Abstract
Spinal cord injuries (SCIs) can lead to significant neurological deficits and lifelong disability, with far-reaching physical, psychological, and economic consequences for affected individuals and their families. Current treatments for SCIs are limited in their ability to restore function, and there is a pressing need for innovative therapeutic approaches. Stem cell therapy has emerged as a promising strategy to promote the regeneration and repair of damaged neural tissue following SCIs. This review article comprehensively discusses the potential of different stem cell types, such as embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), mesenchymal stem cells (MSCs), and neural stem/progenitor cells (NSPCs), in SCI treatment. We provide an in-depth analysis of the unique advantages and challenges associated with each stem cell type, as well as the latest advancements in the field. Furthermore, we address the critical challenges faced in stem cell therapy for SCIs, including safety concerns, ethical considerations, standardization of protocols, optimization of transplantation parameters, and the development of effective outcome measures. We also discuss the integration of novel technologies such as gene editing, biomaterials, and tissue engineering to enhance the therapeutic potential of stem cells. The article concludes by emphasizing the importance of collaborative efforts among various stakeholders in the scientific community, including researchers, clinicians, bioengineers, industry partners, and patients, to overcome these challenges and realize the full potential of stem cell therapy for SCI patients. By fostering such collaborations and advancing our understanding of stem cell biology and regenerative medicine, we can pave the way for the development of groundbreaking therapies that improve the lives of those affected by SCIs.
Keywords: spinal cord injury, stem cell therapy, neural tissue repair, embryonic stem cells, induced pluripotent stem cells, mesenchymal stem cells, regenerative medicine
1. Introduction
Spinal cord injuries (SCIs) are among the most debilitating and life-altering medical conditions, leading to a significant decline in the quality of life and imposing immense physical, psychological, and economic burdens on affected individuals and their families [1]. According to the most recent epidemiological data, the incidence and prevalence of SCIs vary significantly across different regions and demographics, emphasizing the widespread impact of this condition (Table 1). Conventional treatments for SCIs have been limited in their ability to promote meaningful functional recovery [2]. However, stem cell therapy has emerged as a promising avenue to address these limitations, with the potential to transform the landscape of SCI treatment. Recent studies have demonstrated the therapeutic potential of stem cells in treating SCIs while acknowledging the inherent challenges and obstacles that must be overcome [3,4]. In this review article, we explore the promise of stem cell therapy in the context of SCIs, the challenges that researchers face, and the importance of continued research to fully realize the potential of this groundbreaking approach.
Table 1.
Epidemiological data on SCIs: incidence, prevalence, and demographics.
| Data | Country/Region | Age Group Most Affected | Primary Causes of Injury | Notable Trends | Year | Reference | |
|---|---|---|---|---|---|---|---|
| Incidence Rates | 32–50 per million requiring hospital admission | United States | N/A | N/A | N/A | 2000 | [5] |
| 11.4 per million | Spain | 60–69 years | Tumors | N/A | 2012 | [6] | |
| 19.4 per million inhabitants | France | N/A | N/A | N/A | 2005 | [7] | |
| Stable incidence rate, changes in injury etiology | Stockholm, Sweden | N/A | Falls, Transport-related | N/A | 2017 | [8] | |
| 23 cases per 1,000,000 persons | Global | N/A | N/A | N/A | 2007 | [9] | |
| Prevalence | Estimate provided, further refinements needed | Victoria, Australia | N/A | N/A | N/A | 2012 | [10] |
| Demographic Trends | Increased incidence due to falls and motor vehicle accidents | Global | N/A | Falls, Motor Accidents | Increasing | 2010 | [11] |
| Increased incidence in the elderly | Global | Elderly | Falls, Non-traumatic | Increasing | 2010 | [12] | |
| Mean age at injury increased | Finland | N/A | N/A | Increasing | 2008 | [13] | |
| Specific epidemiological characteristics | Xi’an, China | N/A | N/A | N/A | 2020 | [14] | |
| Global Trends | Significant variation in incidence worldwide | Global | N/A | N/A | Varies | 2010 | [11] |
| Variation in etiology, male-to-female ratios, age distributions, and complications | Global | N/A | N/A | Varies | 2004 | [15] |
Note: Some fields are marked as N/A (Not Available) due to the absence of specific information in the cited studies.
Stem cell therapy offers hope for patients suffering from SCI by harnessing the unique regenerative capabilities of stem cells [16]. These cells have the potential to differentiate into various cell types [17], thereby replacing lost neurons, promoting axonal growth, remyelinating damaged axons, modulating immune response, and creating a permissive environment for functional recovery [18,19,20]. By targeting multiple aspects of the SCI pathology, stem cell therapy could overcome the limitations of traditional treatments and significantly improve the lives of affected individuals [21]. Several types of stem cells are currently being investigated for their potential in SCI treatment, including embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), mesenchymal stem cells (MSCs), and neural stem/progenitor cells (NSPCs) [22,23,24,25]. Each of these cell types offers unique advantages and challenges in the context of SCI therapy. ESCs, for instance, are pluripotent and can differentiate into any cell type, but their use raises ethical concerns related to embryo destruction [26]. iPSCs, on the other hand, can be generated from adult cells, avoiding the ethical issues associated with ESCs, but they carry a risk of tumor formation [27,28]. MSCs are known for their immunomodulatory properties and have shown promise in preclinical studies [29], while NSPCs can be derived from various sources and have the inherent ability to generate neural cells [30]. Despite the tremendous potential of stem cell therapy for SCI treatment, researchers still face significant challenges that must be addressed before these therapies can be widely adopted. Ensuring the safety and efficacy of stem cell-based therapies is paramount. For example, the risk of tumor formation, potential for immune rejection, and the need for standardized protocols for stem cell isolation, expansion, and differentiation must be carefully addressed. Additionally, the development of suitable biomaterials and scaffolds to support cell survival, integration, and function is crucial for the successful translation of stem cell therapies from the laboratory to the clinic [31,32,33].
The use of human stem cells, particularly ESCs, raises important ethical concerns [34]. Transparent and robust regulatory frameworks are essential to ensure the safe and ethical application of stem cell therapies for SCIs [35]. Public dialogue and collaboration between researchers, clinicians, policymakers, and society at large are vital for addressing these ethical issues and developing appropriate guidelines. In conclusion, stem cell therapy offers a beacon of hope for patients suffering from SCIs and has the potential to revolutionize treatment by promoting the regeneration and repair of damaged neural tissue. As researchers continue to explore and refine stem cell therapies, we can expect breakthroughs that will transform the way we approach SCI treatment. By overcoming the challenges and addressing ethical concerns, stem cell therapy has the potential to make a significant impact on the lives of those affected by SCIs.
2. The Promise of Stem Cell Therapy for SCIs
Stem cell therapy has the potential to revolutionize the treatment of SCIs by promoting regeneration and repair of damaged neural tissue. By using stem cells, researchers aim to replace lost neurons [36], remyelinate axons [37], modulate the immune response [38], and create a permissive environment for axonal growth and functional recovery [39]. This approach could provide new hope for patients suffering from SCIs, who currently have limited treatment options. Additionally, stem cell therapy can target various aspects of SCI pathology, such as inflammation, apoptosis, and glial scarring [40,41,42]. By addressing these issues, stem cells may mitigate secondary damage and further improve the prospects for neural repair and functional recovery [43]. The versatility of stem cells and their ability to adapt to the specific needs of the injured spinal cord make them ideal candidates for developing tailored therapies that target individual patient requirements.
Recent preclinical studies have shown promising results in animal models of SCI, with stem cell transplantation leading to improvements in locomotor function, reduction in lesion size, and enhanced neural regeneration [44]. These findings highlight the potential of stem cell therapy to translate into clinically meaningful outcomes for SCI patients. Furthermore, ongoing clinical trials are evaluating the safety and efficacy of stem cell-based therapies in human patients with SCIs [45]. These trials represent a critical step toward the development of novel, effective, and safe treatments for individuals suffering from SCIs. As research continues to advance, the integration of novel technologies such as gene editing [22], 3D bioprinting [46,47], and tissue engineering [46,48] may further enhance the potential of stem cell therapy for SCI treatment. These innovations can help optimize stem cell delivery, survival, and integration into the host tissue, ultimately leading to more effective therapies and improved outcomes. Additionally, it is important to note that ongoing clinical trials are actively assessing the safety and effectiveness of stem cell therapies in human patients with SCIs [45]. These clinical trials are a crucial advancement in the field, paving the way for new, effective, and safe treatment options for those affected by SCIs. While the outcomes of these clinical studies have been mixed, they are generally promising. Some studies have even reported improvements in lost motor, sensory, and autonomic nervous system functions, underscoring the potential of stem cell therapies [49,50,51,52]. While it is true that the majority of stem cells differentiate into glial cells rather than neurons, these cells still play crucial roles in structural support, inflammation modulation, and remyelination, all of which contribute to the overall therapeutic effect [53]. Ongoing research is focused on optimizing conditions for neuronal differentiation and integration into existing neural circuits. This research is facilitated by technological advances such as gene editing and 3D bioprinting. For instance, fast-scalable 3D bioprinting has been shown to create biocompatible and biomimetic scaffolds that precisely fit the geometries of spinal cord lesions. These scaffolds promote axonal regeneration and support stem cell grafts, thereby aiding in the recovery from SCIs in rodent models [54]. Despite the bias towards glial cell differentiation, preclinical studies have reported improvements in both locomotor function and lesion size reduction.
2.1. Comparative Analysis of Stem Cell Transplantation and Other Cell-Based Therapies in SCIs
To provide a more comprehensive understanding of the therapeutic options available for SCIs, it is important to compare the advantages and disadvantages of stem cell transplantation with other cell-based therapies such as Schwann cells and olfactory ensheathing glia cells. Below, we outline the key points of this comparison.
2.1.1. Advantages of Stem Cells
Stem cells offer the unique advantage of pluripotency or multipotency, allowing them to differentiate into various cell types, including neurons and glial cells. This makes them versatile candidates for addressing multiple aspects of SCI pathology, such as inflammation, apoptosis, and glial scarring [36,37,38,55].
2.1.2. Disadvantages of Stem Cells
The use of stem cells comes with challenges such as the risk of tumor formation, potential for immune rejection, and ethical concerns, especially in the case of embryonic stem cells [26,27,28].
2.1.3. Advantages of Schwann Cells and Olfactory Ensheathing Glia Cells
These cells are already specialized for roles in the nervous system, potentially reducing the risk of unwanted differentiation. Schwann cells are known for their ability to promote axonal growth, while olfactory ensheathing cells can remyelinate axons and modulate inflammation [50,51].
2.1.4. Disadvantages of Schwann Cells and Olfactory Ensheathing Glia Cells
These cells are more limited in their differentiation potential compared to stem cells, which may restrict their ability to address the multifaceted pathology of SCIs [52].
In summary, stem cell therapy offers a promising and innovative approach to treating SCIs, addressing various aspects of the injury and providing the potential for significant functional recovery [56]. As research continues to progress and collaborations between researchers, clinicians, and patients strengthen, the full potential of stem cell therapy in the treatment of SCIs may soon be realized, offering renewed hope to those affected by these devastating injuries.
2.2. Biomaterials in 3D Stem Cell Constructs for SCI
The field of regenerative medicine has seen significant advancements in the development of biomaterials that can effectively hold 3D tissue-engineered constructs of stem cells. These biomaterials not only serve as scaffolds but also influence stem cell behavior, differentiation, and integration into host tissues. This section explores various types of biomaterials that have shown promise in this regard, including hydrogels, poly(ethylene glycol)-fibrinogen hydrogels, nanomaterials, and microgels.
2.2.1. Hydrogels
Hydrogels such as spatially varying multi-layered hydrogels have been shown to stimulate efficient regeneration of complex tissues from a single stem cell population [57]. Another study demonstrated that hydrogels could be used as 3D cell carriers for the mechanical stimulation of encapsulated stem cells [58].
2.2.2. Poly(ethylene glycol)-Fibrinogen Hydrogels
These hydrogels have been used to directly differentiate human pluripotent stem cells into contracting heart tissues [59].
2.2.3. Nanomaterials
The combination of stem cells and nanomaterials is expected to develop into an important tool in tissue engineering [60].
2.2.4. Microgels
Stem cell-laden microgels with shape-forming properties can be used as smart building blocks for tissue regeneration [61].
In summary, various types of biomaterials, including hydrogels, poly(ethylene glycol)-fibrinogen hydrogels, nanomaterials, and microgels, have shown promise in holding 3D tissue-engineered constructs of stem cells. These biomaterials offer unique advantages in terms of mechanical properties, biocompatibility, and the ability to influence stem cell behavior. Continued research in this area is essential for optimizing these biomaterials for clinical applications in regenerative medicine.
3. The Potential of Different Stem Cell Types
Various types of stem cells are being explored for their potential in SCI treatment. In this review article, we mainly discuss the four cell types, ESCs, iPSCs, MSCs, and NSPCs, which each offer unique advantages and challenges in the context of SCI therapy. By understanding the characteristics and capabilities of each cell type, researchers can optimize their use in regenerative medicine applications. We provide a comparison of the advantages and challenges associated with different stem cell types in the context of SCI treatment (Table 2). In this review, we delve into the therapeutic potential of various stem cell types—ESCs, iPSCs, MSCs, and NSPCs—in the context of SCI treatment. Each stem cell type presents its own set of advantages and limitations. For instance, ESCs offer a broad differentiation spectrum but are fraught with ethical dilemmas and risks such as immune rejection and tumorigenesis [62,63]. iPSCs, while circumventing ethical issues, still pose challenges related to tumor formation and genetic abnormalities [64,65]. MSCs are lauded for their immunomodulatory properties and growth factor secretion but face hurdles in cell survival at the injury site [66,67]. NSPCs, capable of generating neural cells and providing neurotrophic support, also grapple with survival and integration issues [68,69].
Table 2.
Comparison of stem cell types for SCI treatment.
| Stem Cell Type | Advantages | Challenges | References |
|---|---|---|---|
| Embryonic Stem Cells (ESCs) |
|
|
[52,56,57,58] |
| Induced Pluripotent Stem Cells (iPSCs) |
|
|
[66,67,68,69] |
| Mesenchymal Stem Cells (MSCs) |
|
|
[70,71,72,73,74,75] |
| Neural Stem/Progenitor Cells (NSPCs) |
|
|
[76,77,78,79,80] |
To assess the efficacy of these stem cell types, we have incorporated a summary of quantitative data from reputable studies. Metrics such as motor and sensory functional recovery, neural tissue preservation, and the reduction in gliosis and inflammation are considered. Standardized scales such as the Basso, Beattie, and Bresnahan (BBB) scale have been employed in both animal models and clinical trials to gauge locomotor recovery and sensory function [81,82]. Histological analyses provide quantitative measures of neural tissue preservation [83,84], and markers such as GFAP and cytokine levels are used to assess the reduction in gliosis and inflammation [85]. Comparative studies further enrich our understanding, indicating that each stem cell type has its unique set of advantages and limitations [86,87]. Additionally, different types of stem cells have been investigated in various models of SCI, including compression, contusion, section, and hemisection models. ESCs have been particularly effective in contusion models, showing promise in replacing lost neurons and remyelinating axons [88]. iPSCs have been found to be effective in section models, especially in promoting axonal regeneration [65]. MSCs have demonstrated efficacy across multiple models, notably in modulating immune responses and reducing inflammation [89]. NSPCs have been particularly effective in contusion models, where they have contributed to functional recovery and tissue repair [70]. Comparative studies indicate that the type of SCI model can influence the effectiveness of the stem cell type used [71]. Ongoing research aims to tailor stem cell therapies to specific injury types for optimal outcomes [72].
By synthesizing these data, we aim to offer a nuanced, evidence-based evaluation of the therapeutic efficacy of different stem cell types in SCI treatment. This approach not only enhances our understanding of the therapeutic potential of each stem cell type but also provides a robust foundation for future research in regenerative medicine for SCI.
3.1. Embryonic Stem Cells
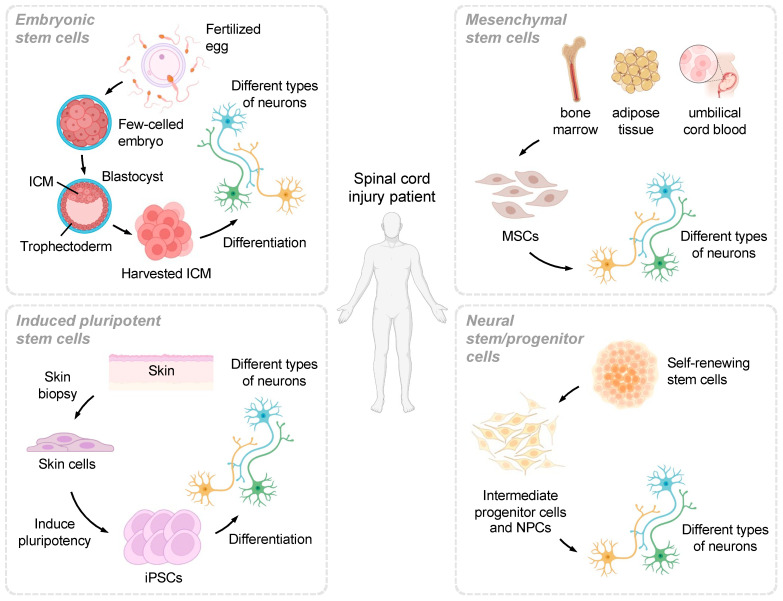
ESCs are pluripotent cells derived from the inner cell mass of blastocysts and have the ability to differentiate into all cell types found in the adult body (Figure 1) [73]. This broad differentiation potential makes them an attractive option for SCI therapy, as they can potentially replace lost neurons [74,75], remyelinate axons [90,91], and facilitate tissue repair following injury [92]. However, the use of ESCs in research and therapy is associated with ethical concerns related to the destruction of embryos during the derivation process. These concerns have sparked debate and led to the implementation of strict guidelines and regulations surrounding ESC research in many countries [76]. Additionally, the use of ESCs for transplantation carries the potential risk of immune rejection, as these cells are allogeneic, meaning they are derived from a genetically different individual [77,78]. This can result in immune responses against the transplanted cells, limiting their therapeutic efficacy.
Figure 1.
Overview of various stem cell types explored for their potential in SCI treatment. This figure provides a visual summary of the four major stem cell types discussed in the review article: embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), mesenchymal stem cells (MSCs), and neural stem/progenitor cells (NSPCs). Each stem cell type is represented by a separate section in the figure, highlighting their unique advantages and potential differentiation into different types of neural cells for SCI therapy. The figure aims to facilitate understanding of the distinct characteristics and capabilities of these stem cell types, enabling researchers to optimize their use in regenerative medicine applications for SCI treatment.
Another challenge associated with ESCs is the potential for tumor formation following transplantation [79]. Since ESCs are pluripotent, they have the capacity to form teratomas, which are tumors composed of a mixture of cell types derived from all three germ layers. This risk must be carefully managed in order to ensure the safety and efficacy of ESC-based therapies. Despite these challenges, researchers are continuing to investigate methods to harness the regenerative potential of ESCs while addressing the associated ethical, safety, and efficacy concerns. Advances in differentiation protocols, cell culture techniques, and transplantation strategies may help pave the way for the successful use of ESCs in SCI therapy [80,93].
3.2. Induced Pluripotent Stem Cells
iPSCs are generated by reprogramming adult somatic cells to a pluripotent state, circumventing the ethical concerns associated with ESCs (Figure 1) [94]. As with ESCs, iPSCs have the ability to differentiate into various cell types and have shown promise in preclinical studies for SCI treatment [95,96]. iPSC-derived neural cells have been used to replace lost neurons, promote axonal regeneration, and provide neurotrophic support, contributing to functional recovery in animal models of SCI [97,98]. However, iPSCs also carry a risk of tumor formation, as their pluripotent nature can lead to uncontrolled cell growth and differentiation [99,100]. Moreover, the reprogramming process may introduce genetic and epigenetic abnormalities that could affect the safety and efficacy of iPSC-based therapies. For instance, the use of viral vectors for reprogramming may result in insertional mutagenesis, which can disrupt normal gene function and increase the risk of tumorigenicity [101].
To address these challenges, researchers are exploring alternative reprogramming methods, such as using non-integrating viral vectors or small molecules, to generate safer iPSCs with reduced risks of genetic and epigenetic alterations [102]. Additionally, strategies to promote the directed differentiation of iPSCs into specific neural cell types and to enhance their integration into the host tissue are being developed to improve the therapeutic potential of iPSCs for SCI treatment [103,104]. Overall, iPSCs offer a promising alternative to ESCs in regenerative medicine for SCI, but further research is needed to overcome the safety concerns and optimize their use in therapy.
3.3. Mesenchymal Stem Cells
MSCs are multipotent cells found in various adult tissues, including bone marrow, adipose tissue, and umbilical cord blood (Figure 1) [105]. MSCs have demonstrated immunomodulatory properties and secrete a variety of growth factors, making them an attractive option for SCI therapy [106,107]. Their immunomodulatory properties help to suppress inflammation and modulate immune responses, which can be beneficial in minimizing secondary damage following SCI [108]. Furthermore, MSCs secrete a variety of growth factors and cytokines that promote angiogenesis, neurogenesis, and axonal growth, which contribute to tissue repair and functional recovery after SCI [109,110]. In preclinical studies, MSC transplantation has been shown to promote functional recovery and tissue repair after SCI, by reducing the lesion size, preserving myelin, and promoting axonal regeneration [111,112]. Additionally, MSCs are relatively easy to isolate and expand in vitro, making them a more accessible source of stem cells for therapeutic applications. They also have a lower risk of immune rejection due to their low expression of major histocompatibility complex molecules and immunosuppressive properties [113]. Moreover, MSCs have a lower risk of tumor formation compared to ESCs and iPSCs, as they are multipotent cells with a more limited differentiation potential [114].
Despite these advantages, there are some challenges associated with MSC-based therapies for SCI. One major challenge is the low survival rate of transplanted MSCs, which can be affected by the harsh microenvironment at the injury site [115]. Researchers are working to enhance MSC survival and engraftment by optimizing transplantation methods, preconditioning MSCs, or using biomaterial scaffolds to support cell survival [116,117,118]. Furthermore, the optimal source of MSCs, as well as the timing, dose, and route of administration, need to be determined to maximize the therapeutic potential of MSCs for SCI treatment. Overall, MSCs hold great promise for SCI therapy, but further research is required to address these challenges and optimize their use in clinical applications.
3.4. Neural Stem/Progenitor Cells
NSPCs can be derived from various sources, including embryonic and adult neural tissue, as well as iPSCs and ESCs (Figure 1) [119]. These cells have the inherent ability to generate neural cells, including neurons, astrocytes, and oligodendrocytes [120,121]. NSPC transplantation has shown promise in preclinical studies, promoting functional recovery, reducing lesion size, and enhancing remyelination in animal models of SCI [36]. Moreover, NSPCs can secrete neurotrophic factors that support the survival and regeneration of host neurons, and modulate the local environment to enhance endogenous repair processes [98]. The use of NSPCs may help overcome some of the challenges associated with other stem cell types, such as ethical concerns and risks of tumor formation [122,123]. As they can be derived from adult neural tissue or iPSCs, the ethical concerns associated with the use of ESCs can be circumvented. Furthermore, since NSPCs are committed to a neural lineage, they are less likely to form tumors compared to pluripotent stem cells such as ESCs and iPSCs [124,125]. However, there are still challenges that need to be addressed in the use of NSPCs for SCI treatment. The survival and integration of transplanted NSPCs into the host tissue can be limited by the harsh microenvironment at the injury site [126]. Strategies to improve cell survival and integration, such as preconditioning, biomaterial scaffolds, and the modulation of the injury microenvironment, are being explored to enhance the therapeutic potential of NSPCs [127,128]. Additionally, the optimal source of NSPCs, as well as the timing, dose, and route of administration, need to be determined for maximal therapeutic benefits.
3.5. Clinical Outcomes and Adverse Events
The application of stem cell transplantation in SCI has garnered significant attention due to its potential for tissue regeneration and functional recovery. However, the approach is not without challenges. This section aims to discuss three critical aspects of stem cell transplantation in SCI: the rate of immune rejection, the incidence of improvement and failure to improve, and the occurrence of cancer or other adverse events.
3.5.1. Immune Rejection
The immune response to stem cell transplantation in SCI is complex and can vary depending on the type of stem cells used. Torres-Espín et al. (2013) found that transplantation of MSCs or OECs leads to an up-regulation of genes related to tissue protection and regeneration. However, there is also a marked increase in the expression of genes associated with foreign body response and adaptive immune response, suggesting a fast rejection response to the grafted cells [129]. This is consistent with other studies that have noted that the immune response in SCI can both exacerbate pathology and promote tissue repair [130].
3.5.2. Rate/Incidence of Improvement and Failure to Improve
While the manuscript initially did not include specific rates of improvement or failure, it is crucial to note that stem cell transplantation has shown promise in various studies. For example, intravenous transplantation of bone marrow mesenchymal stem cells improved motor function in rats with SCI [66]. However, the effectiveness of the therapy can be influenced by the type of stem cells used and the timing of the transplantation [131].
3.5.3. Occurrence of Cancer or Other Adverse Events
The risk of tumor formation is a significant concern, especially with pluripotent stem cells such as ESCs and iPSCs. However, MSCs and NSPCs generally have a lower risk of tumor formation due to their more limited differentiation potential.
3.5.4. Other Adverse Events
The harsh microenvironment at the injury site can affect the survival rate of transplanted stem cells [129]. Moreover, SCI often leads to chronic inflammation and immune dysfunction, which can further complicate the transplantation process [132].
In summary, stem cell transplantation for SCIs holds immense promise for functional recovery, yet it comes with its own set of complex challenges, including immune rejection, inconsistent rates of improvement, and potential adverse events such as tumor formation. Each type of stem cell—ranging from NSPCs to MSCs—brings its own unique advantages and limitations to the table. A nuanced understanding of these characteristics is crucial for optimizing therapeutic strategies and making stem cell transplantation a viable clinical option. As we move forward, the focus of research should be on elucidating the mechanisms of action, refining transplantation protocols, and developing targeted therapies to mitigate these challenges. By doing so, we can pave the way for more effective and safer treatments that have the potential to significantly improve the quality of life for those affected by SCIs. Continued innovation and multidisciplinary research in this field are essential for realizing this potential and overcoming the existing hurdles.
4. Challenges in Stem Cell Therapy for SCI
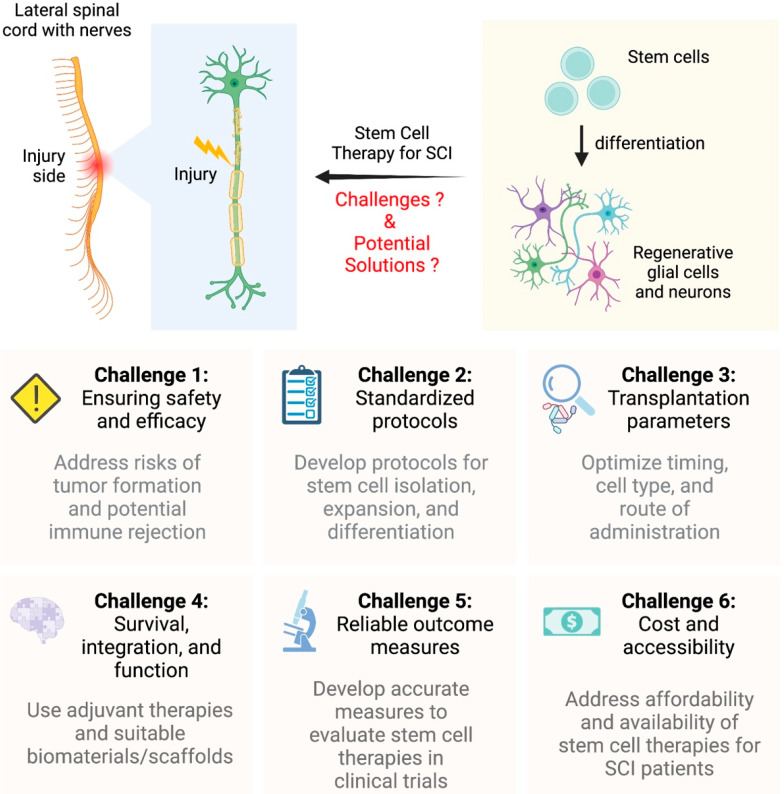
Despite the tremendous potential of stem cell therapy for SCI treatment, the field still faces significant challenges (Figure 2). Ensuring the safety and efficacy of stem cell-based therapies is paramount. For example, the risk of tumor formation and the potential for immune rejection must be carefully addressed [133,134]. Furthermore, the development of standardized protocols for stem cell isolation, expansion, and differentiation is crucial for the successful translation of stem cell therapies from the laboratory to the clinic. In addition to safety concerns, another major challenge is the optimization of stem cell transplantation parameters, such as the timing of transplantation, the choice of cell type, and the most effective route of administration [135,136]. Research has shown that the timing of transplantation can have a significant impact on the therapeutic outcome, as the injured spinal cord undergoes various stages of inflammation, secondary injury, and tissue repair [137,138]. Some studies suggest that transplantation is most effective when carried out 1–2 weeks after injury [139], while others recommend a therapeutic window of 3–4 weeks following injury [140]. These timing considerations are crucial as the injured spinal cord undergoes various stages of inflammation, secondary injury, and tissue repair [137,138]. The choice of cell type is also a critical factor. For example, a study by Dai et al. (2013) confirmed that CT-guided stem cell transplantation is a safe and effective approach for treating sequelae of SCI, offering advantages such as minimal invasion and quicker recovery [141]. Identifying the optimal window for stem cell transplantation may be crucial for maximizing the benefits of stem cell therapy. The choice of cell type is also a critical factor in determining the success of stem cell therapy for SCI. As mentioned earlier, different stem cell types offer unique advantages and challenges [17,28]. Researchers need to determine which cell type or combination of cell types will provide the best therapeutic outcomes for SCI patients while minimizing the risks associated with each cell type.
Figure 2.
Overview of challenges and potential solutions in stem cell therapy for SCIs. This infographic illustrates the main challenges and potential solutions in the development and implementation of stem cell therapies for SCI treatment. Six key challenges are represented in hexagons surrounding a central image of a spinal cord with an overlaying stem cell icon, symbolizing the application of stem cell therapy for SCI. Each hexagon contains an icon representing the challenge, along with a brief description of the corresponding solution. The challenges and solutions addressed in the figure include ensuring safety and efficacy, developing standardized protocols, optimizing transplantation parameters, enhancing survival, integration, and function of transplanted stem cells, establishing reliable outcome measures, and addressing cost and accessibility of stem cell therapies for SCI patients.
Another challenge is the need for effective strategies to enhance the survival, integration, and function of transplanted stem cells within the host tissue [18,142]. This may involve the use of adjuvant therapies, such as growth factors, neurotrophic factors, or immunomodulatory agents, to promote cell survival and improve the local environment for stem cell integration and differentiation. Moreover, the development of suitable biomaterials and scaffolds that support cell survival, integration, and function is critical for the successful translation of stem cell therapies from the laboratory to the clinic [143,144]. There is also a need for the development of reliable and accurate outcome measures to evaluate the efficacy of stem cell therapies in clinical trials [145]. This may include the establishment of standardized functional assessments, imaging techniques, and biomarkers that can provide objective measures of treatment success and facilitate the comparison of results across different trials. Lastly, the cost and accessibility of stem cell therapies for SCI patients must be addressed. Developing effective, safe, and affordable stem cell therapies will be essential in ensuring that these treatments can be made widely available to those who need them.
In conclusion, we have highlighted the major challenges associated with stem cell therapy for SCI and proposed potential solutions to address these issues (Table 3). Overcoming these obstacles is crucial for successfully translating stem cell therapies from the laboratory to clinical settings and ultimately improving the lives of SCI patients.
Table 3.
Stem cell types, advantages, existing challenges, and possible solutions in stem cell therapy for SCI.
| Stem Cell Type | Advantages | Existing Challenges | Possible Solutions |
|---|---|---|---|
| Embryonic Stem Cells (ESCs) |
|
|
|
| Induced Pluripotent Stem Cells (iPSCs) |
|
|
|
| Mesenchymal Stem Cells (MSCs) |
|
|
|
| Neural Stem/Progenitor Cells (NSPCs) |
|
|
|
4.1. Secondary Injury and Stem Cell Transplantation
The impact of secondary injury on stem cell transplantation in SCI is a multifaceted topic that warrants in-depth exploration. This section aims to shed light on how secondary injury mechanisms interact with stem cell transplantation, affecting outcomes such as immune rejection, tissue repair, and overall efficacy.
4.1.1. Prevention of Secondary Damage
Grafted neuroectodermal stem cells have been shown to prevent secondary spinal cord damage and induce significant regeneration through multiple mechanisms [146].
4.1.2. Restriction of Tissue Damage
Resident neural stem cells are required to restrict secondary enlargement of the lesion and further axonal loss after SCI [147].
4.1.3. Optimal Timing
A precise window of opportunity exists for the treatment of complex SCIs with therapeutically plastic somatic stem cells [70].
4.1.4. Microenvironment
The harsh microenvironment at the injury site can affect the survival rate of transplanted stem cells, which is a challenge that needs to be addressed for effective therapy [129].
Understanding the interplay between secondary injury and stem cell transplantation is crucial for optimizing therapeutic strategies in SCI. While stem cells have the potential to mitigate secondary damage and promote tissue repair, challenges such as immune rejection and the harsh microenvironment at the injury site remain. Future research should focus on identifying the optimal timing and conditions for stem cell transplantation to maximize its therapeutic efficacy.
5. Future Directions and Technological Advancements
As the field of stem cell therapy for SCIs continues to evolve, technological advancements are paving the way for more effective treatments. One of the most promising areas of research is the use of gene editing technologies such as CRISPR/Cas9 to modify stem cells for improved therapeutic efficacy, safety, and immunocompatibility [148,149,150].
5.1. Interdisciplinary Approaches to Advancing Stem Cell Therapies for SCIs
5.1.1. Biomaterials and Differentiation
Biomaterials play a significant role in stem cell therapy for SCIs. Advanced scaffolds and biomaterials that mimic the native extracellular matrix can provide a conducive environment for stem cell integration, survival, and differentiation [127,151]. For instance, 3D-printed biomimetic spinal cords have been shown to promote directional differentiation and repair motor function after SCI [152]. Moreover, the mechanical properties of scaffolds can direct stem cell differentiation in vivo [153].
5.1.2. Cell Engineering Techniques
Gene editing techniques such as CRISPR/Cas9 can be used to improve the therapeutic potential of stem cells. These modifications can enhance safety and immunocompatibility, although further research is needed to confirm their efficacy in SCIs [87].
5.1.3. Clinical Translations
Several studies have demonstrated the potential of stem cell therapies in clinical settings. For example, the transplantation of human umbilical cord-mesenchymal stem cells on a collagen scaffold has been shown to promote the recovery of neurological function after acute SCI [154].
5.1.4. Challenges and Solutions
Despite these advancements, challenges such as safety, efficacy, and standardization of protocols remain. Research indicates that the optimal timing for transplantation is crucial, and various approaches such as the pharmacological activation of endogenous stem cells and optogenetics are being explored to improve the application of stem cell therapy [87].
5.1.5. Quality of Life Improvements in SCI Patients
Clinical studies have shown that stem cell transplantation can significantly improve the quality of life for patients with SCI. For example, a study involving six patients with chronic SCI found that autologous co-transplantation of bone marrow mesenchymal stem cells and Schwann cells led to improvements in bladder compliance and axonal regeneration, with no adverse findings over a mean 30 months of follow-up [50]. Another study demonstrated that CT-guided stem cell transplantation was a safe and effective approach for treating sequelae of SCI, offering advantages such as minimal invasion and quicker recovery [141]. Moreover, transplantation of human spinal cord-derived neural stem cells has been shown to improve motor or sensory function in individuals with chronic SCI [49].
In summary, advancements in stem cell therapy for SCIs are accelerating at an unprecedented rate, fueled by technological innovations and multidisciplinary research. The synergy between biomaterial science, genetic engineering, and clinical studies is paving the way for more effective and personalized treatments. However, challenges such as safety protocols, efficacy validation, and treatment standardization remain. As we navigate these complexities, collaborative initiatives across scientific, clinical, and industrial sectors become increasingly vital. These partnerships will be instrumental in overcoming obstacles and unlocking the full therapeutic potential of stem cell treatments for SCIs.
5.2. Challenges and Problems in Non-Traumatic SCI
The application of stem cells for treating SCIs resulting from diseases such as transverse myelitis and neuromyelitis optica presents unique challenges and opportunities. While the underlying pathology may differ from traumatic SCIs, the therapeutic potential and limitations of stem cell interventions remain critical areas of investigation. This section delves into the multifaceted issues surrounding the use of stem cells in non-traumatic SCIs, including safety, timing, microenvironment, ethical concerns, and efficacy.
5.2.1. Safety and Feasibility
Clinical trials have shown the safety of autologous co-transplantation of bone marrow mesenchymal stem cells and Schwann cells, but long-term follow-up is essential [50].
5.2.2. Optimal Timing
A precise window of opportunity exists for stem cell transplantation, which varies depending on the type and severity of the injury [70].
5.2.3. Microenvironment
The harsh microenvironment at the injury site can affect the survival rate of transplanted stem cells [147].
5.2.4. Ethical Concerns
The source of stem cells can raise ethical issues, although some types such as unrestricted somatic stem cells lack such concerns [155].
5.2.5. Efficacy
The effectiveness of stem cell therapies in non-traumatic SCI is still under investigation, and premature application should be discouraged [156].
The challenges in applying stem cells for non-traumatic SCIs are complex and require a multi-faceted approach. While there is promising evidence for the safety and potential efficacy of such treatments, long-term studies are essential. Ethical considerations and the harsh microenvironment at the injury site further complicate the therapeutic landscape. Future research should focus on addressing these challenges to expand the applicability and effectiveness of stem cell therapies in non-traumatic SCIs.
5.3. The Role of Biomaterials, Engineering, and Collaborative Research for SCI Therapies
Biomaterials and tissue engineering techniques can also play a crucial role in the development of more effective stem cell therapies. By designing advanced scaffolds and biomaterials that mimic the native extracellular matrix, researchers can provide a more conducive environment for stem cell integration, survival, and differentiation [127,151]. Moreover, these materials can also be tailored to release growth factors or other bioactive molecules that can further enhance the regenerative process. One emerging approach that holds promise for SCI treatment is the combination of stem cell therapy with electrical stimulation or rehabilitation strategies. The synergistic effects of stem cell transplantation and rehabilitation may lead to improved functional recovery by promoting neural plasticity and enhancing the integration of transplanted cells into the host tissue [157,158]. Further research in this area could lead to the development of more effective and personalized therapeutic interventions for SCI patients. Collaborative efforts between basic scientists, clinicians, engineers, and industry partners will be essential to address the multifaceted challenges in translating stem cell therapies from the laboratory to clinical applications. Additionally, the development of international consortia, multicenter clinical trials, and standardized outcome measures will be crucial in generating high-quality evidence to support the widespread adoption of stem cell therapies for SCIs.
In summary, stem cell therapy for SCIs represents a beacon of hope amid the numerous challenges that need to be addressed. As researchers continue to make strides in overcoming these obstacles and refining stem cell therapies, we can anticipate groundbreaking advancements that will revolutionize the way we approach SCI treatment. By working together to tackle the challenges and address ethical concerns, the potential of stem cell therapy to improve the lives of those affected by SCIs can be fully realized.
6. Conclusions
Stem cell therapy holds promise for SCI treatment by potentially revolutionizing damaged neural tissue repair and regeneration. As research progresses and challenges are addressed, stem cell therapy could significantly impact the lives of those affected by SCIs, offering them hope for a better future. Collaborative efforts among scientists, clinicians, bioengineers, and industry partners are essential for driving progress in stem cell therapies for SCIs. By exploring various stem cell types, developing standardized protocols, and incorporating advancements in gene editing, biomaterials, and tissue engineering, researchers may develop more effective interventions and personalized therapeutic strategies. Furthermore, continued investment in stem cell research and the establishment of international collaborations and networks will facilitate the exchange of knowledge and resources, enabling rapid advancements in the field. Addressing ethical concerns, ensuring regulatory compliance, and fostering public awareness and understanding of stem cell therapies are also crucial to garner support for these potentially life-changing treatments.
In addition to the scientific and technical challenges, clinical trials and long-term follow-up studies will be necessary to evaluate the safety and efficacy of stem cell therapies in the context of SCI treatment. Patient advocacy and involvement will play a crucial role in ensuring that new therapies are developed with the best interests of SCI patients in mind, taking into account their specific needs and desires. Ultimately, realizing the full potential of stem cell therapy for SCI treatment relies on the scientific community’s dedication, passion, and persistence. By working together and pushing the boundaries of our knowledge, we can provide hope and an improved quality of life for countless individuals affected by SCIs, transforming the landscape of SCI treatment for generations to come.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Badenhorst M. Ph.D. Thesis. University of Cape Town; Cape Town, South Africa: 2019. Life after the Game: Consequences of Acute Spinal Cord Injuries in South African Rugby Union Players. [Google Scholar]
- 2.Courtine G., Sofroniew M.V. Spinal cord repair: Advances in biology and technology. Nat. Med. 2019;25:898–908. doi: 10.1038/s41591-019-0475-6. [DOI] [PubMed] [Google Scholar]
- 3.Lin Z., Wu Y., Xu Y., Li G., Li Z., Liu T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. Mol. Cancer. 2022;21:179. doi: 10.1186/s12943-022-01650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chia W.K., Cheah F.C., Abdul Aziz N.H., Kampan N.C., Shuib S., Khong T.Y., Tan G.C., Wong Y.P. A review of placenta and umbilical cord-derived stem cells and the immunomodulatory basis of their therapeutic potential in bronchopulmonary dysplasia. Front. Pediatr. 2021;9:615508. doi: 10.3389/fped.2021.615508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surkin J., Gilbert B.J.C., Harkey III H.L., Sniezek J., Currier M. Spinal cord injury in Mississippi: Findings and evaluation, 1992–1994. Spine. 2000;25:716–721. doi: 10.1097/00007632-200003150-00011. [DOI] [PubMed] [Google Scholar]
- 6.van den Berg M.E., Castellote J.M., Mahillo-Fernandez I., de Pedro-Cuesta J. Incidence of nontraumatic spinal cord injury: A Spanish cohort study (1972–2008) Arch. Phys. Med. Rehabil. 2012;93:325–331. doi: 10.1016/j.apmr.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Albert T., Ravaud J.F. Rehabilitation of spinal cord injury in France: A nationwide multicentre study of incidence and regional disparities. Spinal Cord. 2005;43:357–365. doi: 10.1038/sj.sc.3101717. [DOI] [PubMed] [Google Scholar]
- 8.Joseph C., Andersson N., Bjelak S., Giesecke K., Hultling C. Incidence, aetiology and injury characteristics of traumatic spinal cord injury in Stockholm, Sweden: A prospective, population-based update. J. Rehabil. Med. 2017;49:431–436. doi: 10.2340/16501977-2224. [DOI] [PubMed] [Google Scholar]
- 9.Fitzharris M., Cripps R.A., Lee B.B. Estimating the global incidence of traumatic spinal cord injury. Spinal Cord. 2014;52:117–122. doi: 10.1038/sc.2013.135. [DOI] [PubMed] [Google Scholar]
- 10.New P.W., Farry A., Baxter D., Noonan V.K. Prevalence of non-traumatic spinal cord injury in Victoria, Australia. Spinal Cord. 2013;51:99–102. doi: 10.1038/sc.2012.61. [DOI] [PubMed] [Google Scholar]
- 11.Hagen E.M., Eide G.E., Rekand T., Gilhus N.E., Gronning M. A 50-year follow-up of the incidence of traumatic spinal cord injuries in Western Norway. Spinal Cord. 2010;48:313–318. doi: 10.1038/sc.2009.133. [DOI] [PubMed] [Google Scholar]
- 12.Van den Berg M.E.L., Castellote J.M., Mahillo-Fernandez I., de Pedro-Cuesta J. Incidence of spinal cord injury worldwide: A systematic review. Neuroepidemiology. 2010;34:184–192. doi: 10.1159/000279335. [DOI] [PubMed] [Google Scholar]
- 13.Ahoniemi E., Alaranta H., Hokkinen E.M., Valtonen K., Kautiainen H. Incidence of traumatic spinal cord injuries in Finland over a 30-year period. Spinal Cord. 2008;46:781–784. doi: 10.1038/sc.2008.53. [DOI] [PubMed] [Google Scholar]
- 14.Du J., Hao D., He B., Yan L., Tang Q., Zhang Z., Wang Y., Li H., Cao Y., Jiang C.Q., et al. Epidemiological characteristics of traumatic spinal cord injury in Xi’an, China. Spinal Cord. 2021;59:804–813. doi: 10.1038/s41393-020-00592-3. [DOI] [PubMed] [Google Scholar]
- 15.Ackery A., Tator C., Krassioukov A. A global perspective on spinal cord injury epidemiology. J. Neurotrauma. 2004;21:1355–1370. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- 16.Madl C.M., Heilshorn S.C., Blau H.M. Bioengineering strategies to accelerate stem cell therapeutics. Nature. 2018;557:335–342. doi: 10.1038/s41586-018-0089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajabzadeh N., Fathi E., Farahzadi R. Stem cell-based regenerative medicine. Stem Cell Investig. 2019;6:19. doi: 10.21037/sci.2019.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer I., Dulin J.N., Lane M.A. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat. Rev. Neurosci. 2020;21:366–383. doi: 10.1038/s41583-020-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pajer K., Bellák T., Nógrádi A. Stem Cell secretome for spinal cord repair: Is it more than just a random baseline set of factors? Cells. 2021;10:3214. doi: 10.3390/cells10113214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myatich A., Haque A., Sole C., Banik N.L. Clemastine in remyelination and protection of neurons and skeletal muscle after spinal cord injury. Neural Regen. Res. 2023;18:940–946. doi: 10.4103/1673-5374.355749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman M.M., Islam M.R., Islam M.T., Harun-Or-Rashid M., Islam M., Abdullah S., Uddin M.B., Das S., Rahaman M.S., Ahmed M., et al. Stem cell transplantation therapy and neurological disorders: Current status and future perspectives. Biology. 2022;11:147. doi: 10.3390/biology11010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z., Wang F., Song M. The cell repair research of spinal cord injury: A review of cell transplantation to treat spinal cord injury. J. Neurorestoratology. 2019;7:55–62. doi: 10.26599/JNR.2019.9040011. [DOI] [Google Scholar]
- 23.Chen X., Wang Y., Zhou G., Hu X., Han S., Gao J. The combination of nanoscaffolds and stem cell transplantation: Paving a promising road for spinal cord injury regeneration. Biomed. Pharmacother. 2021;143:112233. doi: 10.1016/j.biopha.2021.112233. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y., Zhou J., Wang Y., Fan F., Liu S., Wang Y. Neural Stem/Progenitor Cell Transplantation in Parkinson’s Rodent Animals: A Meta-Analysis and Systematic Review. Stem Cells Transl. Med. 2022;11:383–393. doi: 10.1093/stcltm/szac006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng C.W. Multipotent Mesenchymal Stem Cell-Based Therapies for Spinal Cord Injury: Current Progress and Future Prospects. Biology. 2023;12:653. doi: 10.3390/biology12050653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonson O.E., Domogatskaya A., Volchkov P., Rodin S. The safety of human pluripotent stem cells in clinical treatment. Ann. Med. 2015;47:370–380. doi: 10.3109/07853890.2015.1051579. [DOI] [PubMed] [Google Scholar]
- 27.Deng J., Zhang Y., Xie Y., Zhang L., Tang P. Cell transplantation for spinal cord injury: Tumorigenicity of induced pluripotent stem cell-derived neural stem/progenitor cells. Stem Cells Int. 2018;2018:5653787. doi: 10.1155/2018/5653787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu G., David B.T., Trawczynski M., Fessler R.G. Advances in pluripotent stem cells: History, mechanisms, technologies, and applications. Stem Cell Rev. Rep. 2020;16:3–32. doi: 10.1007/s12015-019-09935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao C., Tarlé S., Kaigler D. Characterization of the immunomodulatory properties of alveolar bone-derived mesenchymal stem cells. Stem Cell Res. Ther. 2020;11:102. doi: 10.1186/s13287-020-01605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni W., Ramalingam M., Li Y., Park J.H., Dashnyam K., Lee J.H., Bloise N., Fassina L., Visai L., De Angelis M.G.C., et al. Immunomodulatory and Anti-inflammatory effect of Neural Stem/Progenitor Cells in the Central Nervous System. Stem Cell Rev. Rep. 2023;19:866–885. doi: 10.1007/s12015-022-10501-1. [DOI] [PubMed] [Google Scholar]
- 31.Chen F.M., Sun H.H., Lu H., Yu Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials. 2012;33:6320–6344. doi: 10.1016/j.biomaterials.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y., Pal S., Hu Q. Recent advances in biomaterial-assisted cell therapy. J. Mater. Chem. B. 2022;10:7222–7238. doi: 10.1039/D2TB00583B. [DOI] [PubMed] [Google Scholar]
- 33.Li G., Li Z., Li L., Liu S., Wu P., Zhou M., Li C., Li X., Luo G., Zhang J. Stem Cell-Niche Engineering via Multifunctional Hydrogel Potentiates Stem Cell Therapies for Inflammatory Bone Loss. Adv. Funct. Mater. 2023;33:2209466. doi: 10.1002/adfm.202209466. [DOI] [Google Scholar]
- 34.Charitos I.A., Ballini A., Cantore S., Boccellino M., Di Domenico M., Borsani E., Nocini R., Di Cosola M., Santacroce L., Bottalico L. Stem cells: A historical review about biological, religious, and ethical issues. Stem Cells Int. 2021;2021:9978837. doi: 10.1155/2021/9978837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarzeczny A., Atkins H., Illes J., Kimmelman J., Master Z., Robillard J.M., Snyder J., Turner L., Zettler P.J., Caulfield T. The stem cell market and policy options: A call for clarity. J. Law Biosci. 2018;5:743–758. doi: 10.1093/jlb/lsy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao A., Tu S., Lu J., Zhang J. Crosstalk between stem cell and spinal cord injury: Pathophysiology and treatment strategies. Stem Cell Res. Ther. 2019;10:238. doi: 10.1186/s13287-019-1357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duncan G.J., Manesh S.B., Hilton B.J., Assinck P., Plemel J.R., Tetzlaff W. The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury. Glia. 2020;68:227–245. doi: 10.1002/glia.23706. [DOI] [PubMed] [Google Scholar]
- 38.Jiang W., Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:e12712. doi: 10.1111/cpr.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyajima H., Itokazu T., Tanabe S., Yamashita T. Interleukin-17A regulates ependymal cell proliferation and functional recovery after spinal cord injury in mice. Cell Death Dis. 2021;12:766. doi: 10.1038/s41419-021-04064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orr M.B., Gensel J.C. Spinal cord injury scarring and inflammation: Therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15:541–553. doi: 10.1007/s13311-018-0631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang B., Zhang F., Cheng F., Ying L., Wang C., Shi K., Wang J., Xia K., Gong Z., Huang X., et al. Strategies and prospects of effective neural circuits reconstruction after spinal cord injury. Cell Death Dis. 2020;11:439. doi: 10.1038/s41419-020-2620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding Y., Chen Q. mTOR pathway: A potential therapeutic target for spinal cord injury. Biomed. Pharmacother. 2022;145:112430. doi: 10.1016/j.biopha.2021.112430. [DOI] [PubMed] [Google Scholar]
- 43.Pang Q.M., Chen S.Y., Fu S.P., Zhou H., Zhang Q., Ao J., Luo X.P., Zhang T. Regulatory role of mesenchymal stem cells on secondary inflammation in spinal cord injury. J. Inflamm. Res. 2022;15:573–593. doi: 10.2147/JIR.S349572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson L.D., Pickard M.R., Johnson W.E. The comparative effects of mesenchymal stem cell transplantation therapy for spinal cord injury in humans and animal models: A systematic review and meta-analysis. Biology. 2021;10:230. doi: 10.3390/biology10030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin M.C., Medress Z.A., Azad T.D., Doulames V.M., Veeravagu A. Stem cell therapies for acute spinal cord injury in humans: A review. Neurosurg. Focus. 2019;46:E10. doi: 10.3171/2018.12.FOCUS18602. [DOI] [PubMed] [Google Scholar]
- 46.Bedir T., Ulag S., Ustundag C.B., Gunduz O. 3D bioprinting applications in neural tissue engineering for spinal cord injury repair. Mater. Sci. Eng. C. 2020;110:110741. doi: 10.1016/j.msec.2020.110741. [DOI] [PubMed] [Google Scholar]
- 47.Szymoniuk M., Mazurek M., Dryla A., Kamieniak P. The application of 3D-bioprinted scaffolds for neuronal regeneration after traumatic spinal cord injury–A systematic review of preclinical in vivo studies. Exp. Neurol. 2023;363:114366. doi: 10.1016/j.expneurol.2023.114366. [DOI] [PubMed] [Google Scholar]
- 48.Xu Z.X., Zhang L.Q., Zhou Y.N., Chen X.M., Xu W.H. Histological and functional outcomes in a rat model of hemisected spinal cord with sustained VEGF/NT-3 release from tissue-engineered grafts. Artif. Cells Nanomed. Biotechnol. 2020;48:362–376. doi: 10.1080/21691401.2019.1709860. [DOI] [PubMed] [Google Scholar]
- 49.Stower H. Cell therapy for spinal cord injury. Nat. Med. 2018;24:1088. doi: 10.1038/s41591-018-0155-y. [DOI] [PubMed] [Google Scholar]
- 50.Oraee-Yazdani S., Hafizi M., Atashi A., Ashrafi F., Seddighi A.S., Hashemi S.M., Seddighi A., Soleimani M., Zali A. Co-transplantation of autologous bone marrow mesenchymal stem cells and Schwann cells through cerebral spinal fluid for the treatment of patients with chronic spinal cord injury: Safety and possible outcome. Spinal Cord. 2016;54:102–109. doi: 10.1038/sc.2015.142. [DOI] [PubMed] [Google Scholar]
- 51.Moviglia G.A., Fernandez Vina R., Brizuela J.A., Saslavsky J., Vrsalovic F., Varela G., Bastos F., Farina P., Etchegaray G., Barbieri M., et al. Combined protocol of cell therapy for chronic spinal cord injury. Report on the electrical and functional recovery of two patients. Cytotherapy. 2006;8:202–209. doi: 10.1080/14653240600736048. [DOI] [PubMed] [Google Scholar]
- 52.Chhabra H.S., Sarda K., Arora M., Sharawat R., Singh V., Nanda A., Sangodimath G.M., Tandon V. Autologous bone marrow cell transplantation in acute spinal cord injury—An Indian pilot study. Spinal Cord. 2016;54:57–64. doi: 10.1038/sc.2015.134. [DOI] [PubMed] [Google Scholar]
- 53.Zeng C.W. Macrophage–Neuroglia Interactions in Promoting Neuronal Regeneration in Zebrafish. Int. J. Mol. Sci. 2023;24:6483. doi: 10.3390/ijms24076483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koffler J., Zhu W., Qu X., Platoshyn O., Dulin J.N., Brock J., Graham L., Lu P., Sakamoto J., Marsala M., et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019;25:263–269. doi: 10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng C.W. Unraveling the Critical Mechanisms and Functions of Neuroglia in Spinal Cord Injuries. Neuroglia. 2023;4:188–190. doi: 10.3390/neuroglia4030013. [DOI] [Google Scholar]
- 56.Vijayavenkataraman S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020;106:54–69. doi: 10.1016/j.actbio.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen L.H., Kudva A.K., Saxena N.S., Roy K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 2011;32:6946–6952. doi: 10.1016/j.biomaterials.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 58.Wei K., Chen X., Li R., Feng Q., Bian L. Multivalent host–guest hydrogels as fatigue-resistant 3D matrix for excessive mechanical stimulation of encapsulated cells. Chem. Mater. 2017;29:8604–8610. doi: 10.1021/acs.chemmater.7b02196. [DOI] [Google Scholar]
- 59.Kerscher P., Turnbull I.C., Hodge A.J., Kim J., Seliktar D., Easley C.J., Costa K.D., Lipke E.A. Direct hydrogel encapsulation of pluripotent stem cells enables ontomimetic differentiation and growth of engineered human heart tissues. Biomaterials. 2016;83:383–395. doi: 10.1016/j.biomaterials.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao C., Tan A., Pastorin G., Ho H.K. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol. Adv. 2013;31:654–668. doi: 10.1016/j.biotechadv.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Salehi S.S., Shamloo A., Hannani S.K. Microfluidic technologies to engineer mesenchymal stem cell aggregates—Applications and benefits. Biophys. Rev. 2020;12:123–133. doi: 10.1007/s12551-020-00613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corti S., Nizzardo M., Nardini M., Donadoni C., Salani S., Ronchi D., Simone C., Falcone M., Papadimitriou D., Locatelli F., et al. Embryonic stem cell-derived neural stem cells improve spinal muscular atrophy phenotype in mice. Brain. 2010;133:465–481. doi: 10.1093/brain/awp318. [DOI] [PubMed] [Google Scholar]
- 63.Jones I., Novikova L.N., Wiberg M., Carlsson L., Novikov L.N. Human embryonic stem cell–derived neural crest cells promote sprouting and motor recovery following spinal cord injury in adult rats. Cell Transplant. 2021;30:0963689720988245. doi: 10.1177/0963689720988245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nori S., Tsuji O., Okada Y., Toyama Y., Okano H., Nakamura M. Therapeutic potential of induced pluripotent stem cells for spinal cord injury. Brain Nerve = Shinkei Kenkyu No Shinpo. 2012;64:17–27. [PubMed] [Google Scholar]
- 65.Li Y., Shen P.P., Wang B. Induced pluripotent stem cell technology for spinal cord injury: A promising alternative therapy. Neural Regen. Res. 2021;16:1500. doi: 10.4103/1673-5374.303013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang D., He X. A meta-analysis of the motion function through the therapy of spinal cord injury with intravenous transplantation of bone marrow mesenchymal stem cells in rats. PLoS ONE. 2014;9:e93487. doi: 10.1371/journal.pone.0093487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Z., Zhao W., Liu W., Zhou Y., Jia J., Yang L. Transplantation of placenta-derived mesenchymal stem cell-induced neural stem cells to treat spinal cord injury. Neural Regen. Res. 2014;9:2197. doi: 10.4103/1673-5374.147953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McMahon S.S., Albermann S., Rooney G.E., Shaw G., Garcia Y., Sweeney E., Hynes J., Dockery P., O’Brien T., Windebank A.J., et al. Engraftment, migration and differentiation of neural stem cells in the rat spinal cord following contusion injury. Cytotherapy. 2010;12:313–325. doi: 10.3109/14653241003695018. [DOI] [PubMed] [Google Scholar]
- 69.Tang Y., Cui Y.C., Wang X.J., Wu A.L., Hu G.F., Luo F.L., Sun J., Sun J., Wu L. Neural progenitor cells derived from adult bone marrow mesenchymal stem cells promote neuronal regeneration. Life Sci. 2012;91:951–958. doi: 10.1016/j.lfs.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 70.Cusimano M., Biziato D., Brambilla E., Donega M., Alfaro-Cervello C., Snider S., Salani G., Pucci F., Comi G., Garcia-Verdugo J.M., et al. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 2012;135:447–460. doi: 10.1093/brain/awr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo S., Wang L., Xie Y., Luo X., Zhang S., Xiong L., Ai H., Yuan Z., Wang J. Bibliometric and visualized analysis of stem cells therapy for spinal cord injury based on Web of Science and CiteSpace in the last 20 years. World Neurosurg. 2019;132:e246–e258. doi: 10.1016/j.wneu.2019.08.191. [DOI] [PubMed] [Google Scholar]
- 72.Coutts M., Keirstead H.S. Stem cells for the treatment of spinal cord injury. Exp. Neurol. 2008;209:368–377. doi: 10.1016/j.expneurol.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 73.Soto D.A., Navarro M., Zheng C., Halstead M.M., Zhou C., Guiltinan C., Wu J., Ross P.J. Simplification of culture conditions and feeder-free expansion of bovine embryonic stem cells. Sci. Rep. 2021;11:11045. doi: 10.1038/s41598-021-90422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barbuti P.A., Barker R.A., Brundin P., Przedborski S., Papa S.M., Kalia L.V., Mochizuki H. Recent advances in the development of stem-cell-derived dopaminergic neuronal transplant therapies for Parkinson’s disease. Mov. Disord. 2021;36:1772–1780. doi: 10.1002/mds.28628. [DOI] [PubMed] [Google Scholar]
- 75.Ebrahimi T., Abasi M., Seifar F., Eyvazi S., Hejazi M.S., Tarhriz V., Montazersaheb S. Transplantation of stem cells as a potential therapeutic strategy in neurodegenerative disorders. Curr. Stem Cell Res. Ther. 2021;16:133–144. doi: 10.2174/22123946MTA33NzUey. [DOI] [PubMed] [Google Scholar]
- 76.Kossow S. Creating a united front: Harmonizing the united states regulatory policies surrounding human embryonic stem cell research. SMU Sci. Technol. Law Rev. 2022;25:295. doi: 10.25172/smustlr.25.2.7. [DOI] [Google Scholar]
- 77.Otsuka R., Wada H., Murata T., Seino K.I. Immune reaction and regulation in transplantation based on pluripotent stem cell technology. Inflamm. Regen. 2020;40:12. doi: 10.1186/s41232-020-00125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haworth R., Sharpe M. Accept or reject: The role of immune tolerance in the development of stem cell therapies and possible future approaches. Toxicol. Pathol. 2021;49:1308–1316. doi: 10.1177/0192623320918241. [DOI] [PubMed] [Google Scholar]
- 79.Gong Z., Xia K., Xu A., Yu C., Wang C., Zhu J., Huang X., Chen Q., Li F., Liang C., et al. Stem cell transplantation: A promising therapy for spinal cord injury. Curr. Stem Cell Res. Ther. 2020;15:321–331. doi: 10.2174/1574888X14666190823144424. [DOI] [PubMed] [Google Scholar]
- 80.Chang E.A., Jin S.W., Nam M.H., Kim S.D. Human induced pluripotent stem cells: Clinical significance and applications in neurologic diseases. J. Korean Neurosurg. Soc. 2019;62:493–501. doi: 10.3340/jkns.2018.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Basso D.M., Beattie M.S., Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 82.Fehlings M.G., Tator C.H. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp. Neurol. 1995;132:220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 83.McDonald J.W., Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 84.Tetzlaff W., Okon E.B., Karimi-Abdolrezaee S., Hill C.E., Sparling J.S., Plemel J.R., Plunet W.T., Tsai E.C., Baptiste D.C., Smithson L.J., et al. A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma. 2011;28:1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones L.L., Margolis R.U., Tuszynski M.H. The chondroitin sulfate proteoglycans neurocana, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp. Neurol. 2003;182:399–411. doi: 10.1016/S0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 86.Assinck P., Duncan G.J., Hilton B.J., Plemel J.R., Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017;20:637–647. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 87.Gao L., Peng Y., Xu W., He P., Li T., Lu X., Chen G. Progress in stem cell therapy for spinal cord injury. Stem Cells Int. 2020;2020:2853650. doi: 10.1155/2020/2853650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oliveri R.S., Bello S., Biering-Sørensen F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: Systematic review with meta-analyses of rat models. Neurobiol. Dis. 2014;62:338–353. doi: 10.1016/j.nbd.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 89.Yang C., Li X., Sun L., Guo W., Tian W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J. Neural Eng. 2017;14:026005. doi: 10.1088/1741-2552/aa596b. [DOI] [PubMed] [Google Scholar]
- 90.Nagoshi N., Tsuji O., Nakamura M., Okano H. Cell therapy for spinal cord injury using induced pluripotent stem cells. Regen. Ther. 2019;11:75–80. doi: 10.1016/j.reth.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dwivedi S., Choudhary P., Gupta A., Singh S. Therapeutical growth in oligodendroglial fate induction via transdifferentiation of stem cells for neuroregenerative therapy. Biochimie. 2023;211:35–56. doi: 10.1016/j.biochi.2023.02.012. [DOI] [PubMed] [Google Scholar]
- 92.Yu L., Liu S., Wang C., Zhang C., Wen Y., Zhang K., Shang C., Hu H., Liu Y., Wu L., et al. Embryonic stem cell-derived extracellular vesicles promote the recovery of kidney injury. Stem Cell Res. Ther. 2021;12:379. doi: 10.1186/s13287-021-02460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu J.X., Xia T., She L.P., Lin S., Luo X.M. Stem cell therapies for human infertility: Advantages and challenges. Cell Transplant. 2022;31:09636897221083252. doi: 10.1177/09636897221083252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Belviso I., Romano V., Nurzynska D., Castaldo C., Di Meglio F. Biomechanics and Functional Tissue Engineering. IntechOpen; London, UK: 2020. Non-integrating methods to produce induced pluripotent stem cells for regenerative medicine: An overview. [Google Scholar]
- 95.Gorecka J., Kostiuk V., Fereydooni A., Gonzalez L., Luo J., Dash B., Isaji T., Ono S., Liu S., Lee S.R., et al. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res. Ther. 2019;10:87. doi: 10.1186/s13287-019-1185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Y.P., Hsiao Y.J., Chang K.J., Foustine S., Ko Y.L., Tsai Y.C., Tai H.Y., Ko Y.C., Chiou S.H., Lin T.C., et al. Pluripotent stem cells in clinical cell transplantation: Focusing on induced pluripotent stem cell-derived RPE cell therapy in age-related macular degeneration. Int. J. Mol. Sci. 2022;23:13794. doi: 10.3390/ijms232213794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kobayashi Y., Okada Y., Itakura G., Iwai H., Nishimura S., Yasuda A., Nori S., Hikishima K., Konomi T., Fujiyoshi K., et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS ONE. 2012;7:e52787. doi: 10.1371/journal.pone.0052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen H., Fan C., You Z., Xiao Z., Zhao Y., Dai J. Advances in biomaterial-based spinal cord injury repair. Adv. Funct. Mater. 2022;32:2110628. doi: 10.1002/adfm.202110628. [DOI] [Google Scholar]
- 99.Lee A.S., Tang C., Rao M.S., Weissman I.L., Wu J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shamsian A., Sahebnasagh R., Norouzy A., Hussein S.H., Ghahremani M.H., Azizi Z. Cancer cells as a new source of induced pluripotent stem cells. Stem Cell Res. Ther. 2022;13:459. doi: 10.1186/s13287-022-03145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paolini Sguazzi G., Muto V., Tartaglia M., Bertini E., Compagnucci C. Induced pluripotent stem cells (iPSCs) and gene therapy: A new era for the treatment of neurological diseases. Int. J. Mol. Sci. 2021;22:13674. doi: 10.3390/ijms222413674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haridhasapavalan K.K., Borgohain M.P., Dey C., Saha B., Narayan G., Kumar S., Thummer R.P. An insight into non-integrative gene delivery approaches to generate transgene-free induced pluripotent stem cells. Gene. 2019;686:146–159. doi: 10.1016/j.gene.2018.11.069. [DOI] [PubMed] [Google Scholar]
- 103.Huang Z., Powell R., Phillips J.B., Haastert-Talini K. Perspective on schwann cells derived from induced pluripotent stem cells in peripheral nerve tissue engineering. Cells. 2020;9:2497. doi: 10.3390/cells9112497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scesa G., Adami R., Bottai D. iPSC preparation and epigenetic memory: Does the tissue origin matter? Cells. 2021;10:1470. doi: 10.3390/cells10061470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heo J.S., Choi Y., Kim H.S., Kim H.O. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int. J. Mol. Med. 2016;37:115–125. doi: 10.3892/ijmm.2015.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Z., Jiang C.M., An S., Cheng Q., Huang Y.F., Wang Y.T., Gou Y.C., Xiao L., Yu W.J., Wang J., et al. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2014;20:25–34. doi: 10.1111/odi.12086. [DOI] [PubMed] [Google Scholar]
- 107.Kumar P., Kandoi S., Misra R., Vijayalakshmi S., Rajagopal K., Verma R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1–9. doi: 10.1016/j.cytogfr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 108.Shang L., Shao J., Ge S. Immunomodulatory functions of oral mesenchymal stem cells: Novel force for tissue regeneration and disease therapy. J. Leukoc. Biol. 2021;110:539–552. doi: 10.1002/JLB.3MR0321-766R. [DOI] [PubMed] [Google Scholar]
- 109.Lv B., Zhang X., Yuan J., Chen Y., Ding H., Cao X., Huang A. Biomaterial-supported MSC transplantation enhances cell–cell communication for spinal cord injury. Stem Cell Res. Ther. 2021;12:36. doi: 10.1186/s13287-020-02090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Y., Dong N., Hong H., Qi J., Zhang S., Wang J. Mesenchymal stem cells: Therapeutic mechanisms for stroke. Int. J. Mol. Sci. 2022;23:2550. doi: 10.3390/ijms23052550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fan L., Liu C., Chen X., Zheng L., Zou Y., Wen H., Guan P., Lu F., Luo Y., Tan G., et al. Exosomes-Loaded Electroconductive Hydrogel Synergistically Promotes Tissue Repair after Spinal Cord Injury via Immunoregulation and Enhancement of Myelinated Axon Growth. Adv. Sci. 2022;9:2105586. doi: 10.1002/advs.202105586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pang Q.M., Deng K.Q., Zhang M., Wu X.C., Yang R.L., Fu S.P., Lin F.Q., Zhang Q., Ao J., Zhang T. Multiple strategies enhance the efficacy of MSCs transplantation for spinal cord injury. Biomed. Pharmacother. 2023;157:114011. doi: 10.1016/j.biopha.2022.114011. [DOI] [PubMed] [Google Scholar]
- 113.Zheng Q., Zhang S., Guo W.Z., Li X.K. The unique immunomodulatory properties of MSC-derived exosomes in organ transplantation. Front. Immunol. 2021;12:659621. doi: 10.3389/fimmu.2021.659621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Y., Tang Z., Gu P. Stem/progenitor cell-based transplantation for retinal degeneration: A review of clinical trials. Cell Death Dis. 2020;11:793. doi: 10.1038/s41419-020-02955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hu C., Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell. Mol. Med. 2018;22:1428–1442. doi: 10.1111/jcmm.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shafiq M., Jung Y., Kim S.H. Insight on stem cell preconditioning and instructive biomaterials to enhance cell adhesion, retention, and engraftment for tissue repair. Biomaterials. 2016;90:85–115. doi: 10.1016/j.biomaterials.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 117.Chen Z., Chen L., Zeng C., Wang W.E. Functionally improved mesenchymal stem cells to better treat myocardial infarction. Stem Cells Int. 2018;2018:7045245. doi: 10.1155/2018/7045245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.García-Sánchez D., Fernández D., Rodríguez-Rey J.C., Pérez-Campo F.M. Enhancing survival, engraftment, and osteogenic potential of mesenchymal stem cells. World J. Stem Cells. 2019;11:748. doi: 10.4252/wjsc.v11.i10.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu B., Yin M., Yang Y., Zou Y., Liu W., Qiao L., Zhang J., Wang Z., Wu Y., Shen H., et al. Transplantation of neural stem progenitor cells from different sources for severe spinal cord injury repair in rat. Bioact. Mater. 2023;23:300–313. doi: 10.1016/j.bioactmat.2022.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Finkel Z., Esteban F., Rodriguez B., Fu T., Ai X., Cai L. Diversity of adult neural stem and progenitor cells in physiology and disease. Cells. 2021;10:2045. doi: 10.3390/cells10082045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pieczonka K., Fehlings M.G. Incorporating combinatorial approaches to encourage targeted neural stem/progenitor cell integration following transplantation in spinal cord injury. Stem Cells Transl. Med. 2023;12:207–214. doi: 10.1093/stcltm/szad008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jeong S.K., Choi I., Jeon S.R. Current status and future strategies to treat spinal cord injury with adult stem cells. J. Korean Neurosurg. Soc. 2020;63:153–162. doi: 10.3340/jkns.2019.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yavarpour-Bali H., Nakhaei-Nejad M., Yazdi A., Ghasemi-Kasman M. Direct conversion of somatic cells towards oligodendroglial lineage cells: A novel strategy for enhancement of myelin repair. J. Cell. Physiol. 2020;235:2023–2036. doi: 10.1002/jcp.29195. [DOI] [PubMed] [Google Scholar]
- 124.Mothe A.J., Tator C.H. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int. J. Dev. Neurosci. 2013;31:701–713. doi: 10.1016/j.ijdevneu.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 125.Pereira I.M., Marote A., Salgado A.J., Silva N.A. Filling the gap: Neural stem cells as a promising therapy for spinal cord injury. Pharmaceuticals. 2019;12:65. doi: 10.3390/ph12020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yuan T., Liu Q., Kang J., Gao H., Gui S. High-dose neural stem/progenitor cell transplantation increases engraftment and neuronal distribution and promotes functional recovery in rats after acutely severe spinal cord injury. Stem Cells Int. 2019;2019:9807978. doi: 10.1155/2019/9807978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang X., Rivera-Bolanos N., Jiang B., Ameer G.A. Advanced functional biomaterials for stem cell delivery in regenerative engineering and medicine. Adv. Funct. Mater. 2019;29:1809009. doi: 10.1002/adfm.201809009. [DOI] [Google Scholar]
- 128.Liu D., Bobrovskaya L., Zhou X.F. Cell therapy for neurological disorders: The perspective of promising cells. Biology. 2021;10:1142. doi: 10.3390/biology10111142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Torres-Espín A., Hernández J., Navarro X. Gene expression changes in the injured spinal cord following transplantation of mesenchymal stem cells or olfactory ensheathing cells. PLoS ONE. 2013;8:e76141. doi: 10.1371/journal.pone.0076141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brennan F.H., Popovich P.G. Emerging targets for reprograming the immune response to promote repair and recovery of function after spinal cord injury. Curr. Opin. Neurol. 2018;31:334–344. doi: 10.1097/WCO.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 131.Kang E.S., Ha K.Y., Kim Y.H. Fate of transplanted bone marrow derived mesenchymal stem cells following spinal cord injury in rats by transplantation routes. J. Korean Med. Sci. 2012;27:586–593. doi: 10.3346/jkms.2012.27.6.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Allison D.J., Ditor D.S. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord. 2015;53:14–18. doi: 10.1038/sc.2014.184. [DOI] [PubMed] [Google Scholar]
- 133.Zhuang X., Hu X., Zhang S., Li X., Yuan X., Wu Y. Mesenchymal stem cell–based therapy as a new approach for the treatment of systemic sclerosis. Clin. Rev. Allergy Immunol. 2022;64:284–320. doi: 10.1007/s12016-021-08892-z. [DOI] [PubMed] [Google Scholar]
- 134.Hoang D.M., Pham P.T., Bach T.Q., Ngo A.T., Nguyen Q.T., Phan T.T., Nguyen G.H., Le P.T., Hoang V.T., Forsyth N.R., et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022;7:272. doi: 10.1038/s41392-022-01134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rheault-Henry M., White I., Grover D., Atoui R. Stem cell therapy for heart failure: Medical breakthrough, or dead end? World J. Stem Cells. 2021;13:236. doi: 10.4252/wjsc.v13.i4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Attia N., Mashal M., Puras G., Pedraz J.L. Mesenchymal stem cells as a gene delivery tool: Promise, problems, and prospects. Pharmaceutics. 2021;13:843. doi: 10.3390/pharmaceutics13060843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kong X., Gao J. Macrophage polarization: A key event in the secondary phase of acute spinal cord injury. J. Cell. Mol. Med. 2017;21:941–954. doi: 10.1111/jcmm.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zipser C.M., Cragg J.J., Guest J.D., Fehlings M.G., Jutzeler C.R., Anderson A.J., Curt A. Cell-based and stem-cell-based treatments for spinal cord injury: Evidence from clinical trials. Lancet Neurol. 2022;21:659–670. doi: 10.1016/S1474-4422(21)00464-6. [DOI] [PubMed] [Google Scholar]
- 139.Okano H., Ogawa Y., Nakamura M., Kaneko S., Iwanami A., Toyama Y. Seminars in Cell Developmental Biology. Volume 14, Number 3. Academic Press; Cambridge, MA, USA: 2003. Transplantation of neural stem cells into the spinal cord after injury; pp. 191–198. [DOI] [PubMed] [Google Scholar]
- 140.Syková E., Homola A., Mazanec R., Lachmann H., Konrádová Š.L., Kobylka P., Pádr R., Neuwirth J., Komrska V., Vávra V., et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15:675–687. doi: 10.3727/000000006783464381. [DOI] [PubMed] [Google Scholar]
- 141.Dai G., Liu X., Zhang Z., Wang X., Li M., Cheng H., Hua R., Shi J., Wang R., Qin C., et al. Comparative analysis of curative effect of CT-guided stem cell transplantation and open surgical transplantation for sequelae of spinal cord injury. J. Transl. Med. 2013;11:315. doi: 10.1186/1479-5876-11-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kharbikar B.N., Mohindra P., Desai T.A. Biomaterials to enhance stem cell transplantation. Cell Stem Cell. 2022;29:692–721. doi: 10.1016/j.stem.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Smith L.J., Silverman L., Sakai D., Le Maitre C.L., Mauck R.L., Malhotra N.R., Lotz J.C., Buckley C.T. Advancing cell therapies for intervertebral disc regeneration from the lab to the clinic: Recommendations of the ORS spine section. JOR Spine. 2018;1:e1036. doi: 10.1002/jsp2.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Riha S.M., Maarof M., Fauzi M.B. Synergistic effect of biomaterial and stem cell for skin tissue engineering in cutaneous wound healing: A concise review. Polymers. 2021;13:1546. doi: 10.3390/polym13101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vonk L.A., De Windt T.S., Slaper-Cortenbach I.C., Saris D.B. Autologous, allogeneic, induced pluripotent stem cell or a combination stem cell therapy? Where are we headed in cartilage repair and why: A concise review. Stem Cell Res. Ther. 2015;6:94. doi: 10.1186/s13287-015-0086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pajer K., Bellák T., Redl H., Nógrádi A. Neuroectodermal stem cells grafted into the injured spinal cord induce both axonal regeneration and morphological restoration via multiple mechanisms. J. Neurotrauma. 2019;36:2977–2990. doi: 10.1089/neu.2018.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stenudd M., Sabelström H., Frisén J. Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurol. 2015;72:235–237. doi: 10.1001/jamaneurol.2014.2927. [DOI] [PubMed] [Google Scholar]
- 148.Tong S., Moyo B., Lee C.M., Leong K., Bao G. Engineered materials for in vivo delivery of genome-editing machinery. Nat. Rev. Mater. 2019;4:726–737. doi: 10.1038/s41578-019-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee J., Bayarsaikhan D., Bayarsaikhan G., Kim J.S., Schwarzbach E., Lee B. Recent advances in genome editing of stem cells for drug discovery and therapeutic application. Pharmacol. Ther. 2020;209:107501. doi: 10.1016/j.pharmthera.2020.107501. [DOI] [PubMed] [Google Scholar]
- 150.Ferrari G., Thrasher A.J., Aiuti A. Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet. 2021;22:216–234. doi: 10.1038/s41576-020-00298-5. [DOI] [PubMed] [Google Scholar]
- 151.Hussey G.S., Dziki J.L., Badylak S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018;3:159–173. doi: 10.1038/s41578-018-0023-x. [DOI] [Google Scholar]
- 152.Wang J., Kong X., Li Q., Li C., Yu H., Ning G., Xiang Z., Liu Y., Feng S. The spatial arrangement of cells in a 3D-printed biomimetic spinal cord promotes directional differentiation and repairs the motor function after spinal cord injury. Biofabrication. 2021;13:045016. doi: 10.1088/1758-5090/ac0c5f. [DOI] [PubMed] [Google Scholar]
- 153.Zhu Y., Li X., Janairo R.R.R., Kwong G., Tsou A.D., Chu J.S., Wang A., Yu J., Wang D., Li S. Matrix stiffness modulates the differentiation of neural crest stem cells in vivo. J. Cell. Physiol. 2019;234:7569–7578. doi: 10.1002/jcp.27518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Deng W.S., Ma K., Liang B., Liu X.Y., Xu H.Y., Zhang J., Shi H., Sun H., Chen X., Zhang S. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen. Res. 2020;15:1686. doi: 10.4103/1673-5374.276340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schira J., Gasis M., Estrada V., Hendricks M., Schmitz C., Trapp T., Kruse F., Kögler G., Wernet P., Hartung H., et al. Significant clinical, neuropathological and behavioural recovery from acute spinal cord trauma by transplantation of a well-defined somatic stem cell from human umbilical cord blood. Brain. 2012;135:431–446. doi: 10.1093/brain/awr222. [DOI] [PubMed] [Google Scholar]
- 156.Rosenfeld J.V., Gillett G.R. Ethics, stem cells and spinal cord repair. Med. J. Aust. 2004;180:637–639. doi: 10.5694/j.1326-5377.2004.tb06128.x. [DOI] [PubMed] [Google Scholar]
- 157.Hashimoto S., Nagoshi N., Shinozaki M., Nakanishi K., Suematsu Y., Shibata T., Kawai M., Kitagawa T., Ago K., Kamata Y., et al. Microenvironmental modulation in tandem with human stem cell transplantation enhances functional recovery after chronic complete spinal cord injury. Biomaterials. 2023;295:122002. doi: 10.1016/j.biomaterials.2023.122002. [DOI] [PubMed] [Google Scholar]
- 158.Shibata T., Tashiro S., Shibata S., Shinozaki M., Shindo T., Hashimoto S., Kawai M., Kitagawa T., Ago K., Matsumoto M., et al. Rehabilitative training enhances therapeutic effect of human-iPSC-derived neural stem/progenitor cells transplantation in chronic spinal cord injury. Stem Cells Transl. Med. 2023;12:83–96. doi: 10.1093/stcltm/szac089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.