Abstract
Introduction: Unilateral pulmonary edema (UPE) is a potential complication after mitral valve surgery (MVS), and its cause is not yet fully understood. Definitions are inconsistent, and previous studies have reported wide variance in the incidence of UPE. This research aims at the evaluation of the Radiographic Assessment of Lung Edema (RALE) score concerning assessment of UPE after MVS in order to provide an accurate and consistent definition of this pathology. Methods and Results: Postoperative chest X-ray images of 676 patients after MVS (minimally invasive MVS, n = 434; conventional MVS, n = 242) were retrospectively analyzed concerning presence of UPE. UPE was diagnosed only after exclusion of other pathologies up until the eighth postoperative day. RALE values were calculated for each patient. ROC analysis was performed to assess diagnostic performance. UPE was diagnosed in 18 patients (2.8%). UPE occurred significantly more often in the MI-MVS group (p = 0.045; MI-MVS n = 15; C-MVS n = 3). Postoperative RALE values for the right hemithorax (Q1 + Q2) > 12 and the right-to-left RALE difference ((Q1 + Q2) − (Q3 + Q4)) > 13 provide a sensitivity of up to 100% and 94.4% and a specificity of up to 88.4% and 94.2% for UPE detection. Conclusion: The RALE score is a practical tool for assessment of chest X-ray images after MVS with regard to UPE and provides a clear definition of UPE. In addition, it enables objective comparability when assessing of the postoperative course. The given score thresholds provide a sensitivity and specificity of up to 94%. Further, UPE after MVS seems to be a rather rare pathology with an incidence of 2.6%.
Keywords: unilateral pulmonary edema (UPE), Radiographic Assessment of Lung Edema (RALE), mitral valve surgery (MVS), mitral valve surgery complications
1. Introduction
Unilateral pulmonary edema (UPE) is a potentially life-threatening complication observed in the postoperative period following mitral valve surgery (MVS). MVS includes mitral valve reconstruction or replacement as established therapies for mitral regurgitation.
MVS includes two main surgical techniques that differ in their approach to the mitral valve, but both require intraoperative cardiopulmonary bypass. The conventional median sternotomy approach (C-MVS) is associated with a high risk of intra- and postoperative complications, including bleeding in need of transfusion [1]. Alternatively, MVS can be performed minimally invasively (MI-MVS) via a mini-thoracotomy at the right lateral hemithorax to reduce surgical trauma. Previous studies have shown that MI-MVS has a comparatively low mortality rate, and similar perioperative outcomes have been reported [2,3]. In addition, MI-MVS has been documented to have a shorter hospital length of stay, less postoperative pain and better cosmetic results [2,3]. However, a known complication after MI-MVS is the development of UPE, most commonly affecting the right lung. Clinical signs of this complication can be evident in varying degree, manifesting themselves as respiratory or hemodynamic deterioration. In severe cases, oxygenation impairment and cardiac failure can result in requirement of extracorporeal life support, which is associated with an increased mortality rate [4,5]. However, the pathogenesis of UPE after MVS is not well understood. Several studies have discussed different pathogenetic pathways, with pulmonary re-expansion edema being considered the most likely reason; however, inflammatory processes, pulmonary microvasculature abnormalities, and an association with prolonged cardiopulmonary bypass (CPB) have been mentioned [6,7]. If CPB time were to have an influence on the development of UPE, it would seem reasonable to assume that it would also occur after C-MVS. However, to our knowledge, the occurrence of UPE after C-MVS has not yet been mentioned in the literature. Finally, the exact causes and pathogenesis of UPE remain unclear. The reported incidence of UPE after MI-MVS varies from 1.6% up to 28% [4,5,8] (Table S1). This may be due to differences in the patient population, surgical expertise and procedures, or postoperative management. However, the main reason for this discrepancy may be the lack of precise diagnostic criteria for UPE. UPE is primarily diagnosed upon chest X-ray imaging, and previous studies have reported several diagnostic features, including unilateral opacities > 20% in the first 24 h after surgery, as highly suggestive of UPE. However, in the immediate postoperative setting after MI-MVS, various conditions associated with unilateral opacities may mimic UPE and be erroneously defined as such. This may lead to a significant diagnostic bias and distort the incidence of UPE [9,10]. In this study, we investigated the incidence of UPE after MI-MVS and C-MVS on chest X-ray, considering all available imaging studies completed within the first eight postoperative days, and used the Radiographic Assessment of Lung Edema (RALE) score for quantitative assessment. The RALE score was originally validated to assess the severity of lung edema in acute respiratory distress syndrome (ARDS) [11].
2. Material and Methods
This study was conducted according to the declaration of Helsinki and was approved by our local institutional ethics board (No. AZ-D-559/18). All patients gave written informed consent before enrolment.
2.1. Study Population
All patients who underwent elective MVS at our center between 2008 and 2022 were included in this study. Patients were divided into two groups according to the surgical technique of MVS: conventional approach via a median sternotomy (C-MVS) or minimally invasive approach via a mini-thoracotomy (MI-MVS). Demographic and periprocedural data were collected for each group. All patients in both groups were screened for UPE.
2.2. Chest X-ray Analysis and Diagnosis of UPE
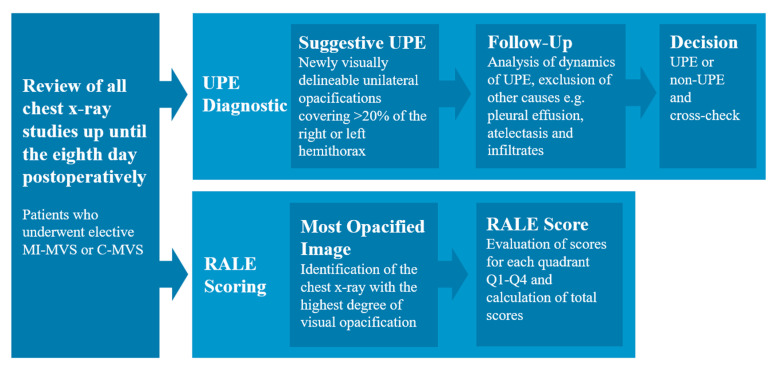
The chest X-ray images were assessed by two radiologists (Karim Mostafa, Svea Seehafer). According to the clinical standard, all patients received a chest X-ray immediately after surgery and at least once a day during the further postoperative course. The chest X-rays were evaluated until the eighth postoperative day. The diagnosis of UPE was made as follows: All available X-ray studies and thoracic CT imaging up until the eighth postoperative day were reviewed. Preoperative imaging studies were used as a baseline for comparison. Any newly visually delineable unilateral opacifications covering >20% of the right or left hemithorax within eight postoperative days were considered as potential evidence of UPE. Next, all available chest X-ray and thoracic CT imaging studies up until the eighth postoperative day were specifically assessed in order to identify other causes such as pleural effusion, atelectasis or pneumonia, and other causes of unilateral opacities, and to assess the dynamics of these conditions. Finally, only cases where the mentioned conditions were excluded and UPE remained as the only possible explanation for the findings were considered as UPE (Figure 1 and Figure 2). Finally, all cases of UPE were cross-checked between the readers to further ensure the accuracy of the diagnosis and to limit false-positive findings.
Figure 1.
Flowchart for diagnosis of UPE and RALE scoring.
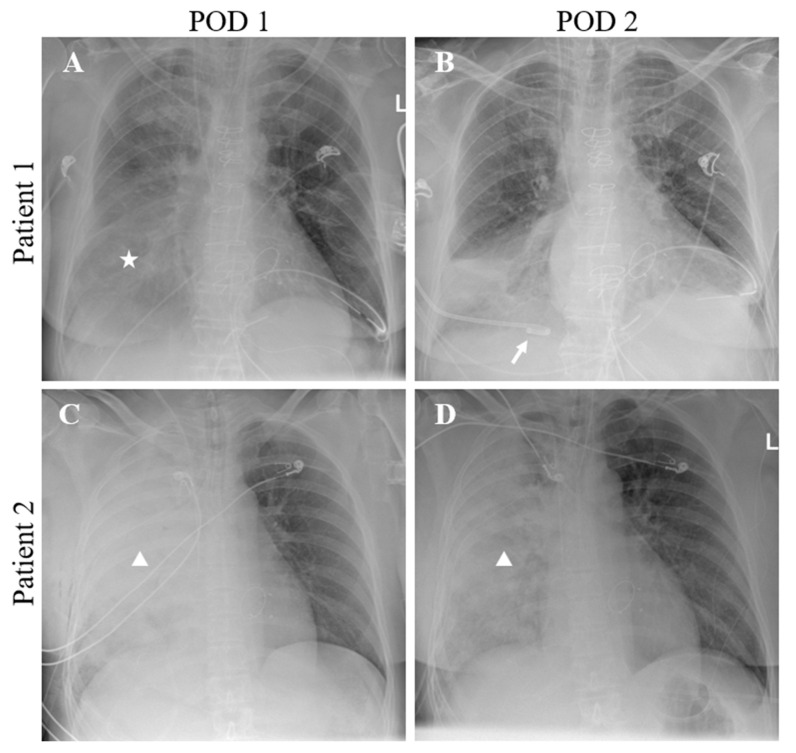
Figure 2.
Two different clinical situations after MVS with unilateral opacities on chest X-ray. The first postoperative chest X-ray (A) of a patient after MVS shows unilateral opacities of the right hemithorax (star), which resolves after insertion of a pleural drain (Arrow, (B)). Therefore, the cause of the opacities can be attributed to pleural effusion with atelectasis rather than UPE. In another patient, chest X-ray on the first (C) and second day (D) after surgery showed a persisting consolidated unilateral opacity of the right hemithorax (triangles), suggesting UPE.
The diagnostic assessment of UPE was performed by reviewing all available imaging studies up to the eighth POD to best ensure that other causes of unilateral opacities on chest X-ray were excluded. The RALE score was then calculated for the most opacified chest X-ray within these 8 PODs.
2.3. Rale Score Evaluation for Diagnosis of UPE
The RALE score divides the chest X-ray into four quadrants using the vertebral column and the first branch of the left main bronchus as a crosshair boundary [11]. The resulting quadrants are described as Q1–Q4, whereby Q1 represents the upper right quadrant, Q2 represents the lower right quadrant, Q3 represents the upper left quadrant, and Q4 represents the lower left quadrant. Scores are calculated for each quadrant by multiplying the degree of consolidation (0–4) by the density of opacities (1–3) and summing the values for each quadrant (Supplementary Figure S1). The maximum possible score is 12 for each quadrant, 24 for each hemithorax, and 48 for the whole thorax. Score values for the whole thorax, each hemithorax, and each quadrant, and score differences between the right and left hemithorax were noted.
In each patient, the RALE score was calculated for the chest X-ray with the highest degree of visual opacities available within the first eight PODs. Receiver operating characteristic (ROC) analysis was then performed to assess the diagnostic performance of the RALE scoring system in patients with known UPE as defined above. We report cut-off values, sensitivity, specificity, and AUC values for the right-sided RALE score and the difference in score values between the right and left hemithorax ((Q1 + Q2) − (Q3 + Q4)). The cut-off values with the highest possible sensitivity and specificity were determined by Youden’s index.
2.4. Statistical Analysis
All statistical analyses were performed using The Jamovi project, version 2.5.5, for Windows (Sydney, Australia, 2021) [12]. Ordinal and non-normally distributed data are presented as medians with interquartiles and were evaluated using the Mann–Whitney U-test as indicated. ROC analysis was performed as described. Statistical significance was set at alpha < 0.05 for all tests.
3. Results
3.1. Study Population
A total of 676 patients were included in this analysis, of whom 434 underwent MI-MVS and 242 underwent C-MVS. Baseline demographic characteristics are summarized in Table 1. We found significant differences between the two groups in the median age of the patients and the duration of the surgical procedures.
Table 1.
Characteristics of the study population with subgroup comparisons. p-values were calculated using the Mann–Whitney U-test.
| Overall MVS (n = 676) |
MI-MVS (n = 434) |
C-MVS (n = 242) |
p-Value | |
|---|---|---|---|---|
| Demographic data | ||||
| Female gender, n (%) | 275 (40.7) | 175 (40.3) | 100 (41.3) | 0.800 |
| Age (years) | 65 [56, 74] | 64 [54, 73] | 68.5 [59, 75] | <0.001 |
| Weight (kg) | 77 [67, 89.3] | 75.5 [66, 89] | 78 [67, 89.8] | 0.308 |
| Height (cm) | 174 [167, 181] | 174 [167, 182] | 172 [166, 180] | 0.328 |
| Duration of procedure (minutes) | 280 [243, 320] | 287 [257, 322] | 261 [214, 313] | <0.001 |
3.2. Chest X-ray Analysis and Diagnosis of UPE
In total, approximately 7000 chest X-ray images were evaluated. Overall, we diagnosed UPE in 18/676 (2.6%) cases using the RALE score. In the MI-MVS group, we found 15/434 (3.5%) cases of UPE, compared to 3/242 (1.2%) in the C-MVS group. Thus, UPE was significantly more common in the MI-MVS group than in the C-MVS group (p = 0.043, Table 2). The three UPE patients in the C-MVS group were treated for moderate and severe mitral valve insufficiency. In one patient, closure of a patent foramen ovale was performed in the same setting. However, beyond arterial hypertension, coronary and peripheral arterial atherosclerosis, and hypercholesterinemia, no common pathology was present that could be suspected as cause for UPE.
Table 2.
Occurrence of UPE in MI-MVS, C-MVS, and in total. p-value was calculated by X2-test.
| Overall MVS (n = 676) | MI-MVS (n = 434) | C-MVS (n = 242) | p-Value | |
|---|---|---|---|---|
| UPE, n (%) | 18 (2.6) | 15 (3.5) | 3 (1.2) | 0.043 |
3.3. RALE Scoring of Chest X-ray and ROC Analysis Results
In 96% of all patients (n = 676), the most opacified chest X-ray was found within the first 72 h postoperatively. The median RALE values for all four quadrants, both hemithoraces, and the RALE values of the whole chest X-ray for the UPE and non-UPE groups are shown in Table 3. In the UPE group, we found significantly higher values in Q1 and Q2, and a greater difference between the right (Q1 + Q2) and left hemithorax (Q3 + Q4) with a p-value of <0.001.
Table 3.
RALE scoring of chest X-ray images. All calculated scores are presented as median with interquartile range for UPE and non-UPE groups. The p-value was calculated using the Mann–Whitney U-test.
| Non-UPE (n = 658) | UPE (n = 18) | p-Value | |
|---|---|---|---|
| Scores | |||
| Q1 | 0 [0, 0] | 12 [12, 12] | <0.001 |
| Q2 | 2 [0, 4] | 12 [12, 12] | <0.001 |
| Q3 | 0 [0, 0] | 0 [0, 0] | 0.543 |
| Q4 | 0 [0, 3] | 0 [0, 3.5] | 0.937 |
| Right hemithorax (Q1 + Q2) | 2 [0, 6] | 24 [24, 24] | 0.001 |
| Left hemithorax (Q3 + Q4) | 0 [0, 4] | 0 [0, 5] | 0.952 |
| RALE score total | 4 [0, 10] | 24 [24, 24] | <0.001 |
| Difference (Q1 + Q2) to (Q3 + Q4) | 0 [0, 4] | 24 [16, 24] | <0.001 |
3.4. ROC Analysis
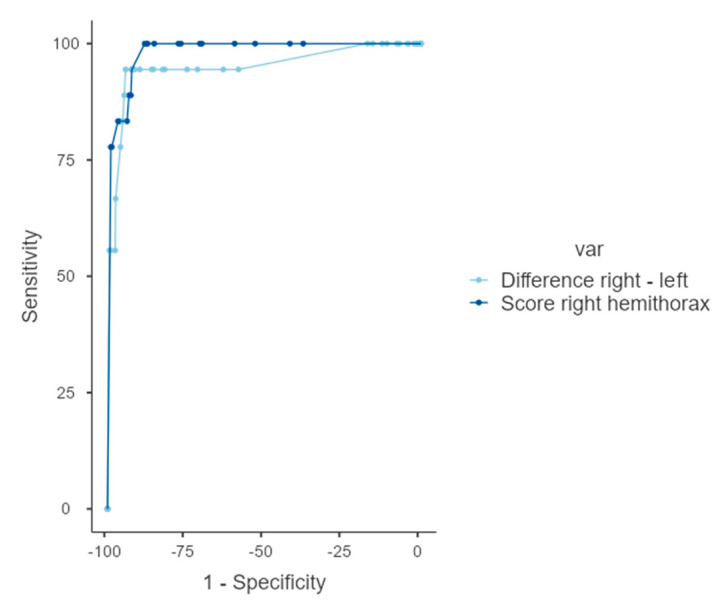
Applied as a diagnostic test, the calculated RALE value of the right hemithorax providing the highest sensitivity and specificity for diagnosis of UPE is 12. Here, we report a sensitivity of 100% and specificity of 88.2%. The threshold value for the difference between both hemithoraces was calculated at 13, providing a sensitivity and specificity of 94% (Figure 3, Table 4).
Figure 3.
ROC curves for RALE score analysis. AUC curves of the difference between the RALE values of both hemithoraces (light blue) and the results of the RALE values of the right hemithorax (dark blue).
Table 4.
ROC analysis to determine RALE score criteria for diagnosing UPE.
| Threshold Value | Sensitivity (%) | Specificity (%) | Youden’s Index | AUC | |
|---|---|---|---|---|---|
| Scores | |||||
| Right hemithorax (Q1 + Q2) |
12 | 100 | 88.2 | 0.88 | 0.980 |
| Difference (Q1 + Q2) to (Q3 + Q4) |
13 | 94.44 | 94.22 | 0.887 | 0.949 |
4. Discussion
The aim of this retrospective study was to evaluate the RALE score for the targeted diagnostic assessment of UPE on postoperative chest X-ray imaging in patients undergoing MVS. In addition, we investigated the incidence of UPE in patients after MI-MVS and C-MVS.
The main results and conclusions of this study are as follows: (1) The incidence of UPE after MVS is 2.6%. (2) UPE was diagnosed significantly more often in the MI-MVS group than in the C-MVS group. (3) With regard to imaging, UPE cannot be diagnosed by a single chest X-ray, as this would cause a high number of false-positive results. (4) The RALE score is a feasible tool for the objective quantification of opacities on chest X-ray images and is therefore suitable for the diagnostic assessment and follow-up of UPE in patients after MVS. (5) In patients with UPE, sensitivity and specificity of up to 94% can be achieved with the presented RALE score cut-off values.
4.1. Diagnosis and Incidence of UPE
In the past decade, studies have reported the incidence of UPE in patients after MVS to range from 1.4% to 25% [5,6,7,13,14,15,16]. As mentioned by Kesävuori et al., the reason for this high range is probably the lack of a standardized objective radiological and clinical definition of this condition [5]. The recent studies by Pühler et al. and Kesävuori et al. defined UPE as unilateral opacities > 20% occurring within the first 24 h after surgery with signs of interstitial thickening, and they also attempted to differentiate between lower and higher grades of UPE by the presence of additional consolidations [5,16]. Both studies reported an incidence of UPE of around 18%. However, as shown by the interobserver agreement of 0.78 reported by Kesävuori et al., these results ultimately depend on the quality of the X-ray image itself and the person reporting the findings. Furthermore, reviewing chest X-ray images only within the first 24 h after MVS may lead to a number of false-positive results, as other pathologies with similar appearance upon imaging cannot be excluded in this short time window. This may artificially increase the incidence of UPE.
In our study, we evaluated all patients’ chest X-rays and other available imaging modalities (e.g., computed tomography) up to the eighth POD to best ensure that pleural effusion, atelectasis, pulmonary hemorrhage, chest wall hematoma, and pneumonia were excluded, as these conditions mimic the imaging findings of UPE upon chest X-ray imaging. This approach enabled us to identify and exclude cases in our study that would otherwise be confused with UPE, and, hence, resulted in an incidence for UPE of 2.6% overall. Moss et al. and Irisawa et al. also reported low incidence of UPE, of 1.4% and 2.5%, respectively, but neither applied a systematic approach to the evaluation of chest X-ray, focusing instead on clinically and radiographically evident cases [13,14]. Thus, Irisawa et al. stated that uneventful cases with ambiguous imaging findings were not included. Our study focused on patients with UPE, which became increasingly likely by excluding other causes of unilateral opacities, by reviewing chest imaging in the 8-day postoperative period in patients after MVS.
The definitive diagnosis of UPE remains the domain of chest imaging, but the currently accepted definition of UPE as postoperative unilateral opacities > 20% is rather vague [5,16]. Although chest X-ray is readily available and provides an overview of the cardiopulmonary status after MVS, there are still limitations in the accurate identification of imaging findings and underlying pathology, particularly due to anterior–posterior image acquisition and the intensive care unit (ICU) setting. In our experience, radiographic imaging acquisition in the ICU setting is often associated with some loss of image quality due to difficult and variable patient positioning and mechanical ventilation with different breathing positions during imaging. This may limit the optimal interpretation of chest X-ray, making it difficult to assess low-grade forms of UPE that are likely to have little or no clinical impact.
4.2. RALE Scoring for UPE Assessment
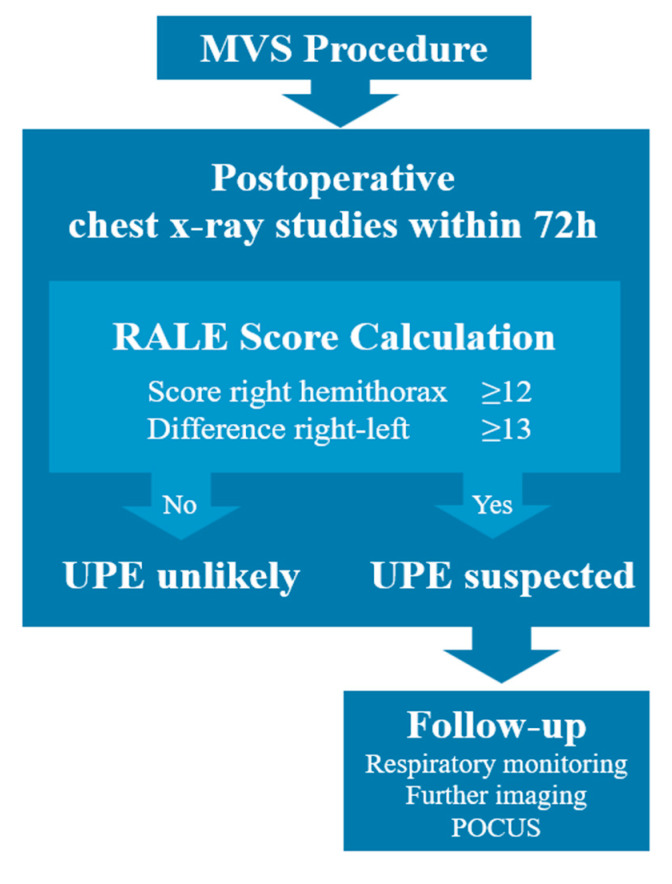
In our study, we investigated, for the first time, the applicability of the RALE score for the diagnostic assessment of UPE after MVS. The RALE score has been well studied for the diagnosis of ARDS, and studies have suggested RALE threshold values of 9 and 10 for the diagnosis of ARDS, which are consistent with the Horowitz index [11,17,18]. These threshold values cannot be applied to UPE because ARDS is a different disease entity. However, RALE soring allows objective quantification of the extent of opacities and lateral differences in opacities on chest X-ray images, both of which may indicate UPE. Our results show that in patients after MVS, RALE values of ≥12 points for the right hemithorax and/or ≥13 points difference between right and left hemithorax indicate UPE. In our study, we were able to confirm the early occurrence of UPE after MVS, and we therefore recommend that RALE scoring be performed especially within the first 72 h after surgery, as almost all images in our study showed the highest degree of opacifications within this period. Another advantage of the RALE score is that it can be used to assess UPE dynamics over time, even with different reviewers. If RALE values are suspicious for UPE, further diagnostic investigations should be performed. In this regard, it is important to consider additional follow-up imaging studies with different modalities and point-of-care ultrasound examinations to further improve diagnostic quality (Figure 4).
Figure 4.
Flowchart for clinical application of the RALE score for assessment of UPE after MVS. (POCUS—point-of-care ultrasound examination).
4.3. Study Limitations
Although our study provides valuable insights into the diagnostic assessment of UPE and the applicability of the RALE score to this condition, the most important limitation is undoubtedly that our main focus was on the examination of chest X-ray images, whereas clinical data were not assessed. This may limit the generalizability of our findings. In future research, identification of patients according to RALE scoring will provide a reliable selection criterion, and, afterwards, clinical and perioperative parameters can be assessed in an attempt to identify the underlying causes of this pathology and to generate a more comprehensive understanding of UPE. Such a study may provide a clinical definition of UPE, while our work focused on providing a chest X-ray imaging definition.
5. Conclusions
The RALE score is a practicable tool to objectively assess chest X-ray images after MVS for UPE. It allows a differentiated assessment of opacities in both hemithoraces, which is crucial for the diagnosis of UPE. The proposed thresholds of the RALE score for the right hemithorax and the difference between the right and left hemithorax provide a sensitivity and specificity for UPE of up to 94%. In addition, our study suggests that UPE is much less common (2.6%) than previously reported, and more common in MI-MVS than in C-MVS.
Abbreviations
| ARDS | Acute respiratory distress syndrome |
| AUC | Area under the curve |
| BMI | Body mass index |
| C-MVS | Conventional mitral valve surgery (median sternotomy) |
| ICU | Intensive care unit |
| MIS | Minimally invasive surgery |
| MVS | Mitral valve surgery |
| MI-MVS | Minimally invasive mitral valve surgery |
| POCUS | Point-of-care ultrasound |
| POD | Postoperative day |
| RALE | Radiographic assessment of lung edema |
| ROC | Receiver operating characteristic |
| UPE | Unilateral pulmonary edema |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12186043/s1, Figure S1. RALE score calculation example. This patient was diagnosed with UPE in the immediate postoperative period. An exemplary calculation of the RALE score is provided, showing diagnostic features suggestive of UPE (right-sided score > 13 and score difference > 12). Table S1. Previous studies investigating incidence of UPE after MVS.
Author Contributions
K.M. and C.W. wrote the main manuscript text, created figures and tables, and managed data. K.M. and C.W. share first authorship. K.M., C.W. and S.S. analyzed the data, prepared the dataset for statistical analysis, and reviewed X-ray imaging. T.P., N.P., G.L. and A.H. (Assad Haneya) were involved in clinical care of the patients, patient selection, data curation, and formal analysis. M.B., O.J. and A.H. (Agreen Horr) were involved in overviewing the manuscript generation process, formulation of research questions, and reviewing the manuscript. P.L. was involved in conceptualizing the study, formulating research questions, and interdisciplinary communications. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted according to the Declaration of Helsinki and was approved by our local institutional ethics board (No. AZ-D-559/18). All patients gave written informed consent before enrolment.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sündermann S.H., Czerny M., Falk V. Open vs. Minimally Invasive Mitral Valve Surgery: Surgical Technique, Indications and Results. Cardiovasc. Eng. Technol. 2015;6:160–166. doi: 10.1007/s13239-015-0210-5. [DOI] [PubMed] [Google Scholar]
- 2.Svensson L.G., Atik F.A., Cosgrove D.M., Blackstone E.H., Rajeswaran J., Krishnaswamy G., Jin U., Gillinov A.M., Griffin B., Navia J.L., et al. Minimally invasive versus conventional mitral valve surgery: A propensity-matched comparison. J. Thorac. Cardiovasc. Surg. 2010;139:926–932.e2. doi: 10.1016/j.jtcvs.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 3.Sündermann S.H., Sromicki J., Biefer H.R.C., Seifert B., Holubec T., Falk V., Jacobs S. Mitral valve surgery: Right lateral minithoracotomy or sternotomy? A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2014;148:1989–1995.e4. doi: 10.1016/j.jtcvs.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 4.López-Baamonde M., Eulufi S., Ascaso M., Arguis M., Navarro-Ripoll R., Rovira I. Unilateral pulmonary edema associated factors after minimally invasive mitral valve surgery. Rev. Esp. Anestesiol. Reanim. 2022;69:134–142. doi: 10.1016/j.redar.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Kesävuori R.I., Vento A.E., Lundbom N.M., Iivonen M.R., Huuskonen A.S., Raivio P.M. Unilateral pulmonary oedema after minimally invasive and robotically assisted mitral valve surgery. Eur. J. Cardio-Thorac. Surg. 2020;57:504–511. doi: 10.1093/ejcts/ezz271. [DOI] [PubMed] [Google Scholar]
- 6.Renner J., Lorenzen U., Borzikowsky C., Schoeneich F., Cremer J., Haneya A., Hensler J., Panholzer B., Huenges K., Broch O. Unilateral pulmonary oedema after minimally invasive mitral valve surgery: A single-centre experience. Eur. J. Cardio-Thoracic Surg. 2017;53:764–770. doi: 10.1093/ejcts/ezx399. [DOI] [PubMed] [Google Scholar]
- 7.Tutschka M.P., Bainbridge D., Chu M.W., Kiaii B., Jones P.M. Unilateral Postoperative Pulmonary Edema After Minimally Invasive Cardiac Surgical Procedures: A Case-Control Study. Ann. Thorac. Surg. 2015;99:115–122. doi: 10.1016/j.athoracsur.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 8.Khalil N.H., Anders R., Forner A.F., Gutberlet M., Ender J. Radiological Incidence of Unilateral Pulmonary Edema After Minimally Invasive Cardiac Surgery. J. Cardiothorac. Vasc. Anesthesia. 2020;34:151–156. doi: 10.1053/j.jvca.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Inotani S., Kubokawa S.-I., Nakaoka Y., Kotani T., Matsuda H., Yamamoto S., Seki S.-I., Kawai K., Hamashige N., Doi Y. Unilateral cardiogenic pulmonary edema. J. Cardiol. Cases. 2017;17:85–88. doi: 10.1016/j.jccase.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handagala R., Ralapanawa U., Jayalath T. Unilateral pulmonary edema: A case report and review of the literature. J. Med. Case Rep. 2018;12:219. doi: 10.1186/s13256-018-1739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren M.A., Zhao Z., Koyama T., Bastarache J.A., Shaver C.M., Semler M.W., Rice T.W., Matthay M.A., Calfee C.S., Ware L.B. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax. 2018;73:840–846. doi: 10.1136/thoraxjnl-2017-211280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Şahin M.D., Aybek E.C. Jamovi: An Easy to Use Statistical Software for the Social Scientists. Int. J. Assess. Tools Educ. 2020;6:670–692. doi: 10.21449/ijate.661803. [DOI] [Google Scholar]
- 13.Moss E., Halkos M.E., Binongo J.N., Murphy D.A. Prevention of Unilateral Pulmonary Edema Complicating Robotic Mitral Valve Operations. Ann. Thorac. Surg. 2016;103:98–104. doi: 10.1016/j.athoracsur.2016.05.100. [DOI] [PubMed] [Google Scholar]
- 14.Irisawa Y., Hiraoka A., Totsugawa T., Chikazawa G., Nakajima K., Tamura K., Yoshitaka H., Sakaguchi T. Re-expansion pulmonary oedema after minimally invasive cardiac surgery with right mini-thoracotomy. Eur. J. Cardio-Thoracic Surg. 2015;49:500–505. doi: 10.1093/ejcts/ezv089. [DOI] [PubMed] [Google Scholar]
- 15.Keyl C., Staier K., Pingpoh C., Pache G., Thoma M., Günkel L., Henschke S., Beyersdorf F. Unilateral pulmonary oedema after minimally invasive cardiac surgery via right anterolateral minithoracotomy. Eur. J. Cardio-Thoracic Surg. 2014;47:1097–1102. doi: 10.1093/ejcts/ezu312. [DOI] [PubMed] [Google Scholar]
- 16.Puehler T., Friedrich C., Lutter G., Kornhuber M., Salem M., Schoettler J., Ernst M., Saad M., Seoudy H., Frank D., et al. Outcome of Unilateral Pulmonary Edema after Minimal-Invasive Mitral Valve Surgery: 10-Year Follow-Up. J. Clin. Med. 2021;10:2411. doi: 10.3390/jcm10112411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valk C.M., Zimatore C., Mazzinari G., Pierrakos C., Sivakorn C., Dechsanga J., Grasso S., Beenen L., Bos L.D.J., Paulus F., et al. The RALE Score Versus the CT Severity Score in Invasively Ventilated COVID-19 Patients—A Retrospective Study Comparing Their Prognostic Capacities. Diagnostics. 2022;12:2072. doi: 10.3390/diagnostics12092072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimatore C., Pisani L., Lippolis V., Warren M.A., Calfee C.S., Ware L.B., Algera A.G., Smit M.R., Grasso S., Schultz M.J. Accuracy of the Radiographic Assessment of Lung Edema Score for the Diagnosis of ARDS. Front. Physiol. 2021;12:672823. doi: 10.3389/fphys.2021.672823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.