Abstract
Candidiasis is one of the most frequent nosocomial infections affecting an increasing number of at-risk patients. Candida albicans remains the most frequent causative agent of candidiasis, but, in the last decade, C. auris has emerged as a formidable multi-drug-resistant pathogen. Both species are fully capable of forming biofilms, which contribute to resistance, increasing the urgency for new effective antifungal therapies. Repurposing existing drugs could significantly accelerate the development of novel therapies against candidiasis. Here, we have screened the Repurposing Hub library from the Broad Institute, containing over 6000 compounds, in search for inhibitors of C. albicans and C. auris biofilm formation. The primary screen identified 57 initial hits against C. albicans and 33 against C. auris. Confirmatory concentration-dependent assays were used to validate the activity of the initial hits and, at the same time, establish their anti-biofilm potency. Based on these results, ebselen, temsirolimus, and compound BAY 11-7082 emerged as the leading repositionable compounds. Subsequent experiments established their spectrum of antifungal activity against yeasts and filamentous fungi. In addition, their in vivo activity was examined in the murine models of hematogenously disseminated C. albicans and C. auris infections. Although promising, further in vitro and in vivo studies are needed to confirm their potential use for the therapy of candidiasis and possibly other fungal infections.
Keywords: Candida spp., biofilm, repurposing, screening, antifungal
1. Introduction
Infections caused by opportunistic pathogenic fungi within the genus Candida represent an increasing threat to an expanding population of immune- and medically compromised patients [1,2]. The limited number of antifungal drugs currently available for the treatment of candidiasis, their limited efficacy, and the emergence of resistance contribute to the high morbidity and mortality rates associated with candidiasis [3]. Approximately 50% of these infections are caused by Candida albicans, but, in recent years, the epidemiology of candidiasis is changing, as infections caused by non-albicans Candida species (NACS) are becoming increasingly common [4]. One NACS, in particular, has recently made headlines for its emergence as a formidable nosocomial pathogen. In 2009, Candida auris was first discovered in an ear infection in Japan [5]. Since then, this opportunistic fungal species has spread simultaneously across multiple continents and has caused outbreaks in several hospitals and healthcare facilities [6,7]. Notably, C. albicans and C. auris are among the fungal priority pathogens identified by the World Health Organization (WHO) as having the greatest threat to public health [8,9].
Both C. albicans and C. auris are fully capable of forming biofilms, which contribute to augmented antifungal resistance, as well as resistance to immunological assaults within the human body [10,11,12]. Given the levels of resistance and high levels of mortality detected with Candida infections associated with a biofilm etiology, it is clear that new antifungal options are desperately needed [3,13].
Repurposing (or repositioning) is the process of finding new therapeutic indications for current existing drugs, which can significantly decrease the time and effort in bringing drugs with novel antifungal activity from the bench to the bedside [14]. In the last decade, this approach has been fueled by the availability of repurposing libraries from different sources which can be used in high-throughput screenings, thereby facilitating the rapid identification of bioactive drugs in a variety of screens for different disease models, including those with antifungal activity [15]. The Broad Institute has recently created the Repurposing Hub, a comprehensive repurposing library and accompanying interactive and curated database [16], consisting of over 6000 compounds, many of which have been approved by the United States Food and Drug Administration (US FDA), and others which are at different stages of clinical development. Here, we report on the high-throughput screening of this chemical library in search for inhibitors of both C. albicans and C. auris biofilm formation.
2. Materials and Methods
2.1. Strains, Cultivation Conditions, and Media
C. albicans SC5314 and C. auris strain 0390, obtained from the U.S. Centers for Disease Control and Prevention (CDC), were used in this study, including primary screens and follow-up experiments. Working cultures were prepared on yeast extract–peptone–dextrose (YPD) (1% (w/v) yeast extract, 2% (w/v) peptone, and 2% (w/v) dextrose) agar plates. From these, the strains were grown by inoculating a loopful of cells in 20 mL of YPD liquid medium in 150 mL flasks and incubating in an orbital shaker at 30 °C overnight. The cells were washed with phosphate-buffered saline (PBS), counted, and adjusted to a final desired final cell density of 2 × 106 cells/mL by diluting in RPMI-140 medium (without sodium bicarbonate, supplemented with L-glutamine, buffered with 165 mM morpholine propane sulfonic acid, and adjusted to pH 6.9). From now on, this medium will be referred to simply as “RPMI medium”.
2.2. Chemical Library
The Repurposing Hub library (Broad Institute, Cambridge, MA, USA) consists of about 6000 different compounds with 663 different therapeutic indications and over 2000 different targets [16]. Drugs in this collection are approved by the US FDA or have undergone testing in at least one phase of clinical trials [16]. Because of this, pharmacodynamics, pharmacokinetics, safety, and toxicity for humans have been characterized for most of these compounds.
2.3. High-Throughput Screen for Inhibitors of C. albicans and C. auris Biofilm Formation
The screening process was based on our previously described 96-well micotiter plate model of Candida biofilm formation [17,18], adapted to the use of 384-well microtiter plates to allow for true high-throughput screening [19]. Briefly, compounds in the Repurposing Hub library were pre-spotted by the Broad Institute in individual wells of 384-well flat-bottom microtiter plates (Corning Incorporated, Corning, NY, USA). Each well contained nanoliter volumes of one individual compound in the library, calculated in a manner that the addition of the cell suspension would result in a final screening concentration of 20 μM. Plates were bar-coded for identification purposes and were shipped to our laboratory as “assay-ready” plates to speed up the screening process. Upon receipt, plates with pre-spotted compounds were stored in our laboratory at −20 °C. In its final format, wells in columns 2 through 22 contained individual pre-spotted compounds, while columns 1 and 24 contained an equivalent volume of DMSO, and selected wells in column 23 contained Amphotericin B (at a final concentration of 4 μg/mL) as a positive control for inhibition. On the day of screening, plates were allowed to thaw and 30 μL of the cell suspension at a concentration of 2 × 106 cells/mL in RPMI medium were added to individual wells in columns 2 through 24, while column 1 served as the sterility control and had only RPMI added. The plates were then incubated at 37 °C for 24 h to allow for biofilm formation. Then, the plates were washed once with 40 µL of PBS per well, and, after washing, 30 µL per well of XTT (2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide)/menadione solution were added, after which the plates were incubated in the dark for 1 h. The plates were then read for absorbance in a microtiter plate reader at 490 nm (from the top) to provide a quantitative measure of inhibition. The data were then normalized and analyzed in comparison with the growth control (in the absence of drug) to determine the percentage of inhibition. An arbitrary threshold of >70% biofilm inhibition was selected in order to identify initial “hits”.
2.4. Concentration-Dependent Assays for Confirmation of Initial Hits and Determination of Their Inhibitory Potency
Concentration-dependent measurements were carried out to reconfirm the activity of initial hits and to assess their potency. We used the same 384-well microtiter plate model for inhibition of Candida biofilm formation, but using a series of concentrations for each individual hit, which were pre-spotted by the Broad Institute prior to shipment to our laboratory as assay-ready plates. Briefly, wells in columns 3 to 22 contained 10-point concentration series of the different hits, in two-fold serial dilutions ranging from 40 µM to 0.078 µM final concentrations, allowing for up to 28 compounds to be tested in a single 384-well microtiter plate. The first column remained empty (to serve as media-only control), while columns 2 and 24 served as growth controls (no inhibition), and selected wells in column 23 contained Amphotericin B (as positive control for inhibition). Then, 30 µL of the standardized yeast cell inoculum were added to columns 2 through 24. After incubation at 37 °C for 24 h to allow for biofilm formation, the plates were washed once with PBS incubated with XTT and read at 490 nm in a microplate reader. Similar to the initial screen, the colorimetric readings were analyzed to determine the percentage of inhibition for each drug at the 10 different concentrations. From these results, the inhibitory concentration required to inhibit 50% of growth (IC50) was determined by fitting normalized results (positive (untreated) and negative (uninoculated) controls arbitrarily set as 100% and 0% growth) to the variable slope Hill equation (an equation that determines the nonlinear drug dose–response relationship) using Prism (version 10.0.2, GraphPad Software Inc., San Diego, CA, USA). Compounds found to inhibit greater than 70% of biofilm growth in the dilution series were considered to be confirmed “hits”.
2.5. Antifungal Susceptibility Testing to Determine the Spectrum of Activity of the Leading Repositionable Drugs Ebselen, Temsirolimus, and BAY 11-7082 against a Panel of Medically Important Fungi
Antifungal susceptibility testing was performed using standard CLSI methods, to examine the activity of the selected leading repositionable compounds ebselen, temsirolimus, and BAY 11-7082 against a panel of medically important fungi, including yeasts and molds. All clinical fungal isolates tested form part of the collection available in the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio. MICs were determined in accordance with the CLSI M27 (for yeast) and M38 (for filamentous fungi) reference standards for antifungal susceptibility testing [20,21]. Stock solutions of resupplied ebselen, temsirolimus, and BAY 11-7082 were prepared by dissolving the powders in DMSO. Further dilutions were prepared in RPMI medium. Fluconazole (for yeasts), and posaconazole or voriconazole (for molds) were used for comparison purposes. The minimum inhibitory concentrations (MICs) were read visually at 50% and/or 100% of growth after 24 to 72 h of incubation for yeasts and filamentous fungi depending upon the species tested against.
2.6. Preliminary Examination of the In Vivo Antifungal Activity of the Leading Repositionable Compounds Ebselen, Temsirolimus, and BAY 11-7082 in Murine Models of Hematogenously Disseminated Candidiasis
All animal experiments were performed following NIH guidelines and in accordance with institutional regulations (IACUC) in AAALAC-certified facilities. Animals were randomly distributed in different cages and allowed a one-week acclimatization period before experiments were started. Throughout the studies, mice were observed multiple times per day to prevent and minimize unnecessary pain and distress that may have occurred with infection. Any animal that appeared moribund was humanely euthanized. Persons monitoring the animals were not blinded as to the identity of the different groups.
The initial assessment of the antifungal activity in vivo of ebselen, temsirolimus, and compound BAY 11-7082 used the well-established model of hematogenously disseminated C. albicans infections, and was performed following methodologies previously described by our group. Briefly, cultures of C. albicans SC5314 strain were grown overnight in YPD broth at 25 °C. Cells were harvested by centrifugation, washed, counted, and diluted appropriately in sterile saline for injection to prepare the infecting inoculum. Then, a final volume of 200 µL containing 3.5 × 105 yeast cells was injected via the lateral tail vein into 6- to 8-week-old female BALB/c mice. The drugs were diluted in 2% DMSO and prepared in saline for injection, with doses of 2.5 mg/kg for temsirolimus and 5 mg/kg for both ebselen and BAY 11-7082 administered intraperitoneally to groups of mice (n = 8). In order to maximize the detection of protective effects, we used a prophylactic regimen with treatment starting 2 days prior to infection, and then continuing once daily until the end of the observational period (typically 14 days). A control group was on the same schedule but received vehicle-only injections.
The C. auris infection model has been previously described [22,23]. Briefly, male ICR mice (10 per group) were rendered neutropenic with a single dose of pharmaceutical-grade 5-fluorouracil (5 mg/mouse) administered 24 h prior to inoculation. To prevent bacterial superinfection and deaths in the immunosuppressed mice, mice received antibacterial prophylaxis consisting of enrofloxacin at 50 ppm in their drinking water beginning 1 day prior to infection. On the day of inoculation (day 0), a clinical isolate of C. auris (strain DI 17-46) was used to infect mice via the lateral tail vein (0.2 mL of a yeast cell inoculum of 1 × 107 cells/mouse). Treatment groups consisted of vehicle control (2% DMSO), ebselen at 5 mg/kg, and temsirolimus at 2.5 mg/kg. Drugs were administered once daily by intraperitoneal injection, starting 2 days prior to infection until day 7 post-infections. Animals were monitored for a total of 21 days post-infection.
To determine the survival curves, days on which the mice died were recorded; for euthanized mice, death was recorded as occurring the next day. Survival was plotted by Kaplan–Meier analysis and differences between groups (treated versus untreated) were analyzed using the log rank test. Analyses were performed using Prism (GraphPad Software, Inc.).
3. Results and Discussion
3.1. High-Throughput Screening of the Drug Repurposing Hub for Inhibitors of C. albicans and C. auris Biofilm Formation
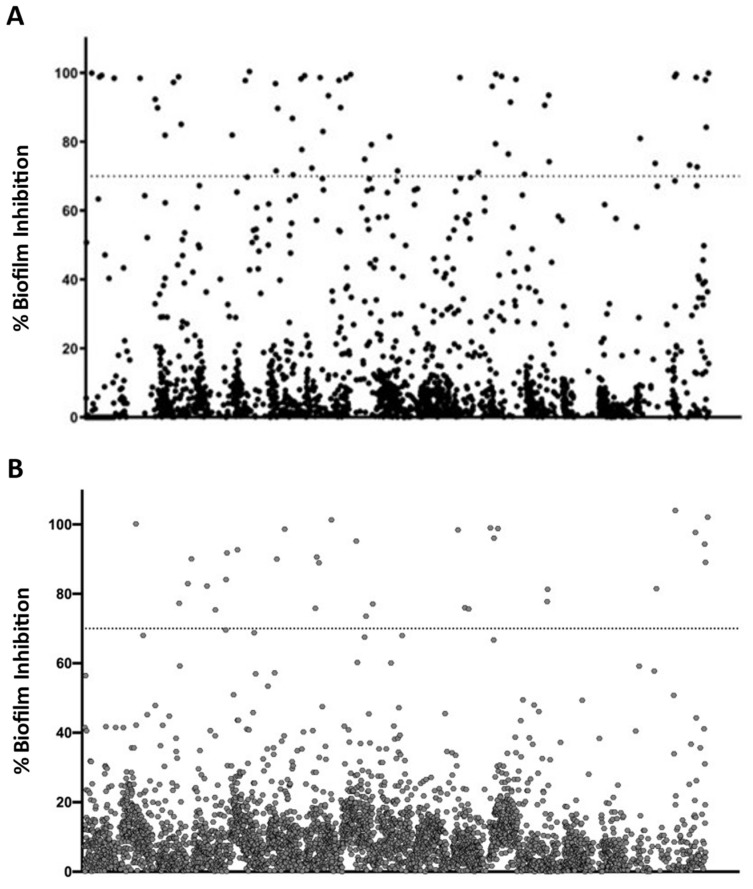
Repurposing represents an auspicious alternative for accelerated drug development, as exploring potential therapeutic utility in indications outside those originally targeted may drastically reduce the effort, time, and money required [14]. This approach is particularly attractive in disease areas with high unmet needs and a paucity of new leads, including antifungal drug development [13]. Most recently, repurposing efforts have been facilitated by the availability of repurposing screening libraries assembled by commercial entities and different organizations [15]. The chemical diversity and known safety profiles of drugs in these libraries (with most having been previously tested in humans) make this a particularly appealing approach with the potential to rapidly advance a candidate into the clinic. To address the shortage of antifungal drugs, particularly those with anti-biofilm activity [3,24], we screened the Repurposing Hub library provided by the Broad Institute [16] in search for inhibitors of biofilm formation of C. albicans strain SC5314 and C. auris strain 0390. Each of the approximately 6000 compounds in the library, which includes FDA-approved drugs and compounds at different stages of clinical trials [16], was screened at a final concentration of 20 μM. The primary screenings against both Candida species were performed using new 384-well microtiter plates that we adapted from our previously described methodology using 96-well microtiter plates [19]. Two sets of compounds from the library (one for each species) were pre-spotted at the appropriate volumes in individual wells of the 384-well microtiter plates, which were then shipped to our laboratory as assay-ready, bar-coded plates. The newly developed 384-well microtiter plate protocol significantly reduced the amount of time and quantity of compound required for the screening, thereby allowing for a true high-throughput screening of the library. After the screening was completed, the percentage of inhibition for each drug or compound was calculated. Figure 1A,B depict the graphical representation of the results from these primary screenings for C. albicans and C. auris respectively. Considering the fact that we screened the library at a relatively high concentration of 20 µM, we arbitrarily set up a threshold of 70% inhibition or higher for the identification of hit compounds. Using this criterion, a total of 57 compounds were identified as inhibitors of C. albicans SC5314 biofilm formation, and 33 compounds were identified as capable of inhibiting biofilm formation in C. auris strain 0390, resulting in initial hit rates of 0.90% for C. albicans and 0.55% for C. auris, which is in agreement with the fact that C. auris biofilms normally display higher levels of resistance compared to their C. albicans counterparts [11].
Figure 1.
Graphical representation of results from the initial screens for inhibitors of biofilm formation against C. albicans (A) and C. auris (B). The dotted lines indicate the 70% arbitrary threshold for initial hit identification.
3.2. Concentration-Dependent Assays to Confirm “Hits” from the Initial Screen and Establish Their Potency
Next, we performed concentration-dependent assays to confirm and establish the potency of the initial hit compounds. These assays were performed using the same 384-well microtiter plate model, except that each compound was tested at 10 different concentrations (serial dilutions), ranging from 40 µM to 0.078 µM. Two sets of concentration dependent plates, one for C. albicans original hits and a second one for C. auris hits, were prepared by the Broad Institute after identifying the hits from each initial screen in the Repurposing-Hub-accompanying database and shipped to us for processing. The yeast cell suspensions were added to wells of these plates according to the plate maps, and the plates incubated for 24 h to allow for biofilm formation. The extent of biofilm formation was calculated based on XTT-colorimetric readings, the percentage of inhibition was calculated, and the results were normalized and analyzed to calculate the IC50 value for each compound tested. As representative examples, the results of these assays for several initial hits showing dose-responsive inhibitory activity against C. auris biofilm formation are provided in Supplementary Figure S1.
In the case of C. albicans, a total of 56 of the original 57 “hits” were confirmed (Table 1), for a 98.2% confirmation rate. The accompanying Repurposing Hub database was used to uncover the identity of the confirmed “hits”, which had a variety of original therapeutic indications: 12 were known antifungals, 18 were antiseptics or antibacterials, and 25 (representing 24 unique compounds) had different primary therapeutic indications (i.e., channel blockers, cytokine inhibitors, enzyme inhibitors, etc.) and could represent repositionable candidates as antifungals. Likewise, a total of 30 of the original 33 “hits” from the C. auris screen were also confirmed (Table 2), resulting in a 90.9% confirmatory rate. Of these confirmed “hits”, 7 were antifungals or fungicides, 12 were antiseptics or antibacterials, and 11 were potentially repositionable compounds. Overall, there were a total of 19 compounds which were hits in both the C. albicans and the C. auris screenings (Supplementary Figure S2), of which 5 were classified as antifungals, 8 were antiseptics, and 6 considered to be “repositionable” compounds.
Table 1.
Identity and biofilm-inhibitory activity of confirmed hit compounds against C. albicans SC314. Information for each compound includes percent inhibition during primary screen, as well as maximum level of inhibition (efficacy) and calculated IC50 values (potency) from concentration-dependent confirmatory experiments.
| Compound Name | % Inhibition Initial Screen | Maximum % Inhibition from Dose–Response Assays | IC50 (µM) from Dose–Response Assays |
|---|---|---|---|
| Antifungals/Fungicides | |||
| Anidulafungin | 92.32 | 99.18 | <0.078 |
| Cerulenin | 98.27 | 96.08 | 4.625 |
| Ciclopirox | 93.51 | 91.77 | 1.200 |
| Flucytosine | 86.81 | 80.51 | 2.936 |
| JIB04 | 74.9 | 76.2 | 21.220 |
| Oligomycin-a | 73.23 | 72.00 | 5.131 |
| Sertaconazole | 81.89 | 68.02 | 4.687 |
| Sulconazole | 71.52 | 73.51 | 0.114 |
| Terbinafine | 71.14 | 68.89 | 22.400 |
| Terconazole | 69.58 | 69.37 | 1.304 |
| Toyocamycin | 98.45 | 95.38 | 1.582 |
| Voriconazole | 70.42 | 65.32 | <0.078 |
| Antiseptics/Antibacterials | |||
| Alexidine | 98.69 | 99.59 | 1.246 |
| Benzethonium | 99.62 | 100 | 2.436 |
| Benzyldimethylhexadecylammonium | 98.58 | 99.88 | 18.180 |
| Bithionol | 84.19 | 81.24 | 7.344 |
| Brilliant-green | 99.29 | 96.25 | 1.183 |
| Cetrimonium | 99.01 | 99.59 | 4.759 |
| Cetylpyridinium | 100.42 | 99.77 | 6.073 |
| Chlorhexidine | 98.91 | 98.42 | 11.520 |
| Chloroxine | 91.52 | 97.49 | 2.273 |
| Clioquinol | 89.7 | 84.5 | 0.871 |
| Crystal-violet | 98.65 | 96.47 | 1.346 |
| Dequalinium | 98.15 | 96.47 | 15.440 |
| Domiphen | 99.94 | 97.14 | 12.880 |
| Octenidine | 97.93 | 95.02 | 9.014 |
| Oritavancin | 81.94 | 83.87 | 15.460 |
| Phenylmercuric acetate | 99.89 | 98.38 | <0.078 |
| Thiomersal | 99.53 | 96.86 | 0.448 |
| Triclosan | 98.65 | 98.48 | 6.220 |
| Repositionable Compounds | |||
| Anisomycin | 85.08 | 82.3 | 2.973 |
| Atiprimod | 72.38 | 73.22 | 19.450 |
| BAY 11-7082 | 99.67 | 98.01 | 6.733 |
| BAY 11-7085 | 98.84 | 96.81 | 12.180 |
| Broxaldine | 93.36 | 88.35 | 1.708 |
| Ceritinib | 96.09 | 83.04 | 18.650 |
| Clomifene | 79.4 | 100 | 9.279 |
| Darapladib | 99.16 | 99.12 | 18.640 |
| Enasidenib | 89.91 | 99.5 | 11.710 |
| Fingolimod | 72.69 | 93.11 | 9.600 |
| K145 | 96.85 | 96.43 | 18.420 |
| NSC-319726 | 90.59 | 92.05 | 1.993 |
| Otilonium | 97.28 | 97.43 | 6.750 |
| Pinaverium | 76.42 | 75.02 | 25.970 |
| Pitavastatin | 79.18 | 95.24 | 4.015 |
| Plurisin-#1 | 74.23 | 70.88 | 27.760 |
| Sanguinarium-chloride | 97.85 | 96.96 | 4.928 |
| SC-144 | 77.72 | 84.39 | 9.163 |
| Semapimod | 98.83 | 98.71 | 9.055 |
| Sirolimus * | 80.96 | 88.30 | 0.606 |
| Sirolimus * | 73.73 | 85.61 | 0.614 |
| Temsirolimus | 71.54 | 84.43 | 0.376 |
| Toremifene | 81.49 | 84.88 | 4.952 |
| Triclabendazole | 70.53 | 62.4 | 15.740 |
| U-18666A | 97.78 | 95.35 | 4.792 |
* Indicates Sirolimus from two different sources represented in the library and tested independently.
Table 2.
Identity and biofilm-inhibitory activity of confirmed hit compounds against C. auris strain 0390. Information for each compound includes percent inhibition during primary screen, as well as maximum level of inhibition (efficacy) and calculated IC50 values (potency) from concentration-dependent confirmatory experiments.
| Compound Name | % Inhibition Initial Screen | Maximum % Inhibition from Dose–Response Assays | IC50 (µM) from Dose–Response Assays |
|---|---|---|---|
| Antifungals/Fungicides | |||
| Anidulafungin | 77.09% | 95.08% | 3.621 |
| Cerulenin | 82.94% | 87.71% | 30.640 |
| Ciclopirox | 77.78% | 77.67% | 9.779 |
| Flucytosine | 77.25% | 74.40% | 10.600 |
| Ketoconazole | 76.00% | 57.90% | 10.720 |
| Tavaborole | 91.78% | 96.11% | 1.075 |
| Terconazole | 75.64% | 60.70% | 7.146 |
| Antiseptics/Antibacterials | |||
| Alexidine | 97.69% | 87.30% | 3.610 |
| Benzethonium chloride | 104.00% | 89.55% | 8.898 |
| Brilliant-green | 88.94% | 88.08% | 4.603 |
| Cetylpyridinium | 100.19% | 81.16% | 17.060 |
| Crystal-violet | 82.26% | 93.86% | 2.707 |
| Cycloheximide | 73.54% | 86.69% | 2.574 |
| Hexachlorophene | 81.50% | 95.90% | 4.989 |
| Octenidine | 94.32% | 77.06% | 8.423 |
| Phenylmercuric acetate | 102.10% | 95.49% | 0.210 |
| Thiomersal | 92.70% | 92.83% | 0.541 |
| Thonzonium | 101.31% | 91.40% | 8.406 |
| Triclosan | 98.40% | 77.83% | 5.020 |
| Repositionable Compounds | |||
| BAY 11-7082 | 96.07% | 93.24% | 17.460 |
| Bithionol | 89.07% | 74.40% | 5.367 |
| Darapladib | 90.05% | 82.38% | 18.890 |
| Ebselen | 95.21% | 82.59% | 16.910 |
| KHK-IN-1 | 98.80% | 94.47% | 37.140 |
| Plurisin-#1 | 81.30% | 73.37% | 38.860 |
| Semapimod | 90.63% | 96.31% | 11.370 |
| Temsirolimus | 98.62% | 91.40% | 0.965 |
| Toremifene | 90.00% | 96.09% | 14.470 |
| Tribomsalan | 69.57% | 62.93% | 25.560 |
| Zotarolimus | 75.83% | 90.58% | 2.777 |
We note that, although the main focus of this particular screen was to identify compounds that can be potentially repurposed as antifungals, the “existing” antifungal hits helped to confirm the reliability and accuracy of this screen, while, at the same time, antiseptic hits can perhaps identify potential compounds that can prevent or eliminate the contamination of skin and/or environmental surfaces with C. auris [6,7]. For example, benzethonium chloride, hexachlorophene, and ciclopirox were previously identified by our group in the screen of the Prestwick Chemical Library [25] as effective antiseptics or antibacterials against C. auris. The potent antibiofilm and antifungal activity of alexidine has been described before in screens of the Prestwick library [26,27]. In another screen of the Calibr ReFRAME library against C. auris 0390 biofilm formation by our lab, cycloheximide was also identified as having efficacy in the inhibition of biofilm formation [28]. Hexachlorophene and thonzonium were identified recently as having broad-spectrum activity against Aspergillus calidoustus, Fusarium oxysporum, Fusarium solani, Rhizopus oryzae, Lomentospora prolificans, and Lichtheimia corymbifera, which are highly resistant fungi [29].
Since the main objective of these particular screens was to identify compounds with novel inhibitory activity against Candida biofilms, we focused our attention in those drugs with original therapeutic indications other than antifungals or antiseptics, which are designated in Table 1 and Table 2 as “repositionable compounds”. Several of these drugs, particularly those active against both species, merit some further discussion. Toremifene is a selective estrogen receptor modulator that is used in the treatment of estrogen-receptor-positive breast cancer, and it is thought that this compound exhibits antiestrogen behavior in order to inhibit tumor growth [30,31]. Semapimod is known as an anti-inflammatory drug that suppresses inflammatory cytokine production [32]. It has undergone phase II clinical trials for efficacy in the treatment of Crohn’s disease; however, it has yet to move forward into phase III [33]. Plurisin #1 is a pluripotent cell-specific inhibitor that induces apoptosis, and it prevents undifferentiated cells from developing into tumors when tissues are regenerated [34]. Darapladib is known to inhibit lipoprotein-associated phospholipase A2, an indicator of atherosclerosis in patients with coronary heart disease [35]. Although this compound went into phase III clinical trials, it was not able to reduce the risk of cardiovascular events in patients with chronic coronary heart disease; thereby, its development was discontinued for this purpose [36]. Tribomsalan is a photosensitizing agent that was previously used in over-the-counter drugs and cosmetic products, but it has since been removed because photosensitizing agents are thought to cause higher risk of non-melanoma skin cancer [37]. KHK-IN-1 is a ketohexokinase inhibitor, but it has so far not been approved to treat any specific disease [38]. Bithionol is an antihelminthic, especially used in treating liver flukes, and it has been reported to have antibacterial activity [39]. This compound was previously used in topical products; however, it was removed from the market in the U.S. because of skin disorders that occurred, although it is currently used in other countries to treat different types of helminth infections [40]. Although its removal from the U.S. market presents a problem for future repositioning, this drug could be used perhaps to coat medical equipment like catheters to prevent the growth of C. auris.
As seen in Table 1 and Table 2, we identified rapamycin (also referred to as sirolimus) and different rapalogs as the drugs displaying the lowest IC50 value (approaching the picomolar range) among all confirmed compounds. This is not surprising, as rapamycin is a highly potent immunosuppressant also known to display both antifungal and antineoplastic properties [41,42,43]. Temsirolimus, a common hit to both C. albicans and C. auris screens, is currently approved to treat renal cell carcinoma, a type of kidney cancer [44,45], whereas zotarolimus, which was a hit against C. auris only, is used in drug-eluting stents to treat cardiac restenosis, the narrowing of blood vessels [46]. We recently reported on another rapalog, everolimus, as our main leading repositionable compound from the Pandemic Response Box, also with potent biofilm-inhibitory activity against these two Candida species [47]. Temsirolimus is actually an ester analog of rapamycin, and, after administration in humans, it is converted to its major metabolite (rapamycin) via enzymatic hydrolysis, which results in improved solubility and pharmacokinetic properties [48,49]. Like rapamycin, temsirolimus is also a highly specific inhibitor of the mammalian target of rapamycin (mTOR), which has been implicated in multiple tumor-promoting intracellular signaling pathways [50,51]. Temsirolimus was the first mTOR inhibitor to be approved as an anticancer agent; more specifically, it was approved by the FDA for the treatment of advanced renal cell carcinoma in May 2007 [52], and displays promising activity in other cancers, including lymphomas, as well as breast, endometrial, and neuroendocrine cancers [53]. Interestingly, temsirolimus seems to display much more potent antifungal activity compared to other rapamycin analogs (“rapalogs”) such as tacrolimus (FK 506) [47]. Much less is known about the NFκB inhibitor BAY 11-7082 (identified as a hit in both C. albicans and C. auris screens) and its analog BAY 11-7085 (a hit in the C. albicans screen only), which were also among the top leading repositionable candidates. In mammalian cells, they inhibit IκB-α phosphorylation and are known to regulate cytokine function and, specifically, inflammation [54]; as a result, they exhibit broad-spectrum anti-inflammatory activity against multiple targets [55]. They display pharmacological activities that include anticancer, neuroprotective, and anti-inflammatory effects, but have been primarily used as a bioactive small molecule for gene regulation research [54,55]. BAY 11-7082 has also undergone preclinical studies to examine its effect in preventing inflammation after hematopoietic stem cell transplants in mice, its anti-tumor activity, and its efficacy in protecting against psoriasis [56,57,58]. Interestingly, compounds BAY 11-7082 and BAY 11-7085 were among our top hits in a screening of Sigma’s LOPAC library for inhibitors of C. albicans biofilm formation [59], and were also among the main hits reported by Watamoto et al. when they screened the same LOPAC library for antifungal activity against C. albicans [60]. Escobar et al. reported on the activity of BAY 11-7085 against C. albicans single- and mixed-species biofilms (i.e., Staphylococcus aureus and C. albicans) [61], and, most recently, both BAY 11-7085 and BAY 11-7082 were identified as inhibitors of C. albicans filamentation during a high-content imaging screen [62]. From our initial screening and concentration-dependent assays, among these two compounds, BAY 11-7082 displayed higher efficacy (maximum percent inhibition) and potency (lower IC50), and was selected for follow-up studies.
In addition, the antifungal activity of ebselen, which was one of the main hits against C. auris only, has been previously reported by our lab [25] and others [63]. We identified this organoselenium compound as a highly effective inhibitor of planktonic growth of C. auris 0390 in a screen of the Prestwick Chemical Library, with follow-up studies indicating its activity against all other C. auris strains in the CDC panel, several other Candida spp., and different pathogenic fungi [25]. Ebselen is known to have anti-inflammatory and anti-oxidant properties, and it acts as a glutathione peroxidase mimic, which allows it to prevent damage from reactive oxygen species [64]. It has been through phase II clinical trials for its ability to prevent noise-induced hearing loss [65] as well as other diseases. In spite of these trials, ebselen has not yet been approved for the treatment of any disease.
Thereby, we selected temsirolimus, ebselen, and compound BAY 11-7082 (Supplementary Figure S3) as our main repositionable compounds for follow-up studies characterizing their in vitro and in vivo antifungal activity.
3.3. Determination of the Spectrum of Antifungal Activity of the Leading Repositionable Compounds Ebselen, Temsirolimus, and BAY 11-7082
Once their anti-biofilm activity against Candida spp. was fully established, we were interested in testing the activity of the selected leading repositionable compounds temsirolimus and BAY 11-7082 against a panel of medically important fungi, and, in doing so, determining their spectrum of antifungal activity. We have previously reported on similar experiments with ebselen, at a much more limited scale [25], and we wanted to expand our investigations into the spectrum of its antifungal activity here. These assays were performed by the Fungus Testing Laboratory utilizing standardized CLSI methodologies for antifungal susceptibility testing against yeasts and molds [20,21], with MICs determined at both 50% and 100% inhibitory endpoints. Table 3 and Table 4 summarize the in vitro activity of the selected compounds against yeasts and filamentous fungi.
Table 3.
MIC values of the leading repositionable compounds ebselen, temsirolimus, and BAY 11-7082 against multiple clinical isolates belonging to different species of yeast, in comparison to fluconazole. Values are in μg/mL.
| Species | Isolate | Ebselen | Temsirolimus | BAY 11-7082 | Fluconazole | |||
|---|---|---|---|---|---|---|---|---|
| 50% | 100% | 50% | 100% | 50% | 100% | 50% | ||
| C. parapsilosis QC | ATCC 22019 | 0.5 | 2 | 1 | 1 | 4 | 8 | 1 |
| C. krusei QC | ATCC 6258 | 1 | 4 | 1 | 2 | 0.5 | 0.5 | 16 |
| C. albicans | ATCC 90028 | 1 | 2 | 1 | 1 | 1 | 2 | 0.25 |
| SC5314 | 2 | 2 | 1 | 1 | 1 | 2 | ≤0.125 | |
| Ca-1 | 1 | 2 | 1 | 1 | 1 | 1 | 0.5 | |
| C. auris | Cau-1 | 0.125 | 0.25 | 1 | 1 | 2 | 4 | >64 |
| Cau-2 | 0.125 | 1 | 1 | 1 | 0.25 | 1 | >64 | |
| Cau-3 | 0.25 | 0.25 | 1 | 1 | 2 | 4 | 2 | |
| C. glabrata | Cg-1 | 1 | 2 | 0.5 | 1 | 1 | 2 | 64 |
| Cg-2 | 1 | 2 | 0.5 | 1 | 1 | 2 | 4 | |
| Cg-3 | 0.5 | 2 | 1 | 1 | 0.5 | 1 | 0.5 | |
| C. parapsilosis | Cp-1 | 0.25 | 2 | 1 | 1 | 2 | 4 | 0.5 |
| Cp-2 | 0.25 | 2 | 0.5 | 1 | 2 | 4 | 0.25 | |
| Cp-3 | 0.5 | 2 | 1 | 1 | 2 | 4 | 0.5 | |
| Cryptococcus neoformans | Cn-1 | 2 | 2 | 1 | >32 | 1 | 1 | 64 |
| USC1597 | 2 | 4 | 1 | >32 | 1 | 1 | 4 | |
| H99 | 2 | 2 | 1 | >32 | 1 | 1 | 16 | |
Table 4.
MIC values of the leading repositionable compounds ebselen, temsirolimus, and BAY 11-7082 against multiple clinical isolates belonging to different species of filamentous fungi, in comparison to voriconazole and/or posaconazole. Values are in μg/mL.
| Species | Isolate | Ebselen | Temsirolimus | BAY 11-7082 | Voriconazole | Posaconazole | |||
|---|---|---|---|---|---|---|---|---|---|
| 50% | 100% | 50% | 100% | 50% | 100% | 100% | 100% | ||
| P. variotii QC | MYA-3630 | 4 | 4 | 16 | >32 | 1 | 1 | 0.125 | ≤0.03 |
| Rhizopus arrhizus | Rh-1 | 4 | >32 | >32 | >32 | 4 | 4 | - | 1 |
| Rh-2 | 8 | >32 | >32 | >32 | 4 | 4 | - | 0.5 | |
| Rh-3 | 2 | >32 | >32 | >32 | 0.5 | 1 | - | 0.5 | |
| Mucor spp. | Mu-1 | 8 | >32 | >32 | >32 | 4 | 4 | - | 2 |
| Mu-2 | 8 | >32 | >32 | >32 | 2 | 4 | - | 1 | |
| Mu-3 | 4 | >32 | 0.125 | >32 | 2 | 4 | - | 2 | |
| Aspergillus flavus | ATCC204304 | 4 | 4 | >32 | >32 | 4 | 4 | 1 | - |
| Afl-1 | 4 | 4 | >32 | >32 | 2 | 4 | 1 | - | |
| Afl-2 | 4 | 4 | >32 | >32 | 2 | 4 | 1 | - | |
| Aspergillus fumigatus | AF293 | 4 | 4 | >32 | >32 | 1 | 1 | 0.5 | - |
| Af-1 | 4 | 4 | >32 | >32 | 1 | 1 | >16 | - | |
| Af-2 | 4 | 4 | >32 | >32 | 1 | 1 | 4 | - | |
| Fusarium spp. | Fu-1 | 4 | 4 | 2 | >32 | 1 | 1 | >16 | - |
| Fu-2 | 4 | 8 | 2 | >32 | 0.5 | 1 | >16 | - | |
| Fu-3 | 4 | 8 | 1 | >32 | 1 | 2 | >16 | - | |
| Lomentospora prolificans | Sc-1 | 4 | 8 | 0.5 | >32 | 0.5 | 0.5 | >16 | - |
| Scedosporium spp. | Sc-2 | 4 | 4 | 1 | >32 | 0.5 | 0.5 | 2 | - |
| Sc-3 | 2 | 2 | 0.5 | >32 | 0.25 | 0.5 | 1 | - | |
| Altenaria | Al-1 | 0.5 | 2 | >32 | >32 | 0.5 | 1 | 1 | - |
| Curvularia | Cu-1 | 1 | 2 | 16 | >32 | 0.5 | 1 | 0.5 | - |
| Exserohilum | Ex-1 | 1 | 4 | 16 | >32 | 0.5 | 1 | 2 | - |
As previously indicated by our group [25], these results confirmed the relatively broad spectrum of antifungal activity of ebselen against yeasts and filamentous fungi, perhaps with the exception of the Mucorales. As seen in the tables, the MIC values determined for temsirolimus against different species of yeasts indicate the potent antifungal activity of this rapalog against all clinical isolates belonging to the different species of Candida, including C. albicans, C. auris, C. parapsilosis, C. glabrata, and C. krusei, irrespective of their fluconazole resistance. Importantly, these inhibitory concentrations are within the range of the clinically achievable concentration of the drug in blood from patients treated with a conventional dosing regimen of temsirolimus [49]. However, temsirolimus showed limited antifungal activity against Cryptococcus neoformans, with MIC values of 1 μg/mL detected for most isolates when using the 50% reading endpoint, but MIC > 32 μg/mL (the highest concentration used in these assays) for all clinical isolates tested when using the 100% reading endpoint. Likewise, all filamentous fungi tested were not inhibited by temsirolimus, with MIC > 32 μg/mL for all clinical isolates tested belonging to different species of molds when using the 100% reading endpoint, although some lower MIC values when using the 50% reading endpoint seem to indicate some the limited antifungal activity of this rapalog against molds. In contrast, BAY 11-7082 displayed a remarkable broad spectrum of antifungal activity with potent in vitro activity against both yeasts and filamentous fungi. MIC values ranging from 0.25 to 4 μg/mL (at 100% inhibition) were detected for all yeast clinical isolates tested, including all different Candida spp. and C. neoformans, which compared favorably to fluconazole MICs. But perhaps most interesting is the fact that BAY 11-7082 seems to display potent activity also against all species of filamentous fungi tested, including the Mucorales (both Rhizopus and Mucor species), Aspergillus spp., Scedosporium spp., Lomentospora prolificans, Fusarium spp., Alternaria, Curvularia, and Exserohilum spp., with MICs which, in most instances, compared quite favorably with their corresponding MIC values for voriconazole and/or posaconazole. Of note, infections caused by non-Aspergillus molds are becoming increasingly frequent and are difficult to treat, as many of these species are remarkably recalcitrant to most current existing antifungals, often leading to very poor outcomes in patients suffering from these devastating infections [24]. As such, developing antifungals with activity against these filamentous fungi represents one of the most pressing needs in the field of medical mycology.
3.4. In Vivo Efficacy of Ebselen, Temsirolimus, and BAY 11-7082 in the Murine Models of Hematogenously Disseminated Candidiasis
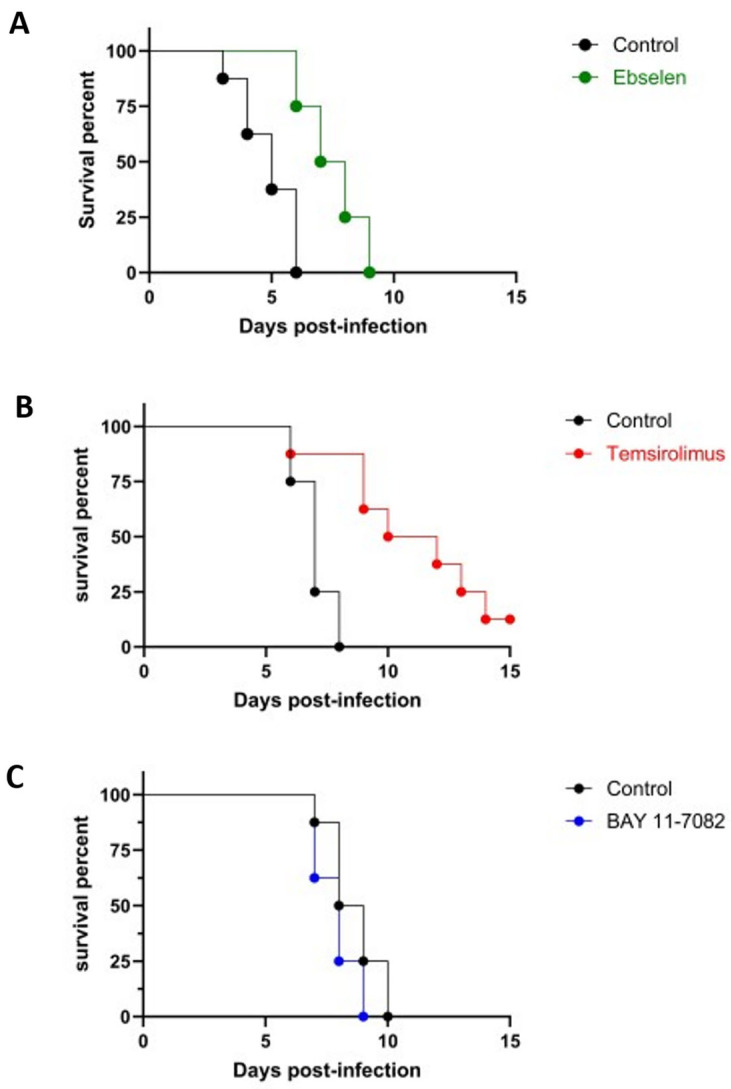
In an initial set of experiments, we proceeded to preliminarily examine the potential in vivo antifungal activity of ebselen, temsirolimus, and compound BAY 11-7082, for which we used the well-established mice model of hematogenously disseminated C. albicans. The resulting survival curves are shown in Figure 2. Treatment with ebselen and temsirolimus increased the median survival of animals infected with C. albicans compared the untreated control group, and these differences were statistically significant (p = 0.0011 and p = 0.0072, respectively). In contrast, under the specific parameters (i.e., infecting inoculum, dose, regimen, etc.) used in this set of experiments, mice treated with compound BAY 11-7082 did not exhibit any significant differences in survival compared to the control group. Although these represent preliminary results, this lack of activity in vivo, as opposed to its potent antifungal effects in vitro, points potentially to the need to improve the drug-like properties of this class of compounds [55].
Figure 2.
Evaluation of protective effects of treatment with ebselen (A), temsirolimus (B), and compound BAY 11-7082 (C) in the murine model of hematogenously disseminated infection by C. albicans.
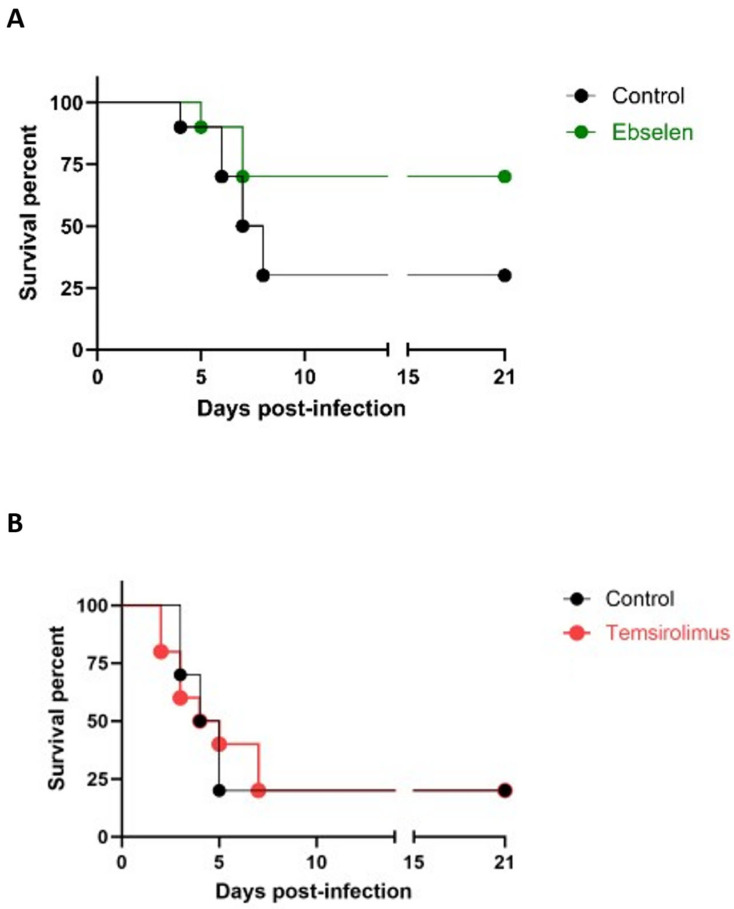
Having demonstrated their in vivo activity in the C. albicans model, we then proceeded to evaluate the protective effects of ebselen and temsirolimus treatment in the murine model of hematogenously disseminated candidiasis caused by C. auris. As shown in Figure 3A, treatment with ebselen increased the median survival of animals from 7.5 days (for untreated control) to over 21 days. When the resulting survival curves were analyzed, there was a trend towards improved survival against C. auris infection, although the differences did not achieve statistical significance (p = 0.0645). Moreover, under the specific conditions used in this set of experiments, we were unable to detect any protective effects of treatment with temsirolimus, with the resulting survival curves virtually overlapping those obtained for the control (untreated) group (Figure 3B). Compared to the C. albicans model, this is a more demanding model since it uses immunosuppressed mice [22,23], which could be partially responsible for the more limited protective effects observed in this model. In future experiments, further assessment of the potential protective effects of treeatment with ebselen and temsirolimus in this model may involve the evaluation of different parameters of both the infection (i.e., infecting inocula) and treatment (i.e., dose, frequency and route of administration, etc.).
Figure 3.
Evaluation of protective effects of treatment with ebselen (A) and temsirolimus (B) in the murine model of hematogenously disseminated infection by C. auris.
In summary, this screen identified compounds in the Repurposing Hub library that inhibited 70% or more of biofilm formation by C. albicans and/or C. auris. Besides known antifungals and antiseptics, several other drugs were identified, with a variety of original therapeutic indications, modes of action, and clinical trial records. Further in vitro and in vivo characterization of the antifungal activity was performed for the leading repositionable compounds ebselen, temsirolimus, and BAY 11-7082. It is likely that some of these compounds have direct effects on the fungal cell, presumably “off-target” relative to their original clinical indications or biological activities on human cells. Despite some promising initial results, further experiments are needed to evaluate and confirm their promise to be repurposed as antifungal drugs for the prevention and treatment of C. albicans and C. auris infections, as well as for potentially other fungal infections, for which there is a dire and urgent need.
Acknowledgments
We thank the Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention (CDC), Clinical and Environmental Microbiology Branch for providing the Candida auris strain (recipient J.L.L.-R.).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof9090879/s1. Figure S1: Representative results of concentration-dependent experiments to confirm the activity and establish the potency of initial hits against C. auris biofilm formation. Figure S2: Venn diagram depicting numbers of confirmed inhibitory compounds against either or both C. albicans and C. auris biofilm formation. Figure S3: Chemical structures and properties of the leading repositionable compounds ebselen (A), temsirolimus (B), and compound BAY 11-082 (C).
Author Contributions
O.H.A., G.W., B.V.B. and L.A.M.D. performed the majority of the experiments and initial data analysis, and contributed to writing the initial draft of the manuscript. H.P.P. contributed to the susceptibility testing experiments. A.K.C. and L.K.N. performed the animal experiments. F.L.W.J., N.P.W., T.F.P. and J.L.L.-R. contributed to the original design of the experiments, supervised the performance of the experiments, analyzed the data, edited the manuscript, and secured funding. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Most of the data are contained within the article or Supplementary Material. Data from primary screenings and dose–response experiments are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This work was supported by NIH grants R33AI140823 and R21AI156100 from the National Institute of Allergy and Infectious Diseases. Additional support was provided by the Margaret Batts Tobin Foundation, San Antonio, TX, USA. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript, and the content is solely the responsibility of the authors.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Thomas-Ruddel D.O., Schlattmann P., Pletz M., Kurzai O., Bloos F. Risk factors for invasive Candida infection in critically ill patients: A systematic review and meta-analysis. Chest. 2022;161:345–355. doi: 10.1016/j.chest.2021.08.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsay S.V., Mu Y., Williams S., Epson E., Nadle J., Bamberg W.M., Barter D.M., Johnston H.L., Farley M.M., Harb S., et al. Burden of candidemia in the United States, 2017. Clin. Infect. Dis. 2020;71:e449–e453. doi: 10.1093/cid/ciaa193. [DOI] [PubMed] [Google Scholar]
- 3.Wall G., Lopez-Ribot J.L. Current antimycotics, new prospects, and future approaches to antifungal therapy. Antibiotics. 2020;9:445. doi: 10.3390/antibiotics9080445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quindos G., Marcos-Arias C., San-Millan R., Mateo E., Eraso E. The continuous changes in the aetiology and epidemiology of invasive candidiasis: From familiar Candida albicans to multiresistant Candida auris. Int. Microbiol. 2018;21:107–119. doi: 10.1007/s10123-018-0014-1. [DOI] [PubMed] [Google Scholar]
- 5.Satoh K., Makimura K., Hasumi Y., Nishiyama Y., Uchida K., Yamaguchi H. Candida auris sp. Nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a japanese hospital. Microbiol. Immunol. 2009;53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti A., Singh S. Multidrug-resistant Candida auris: An epidemiological review. Expert Rev. Anti Infect. Ther. 2020;18:551–562. doi: 10.1080/14787210.2020.1750368. [DOI] [PubMed] [Google Scholar]
- 7.Kean R., Brown J., Gulmez D., Ware A., Ramage G. Candida auris: A decade of understanding of an enigmatic pathogenic yeast. J. Fungi. 2020;6:30. doi: 10.3390/jof6010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher M.C., Denning D.W. The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023;21:211–212. doi: 10.1038/s41579-023-00861-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. 2022. [(accessed on 17 August 2023)]. Available online: https://www.who.int/publications/i/item/9789240060241.
- 10.Ramage G., Martinez J.P., Lopez-Ribot J.L. Candida biofilms on implanted biomaterials: A clinically significant problem. FEMS Yeast Res. 2006;6:979–986. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 11.Sherry L., Ramage G., Kean R., Borman A., Johnson E.M., Richardson M.D., Rautemaa-Richardson R. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg. Infect. Dis. 2017;23:328–331. doi: 10.3201/eid2302.161320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramage G., Borghi E., Rodrigues C.F., Kean R., Williams C., Lopez-Ribot J. Our current clinical understanding of Candida biofilms: Where are we two decades on? APMIS. 2023 doi: 10.1111/apm.13310. in print . [DOI] [PubMed] [Google Scholar]
- 13.Ajetunmobi O.H., Badali H., Romo J.A., Ramage G., Lopez-Ribot J.L. Antifungal therapy of Candida biofilms: Past, present and future. Biofilm. 2023;5:100126. doi: 10.1016/j.bioflm.2023.100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farha M.A., Brown E.D. Drug repurposing for antimicrobial discovery. Nat. Microbiol. 2019;4:565–577. doi: 10.1038/s41564-019-0357-1. [DOI] [PubMed] [Google Scholar]
- 15.Wall G., Lopez-Ribot J.L. Screening repurposing libraries for identification of drugs with novel antifungal activity. Antimicrob. Agents Chemother. 2020;64:e00924-20. doi: 10.1128/AAC.00924-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corsello S.M., Bittker J.A., Liu Z., Gould J., McCarren P., Hirschman J.E., Johnston S.E., Vrcic A., Wong B., Khan M., et al. The drug repurposing hub: A next-generation drug library and information resource. Nat. Med. 2017;23:405–408. doi: 10.1038/nm.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierce C.G., Uppuluri P., Tristan A.R., Wormley F.L., Jr., Mowat E., Ramage G., Lopez-Ribot J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008;3:1494–1500. doi: 10.1038/nprot.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramage G., Vande Walle K., Wickes B.L., Lopez-Ribot J.L. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 2001;45:2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ajetunmobi O.H., Wall G., Bonifacio B.V., Montelongo-Jauregui D., Lopez-Ribot J.L. A 384-well microtiter plate model for Candida biofilm formation and its application to high-throughput screening. Methods Mol. Biol. 2023;2658:53–64. doi: 10.1007/978-1-0716-3155-3_5. [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute . Approved Standard. 2nd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Clsi Document M27-A3. [Google Scholar]
- 21.Clinical and Laboratory Standards Institute . Approved Standard. 2nd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Clsi Document M38-A2. [Google Scholar]
- 22.Wiederhold N.P., Lockhart S.R., Najvar L.K., Berkow E.L., Jaramillo R., Olivo M., Garvey E.P., Yates C.M., Schotzinger R.J., Catano G., et al. The fungal cyp51-specific inhibitor VT-1598 demonstrates in vitro and in vivo activity against Candida auris. Antimicrob. Agents Chemother. 2019;63:e02233-18. doi: 10.1128/AAC.02233-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiederhold N.P., Najvar L.K., Jaramillo R., Olivo M., Patterson H., Connell A., Fukuda Y., Mitsuyama J., Catano G., Patterson T.F. The novel arylamidine T-2307 demonstrates in vitro and in vivo activity against Candida auris. Antimicrob. Agents Chemother. 2020;64:e02198-19. doi: 10.1128/AAC.02198-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiederhold N.P., Patterson T.F. What’s new in antifungals: An update on the in-vitro activity and in-vivo efficacy of new and investigational antifungal agents. Curr. Opin. Infect. Dis. 2015;28:539–545. doi: 10.1097/QCO.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 25.Wall G., Chaturvedi A.K., Wormley F.L., Jr., Wiederhold N.P., Patterson H.P., Patterson T.F., Lopez-Ribot J.L. Screening a repurposing library for inhibitors of multidrug-resistant Candida auris identifies ebselen as a repositionable candidate for antifungal drug development. Antimicrob. Agents Chemother. 2018;62:e01084-18. doi: 10.1128/AAC.01084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mamouei Z., Alqarihi A., Singh S., Xu S., Mansour M.K., Ibrahim A.S., Uppuluri P. Alexidine dihydrochloride has broad-spectrum activities against diverse fungal pathogens. mSphere. 2018;3:e00539-18. doi: 10.1128/mSphere.00539-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siles S.A., Srinivasan A., Pierce C.G., Lopez-Ribot J.L., Ramasubramanian A.K. High-throughput screening of a collection of known pharmacologically active small compounds for identification of Candida albicans biofilm inhibitors. Antimicrob. Agents Chemother. 2013;57:3681–3687. doi: 10.1128/AAC.00680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wall G., Chen E., Hull M.V., Lopez-Ribot J.L. Screening the CALIBR ReFRAME library in search for inhibitors of Candida auris biofilm formation. Front. Cell Infect. Microbiol. 2020;10:597931. doi: 10.3389/fcimb.2020.597931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yousfi H., Ranque S., Cassagne C., Rolain J.M., Bittar F. Identification of repositionable drugs with novel antimycotic activity by screening the Prestwick Chemical Library against emerging invasive moulds. J. Glob. Antimicrob. Resist. 2020;21:314–317. doi: 10.1016/j.jgar.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Hong J., Huang J., Shen L., Zhu S., Gao W., Wu J., Huang O., He J., Zhu L., Chen W., et al. A prospective, randomized study of toremifene vs. Tamoxifen for the treatment of premenopausal breast cancer: Safety and genital symptom analysis. BMC Cancer. 2020;20:663. doi: 10.1186/s12885-020-07156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mustonen M.V., Pyrhonen S., Kellokumpu-Lehtinen P.L. Toremifene in the treatment of breast cancer. World J. Clin. Oncol. 2014;5:393–405. doi: 10.5306/wjco.v5.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J., Grishin A.V., Ford H.R. Experimental anti-inflammatory drug semapimod inhibits TLR signaling by targeting the TLR chaperone gp96. J. Immunol. 2016;196:5130–5137. doi: 10.4049/jimmunol.1502135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dotan I., Rachmilewitz D., Schreiber S., Eliakim R., van der Woude C.J., Kornbluth A., Buchman A.L., Bar-Meir S., Bokemeyer B., Goldin E., et al. A randomised placebo-controlled multicentre trial of intravenous semapimod HCL for moderate to severe crohn’s disease. Gut. 2010;59:760–766. doi: 10.1136/gut.2009.179994. [DOI] [PubMed] [Google Scholar]
- 34.Ben-David U., Gan Q.F., Golan-Lev T., Arora P., Yanuka O., Oren Y.S., Leikin-Frenkel A., Graf M., Garippa R., Boehringer M., et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12:167–179. doi: 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Riley R.F., Corson M.A. Darapladib, a reversible lipoprotein-associated phospholipase a2 inhibitor, for the oral treatment of atherosclerosis and coronary artery disease. IDrugs. 2009;12:648–655. [PubMed] [Google Scholar]
- 36.Mullard A. Gsk’s darapladib failures dim hopes for anti-inflammatory heart drugs. Nat. Rev. Drug Discov. 2014;13:481–482. doi: 10.1038/nrd4381. [DOI] [PubMed] [Google Scholar]
- 37.Robinson S.N., Zens M.S., Perry A.E., Spencer S.K., Duell E.J., Karagas M.R. Photosensitizing agents and the risk of non-melanoma skin cancer: A population-based case-control study. J. Investig. Dermatol. 2013;133:1950–1955. doi: 10.1038/jid.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maryanoff B.E., O’Neill J.C., McComsey D.F., Yabut S.C., Luci D.K., Jordan A.D., Jr., Masucci J.A., Jones W.J., Abad M.C., Gibbs A.C., et al. Inhibitors of ketohexokinase: Discovery of pyrimidinopyrimidines with specific substitution that complements the atp-binding site. ACS Med. Chem. Lett. 2011;2:538–543. doi: 10.1021/ml200070g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaliszewicz S., Swiezawska E. Bithionol as an antibacterial, antifungal and antihelminthic agent. Pol. Tyg. Lek. 1968;23:1982–1984. [PubMed] [Google Scholar]
- 40.Delahanty J.N., Evans J.C., Rowlands C.C., Pendlington R.U., Barratt M.D. The photochemical binding of bithionol to soluble proteins and peptides. Biochem. Pharmacol. 1989;38:3879–3883. doi: 10.1016/0006-2952(89)90599-6. [DOI] [PubMed] [Google Scholar]
- 41.Li J., Kim S.G., Blenis J. Rapamycin: One drug, many effects. Cell Metab. 2014;19:373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sehgal S.N., Baker H., Vezina C. Rapamycin (ay-22,989), a new antifungal antibiotiC. II. Fermentation, isolation and characterization. J. Antibiot. 1975;28:727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- 43.Vezina C., Kudelski A., Sehgal S.N. Rapamycin (ay-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 44.Gore M.E. Temsirolimus in the treatment of advanced renal cell carcinoma. Ann. Oncol. 2007;18((Suppl. S9)):ix87–ix88. doi: 10.1093/annonc/mdm299. [DOI] [PubMed] [Google Scholar]
- 45.Zanardi E., Verzoni E., Grassi P., Necchi A., Giannatempo P., Raggi D., De Braud F., Procopio G. Clinical experience with temsirolimus in the treatment of advanced renal cell carcinoma. Ther. Adv. Urol. 2015;7:152–161. doi: 10.1177/1756287215574457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J.X., Yeh R.W. Safety and effectiveness of zotarolimus-eluting stents for percutaneous coronary intervention: A systematic review. Future Cardiol. 2018;14:251–267. doi: 10.2217/fca-2017-0091. [DOI] [PubMed] [Google Scholar]
- 47.Ajetunmobi O.H., Chaturvedi A.K., Badali H., Vaccaro A., Najvar L., Wormley F.L., Jr., Wiederhold N.P., Patterson T.F., Lopez-Ribot J.L. Screening the medicine for malaria venture’s pandemic response box to identify novel inhibitors of Candida albicans and Candida auris biofilm formation. APMIS. 2023 doi: 10.1111/apm.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma W.W., Jimeno A. Temsirolimus. Drugs Today. 2007;43:659–669. doi: 10.1358/dot.2007.43.10.1148059. [DOI] [PubMed] [Google Scholar]
- 49.Mizuno T., Fukuda T., Christians U., Perentesis J.P., Fouladi M., Vinks A.A. Population pharmacokinetics of temsirolimus and sirolimus in children with recurrent solid tumours: A report from the children’s oncology group. Br. J. Clin. Pharmacol. 2017;83:1097–1107. doi: 10.1111/bcp.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malizzia L.J., Hsu A. Temsirolimus, an mtor inhibitor for treatment of patients with advanced renal cell carcinoma. Clin. J. Oncol. Nurs. 2008;12:639–646. doi: 10.1188/08.CJON.639-646. [DOI] [PubMed] [Google Scholar]
- 51.Rini B.I. Temsirolimus, an inhibitor of mammalian target of rapamycin. Clin. Cancer Res. 2008;14:1286–1290. doi: 10.1158/1078-0432.CCR-07-4719. [DOI] [PubMed] [Google Scholar]
- 52.Kwitkowski V.E., Prowell T.M., Ibrahim A., Farrell A.T., Justice R., Mitchell S.S., Sridhara R., Pazdur R. FDA approval summary: Temsirolimus as treatment for advanced renal cell carcinoma. Oncologist. 2010;15:428–435. doi: 10.1634/theoncologist.2009-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trivedi N.D., Armstrong S., Wang H., Hartley M., Deeken J., Ruth He A., Subramaniam D., Melville H., Albanese C., Marshall J.L., et al. A phase I trial of the mtor inhibitor temsirolimus in combination with capecitabine in patients with advanced malignancies. Cancer Med. 2021;10:1944–1954. doi: 10.1002/cam4.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pierce J.W., Schoenleber R., Jesmok G., Best J., Moore S.A., Collins T., Gerritsen M.E. Novel inhibitors of cytokine-induced ikappabalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 55.Lee J., Rhee M.H., Kim E., Cho J.Y. Bay 11-7082 is a broad-spectrum inhibitor with anti-inflammatory activity against multiple targets. Mediat. Inflamm. 2012;2012:416036. doi: 10.1155/2012/416036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irrera N., Vaccaro M., Bitto A., Pallio G., Pizzino G., Lentini M., Arcoraci V., Minutoli L., Scuruchi M., Cutroneo G., et al. BAY 11-7082 inhibits the NF-kappab and NLRP3 inflammasome pathways and protects against IMQ-induced psoriasis. Clin. Sci. 2017;131:487–498. doi: 10.1042/CS20160645. [DOI] [PubMed] [Google Scholar]
- 57.Kim Y.S., Kim J.S., Kwon J.S., Jeong M.H., Cho J.G., Park J.C., Kang J.C., Ahn Y. Bay 11-7082, a nuclear factor-kappab inhibitor, reduces inflammation and apoptosis in a rat cardiac ischemia-reperfusion injury model. Int. Heart J. 2010;51:348–353. doi: 10.1536/ihj.51.348. [DOI] [PubMed] [Google Scholar]
- 58.White D.E., Burchill S.A. Bay 11-7082 induces cell death through nf-kappab-independent mechanisms in the ewing’s sarcoma family of tumours. Cancer Lett. 2008;268:212–224. doi: 10.1016/j.canlet.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 59.Martinez-Delgado L. Master’s Thesis. The University of Texas at San Antonio; San Antonio, TX, USA: 2018. Repurposing for Antifungal Drug Development: Large-Scale Screening of the Library of Pharmacologically Active Compounds (LOPAC®1280) for Identification of Candida albicans Biofilm Inhibitors. [Google Scholar]
- 60.Watamoto T., Egusa H., Sawase T., Yatani H. Screening of pharmacologically active small molecule compounds identifies antifungal agents against Candida biofilms. Front. Microbiol. 2015;6:1453. doi: 10.3389/fmicb.2015.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Escobar I.E., Possamai Rossatto F.C., Kim S.M., Kang M.H., Kim W., Mylonakis E. Repurposing kinase inhibitor BAY 11-7085 to combat Staphylococcus aureus and Candida albicans biofilms. Front. Pharmacol. 2021;12:675300. doi: 10.3389/fphar.2021.675300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Metzner K., O’Meara M.J., Halligan B., Wotring J.W., Sexton J.Z., O’Meara T.R. Imaging-based screening identifies modulators of the eif3 translation initiation factor complex in Candida albicans. Antimicrob. Agents Chemother. 2023;67:e0050323. doi: 10.1128/aac.00503-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Oliveira H.C., Monteiro M.C., Rossi S.A., Peman J., Ruiz-Gaitan A., Mendes-Giannini M.J.S., Mellado E., Zaragoza O. Identification of off-patent compounds that present antifungal activity against the emerging fungal pathogen Candida auris. Front. Cell Infect. Microbiol. 2019;9:83. doi: 10.3389/fcimb.2019.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azad G.K., Tomar R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014;41:4865–4879. doi: 10.1007/s11033-014-3417-x. [DOI] [PubMed] [Google Scholar]
- 65.Kil J., Lobarinas E., Spankovich C., Griffiths S.K., Antonelli P.J., Lynch E.D., Le Prell C.G. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2017;390:969–979. doi: 10.1016/S0140-6736(17)31791-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Most of the data are contained within the article or Supplementary Material. Data from primary screenings and dose–response experiments are available from the corresponding author upon request.