Abstract
Aims
Recurrence of arrhythmia after catheter ablation of atrial fibrillation (AF) in the form of atypical atrial flutter (AFL) is common among a significant number of patients and often requires redo ablation with limited success rates. Identifying patients at high risk of AFL after AF ablation could aid in patient selection and personalized ablation approach. The study aims to assess the relationship between pre-existing atrial cardiomyopathy and the occurrence of AFL following AF ablation.
Methods and results
We analysed a cohort of 1007 consecutive AF patients who underwent catheter ablation and were included in a prospective registry. Patients who did not have baseline cardiac magnetic resonance imaging and late gadolinium enhancement (LGE-CMR) or did not experience any recurrences were excluded. A total of 166 patients were included gathering 56 patients who underwent re-ablation due to AFL recurrences and 110 patients who underwent re-ablation due to AF recurrences (P = 0.11). A multiparametric assessment of atrial cardiomyopathy was based on basal LGE-CMR, including left atrial (LA) volume, LA sphericity, and global and segmental LA fibrosis using semiautomated post-processing software. Out of the initial cohort of 1007 patients, AFL and AF occurred in 56 and 110 patients, respectively. An age higher than 65 [odds ratio (OR) = 5.6, 95% confidence interval (CI): 2.2–14.4], the number of previous ablations (OR = 3.0, 95% CI: 1.2–7.8), and the management of ablation lines in the index procedure (OR = 2.5, 95% CI: 1.0–6.3) were independently associated with AFL occurrence. Furthermore, several characteristics assessed by LGE-CMR were identified as independent predictors of AFL recurrence after the index ablation for AF, such as enhanced LA sphericity (OR = 1.3, 95% CI: 1.1–1.6), LA global fibrosis (OR = 1.03, 95% CI: 1.01–1.07), and increased fibrosis in the lateral wall (OR = 1.03, 95% CI: 1.01–1.04).
Conclusion
Advanced atrial cardiomyopathy assessed by LGE-CMR, such as increased LA sphericity, global LA fibrosis, and fibrosis in the lateral wall, is independently associated with arrhythmia recurrence in the form of AFL following AF ablation.
Keywords: Atrial fibrillation, Atrial cardiomyopathy, Atypical atrial flutter, Fibrosis, Atrial remodelling
Graphical Abstract
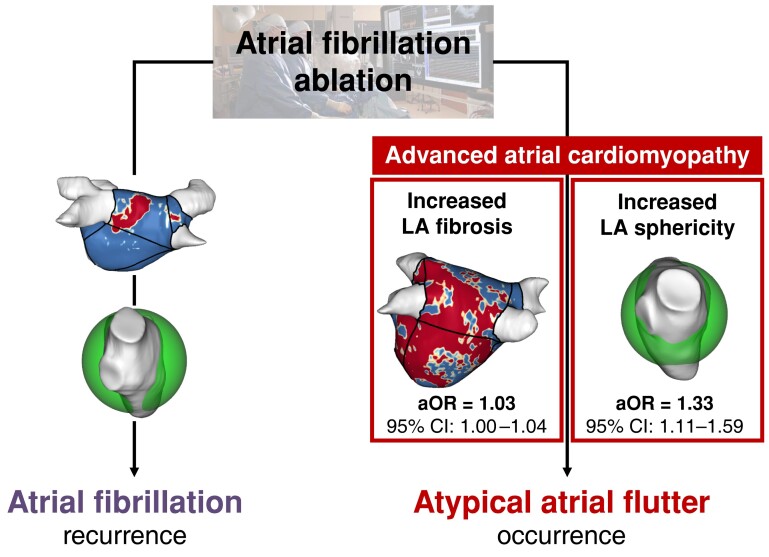
Graphical Abstract.
Advanced atrial cardiomyopathy assessed by LGE-CMR as a predictor of atypical atrial flutter occurrence following atrial fibrillation ablation. Two aspects of atrial cardiomyopathy, specifically increased LA sphericity and LA global fibrosis, are independent predictors of atypical atrial flutter onset compared to atrial fibrillation recurrence following catheter ablation. aOR, adjusted odds ratio; CI, confidence interval; LA, left atrium; LGE-CMR, late gadolinium enhancement cardiac magnetic resonance.
What’s new?
The study highlights the impact of pre-existing structural atrial remodelling on the occurrence of atypical atrial flutter after ablation for atrial fibrillation.
Late gadolinium enhancement cardiac magnetic resonance provides relevant assessment of structural remodelling in these patients, with left atrial sphericity, global fibrosis, and fibrosis in the lateral part of the left atrium being independent predictors of the onset of atypical atrial flutter.
The study emphasizes the crucial role of advanced atrial cardiomyopathy in the occurrence of atypical atrial flutter following ablation for atrial fibrillation.
Introduction
Catheter ablation (CA) of atrial fibrillation (AF) is an essential procedure to maintain sinus rhythm in patients facing paroxysmal and persistent AF.1 Despite technological improvements, arrhythmia recurrence still occurs in several patients, especially in persistent AF forms.2 Atypical atrial flutter (AFL) is a common type of arrhythmia recurrence that is seen in about 10–20% of cases.3 Atypical atrial flutter recurrence is usually more symptomatic than AF and is often associated with tachycardiomyopathy and heart failure. Managing CA for AFL is usually difficult and typically requires re-ablation.4 Identifying patients at risk of AFL post-AF ablation may help in patient selection, ablation design, and specific follow-up. Procedure features of the index CA have been associated with AFL recurrences, such as longer ablation time, additional linear lesions associated with the absence of bidirectional line of block at the end of the procedure, and ablation of complex fractionated atrial electrograms.5,6 However, these factors may simply indicate a more extensive underlying atrial cardiomyopathy (ACM). The role of late gadolinium enhancement cardiac magnetic resonance (LGE-CMR) in exploring the underlying ACM and analysing the risk for AFL has been less explored.7,8 The aim of this study is to assess the relationship between LGE-CMR parameters of ACM and the occurrence AFL following AF ablation.
Methods
Study population
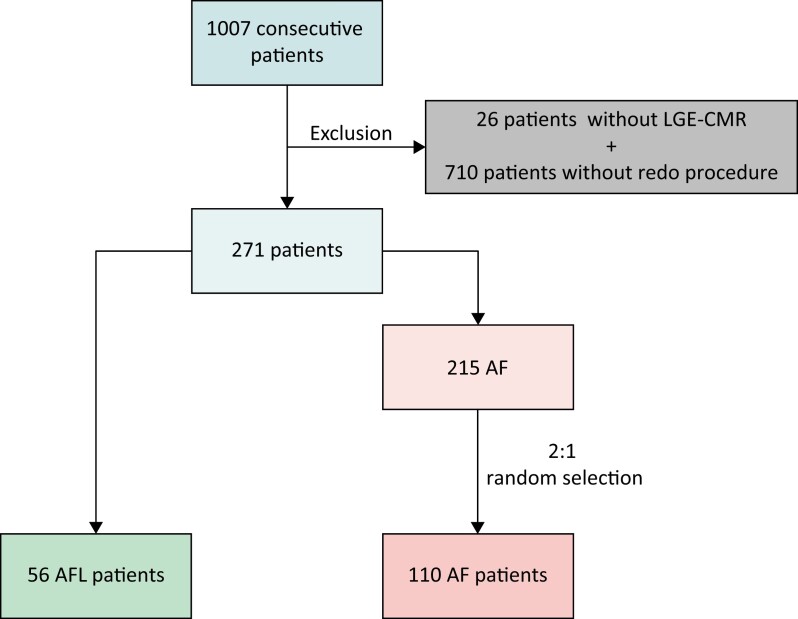
A cohort of 1007 consecutive AF patients included in the AF ablation registry at the Hospital Clínic de Barcelona, University of Barcelona (Spain), between November 2011 and October 2021 were retrospectively screened. Patients without redo procedures (710), as well as those without basal LGE-CMR scans (26), were excluded (Figure 1). A total of 56 patients with post-procedure AFL were identified and compared to a randomly selected group of 110 patients with AF recurrence in a 2:1 ratio. Clinical, procedural, and LGE-CMR data were systematically extracted using electronic medical records. The research protocol was approved by the ethics committee of the Hospital Clínic de Barcelona (HCB/2022/0123).
Figure 1.
Flow chart of the observational retrospective cohort study. Consecutive patients from 2011 to 2021 were screened and pre-selected based on the presence of baseline LGE-CMR and a redo procedure. Fifty-six patients who developed AFL following AF ablation were included in the study. After a 2:1 randomization, 110 patients were included from the pre-selection list of 215 patients with AF recurrence. AF, atrial fibrillation; AFL, atypical atrial flutter; LGE-CMR, late gadolinium enhancement cardiac magnetic resonance.
Late gadolinium enhancement cardiac magnetic resonance acquisition protocol
Late gadolinium enhancement cardiac magnetic resonance was performed according to previously described methods.9 Briefly, the studies were conducted in sinus rhythm and after external electrical cardioversion using two different 3 T scanners: Magnetom Prisma (Siemens Healthineers, Erlangen, Germany) and Signa Architect (General Electric, Chicago, IL), both equipped with a 32-channel phased array cardiovascular coil. Inversion recovery–prepared T1-weighted gradient-echo sequences were acquired in axial orientation using electrocardiogram (ECG) gating and a free-breathing 3D navigator, 20 min after administering an intravenous bolus of 0.2 mmol/kg of gadobutrol (Bayer, Leverkusen, Germany). A free-breathing 3D navigator and ECG-gated inversion recovery gradient-echo sequence were applied in axial projection. The sequence parameters for the Magnetom Prisma scanner were as follows: repetition time of 2.3 ms, echo time of 1.4 ms, flip angle of 11°, bandwidth of 460 Hz/pixel, inversion time of 280–380 ms, and acquired voxel size of 1.25 × 1.25 × 2.5 mm. The sequence parameters for the Signa Architect scanner were as follows: repetition time of 6.4 ms, echo time of 2.2 ms, flip angle of 20°, bandwidth of 244 Hz/pixel, inversion time of 280–380 ms, and acquired voxel size of 1.25 × 1.25 × 2.4 mm. A complete left atrial (LA) coverage was typically obtained with 60 slices.
Late gadolinium enhancement cardiac magnetic resonance post-processing protocol
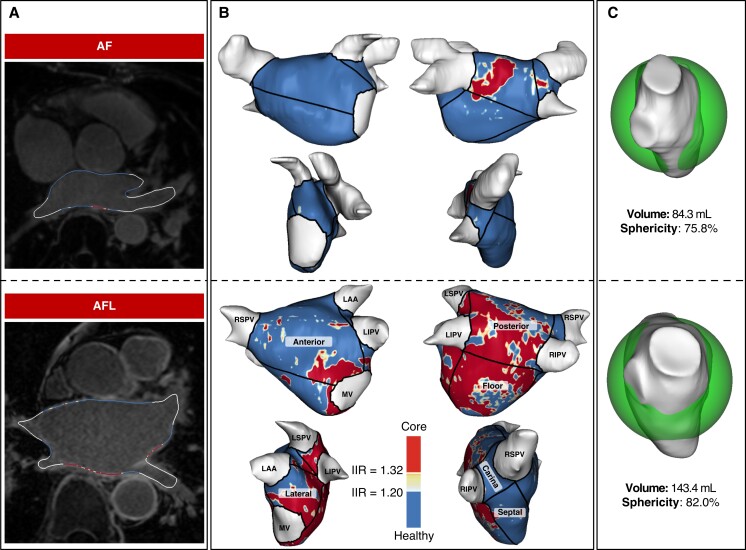
Late gadolinium enhancement cardiac magnetic resonance post-processing was carried out using Adas3D software (Galgo Medical SL, Barcelona, Spain). For the semiautomatic 3D reconstruction of LA and right atrial (RA), the atrial wall was manually traced on each axial plane slice and automatically adjusted to create a 3D shell. Late gadolinium enhancement was quantified based on voxel signal intensities relative to the mean blood pool signal intensity, using a previously validated signal intensity ratio threshold of ≥1.2 to define LGE indicative of fibrotic tissue9,10 (Figure 2). The 3D reconstructions were colour coded accordingly after the exclusion of extra-cardiac structures, such as pulmonary veins (PV), LA and RA appendages. The mitral valve leaflets were used as landmarks to separate LA from the left ventricle, and the tricuspid valve leaflets were used to separate RA from the right ventricle. The LA was automatically divided into seven standardized regions11: anterior, posterior, lateral, and septal wall, floor, and right and left carinas (Figure 2). The LA sphericity was assessed as previously described,8 which evaluates the variation between the LA and the sphere that best fits the LA shape. The radius of this sphere is calculated as the mean distance between all points of the LA wall and the centre of mass. Index LA and RA volumes were indexed by dividing the volumes by the estimated body surface area, using the Dubois and Dubois formula.
Figure 2.
Graphical examples of the differential level of atrial cardiomyopathy between patients with onset of atypical atrial flutter following ablation of atrial fibrillation and patients with AF recurrence. Based on post-processing LGE-CMR data, different parameters of morphological and structural left atrial remodelling predict the arrhythmia recurrence in the form of atypical atrial flutter compared with atrial fibrillation. (A) Overlay of the T1-weighted image with the LGE colour coding based on signal intensity ratios applying thresholds for fibrotic tissue (yellow ≥ 1.2; red > 1.32) using ADAS 3D software (Adas3D Medical, Barcelona, Spain). (B) 3D reconstruction of the left atrium with the automatized regionalization: anterior and posterior wall, floor, septum, lateral wall, and right and left carinas. (C) Assessment of LA volume and LA sphericity using ADAS 3D software (Adas3D Medical, Barcelona, Spain). AF, atrial fibrillation; AFL, atypical atrial flutter; LAA, left atrial appendage; LGE-CMR, late gadolinium enhancement cardiac magnetic resonance; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MV, mitral valve; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Catheter ablation
The cohort of patients in question underwent an index AF CA using either radiofrequency or cryoablation,12 with systematic pulmonary vein isolation (PVI) and confirmation of entrance and exit conduction block. For long-standing persistent AF patients, additional linear lesions were performed based on physician discretion. These included the LA roof, box of the posterior wall, and complex fractionated atrial electrograms, with bidirectional conduction block confirmation for each additional ablation line.
During a subsequent redo procedure, the presence of AFL was evaluated using activation and propagation mapping with electroanatomical mapping systems and multipolar mapping catheters, such as LassoNav and Pentaray using CARTO (Biosense Webster, Irvine, CA), IntellaMap Orion using RYHTMIA (Boston Scientific Inc., Marlborough, MA), and Advisor HD Grid using ENSITEX (Abbott, Chicago, IL). The presence of a macro-rentry during the redo procedure was mandatory to confirm the diagnosis of AFL onset during the follow-up.
Follow-up
After the initial AF ablation procedure, antiarrhythmic medications were discontinued after the 3-month blanking period in the absence of AF recurrence. A systematic clinical follow-up including a 12-lead ECG and a 24-h Holter ECG was performed at 3, 6, and 12 months after ablation, then annually. Atrial fibrillation recurrence and AFL occurrence during the long-term follow-up were defined using 12-lead ECG and 24-h Holter ECG leading to indication for a redo procedure.
Statistical analysis
Continuous variables were presented as mean ± standard deviation, while median–interquartile range was used as appropriate. Categorical variables were expressed as total numbers and percentages. Logistic regression analysis was used to investigate the impact of baseline characteristics on AFL occurrence, with a significance level of P < 0.05. Forward stepwise selection algorithms were employed for constructing the multivariate logistic regression model, where covariates with a P < 0.10 were retained in the final model. The odds ratio (OR) and 95% confidence interval (CI) were also calculated. Furthermore, receiver operating characteristic (ROC) methodology was used to evaluate the predictive capacity of various variables for arrhythmia recurrence in the form of AFL.
All tests used a two-sided type I error of 5%. R software for Windows version 4.2.1 (R Project for Statistical Computing, Vienna, Austria) was used for statistical analysis.
Results
Baseline characteristics
The final cohort included 166 patients, comprising 56 patients with AFL occurrence following AF ablation and 110 patients with AF recurrence. The median duration between the index ablation and the redo procedure due to arrhythmia recurrence was 20 months (interquartile range: 11–37). Table 1 provides a summary of the patients’ baseline characteristics. The mean age of the patients was 60 years, 72.0% of whom were men, and 65.5% had paroxysmal AF at index ablation. The mean left ventricular ejection fraction was 54.9%, while the mean LA and RA indexed volumes were 53.5 and 59.6 mL/m2, respectively, indicating biatrial dilation. The mean amount of LA fibrosis was 16.1%, corresponding to Stage 2 of the Utah classification.13 Additional LA lines were reported in 30.7% of the patients (n = 51), with the roof line being the most frequent (21.7%, n = 36), followed by the posterior box (12.0%, n = 20), mitral line (15.0%, n = 9), and complex fractionated atrial electrogram ablation (11.4%, n = 19).
Table 1.
Baseline characteristics of patients with arrhythmia recurrence following AF ablation
| All (n = 166) | |
|---|---|
| Clinical features | |
| Age (years) | 56.97 ± 10.45 |
| Age > 65 | 44 (26.5%) |
| Male gender | 120 (72.0%) |
| Body mass index (kg/m2) | 27.5 (25.0–30.8) |
| Hypertension | 87 (52.4%) |
| Diabetes mellitus | 19 (11.4%) |
| Obstructive sleep apnoea | 24 (14.5%) |
| Initial AF subtype | |
| Paroxysmal | 107 (65.5%) |
| Persistent | 58 (34.9%) |
| CHA2DS2 VASc score | 1 (0–2) |
| EHRA score | |
| I | 8 (4.8%) |
| II | 131 (78.9%) |
| III | 24 (14.5%) |
| IV | 2 (1.2%) |
| Previous ablation features | |
| Number of previous catheter ablation | 2 (2–2) |
| Delay from previous catheter ablation (years) | 1.66 (0.94–3.07) |
| History of additional lines | 51 (30.7%) |
| History of CFAE | 19 (11.4%) |
| CMR features | |
| Left ventricular ejection fraction (%) | 54.92 ± 7.97 |
| LA volume index (mL/m2) | 53.50 ± 14.98 |
| LA fibrosis (%) | 16.10 ± 14.94 |
| RA volume index (mL/m2) | 59.64 ± 14.46 |
| RA fibrosis (%) | 20.07 ± 12.51 |
AF, atrial fibrillation; CFAE, complex fractionated atrial electrogram; CMR, cardiac magnetic resonance; EHRA, European Heart Rhythm Association; LA, left atrium; RA, right atrium.
Predictors of post-atrial fibrillation ablation atypical atrial flutter occurrence
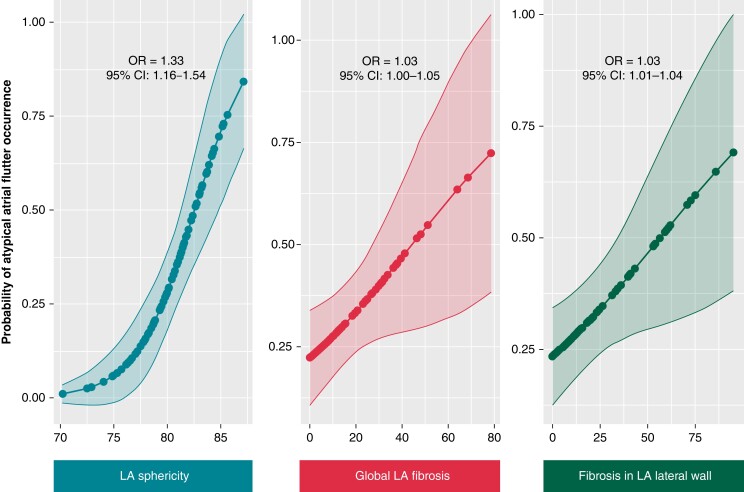
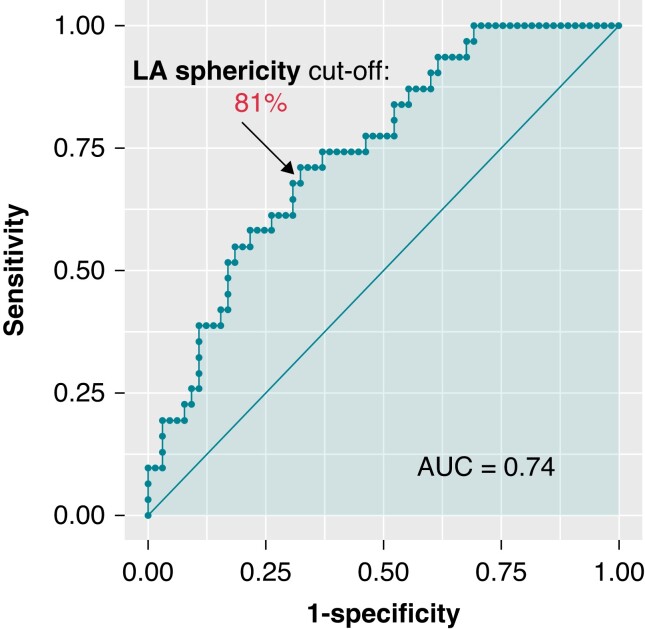
In this study, several factors were found to be associated with AFL occurrence after AF ablation. Univariate analysis showed that age over 65, diabetes mellitus, obstructive sleep apnoea, persistent AF, higher CHA2DS2 VASc score, and a higher number of previous AF ablation procedures were linked to AFL recurrence. Additionally, the presence of additional lines and complex fractionated atrial electrogram ablation, higher LA indexed volume, sphericity, and overall fibrosis were also associated with AFL occurrence. The energy used during the indexed ablation procedure (weather cryotherapy, radiofrequency, or laser) was not associated with AFL onset compared with AF recurrence. Multivariate analyses identified several independent predictors of AFL occurrence (Table 2), including age over 65 (OR = 5.61, 95% CI: 2.18–14.41), a high number of previous CA procedures (OR = 3.03, 95% CI: 1.17–7.81), a history of additional lines (2.48, 95% CI: 1.01–6.27), higher LA sphericity (OR = 1.33, 95% CI: 1.11–1.59), and LA overall fibrosis (OR = 1.03, 95% CI: 1.00–1.07). A graphical representation of the association between two ACM features, namely LA sphericity and LA overall fibrosis, and AFL occurrence after AF ablation is shown in Figure 3. The study found that a baseline LA sphericity higher than 80.7% was associated with AFL recurrence with 71% specificity and 68% specificity (c-statistic 0.74, Figure 4). The initial subtype of AF was not found to have an independent association with AFL occurrence.
Table 2.
Univariate and multivariate logistic regression models to predict arrhythmia recurrence in the form of atypical atrial flutter following ablation of atrial fibrillation
| AF recurrence (n = 110) | AFL occurrence (n = 56) | Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |||
| Clinical features | ||||||||
| Age > 65 | 18 (16.4%) | 26 (46.4%) | 4.43 | (2.14–9.18) | <0.01 | 5.61 | (2.18–14.41) | <0.01 |
| Male gender | 82 (74.5%) | 37 (66.1%) | 1.50 | (0.75–3.03) | 0.67 | |||
| BMI | 27.2 (24.8–29.7) | 29.1 (25.7–32.8) | 1.00 | (0.98–1.01) | 0.82 | |||
| Hypertension | 52 (47.3%) | 34 (60.7%) | 1.72 | (0.90–3.32) | 0.10 | |||
| Diabetes mellitus | 8 (7.3%) | 10 (17.9%) | 2.77 | (1.03–7.48) | 0.04 | 3.02 | (0.84–10.81) | 0.09 |
| Obstructive sleep apnoea | 10 (9.1%) | 13 (23.2%) | 3.02 | (1.23–7.43) | 0.02 | – | – | – |
| AF features | ||||||||
| Persistent AF | 33 (30%) | 23 (41.8%) | 2.07 | (1.14–3.76) | 0.02 | – | – | – |
| CHADS VASc score | 1 (0–2) | 2 (2–3) | 1.54 | (1.18–2.03) | <0.01 | – | – | – |
| Previous catheter ablation features | ||||||||
| Number of previous catheter ablation procedures | 2 (2–2) | 2 (2–3) | 3.26 | (1.56–6.78) | <0.01 | 3.03 | (1.17–7.81) | 0.02 |
| Delay from previous catheter ablation | 1.7 (1.0–2.9) | 1.6 (0.6–4.1) | 1.08 | (0.94–1.24) | 0.31 | |||
| History of additional lines | 23 (20.9%) | 28 (50.0%) | 3.78 | (1.88–7.59) | <0.01 | 2.48 | (1.01–6.27) | 0.05 |
| History of CFAE | 8 (7.3%) | 11 (19.6%) | 3.12 | (1.17–8.27) | 0.02 | – | – | – |
| CMR features | ||||||||
| Left ventricular ejection fraction | 55 ± 8 | 54 ± 8 | 0.98 | (0.94–1.03) | 0.47 | |||
| LA indexed volume | 50.9 ± 14.5 | 58.9 ± 15.7 | 1.04 | (1.01–1.06) | <0.01 | – | – | – |
| LA sphericity | 79.5 ± 3.1 | 81.7 ± 2.6 | 1.33 | (1.16–1.54) | <0.01 | 1.33 | (1.11–1.59) | <0.01 |
| LA fibrosis | 14.1 ± 12.9 | 20.1 ± 17.9 | 1.03 | (1.00–1.05) | 0.03 | 1.03 | (1.00–1.07) | 0.04 |
| RA indexed volume | 59.6 ± 13.9 | 59.0 ± 15.7 | 1.00 | (0.98–1.03) | 0.92 | |||
| RA sphericity | 79.8 ± 2.4 | 79.6 ± 2.2 | 0.97 | (0.84–1.12) | 0.65 | |||
| RA fibrosis | 19.5 ± 11.6 | 21.3 ± 14.3 | 1.01 | (0.99–1.04) | 0.40 | |||
Forward stepwise selection algorithms were used for building up the multivariate logistic regression model. In bold: statistacally significant results, defined as P < 0.05.
AF, atrial fibrillation; AFL, atypical atrial flutter; BMI, body mass index; CFAE, complex fractionated atrial electrogram; CI, confidence interval; CMR, cardiac magnetic resonance; EHRA, European Heart Rhythm Association; LA, left atrium; OR, odds ratio; RA, right atrium.
Figure 3.
Graphical representation of the main predictors of atypical atrial flutter occurrence using a univariate logistic regression model. These graphs represent the probability of atrial flutter onset compared with the recurrence of atrial flutter following catheter ablation for atrial fibrillation concerning three main components of atrial cardiomyopathy assessed by LGE-CMR: left atrial sphericity, global fibrosis, and fibrosis among the lateral wall. CI, confidence interval; LA, left atrium; LGE-CMR, late gadolinium enhancement cardiac magnetic resonance; OR, odds ratio.
Figure 4.
Receiver operating characteristic curve of left atrial sphericity for prediction of post-atrial fibrillation ablation atypical atrial flutter. AUC, area under the curve; LA, left atrium.
Left atrial fibrosis as a predictor of atypical atrial flutter occurrence following atrial fibrillation ablation
As LA global fibrosis was found to be an independent predictor of AFL occurrence after AF CA procedure, a regional analysis of fibrosis distribution was conducted and presented in Table 3. The level of fibrosis in the lateral aspect, below the LA appendage, was the only independent predictor of AFL occurrence after AF ablation compared with AF recurrence (OR = 1.03, 95% CI: 1.01–1.04), as shown in Figure 3. Although not statistically significant, the distribution heterogeneity of LA fibrosis was slightly higher in patients with AFL occurrence after AF CA.
Table 3.
Univariate and multivariate logistic regression models to predict arrhythmia recurrence in the form of atypical atrial flutter following ablation of atrial fibrillation regarding regional distribution of LA fibrosis
| AF recurrence (n = 110) | AFL occurrence (n = 56) | Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |||
| Regional distribution | ||||||||
| LA floor fibrosis | 16.8 ± 26.1 | 26.1 ± 15.4 | 1.02 | (1.00–1.03) | 0.02 | – | – | – |
| LA anterior fibrosis | 6.8 ± 10.5 | 13.8 ± 18.7 | 1.04 | (1.01–1.07) | 0.01 | – | – | – |
| LA lateral fibrosis | 16.2 ± 19.2 | 29.2 ± 27.2 | 1.03 | (1.01–1.04) | <0.01 | 1.03 | (1.01–1.04) | <0.01 |
| Posterior LA fibrosis | 26.4 ± 22.4 | 32.2 ± 24.1 | 1.01 | (0.99–1.03) | 0.16 | |||
| LA carina fibrosis | 17.3 ± 20.3 | 17.1 ± 19.6 | 0.99 | (0.98–1.02) | 0.94 | |||
| LA septal fibrosis | 7.7 ± 11.0 | 10.1 ± 15.4 | 1.02 | (0.99–1.04) | 0.28 | |||
| Heterogeneity of distribution | ||||||||
| SD regions | 11.7 ± 7.9 | 14.6 ± 8.9 | 1.04 | (0.99–1.08) | 0.05 | – | – | – |
Forward stepwise selection algorithms were used for building up the multivariate logistic regression model. In bold: statistacally significant results, defined as P < 0.05.
AF, atrial fibrillation; AFL, atypical atrial flutter; CI, confidence interval; LA, left atrium; OR, odds ratio; SD, standard deviation.
Discussion
Main findings
This study is the first to investigate the impact of pre-existing structural atrial remodelling on AFL occurrence after AF ablation. The study found that various aspects of ACM evaluated by LGE-CMR were independent predictors of AFL occurrence, including increased LA sphericity, LA global fibrosis, and elevated fibrosis in the lateral wall. The results underscore the crucial role of advanced ACM in AFL occurrence following AF ablation.
Atrial cardiomyopathy as a key element for atypical atrial flutter occurrence following atrial fibrillation ablation
Atypical atrial flutter is a frequent and challenging complication following AF ablation due to its persistent and symptomatic presentation, along with diagnostic and therapeutic management.14 Therefore, defining the risk factors of AFL occurrence after AF ablation may aid in patient selection and procedure design to improve results. Previous extensive LA ablation, which includes iterative CA procedures, longer ablation time, and the management of additional LA lines,15 is an established factor for post-AF ablation AFL occurrence.5,6 Our study confirms the role of extensive LA ablation in AFL occurrence. However, the need for extensive ablation is likely just a marker of more extensive underlying ACM. The involvement of pre-existing atrial remodelling in the physiology of post-CA AFL is less known.16 Morphological LA abnormalities, such as dilated LA, have been found to independently predict AFL onset after CA for AF.3 But other features of the extension of ACM assessed by LGE-CMR may be more efficient in predicting AFL occurrence. Atrial fibrosis likely creates slow conduction and structural barriers that enable the development of critical isthmus and AFL onset.17 Our study itemizes, for the first time, the central role of LA fibrosis in AFL onset after CA for AF. An increased LA sphericity was also strongly and independently associated with AFL onset. Left atrial sphericity is a parameter that assesses LA morphological abnormality and has two major significant strengths: this ACM marker, although characterizing a morphological atrial abnormality, is strongly correlated with fibrosis burden.8 Furthermore, this marker is assessable in computerized tomography scans and CMR, without injection of contrast or dedicated acquisition protocol. Of note, LA sphericity is an independent predictor of AF recurrence after CA.18 The present study confirms the crucial role of ACM in long-term complications following CA for AF.
The central role of late gadolinium enhancement cardiac magnetic resonance in the characterization of atrial cardiomyopathy and atrial fibrillation management
Atrial cardiomyopathy is an emerging concept aimed at improving the management of atrial disease, including arrhythmia or cardioembolic stroke.19 The ongoing challenge is to efficiently and non-invasively assess the different components of ACM, namely morphological, functional, electrical, and structural LA remodelling, to improve patient management.20 Late gadolinium enhancement cardiac magnetic resonance is a central diagnostic tool for evaluating ACM. This non-invasive imaging tool enables the itemization of LA morphological abnormalities (such as size3 and sphericity8), functional abnormalities (global strain21,22), and structural abnormalities via the assessment of fibrosis using LGE.23 Regarding the assessment of structural remodelling, the novel automated tool to regionalize the LA11 and assess quantitatively the amount of fibrosis reduces inter- and intraobserver variability, making this tool more reproducible. The non-invasive assessment of global and regional fibrosis using LGE-CMR has already been validated in comparison with the invasive assessment of low-voltage areas using electroanatomical mapping24 and evaluates the crucial impact of fibrosis in arrhythmia recurrence following AF ablation.25
The presence of advanced ACM is a validated predictor of AF recurrence after CA ablation. Both dilated LA,26 LA functional impairment,27 and LA global fibrosis7 are predictors assessed by CMR of AF recurrence. This is consistent with our results. In conclusion, advanced ACM is a relevant predictor of worse outcomes after CA, both in terms of AF recurrence and AFL occurrence. Pre-procedural assessment of ACM based on LGE-CMR seems to be useful in selecting patients who would benefit the most from CA for AF.28 Late gadolinium enhancement cardiac magnetic resonance could also be helpful to tailor the ablation procedure for arrhythmia recurrence.9,29
Limitations
The observational and retrospective design of the study has significant limitations. The selection criteria used to set up the cohort may have introduced a selection bias, as only patients with an indication for a redo procedure were included for the assessment of AFL occurrence and AF recurrence. Therefore, patients who experienced arrhythmia recurrence after CA for AF without undergoing a redo procedure were not included in the cohort. Consequently, we cannot evaluate the incidence of AF and AFL onset during the follow-up among the whole cohort. Cofounders cannot be excluded, as the study aims to identify predictors of AFL occurrence compared with AF recurrence, but the causative role of the studied parameters cannot be established despite multivariate analyses.
Clinical perspectives
This study highlights that advanced ACM assessed by LGE-CMR is predictive of AFL occurrence after AF ablation. Two perspectives can be drawn: on the one hand, early management of AF patients, to avoid advanced ACM, seems to be crucial in improving clinical outcomes.30 On the other hand, selecting patients with the best clinical net benefit regarding endocavity ablation for AF is a key point in avoiding post-procedural complications. It is important to note that the assessment of ACM by LGE-CMR outperformed classical predictors of arrhythmia recurrence after CA for AF, such as persistent AF subtype and LA dilation. Selecting AF patient before CA is crucial in improving post-procedural outcomes and reducing arrhythmia recurrence. A randomized clinical trial is needed to assess the benefit of tailoring the lesion set based on the evaluation of the advanced ACM during CA for AF.
Conclusions
Advanced ACM, assessed by LGE-CMR, such as increased LA sphericity, global LA fibrosis, and fibrosis into the lateral wall, is independently associated with arrhythmia recurrence in the form of AFL following AF ablation. These results suggest the usefulness of pre-procedural ACM assessment in selecting AF patients with an optimal benefit–risk balance.
Acknowledgements
The authors would like to thank Neus Portella and Carolina Sanroman for their secretarial assistance with the manuscript, as well as the nurse team of the arrhythmia unit of Hospital Clínic de Barcelona for their invaluable clinical support. J.B.G. thanks the French Federation of Cardiology (FFC) for their institutional grant support.
Contributor Information
Elisenda Ferró, Institut Clínic Cardiovascular, Hospital Clínic, Universitat de Barcelona, Carrer Villaroel, 170, 08036 Barcelona, Catalonia, Spain; Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Carrer del Rosselló, 149, 08036 Barcelona, Catalonia, Spain.
Núria Pérez, Institut Clínic Cardiovascular, Hospital Clínic, Universitat de Barcelona, Carrer Villaroel, 170, 08036 Barcelona, Catalonia, Spain.
Till Althoff, Institut Clínic Cardiovascular, Hospital Clínic, Universitat de Barcelona, Carrer Villaroel, 170, 08036 Barcelona, Catalonia, Spain; Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Carrer del Rosselló, 149, 08036 Barcelona, Catalonia, Spain; Department of Cardiology and Angiology, Charité-University Medicine, Berlin, Germany.
Eduard Guasch, Institut Clínic Cardiovascular, Hospital Clínic, Universitat de Barcelona, Carrer Villaroel, 170, 08036 Barcelona, Catalonia, Spain; Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Carrer del Rosselló, 149, 08036 Barcelona, Catalonia, Spain.
Susana Prat, Institut Clínic Cardiovascular, Hospital Clínic, Universitat de Barcelona, Carrer Villaroel, 170, 08036 Barcelona, Catalonia, Spain; Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Carrer del Rosselló, 149, 08036 Barcelona, Catalonia, Spain.
Adelina Doltra, Institut Clínic Cardiovascular, Hospital Clínic, Universitat de Barcelona, Carrer Villaroel, 170, 08036 Barcelona, Catalonia, Spain; Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Carrer del Rosselló, 149, 08036 Barcelona, Catalonia, Spain.
Roger Borrás, Institut Clínic Cardiovascular, Hospital Clínic, Universitat de Barcelona, Carrer Villaroel, 170, 08036 Barcelona, Catalonia, Spain; Centro de Investigación Biomédica en Red e Salud Mental (CIBERSAM), Madrid, Spain.
José María Tolosana, Institut Clínic Cardiovascular, Hospital Clínic, Universitat de Barcelona, Carrer Villaroel, 170, 08036 Barcelona, Catalonia, Spain; Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Carrer del Rosselló, 149, 08036 Barcelona, Catalonia, Spain.
Elena Arbelo, Institut Clínic Cardiovascular, Hospital Clínic, Universitat de Barcelona, Carrer Villaroel, 170, 08036 Barcelona, Catalonia, Spain; Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Carrer del Rosselló, 149, 08036 Barcelona, Catalonia, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Avinguda Monforte de Lemos, 3-5, 28029 Madrid, Spain.
Marta Sitges, Institut Clínic Cardiovascular, Hospital Clínic, Universitat de Barcelona, Carrer Villaroel, 170, 08036 Barcelona, Catalonia, Spain; Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Carrer del Rosselló, 149, 08036 Barcelona, Catalonia, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Avinguda Monforte de Lemos, 3-5, 28029 Madrid, Spain.
Andreu Porta-Sánchez, Institut Clínic Cardiovascular, Hospital Clínic, Universitat de Barcelona, Carrer Villaroel, 170, 08036 Barcelona, Catalonia, Spain.
Ivo Roca-Luque, Institut Clínic Cardiovascular, Hospital Clínic, Universitat de Barcelona, Carrer Villaroel, 170, 08036 Barcelona, Catalonia, Spain; Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Carrer del Rosselló, 149, 08036 Barcelona, Catalonia, Spain.
Lluís Mont, Institut Clínic Cardiovascular, Hospital Clínic, Universitat de Barcelona, Carrer Villaroel, 170, 08036 Barcelona, Catalonia, Spain; Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Carrer del Rosselló, 149, 08036 Barcelona, Catalonia, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Avinguda Monforte de Lemos, 3-5, 28029 Madrid, Spain.
Jean-Baptiste Guichard, Institut Clínic Cardiovascular, Hospital Clínic, Universitat de Barcelona, Carrer Villaroel, 170, 08036 Barcelona, Catalonia, Spain; Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Carrer del Rosselló, 149, 08036 Barcelona, Catalonia, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Avinguda Monforte de Lemos, 3-5, 28029 Madrid, Spain; INSERM, SAINBIOSE U1059, University Hospital of Saint-Étienne, 10 rue de la Marandinière, 42270 Saint-Priest-enJarez, France; Cardiology Department, University Hospital of Saint-Étienne, 42 Avenue Albert Raimond, 42270 Saint-Priest-en-Jarez, France.
Funding
This work is supported in part by grants from the Instituto de Salud Carlos III, Spanish Government, Madrid, Spain (FIS_PI16/00435 – FIS_CIBER16; to L.M.).
Conflict of interest: E.F. reports receiving full salary funding from Medtronic. T.F.A. has received research grants for investigator-initiated trials from Biosense Webster. A.P.-S. has received honoraria as a consultant and lecturer from Abbott, Biosense Webster, and Boston Scientific. E.A. reports honoraria as consultant and lecturer from Biosense Webster and Bayer. M.S. has received grants, consulting honoraria, and speakers’ fees from General Electric, Edwards Lifesciences, Abbott Medical, and Medtronic. L.M. reports honoraria as a consultant, lecturer, and advisory board member from Boston Scientific, Abbott Medical, Johnson & Johnson, and Medtronic. He is also a shareholder of Galgo Medical SL. J.B.G. reports honoraria as a consultant from Microport CRM, a lecturer from Microport CRM and Abbott, and unrestricted grant support for a fellowship from Abbott Labs. All remaining authors have declared no conflicts of interest.
Data availability
The authors agree to make data and materials supporting the results or analyses presented in their paper available upon reasonable request.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498.. [DOI] [PubMed] [Google Scholar]
- 2. Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HSet al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gucuk Ipek E, Marine J, Yang E, Habibi M, Chrispin J, Spragg Det al. Predictors and incidence of atrial flutter after catheter ablation of atrial fibrillation. Am J Cardiol 2019;124:1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga Let al. HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoyt H, Bhonsale A, Chilukuri K, Alhumaid F, Needleman M, Edwards Det al. Complications arising from catheter ablation of atrial fibrillation: temporal trends and predictors. Heart Rhythm 2011;8:1869–74. [DOI] [PubMed] [Google Scholar]
- 6. Chae S, Oral H, Good E, Dey S, Wimmer A, Crawford Tet al. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol 2007;50:1781–7. [DOI] [PubMed] [Google Scholar]
- 7. Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski Fet al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 8. Bisbal F, Guiu E, Calvo N, Marin D, Berruezo A, Arbelo Eet al. Left atrial sphericity: a new method to assess atrial remodeling. Impact on the outcome of atrial fibrillation ablation. J Cardiovasc Electrophysiol 2013;24:752–9. [DOI] [PubMed] [Google Scholar]
- 9. Padilla-Cueto D, Ferro E, Garre P, Prat S, Guichard JB, Perea RJet al. Non-invasive assessment of pulmonary vein isolation durability using late gadolinium enhancement magnetic resonance imaging. Europace 2023;25:360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gunturiz-Beltrán C, Borràs R, Alarcón F, Garre P, Figueras I Ventura RM, Benito EMet al. Quantification of right atrial fibrosis by cardiac magnetic resonance: verification of the method to standardize thresholds. Rev Esp Cardiol (Engl Ed) 2023;76:173–82. [DOI] [PubMed] [Google Scholar]
- 11. Benito EM, Cabanelas N, Nuñez-Garcia M, Alarcón F, Figueras I Ventura RM, Soto-Iglesias Det al. Preferential regional distribution of atrial fibrosis in posterior wall around left inferior pulmonary vein as identified by late gadolinium enhancement cardiac magnetic resonance in patients with atrial fibrillation. Europace 2018;20:1959–65. [DOI] [PubMed] [Google Scholar]
- 12. Kuck K-H, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KRet al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. [DOI] [PubMed] [Google Scholar]
- 13. Mahnkopf C, Badger TJ, Burgon NS, Daccarett M, Haslam TS, Badger CTet al. Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed-enhanced MRI: implications for disease progression and response to catheter ablation. Heart Rhythm 2010;7:1475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bun S-S, Latcu DG, Marchlinski F, Saoudi N. Atrial flutter: more than just one of a kind. Eur Heart J 2015;36:2356–63. [DOI] [PubMed] [Google Scholar]
- 15. Sawhney N, Anousheh R, Chen W, Feld GK. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:243–8. [DOI] [PubMed] [Google Scholar]
- 16. Hussein AA, Saliba WI, Martin DO, Bhargava M, Sherman M, Magnelli-Reyes Cet al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4:271–8. [DOI] [PubMed] [Google Scholar]
- 17. Cosío FG. Atrial flutter, typical and atypical: a review. Arrhythm Electrophysiol Rev 2017;6:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bisbal F, Guiu E, Cabanas P, Calvo N, Berruezo A, Tolosana JMet al. Reversal of spherical remodelling of the left atrium after pulmonary vein isolation: incidence and predictors. Europace 2014;16:840–7. [DOI] [PubMed] [Google Scholar]
- 19. Guichard J-B, Nattel S. Atrial cardiomyopathy: a useful notion in cardiac disease management or a passing fad? J Am Coll Cardiol 2017;70:756–65. [DOI] [PubMed] [Google Scholar]
- 20. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SAet al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Heart Rhythm 2017;14:e3–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gucuk Ipek E, Marine JE, Habibi M, Chrispin J, Lima J, Rickard Jet al. Association of left atrial function with incident atypical atrial flutter after atrial fibrillation ablation. Heart Rhythm 2016;13:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar Set al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 2010;3:231–9. [DOI] [PubMed] [Google Scholar]
- 23. Akoum N, Daccarett M, McGann C, Segerson N, Vergara G, Kuppahally Set al. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J Cardiovasc Electrophysiol 2011;22:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bisbal F, Andreu D, Berruezo A. Simplified mapping and ablation of a scar-related atrial tachycardia using magnetic resonance imaging tissue characterization. Europace 2015;17:186. [DOI] [PubMed] [Google Scholar]
- 25. Ravelli F, Masè M, Cristoforetti A, Avogaro L, D’Amato E, Tessarolo Fet al. Quantitative assessment of transmural fibrosis profile in the human atrium: evidence for a three-dimensional arrhythmic substrate by slice-to-slice histology. Europace 2023;25:739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montefusco A, Biasco L, Blandino A, Cristoforetti Y, Scaglione M, Caponi Det al. Left atrial volume at MRI is the main determinant of outcome after pulmonary vein isolation plus linear lesion ablation for paroxysmal-persistent atrial fibrillation. J Cardiovasc Med (Hagerstown) 2010;11:593–8. [DOI] [PubMed] [Google Scholar]
- 27. Hammerstingl C, Schwekendiek M, Momcilovic D, Schueler R, Sinning J-M, Schrickel JWet al. Left atrial deformation imaging with ultrasound based two-dimensional speckle-tracking predicts the rate of recurrence of paroxysmal and persistent atrial fibrillation after successful ablation procedures. J Cardiovasc Electrophysiol 2012;23:247–55. [DOI] [PubMed] [Google Scholar]
- 28. Bisbal F, Baranchuk A, Braunwald E, Bayés de Luna A, Bayés-Genís A. Atrial failure as a clinical entity: JACC review topic of the week. J Am Coll Cardiol 2020;75:222–32. [DOI] [PubMed] [Google Scholar]
- 29. Quinto L, Cozzari J, Benito E, Alarcón F, Bisbal F, Trotta Oet al. Magnetic resonance-guided re-ablation for atrial fibrillation is associated with a lower recurrence rate: a case-control study. Europace 2020;22:1805–11. [DOI] [PubMed] [Google Scholar]
- 30. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan Aet al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors agree to make data and materials supporting the results or analyses presented in their paper available upon reasonable request.