(01)61808-1. [DOI] [PubMed] [Google Scholar]
- 49.Marquetand C., Lamprecht P., Dressler F.F., Perner S., Holl-Ulrich K., Stierle U., Aboud A., Reil J.-C. GPA-Induced Granulomatous Endocarditis Mimicking a Thrombotic Mitral Valve Stenosis. JACC Case Rep. 2020;2:2151–2155. doi: 10.1016/j.jaccas.2020.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karthikeyan K., Balla S., Alpert M.A. Non-Infectious Aortic and Mitral Valve Vegetations in a Patient with Eosinophilic Granulomatosis with Polyangiitis. BMJ Case Rep. 2019;12:e225947. doi: 10.1136/bcr-2018-225947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muñoz-Grajales C., Chavarriaga J.C., Márquez J.D., Pinto L.F. Noninfective Endocarditis in Microscopic Polyangiitis: Report of a Case with a Successful Response to Immunosuppressive Therapy. Reumatol. Clin. (Engl. Ed.) 2019;15:e21–e23. doi: 10.1016/j.reuma.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Hesselink D.A., van der Klooster J.M., Schelfhout L.J.D.M., Scheffer M.G. Non-Bacterial Thrombotic (Marantic) Endocarditis Associated with Giant-Cell Arteritis. Eur. J. Intern. Med. 2001;12:454–458. doi: 10.1016/S0953-6205(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 53.Terré A., Lidove O., Georges O., Mesnildrey P., Chennebault H., Ziza J.-M. Non-Infective Endocarditis: Expanding the Phenotype of Giant Cell Arteritis. Jt. Bone Spine. 2019;86:115–116. doi: 10.1016/j.jbspin.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Sheagren J.N., Tuazon C.U., Griffin C., Padmore N. Rheumatoid Factor in Acute Bacterial Endocarditis. Arthritis Rheum. 1976;19:887–890. doi: 10.1002/art.1780190509. [DOI] [PubMed] [Google Scholar]
- 55.Kupferwasser L.I., Hafner G., Mohr-Kahaly S., Erbel R., Meyer J., Darius H. The Presence of Infection-Related Antiphospholipid Antibodies in Infective Endocarditis Determines a Major Risk Factor for Embolic Events. J. Am. Coll. Cardiol. 1999;33:1365–1371. doi: 10.1016/S0735-1097(99)00024-8. [DOI] [PubMed] [Google Scholar]
- 56.Kim H.S., Suzuki M., Lie J.T., Titus J.L. Nonbacterial Thrombotic Endocarditis (NBTE) and Disseminated Intravascular Coagulation (DIC): Autopsy Study of 36 Patients. Arch. Pathol. Lab Med. 1977;101:65–68. [PubMed] [Google Scholar]
- 57.Nakanishi K., Tajima F., Nakata Y., Osada H., Sugiyama K., Maruta H., Kawai T., Suzuki M., Torikata C. Hypercoagulable State in a Hypobaric, Hypoxic Environment Causes Non-Bacterial Thrombotic Endocarditis in Rats. J. Pathol. 1997;181:338–346. doi: 10.1002/(SICI)1096-9896(199703)181:3<338::AID-PATH773>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 58.Nakanishi K., Tajima F., Nakata Y., Osada H., Ogata K., Kawai T., Torikata C., Suga T., Takishima K., Aurues T., et al. Tissue Factor Is Associated with the Nonbacterial Thrombotic Endocarditis Induced by a Hypobaric Hypoxic Environment in Rats. Virchows Arch. 1998;433:375–379. doi: 10.1007/s004280050262. [DOI] [PubMed] [Google Scholar]
- 59.Asopa S., Patel A., Khan O.A., Sharma R., Ohri S.K. Non-Bacterial Thrombotic Endocarditis. Eur. J. Cardiothorac. Surg. 2007;32:696–701. doi: 10.1016/j.ejcts.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 60.Steiner I. Nonbacterial Thrombotic versus Infective Endocarditis: A Necropsy Study of 320 Cases. Cardiovasc. Pathol. 1995;4:207–209. doi: 10.1016/1054-8807(95)00001-L. [DOI] [PubMed] [Google Scholar]
- 61.Stevens S.M., Woller S.C., Bauer K.A., Kasthuri R., Cushman M., Streiff M., Lim W., Douketis J.D. Guidance for the Evaluation and Treatment of Hereditary and Acquired Thrombophilia. J. Thromb. Thrombolysis. 2016;41:154–164. doi: 10.1007/s11239-015-1316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vannucchi A.M., Guglielmelli P. JAK2 Mutation-Related Disease and Thrombosis. Semin. Thromb. Hemost. 2013;39:496–506. doi: 10.1055/s-0033-1343890. [DOI] [PubMed] [Google Scholar]
- 63.Arber D.A., Orazi A., Hasserjian R.P., Borowitz M.J., Calvo K.R., Kvasnicka H.-M., Wang S.A., Bagg A., Barbui T., Branford S., et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating Morphologic, Clinical, and Genomic Data. Blood. 2022;140:1200–1228. doi: 10.1182/blood.2022015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roldan C.A., Tolstrup K., Macias L., Qualls C.R., Maynard D., Charlton G., Sibbitt W.L. Libman-Sacks Endocarditis: Detection, Characterization, and Clinical Correlates by Three-Dimensional Transesophageal Echocardiography. J. Am. Soc. Echocardiogr. 2015;28:770–779. doi: 10.1016/j.echo.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deppisch L.M., Fayemi A.O. Non-Bacterial Thrombotic Endocarditis: Clinicopathologic Correlations. Am. Heart J. 1976;92:723–729. doi: 10.1016/S0002-8703(76)80008-7. [DOI] [PubMed] [Google Scholar]
- 66.Fournier P.-E., Thuny F., Richet H., Lepidi H., Casalta J.-P., Arzouni J.-P., Maurin M., Célard M., Mainardi J.-L., Caus T., et al. Comprehensive Diagnostic Strategy for Blood Culture-Negative Endocarditis: A Prospective Study of 819 New Cases. Clin. Infect. Dis. 2010;51:131–140. doi: 10.1086/653675. [DOI] [PubMed] [Google Scholar]
- 67.Waller B.F., Knapp W.S., Edwards J.E. Marantic Valvular Vegetations. Circulation. 1973;48:644–650. doi: 10.1161/01.CIR.48.3.644. [DOI] [PubMed] [Google Scholar]
- 68.Dafer R.M. Handbook of Clinical Neurology. Volume 177. Elsevier; Amsterdam, The Netherlands: 2021. Neurologic Complications of Nonbacterial Thrombotic Endocarditis; pp. 135–141. [DOI] [PubMed] [Google Scholar]
- 69.Singhal A.B., Topcuoglu M.A., Buonanno F.S. Acute Ischemic Stroke Patterns in Infective and Nonbacterial Thrombotic Endocarditis: A Diffusion-Weighted Magnetic Resonance Imaging Study. Stroke. 2002;33:1267–1273. doi: 10.1161/01.STR.0000015029.91577.36. [DOI] [PubMed] [Google Scholar]
- 70.Fayemi A.O., Deppisch L.M. Coronary Embolism and Myocardial Infarction Associated with Nonbacterial Thrombotic Endocarditis. Am. J. Clin. Pathol. 1977;68:393–396. doi: 10.1093/ajcp/68.3.393. [DOI] [PubMed] [Google Scholar]
- 71.Roldan C.A., Sibbitt W.L., Qualls C.R., Jung R.E., Greene E.R., Gasparovic C.M., Hayek R.A., Charlton G.A., Crookston K. Libman-Sacks Endocarditis and Embolic Cerebrovascular Disease. JACC Cardiovasc. Imaging. 2013;6:973–983. doi: 10.1016/j.jcmg.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reisner S.A., Brenner B., Haim N., Edoute Y., Markiewicz W. Echocardiography in Nonbacterial Thrombotic Endocarditis: From Autopsy to Clinical Entity. J. Am. Soc. Echocardiogr. 2000;13:876–881. doi: 10.1067/mje.2000.106070. [DOI] [PubMed] [Google Scholar]
- 73.Kuramoto K., Matsushita S., Yamanouchi H. Nonbacterial Thrombotic Endocarditis as a Cause of Cerebral and Myocardial Infarction. Jpn. Circ. J. 1984;48:1000–1006. doi: 10.1253/jcj.48.1000. [DOI] [PubMed] [Google Scholar]
- 74.Sethi K., Buckley J., de Wolff J. Splinter Haemorrhages, Osler’s Nodes, Janeway Lesions and Roth Spots: The Peripheral Stigmata of Endocarditis. Br. J. Hosp. Med. 2013;74:C139–C142. doi: 10.12968/hmed.2013.74.Sup9.C139. [DOI] [PubMed] [Google Scholar]
- 75.Cahill T.J., Prendergast B.D. Infective Endocarditis. Lancet. 2016;387:882–893. doi: 10.1016/S0140-6736(15)00067-7. [DOI] [PubMed] [Google Scholar]
- 76.Chambers H.F., Bayer A.S. Native-Valve Infective Endocarditis. N. Engl. J. Med. 2020;383:567–576. doi: 10.1056/NEJMcp2000400. [DOI] [PubMed] [Google Scholar]
- 77.Kiriakidou M., Ching C.L. Systemic Lupus Erythematosus. Ann. Intern. Med. 2020;172:ITC81–ITC96. doi: 10.7326/AITC202006020. [DOI] [PubMed] [Google Scholar]
- 78.Mosca M., Tani C., Vagnani S., Carli L., Bombardieri S. The Diagnosis and Classification of Undifferentiated Connective Tissue Diseases. J. Autoimmun. 2014;48:50–52. doi: 10.1016/j.jaut.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 79.Eiken P.W., Edwards W.D., Tazelaar H.D., McBane R.D., Zehr K.J. Surgical Pathology of Nonbacterial Thrombotic Endocarditis in 30 Patients, 1985–2000. Mayo Clin. Proc. 2001;76:1204–1212. doi: 10.4065/76.12.1204. [DOI] [PubMed] [Google Scholar]
- 80.Fournier P.-E., Gouriet F., Casalta J.-P., Lepidi H., Chaudet H., Thuny F., Collart F., Habib G., Raoult D. Blood Culture-Negative Endocarditis: Improving the Diagnostic Yield Using New Diagnostic Tools. Medicine. 2017;96:e8392. doi: 10.1097/MD.0000000000008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Subedi S., Jennings Z., Chen S.C.-A. Laboratory Approach to the Diagnosis of Culture-Negative Infective Endocarditis. Heart Lung Circ. 2017;26:763–771. doi: 10.1016/j.hlc.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 82.Agmon-Levin N., Damoiseaux J., Kallenberg C., Sack U., Witte T., Herold M., Bossuyt X., Musset L., Cervera R., Plaza-Lopez A., et al. International Recommendations for the Assessment of Autoantibodies to Cellular Antigens Referred to as Anti-Nuclear Antibodies. Ann. Rheum. Dis. 2014;73:17–23. doi: 10.1136/annrheumdis-2013-203863. [DOI] [PubMed] [Google Scholar]
- 83.Pisetsky D.S. Standardization of Anti-DNA Antibody Assays. Immunol. Res. 2013;56:420–424. doi: 10.1007/s12026-013-8415-x. [DOI] [PubMed] [Google Scholar]
- 84.Damoiseaux J., Csernok E., Rasmussen N., Moosig F., van Paassen P., Baslund B., Vermeersch P., Blockmans D., Cohen Tervaert J.-W., Bossuyt X. Detection of Antineutrophil Cytoplasmic Antibodies (ANCAs): A Multicentre European Vasculitis Study Group (EUVAS) Evaluation of the Value of Indirect Immunofluorescence (IIF) versus Antigen-Specific Immunoassays. Ann. Rheum. Dis. 2017;76:647–653. doi: 10.1136/annrheumdis-2016-209507. [DOI] [PubMed] [Google Scholar]
- 85.Asherson R.A., Tikly M., Staub H., Wilmshurst P.T., Coltart D.J., Khamashta M., Hughes G.R. Infective Endocarditis, Rheumatoid Factor, and Anticardiolipin Antibodies. Ann. Rheum. Dis. 1990;49:107–108. doi: 10.1136/ard.49.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zaratzian C., Gouriet F., Tissot-Dupont H., Casalta J.-P., Million M., Bardin N., Grisoli D., Habib G., Raoult D. Antiphospholipid Antibodies Proposed in the Diagnosis of Infective Endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36:1159–1162. doi: 10.1007/s10096-017-2903-1. [DOI] [PubMed] [Google Scholar]
- 87.Selton-Suty C., Maigrat C.-H., Devignes J., Goehringer F., Erpelding M.-L., Alla F., Thivilier C., Huttin O., Venner C., Juilliere Y., et al. Possible Relationship between Antiphospholipid Antibodies and Embolic Events in Infective Endocarditis. Heart. 2018;104:509–516. doi: 10.1136/heartjnl-2017-312359. [DOI] [PubMed] [Google Scholar]
- 88.Ying C.-M., Yao D.-T., Ding H.-H., Yang C.-D. Infective Endocarditis with Antineutrophil Cytoplasmic Antibody: Report of 13 Cases and Literature Review. PLoS ONE. 2014;9:e89777. doi: 10.1371/journal.pone.0089777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Messias-Reason I.J., Hayashi S.Y., Nisihara R.M., Kirschfink M. Complement Activation in Infective Endocarditis: Correlation with Extracardiac Manifestations and Prognosis. Clin. Exp. Immunol. 2002;127:310–315. doi: 10.1046/j.1365-2249.2002.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bai A.D., Steinberg M., Showler A., Burry L., Bhatia R.S., Tomlinson G.A., Bell C.M., Morris A.M. Diagnostic Accuracy of Transthoracic Echocardiography for Infective Endocarditis Findings Using Transesophageal Echocardiography as the Reference Standard: A Meta-Analysis. J. Am. Soc. Echocardiogr. 2017;30:639–646.e8. doi: 10.1016/j.echo.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 91.Langston M.C., Zack C.J., Fender E.A. Non-Bacterial Thrombotic Endocarditis: Manifestations and Diagnosis in the Age of Echocardiography. Heart. 2022;108:1590–1591. doi: 10.1136/heartjnl-2022-321223. [DOI] [PubMed] [Google Scholar]
- 92.Wong D., Rubinshtein R., Keynan Y. Alternative Cardiac Imaging Modalities to Echocardiography for the Diagnosis of Infective Endocarditis. Am. J. Cardiol. 2016;118:1410–1418. doi: 10.1016/j.amjcard.2016.07.053. [DOI] [PubMed] [Google Scholar]
- 93.Khalique O.K., Veillet-Chowdhury M., Choi A.D., Feuchtner G., Lopez-Mattei J. Cardiac Computed Tomography in the Contemporary Evaluation of Infective Endocarditis. J. Cardiovasc. Comput. Tomogr. 2021;15:304–312. doi: 10.1016/j.jcct.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 94.Ferreira V.M., Schulz-Menger J., Holmvang G., Kramer C.M., Carbone I., Sechtem U., Kindermann I., Gutberlet M., Cooper L.T., Liu P., et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 95.Vahanian A., Beyersdorf F., Praz F., Milojevic M., Baldus S., Bauersachs J., Capodanno D., Conradi L., De Bonis M., De Paulis R., et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease: Developed by the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 96.Dursun M., Yılmaz S., Yılmaz E., Yılmaz R., Onur İ., Oflaz H., Dindar A. The Utility of Cardiac MRI in Diagnosis of Infective Endocarditis: Preliminary Results. Diagn. Interv. Radiol. 2015;21:28–33. doi: 10.5152/dir.2014.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elagha A., Mohsen A. Cardiac MRI Clinches Diagnosis of Libman-Sacks Endocarditis. Lancet. 2019;393:e39. doi: 10.1016/S0140-6736(19)30770-6. [DOI] [PubMed] [Google Scholar]
- 98.Vincent L.L., Otto C.M. Infective Endocarditis: Update on Epidemiology, Outcomes, and Management. Curr. Cardiol. Rep. 2018;20:86. doi: 10.1007/s11886-018-1043-2. [DOI] [PubMed] [Google Scholar]
- 99.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., Gentile F., Jneid H., Krieger E.V., Mack M., McLeod C., et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 100.Yeh C.-L., Liou J.-Y., Chen S.-W., Chen Y.-K. Infective Endocarditis Detected by 18F-Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography/Computed Tomography in a Patient with Occult Infection. Kaohsiung J. Med. Sci. 2011;27:528–531. doi: 10.1016/j.kjms.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Erba P.A., Conti U., Lazzeri E., Sollini M., Doria R., De Tommasi S.M., Bandera F., Tascini C., Menichetti F., Dierckx R.A.J.O., et al. Added Value of 99mTc-HMPAO-Labeled Leukocyte SPECT/CT in the Characterization and Management of Patients with Infectious Endocarditis. J. Nucl. Med. 2012;53:1235–1243. doi: 10.2967/jnumed.111.099424. [DOI] [PubMed] [Google Scholar]
- 102.Dahl A., Schaadt B.K., Santoni-Rugiu E., Bruun N.E. Molecular Imaging in Libman-Sacks Endocarditis. Infect. Dis. 2015;47:263–266. doi: 10.3109/00365548.2014.984323. [DOI] [PubMed] [Google Scholar]
- 103.Deharo F., Arregle F., Bohbot Y., Tribouilloy C., Cosyns B., Donal E., Di Lena C., Selton Suty C., Bourg C., Hubert S., et al. Multimodality Imaging in Marantic Endocarditis Associated with Cancer: A Multicentric Cohort Study. Eur. Heart J. Cardiovasc. Imaging. 2023:jead139. doi: 10.1093/ehjci/jead139. [DOI] [PubMed] [Google Scholar]
- 104.Kuipers R.S., Berghuis M.A.T., Ogilvie A.C., van Wissen S.A., Riezebos R.K. Non-Bacterial Thrombotic Endocarditis Manifested by Ventricular Fibrillation in a Patient with Low Grade Ovarian Carcinoma: Case Report and Literature Review. Eur. Heart J. Case Rep. 2021;5:ytab120. doi: 10.1093/ehjcr/ytab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kurmann R., El-Am E., Ahmad A., Abbasi M.A., Mazur P., Akiki E., Anand V., Herrmann J., Casanegra A.I., Young P., et al. Cardiac Masses Discovered by Echocardiogram; What to Do Next? Struct. Heart. 2023;7:100154. doi: 10.1016/j.shj.2022.100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alhuarrat M.A.D., Garg V., Borkowski P., Nazarenko N., Alhuarrat M.R., Abushairah A., Zyoud B.A., Bitsis E.-M., Barzallo D., Alemu R., et al. Epidemiologic and Clinical Characteristics of Marantic Endocarditis: A Systematic Review and Meta-Analysis of 416 Reports. Curr. Probl. Cardiol. 2023;49:102027. doi: 10.1016/j.cpcardiol.2023.102027. [DOI] [PubMed] [Google Scholar]
- 107.Mercadante S., Gebbia V., Marrazzo A., Filosto S. Anaemia in Cancer: Pathophysiology and Treatment. Cancer Treat. Rev. 2000;26:303–311. doi: 10.1053/ctrv.2000.0181. [DOI] [PubMed] [Google Scholar]
- 108.Pang W.W., Abdul-Rahman P.S., Wan-Ibrahim W.I., Hashim O.H. Can the Acute-Phase Reactant Proteins Be Used as Cancer Biomarkers? Int. J. Biol. Markers. 2010;25:1–11. doi: 10.1177/172460081002500101. [DOI] [PubMed] [Google Scholar]
- 109.Clarke A.E., Pooley N., Marjenberg Z., Langham J., Nicholson L., Langham S., Embleton N., Wang X., Desta B., Barut V., et al. Risk of Malignancy in Patients with Systemic Lupus Erythematosus: Systematic Review and Meta-Analysis. Semin. Arthritis Rheum. 2021;51:1230–1241. doi: 10.1016/j.semarthrit.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 110.Reinstein E., Shoenfeld Y. Antiphospholipid Syndrome and Cancer. Clin. Rev. Allergy Immunol. 2007;32:184–187. doi: 10.1007/s12016-007-0003-1. [DOI] [PubMed] [Google Scholar]
- 111.Islam M.A. Antiphospholipid Antibodies and Antiphospholipid Syndrome in Cancer: Uninvited Guests in Troubled Times. Semin. Cancer Biol. 2020;64:108–113. doi: 10.1016/j.semcancer.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 112.Davierwala P.M., Marin-Cuartas M., Misfeld M., Borger M.A. The Value of an “Endocarditis Team”. Ann. Cardiothorac. Surg. 2019;8:621–629. doi: 10.21037/acs.2019.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Itzhaki Ben Zadok O., Spectre G., Leader A. Cancer-Associated Non-Bacterial Thrombotic Endocarditis. Thromb. Res. 2022;213((Suppl. S1)):S127–S132. doi: 10.1016/j.thromres.2021.11.024. [DOI] [PubMed] [Google Scholar]
- 114.Smythe M.A., Parker D., Garwood C.L., Cuker A., Messé S.R. Timing of Initiation of Oral Anticoagulation after Acute Ischemic Stroke in Patients with Atrial Fibrillation. Pharmacotherapy. 2020;40:55–71. doi: 10.1002/phar.2345. [DOI] [PubMed] [Google Scholar]
- 115.Levi M., Ten Cate H. Disseminated Intravascular Coagulation. N. Engl. J. Med. 1999;341:586–592. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- 116.Lee A.Y.Y., Levine M.N., Baker R.I., Bowden C., Kakkar A.K., Prins M., Rickles F.R., Julian J.A., Haley S., Kovacs M.J., et al. Low-Molecular-Weight Heparin versus a Coumarin for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 117.Lyman G.H., Carrier M., Ay C., Di Nisio M., Hicks L.K., Khorana A.A., Leavitt A.D., Lee A.Y.Y., Macbeth F., Morgan R.L., et al. American Society of Hematology 2021 Guidelines for Management of Venous Thromboembolism: Prevention and Treatment in Patients with Cancer. Blood Adv. 2021;5:927–974. doi: 10.1182/bloodadvances.2020003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schrag D., Uno H., Rosovsky R., Rutherford C., Sanfilippo K., Villano J.L., Drescher M., Jayaram N., Holmes C., Feldman L., et al. Direct Oral Anticoagulants vs Low-Molecular-Weight Heparin and Recurrent VTE in Patients with Cancer: A Randomized Clinical Trial. JAMA. 2023;329:1924–1933. doi: 10.1001/jama.2023.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pastori D., Menichelli D., Cammisotto V., Pignatelli P. Use of Direct Oral Anticoagulants in Patients with Antiphospholipid Syndrome: A Systematic Review and Comparison of the International Guidelines. Front. Cardiovasc. Med. 2021;8:715878. doi: 10.3389/fcvm.2021.715878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Van der Hulle T., den Exter P.L., van den Hoven P., van der Hoeven J.J., van der Meer F.J.M., Eikenboom J., Huisman M.V., Klok F.A. Cohort Study on the Management of Cancer-Associated Venous Thromboembolism Aimed at the Safety of Stopping Anticoagulant Therapy in Patients Cured of Cancer. Chest. 2016;149:1245–1251. doi: 10.1016/j.chest.2015.10.069. [DOI] [PubMed] [Google Scholar]
- 121.Steffel J., Collins R., Antz M., Cornu P., Desteghe L., Haeusler K.G., Oldgren J., Reinecke H., Roldan-Schilling V., Rowell N., et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. EP Eur. 2021;23:1612–1676. doi: 10.1093/europace/euab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Larsen T.-L., Garresori H., Brekke J., Enden T., Frøen H., Jacobsen E.M., Quist-Paulsen P., Porojnicu A.C., Ree A.H., Torfoss D., et al. Low Dose Apixaban as Secondary Prophylaxis of Venous Thromboembolism in Cancer Patients—30 Months Follow-Up. J. Thromb. Haemost. 2022;20:1166–1181. doi: 10.1111/jth.15666. [DOI] [PubMed] [Google Scholar]
- 123.Roldan C.A., Sibbitt W.L., Greene E.R., Qualls C.R., Jung R.E. Libman-Sacks Endocarditis and Associated Cerebrovascular Disease: The Role of Medical Therapy. PLoS ONE. 2021;16:e0247052. doi: 10.1371/journal.pone.0247052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.