Abstract
A reverse transcription-PCR (RT-PCR) technique was used to detect La Crosse (LAC) virus RNA in the central nervous system (CNS) tissues of two patients who died of LAC encephalitis in 1960 and 1978. Viral RNA was readily detected by RT-PCR although the tissues had been stored frozen for up to 37 years. LAC virus was detected in the cerebral cortex but not in other CNS tissues. RT-PCR allowed detection of replicative forms of the virus, indicating that the virus was actively replicating in the specific CNS tissues. The small (S) RNA segments of the viruses from the CNS samples were demonstrated to be genetically similar by single-strand conformation polymorphism analyses. These S RNA segments were then sequenced; only two base changes were demonstrated between the 1960 and the 1978 samples, suggesting that LAC virus is genetically stable in areas of endemicity. The RT-PCR analyses of analyte directly from CNS tissues allows study of the virus without passage in cell culture.
La Crosse (LAC) virus is the causative agent of La Crosse encephalitis, a vector-borne disease of children in the United States. LAC virus is a member of the family Bunyaviridae, in the genus Bunyavirus (5). LAC virus was first identified in 1965, when it was isolated from the brain of a child who had died of meningoencephalitis in 1960 (30). At that time it was recognized as a California serogroup virus but distinct from California encephalitis virus, the prototype virus of the serogroup. It was subsequently named LAC virus.
LAC virus is distributed throughout the eastern half of the United States (5, 6). Human infections with LAC virus occur frequently (11); however, less than 1.5% of LAC virus infections are clinically apparent (11, 31). Nonetheless, LAC encephalitis is the most commonly reported cause of pediatric arboviral encephalitis in the United States (5, 10). Approximately 75 to 100 cases are reported each year (10, 12, 31). Although the virus is quite widely distributed in the United States, most (>90%) of the reported cases of LAC encephalitis occur in Minnesota, Wisconsin, Iowa, Ohio, Illinois, and Indiana (1, 12, 15, 31). It is not known whether the differences in disease incidence are due to differences in viruses (virulence phenotypes), recognition and reporting of disease, or other factors. Genetic analyses of LAC virus isolates from different geographic areas will be required to determine whether phenotypic differences between viruses account for differences in disease incidence (3, 16).
LAC encephalitis is currently diagnosed by classical serological techniques such as immunoglobulin G and immunoglobulin M detection (2). Recently, reverse transcription-PCR (RT-PCR) techniques have been used to detect California serogroup viruses, including LAC virus (4, 7, 9, 17, 33, 34). RT-PCR techniques are sensitive and specific when used for the detection of these viruses, but they have not been used for diagnosis of California serogroup virus infections from post mortem tissues.
In this paper, we report the development and use of a LAC virus-specific RT-PCR technique to detect LAC virus RNA species in autopsied central nervous system (CNS) tissues of patients who died from LAC encephalitis in 1960 and 1978. The LAC virus RT-PCR technique also identified the specific tissues in which the virus was replicating and allowed us to genetically characterize the viruses without passage of the virus in mice or cell culture. The viruses were then genetically compared by single-strand conformation polymorphism (SSCP) and sequence analyses.
MATERIALS AND METHODS
Virus stocks.
Prototype LAC virus was obtained from the Yale Arbovirus Research Unit, New Haven, Conn. This virus was originally isolated from the brain of a patient who died of encephalitis in 1960 (30). The virus had been passaged three times in suckling mice and six times in BHK-21 cells. Stock virus was prepared by standard techniques (2).
Human CNS tissues.
Human CNS tissues had been obtained at autopsy from two patients who died of LAC encephalitis. One of the samples was from a patient who died in 1960 and was the sample from which the prototype virus was originally isolated in 1965 (30). The second CNS sample was from a patient who died in 1978 (A-78-134). The CNS samples from patient A-78-134 had previously been separated into discrete tissues: medulla, temporal lobe, cerebral cortex, spinal cord, cerebellum, and basal ganglion. Each was stored separately. All CNS materials had been stored at −70°C.
Extraction of total cellular RNA from infected BHK-21 cells or from CNS tissue.
For positive controls, prototype LAC virus was inoculated into BHK-21 cells and RNA was extracted 24 h postinfection. For negative controls, total BHK-21 cellular RNA was extracted from noninfected cells. Approximately 107 BHK-21 cells were washed with sterile phosphate-buffered saline (PBS) and then pelleted and subjected to the RNA extraction procedure. For RNA extraction from the CNS tissues, six to eight small pieces of tissue (approximately 100 mg each) were cut from the sample with a sterile scalpel blade.
Total cellular RNA was extracted from BHK-21 cells or from CNS tissue using an RNaid kit (Bio 101, Inc., La Jolla, Calif.). Samples were either frozen at −70°C or used immediately for RT-PCR analysis.
Primers for RT-PCR.
Primers were designed to prime first-strand cDNA synthesis from LAC S genomic, mRNA, or virion complementary RNA (vcRNA) (34). The primers were then used in pairs for PCR. Primer sequences and expected product sizes are presented in Table 1.
TABLE 1.
Primers for RT-PCR of LAC virus S RNA
| RT primer | Sequence (5′→3′) | Bases represented | Paired primer for PCR | LAC virus RNA detected | Product size (bp) |
|---|---|---|---|---|---|
| LNF | TCAAGAGTGTGATGTCGGATTTGG | 71–95 of LAC S vcRNA | LVC | S genome | 860 |
| LNR | GGAAGCCTGATGCCAAATTTCTG | 763–785 of LAC S genomic RNA | LNF | S mRNA and vcRNA | 715 |
| LVC | TTTTGCTGTCCCCTACCACC | 911–931 of LAC S genomic RNA | LNF | S vcRNA | 860 |
| LNF2 | CAAATTCTACCCGCTGAC | 550–568 of LAC S genomic RNA | LNR | S genome | 214 |
| LNR 2 | ATGGTCAGCGGGTAGAAT | 553–571 of LAC S genomic RNA | LNF | S genome | 482 |
RT-PCR.
For RT synthesis of first-strand cDNA, total cellular RNA (approximately 0.5 μg of RNA in 5 μl) was mixed with 15 pmol of the appropriate primer. Samples were incubated at 70°C for 10 min to denature secondary structure in the RNA and then cooled to 20°C for primer annealing. Following priming, reverse transcription was performed by adding 4 μl of 5× buffer, 2 μl of 0.1 mM dithiothreitol, 4 μl of deoxynucleoside triphosphate mix (equal parts of each nucleotide at 10 mM), and 1.5 U of Superscript II reverse transcriptase (Gibco-BRL). Synthesis of cDNA was performed by incubating at 40°C for 1 h, and then the RT reaction was terminated by heating the tubes to 95°C for 10 min.
For PCR, 5 μl of cDNA was removed from each reaction mixture and added to 40 μl of 1× PCR buffer containing 200 pmol of the appropriate primers. The solution was overlaid with 50 μl of mineral oil (Perkin-Elmer), and samples were heated to 80°C. While samples were held at 80°C, 10 μl of 1× PCR buffer containing 1.5 U of Taq DNA polymerase (Promega, Madison, Wis.) was added to each tube. The samples were then thermocycled as follows: 92°C for 1 min, 56°C for 1 min, and 72°C for 2 min for 25 cycles and a final extension step at 70°C for 7 min. After amplification, samples were held at 4°C for 12 to 16 h. PCR products were analyzed by electrophoresis in 1.5% agarose.
As a positive control for the PCR, a plasmid containing a full-length cDNA copy of the LAC small (S) RNA segment (20) was used. Reaction tubes containing water in place of RT reaction mixtures were included as negative controls for the PCR.
SSCP analysis of PCR products.
SSCP analysis was performed as previously described for LAC virus (4). Immediately following the PCR, 4 μl of PCR product was removed and mixed with 1.5 μl of SSCP loading buffer (10 mM NaOH, 95% formamide, 0.05% bromophenol blue, and 0.05% xylene cyanol). The DNA was denatured by heating at 95°C for 2 min and then quickly cooled in ice to allow intrastrand reannealing of the single strands. Samples were immediately loaded onto a 12% polyacrylamide gel and electrophoresed in 1× TBE buffer (8.9 mM Tris, 8.9 mM boric acid, 0.2 mM EDTA [pH 8.0]) for 18 h at 6 mA and 4°C. Following electrophoresis, the gel was fixed for 1 h in 10% acetic acid and stained for 30 min at room temperature in 0.15% (wt/vol) silver nitrate–0.055% (vol/vol) 37% formaldehyde in distilled H2O. Following staining, the bands were visualized by developing the gel in a 3% sodium carbonate–0.0002% sodium thiosulfate–0.15% formaldehyde solution. Development was stopped with 10% acetic acid, and the gel was rinsed in water and allowed to dry overnight.
Sequence analyses.
PCR products were cloned into the plasmid vector pCR2.1 with a TA cloning kit (Invitrogen, San Diego, Calif.). The insert was amplified by using M13 primers, and the PCR product was purified with a Wizard Kit (Promega). Sequencing was performed by MacroMolecular Resources (Colorado State University, Fort Collins) by using an ABI Prism automated sequencing apparatus. M13 primers and internal primers (LNF2 and LNR2) were used to sequence three clones per sample. The internal primers were designed by using OLIGO 4.0 (National Biosciences, Plymouth, Minn.). Internal primer sequences and expected product sizes are listed in Table 1. Nucleotide sequences were aligned by using SEQAID II (22) and CLUSTAL W version 1.6 (29).
Virus isolation from CNS tissue.
Virus isolation from the A-78-134 cortex tissue was attempted by following standard procedures (2). Small pieces of tissue (approximately 100 mg each) were removed aseptically and homogenized in sterile PBS containing 10% fetal bovine serum. The homogenate was centrifuged (4,000 × g for 10 min at 4°C), and the supernatant was inoculated intracranially into 2-day-old suckling mice (approximately 0.025 ml per mouse). Three different samples were prepared, and each was inoculated into one litter of mice. A total of 32 suckling mice were inoculated. Virus isolation from the 1960 CNS sample was not attempted.
Confirmation of virus identity.
Fluorescent-antibody testing and RT-PCR were used to confirm the identity of the virus isolated from the CNS tissues. Virus isolated in the suckling mouse brain was diluted 1:100 and inoculated onto Vero cells. Virus was allowed to replicate for 24 h, at which time the cells were fixed in cold (40°C) acetone for 20 min. A LAC virus-specific monoclonal antibody (807-09) was obtained from Francisco Gonzalez- Scarano, University of Pennsylvania (8). Antibody was used at a working dilution of 1:500. The indirect fluorescent-antibody test was performed as described previously (2), using fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin as the detector antibody. For identification of the isolated virus by RT-PCR, RNA was extracted from the mouse-passaged material. This RNA was subjected to RT-PCR with the LAC virus-specific primers. Following amplification, the material was sequenced as described above.
Nucleotide sequence accession numbers.
The sequences of the 668-bp S segment fragments from the 1960 and 1978 viruses have been submitted to GenBank and given accession no. AFO25478 and AFO25479, respectively.
RESULTS
Detection of LAC virus RNA in CNS extracts.
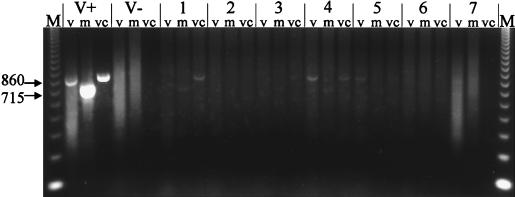
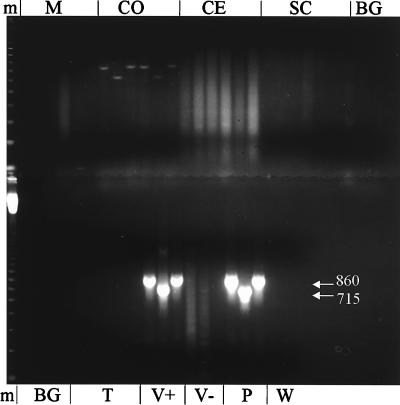
LAC virus RNA was detected by RT-PCR in the CNS tissues of patients who had died in 1960 and 1978 (Fig. 1 and 2, respectively). In addition to genomic RNA, mRNA and vcRNA were detected, indicating that the virus had replicated in the CNS of the patients. For patient A-78-134, the exact location of viral replication in the CNS was determined. RNA was extracted from six different areas of the brain; viral RNA was detected only in the cerebral cortex tissue (Fig. 2). When RNA extracted from LAC virus-infected BHK-21 cells was subjected to RT-PCR, specific bands of either 715 or 860 bp were obtained (Table 1; Fig. 1). No bands were obtained when RNA extracted from noninfected cells was subjected to RT-PCR (Fig. 1).
FIG. 1.
RT-PCR detection of LAC virus RNA in CNS tissues of a patient from 1960. Total cellular RNA extracted from CNS tissues was subjected to RT-PCR with primers specific for LAC virus genomic and replicative forms of RNA. V+ and V− are control reaction mixtures containing RNA extracted from BHK-21 cells inoculated with LAC virus (V+) or from noninfected BHK-21 cells (V−). Lane groups 1 through 7 contain seven different tissue samples from CNS of patient from 1960. Each group contains material from RT-PCR using primers specific for viral genomic (v), mRNA, or vcRNA. RT-PCRs were performed as described in the text. M, molecular weight markers (123-bp DNA ladder). Numbers on the left are base pairs.
FIG. 2.
RT-PCR detection of LAC virus RNA in CNS tissues of a patient from 1978. Total cellular RNA was extracted from the medulla (M), cerebral cortex (CO), cerebellum (CE), spinal cord (SC), basal ganglion (BG), or temporal lobe (T), each containing two sets of tissues subjected to RT-PCR with primers specific for viral genomic, mRNA, or vcRNA as described for Fig. 1. V+ and V− are control reaction mixtures containing RNA extracted from BHK-21 cells inoculated with LAC virus (V+) or from noninfected BHK-21 cells (V−). P and W, plasmid (positive) and water (negative) PCR controls. m, molecular weight markers (123-bp DNA ladder). Numbers in white are base pairs.
Analysis of genetic heterogeneity by using SSCP.
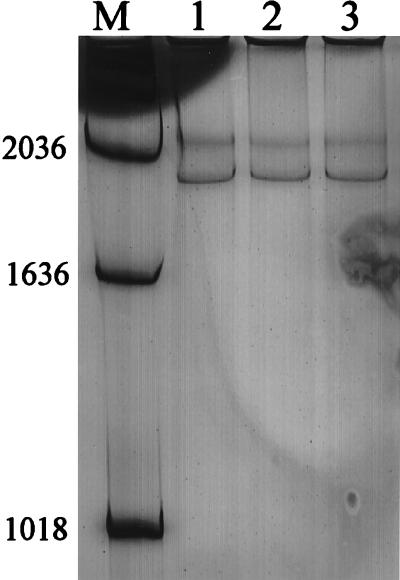
SSCP was used to investigate genetic heterogeneity between the prototype LAC virus and the viral RNA detected in the CNS tissues. SSCP analysis was performed only on the PCR products obtained with the primer that amplified genomic RNA. All three viruses yielded the same SSCP pattern (Fig. 3). Thus, the prototype laboratory virus that had been isolated 30 years earlier from the original brain specimen assayed in these experiments and had been passaged in the laboratory many times did not differ substantially from the virus in the 1960 CNS tissue or from the virus in the 1978 CNS tissue. These results indicated that although the encephalitis cases occurred 18 years and approximately 50 miles apart, the viruses causing the infections were genetically similar.
FIG. 3.
SSCP analysis of PCR products obtained from control RNA (lane 1), the 1960 CNS sample (lane 2), and the 1978 CNS sample (lane 3). M, molecular weight markers. The PCRs were performed with the LNF and LNR primer set (product size = 715 bp), which detects genomic RNA. Numbers on the left are base pairs.
Sequence analysis.
To investigate nucleotide sequence differences between the 1960 virus and the 1978 virus from the CNS tissues, a 668-bp fragment of the S segment was sequenced. The laboratory strain of prototype LAC virus was also sequenced and compared to GenBank sequences of prototype LAC virus S segment (accession no. K00108) to determine if nucleotide changes occurred during laboratory passage. Analysis was performed only on the PCR products obtained by using the primer that amplified genomic RNA. Nucleotide sequences from the prototype LAC virus (those obtained from GenBank and from sequencing of laboratory prototype LAC virus) were identical to those obtained from the 1960 CNS tissues. Two base changes were seen in the virus from the 1978 case compared to the 1960 sample. One occurred at position 180, resulting in an amino acid change from serine to leucine in the N protein and a change from histidine to tyrosine in the NSs protein. A second base change occurred at position 481, but this did not result in an amino acid change in the N protein and did not affect the coding of the NSs protein, which terminates at base 377. Thus, although the two encephalitis cases occurred 18 years and 50 miles apart, the viruses were genetically similar.
Virus isolation and identification.
A total of 32 suckling mice were inoculated with the 1978 CNS homogenate, and 5 mice showed signs of encephalitis at day 3 or 4 postinoculation. The brains of these mice were removed aseptically, pooled, and homogenized in PBS–10% fetal bovine serum. The homogenate was centrifuged, and the supernatant was aliquoted. This virus was called passage 1. The mouse-passaged material was propagated in Vero cells, and identity of the virus isolate was confirmed as LAC virus by using monoclonal antibody (data not shown). RNA was extracted from a portion of the first-passage material, and RT-PCR revealed a specific band, confirming the identity of the virus as LAC virus (data not shown). The mouse passage of the virus was also sequenced; no nucleotide changes were detected.
DISCUSSION
LAC viral RNA was detected by RT-PCR in CNS tissues from two children who died of encephalitis in 1960 and in 1978. Although the CNS tissues had been stored frozen at −70°C for 37 and 19 years, respectively, viral RNA was readily detected in both by using RT-PCR. In addition, LAC virus was isolated from the cerebral cortex samples of patient A-78-134. RT-PCR may be a more sensitive technique for detection of virus in CNS tissues than virus isolation, and it is certainly more rapid. RT-PCR revealed that LAC virus analyte was restricted to the cerebral cortex. It is possible that other brain tissues were infected but that extraction of analyte from these tissues did not yield amplifiable LAC RNA. However, all samples did contain abundant RNA as determined by spectrophotometry (data not shown).
Thus, the RT-PCR technique has potential as a tool for diagnosis of LAC virus infections. Because this disease can now be treated with ribavirin (18), early and rapid diagnosis of LAC viral encephalitis is important. RT-PCR has been successfully used for rapid diagnosis of several viral CNS infections, such as enterovirus (13) and herpesvirus (19) infections. RT-PCR has the potential to become a routine method for diagnosis of viral CNS infections (21, 32). Further studies will be necessary to determine if the LAC virus RT-PCR has potential for diagnosis of LAC infections by detection of specific analyte in cerebrospinal fluid.
Considerable effort is now being applied to the study of molecular determinants of the distribution and other determinants of disease and to the emergence of diseases. RT-PCR and genetic sequencing techniques have become important tools in molecular epidemiology studies. For example, sequence data on viruses that have circulated in the past can be used in comparison with data on viruses that are currently circulating to investigate the genetic basis of emergence, virulence changes, or other important epidemiologic data (27). Our studies illustrate the potential application of molecular techniques to the study of LAC viral epidemiology. By using direct detection of LAC virus RNA in the CNS tissues, rapid genetic analysis without passage of the virus is possible. This precludes genetic variation that may occur with bioamplification.
The SSCP analyses of the LAC viral S segment RNA revealed that although the two encephalitis cases occurred 18 years and 50 miles apart, the viruses were genetically similar. Sequence analyses confirmed the SSCP results and revealed only two nucleotide changes between the two isolates in the 668-bp fragment. Interestingly, one amino acid change occurred in the conserved overlapping reading frame of the S segment that codes for the N and NSs proteins.
The similarity of the virus sequences was somewhat surprising, since the cases were separated temporally and spatially. A similar lack of heterogeneity in the M RNA segment of the same LAC virus isolates was recently demonstrated (14); indeed, these investigators speculated that fatal LAC encephalitis may result from infections with a limited range of virus genotypes. The genome of Ebola virus, which also has a negative-sense RNA genome, has also been shown to exhibit remarkable genomic stability over time and space (28). Genetic stability of other RNA viruses, such as measles virus, has also been demonstrated (23–26). The epidemiological significance of LAC virus genomic stability remains to be determined.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI 19688 from the National Institutes of Health.
The expert technical assistance of Bernard Kalfayan and William Sweeney is acknowledged.
REFERENCES
- 1.Beaty B J, Calisher C H. Bunyaviridae—natural history. Curr Top Microbiol Immunol. 1991;169:27–78. [PubMed] [Google Scholar]
- 2.Beaty B J, Calisher C H, Shope R E. Arboviruses. In: Schmidt N J, Emmons R W, editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. 7th ed. Washington, D.C: American Public Health Association; 1995. pp. 189–212. [Google Scholar]
- 3.Beaty B J, Trent D W, Roehrig J T. Virus variation and evolution: mechanisms and epidemiological significance. In: Monath T P, editor. The arboviruses: epidemiology and ecology. I. Boca Raton, Fla: CRC Press; 1988. pp. 59–85. [Google Scholar]
- 4.Black W C, Vanlandingham D L, Sweeney W P, Wasieloski L P, Calisher C H, Beaty B J. Typing of LaCrosse, snowshoe hare, and Tahyna viruses by analyses of single-strand conformation polymorphisms of the small RNA segments. J Clin Microbiol. 1995;33:3179–3182. doi: 10.1128/jcm.33.12.3179-3182.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calisher C H. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev. 1994;7:89–116. doi: 10.1128/cmr.7.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calisher C H. Taxonomy, classification, and geographic distribution of California serogroup bunyaviruses. A. R. New York, N.Y: Liss; 1983. [PubMed] [Google Scholar]
- 7.Campbell W P, Huang C. Detection of California serogroup viruses using universal primers and reverse transcription-polymerase chain reaction. J Virol Methods. 1995;53:55–61. doi: 10.1016/0166-0934(94)00176-h. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Scarano F, Shope R E, Calisher C H, Nathanson N. Characterization of monoclonal antibodies against the G1 and N proteins of La Crosse and Tahyna, two California serogroup bunyaviruses. Virology. 1982;120:42–53. doi: 10.1016/0042-6822(82)90005-8. [DOI] [PubMed] [Google Scholar]
- 9.Grady L J, Campbell W P. Amplification of large RNAs (>1.5 kb) by polymerase chain reaction. BioTechniques. 1989;7:798–791. [PubMed] [Google Scholar]
- 10.Grimstad P R. California group virus disease. In: Monath T P, editor. The arboviruses: epidemiology and ecology. II. Boca Raton, Fla: CRC Press; 1988. pp. 99–136. [Google Scholar]
- 11.Grimstad P R, Barrett C L, Humphrey R L, Sinsko M J. Serologic evidence for widespread infection with La Crosse and St. Louis encephalitis viruses in the Indiana human population. Am J Epidemiol. 1984;119:913–930. doi: 10.1093/oxfordjournals.aje.a113814. [DOI] [PubMed] [Google Scholar]
- 12.Griot C R, Tselis A, Gonzalez-Scarano F, Nathanson N, Tsai T F. Bunyavirus diseases. In: McKendall R R, Stroop W G, editors. Handbook of neurovirology. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 439–454. [Google Scholar]
- 13.Hosoya M, Honzumi K, Suzuki H. Detection of enterovirus by polymerase chain reaction and culture in cerebrospinal fluid of children with transient neurologic complications associated with acute febrile illness. J Infect Dis. 1997;175:700–703. doi: 10.1093/infdis/175.3.700. [DOI] [PubMed] [Google Scholar]
- 14.Huang C, Thompson W H, Karabatsos N, Grady L, Campbell W P. Evidence that fatal human infections with La Crosse virus may be associated with a narrow range of genotypes. Virus Res. 1997;48:143–148. doi: 10.1016/s0168-1702(97)01437-8. [DOI] [PubMed] [Google Scholar]
- 15.Kappus K D, Monath T P, Kaminski R M, Calisher C H. Reported encephalitis associated with California serogroup virus infections in the United States, 1963–1981. A.R. New York, N.Y: Liss; 1983. [PubMed] [Google Scholar]
- 16.Klimas R, Thompson W H, Calisher C H, Clark G G, Grimstad P R, Bishop D H L. Genotypic varieties of La Crosse virus isolated from different geographic regions of the continental United States and evidence for a naturally occurring intertypic recombinant La Crosse virus. Am J Epidemiol. 1981;114:112–131. doi: 10.1093/oxfordjournals.aje.a113158. [DOI] [PubMed] [Google Scholar]
- 17.Kuno G, Mitchell C J, Chang G-J J, Smith G C. Detecting bunyaviruses of the Bunyamwera and California serogroups by a PCR technique. J Clin Microbiol. 1996;34:1184–1188. doi: 10.1128/jcm.34.5.1184-1188.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McJunkin J E, Khan R, de los Reyes E C, Parsons D L, Minnich L L, Ashley R G, Tsai T F. Treatment of severe La Crosse encephalitis with intravenous ribavirin following diagnosis by brain biopsy. Pediatrics. 1997;99:261–267. doi: 10.1542/peds.99.2.261. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell P S, Espy M J, Smith T F, Toal D R, Rys P N, Berbari E F, Osmon D R, Persing D H. Laboratory diagnosis of central nervous system infections with herpes simplex virus by PCR performed with cerebrospinal fluid specimens. J Clin Microbiol. 1997;35:2873–2877. doi: 10.1128/jcm.35.11.2873-2877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powers A M, Olson K E, Higgs S, Carlson J O, Beaty B J. Intracellular immunization of mosquito cells to La Crosse virus using a recombinant Sindbis virus vector. Virus Res. 1994;32:57–67. doi: 10.1016/0168-1702(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 21.Read S J, Jeffery K J M, Bangham C R M. Aseptic meningitis and encephalitis: the role of PCR in the diagnostic laboratory. J Clin Microbiol. 1997;35:691–696. doi: 10.1128/jcm.35.3.691-696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhoads D D, Roufa D J. SEQAID II user’s manual, version 3.81. Manhattan: Kansas State University; 1991. [Google Scholar]
- 23.Rima B K, Earle J A P, Baczko K, Rota P A, Bellini W J. Measles virus strain variations. Curr Top Microbiol Immunol. 1995;191:65–83. doi: 10.1007/978-3-642-78621-1_5. [DOI] [PubMed] [Google Scholar]
- 24.Rima B K, Earle J A P, Yeo R P, Herlihy L, Baczko K, ter Meulen V, Carabana J, Caballero M, Celma M L, Fernandez-Munoz R. Temporal and geographical distribution of measles virus genotypes. J Gen Virol. 1995;76:1173–1180. doi: 10.1099/0022-1317-76-5-1173. [DOI] [PubMed] [Google Scholar]
- 25.Rota J S, Heath J L, Rota P A, King G E, Celma M L, Carabana J, Fernandez-Munoz R, Brown D, Jin L, Bellini W J. Molecular epidemiology of measles virus: identification of pathways of transmission and implications for measles elimination. J Infect Dis. 1996;173:32–37. doi: 10.1093/infdis/173.1.32. [DOI] [PubMed] [Google Scholar]
- 26.Rota J S, Rota P A, Redd S B, Redd S C, Pattamadilok S, Bellini W J. Genetic analysis of measles viruses isolated in the United States, 1995–1996. J Infect Dis. 1997;177:204–208. doi: 10.1086/513825. [DOI] [PubMed] [Google Scholar]
- 27.Sable C A, Mandell G L. The role of molecular techniques in the understanding of emerging infections. Mol Med Today. 1996;2(3):120–128. doi: 10.1016/1357-4310(96)88722-3. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez A, Trappier S G, Mahy B W J, Peters C J, Nichol S T. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc Natl Acad Sci USA. 1996;93:3602–3607. doi: 10.1073/pnas.93.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson W H, Kalfayan B, Anslow R O. Isolation of California encephalitis group virus from a fatal human illness. Am J Epidemiol. 1965;81:245–253. doi: 10.1093/oxfordjournals.aje.a120512. [DOI] [PubMed] [Google Scholar]
- 31.Tsai T F. Arboviral infections in the United States. Infect Dis Clin N Am. 1991;5:73–102. [PubMed] [Google Scholar]
- 32.Tyler K L. Polymerase chain reaction and the diagnosis of viral central nervous system diseases. Ann Neurol. 1994;36:809–811. doi: 10.1002/ana.410360602. [DOI] [PubMed] [Google Scholar]
- 33.Vodkin M H, Streit T, Mitchell C J, McLaughlin G L, Novak R J. PCR-based detection of arboviral RNA from mosquitoes homogenized in detergent. BioTechniques. 1994;17:114–116. [PubMed] [Google Scholar]
- 34.Wasieloski L P, Rayms-Keller A, Curtis L A, Blair C D, Beaty B J. Reverse transcription-PCR detection of La Crosse virus in mosquitoes and comparison with enzyme immunoassay and virus isolation. J Clin Microbiol. 1994;32:2076–2080. doi: 10.1128/jcm.32.9.2076-2080.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]