Abstract
Disseminated infection with Aspergillus terreus is a rare disease that affects only the immunocompromised host. We report a case of systemic infection with A. terreus resulting in endocarditis, aortic embolization, and splenic infarction in a patient with acute lymphoblastic leukemia. Diagnosis through peripheral blood culture, lack of pulmonary involvement, and onset of disease during complete remission from leukemia constitute uncommon features of this case.
Aspergilli are widespread molds populating virtually every site of organic debris (9). In the case of a normal human immune system, Aspergillus species do not cause systemic disease, which is restricted to the immunocompromised host (2, 12). More than 200 different species of Aspergillus are known, but only a few are consistently pathogenic. A. fumigatus and A. flavus are the most frequently isolated species (2), whereas systemic infection with A. terreus is a rare disease, and fewer than a dozen cases have been reported elsewhere (4, 6, 8, 10, 15, 16). Local infections such as keratitis and otitis media (11, 14), as well as limited invasive disease in three patients with endocarditis, have been described elsewhere (3, 7, 13). Systemic infections occurred only in severely immunocompromised patients and always included a pulmonary involvement in these reports, and their outcome was fatal. We report a case of systemic infection with A. terreus in a patient with acute lymphoblastic leukemia and describe the uncommon features of this case.
Case report.
A 60-year-old Caucasian woman was diagnosed with Philadelphia chromosome-positive (bcr-abl translocation-positive), acute lymphoblastic leukemia in February 1997. Polychemotherapy according to the German ALL Study Group protocol (5) was initiated, and complete remission was achieved after phases I and II of induction chemotherapy (June 1997). Apart from an episode of Pseudomonas aeruginosa sepsis in February, the chemotherapy was well tolerated. In October 1997, the patient was hospitalized due to persistent fever (38.5°C) starting 6 weeks previously. Repeated outpatient examinations did not show any sign of pulmonary or urinary tract infection, and empirical antibiotic therapy with amoxicillin, clarithromycin, ciprofloxacin, and clindamycin did not result in cessation of fever.
Upon admission, the patient was in relatively good general condition but complained of fatigue, lack of appetite, and fever (as high as 38.5°C). Physical examination revealed arrhythmia due to atrial fibrillation, mild tachycardia, a faint systolic heart murmur over the mitral valve, and bilaterally weakened leg pulses. Laboratory investigation disclosed moderate anemia (hemoglobin, 10.0 g/dl) and thrombocytopenia (80,000/μl) but normal leukocyte counts (6,000/μl). Differential blood counting showed 78% granulocytes, 14% lymphocytes, and 8% monocytes. Acute-phase parameters (C-reactive protein, 4.6 mg/dl; blood sedimentation rate, 35/70; fibrinogen, 432 mg/dl) were elevated, and a mild cholestasis (alkaline phosphatase, 339 U/liter; γ-glutamyl transpeptidase, 35 U/liter) was detected. Bone marrow biopsy did not reveal signs of leukemic infiltration. Repeated analysis of urine specimens showed no signs of infection.
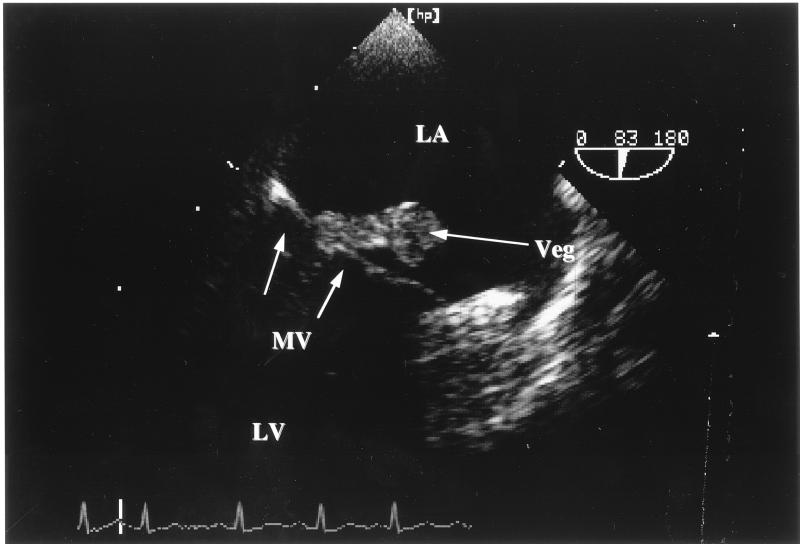
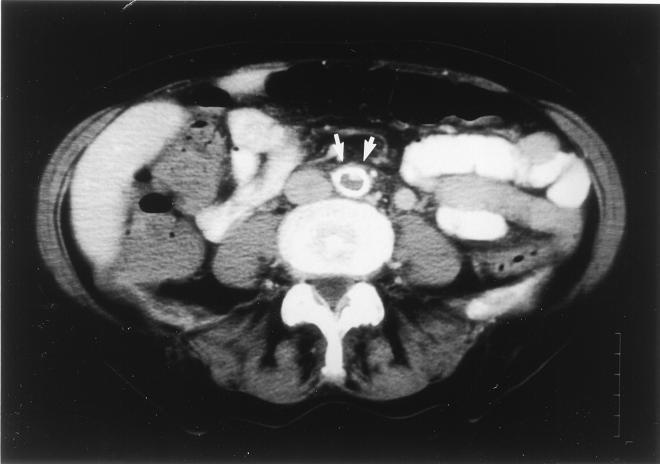
Multiple blood cultures were performed on days 1, 2, 3, 6, 7, 9, and 11 (after admission), and growth of A. terreus was observed on days 1 (three positive cultures), 2, 3, 7, and 9 (Fig. 1). Chest X-irradiation was normal, i.e., did not show any signs of inflammatory infiltrates. Transesophageal echocardiography disclosed vegetation (3.7 by 1.2 cm in diameter) on the posterior part of the mitral valve with protrusions into the left atrium and infiltrations of the subvalvular and ventricular endocardium (Fig. 2), causing mild mitral valve insufficiency. Abdominal computed tomography (CT) revealed a massive aortic embolization with a thrombus located in the lower abdominal aorta and left iliac artery, with almost complete occlusion of the vasal lumen in these segments (Fig. 3). Despite massive embolization, clinical signs of hypoperfusion or ischemia of the lower extremities were confined to bilaterally weakened leg pulses. In addition, embolic infarctions in the spleen and kidneys were detected by means of CT examination.
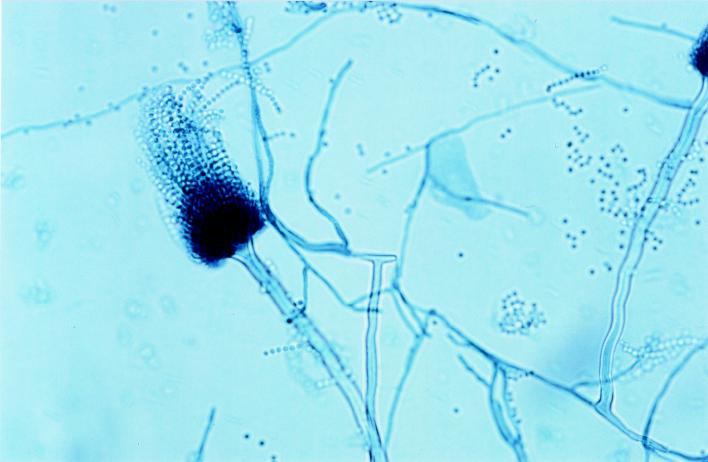
FIG. 1.
Lactophenol blue stain of A. terreus hyphae from a peripheral blood culture. Magnification, ×100.
FIG. 2.
Transesophageal echocardiography of valvular vegetations (veg) caused by A. terreus; protrusion of mitral valve (MV) fungal vegetations into the left atrium (LA). LV, left ventricle.
FIG. 3.
CT scan 2 cm above the aortal bifurcation. Arrows, aorta; white ring, perfused lumen; gray area, central blockage of perfusion due to a thrombus.
Drug therapy with amphotericin B (1 mg/kg of body weight) was initiated 8 days after admission, and heart surgery with excision of vegetations from the valve and subsequent valve reconstruction were performed 1 day later. Histologic assessment showed fungal endocarditis, with excessive hyphal spreading and massive destruction (Fig. 4). Cultivation of valvular tissue on Sabouraud dextrose agar confirmed the growth of A. terreus. The patient required intensive-care management after heart surgery; after 3 days, recolonization of the mitral valve with fungal vegetations was detected by echocardiography. The patient deteriorated rapidly, showing signs of fungal sepsis, including massive increases in leukocyte count, acute-phase parameters, and liver enzymes, along with decreases in erythrocyte and thrombocyte values to counts even lower than those observed upon her admission. The patient died on day 15 despite intensive-care management. Postmortem examination confirmed recurrence of fungal endocarditis, as well as fungal aortic embolization and multiple splenic and renal infarctions.
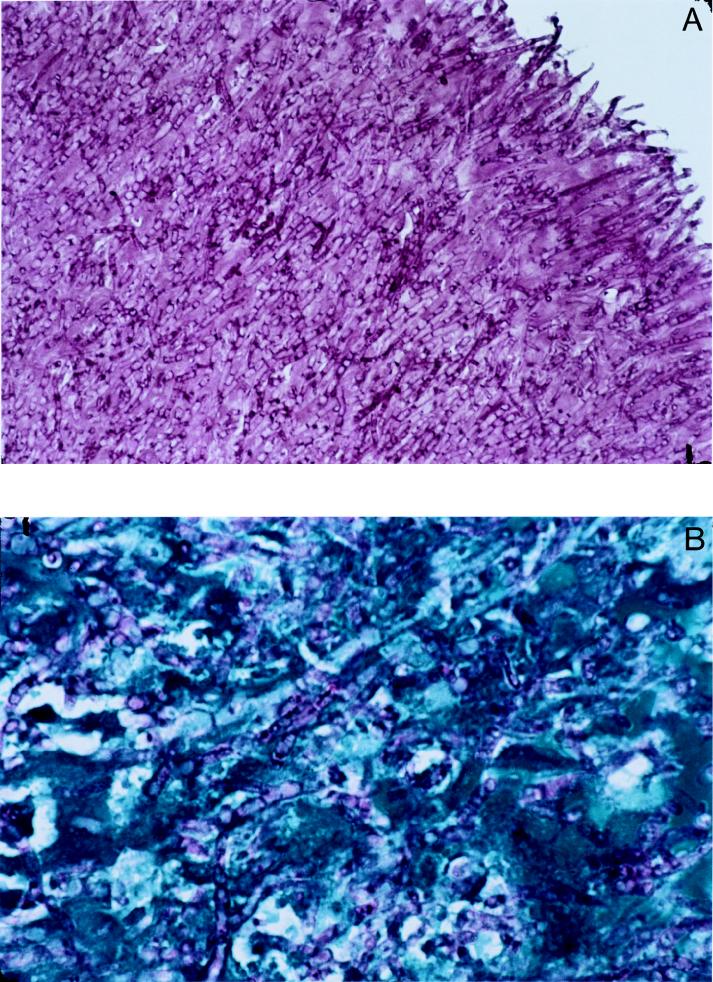
FIG. 4.
(A) Periodic acid-Schiff stain of mitral valve tissue showing complete infiltration with broad hyphae of A. terreus. Magnification, ×40. (B) McManus stain of mitral valve tissue (blue) showing infiltration of A. terreus hyphae (violet). Magnification, ×100.
Mycologic results.
During a period of 11 days, 19 blood cultures were inoculated. In each case, two bottles, both aerobic and anaerobic, were filled with 10 ml of blood and incubated at 35.5°C in a VITAL system (bioMérieux, Marcy l’Etoile, France), a fully automated instrument for the detection of growing microorganisms. Readings were taken every 15 min. No positive signal was given by the detection system at any time. However, all of the bottles were also checked visually; seven of them showed hyphal growth after 5 days of culture. A certain amount of each positive blood culture was inoculated onto Sabouraud glucose agar (SAB), and the inoculation was incubated at 35°C. After a few days, the colonies were examined macro- and microscopically. Material obtained from the mitral valve was also cultured on SAB at 35°C.
In total, 7 blood cultures were positive, revealing growth of a mold, while 12 cultures remained sterile. After subcultivation of the positive cultures, cinnamon-brown colonies with granular to velvety textures were observed on SAB. Microscopically, the colonies showed long slender and smooth conidiophores, as well as columnar conidium heads with biseriate phialides, which were indicative of the presence of A. terreus (Fig. 1). In addition, culture of the mitral valve yielded growth of A. terreus.
Histology.
Mitral valve tissue samples were obtained during surgery and were fixed in formalin. Paraffin sections (4 μm) were obtained and stained with periodic acid-Schiff (Fig. 4A), McManus (Fig. 4B), and Grocott (not shown) stains to demonstrate dichotomously branched septate hyphae of A. terreus within the tissue.
Discussion.
Systemic infection with A. terreus is a rare event, and only nine cases have been described in the literature. The case described herein shows remarkable differences regarding diagnostic, clinical, and pathological features compared with the cases reported previously.
First, primary diagnosis of aspergillosis was achieved by isolation of the fungi from blood cultures. Isolation of Aspergillus species from peripheral blood cultures is highly uncommon, even in the case of disseminated disease, and A. terreus has not been isolated from blood cultures of living patients before, while one case of postmortem isolation from peripheral blood has been reported elsewhere (9). Even in the case of generalized disease, blood cultures have remained persistently negative (1, 4, 6, 8, 10, 15, 16). Special localizations of A. terreus growth directly exposed to the blood flow, as in the case of the valvular endocardium, may lead to persistent detachment and embolization of fungi, favoring detection by blood culture.
Second, the lack of pulmonary involvement in the presence of generalized infection with A. terreus is unique, since all earlier cases reported to date included severe pulmonary disease. None of the nine chest X rays revealed the presence of aspergilloma or invasive pulmonary aspergillosis. Furthermore, postmortem macro- and microscopic assessments of serial lung sections showed no signs of pulmonary disease. Despite the lack of clinical and pathological signs of a pulmonary port of entry, infection through the respiratory tract cannot be ruled out, since the respiratory tract is considered the usual port of entry for these organisms (16), no venous access device had been in place during the onset of fever, and preceding bacterial sepsis with pneumonia might have caused pulmonary tissue damage, facilitating fungal entry.
Third, the strongest risk factor for disseminated aspergillosis is prolonged granulocytopenia in the context of immunosuppressive drug therapies (17). Five of nine cases of generalized A. terreus infections occurred in the complete absence of granulocytes. In contrast, our patient presented in complete remission from leukemia, and leukocyte and granulocyte counts were normal at the onset of infection. Maintenance chemotherapy with oral methotrexate and purinethole, which was administered during the onset of fever, may have triggered fungal infection. Alternatively, tissue damage caused by bacterial infection is known to favor aspergillosis (2), and the episode of P. aeruginosa sepsis during induction chemotherapy may have entailed colonization with A. terreus. However, echocardiographic examination, as well as assessment of Aspergillus antigen and antibodies, was negative at that time. Despite discontinuation of maintenance chemotherapy and despite the presence of normal peripheral blood counts, the fever persisted and disease progressed.
In accordance with the cases of disseminated A. terreus infection described earlier, the outcome in our patient was also fatal, with a survival of only 15 days after the first positive blood culture. Although amphothericin B was administered in high doses and in liposomal form, it did not influence the course of disease. In addition, heart surgery performed to remove fungal vegetations was not successful, since fulminant recolonization occurred.
We conclude that A. terreus infection can occur in nongranulocytopenic patients after chemotherapy and that blood cultures may detect the presence of fungal growth, while lack of pulmonary aspergillosis does not necessarily exclude a systemic infection with A. terreus.
REFERENCES
- 1.Bhatnagar N K, Dhasmana J P, Russell G A, Jordan S C. Aspergillus ball thrombus occluding a homograft conduit. Eur J Cardiothorac Surg. 1989;3:270–272. doi: 10.1016/1010-7940(89)90078-x. [DOI] [PubMed] [Google Scholar]
- 2.Bodey G P, Vartivarian S. Aspergillosis. Eur J Clin Microbiol Infect Dis. 1989;8:413–437. doi: 10.1007/BF01964057. [DOI] [PubMed] [Google Scholar]
- 3.Drexler L, Rytel M, Keelan M, Bonchek L I, Olinger G N. Aspergillus terreus infective endocarditis on a porcine heterograft valve. J Thorac Cadiovasc Surg. 1980;79:269–274. [PubMed] [Google Scholar]
- 4.Hara K S, Ryu J H, Lie J T, Roberts G D. Disseminated Aspergillus terreus infection in immunocompromised hosts. Mayo Clin Proc. 1989;64:770–775. doi: 10.1016/s0025-6196(12)61749-2. [DOI] [PubMed] [Google Scholar]
- 5.Hoelzer D, Thiel E, Ludwig W D, Loffler H, Buchner T, Freund M, Heil G, Hiddemann W, Maschmeyer G, Volkers B, Aydemir U for The German ALL Study Group. The German multicenter trials for treatment of acute lymphoblastic leukemia in adults. The German Adult ALL Study Group. Leukemia. 1992;6:175–177. [PubMed] [Google Scholar]
- 6.Kalina P H, Campbell R J. Aspergillus terreus endophthalmitis in a patient with chronic lymphocytic leukemia. Arch Ophthalmol. 1991;109:102–103. doi: 10.1001/archopht.1991.01080010104040. [DOI] [PubMed] [Google Scholar]
- 7.Laham M N, Carpenter J L. Aspergillus terreus, a pathogen capable of causing infective endocarditis, pulmonary mycetoma, and allergic bronchopulmonary aspergillosis. Am Rev Respir Dis. 1982;125:769–772. doi: 10.1164/arrd.1982.125.6.769. [DOI] [PubMed] [Google Scholar]
- 8.Neumeister B, Hartmann W, Oethinger M, Heymer B, Marre R. A fatal infection with Alternaria alternata and Aspergillus terreus in a child with agranulocytosis of unknown origin. Mycoses. 1994;37:181–185. doi: 10.1111/j.1439-0507.1994.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 9.Rinaldi M G. Invasive aspergillosis. Rev Infect Dis. 1983;5:1061–1077. doi: 10.1093/clinids/5.6.1061. [DOI] [PubMed] [Google Scholar]
- 10.Russack V. Aspergillus terreus myocarditis: report of a case and review of the literature. Am J Cardiovasc Pathol. 1990;3:275–279. [PubMed] [Google Scholar]
- 11.Singh S M, Sharma S, Chatterjee P K. Clinical and experimental mycotic keratitis caused by Aspergillus terreus and the effect of subconjunctival oxiconazole treatment in an animal model. Mycopathologia. 1990;112:127–137. doi: 10.1007/BF00436642. [DOI] [PubMed] [Google Scholar]
- 12.Stein D K, Sugar A M. Fungal infections in immunocompromised host. Diagn Microbiol Infect Dis. 1989;12:221S–228S. doi: 10.1016/0732-8893(89)90140-5. [DOI] [PubMed] [Google Scholar]
- 13.Thamlikitkul V, Prachuabmoh K, Sukroongreung S, Danchaivijitr S. Aspergillus terreus endocarditis—a case report. J Med Assoc Thail. 1983;66:723–726. [PubMed] [Google Scholar]
- 14.Tiwari S, Singh S M, Jain S. Chronic bilateral suppurative otitis media caused by Aspergillus terreus. Mycoses. 1995;38:297–300. doi: 10.1111/j.1439-0507.1995.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 15.Tracy S L, McGinnis M R, Peacock J E, Jr, Cohen M S, Walker D H. Disseminated infection with Aspergillus terreus. Am J Clin Pathol. 1983;80:728–733. doi: 10.1093/ajcp/80.5.728. [DOI] [PubMed] [Google Scholar]
- 16.Tritz D M, Woods G L. Fatal disseminated infection with Aspergillus terreus in immunocompromised hosts. Clin Infect Dis. 1993;16:118–122. doi: 10.1093/clinids/16.1.118. [DOI] [PubMed] [Google Scholar]
- 17.Wingard J R, Beals S U, Santos G W, Mirz W G, Saral R. Aspergillus infection in bone marrow transplant recipients. Bone Marrow Transplant. 1987;2:175–181. [PubMed] [Google Scholar]