Abstract
Background: The potentially harmful effects of air pollution on the human health have been already presented in epidemiological studies, suggesting a strong association with increased morbidity and mortality. The aim of the study was to evaluate a possible relationship between coronary artery lesion progression related to habitation place (cities vs. villages) and air pollution. Methods: There were 148 (101 men and 47 women) patients with a median age of 70 (63–74) years enrolled into retrospective analysis based on the coronary angiography results and their habitation place. Patients with stable coronary syndrome, who underwent repeated percutaneous coronary interventions were enrolled into the analysis based on demographical and clinical characteristics combined with annual exposure to air pollution (PM2.5, PM10, and NO2). Results: The results of multivariable regression analysis showed a significant relationship between coronary artery lesion progression requiring percutaneous intervention and NO2 chronic exposure in patients living in cities of Poland (OR 2.00, 95% CI: 0.41–9.62, p < 0.001). The predictive value of air pollution exposure at habitation place for coronary artery lesion progression requiring percutaneous intervention was evaluated by receiver-operator curve analysis, which revealed an area under the curve of 0.939, yielding a sensitivity of 87.1% and specificity of 90.7%. Conclusions: Coronary artery lesion progression can be related to chronic exposure to NO2 air pollution in patients living in cities in Poland.
Keywords: NO2, air pollution, CAD, PM2.5, PM10
1. Introduction
Coronary artery disease is still a major cardiovascular diseases and a leading cause of mortality affecting modern population, though over the last decade, morbidity has declined [1]. A growing body of studies has investigated possible modifiable risk factors like lifestyle or physical activity that may have potential influence on patients’ prognosis [2]. The heterogeneity of coronary artery disease risk factors secondary to age-related co-morbidities were postulated by Simonetto et al. [3].
The traditional risk factors include arterial hypertension, diabetes mellitus, hyperlipidemia, homocystinuria, and psychosocial stress [4]. Overweight/obesity is considered as non-traditional and an independent risk factor for coronary artery disease development [5]. The gender differences in ischemic disease epidemiology were postulated [6,7], and gender-related differences in therapeutic approaches in the BASKET-SMALL 2 trial were presented [8]. Although more than 70% of patients referred for surgical revascularization due to multivessel disease are males [9], female gender is claimed to be associated with higher risk for greater complications and early mortality following surgery [10].
The potentially harmful effects of air pollution on the human health have already been presented in epidemiological studies suggesting the strong association with increased morbidity and mortality [11,12,13]. There is an escalating body of evidence indicating the role of air pollution exposure in the development of cardiovascular disease [14]. In the U.K. BioBank study, a healthy diet and annual average air pollutant concentrations of particulate matter (PM) with diameters ≤ 2.5 (PM2.5) and ≤10 (PM10) and also nitrogen oxides (NO2) were found related to the risk of all-cause and cardiovascular morbidity and mortality [15]. The results of the ELAPSE project revealed an association between PM2.5 exposure and increased incidence of stroke, followed by the association between coronary heart disease and nitrogen oxides [16].
Though the negative effect of air pollution on cardiovascular health is already known, the data on its relationship with the progression of coronary artery disease in individuals are lacking. The aim of the study was to evaluate the possible relationship between coronary artery lesion progression, evaluated by the need for repeated coronary angiography and angioplasty due to the occurrence of clinical symptoms, and air pollution in habitation place based on its measurements.
2. Materials and Methods
There were 148 (101 men and 47 women) patients with a median age of 70 (63–74) years enrolled into the study.
This was a single-center, retrospective analysis performed based on data obtained from patients who underwent repetitive hospitalizations due to stable coronary disease syndromes between 2018 and 2022.
Demographic and clinical characteristics, including echocardiographic and laboratory results, were collected. Results of the first and consecutive coronary angiography examinations were analyzed. The atherosclerotic culprit lesion was estimated as significant for atherosclerosis on angiography as narrowing above 30%. The culprit lesion suitable for PCI intervention was defined as a stenosis above 70% or 50% in cases of non- and left-main coronary artery disease, respectively.
The progression was estimated by progression of previous mild stenosis or a new stenosis and the requirement for percutaneous coronary intervention.
Participants with acute cardiovascular syndromes and those with congestive heart failure or hematological, oncological, thyroid, liver, and kidney diseases or corticosteroid treatment were excluded from the analysis.
The basis for assessing the level of individual exposure for air pollution for particulate matter (PM) with diameters ≤ 2.5 (PM2.5) and ≤10 (PM10) and nitrogen dioxides (NO2) of each of the patients comprised spatial distributions of air concentration fields for Poland provided by the Chief Inspectorate of Environmental Protection [17]. The maps were based on the results for the national air quality modelling system elaborated by the Institute of Environmental Protection—National Research Institute in Poland (IEP-NRI) in accordance with the legal obligation set out in Environmental Protection Act in Poland (Art 66, paragraph 6).
The national air quality modelling system in IEP-NRI, which is based on two procedures, namely (1) elaboration of yearly high-resolution bottom-up emission inventory for Poland and (2) elaboration air quality maps based on GEM-AQ model, operates in the Copernicus Atmosphere Monitoring Service—Regional Production (CAMS2_40) [18].
High-resolution bottom-up emission inventory data for Poland are developed and maintained by IEP-NRI and stored in the Central Emission Database [19]. All annual emissions data were elaborated based on Standard Nomenclature for Air Pollution (SNAP) categories [20], including main air pollutants emission sectors in Poland, like residential emission [21], energy production, industry, transport, or agriculture. GEM-AQ is a semi-Lagrangian chemical weather model developed at Environment Canada in which air quality processes and tropospheric chemistry are implemented in a weather prediction model, the Global Environmental Multiscale (GEM) [22]. In the GEM-AQ, the air concentration fields are performed using a 0.025-degree resolution grid.
Personal exposure was estimated using patients’ home addresses and air quality concentration downscaling statistical methods based on an expert-in-the-loop stepwise regression procedure elaborated upon in the Neurosmog project [23], validated experimentally for real-life data from various sources aiming at predicting air pollution [24].
Electrocardiography (ECG) and transthoracic echocardiography (TTE) were performed in each patient before the procedures. Blood samples were collected before the procedures. Whole-blood analysis was measured with routine hematology analyzer (Sysmex Euro GmbH, Norderstedt, Germany). GFR was calculated by simplified modification of diet in renal disease (MDRD) formula.
Patients were divided into subgroups according to the habitation place. Group 1 was composed of 90 patients with a median age of 68 (57–73) years living in villages and small towns (below 50,000 citizens) compared with 58 patients with a median age of 72 (65–77) years living in a city agglomeration. The groups were matched for demographical characteristics.
Statistical Analysis
Continuous variables were reported as medians and interquartile ranges (Q1–Q3) since data did not follow normal distribution. Categorical data are presented as numbers and percentages. The comparison of interval parameters between proximal and non-proximal groups was performed by Mann–Whitney test. Categorical data were compared by chi-square test of independence. A logistic regression analysis was performed to identify potential predictors of coronary artery disease culprit lesion. Both univariate and multivariable models were used. The multivariable model was assessed by best subset method. The results are presented as odds ratio (OR) and its 95% confidence intervals (95%CI). Additionally, a receiver-operator characteristic (ROC) curve was determined for the predict score of the significant model including factor, which occurred as predictive in the multivariable analysis.
3. Results
All patients presented with chronic anginal symptoms and were hospitalized for planned coronary angiography. The first examination was performed before 2019, and second admission was carried out after 2021. The median interval between repeated examination was 1224 (680–1513) days.
There were 114 (77%) vs. 34 (23%) patients presenting significant vs non-significant atherosclerotic lesions on initial assessment, respectively. From the mentioned 34 patients, there were 33 normal angiograms and 1 presenting lumen coronary artery stenosis < 30%.
On repeated examination from 34 patients, normal angiograms were found in 4 (12%), and non-significant progression of atherosclerotic lesions (<30%) was noticed in 6 (18%) patients. From 34 initially normal angiograms, 24 (71%) patients required percutaneous intervention on repeated examination.
On initial angiography, stent implantation was required in 109 patients; among them, the percutaneous angioplasty in 65 (60%) patients was performed during repeated angiography in comparison to 44 (40%) subjects with normal consecutive angiograms.
The majority of patients presented with co-morbidities (Table 1). Laboratory and imaging examinations results were evaluated. Both subgroups represented similar populations except for differences in left ventricular diameter and red blood cells width (RDW), which were both higher in group 2 (Table 1).
Table 1.
Clinical and demographical characteristics.
| Parameter | Group 1 | Group 2 | p |
|---|---|---|---|
| n = 90 | n = 58 | ||
| Sex (male (%)/female (%)) | 66 (73)/24 (27) | 36 (62)/22 (38) | 0.657 |
| Age (years) (median (Q1–Q3)) | 68 (57–73) | 72 (65–77) | 0.074 |
| Weight (kg) (median (Q1–Q3)) | 93 (92–94) | 90 (67–94) | 0.434 |
| Height (cm) (median (Q1–Q3)) | 168 (162–175) | 171 (161–176) | 0.703 |
| Body mass index (median (Q1–Q3)) | 29.7 (27.2–33.8) | 29.1 (25.7–31.5) | 0.398 |
| Co-morbidities: | |||
| Arterial hypertension (n(%)) | 47 (52) | 34 (59) | 0.445 |
| Diabetes mellitus (n(%)) | 26 (29) | 14 (24) | 0.525 |
| Hypercholesterolemia (n(%)) | 48 (53) | 30 (52) | 0.848 |
| COPD (n(%)) | 5 (6) | 3 (5) | 0.919 |
| Thyroid disease (n(%)) | 10 (11) | 5 (9) | 0.624 |
| Atrial fibrillation (n(%)) | 8 (9) | 4 (7) | 0.665 |
| Current nicotinism (n(%)) | 3 (3) | 1 (2) | 0.556 |
| Stroke (n(%)) | 3 (3) | 3 (5) | 0.58 |
| PAD (n(%)) | 9 (10) | 5 (9) | 0.78 |
| Current nicotinism (n(%)) | 3 (3) | 1 (2) | 0.556 |
| Echocardiographic results | |||
| Left ventricular diameter (mm) (median (Q1–Q3)) | 40 (38–43) | 47 (44–50) | 0.044 * |
| Left atrium diameter (mm) (median (Q1–Q3)) | 35 (30–37) | 39 (37–42) | 0.137 |
| Left ventricular ejection fraction (%)(median (Q1–Q3)) | 65 (60–65) | 55 (55–60) | 0.199 |
| Laboratory results | |||
| WBC (×109/L) (median (Q1–Q3)) | 7.48 (7.29–8.41) | 7.44 (6.13–8.67) | 0.669 |
| Neutrophils (×109/L) (median (Q1–Q3)) | 5.28 (4.90–5.64) | 4.14 (3.54–5.58) | 0.269 |
| Lymphocyte (×109/L) (median (Q1–Q3)) | 1.70 (1.54–2.11) | 1.98 (1.45–2.20) | 0.88 |
| Monocyte (×109/L) (median (Q1–Q3)) | 0.50 (0.42–0.52) | 0.46 (0.41–0.57) | 0.801 |
| Platelets (×109/L) (median (Q1–Q3)) | 218 (216–231) | 218 (178–255) | 0.88 |
| Hemoglobin (mmol/L) (median (Q1–Q3)) | 7.70 (7.55–8.30) | 8.60 (8.25–8.98) | 0.19 |
| Hematocrit (%) (median (Q1–Q3)) | 36 (35–40) | 38 (34–43) | 0.21 |
| MPV (fl) (median (Q1–Q3)) | 9.1 (8.5–9.4) | 8.4 (7.9–9.2) | 0.58 |
| MCV (median (Q1–Q3)) | 90 (89–92) | 93 (90–97) | 0.313 |
| MCHC (mmol/L) (median (Q1–Q3)) | 20.87 (20.82–20.97) | 20.96 (20.59–21.40) | 0.802 |
| RDW (%) (median (Q1–Q3)) | 12.60 (12.55–12.90) | 13.80 (13.18–14.18) | 0.030 * |
| Creatinine (median (Q1–Q3)) | 90 (83–108) | 92 (71–108) | 0.725 |
| GFR (umol/l) (median (Q1–Q3)) | 59 (57–65) | 74 (60–86) | 0.242 |
| Ureic acid (median (Q1–Q3)) | 296 (272–352) | 363 (317–403) | 0.634 |
Abbreviations: COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MPV, mean platelets volume; PAD, peripheral artery disease; RDW, red cells distribution width; * statistically significant.
The angiographic characteristics are presented in Table 2, indicating significant difference (p = 0.049) in culprit lesions in repeated coronary angiography between both groups. The numbers of two-stents procedures (p = 0.021) and bifurcation culprit lesion (p = 0.023) were statistically different.
Table 2.
Comparison of coronary angiographic results within both groups.
| Group 1 | Group 2 | p | |
|---|---|---|---|
| n = 90 | n = 58 | ||
| First angiographic results | |||
| 1. Disease (>30% stenosis): | |||
| 1. LMCA disease (n,%) | 6 (7) | 4 (7) | 0.96 |
| 2. LAD disease (n,%) | 52 (58) | 33 (57) | 0.918 |
| 3. Cx disease (n,%) | 29 (32) | 16 (28) | 0.552 |
| 4. RCA disease (n,%) | 42 (47) | 23 (40) | 0.404 |
| 2. Normal angiogram (n,%) | 21 (23) | 11 (19) | 0.532 |
| 3. Culprit lesion > 70% (n,%) | 75 (83) | 56 (97) | 0.179 |
| 4. Performed procedures: | |||
| 1. Single PCIs (n,%) | 55 (61) | 37 (64) | 0.645 |
| 2. Two stents (n,%) | 10 (11) | 7 (12) | 0.773 |
| 3. On bifurcation (n,%) | 5 (6) | 5 (9) | 0.84 |
| Second angiographic results | |||
| 1. Disease (>30% stenosis): | |||
| 1. LMCA disease (n,%) | 7 (8) | 5 (9) | 0.858 |
| 2. LAD disease (n,%) | 41 (46) | 26 (45) | 0.933 |
| 3. Cx disease (n,%) | 29 (32) | 11 (19) | 0.078 |
| 4. RCA disease (n,%) | 33 (37) | 26 (45) | 0.325 |
| 2. Normal angiogram (n,%) | 3 (3) | 1 (2) | 0.079 |
| 3. Culprit lesion >70% (n,%) | 46 (51) | 55 (9) | 0.049 * |
| 4. Performed procedures: | |||
| 1. Single PCIs (n,%) | 44 (49) | 26 (45) | 0.732 |
| 2. Two stents (n,%) | 1 (1) | 11 (19) | 0.021 * |
| 3. Bifurcation (n,%) | 0 (0) | 7 (12) | 0.023 * |
| 5. Culprit lesion change (%) | 55 (10–90) | 68 (15–97) | 0.383 |
Abbreviations: Cx, circumflex artery; LAD, left descending artery; LMCA, left main coronary artery; PCI, percutaneous intervention; RCA, right coronary artery. * statistically significant.
The significant differences in mean annular exposure to air pollutants between groups are presented in Table 3.
Table 3.
Exposure to air pollution.
| Parameter | Group 1 | Group 2 | p |
|---|---|---|---|
| n = 90 | n = 58 | ||
| Particulate Matter < 2.5 (PM < 2.5) | |||
| Mean exposure in 2019 (median (Q1–Q3)) | 17.5 (15.5–19.1) | 17.5 (16.6–18.7) | 0.736 |
| Mean exposure in 2020 (median (Q1–Q3)) | 12.6 (11.0–15.1) | 16.4 (14.7–19.1) | <0.001 |
| Mean exposure in 2021 (median (Q1–Q3)) | 14.6 (13.4–16.3) | 17.7 (15.8–19.2) | <0.001 |
| Mean exposure 2019–2021 (median (Q1–Q3)) | 15.8 (13.6–16.6) | 17.2 (16.0–18.9) | <0.001 |
| Maximal exposure (median (Q1–Q3)) | 18 (15.5–19.3) | 18.9 (16.9–20.0) | 0.269 |
| Particulate Matter < 10 (PM < 10) | |||
| Mean exposure in 2019 (median (Q1–Q3)) | 23.4 (21.8–25.0) | 26.4 (24.6 28.6) | <0.001 |
| Mean exposure in 2020 (median (Q1–Q3)) | 20.2 (18.4–22.0) | 23.7 (21.9–28.1) | <0.001 |
| Mean exposure in 2021 (median (Q1–Q3)) | 23.3 (22.1–28.1) | 25.9 (23.6–29.7) | <0.001 |
| Mean exposure 2019–2021 (median (Q1–Q3)) | 22.2 (21.5–23.5) | 25.4 (23.6 28.7) | <0.001 |
| Maximal exposure (median (Q1–Q3)) | 24.0 (22.6–26.00) | 26.6 (25.2–30.1) | <0.001 |
| Nitrogen Oxides (NO2) | |||
| Mean exposure in 2019 (median (Q1–Q3)) | 12.2 (10.5–14.2) | 22.4 (19.4–24.00) | <0.001 |
| Mean exposure in 2020 (median (Q1–Q3)) | 11.2 (10.2–13.1) | 17.8 (16.5–18.7) | <0.001 |
| Mean exposure in 2021 (median (Q1–Q3)) | 12.6 (12.00–13.8) | 18.1 (17.0–19.5) | <0.001 |
| Mean exposure 2019–2021 (median (Q1–Q3)) | 12.1 (11.0–13.6) | 19.4 (17.8–20.7) | <0.001 |
| Maximal exposure (median (Q1–Q3)) | 13.0 (12.0–14.2) | 22.5 (19.7–24.0) | <0.001 |
3.1. Uni- and Multivariable Analysis for CAD Prediction in Patients’ Living in Cities
The univariable and multivariable analysis was performed to point out possible risk factors for culprit lesion progression and presented in Table 4.
Table 4.
Univariable and multivariable analysis for NO2 exposure related to CAD progression.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Demographical factors: | - | - | - | |||
| Sex | 1.24 | 0.42–1.84 | <0.001 * | |||
| Age | 1.05 | 0.10–1.13 | 0.055 | |||
| Co-morbidities | - | - | - | |||
| Arterial hypertension | 5.43 | 0.42–29.63 | 0.009 * | |||
| DM | 2.83 | 0.01–2.92 | 0.073 | |||
| Hyperlipidemia | 2.65 | 0.50–14.55 | <0.001 * | |||
| Thyroid disease | 1.99 | 0.14–1.15 | 0.418 | |||
| Laboratory results: | - | - | - | |||
| WBC | 0.66 | 0.03–0.21 | 0.197 | |||
| Hemoglobin | 2.06 | 0.61–2.05 | 0.286 | |||
| NLR | 1.09 | 0.77–1.09 | 0.557 | |||
| MLR | 4.83 | 1.67–4.82 | 0.342 | |||
| SIRI | 2.23 | 1.14–2.74 | 0.42 | |||
| MCHC | 0.77 | 0.61–2.43 | 0.243 | |||
| Creatinine | 1.01 | 0.03–0.53 | 0.486 | |||
| Ureic acid | 1 | 0.03–1.02 | 0.734 | |||
| Air pollution—PM < 2.5 | - | - | - | |||
| PM < 2.5 in 2019 | 0.98 | 0.796–1.722 | 0.859 | |||
| PM < 2.5 in 2020 | 4.13 | 0.23–6.42 | <0.001 | |||
| PM < 2.5 in 2021 | 1.53 | 0.20–6.58 | <0.001 | |||
| Mean PM < 2.5 exposure | 1.44 | 0.21–5.17 | <0.001 | |||
| Max PM < 2.5 exposure | 1.15 | 0.01–2.76 | 0.033 | |||
| Air pollution—PM < 10 | - | - | - | |||
| PM < 10 in 2019 | 1.39 | 0.20–4.55 | <0.001 | |||
| PM < 10 in 2020 | 1.43 | 0.23–4.87 | <0.001 | |||
| PM < 10 in 2021 | 1.28 | 0.13–3.55 | <0.001 | |||
| Mean PM < 10 exposure | 1.44 | 0.23–5.01 | <0.001 | |||
| Max PM < 10 exposure | 1.33 | 0.17–4.04 | <0.001 | |||
| Air pollution—NO2 | ||||||
| NO2 in 2019 | 1.57 | 0.33–5.80 | <0.001 | |||
| NO2 in 2020 | 1.99 | 0.49–8.80 | <0.001 | |||
| NO2 in 2021 | 2.35 | 0.61–1.10 | <0.001 | |||
| Mean | 1.93 | 0.48–8.45 | <0.001 | 2 | 0.41–9.62 | <0.001 |
| Max | 1.76 | 0.41–7.27 | <0.001 | |||
Abbreviations: DM, diabetes mellitus; max, maximum; MCHC, mean corpuscular hemoglobin concentration; NO2, nitrogen oxides; OR, odds ratio; RDW, red cells distribution width; WBC, white blood count. * statistically significant.
3.2. Receiver-Operator Curve Prediction for Coronary Artery Disease in Patients Living in Cities
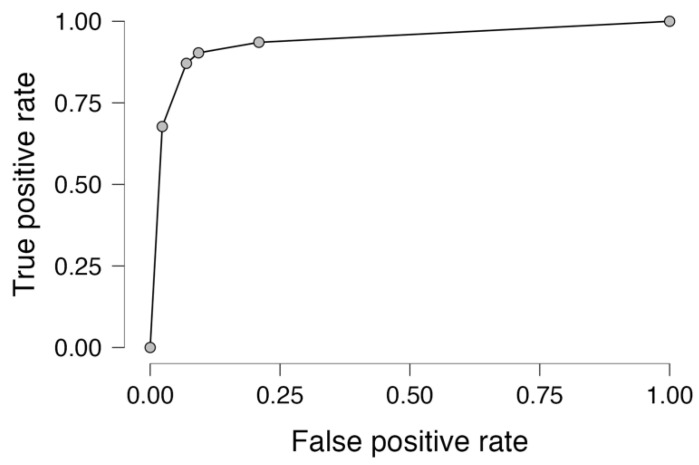
The predictive value of the significant factor in multivariable analysis, mean NO2, for coronary artery lesion progression related to air pollution exposure related to habitation place was evaluated by receiver-operator curve, revealing an area under the curve of 0.939 and yielding a sensitivity of 87.1% and specificity of 90.7%, as presented in Figure 1.
Figure 1.
Receiver-operator curve for mean NO2 exposure in coronary artery disease patients’ living in cities.
4. Discussion
Our study presents the relationship between coronary artery lesion disease progression and mean values of nitrogen-oxides-related air pollution in patients living in cities in one of Europe’s country regions. The analysis was performed in a country where the average density population is estimated at 117 vs. 1222/km2 in non-cities vs. cities areas, respectively. The results of our study revealed significant differences in polluted air components, such as PM2.5, PM10, and NO2, in patients living in cities in comparison to rural areas. Moreover, we found significant differences in coronary angiography results related to culprit lesion progression in patients exposed to polluted air. Chen et al. [25] in their analysis found increased mortality related to PM2.5 air pollution. In the analysis of Pasada-Sanchez et al. [26], ozone and PM2.5 exposure were found associated with premature coronary artery disease in metropolitan areas.
Not only long-term but also short-term exposure to PM2.5 and PM10 were found significant for morbidity, including lung diseases [27]. The necessity for better and more adequate air control is under continuous investigation, and new devices are under development [28].
We present the relationship between chronic coronary artery disease progression and annual exposure to air pollution. Previous studies indicated the relationship between acute coronary syndromes (ACS) and short-term exposure to air pollutants. In the study of Jiang et al. [29], a greater admission risk from acute cardiovascular events was observed under high-ozone-pollution days. Dynamic processes that occur in acute coronary syndromes that involve plaque vulnerability, fibrinolytic function, and platelet activation responsible for acute event were found related to transient exposure to environmental air in the work of Chen et al. [30]. The relationship between weather changes and increased risk for ST-segment elevated acute coronary syndromes was presented by Biondi-Zoccai et al. [31]. The impact of short-term air pollution in industrialized and non-industrialized areas on ACS was found in Kuźma et al.’s analysis [32].
Our analysis points out the significance of nitrogen oxides for coronary artery lesion progression. Kim et al. [33] in the Meta-Air study revealed an association between NO2 air pollution and serum lipid measurements that can explain a possible link with atherosclerosis progression. Tian et al. [34] in their study presented an association with the transition from healthy status to incident stroke with each 5 μg/m3 increase in nitrogen oxides in the air. Dominski et al. [35] in their review pointed out the primary relationship between air pollution and respiratory diseases (mainly asthma and COPD), followed by cardiovascular outcomes (mainly stroke).
It is widely known that over 90% of the pollutant mass is represented by the mixture of ozone, nitrogen oxide, volatile organic compounds, and sulfur dioxide (SO2) [36]. Previous reviews presented the pathophysiological mechanisms that link atherosclerosis progression and explained increased acute coronary syndromes risk in patients exposed to PM2.5 [37]. Air’s polluted particles are claimed to interfere with endothelial function, activate cytokines, and derange lipid profiles to provoke atherosclerosis progression [38,39]. Moreover, the Zhang et al. [40] presented the increased risk for secondary acute coronary syndrome among patients with short- or long-term air pollution exposure, including nitrogen oxide.
Our study points out the significance of nitrogen oxide in comparison to previous reports that the main constituent of highly polluted air is particulate matter, which is claimed as responsible for the production of reactive oxygen radicals and the alteration of calcium levels [41]. Chen et al. [42] in their recent analysis found the relationship between NO exposure and subclinical atherosclerosis among young adults. Most importantly, Brunekreef et al. [43] in their meta-analysis found an association between PM2.5 and NO long-term exposure and increased all-cause mortality that was below current limits.
The presented results from our analysis correlate nitric oxide air pollution with chronic coronary syndrome, indicating progressive atherosclerotic plaque development. This novel finding broadens the scientific perspective on the pathophysiology of coronary culprit lesion and, in contrary to previous reports, suggests the relationship between meteorological factors and acute coronary syndrome risk [44,45]. More interestingly, Diaz-Chiron et al. [46] presented the results of myocardial infarct size in relation to nitric oxide air and pollution and inflammatory activation measured by the neutrophil-to-lymphocyte ratio. It is worth adding that in our analysis, none of the inflammatory indexes obtained from whole-blood-count analysis showed such a correlation.
Nitrogen oxides pollution was found to be influenced by road transport and the industrial combustion and processes sectors [47]. Kiesseweter et al. in their analysis attributed the contribution of increased NO2 concentrations to the high shares of diesel cars [48]. Newell et al. [49] in their meta-analysis found the association between nitric oxide and chronic obstructive pulmonary disease (COPD), followed by increased mortality.
In our analysis, the mean annual NO2 exposure was above the levels suggested by World Health Organization [50]. Its seasonal variations were presented by Wang et al. [51] as a possible morbidity risk factor related to maximal exposure, as Chen et al. found a relationship between hourly air pollution changes and risk for acute coronary syndromes [30]. The significant relationship between all-cause mortality and air pollution, especially when combined with increased concentrations of PM2.5 and nitric oxide, was presented in Liu et al.’s study [52].
Study Limitation
The study was performed on a relatively low-volume population and assumed that patients did not change their habitation place for a long period of time (except for short-term vacations), according to interview protocol. The study was based on one region in an EU country that accounts for 3.5 million citizens.
5. Conclusions
Coronary artery lesion progression can be related to chronic exposure to NO2 air pollution in patients living in cities in Poland.
Author Contributions
Conceptualization, T.U. and K.S. (Krzysztof Skotak); methodology, T.U., K.S.(Krzysztof Skotak), and K.S. (Krystian Szczepański); software, K.S. (Krystian Szczepański); validation, T.U., A.O.-W. and K.S. (Krzysztof Skotak); formal analysis, T.U.; investigation, T.U., A.O.-W., M.W. and J.S.; resources, T.U., A.O.-W., M.W. and J.S.; data curation, T.U., M.W. and J.S.; writing—original draft preparation, T.U.; writing—review and editing, T.U., A.O.-W., K.S.(Krystian Szczepański) and K.J.F.; visualization, T.U.; supervision, K.J.F., A.T. and M.J.; project administration, T.U. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Poznan University of Medical Sciences, Poznan, Poland (protocol code 55/20 from 16 January 2020), for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data will be available for 3 years following the publication after reasonable request is presented in e-mail correspondence to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Duggan J.P., Peters A.S., Trachiotis G.D., Antevil J.L. Epidemiology of Coronary Artery Disease. Surg. Clin. N. Am. 2022;102:499–516. doi: 10.1016/j.suc.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Cleven L., Krell-Roesch J., Nigg C.R., Woll A. The association between physical activity with incident obesity, coronary heart disease, diabetes and hypertension in adults: A systematic review of longitudinal studies published after 2012. BMC Public Health. 2020;20:726–741. doi: 10.1186/s12889-020-08715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonetto C., Rospleszcz S., Kaiser J.C., Furukawa K. Heterogeneity in coronary heart disease risk. Sci. Rep. 2022;12:10131–10140. doi: 10.1038/s41598-022-14013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malakar A.K., Choudhury D., Halder B., Paul P., Uddin A., Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell Physiol. 2019;234:16812–16823. doi: 10.1002/jcp.28350. [DOI] [PubMed] [Google Scholar]
- 5.Katta N., Loethen T., Lavie C.J., Alpert M.A. Obesity and Coronary Heart Disease: Epidemiology, Pathology, and Coronary Artery Imaging. Curr. Probl. Cardiol. 2021;46:100655–100681. doi: 10.1016/j.cpcardiol.2020.100655. [DOI] [PubMed] [Google Scholar]
- 6.Shufelt C.L., Pacheco C., Tweet M.S., Miller V.M. Sex-Specific Physiology and Cardiovascular Disease. Adv. Exp. Med. Biol. 2018;1065:433–454. doi: 10.1007/978-3-319-77932-4_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khamis R.Y., Ammari T., Mikhail G.W. Gender differences in coronary heart disease. Heart. 2016;102:1142–1149. doi: 10.1136/heartjnl-2014-306463. [DOI] [PubMed] [Google Scholar]
- 8.Gimenez M.R., Scheller B., Farah A., Ohlow M.-A., Mangner N., Weilenmann D., Wöhrle J., Cuculi F., Leibundgut G., Möbius-Winkler S., et al. Sex-specific inequalities in the use of drug-coated balloons for small coronary artery disease: A report from the BASKET-SMALL 2 trial. Clin. Res. Cardiol. 2023 doi: 10.1007/s00392-023-02249-6. epub ahead of print . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbanowicz T., Michalak M., Gąsecka A., Perek B., Rodzki M., Bociański M., Straburzyńska-Migaj E., Jemielity M. Postoperative Neutrophil to Lymphocyte Ratio as an Overall Mortality Midterm Prognostic Factor following OPCAB Procedures. Clin. Pract. 2021;11:587–597. doi: 10.3390/clinpract11030074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim C., Redberg R.F., Pavlic T., Eagle K.A. A systematic review of gender differences in mortality after coronary artery bypass graft surgery and percutaneous coronary interventions. Clin. Cardiol. 2007;30:491–495. doi: 10.1002/clc.20000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahrbaf M.A., Akbarzadeh M.A., Tabary M., Khaheshi I. Air Pollution and Cardiac Arrhythmias: A Comprehensive Review. Curr. Probl. Cardiol. 2021;46:100649–100667. doi: 10.1016/j.cpcardiol.2020.100649. [DOI] [PubMed] [Google Scholar]
- 12.de Bont J., Jaganathan S., Dahlquist M., Persson Å., Stafoggia M., Ljungman P. Ambient air pollution and cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. J. Intern. Med. 2022;291:779–800. doi: 10.1111/joim.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manisalidis I., Stavropoulou E., Stavropoulos A., Bezirtzoglou E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health. 2020;8:14. doi: 10.3389/fpubh.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin B.A., Brook R., Arden Pope C., 3rd Air pollution and cardiovascular disease. Curr. Probl. Cardiol. 2015;40:207–238. doi: 10.1016/j.cpcardiol.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Wang M., Zhou T., Song Q., Ma H., Hu Y., Heianza Y., Qi L. Ambient air pollution, healthy diet and vegetable intakes, and mortality: A prospective UK Biobank study. Int. J. Epidemiol. 2022;51:1243–1253. doi: 10.1093/ije/dyac022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf K., Hoffmann B., Andersen Z.J., Atkinson R.W., Bauwelinck M., Bellander T., Brandt J., Brunekreef B., Cesaroni G., Chen J., et al. Long-term exposure to low-level ambient air pollution and incidence of stroke and coronary heart disease: A pooled analysis of six European cohorts within the ELAPSE project. Lancet Planet Health. 2021;5:e620–e632. doi: 10.1016/S2542-5196(21)00195-9. [DOI] [PubMed] [Google Scholar]
- 17. [(accessed on 1 July 2023)]; Available online: https://powietrze.gios.gov.pl/pjp/maps/modeling.
- 18.European Air Quality, Copernicus, Atmosphere Monitoring Service. [(accessed on 27 December 2022)]. Available online: https://www.regional.atmosphere.copernicus.eu/
- 19. [(accessed on 19 July 2023)]. Available online: www.kobize.pl/en/article/national-database-on-greenhouse-gases-and-other-substances-emissions/id/1232/general-information.
- 20.Tagaris E., Sotiropoulou R.E.P., Gounaris N., Andronopoulos S., Vlachogiannis D. Effect of the Standard Nomenclature for Air Pollution (SNAP) categories on air quality over Europe. Atmosphere. 2015;6:1119–1128. doi: 10.3390/atmos6081119. [DOI] [Google Scholar]
- 21.Gawuc L., Szymankiewicz K., Kawicka D., Mielczarek E., Marek K., Soliwoda M., Maciejewska J. Bottom–Up Inventory of Residential Combustion Emissions in Poland for National Air Quality Modelling: Current Status and Perspectives. Atmosphere. 2021;12:1460. doi: 10.3390/atmos12111460. [DOI] [Google Scholar]
- 22.Kaminski J.W., Neary L., Struzewska J., McConnell J.C., Lupu A., Jarosz J., Toyota K., Gong S.L., Côté J., Liu X. GEM-AQ, an online global multiscale chemical weather modelling system: Model description and evaluation of gas phase chemistry processes. Atmos. Chem. Phys. 2008;8:3255–3281. doi: 10.5194/acp-8-3255-2008. [DOI] [Google Scholar]
- 23.Markevych I., Orlov N., Grellier J., Kaczmarek-Majer K., Lipowska M., Sitnik-Warchulska K., Mysak Y., Baumbach C., Wierzba-Łukaszyk M., Soomro M.H., et al. NeuroSmog: Determining the impact of air pollution on the developing brain: Project protocol. Int. J. Environ. Res. Public Health. 2022;19:310. doi: 10.3390/ijerph19010310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraszczyk M., Kaczmarek-Majer K., Hryniewicz O., Skotak K., Degorska A. Expert-in-the-loop Stepwise Regression and its Application in Air Pollution Modeling; Proceedings of the 2022 IEEE 11th International Conference on Intelligent Systems (IS); Warsaw, Poland. 12–14 October 2022; pp. 1–7. [Google Scholar]
- 25.Chen R., Yin P., Meng X., Liu C., Wang L., Xu X., Ross J.A., Tse L.A., Zhao Z., Kan H., et al. Fine Particulate Air Pollution and Daily Mortality. A Nationwide Analysis in 272 Chinese Cities. Am. J. Respir. Crit. Care Med. 2017;196:73–81. doi: 10.1164/rccm.201609-1862OC. [DOI] [PubMed] [Google Scholar]
- 26.Posadas-Sánchez R., Vargas-Alarcón G., Cardenas A., Texcalac-Sangrador J.L., Osorio-Yáñez C., Sanchez-Guerra M. Long-Term Exposure to Ozone and Fine Particulate Matter and Risk of Premature Coronary Artery Disease: Results from Genetics of Atherosclerotic Disease Mexican Study. Biology. 2022;11:1122. doi: 10.3390/biology11081122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang N., Mengersen K., Tong S., Kimlin M., Zhou M., Wang L., Yin P., Xu Z., Cheng J., Zhang Y., et al. Short-term association between ambient air pollution and lung cancer mortality. Environ. Res. 2019;179:108748–108758. doi: 10.1016/j.envres.2019.108748. [DOI] [PubMed] [Google Scholar]
- 28.Shakhov V., Materukhin A., Sokolova O., Koo I. Optimizing Urban Air Pollution Detection Systems. Sensors. 2022;22:4767. doi: 10.3390/s22134767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Y., Huang J., Li G., Wang W., Wang K., Wang J., Wei C., Li Y., Deng F., Baccarelli A., et al. Ozone pollution and hospital admissions for cardiovascular events. Eur. Heart J. 2023;44:1622–1632. doi: 10.1093/eurheartj/ehad091. [DOI] [PubMed] [Google Scholar]
- 30.Chen R., Jiang Y., Hu J., Chen H., Li H., Meng X., Ji J.S., Gao Y., Wang W., Liu C., et al. Hourly Air Pollutants and Acute Coronary Syndrome Onset in 1.29 Million Patients. Circulation. 2022;145:1749–1760. doi: 10.1161/CIRCULATIONAHA.121.057179. [DOI] [PubMed] [Google Scholar]
- 31.Biondi-Zoccai G., Frati G., Gaspardone A., Mariano E., Di Giosa A.D., Bolignano A., Giudici A.D., Calcagno S., Scappaticci M., Sciarretta S., et al. Impact of environmental pollution and weather changes on the incidence of ST-elevation myocardial infarction. Eur. J. Prev. Cardiol. 2021;28:1501–1507. doi: 10.1177/2047487320928450. [DOI] [PubMed] [Google Scholar]
- 32.Kuźma Ł., Wańha W., Kralisz P., Kazmierski M., Bachórzewska-Gajewska H., Wojakowski W., Dobrzycki S. Impact of short-term air pollution exposure on acute coronary syndrome in two cohorts of industrial and non-industrial areas: A time series regression with 6,000,000 person-years of follow-up (ACS—Air Pollution Study) Environ. Res. 2021;197:111154–111165. doi: 10.1016/j.envres.2021.111154. [DOI] [PubMed] [Google Scholar]
- 33.Kim J.S., Chen Z., Alderete T.L., Toledo-Corral C., Lurmann F., Berhane K., Gilliland F.D. Associations of air pollution, obesity and cardiometabolic health in young adults: The Meta-AIR study. Environ. Int. 2019;133:105180–105205. doi: 10.1016/j.envint.2019.105180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian F., Cai M., Li H., Qian Z.M., Chen L., Zou H., Zhang Z., Wang C., Xian H., McMillin S.E., et al. Air Pollution Associated with Incident Stroke, Poststroke Cardiovascular Events, and Death: A Trajectory Analysis of a Prospective Cohort. Neurology. 2022;99:e2474–e2484. doi: 10.1212/WNL.0000000000201316. [DOI] [PubMed] [Google Scholar]
- 35.Dominski F.H., Lorenzetti Branco J.H., Buonanno G., Stabile L., Gameiro da Silva M., Andrade A. Effects of air pollution on health: A mapping review of systematic reviews and meta-analyses. Environ. Res. 2021;201:111487–111501. doi: 10.1016/j.envres.2021.111487. [DOI] [PubMed] [Google Scholar]
- 36.Münzel T., Gori T., Al-Kindi S., Deanfield J., Lelieveld J., Daiber A., Rajagopalan S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart J. 2018;39:3543–3550. doi: 10.1093/eurheartj/ehy481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bevan G.H., Al-Kindi S.G., Brook R., Rajagopalan S. Ambient Air Pollution and Atherosclerosis: Recent Updates. Curr. Atheroscler. Rep. 2021;23:63–73. doi: 10.1007/s11883-021-00958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Utell M.J., Frampton M.W., Zareba W., Devlin R.B., Cascio W.E. Cardiovascular effects associated with air pollution: Potential mechanisms and methods of testing. Inhal. Toxicol. 2002;14:1231–1247. doi: 10.1080/08958370290084881. [DOI] [PubMed] [Google Scholar]
- 39.Liang S., Zhang J., Ning R., Du Z., Liu J., Batibawa J.W., Duan J., Sun Z. The critical role of endothelial function in fine particulate matter-induced atherosclerosis. Part Fibre Toxicol. 2020;17:61–85. doi: 10.1186/s12989-020-00391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H., Yi M., Wang Y., Zhang Y., Xiao K., Si J., Shi N., Sun L., Miao Z., Zhao T., et al. Air pollution and recurrence of cardiovascular events after ST-segment elevation myocardial infarction. Atherosclerosis. 2022;342:1–8. doi: 10.1016/j.atherosclerosis.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Ain N.U., Qamar S.U.R. Particulate Matter-Induced Cardiovascular Dysfunction: A Mechanistic Insight. Cardiovasc. Toxicol. 2021;21:505–516. doi: 10.1007/s12012-021-09652-3. [DOI] [PubMed] [Google Scholar]
- 42.Chen S.Y., Hwang J.S., Chan C.C., Wu C.F., Wu C., Su T.C. Urban Air Pollution and Subclinical Atherosclerosis in Adolescents and Young Adults. J. Adolesc. Health. 2022;71:233–238. doi: 10.1016/j.jadohealth.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Brunekreef B., Strak M., Chen J., Andersen Z.J., Atkinson R., Bauwelinck M., Bellander T., Boutron M.-C., Brandt J., Carey I., et al. Mortality and Morbidity Effects of Long-Term Exposure to Low-Level PM2.5, BC, NO2, and O3: An Analysis of European Cohorts in the ELAPSE Project. Res. Rep. Health Eff. Inst. 2021;208:1–127. [PMC free article] [PubMed] [Google Scholar]
- 44.Rus A.A., Mornoş C. The Impact of Meteorological Factors and Air Pollutants on Acute Coronary Syndrome. Curr. Cardiol. Rep. 2022;24:1337–1349. doi: 10.1007/s11886-022-01759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaluzna-Oleksy M., Aunan K., Rao-Skirbekk S., Kjellstrom T., Ezekowitz J.A., Agewall S., Atar D. Impact of climate and air pollution on acute coronary syndromes: An update from the European Society of Cardiology Congress 2017. Scand Cardiovasc. J. 2018;52:1–3. doi: 10.1080/14017431.2017.1405069. [DOI] [PubMed] [Google Scholar]
- 46.Díaz-Chirón L., Negral L., Megido L., Suárez-Peña B., Domínguez-Rodríguez A., Rodríguez S., Abreu-Gonzalez P., Pascual I., Moris C., Avanzas P. Relationship Between Exposure to Sulphur Dioxide Air Pollution, White Cell Inflammatory Biomarkers and Enzymatic Infarct Size in Patients With ST-segment Elevation Acute Coronary Syndromes. Eur. Cardiol. 2021;16:e50–e55. doi: 10.15420/ecr.2021.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coelho S., Ferreira J., Rodrigues V., Lopes M. Source apportionment of air pollution in European urban areas: Lessons from the ClairCity project. J. Environ. Manag. 2022;320:115899–115909. doi: 10.1016/j.jenvman.2022.115899. [DOI] [PubMed] [Google Scholar]
- 48.Kiesewetter G., Borken-Kleefeld J., Schöpp W., Heyes C., Thunis P., Bessagnet B., Terrenoire E., Gsella A., Amann M. Modelling NO2 concentrations at the street level in the GAINS integrated assessment model: Projections under current legislation. Atmos. Chem. Phys. 2014;14:813–829. doi: 10.5194/acp-14-813-2014. [DOI] [Google Scholar]
- 49.Newell K., Kartsonaki C., Lam K.B.H., Kurmi O. Cardiorespiratory health effects of gaseous ambient air pollution exposure in low and middle income countries: A systematic review and meta-analysis. Environ. Health. 2018;17:41–55. doi: 10.1186/s12940-018-0380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization . WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. World Health Organization; Geneva, Switzerland: 2021. [PubMed] [Google Scholar]
- 51.Wang W., Fecht D., Beevers S., Gulliver J. Predicting daily concentrations of nitrogen dioxide, particulate matter and ozone at fine spatial scale in Great Britain. Atmos. Pollut. Res. 2022;13:101506–101512. doi: 10.1016/j.apr.2022.101506. [DOI] [Google Scholar]
- 52.Liu C., Chen R., Sera F., Vicedo-Cabrera A.M., Guo Y., Tong S., Coelho M.S.Z.S., Saldiva P.H.N., Lavigne E., Matus P., et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019;381:705–715. doi: 10.1056/NEJMoa1817364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be available for 3 years following the publication after reasonable request is presented in e-mail correspondence to the corresponding author.