Abstract
A new immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA) based on the recombinant Epstein-Barr virus (EBV) matrix protein was developed. Compared to indirect immunofluorescence for the detection of IgM antibody to the EBV capsid antigen on clinical specimens, the sensitivity and specificity of the new IgM ELISA were 96 and 96%, respectively.
Primary Epstein-Barr virus (EBV) infection in childhood is usually mild but in later life usually manifests as infectious mononucleosis. Heterophile antibody detection is frequently used for confirmation of a diagnosis of acute primary EBV infection but leads to false-positive (5%) and false-negative (10 to 20%) results in adults (2). False-negative rates are even higher in children, especially those under 4 years old (8, 9). Slide agglutination tests for heterophile antibody, though simpler to perform, do not improve sensitivity or specificity (3, 8). Immunoglobulin M (IgM) enzyme-linked immunosorbent assays (ELISAs) may overcome these problems. The objective of this study was to develop and evaluate an EBV IgM ELISA based on a purified recombinant fusion protein of the 18-kDa EBV matrix protein.
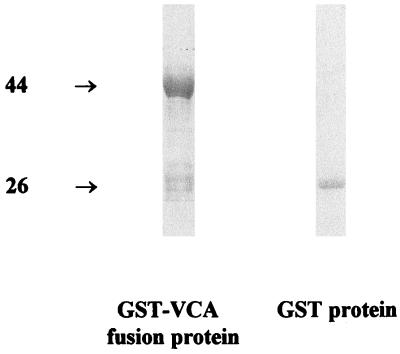
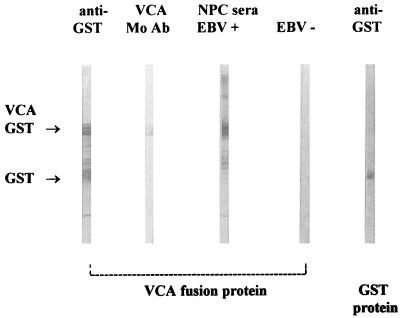
EBV BFRF3 mRNA from the lymphoid cell line B95-8 was reverse transcribed, and the cDNA was amplified by PCR with primers containing EcoRI/BamHI restriction sites at their 5′ ends (sense primer CGCGGATCCGCACGCCGGCTGCCC and antisense primer TGCCTTAAGCTGTTTCTTACGT) (9, 10). The PCR amplicons were ligated to the glutathione transferase (GST) gene on the EcoRI/BamHI-digested pGEX2T plasmid vector (Pharmacia, Uppsala, Sweden). Escherichia coli cells were transformed with the recombinant vector. The transformants were induced by the addition of the lactose analogue IPTG (isopropyl-β-d-thiogalactopyranoside), and the harvested fusion protein was purified by GST-glutathione affinity chromatography and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Two major bands, of 44 and 26 kDa, were seen, corresponding to the expected sizes of GST-virus capsid antigen (VCA) fusion protein and the GST protein, respectively (Fig. 1). Immunoblotting showed that both the 44- and 26-kDa proteins reacted with anti-GST rabbit antiserum. However, only the 44-kDa protein was reactive with the EBV-VCA p-18 antibody and the nasopharyngeal carcinoma (NPC) serum pool. An EBV antibody-negative serum reacted with neither protein (Fig. 2). These results indicated that the 18-kDa matrix protein was encoded as a GST fusion protein of BFRF3.
FIG. 1.
SDS-PAGE analysis of affinity-purified GST-VCA fusion protein. The induced bacterial cells containing fusion protein were lysed by 0.1% Triton X-100 detergent in Tris-HCl buffer with mild sonication, clarified by centrifugation at 10,000 × g for 15 min, and purified by GST-glutathione affinity chromatography with glutathione-Sepharose 4B. The bound GST fusion proteins were eluted with glutathione elution buffer. The samples were loaded onto an SDS–10% polyacrylamide gel and stained with 0.5% Coomassie blue to visualize the fusion protein and control parental GST protein (made in control cells carrying the parental pGEX vector only). Masses are shown on the left, in kilodaltons.
FIG. 2.
Characterization of VCA fusion protein by immunoblotting. Strips were stained with anti-GST antibody, antibody (Mo Ab) to VCA p-18 (rat antibody EBV.OT-15E, kindly supplied by J. M. Middeldorp), a serum pool from NPC patients, and EBV-negative serum.
Microtiter plates (96 wells) (Nunc) were coated overnight with 3 μg of purified fusion protein in coating buffer (0.05 M sodium carbonate-bicarbonate buffer, pH 9.6). After a wash (0.05% Tween 20 in phosphate-buffered saline), the wells were blocked with 2% bovine serum albumin (37°C; 1 h) and filled with 100 μl of patient’s serum (diluted 1 in 100) and diluted alkaline phosphatase-conjugated goat anti-human IgM antibody (BioSource International, Camarillo, Calif.), with a 1-h incubation at 37°C and a wash each time. Finally, 100 μl of phosphatase substrate (Sigma Chemical Co., St. Louis, Mo.) was added, and colour development was stopped after 30 min by addition of 1 M sodium hydroxide. The absorbance was measured at 405 nm. Blank wells and negative and positive control sera were included on each plate. Cutoff values were defined as twice the optical density of the mean of the negative controls. To avoid false-negative IgM results due to competition from high concentrations of EBV-specific IgG antibodies and false-positive results due to rheumatoid factor (6), the patient sera were treated with goat anti-human IgG serum (GullSORB; Gull Laboratories, Salt Lake City, Utah) prior to testing.
Heterophile antibodies were determined by a commercial latex agglutination kit (Monolex; Gull Laboratories) according to the manufacturer’s instructions.
One hundred eighteen sequential patient sera submitted to the Department of Microbiology of the Queen Mary Hospital, Hong Kong, for diagnosis of primary EBV infection were used for this study. One serum sample, from a 2-month-old child, was excluded from further analysis because passive transfer of maternal antibody could potentially confound the serological interpretation of the EBNA and VCA IgG antibody profiles. Comparisons were performed on the remaining 117 sera.
Two standard serological tests for diagnosing recent primary EBV infection, (i) the VCA IgG-EBNA antibody profile and (ii) detection of VCA IgM by indirect immunofluorescence (IF) were used as our reference assays for evaluating the new VCA IgM ELISA test (2, 8). Primary infection was defined as the presence of IgM antibody to EBV-VCA or the presence of IgG antibody to EBV-VCA in the absence of antibody to EBNA. Past infection was defined as the presence of antibodies to both EBV-VCA and EBNA (with no significant changes in antibody titer in subsequent sera, when available). Seronegative individuals were those who had no antibodies against either EBV-VCA or EBNA.
It has been reported that 24% of children with EBV infection many months previously may still be EBNA antibody negative and would therefore be wrongly diagnosed as having had recent primary EBV infection. Conversely, around 7% of children may have detectable EBNA antibodies within 2 weeks of primary EBV infection and therefore be falsely diagnosed as having had past EBV infection (8). Furthermore, 12 to 17% of children with primary EBV infection may have undetectable VCA IgM antibody by IF. In the absence of a single serological “gold standard” for diagnosing primary EBV infections in children, we have compared the new VCA IgM ELISA with each of these reference tests independently and also against a panel of sera giving concordant results by both reference assays.
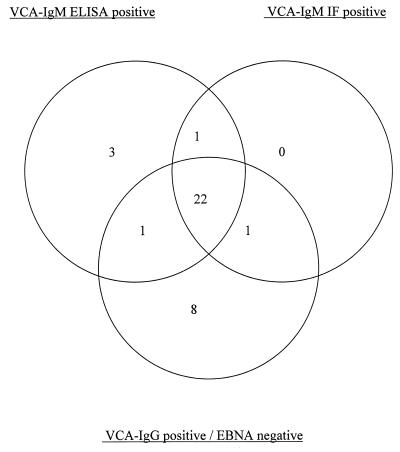
The correlations of the three assays with each other and with the heterophile antibody test are shown in Table 1. The reference assays were concordant for 107 specimens and discordant (diagnosed as recent infection by one reference assay only) for 10 patients. One or more of the tests indicative of recent infection (two reference tests or the new IgM ELISA) were positive for 36 sera, and the overlaps of their reaction profiles are shown in Fig. 3.
TABLE 1.
Evaluation of IgM ELISA based on recombinant EBV 18-kDa matrix protein antigen for the diagnosis of primary EBV infection and comparison with reference assays and heterophile antibody tests
| Results of reference tests
|
No. of sera | Consensus interpretation of reference test results | No. (%) of sera positive by:
|
|||
|---|---|---|---|---|---|---|
| EBV VCA IgM IF | EBV VCA IgG IF | EBNA antibody | EBV VCA IgM ELISA | Heterophile antibody test | ||
| Concordant results | ||||||
| Positive | Positive | Negative | 23 | Recent primary EBV infection | 22 (96) | 9 (39) |
| Negative | Positive | Positive | 56 | Past EBV infection | 3 (5) | 1 (2) |
| Negative | Negative | Negative | 28 | No evidence of recent or past EBV infection | 0 (0) | 1 (4) |
| Discordant results | ||||||
| Negative | Positive | Negative | 9 | Discordant results in reference testsa | 1 (11) | 1 (11) |
| Positive | Positive | Positive | 1 | Discordant results in reference testsb | 1 | 0 |
VCA IgG-EBNA profile suggested recent infection, but no VCA IgM was detected by IF test.
VCA IgM IF test indicated recent infection, but positive EBNA antibody suggested past infection.
FIG. 3.
Reaction profiles of 36 sera, giving results suggestive of recent primary EBV infection by one or more tests (VCA IgM IF, VCA IgM ELISA, and VCA IgG-EBNA profiling).
With the VCA IgM IF as the gold standard, the sensitivity of the VCA IgM ELISA was similar (96%; P = 1.0) while that of the heterophile antibody test was significantly lower (38%; P = 0.009). The specificities of the two tests were similar, at 96 and 97%, respectively. In comparison to the VCA IgG-EBNA profile of acute infection, the sensitivity of the VCA IgM ELISA was 72% (P = 0.2) and that of the heterophile antibody test was 31% (P = 0.0003); the specificities were 95 and 97%, respectively. Excluding specimens with discordant reactivities in the two reference assays from the analysis, the sensitivity and specificity of the IgM ELISA on the remaining 107 sera were 96 and 96%, respectively.
The sensitivity and specificity of the VCA IgM IF test compared to the VCA IgG-EBNA profile of acute infection were 72 and 99%, respectively. Conversely, with the VCA IgM IF taken as the gold standard, the sensitivity and specificity of the other reference test (VCA IgG-EBNA antibody profile) were 96 and 90%, respectively.
For patients older than 12 years, all five VCA IgM IF-positive sera were positive by the VCA IgM ELISA and the heterophile antibody assay. However, while 17 of 18 (94%) sera from VCA IgM IF-positive sera of children younger than 12 years were VCA IgM ELISA positive, only 5 (28%) of them had detectable heterophile antibody (P = 0.0002).
In agreement with previous reports (8), our results showed that only 36% of children under 12 years with primary EBV infection (VCA IgM IF positive) had detectable heterophile antibody. The majority of primary EBV infections in Hong Kong and other parts of Asia occur in childhood, with 60% of children in Hong Kong being infected by the age of 2 years (1). Thus, heterophile antibody tests have limited use in the serodiagnosis of primary EBV infections in this region.
The preliminary evaluation of the VCA IgM ELISA suggests that it may provide a sensitive and convenient alternative for diagnosis of primary EBV infection in children and that larger “in-use” studies are warranted. It correlates well with the detection of VCA IgM by IF tests but has lower sensitivity than VCA IgG-EBNA profiling. The possibility of false-positive results when profiles of VCA IgG-EBNA antibodies are used for diagnosing recent primary infection has been alluded to previously. Excluding sera giving discordant results in the two reference assays, the sensitivity and specificity of the new IgM ELISA are excellent.
Three patients with no evidence of recent EBV infection by either of the reference assays had detectable VCA IgM by ELISA and are considered false positives. Two of these sera were available for further analysis and were shown to have high-affinity IgG antibody (an independent marker of past infection) (4). It is unlikely that any of these IgM-positive sera were due to rheumatoid factor, because IgG adsorption procedures were carried out on the sera. Further, none of the 12 sera containing rheumatoid factor and seropositive for EBV VCA IgG reacted in the IgM ELISA. The false positives could be the result of EBV reactivation due to cross-reactions with IgM of other viruses or to the reappearance of EBV IgM due to polyclonal activation induced by cytomegalovirus, adenovirus, hepatitis A virus, Toxoplasma gondii, rubella virus, or other as yet unknown agents that produce an infectious mononucleosis-like disease (5, 7). Whereas none of the six sera containing toxoplasma IgM antibody were positive in the EBV IgM ELISA, three of five cytomegalovirus IgM-positive sera gave a positive result. The false-positive EBV IgM results may be cross-reacting cytomegalovirus IgM. Alternatively, contaminating E. coli protein in our antigen preparation may have contributed to some of the false-positive reactivity. Some E. coli proteins (approximately 200 kDa) copurify with the fusion protein during glutathione-Sepharose 4B affinity chromatography, as can be seen in Fig. 2.
The presently available reference tests for recent EBV infection, i.e., the VCA IgG-EBNA profile and the VCA IgM IF test, are immunofluorescence-based assays that are technically demanding, subjective in interpretation, and not readily automated. IgM ELISA methods for diagnosis of primary EBV infection are adaptable to large-scale testing, provide objective endpoints, and are technically less demanding. Furthermore, EBV-specific IgM antibody tests would be useful in diagnosing primary infection in children below the age of 6 months (when maternal antibody may give rise to detectable EBNA antibody and lead to a false diagnosis of past EBV infection) and in immunosuppressed patients who are often unable to mount an EBNA-1 antibody response, making differentiation of acute and past infections difficult. A number of commercially available ELISAs for the detection of EBV-specific IgM have recently been evaluated and shown to possess varying degrees of sensitivity and specificity (11). The VCA IgM ELISA we describe would be a useful alternative to presently available ELISAs for the investigation of acute EBV disease.
Acknowledgments
This study was partly supported by the Croucher Foundation and the Industrial Support Fund of the government of Hong Kong.
REFERENCES
- 1. Chan, K. H. Unpublished data.
- 2.Evans A S, Niederman J C, Cenabre L C, West B, Richards V A. A prospective evaluation of heterophile and Epstein-Barr virus-specific IgM antibody tests in clinical and subclinical infectious mononucleosis: specificity and sensitivity of the tests and persistence of antibody. J Infect Dis. 1975;132:546–554. doi: 10.1093/infdis/132.5.546. [DOI] [PubMed] [Google Scholar]
- 3.Fleisher G, Lennette E T, Henle G, Henle W. Incidence of heterophile antibody responses in children with infectious mononucleosis. J Pediatr. 1979;94:723–728. doi: 10.1016/s0022-3476(79)80138-9. [DOI] [PubMed] [Google Scholar]
- 4.Gray J J, Wreghitt T G. Immunoglobulin G avidity in Epstein-Barr virus infections in organ transplant recipients. Serodiagn Immunother Infect Dis. 1989;3:389–393. [Google Scholar]
- 5.Harnett G B, Palmer C A, Bucens M R. A modified immunofluorescence test for Epstein-Barr virus-specific IgM antibody. J Virol Methods. 1985;12:25–30. doi: 10.1016/0166-0934(85)90004-7. [DOI] [PubMed] [Google Scholar]
- 6.Henle G, Lenette E T, Alspaugh M A, Henle W. Rheumatoid factor as a course of positive reaction in tests for Epstein-Barr virus-specific IgM antibodies. Clin Exp Immunol. 1979;36:415–422. [PMC free article] [PubMed] [Google Scholar]
- 7.Matheson B A, Chisholm S M, Ho-Yen D O. Assessment of rapid ELISA test for detection of Epstein-Barr virus infection. J Clin Pathol. 1990;43:691–693. doi: 10.1136/jcp.43.8.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumaya C V, Ench Y. Epstein-Barr virus infectious mononucleosis in children. II. Heterophile antibody and viral-specific response. Pediatrics. 1985;75:1011–1019. [PubMed] [Google Scholar]
- 9.Van Grunsven W M J, Van Heerde E C, De Haard H J W, Spaan W J M, Middeldorp J M. Gene mapping and expression of two immunodominant Epstein-Barr virus capsid proteins. J Virol. 1993;67:3908–3916. doi: 10.1128/jvi.67.7.3908-3916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Grunsven W M J, Spaan W J M. Localization and diagnostic application of immunodominant domains of the BFRF3-encoded Epstein-Barr virus capsid protein. J Infect Dis. 1994;170:13–19. doi: 10.1093/infdis/170.1.13. [DOI] [PubMed] [Google Scholar]
- 11.Weber B, Brunner M, Preiser W, Doerr H W. Evaluation of 11 enzyme immunoassays for the detection of immunoglobulin M antibodies to Epstein-Barr virus. J Virol Methods. 1996;57:87–93. doi: 10.1016/0166-0934(95)01971-5. [DOI] [PubMed] [Google Scholar]