Abstract
The present systematic review addresses the influence of occupational exposures on prostate cancer risk. Eleven studies were analyzed for a range of occupational exposures, including but not limited to firefighting, physical activity, night shift work, chemical exposure, and solar ultraviolet radiation. The results of the review reveal that firefighters exposed to harmful substances, individuals engaged in physically strenuous work, and workers with chronic night shift routines showed an increased likelihood of developing prostate cancer. Moreover, the review identified an increased risk associated with exposure to certain chemicals, including alkylphenolic compounds and benzene-related substances. The evidence underscores the importance of considering the cumulative effect of multiple risk factors in a comprehensive risk assessment. However, the conclusions indicate the necessity for further research to deepen these relationships and develop more effective strategies for the prevention of prostate cancer.
Keywords: prostate cancer; occupational exposure; risk factors; systematic review; firefighters; night shift work; physical activity; chemical exposure; total worker health; predictive, preventive, personalized, participatory (4P) medicine
1. Introduction
Prostate cancer (PCa) is the second most common cancer among men worldwide and the fifth leading cause of cancer-related death [1]. The incidence of PCa varies geographically, with the highest rates found in developed countries such as the United States, Western Europe, and Australia [1,2].
The introduction of the prostate-specific antigen (PSA) test in the late 1980s led to a substantial increase in the detection of early-stage PCa cases [3]. However, concerns have been raised about the potential overdiagnosis and overtreatment of indolent tumors that may not pose a significant threat to a patient’s health [4]. As a result, guidelines for PCa screening have evolved over time, with a greater emphasis on shared decision making between patients and healthcare providers [5]. Overall, the 5-year survival rate for PCa is high, at around 98% [6]. However, survival rates can vary depending on the stage at diagnosis and other factors, such as age, race, and overall health [6].
The risk of developing PCa increases with age, particularly after the age of 50, with the majority of cases diagnosed in men over 65 years of age [6]. The incidence of PCa varies among racial and ethnic groups; African-American men and Caribbean men of African descent have a higher risk compared to white men, while Asian-American and Hispanic/Latino men have a lower risk [6]. Family history plays a significant role in the development of prostate cancer; men with a father or brother who had PCa are more than twice as likely to develop the disease [7]. This risk increases if several family members have been affected, particularly if they were diagnosed at a young age [8]. Genetic factors also contribute to risk, with several gene mutations linked to an increased risk of prostate cancer, including BRCA1, BRCA2, and HOXB13 [9,10,11]. Dietary habits have been associated with PCa risk, with a diet high in red and processed meats and dairy products and low in fruits and vegetables potentially increasing risk [12,13]. Obesity is another factor, as men who are overweight or obese have a slightly higher risk of developing prostate cancer, with the association being stronger for more aggressive forms of the disease [14]. Chronic inflammation of the prostate (prostatitis) may also increase the risk of PCa [15]. Hormone levels, such as higher levels of testosterone and insulin-like growth factor 1 (IGF-1), have been linked to an increased risk of PCa [16,17]. Lastly, environmental factors, including exposure to certain chemicals like pesticides and herbicides, may be associated with an increased risk of PCa [18,19].
Occupational exposure to certain chemicals, substances, and work environments may contribute to an increased risk of prostate cancer. Several studies have investigated the link between various occupations and PCa risk, with varying degrees of evidence. For instance, agricultural workers, particularly farmers and pesticide applicators, may have a higher risk of PCa due to their exposure to pesticides and herbicides [18]. A meta-analysis by Van Maele-Fabry et al. [19] reported a weak but significant association between pesticide exposure and PCa risk. Workers exposed to polycyclic aromatic hydrocarbons (PAHs), such as firefighters, aluminum smelter workers, and coke oven workers, may have an increased risk of PCa [20,21,22,23]. PAHs are generated during the incomplete combustion of organic materials and are known carcinogens. Men engaged in shift work, particularly those working night shifts, may also have an increased risk of PCa [24,25]. This may be due to disruptions in the circadian rhythm and the suppression of melatonin production, which has been hypothesized to have a protective effect against cancer development [26].
The International Agency for Research on Cancer (IARC) has already recognized several agents with limited evidence in humans as being potentially carcinogenic with respect to prostate cancer. These agents include androgenic (anabolic) steroids, arsenic and inorganic arsenic compounds, cadmium and cadmium compounds, occupational exposure as a firefighter, malathion, night shift work, consumption of red meat, the rubber manufacturing industry, thorium-232 and its decay products, and X and gamma radiation [27,28,29,30,31,32,33].
However, the evidence at hand, while compelling, necessitates further examination and substantiation through additional research to firmly establish a clear link between occupational exposure and PCa risk. The interplay between occupational risk factors and PCa is a multifaceted issue that is likely influenced by a host of other factors, including genetic predisposition, lifestyle elements, and the duration and intensity of exposure.
Therefore, additional research is paramount to not only illuminate these intricate connections but also to drive the creation and implementation of effective prevention strategies. It is critical to continue to deepen our understanding, and this urgency underscores the value and need for thorough, systematic reviews such as this one.
2. Materials and Methods
2.1. Search Strategy
The primary objective of this review was to evaluate case–control studies concerning workers with a confirmed diagnosis of PCa.
This review is exclusively centered on case–control studies due to their distinctive advantages when investigating rare outcomes, such as specific cancers linked to occupational factors. These studies offer an in-depth analysis of potential risk factors among individuals diagnosed with the disease, facilitating a more targeted investigation of occupational risks in relation to PCa. Moreover, in terms of both time and cost, case–control studies are typically more efficient compared to cohort studies.
A comprehensive search was performed in four electronic databases: PubMed, Web of Science, Scopus, and the Cochrane Library. The search strategy combined medical subject headings (MeSH) and relevant keywords. The search string comprised terms such as “occupational risk factors”, “work exposure”, “employment”, “prostate cancer”, and “neoplasms”. Additional filters were added to refine the search, which included human studies, English language, and peer-reviewed articles [34].
2.2. Selection Criteria
Included studies had to meet the following criteria: (1) original research articles, (2) case–control studies focusing on specific occupational risk factors and prostate cancer, and (3) studies published in English. Exclusion criteria were (1) reviews, case reports, commentaries, conference papers, and editorials, as well as (2) studies that did not provide sufficient data for analysis. The study selection followed the PRISMA guidelines [35].
In this systematic review, the definition of a ‘case’ was directly sourced from the original authors of each included study. These researchers specifically considered patients diagnosed with PCa based on their established criteria and methodologies.
2.3. Data Extraction
Data were extracted by two independent reviewers using a predefined extraction form. The data included authors, year of publication, study design, sample size, type of occupational exposure, assessment technique, and main findings. Disagreements between the reviewers were resolved through discussion or consultation with a third reviewer.
3. Results
3.1. Characteristics of Eligible Studies
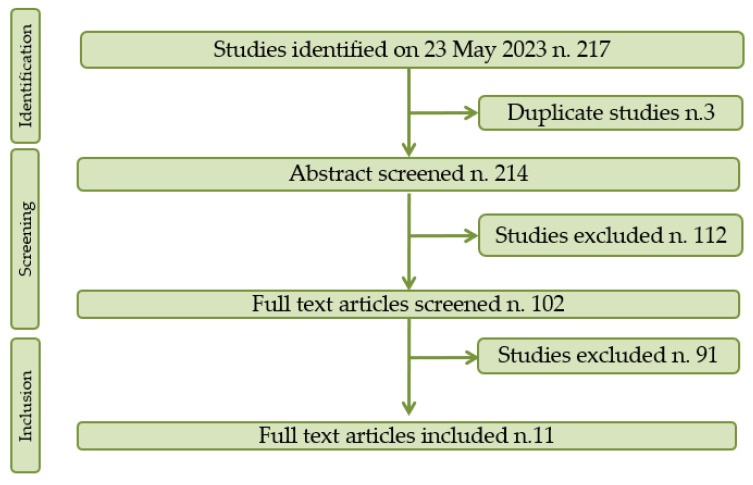
Following a search of the relevant databases, 214 documents were identified, of which 112 were excluded after review of the title and abstract and 91 of which were excluded after evaluation of the manuscript. Ultimately, eleven studies satisfied the inclusion criteria and were included in the systematic review. A flow chart depicting the study selection process is shown in Figure 1.
Figure 1.
PRISMA flow chart of the selection process.
3.2. Results of Eligible Studies
The data presented in the Table 1 concern various studies conducted in different countries, investigating the possible association between occupational exposure to different risk factors and PCa.
Table 1.
Studies evaluating association between occupational exposure and prostate cancer.
| Author (Year) | Country | Study Size | Occupation | Exposure | Assessment Technique | Adjustment Level | Effect Estimate OR 95%: CI Inf–CI Sup |
|
|---|---|---|---|---|---|---|---|---|
| Cases | Control | |||||||
| Blanc-Lapierre et al. (2017)a [36] | Canada | 328 | 400 | Various | Perceived stress at work | Questionnaire and interviews | Age, ethnicity, education level, family income, respondent status, and site-specific non-occupational and occupational covariates | 1.26 (0.95–1.68) |
| Blanc-Lapierre et al. (2017)b [37] | Canada | 1.933 | 1.994 | Various | Perceived stress at work before age 65 | Questionnaire and interviews | Age, ancestry, first-degree family history of PCa, family income, education, marital status, body mass index, type 2 diabetes, depression treated with medication, alcohol consumption, smoking, physical activity at work, and frequency of fruit and vegetable intake | 1.12 (1.04–1.20) |
| Blanc-Lapierre et al. (2018) [38] | Canada | 1920 | 1989 | Various | Benzene, toluene, xylene (BTX), and styrene | Canadian occupational classification | Age, ancestry, first-degree family history of PCa, household income, education, body mass index, type 2 diabetes, alcohol consumption, smoking, and physical activity at work | BTX 1.27 (1.05–1.35) Styrene 1.19 (0.74–1.91) |
| Doolan et al. (2014) [39] | Australia | 1.436 | 1.436 | Various | Physical activity at work | Questionnaire | Age, family history, and SEIFA index of economic resources | 1.15 (0.95–1.40) |
| Lee et al. (2020) [40] | U.S.A. | 1.119 | 63.912 | Firefighters | Firefighting | Firefighter registry | Age at cancer diagnosis | 1.36 (1.27–1.46) |
| Papantoniou et al. (2015) [25] | Spain | 1.095 | 1.388 | Various | Night shift work (NSW) | Nested data from the MCC-Spain study | Age, center, educational level, family history of prostate cancer, physical activity over the past decade, smoking status, past sun exposure, and daily meat consumption | Ever NSW 1.14 (0.94–1.37) Permanent NSW 1.10 (0.85–1.43) Rotating NSW 1.16 (0.95–1.46) |
| Peremiquel-Trillas et al. (2019) [41] | Spain | 1.095 | 1.480 | Various | Alkylphenolic compounds | Job exposure matrix (JEM) | Age, region, education level, BMI, smoking, alcohol consumption, occupational shift, exposure to pesticides, and exposure to solvents | 1.09 (0.89–1.33) |
| Peters et al. (2016) [42] | Canada | 1.638 | 1.697 | Outdoor workers | Solar ultraviolet radiation | Job exposure matrix (JEM) | Relationship status, smoking, education, and fruit and vegetable consumption | 0.68 (0.51–0.92) |
| Sharma et al. (2015) [43] | Canada | 114 | 2.824 | Farming job | Farm risk | Questionnarie | Residence and family history of cancer | Farming job 1.43 (0.70–2.92) Farm residence 1.86 (1.07–3.25) Insecticide 1.31 (0.55–3.15) Fungicides 0.98 (0.26–3.63) Both pesticides 2.23 (1.15–4.33) Radiation 1.97 (1.04–3.74) |
| Tsai et al. (2015) [44] | U.S.A. | 1.397 | 48.825 | Firefighters | Firefighting | Firefighter registry | Age at cancer diagnosis | 1.45 (1.25–1.69) |
| Wendeu-Foyet et al. (2018) [45] | France | 818 | 875 | Various | Night shift work (NSW) | Questionnaire and interviews | Age, family history of prostate cancer, race, and education level | Evening chronotype 1.83 (1.05–3.19) 20 yr permanent NSW 1.76 (1.13–2.75) >10 h NSW 4.64 (1.78–12.13) ≥6 consecutive nights 2.43 (1.32–4.47) |
A plurality of studies were conducted in Canada (5 of 11 studies, which equates to 45.5%) [36,37,38,42,43]. The United States contributed two studies, making up 18.2% of reviewed studies [40,44]. These studies primarily targeted firefighters, indicating an interest in the risks faced by this occupational group within the American context.
Spain also contributed two studies, constituting 18.2% of the total [25,41]. These studies investigated various risk factors, including night shift work and exposure to alkylphenolic compounds, highlighting diverse areas of interest within the Spanish research community.
Single studies were conducted in Australia and France, each representing 9.1% of the total studies. They explored various risk factors, from physical activity at work in Australia to different patterns of night shift work in France [39,45].
Regarding the study size, there was significant variation, with smaller studies consisting of as few as 114 cases [43] and larger studies including up to 1933 cases [37]. Similarly, the control groups varied widely in size, from 400 in one of the Canadian studies [37] to a considerable 63,912 [40] in one of the U.S. studies examining firefighters.
A diverse range of occupations and risk factors was studied, including perceived work stress, physical activity at work, exposure to certain chemicals, firefighting, night shift work, solar ultraviolet radiation, and farming and firefighting activities. Across the 11 studies under review, a variety of methods was employed to assess the outcome measure—specifically, the incidence of prostate cancer. In several studies, such as those conducted by Blanc-Lapierre et al. in 2017 and Doolan et al. in 2014, data were collected via questionnaires and/or interviews [36,37,39,43,45]. A study conducted by Blanc-Lapierre et al. in 2018 utilized the Canadian occupational classification [38]. The studies by Lee et al. in 2020 and Tsai et al. in 2015, which focused on firefighters, employed a firefighter registry to track the incidence of PCa [40,44]. The research conducted by Papantoniou et al. in 2015 took a different approach, using data nested from the MCC-Spain study, a comprehensive population-based case–control study [25]. This research project collected a wide range of data on various health outcomes, including prostate cancer and associated risk factors, enabling researchers to draw from a vast pool of pre-existing data. Meanwhile, the studies conducted by Peremiquel-Trillas et al. in 2019 and Peters et al. in 2016 utilized a job exposure matrix (JEM) [41,42].
The study conducted by Blanc-Lapierre et al. in 2017 [36] in Canada found a weak association between perceived stress at work and PCa, with an odds ratio (OR) of 1.26 (95% confidence interval (CI): 0.95–1.68). Their later study in 2017 [37], with a larger sample size, reinforced the same finding, with an OR of 1.12 (95% CI: 1.04–1.20), indicating a slightly increased risk of PCa with work stress before age 65. In 2018, the same group [38] reported that exposure to benzene, toluene, xylene (BTX), and styrene, common substances in various industrial processes, was associated with increased PCa risk (OR for BTX: 1.27; 95% CI: 1.05–1.35; OR for styrene: 1.19, 95% CI: 0.74–1.91).
In an Australian study by Doolan et al. (2014) [39], physical activity at work was evaluated, showing a marginally increased risk (OR: 1.15; 95% CI: 0.95–1.40). Meanwhile, studies in the USA and Canada focusing on firefighters [40,44] observed a more significant increase in PCa risk related to firefighting activities, with ORs of 1.36 (95% CI: 1.27–1.46) and 1.45 (95% CI: 1.25–1.69), respectively.
Two separate Spanish studies [25,41] exploring the impact of night shift work (NSW) and exposure to alkylphenolic compounds, respectively, reported ORs close to 1, indicating no significant increase in PCa risk. However, another study conducted in France by Wendeu-Foyet et al. (2018) [45] showed a more pronounced association between NSW and PCa, especially for long hours and consecutive nights.
Another interesting finding was reported in a Canadian study by Peters et al. (2016) [42], suggesting that solar ultraviolet radiation, a risk factor for skin cancer, could potentially lower the risk of PCa (OR: 0.68, 95% CI: 0.51–0.92) in outdoor workers.
A comprehensive study by Sharma et al. (2015) [43] explored several risk factors associated with farming jobs, including residence and pesticide use. The study revealed variable risk levels depending on the specific exposure, with farm residence and combined pesticide use showing higher ORs.
4. Discussion
In this systematic review, we investigated a collection of 11 studies from various geographical locations exploring the relationship between different occupational exposures and the risk of prostate cancer. The presented studies demonstrate the potential for various occupational exposures to influence PCa risk. Several studies focused on perceived stress at work; physical activity at work; and exposure to specific substances such as benzene, toluene, xylene (BTX), styrene, and alkylphenolic compounds. Others evaluated more specific occupational contexts, such as firefighting, farming, and night shift work or environmental exposure, like solar ultraviolet radiation. The outcome measures used to quantify the association between exposure and PCa also varied, most commonly employing a combination of questionnaires, interviews, occupational classifications, registries, and job exposure matrices (JEMs).
The review found a diverse range of associations between occupational exposure and PCa risk, supporting previous research suggesting that the work environment can have profound implications for employee health, especially with respect to chronic conditions like prostate cancer.
The studies conducted by Blanc-Lapierre et al. in 2017 highlighted perceived work stress as a potential risk factor for PCa [36,37]. This aligns with previous research linking chronic psychological stress to various health complications, including the initiation and progression of multiple cancer types. Such mechanisms might involve stress-induced changes in gene expression or immunosuppression, which may promote cancer development and progression [46,47,48,49].
Blanc-Lapierre et al. (2018) investigated the impact of exposure to benzene, toluene, xylene (BTX), and styrene [38]. In this particular study, BTX exposure was found to have a significant association with an increased risk of prostate cancer, reinforcing previous findings that underscored the potential carcinogenic effects of these substances. Long-term exposure to BTX, especially in high concentrations, has been linked to various health complications, including hematological malignancies and other cancers [50,51]. Extending this risk to PCa signifies an important direction for future occupational health research.
On the other hand, our study found no significant association between exposure to styrene and the risk of prostate cancer. This is particularly intriguing, given that styrene has been classified as a possible human carcinogen and is known to cause a range of health problems upon long-term exposure [52]. However, the effect of styrene exposure on the risk of PCa specifically remains ambiguous and requires further investigation. The findings reported by Blanc-Lapierre et al. (2018) contribute to this ongoing discourse, suggesting that the relationship might be more complex than initially anticipated [38].
The risk posed by firefighting as an occupation, as investigated in studies by Lee et al. (2020) and Tsai et al. (2015), has been previously documented [40,44]. Firefighters are exposed to a variety of carcinogens during the course of their duties, which could explain the elevated incidence of not only prostate cancer but also other types of cancer. This reinforces the importance of protective measures, including appropriate use of personal protective equipment and hygiene practices, to reduce exposure [28,33,53,54].
Interestingly, a study by Peters et al. (2016) found an inverse relationship between outdoor workers’ exposure to solar ultraviolet radiation and PCa [42]. This could potentially be explained by the protective role of vitamin D synthesized in skin upon exposure to ultraviolet-B radiation. Vitamin D has been implicated in the regulation of cell growth and immune function, potentially offering some protection against cancer [55,56,57,58].
According to Doolan et al. (2014) [39], physical activity at work was associated with PCa risk, with modestly increasing odds ratios, although the association was non-significant. While exercise is generally seen as protective against numerous health issues, the specific nature and conditions of physical work may lead to different effects, possibly related to stress and exhaustion factors [59,60].
Sharma et al. (2015) presented a complex set of findings within the farming context [43]. While residing on a farm was found to be associated with an elevated PCa risk, the role of exposure to specific agricultural chemicals, such as insecticides and fungicides, was less clear. Some types of pesticides were associated with increased risk, while others showed no significant effect. These results might be reflective of the heterogeneous nature of agricultural practices and exposures and underline the need for more specific investigations into the role of individual pesticides and other farming-related exposures in PCa development [61,62,63,64,65,66].
Moreover, night shift work, with its inherent disruption of circadian rhythms, has been hypothesized to contribute to cancer risk. The studies by Papantoniou et al. (2015) and Wendeu-Foyet et al. (2018) provide some support for this theory, especially among those with long-term and heavy exposure to night work [25,45]. Night work, particularly when it involves frequent or extended hours, can disrupt circadian rhythms and other biological processes, which, in turn, can have a variety of health impacts.
Circadian disruption due to night shift work has been hypothesized to contribute to increased cancer risk due to several potential mechanisms, including the suppression of melatonin production, which normally occurs at night. Melatonin is an endogenous hormone known for its antioxidant properties and its role in regulating the immune system, among other functions. Reduced melatonin levels have been associated with an increased risk of several types of cancer, including PCa [67,68,69,70,71]. A perturbative effect of shift work on testosterone serum levels was evidenced in a sample of male night shift workers [72]. Moreover, shift workers were found to have significantly lower levels of vitamin D [73].
Circadian disruption can also lead to sleep deprivation, which has been associated with inflammation, immune suppression, and other physiological changes that can potentially increase cancer risk [74,75,76].
Importantly, the study by Wendeu-Foyet et al. (2018) suggests that the duration and intensity of night work exposure may play a critical role in influencing PCa risk [45]. This implies that all night shift work may not carry the same level of risk and that the specific conditions of work (e.g., the frequency of night shifts, the number of consecutive night shifts, and the duration of exposure over a lifetime) can moderate this association.
However, as the mechanisms linking night shift work and cancer are still not fully understood, further research is needed to corroborate these findings and elucidate the biological pathways involved. Moreover, future studies should consider potential confounding factors, such as lifestyle behaviors or other occupational exposures, that could influence the observed associations [72,77,78,79,80].
The study by Peremiquel-Trillas et al. (2019) broadens our perspective to the industrial environment, looking at exposure to alkylphenolic compounds [41]. While these substances have endocrine-disrupting properties and therefore could theoretically contribute to PCa risk, the study did not find a significant association. This underlines the complexities of investigating associations with endocrine disruptors, which can depend on a multitude of factors, including timing, dosage, and individual susceptibility [81,82,83].
The findings of this systematic review, encompassing eleven studies, underscore the important implications of occupational exposures for PCa risk. The potential risks associated with specific chemical substances, such as BTX [38] and alkylphenolic compounds [41], as well as the links of physical activity at work [39], stress at work [36,37], and night shift work [25,45] with prostate cancer, offer new avenues for prevention and control strategies in occupational health.
For instance, the findings related to chemical exposures might encourage the implementation of better industrial hygiene practices and the development of safer alternatives to hazardous substances. Furthermore, the research on night shift work suggests the importance of maintaining healthy circadian rhythms, possibly by incorporating regular breaks and rotating schedules for shift workers.
The results also demonstrate the necessity for broader public health interventions. For instance, the significant findings related to firefighting activities [40,44] and farming practices [43] may imply the need for improved protective measures and regulations within these professions, including enhanced protective equipment and regular health screenings. It is well-established that early detection through PSA screenings can lead to better prognosis and outcomes for PCa patients [84]. Given the evidence suggesting increased risk of PCa in certain occupational groups, it seems prudent to consider enhanced surveillance strategies for these groups. Tailored screening, possibly starting at a younger age or with more frequent intervals, could be beneficial for individuals with prolonged exposure to known occupational risk factors. For instance, firefighters, who are repeatedly exposed to carcinogens during their service, might benefit from such a tailored approach [85]. Similarly, those involved in jobs with consistent exposure to chemicals like benzene or toluene could also be considered for enhanced surveillance [38]. However, it is essential that any recommendations for altered screening strategies be made in alignment with the broader clinical and epidemiological evidence and in consideration of potential overdiagnosis or overtreatment [86,87,88].
The studies under review underscore the fact that the relationship between occupational exposure and the risk of developing PCa is multifaceted, often interacting with other factors. For instance, age, ancestry, family history of prostate cancer, education, income, marital status, body mass index, type 2 diabetes, alcohol consumption, smoking, physical activity, and dietary habits have all been controlled for in various studies, highlighting their potential role in modulating the effect of occupational exposure on PCa risk [89,90].
Ancestry and family history of prostate cancer, in particular, are well-documented risk factors for prostate cancer, and their interaction with occupational exposure might influence individual susceptibility to this disease [91].
Body mass index (BMI) and type 2 diabetes, which are indicative of overall metabolic health, may also interact with occupational risk factors. Obesity is associated with chronic low-grade inflammation and hormonal changes, which may amplify the carcinogenic effects of certain occupational exposures [92,93]. Similarly, type 2 diabetes may increase PCa risk by influencing insulin and insulin-like growth factor pathways, potentially enhancing the carcinogenic impact of certain workplace exposures [94].
Lifestyle factors such as smoking and alcohol consumption, which can independently increase the risk of various cancers, might also interact with occupational exposure. For instance, they may contribute to a higher burden of overall oxidative stress and DNA damage, increasing the likelihood of carcinogenic transformations in the presence of occupational hazards [95,96].
Physical activity, either at work or during leisure time, and dietary habits, especially the intake of fruits and vegetables, may also modulate PCa risk; hence, their interaction with occupational exposure warrants further research [97,98,99,100].
Exposure to specific carcinogens, such as BTX, styrene, and alkylphenolic compounds, among others, may interact synergistically or additively with other factors, increasing the overall risk of PCa [101,102].
Moreover, understanding the mechanisms through which occupational exposures contribute to prostate cancer is fundamental to clarify the etiological pathways. First, firefighting exposures often involve contact with carcinogenic substances like PAHs.
PAHs are known to form DNA adducts, leading to mutations that may drive carcinogenesis in the prostate [103]. Moreover, firefighters are exposed to other carcinogens such as formaldehyde and acrolein, which can cause DNA crosslinking and contribute to genomic instability [104].
Physical activity has predominantly been associated with protective effects against various cancers due to its anti-inflammatory effects, improved insulin sensitivity, and modulation of sex hormone levels [105]. However, excessive physical activity might lead to hormonal imbalances, particularly in testosterone levels, which are implicated in prostate cancer progression [106].
Night shift work, with its associated circadian disruption, affects melatonin secretion. Melatonin is a potent antioxidant that also modulates the immune response and apoptosis [107]. Circadian disruption may lead to increased oxidative stress and immunosuppression, providing a favorable environment for prostate tumorigenesis [108].
Occupational exposure to chemicals like benzene, toluene, and xylene can impact various cellular processes. Benzene metabolites can induce oxidative stress, causing DNA strand breaks and chromosomal aberrations [109]. Chronic exposure to these chemicals may also cause epigenetic alterations, leading to abnormal gene expression patterns associated with prostate cancer [110].
Solar ultraviolet (UV) radiation indirectly affects prostate cancer through vitamin D synthesis. Vitamin D exerts its effects through the vitamin D receptor (VDR), which is involved in cell differentiation, proliferation, and apoptosis [111]. Altered VDR expression or vitamin D deficiency may contribute to prostate cancer progression by disrupting these cellular processes [112].
These molecular alterations collectively provide insight into the potential mechanisms linking occupational exposures to prostate cancer risk.
In relation to the potential biomarkers linked to occupational exposures and prostate cancer, some biomarkers of exposure should be used.
For instance, firefighters’ exposure to PAHs can lead to the formation of PAH-DNA adducts, serving as biomarkers for genotoxic exposure [103]. Workers exposed to chemical agents might display elevated levels of urinary benzene metabolites, which are indicative of organic solvent exposure, which could indirectly point to prostate cancer susceptibility [113]. Additionally, night shift workers experiencing circadian rhythm disruptions often present with altered melatonin levels, which have been postulated as a potential biomarker for various cancers, including prostate cancer [114]. For those exposed to UV radiation, serum vitamin D levels might act as a potential biomarker, considering the pivotal role of vitamin D in cellular processes and its association with prostate cancer [111].
An inherent limitation of our review is the variability in exposure classifications across the referenced studies. Some studies provided specific thresholds or criteria for classifying exposure, while others adopted a broader definition. This inconsistency can introduce potential uncertainties in interpreting the observed associations, especially when comparing results across different studies.
In light of the findings, it is abundantly clear that investing in the four Ps of medicine—predictive, preventive, personalized, and participatory—could significantly contribute to addressing the multifaceted issue of PCa risk in various occupations by harnessing the protective factors and mitigating the risk factors identified in the studies.
Moreover, a cumulative risk assessment approach that considers the combined effect of multiple risk factors rather than their individual impact can likely provide a more accurate measure of PCa risk. This shift in perspective from a single risk factor to a cumulative risk approach would not only improve our understanding of PCa epidemiology but also enhance our ability to design and implement effective prevention strategies.
Furthermore, the adoption of Total Worker Health® approaches aimed at improving the well-being of workers by eliminating modifiable risk factors could prove invaluable. This includes reducing exposure to harmful substances, promoting healthy habits like regular physical activity and a balanced diet, and ensuring regular health screenings.
Lastly, the findings emphasize the importance of continued epidemiological research to elucidate the relationships between various occupational exposures and PCa risk. Improved understanding of these relationships can inform preventive measures, aid in early detection efforts, and contribute to more effective treatments, thereby reducing the burden of prostate cancer.
5. Conclusions
This systematic review underscores the complex interplay of occupational exposures and other risk factors in the etiology of PCa. It is clear from the gathered evidence that certain occupational exposures, such as firefighting; physical activity; night shift work; and exposure to alkylphenolic compounds, solar ultraviolet radiation, and certain chemicals, can significantly influence the risk of developing prostate cancer. Importantly, the risk conferred by these factors can be further modulated by various individual and lifestyle factors, necessitating a comprehensive approach to risk assessment.
The evidence from these studies strongly advocates for the adoption of the four Ps of medicine, i.e., predictive, preventive, personalized, and participatory healthcare strategies.
Moreover, the review emphasizes the necessity of adopting a cumulative risk assessment approach, taking into account the combined effects of multiple risk factors rather than their individual contributions. Our review also highlights the importance of a Total Worker Health® approach with the aim of improving worker health by eliminating modifiable risk factors and promoting overall well-being.
Despite the significant strides made in understanding the occupational risk factors for PCa, there remain gaps in our knowledge, especially concerning the nuanced molecular mechanisms at play and the exact role of potential biomarkers in signaling exposure-related risks. Further studies are needed to delve deeper into exposure duration, intensity, and latency periods, which might modulate PCa risk. There is also a significant need to study synergistic interactions among various occupational exposures and to evaluate if certain subpopulations are more genetically predisposed to PCa when subjected to specific occupational risks.
Finally, further research is required to fully elucidate these relationships and the mechanisms underlying them. We hope that continued advancements in this area will pave the way for the development of more effective strategies for preventing and managing PCa, ultimately improving the lives of workers worldwide.
Author Contributions
Conceptualization, C.L.; methodology, C.L. and V.R.; validation, A.S.; formal analysis, C.L. and V.R.; data curation, C.L.; writing—original draft preparation, C.L., G.M. and G.S.; writing—review and editing, M.B. and D.C.; supervision, V.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Tran K.B., Lang J.J., Compton K., Xu R., Acheson A.R., Henrikson H.J., Kocarnik J.M., Penberthy L., Aali A., Abbas Q., et al. The Global Burden of Cancer Attributable to Risk Factors, 2010–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400:563–591. doi: 10.1016/S0140-6736(22)01438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyer V.A. Screening for Cervical Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2012;156:880. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg M.R., Broering J.M., Carroll P.R. Time Trends and Local Variation in Primary Treatment of Localized Prostate Cancer. JCO. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenton S.A.M., Veldhuijzen Van Zanten J.J.C.S., Duda J.L., Metsios G.S., Kitas G.D. Sedentary Behaviour in Rheumatoid Arthritis: Definition, Measurement and Implications for Health. Rheumatology. 2018;57:213–226. doi: 10.1093/rheumatology/kex053. [DOI] [PubMed] [Google Scholar]
- 6.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 7.Eeles R., Goh C., Castro E., Bancroft E., Guy M., Olama A.A.A., Easton D., Kote-Jarai Z. The Genetic Epidemiology of Prostate Cancer and Its Clinical Implications. Nat. Rev. Urol. 2014;11:18–31. doi: 10.1038/nrurol.2013.266. [DOI] [PubMed] [Google Scholar]
- 8.Ewing C.M., Ray A.M., Lange E.M., Zuhlke K.A., Robbins C.M., Tembe W.D., Wiley K.E., Isaacs S.D., Johng D., Wang Y., et al. Germline Mutations in HOXB13 and Prostate-Cancer Risk. N. Engl. J. Med. 2012;366:141–149. doi: 10.1056/NEJMoa1110000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The UKGPCS Collaborators. Leongamornlert D., Mahmud N., Tymrakiewicz M., Saunders E., Dadaev T., Castro E., Goh C., Govindasami K., Guy M., et al. Germline BRCA1 Mutations Increase Prostate Cancer Risk. Br. J. Cancer. 2012;106:1697–1701. doi: 10.1038/bjc.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The UKGPCS Collaborators. Kote-Jarai Z., Leongamornlert D., Saunders E., Tymrakiewicz M., Castro E., Mahmud N., Guy M., Edwards S., O’Brien L., et al. BRCA2 Is a Moderate Penetrance Gene Contributing to Young-Onset Prostate Cancer: Implications for Genetic Testing in Prostate Cancer Patients. Br. J. Cancer. 2011;105:1230–1234. doi: 10.1038/bjc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro E., Goh C., Olmos D., Saunders E., Leongamornlert D., Tymrakiewicz M., Mahmud N., Dadaev T., Govindasami K., Guy M., et al. Germline BRCA Mutations Are Associated With Higher Risk of Nodal Involvement, Distant Metastasis, and Poor Survival Outcomes in Prostate Cancer. JCO. 2013;31:1748–1757. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan J.M., Stampfer M.J., Ma J., Gann P.H., Gaziano J.M., Giovannucci E.L. Dairy Products, Calcium, and Prostate Cancer Risk in the Physicians’ Health Study. Am. J. Clin. Nutr. 2001;74:549–554. doi: 10.1093/ajcn/74.4.549. [DOI] [PubMed] [Google Scholar]
- 13.Aune D., Navarro Rosenblatt D.A., Chan D.S., Vieira A.R., Vieira R., Greenwood D.C., Vatten L.J., Norat T. Dairy Products, Calcium, and Prostate Cancer Risk: A Systematic Review and Meta-Analysis of Cohort Studies. Am. J. Clin. Nutr. 2015;101:87–117. doi: 10.3945/ajcn.113.067157. [DOI] [PubMed] [Google Scholar]
- 14.Cao Y., Ma J. Body Mass Index, Prostate Cancer–Specific Mortality, and Biochemical Recurrence: A Systematic Review and Meta-Analysis. Cancer Prev. Res. 2011;4:486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dennis L.K., Lynch C.F., Torner J.C. Epidemiologic Association between Prostatitis and Prostate Cancer. Urology. 2002;60:78–83. doi: 10.1016/S0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 16.Travis R.C., Spencer E.A., Allen N.E., Appleby P.N., Roddam A.W., Overvad K., Johnsen N.F., Olsen A., Kaaks R., Linseisen J., et al. Plasma Phyto-Oestrogens and Prostate Cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer. 2009;100:1817–1823. doi: 10.1038/sj.bjc.6605073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roddam A.W., Allen N.E., Appleby P., Key T.J., Ferrucci L., Carter H.B., Metter E.J., Chen C., Weiss N.S., Fitzpatrick A., et al. Insulin-like Growth Factors, Their Binding Proteins, and Prostate Cancer Risk: Analysis of Individual Patient Data from 12 Prospective Studies. Ann. Intern. Med. 2008;149:461–471. doi: 10.7326/0003-4819-149-7-200810070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alavanja M.C.R. Use of Agricultural Pesticides and Prostate Cancer Risk in the Agricultural Health Study Cohort. Am. J. Epidemiol. 2003;157:800–814. doi: 10.1093/aje/kwg040. [DOI] [PubMed] [Google Scholar]
- 19.Van Maele-Fabry G., Libotte V., Willems J., Lison D. Review and Meta-Analysis of Risk Estimates for Prostate Cancer in Pesticide Manufacturing Workers. Cancer Causes Control. 2006;17:353–373. doi: 10.1007/s10552-005-0443-y. [DOI] [PubMed] [Google Scholar]
- 20.Vinceti M., Venturelli M., Sighinolfi C., Trerotoli P., Bonvicini F., Ferrari A., Bianchi G., Serio G., Bergomi M., Vivoli G. Case-Control Study of Toenail Cadmium and Prostate Cancer Risk in Italy. Sci. Total Environ. 2007;373:77–81. doi: 10.1016/j.scitotenv.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Rapisarda V., Miozzi E., Loreto C., Matera S., Fenga C., Avola R., Ledda C. Cadmium Exposure and Prostate Cancer: Insights, Mechanisms and Perspectives. Front. Biosci. 2018;23:1687–1700. doi: 10.2741/4667. [DOI] [PubMed] [Google Scholar]
- 22.Parent M.-E., Siemiatycki J. Occupation and Prostate Cancer. Epidemiol. Rev. 2001;23:138–143. doi: 10.1093/oxfordjournals.epirev.a000779. [DOI] [PubMed] [Google Scholar]
- 23.Fajersztajn L., Veras M., Barrozo L.V., Saldiva P. Air Pollution: A Potentially Modifiable Risk Factor for Lung Cancer. Nat. Rev. Cancer. 2013;13:674–678. doi: 10.1038/nrc3572. [DOI] [PubMed] [Google Scholar]
- 24.Kubo T., Ozasa K., Mikami K., Wakai K., Fujino Y., Watanabe Y., Miki T., Nakao M., Hayashi K., Suzuki K., et al. Prospective Cohort Study of the Risk of Prostate Cancer among Rotating-Shift Workers: Findings from the Japan Collaborative Cohort Study. Am. J. Epidemiol. 2006;164:549–555. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 25.Papantoniou K., Castaño-Vinyals G., Espinosa A., Aragonés N., Pérez-Gómez B., Burgos J., Gómez-Acebo I., Llorca J., Peiró R., Jimenez-Moleón J.J., et al. Night Shift Work, Chronotype and Prostate Cancer Risk in the MCC-Spain Case-Control Study: MCC-Spain Case-Control Study. Int. J. Cancer. 2015;137:1147–1157. doi: 10.1002/ijc.29400. [DOI] [PubMed] [Google Scholar]
- 26.Stevens C., Hightower A., Buxbaum S.G., Falzarano S.M., Rhie S.K. Genomic, Epigenomic, and Transcriptomic Signatures of Prostate Cancer between African American and European American Patients. Front. Oncol. 2023;13:1079037. doi: 10.3389/fonc.2023.1079037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.IARC . In: A Review of Human Carcinogens. Centre International de Recherche sur le Cancer, editor. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer; Lyon, France: 2012. [Google Scholar]
- 28.IARC . In: Painting, Firefighting, and Shiftwork: This Publication Represents the Views and Expert Opinions of an IARC Monographs Working Group on the Evaluation of Carcinogenic Risks to Humans, Which Met in Lyon, 2–9 October 2007. International Agency for Research on Cancer, editor. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC; Lyon, France: 2010. [Google Scholar]
- 29.IARC . In: Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42: This Publication Represents the Views and Expert Opinions of an IARC Ad-Hoc Working Group on the Evaluation of the Carcinogenic Risks to Humans Which Met in Lyon, 10–18 March 1987. International Agency for Research on Cancer, Weltgesundheitsorganisation, editor. IARC Monographs on the Evaluation of Carcinogenic Risks of Chemicals to Humans Supplement; Internat; International Agency for Research on Cancer; Lyon, France: 1987. [Google Scholar]
- 30.IARC . In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 114; Red Meat and Processed Meat. International Agency for Research on Cancer, Weltgesundheitsorganisation, editor. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press; Lyon, France: 2018. [Google Scholar]
- 31.IARC . Some Organophosphate Insecticides and Herbicides. International Agency for Research on Cancer; Lyon, France: 2017. [PubMed] [Google Scholar]
- 32.IARC . Night Shift Work. International Agency for Research on Cancer; Lyon, France: 2021. [Google Scholar]
- 33.Demers P.A., DeMarini D.M., Fent K.W., Glass D.C., Hansen J., Adetona O., Andersen M.H., Freeman L.E.B., Caban-Martinez A.J., Daniels R.D., et al. Carcinogenicity of Occupational Exposure as a Firefighter. Lancet Oncol. 2022;23:985–986. doi: 10.1016/S1470-2045(22)00390-4. [DOI] [PubMed] [Google Scholar]
- 34.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A., Altman D.G., Booth A., et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanc-Lapierre A., Rousseau M.-C., Weiss D., El-Zein M., Siemiatycki J., Parent M.-É. Lifetime Report of Perceived Stress at Work and Cancer among Men: A Case-Control Study in Montreal, Canada. Prev. Med. 2017;96:28–35. doi: 10.1016/j.ypmed.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Blanc-Lapierre A., Rousseau M.-C., Parent M.-E. Perceived Workplace Stress Is Associated with an Increased Risk of Prostate Cancer before Age 65. Front. Oncol. 2017;7:269. doi: 10.3389/fonc.2017.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanc-Lapierre A., Sauvé J.-F., Parent M.-E. Occupational Exposure to Benzene, Toluene, Xylene and Styrene and Risk of Prostate Cancer in a Population-Based Study. Occup. Environ. Med. 2018;75:562–572. doi: 10.1136/oemed-2018-105058. [DOI] [PubMed] [Google Scholar]
- 39.Doolan G.W., Benke G., Giles G.G., Severi G., Kauppinen T. A Case Control Study Investigating the Effects of Levels of Physical Activity at Work as a Risk Factor for Prostate Cancer. Environ. Health Glob. Access Sci. Source. 2014;13:64. doi: 10.1186/1476-069X-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee D.J., Koru-Sengul T., Hernandez M.N., Caban-Martinez A.J., McClure L.A., Mackinnon J.A., Kobetz E.N. Cancer Risk among Career Male and Female Florida Firefighters: Evidence from the Florida Firefighter Cancer Registry (1981–2014) Am. J. Ind. Med. 2020;63:285–299. doi: 10.1002/ajim.23086. [DOI] [PubMed] [Google Scholar]
- 41.Peremiquel-Trillas P., Benavente Y., Martín-Bustamante M., Casabonne D., Pérez-Gómez B., Gómez-Acebo I., Oliete-Canela A., Diéguez-Rodríguez M., Tusquets I., Amiano P., et al. Alkylphenolic Compounds and Risk of Breast and Prostate Cancer in the MCC-Spain Study. Environ. Int. 2019;122:389–399. doi: 10.1016/j.envint.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Peters C.E., Demers P.A., Kalia S., Hystad P., Villeneuve P.J., Nicol A.-M., Kreiger N., Koehoorn M.W. Occupational Exposure to Solar Ultraviolet Radiation and the Risk of Prostate Cancer. Occup. Environ. Med. 2016;73:742–748. doi: 10.1136/oemed-2016-103567. [DOI] [PubMed] [Google Scholar]
- 43.Sharma M., Lawson J.A., Kanthan R., Karunanayake C., Hagel L., Rennie D., Dosman J.A., Pahwa P., Gordon J., Chen Y., et al. Factors Associated With the Prevalence of Prostate Cancer in Rural Saskatchewan: The Saskatchewan Rural Health Study. J. Rural. Health. 2016;32:125–135. doi: 10.1111/jrh.12137. [DOI] [PubMed] [Google Scholar]
- 44.Tsai R.J., Luckhaupt S.E., Schumacher P., Cress R.D., Deapen D.M., Calvert G.M. Risk of Cancer among Firefighters in California, 1988-2007. Am. J. Ind. Med. 2015;58:715–729. doi: 10.1002/ajim.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wendeu-Foyet M.G., Bayon V., Cénée S., Trétarre B., Rébillard X., Cancel-Tassin G., Cussenot O., Lamy P.-J., Faraut B., Ben Khedher S., et al. Night Work and Prostate Cancer Risk: Results from the EPICAP Study. Occup. Environ. Med. 2018;75:573–581. doi: 10.1136/oemed-2018-105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang T., Qiao Y., Xiang S., Li W., Gan Y., Chen Y. Work Stress and the Risk of Cancer: A Meta-Analysis of Observational Studies. Int. J. Cancer. 2019;144:2390–2400. doi: 10.1002/ijc.31955. [DOI] [PubMed] [Google Scholar]
- 47.Antoni M.H., Lutgendorf S.K., Cole S.W., Dhabhar F.S., Sephton S.E., McDonald P.G., Stefanek M., Sood A.K. The Influence of Bio-Behavioural Factors on Tumour Biology: Pathways and Mechanisms. Nat. Rev. Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cannizzaro E., Cirrincione L., Mazzucco W., Scorciapino A., Catalano C., Ramaci T., Ledda C., Plescia F. Night-Time Shift Work and Related Stress Responses: A Study on Security Guards. IJERPH. 2020;17:562. doi: 10.3390/ijerph17020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramaci T., Pellerone M., Ledda C., Rapisarda V. Health Promotion, Psychological Distress, and Disease Prevention in the Workplace: A Cross-Sectional Study of Italian Adults. Risk Manag. Healthc. Policy. 2017;10:167–175. doi: 10.2147/RMHP.S139756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masekameni M., Moolla R., Gulumian M., Brouwer D. Risk Assessment of Benzene, Toluene, Ethyl Benzene, and Xylene Concentrations from the Combustion of Coal in a Controlled Laboratory Environment. IJERPH. 2018;16:95. doi: 10.3390/ijerph16010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z., Liu X., Guo C., Zhang X., Zhang Y., Deng N., Lai G., Yang A., Huang Y., Dang S., et al. Hematological Effects and Benchmark Doses of Long-Term Co-Exposure to Benzene, Toluene, and Xylenes in a Follow-Up Study on Petrochemical Workers. Toxics. 2022;10:502. doi: 10.3390/toxics10090502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huff J., Infante P.F. Styrene Exposure and Risk of Cancer. Mutagenesis. 2011;26:583–584. doi: 10.1093/mutage/ger033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daniels R.D., Kubale T.L., Yiin J.H., Dahm M.M., Hales T.R., Baris D., Zahm S.H., Beaumont J.J., Waters K.M., Pinkerton L.E. Mortality and Cancer Incidence in a Pooled Cohort of US Firefighters from San Francisco, Chicago and Philadelphia (1950–2009) Occup. Environ. Med. 2014;71:388–397. doi: 10.1136/oemed-2013-101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daniels R.D., Bertke S., Dahm M.M., Yiin J.H., Kubale T.L., Hales T.R., Baris D., Zahm S.H., Beaumont J.J., Waters K.M., et al. Exposure-Response Relationships for Select Cancer and Non-Cancer Health Outcomes in a Cohort of Us Firefighters from San Francisco, Chicago and Philadelphia (1950-2009) Occup. Environ. Med. 2015;72:699–706. doi: 10.1136/oemed-2014-102671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilbert R., Martin R.M., Beynon R., Harris R., Savovic J., Zuccolo L., Bekkering G.E., Fraser W.D., Sterne J.A.C., Metcalfe C. Associations of Circulating and Dietary Vitamin D with Prostate Cancer Risk: A Systematic Review and Dose–Response Meta-Analysis. Cancer Causes Control. 2011;22:319–340. doi: 10.1007/s10552-010-9706-3. [DOI] [PubMed] [Google Scholar]
- 56.Travis R.C., Perez-Cornago A., Appleby P.N., Albanes D., Joshu C.E., Lutsey P.L., Mondul A.M., Platz E.A., Weinstein S.J., Layne T.M., et al. A Collaborative Analysis of Individual Participant Data from 19 Prospective Studies Assesses Circulating Vitamin D and Prostate Cancer Risk. Cancer Res. 2019;79:274–285. doi: 10.1158/0008-5472.CAN-18-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H., Stampfer M.J., Hollis J.B.W., Mucci L.A., Gaziano J.M., Hunter D., Giovannucci E.L., Ma J. A Prospective Study of Plasma Vitamin D Metabolites, Vitamin D Receptor Polymorphisms, and Prostate Cancer. PLoS Med. 2007;4:e103. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tio M., Andrici J., Cox M.R., Eslick G.D. Folate Intake and the Risk of Prostate Cancer: A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2014;17:213–219. doi: 10.1038/pcan.2014.16. [DOI] [PubMed] [Google Scholar]
- 59.Schultchen D., Reichenberger J., Mittl T., Weh T.R.M., Smyth J.M., Blechert J., Pollatos O. Bidirectional Relationship of Stress and Affect with Physical Activity and Healthy Eating. Br. J. Health Psychol. 2019;24:315–333. doi: 10.1111/bjhp.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stults-Kolehmainen M.A., Sinha R. The Effects of Stress on Physical Activity and Exercise. Sports Med. 2014;44:81–121. doi: 10.1007/s40279-013-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Béranger R., Pérol O., Bujan L., Faure E., Blain J., Cornet C.L., Flechon A., Charbotel B., Philip T., Schüz J., et al. Studying the Impact of Early Life Exposures to Pesticides on the Risk of Testicular Germ Cell Tumors during Adulthood (TESTIS Project): Study Protocol. BMC Cancer. 2014;14:563. doi: 10.1186/1471-2407-14-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koutros S., Alavanja M.C.R., Lubin J.H., Sandler D.P., Hoppin J.A., Lynch C.F., Knott C., Blair A., Freeman L.E.B. An Update of Cancer Incidence in the Agricultural Health Study. J. Occup. Environ. Med. 2010;52:1098–1105. doi: 10.1097/JOM.0b013e3181f72b7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ragin C., Davis-Reyes B., Tadesse H., Daniels D., Bunker C.H., Jackson M., Ferguson T.S., Patrick A.L., Tulloch-Reid M.K., Taioli E. Farming, Reported Pesticide Use, and Prostate Cancer. Am. J. Mens Health. 2013;7:102–109. doi: 10.1177/1557988312458792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ledda C., Bracci M., Lovreglio P., Senia P., Larrosa M., Martínez-Jarreta B., Rapisarda V. Pesticide Exposure and Gender Discrepancy in Breast Cancer. Eur. Rev. Med. Pharmacol. Sci. 2021;25:2898–2915. doi: 10.26355/eurrev_202104_25543. [DOI] [PubMed] [Google Scholar]
- 65.Ledda C., Cannizzaro E., Cinà D., Filetti V., Vitale E., Paravizzini G., Di Naso C., Iavicoli I., Rapisarda V. Oxidative Stress and DNA Damage in Agricultural Workers after Exposure to Pesticides. J. Occup. Med. Toxicol. 2021;16:1. doi: 10.1186/s12995-020-00290-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rapisarda V., Ledda C., Matera S., Fago L., Arrabito G., Falzone L., Marconi A., Libra M., Loreto C. Absence of t(14;18) Chromosome Translocation in Agricultural Workers after Short-Term Exposure to Pesticides. Mol. Med. Rep. 2017;15:3379–3382. doi: 10.3892/mmr.2017.6385. [DOI] [PubMed] [Google Scholar]
- 67.Meléndez-Fernández O.H., Liu J.A., Nelson R.J. Circadian Rhythms Disrupted by Light at Night and Mistimed Food Intake Alter Hormonal Rhythms and Metabolism. IJMS. 2023;24:3392. doi: 10.3390/ijms24043392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.James S.M., Honn K.A., Gaddameedhi S., Van Dongen H.P.A. Shift Work: Disrupted Circadian Rhythms and Sleep—Implications for Health and Well-Being. Curr. Sleep Med. Rep. 2017;3:104–112. doi: 10.1007/s40675-017-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davis S., Mirick D.K. Circadian Disruption, Shift Work and the Risk of Cancer: A Summary of the Evidence and Studies in Seattle. Cancer Causes Control. 2006;17:539–545. doi: 10.1007/s10552-005-9010-9. [DOI] [PubMed] [Google Scholar]
- 70.Manzella N., Bracci M., Strafella E., Staffolani S., Ciarapica V., Copertaro A., Rapisarda V., Ledda C., Amati M., Valentino M., et al. Circadian Modulation of 8-Oxoguanine DNA Damage Repair. Sci. Rep. 2015;5:13752. doi: 10.1038/srep13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bracci M., Ciarapica V., Zabaleta M.E., Tartaglione M.F., Pirozzi S., Giuliani L., Piva F., Valentino M., Ledda C., Rapisarda V., et al. BRCA1 and BRCA2 Gene Expression: Diurnal Variability and Influence of Shift Work. Cancers. 2019;11:1146. doi: 10.3390/cancers11081146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bracci M., Zingaretti L., Martelli M., Lazzarini R., Salvio G., Amati M., Milinkovic M., Ulissi A., Medori A.R., Vitale E., et al. Alterations in Pregnenolone and Testosterone Levels in Male Shift Workers. Int. J. Environ. Res. Public Health. 2023;20:3195. doi: 10.3390/ijerph20043195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martelli M., Salvio G., Santarelli L., Bracci M. Shift Work and Serum Vitamin D Levels: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health. 2022;19:8919. doi: 10.3390/ijerph19158919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amidi A., Wu L.M. Circadian Disruption and Cancer- and Treatment-Related Symptoms. Front. Oncol. 2022;12:1009064. doi: 10.3389/fonc.2022.1009064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McEwen B.S., Karatsoreos I.N. Sleep Deprivation and Circadian Disruption. Sleep Med. Clin. 2015;10:1–10. doi: 10.1016/j.jsmc.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shafi A.A., McNair C.M., McCann J.J., Alshalalfa M., Shostak A., Severson T.M., Zhu Y., Bergman A., Gordon N., Mandigo A.C., et al. The Circadian Cryptochrome, CRY1, Is a pro-Tumorigenic Factor That Rhythmically Modulates DNA Repair. Nat. Commun. 2021;12:401. doi: 10.1038/s41467-020-20513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strohmaier S., Devore E.E., Zhang Y., Schernhammer E.S. A Review of Data of Findings on Night Shift Work and the Development of DM and CVD Events: A Synthesis of the Proposed Molecular Mechanisms. Curr. Diab. Rep. 2018;18:132. doi: 10.1007/s11892-018-1102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fritschi L., Glass D.C., Heyworth J.S., Aronson K., Girschik J., Boyle T., Grundy A., Erren T.C. Hypotheses for Mechanisms Linking Shiftwork and Cancer. Med. Hypotheses. 2011;77:430–436. doi: 10.1016/j.mehy.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 79.Bracci M., Copertaro A., Ciarapica V., Barbaresi M., Esposito S., Albanesi A., Valentino M., Ledda C., Rapisarda V., Santarelli L. NOCTURNIN Gene Diurnal Variation in Healthy Volunteers and Expression Levels in Shift Workers. BioMed Res. Int. 2019;2019:7582734. doi: 10.1155/2019/7582734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bracci M., Zabaleta M.E., Tartaglione M.F., Ledda C., Rapisarda V., Santarelli L. Exosomal MiR-92a Concentration in the Serum of Shift Workers. Appl. Sci. 2020;10:430. doi: 10.3390/app10020430. [DOI] [Google Scholar]
- 81.De Falco M., Laforgia V. Combined Effects of Different Endocrine-Disrupting Chemicals (EDCs) on Prostate Gland. IJERPH. 2021;18:9772. doi: 10.3390/ijerph18189772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lacouture A., Lafront C., Peillex C., Pelletier M., Audet-Walsh É. Impacts of Endocrine-Disrupting Chemicals on Prostate Function and Cancer. Environ. Res. 2022;204:112085. doi: 10.1016/j.envres.2021.112085. [DOI] [PubMed] [Google Scholar]
- 83.Prins G.S. Endocrine Disruptors and Prostate Cancer Risk. Endocr. Relat. Cancer. 2008;15:649–656. doi: 10.1677/ERC-08-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith R.A., Andrews K.S., Brooks D., Fedewa S.A., Manassaram-Baptiste D., Saslow D., Brawley O.W., Wender R.C. Cancer Screening in the United States, 2018: A Review of Current American Cancer Society Guidelines and Current Issues in Cancer Screening: Cancer Screening in the US, 2018. CA Cancer J. Clin. 2018;68:297–316. doi: 10.3322/caac.21446. [DOI] [PubMed] [Google Scholar]
- 85.LeMasters G.K., Genaidy A.M., Succop P., Deddens J., Sobeih T., Barriera-Viruet H., Dunning K., Lockey J. Cancer Risk Among Firefighters: A Review and Meta-Analysis of 32 Studies. J. Occup. Environ. Med. 2006;48:1189–1202. doi: 10.1097/01.jom.0000246229.68697.90. [DOI] [PubMed] [Google Scholar]
- 86.Ledda C., Rapisarda V. Biomarkers and Human Biomonitoring in Occupational Medicine. Appl. Sci. 2020;10:6181. doi: 10.3390/app10186181. [DOI] [Google Scholar]
- 87.Ledda C., Rapisarda V. Occupational and Environmental Carcinogenesis. Cancers. 2020;12:2547. doi: 10.3390/cancers12092547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ledda C. Epidemiological Research on Occupational and Environmental Carcinogens. Int. J. Environ. Res. Public Health. 2021;18:2215. doi: 10.3390/ijerph18052215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hackshaw-Mcgeagh L.E., Penfold C.M., Walsh E., Donovan J.L., Hamdy F.C., Neal D.E., Jeffreys M., Martin R.M., Lane J.A. Physical Activity, Alcohol Consumption, BMI and Smoking Status before and after Prostate Cancer Diagnosis in the ProtecT Trial: Opportunities for Lifestyle Modification. Int. J. Cancer. 2015;137:1509–1515. doi: 10.1002/ijc.29514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Demoury C., Karakiewicz P., Parent M.-E. Association between Lifetime Alcohol Consumption and Prostate Cancer Risk: A Case-Control Study in Montreal, Canada. Cancer Epidemiol. 2016;45:11–17. doi: 10.1016/j.canep.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 91.Madersbacher S., Alcaraz A., Emberton M., Hammerer P., Ponholzer A., Schröder F.H., Tubaro A. The Influence of Family History on Prostate Cancer Risk: Implications for Clinical Management: Family History and Prostate Cancer Risk. BJU Int. 2011;107:716–721. doi: 10.1111/j.1464-410X.2010.10024.x. [DOI] [PubMed] [Google Scholar]
- 92.Scully T., Ettela A., LeRoith D., Gallagher E.J. Obesity, Type 2 Diabetes, and Cancer Risk. Front. Oncol. 2021;10:615375. doi: 10.3389/fonc.2020.615375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ledda C., Cinà D., Matera S., Mucci N., Bracci M., Rapisarda V. High HOMA-IR Index in Healthcare Shift Workers. Medicina. 2019;55:186. doi: 10.3390/medicina55050186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sousa A.P., Costa R., Alves M.G., Soares R., Baylina P., Fernandes R. The Impact of Metabolic Syndrome and Type 2 Diabetes Mellitus on Prostate Cancer. Front. Cell Dev. Biol. 2022;10:843458. doi: 10.3389/fcell.2022.843458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anand P., Kunnumakara A.B., Sundaram C., Harikumar K.B., Tharakan S.T., Lai O.S., Sung B., Aggarwal B.B. Cancer Is a Preventable Disease That Requires Major Lifestyle Changes. Pharm. Res. 2008;25:2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharifi-Rad M., Anil Kumar N.V., Zucca P., Varoni E.M., Dini L., Panzarini E., Rajkovic J., Tsouh Fokou P.V., Azzini E., Peluso I., et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shephard R.J. Physical Activity and Prostate Cancer: An Updated Review. Sports Med. 2017;47:1055–1073. doi: 10.1007/s40279-016-0648-0. [DOI] [PubMed] [Google Scholar]
- 98.Liu F., Wang J., Wu H.-L., Wang H., Wang J.-X., Zhou R., Zhu Z. Leisure Time Physical Activity and Risk of Prostate Cancer: A Dose-Response Meta-Analysis. Minerva Urol. Nephrol. 2018;70:152–161. doi: 10.23736/S0393-2249.17.02874-0. [DOI] [PubMed] [Google Scholar]
- 99.Rapisarda V., Cannizzaro E., Barchitta M., Vitale E., Cinà D., Minciullo F., Matera S., Bracci M., Agodi A., Ledda C. A Combined Multidisciplinary Intervention for Health Promotion in the Workplace: A Pilot Study. JCM. 2021;10:1512. doi: 10.3390/jcm10071512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rapisarda V., Loreto C., De Angelis L., Simoncelli G., Lombardo C., Resina R., Mucci N., Matarazzo A., Vimercati L., Ledda C. Home Working and Physical Activity during SARS-CoV-2 Pandemic: A Longitudinal Cohort Study. IJERPH. 2021;18:13021. doi: 10.3390/ijerph182413021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei C., Chen Y., Yang Y., Ni D., Huang Y., Wang M., Yang X., Chen Z. Assessing Volatile Organic Compounds Exposure and Prostate-Specific Antigen: National Health and Nutrition Examination Survey, 2001–2010. Front. Public Health. 2022;10:957069. doi: 10.3389/fpubh.2022.957069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rybicki B.A., Neslund-Dudas C., Nock N.L., Schultz L.R., Eklund L., Rosbolt J., Bock C.H., Monaghan K.G. Prostate Cancer Risk from Occupational Exposure to Polycyclic Aromatic Hydrocarbons Interacting with the GSTP1 Ile105Val Polymorphism. Cancer Detect. Prev. 2006;30:412–422. doi: 10.1016/j.cdp.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baird W.M., Hooven L.A., Mahadevan B. Carcinogenic Polycyclic Aromatic Hydrocarbon-DNA Adducts and Mechanism of Action. Environ. Mol. Mutagen. 2005;45:106–114. doi: 10.1002/em.20095. [DOI] [PubMed] [Google Scholar]
- 104.Ma Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moore S.C., Lee I.-M., Weiderpass E., Campbell P.T., Sampson J.N., Kitahara C.M., Keadle S.K., Arem H., Berrington De Gonzalez A., Hartge P., et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016;176:816. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grossmann M., Wittert G. Androgens, Diabetes and Prostate Cancer. Endocr.-Relat. Cancer. 2012;19:F47–F62. doi: 10.1530/ERC-12-0067. [DOI] [PubMed] [Google Scholar]
- 107.Reiter R.J., Mayo J.C., Tan D.-X., Sainz R.M., Alatorre-Jimenez M., Qin L. Melatonin as an Antioxidant: Under Promises but over Delivers. J. Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 108.Cadenas C., Van De Sandt L., Edlund K., Lohr M., Hellwig B., Marchan R., Schmidt M., Rahnenführer J., Oster H., Hengstler J.G. Loss of Circadian Clock Gene Expression Is Associated with Tumor Progression in Breast Cancer. Cell Cycle. 2014;13:3282–3291. doi: 10.4161/15384101.2014.954454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith M.T. Advances in Understanding Benzene Health Effects and Susceptibility. Annu. Rev. Public Health. 2010;31:133–148. doi: 10.1146/annurev.publhealth.012809.103646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Landi M.T., Bertazzi P.A., Shields P.G., Clark G., Lucier G.W., Garte S.J., Cosma G., Caporaso N.E. Association between CYP1A1 Genotype, MRNA Expression and Enzymatic Activity in Humans. Pharmacogenetics. 1994;4:242–246. doi: 10.1097/00008571-199410000-00002. [DOI] [PubMed] [Google Scholar]
- 111.Feldman D., Krishnan A.V., Swami S., Giovannucci E., Feldman B.J. The Role of Vitamin D in Reducing Cancer Risk and Progression. Nat. Rev. Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 112.Hendrickson W.K., Flavin R., Kasperzyk J.L., Fiorentino M., Fang F., Lis R., Fiore C., Penney K.L., Ma J., Kantoff P.W., et al. Vitamin D Receptor Protein Expression in Tumor Tissue and Prostate Cancer Progression. JCO. 2011;29:2378–2385. doi: 10.1200/JCO.2010.30.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rappaport S.M., Kim S., Lan Q., Vermeulen R., Waidyanatha S., Zhang L., Li G., Yin S., Hayes R.B., Rothman N., et al. Evidence That Humans Metabolize Benzene via Two Pathways. Environ. Health Perspect. 2009;117:946–952. doi: 10.1289/ehp.0800510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mirick D.K., Davis S. Melatonin as a Biomarker of Circadian Dysregulation. Cancer Epidemiol. Biomark. Prev. 2008;17:3306–3313. doi: 10.1158/1055-9965.EPI-08-0605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.